Abstract
Renal medullary blood flow is relatively insensitive to angiotensin II (Ang II)-induced vasoconstriction, due partly to AT1-mediated release of nitric oxide and/or prostaglandins. AT2-receptor activation appears to blunt AT1-mediated vasodilatation within the medullary circulation. This could affect long-term efficacy of antihypertensive pharmacotherapies targeting the renin/angiotensin system, particularly in Ang II-dependent forms of hypertension.
We tested the effects of AT1- and AT2-receptor blockade on basal cortical and medullary laser Doppler flux (CLDF and MLDF), and on responses to renal arterial infusion of Ang II, in rats with 2 kidney, 1 clip (2K1C) hypertension and sham-operated controls. Studies were carried out in thiobutabarbital (175 mg kg−1, i.p.) anaesthetised rats, 4 weeks after clipping, or sham surgery (n=6 in each of eight groups).
Candesartan (10 μg kg−1 h−1, intravenous (i.v.)) reduced mean arterial pressure (∼17%) and increased CLDF (∼24%), similarly in both sham and 2K1C rats, but did not significantly affect MLDF. PD123319 (1 mg kg−1 h−1, i.v.) increased basal MLDF (19%) in 2K1C but not sham rats, without significantly affecting other variables.
In sham rats, renal arterial infusion of Ang II (1–100 ng kg−1 min−1) dose dependently decreased CLDF (up to 44%), but did not significantly affect MLDF. These effects were markedly blunted in 2K1C rats. After PD123319, Ang II dose dependently increased MLDF (up to 38%) in sham but not 2K1C rats. Candesartan abolished all effects of Ang II, including those seen after PD123319.
Our data indicate that AT1 receptors mediate medullary vasodilatation, which is opposed by AT2-receptor activation. In 2K1C hypertension, AT2-receptor activation tonically constricts the medullary circulation.
Keywords: Angiotensin receptors, kidney, laser Doppler flowmetry, rat, hypertension–renal, renal circulation
Introduction
Angiotensin II (Ang II) is the main effector peptide of the renin–angiotensin system (RAS), acting at two main receptor subtypes: type 1 (AT1) and type 2 (AT2) (Carey et al., 2000). In rats and rabbits, infusions of Ang II reduce total renal blood flow (RBF) and cortical perfusion measured by laser Doppler flowmetry (cortical laser Doppler flux; CLDF). However, medullary perfusion is relatively insensitive to the vasoconstrictor effects of Ang II under most experimental conditions (Cupples et al., 1988; Parekh et al., 1996; Walker et al., 1999; Evans et al., 2000; 2004; Badzynska et al., 2002; 2003; Oliver et al., 2002; Rajapakse et al., 2002; Duke et al., 2003). The explanation for these observations seems to be that, although AT1-receptor activation causes vasoconstriction within vascular elements controlling medullary blood flow, it can also cause vasodilatation. This latter effect appears to be mediated by release of nitric oxide and/or prostaglandins (Zou et al., 1998; Oliver et al., 2002; Rajapakse et al., 2002; Badzynska et al., 2003; Evans et al., 2004). The contributions of AT2 receptors to the control of medullary blood flow are less clear. However, our recent observations in anaesthetised rabbits suggest that AT2-receptor activation counteracts AT1-mediated vasodilatation in the renal medulla, as the AT2 antagonist PD123319 revealed dose-dependent increases in medullary laser Doppler flux (MLDF) during renal arterial infusion of Ang II (Duke et al., 2003). This observation is at odds with the conventional view that AT2 receptors mediate vasodilatation (Carey et al., 2000; Bautista et al., 2001; Widdop et al., 2002).
There is now strong evidence that the level of renal medullary blood flow has a major impact on urine flow and sodium excretion, and so the long-term control of blood pressure (Cowley, 1997; Cowley et al., 2003; Mattson, 2003; Pallone et al., 2003). Thus, interactions between AT1 and AT2 receptors in the control of medullary blood flow could have important implications for the long-term efficacy of antihypertensive therapies targeting the RAS, and for the ongoing debate on the relative merits of AT1-receptor blockade versus Ang-converting enzyme inhibition, or their combination (Laverman et al., 2004). In our previous study in anaesthetised rabbits, neither candesartan nor PD123319 altered basal MLDF, suggesting that neither AT1 nor AT2 receptors contribute significantly to setting the basal level of medullary blood flow in normotensive animals (Duke et al., 2003). However, this might not be the case in hypertension, since intrarenal and/or circulating levels of Ang II are increased in most forms of hypertension, particularly that of renovascular origin (Navar et al., 2002). Therefore, the chief aim of the present study was to determine whether endogenous Ang II, acting at AT1 and AT2 receptors, contributes to the regulation of medullary blood flow in a renin-dependent form of hypertension. Therefore, we tested the effects of candesartan and PD123319, both on basal regional kidney perfusion and on responses to renal arterial administration of Ang II, in both 2 kidney, 1 clip (2K1C) hypertensive rats and sham-operated control rats. This also allowed us to confirm our findings in the rabbit, and in the most commonly used species for studies of the impact of Ang II on the renal circulation, the rat.
Methods
Animals
Male Sprague–Dawley rats (n=48, Biological Research Laboratories, Baker Heart Institute, Victoria, Australia) were used. Procedures were approved by the Monash University Department of Physiology Animal Ethics Committee, and accorded with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Surgery
To establish 2K1C hypertension, 4-week-old rats (100–150 g) were anaesthetised with isofluorane (1–3%, Abbott Australasia Pty Ltd, Kurnell, Australia) and a U-shaped silver clip (0.2 mm ID) was placed on the right renal artery (Li & Widdop, 1995). A control group underwent sham surgery. Tail-cuff systolic blood pressure (SBP) was measured in both groups at weekly intervals (Li & Widdop, 1995). Only 2K1C rats with SBP greater than 160 mmHg were used. At 3 to 4 weeks after this initial surgery, rats were anaesthetised (Inactin; thiobutabarbital, 175 mg kg−1 i.p.; Sigma, St Louis, MO, U.S.A.) and catheters were implanted into the jugular vein, and carotid and femoral arteries as described previously (Edgley et al., 2002). To maintain renal arterial pressure (measured from the femoral artery) at control levels during renal arterial infusion of Ang II, a clamp was placed around the aorta above the level of the renal arteries. Bovine serum albumin (2% (w v−1) in 154 mM NaCl, Sigma) was infused intravenously (i.v.) throughout surgery (6 ml kg−1 h−1) and the remainder of the experiment (1.5 ml kg−1 h−1). The left kidney was placed in a stable cup, and a renal artery was catheterised (Edgley et al., 2002). Left kidney RBF was monitored by transit-time ultrasound flowmetry (type 0.7VB; Transonic Systems, Ithaca, NY, U.S.A.). For measurement of MLDF, a 26-gauge needle-type laser Doppler flowprobe (MNPIIOXP, Oxford Optronix, Oxford, U.K.) was inserted into the left kidney using a micromanipulator (Narashige, Tokyo, Japan). To allow for differences in kidney size between groups, the tip of the probe was placed ∼5 mm below the midregion of the lateral kidney surface in sham rats and ∼6 mm below the surface in 2K1C rats. We confirmed post mortem that this placed the tip of the probe at the margin of the inner and outer medulla. CLDF was measured with a miniature surface probe (MSP310XP, Oxford Optronix, Oxford, U.K.) placed on the dorsal surface of the kidney.
Experimental protocol
In both 2K1C rats (n=24) and sham rats (n=24), we tested the effects of (1) saline vehicle (n=6), (2) AT1-receptor blockade (n=6), (3) AT2-receptor blockade (n=6) or (4) combined AT1-/AT2-receptor blockade (n=6). Each rat then received renal arterial infusions of Ang II. Candesartan (Astra Zeneca, Switzerland) and PD123319 (synthesized as described by Cundy et al., 2000) were used for blockade of AT1 and AT2 receptors, respectively. Candesartan is a highly selective and insurmountable AT1-receptor antagonist (Shibouta et al., 1993). PD123319 has 3500-fold greater affinity for AT2 receptors relative to AT1 receptors (Timmermans et al., 1991).
Effects of i.v. antagonist infusion
After a 60–90 min equilibration period and a 10 min control period, rats were administered either candesartan (10 μg kg−1 plus 10 μg kg−1 h−1), PD123319 (1 mg kg−1 plus 1 mg kg−1 h−1), both antagonists or saline vehicle (1 ml kg−1 plus 1 ml kg−1 h−1 154 mmol l−1 NaCl) i.v. The antagonist infusions continued for the whole experiment.
Effects of renal arterial infusion of Ang II
At 30 min after commencing antagonist treatments, a series of renal arterial infusions of Ang II (0, 1, 3, 10, 30 and 100 ng kg−1 min−1) commenced. Each dose was administered over a 15 min period, and the final dose was followed by a 15 min recovery period.
Statistical methods
Data are expressed as mean±s.e.m. Values of P⩽0.05 were considered statistically significant. Paired and unpaired t-tests, ANOVA, and where appropriate, repeated measures ANOVA (Ludbrook, 1994) were used to evaluate the effects of the various treatments.
Results
Tail-cuff SBP in conscious rats
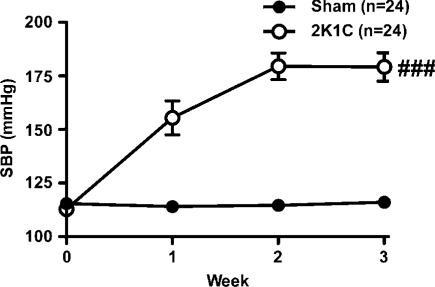
SBP of sham rats remained constant (115±2 mmHg) over the 3–4 week measurement period. In contrast, SBP of 2K1C rats rose gradually to 180±6 mmHg, 3 weeks after surgery (Figure 1).
Figure 1.
Tail-cuff SBP was recorded before (week 0) and at weekly intervals after surgery in 2K1C rats (n=24) and sham-operated rats (n=24). Lines and error bars represent mean±s.e.m. ###P<0.001 2K1C versus sham group (analysis of variance).
Baseline measurements in anaesthetised rats
Arterial pressure (systolic, diastolic and mean) and nonclipped (left) kidney dry weight were greater in 2K1C than sham rats (Table 1). In contrast, RBF, CLDF, MLDF and body weight were indistinguishable in the two groups. Renal vascular resistance (RVR) in the nonclipped kidney tended to be greater in 2K1C than sham rats, but this did not reach statistical significance (P=0.07; Table 1). In both groups, the levels of all haemodynamic variables did not vary significantly according to the treatment that followed.
Table 1.
Mean baseline levels of haemodynamic and renal variables before antagonist treatment in anaesthetised rats
| Variable | Sham-operated rats | 2K1C rats | P-value |
|---|---|---|---|
| SBP (mmHg) | 125±5 | 155±5 | <0.001 |
| DBP (mmHg) | 93±5 | 108±3 | 0.008 |
| MAP (mmHg) | 110±3 | 129±4 | <0.001 |
| HR (beats min−1) | 353±8 | 368±8 | 0.2 |
| RBF (ml min−1 g−1 dry kidney wt) | 17±1 | 16±1 | 0.5 |
| RVR (mmHg ml min−1 g−1 dry kidney wt) | 7.3±0.5 | 8.5±0.4 | 0.07 |
| CLDF (U) | 1526±51 | 1599±39 | 0.3 |
| MLDF (U) | 645±47 | 659±42 | 0.8 |
| Body weight (g) | 392±9 | 367±12 | 0.1 |
| Left kidney dry weight (g) | 0.341±0.01 | 0.394±0.01 | <0.001 |
Data are the mean±s.e.m. (n=24 per group) of levels during the 10 min period before i.v. administration of antagonist treatments. SBP, systolic blood pressure; DBP, diastolic blood pressure, MAP, mean arterial pressure; HR, heart rate; RBF, total renal blood flow; RVR, renal vascular resistance; CLDF, cortical laser Doppler flux; MLDF, medullary laser Doppler flux. P-values represent the outcomes of Student's unpaired t-test, testing whether these variables differed between the two groups. Note that analysis of variance within each group showed that none of these variables differed significantly according to the antagonist treatment that followed.
Responses to candesartan and PD123319
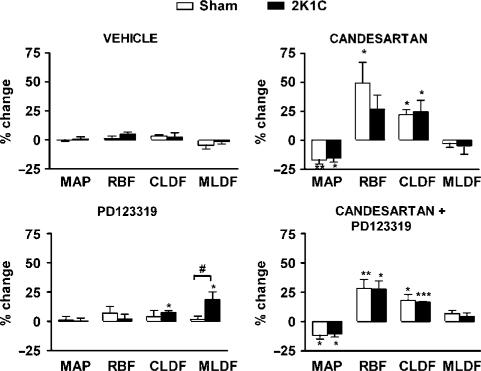
In sham rats, mean arterial pressure (MAP), heart rate (HR), RBF, CLDF and MLDF were not significantly affected by vehicle or PD123319 treatment. In contrast, after candesartan, MAP was decreased by 17±4%, and this was accompanied by increases in RBF (49±18%) and CLDF (22±5%). Candesartan did not significantly affect MLDF or HR in sham rats. When candesartan was coadministered with PD123319, responses of HR, MAP, RBF, CLDF and MLDF were indistinguishable from those of rats receiving candesartan alone (Figure 2).
Figure 2.
Effects of antagonist treatments on systemic and renal haemodynamics. Data indicate percentage differences between levels during a 10 min control period and those 20–30 min after the antagonist treatments commenced. Columns and error bars represent mean±s.e.m. (n=6 per group). MAP, mean arterial pressure; RBF, total renal blood flow; CLDF, cortical laser Doppler flux; MLDF, medullary laser Doppler flux. *P<0.05, **P<0.01, ***P<0.001 for change from control (paired t-test) and #P<0.05 for sham versus 2K1C hypertensive rats (unpaired t-test).
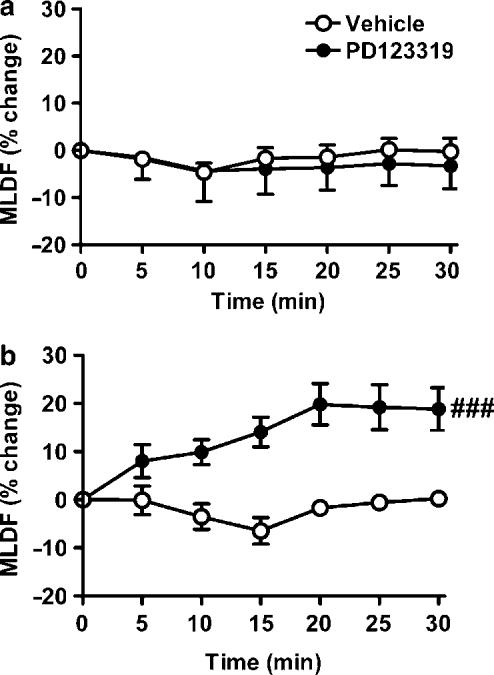
In 2K1C rats, neither vehicle nor PD123319 treatment significantly affected MAP, HR or RBF. After candesartan, MAP of 2K1C rats was decreased by 16±3%, and this was accompanied by increased CLDF (25±10%). RBF also tended to increase (by 27±12%), but this was not statistically significant. Candesartan did not significantly affect MLDF or HR (Figure 2). In contrast to sham rats, in 2K1C rats PD123319 caused a small increase in CLDF (8±2%), although this response did not differ significantly from that in sham rats (Figure 2). Furthermore, in 2K1C rats, PD123319 increased MLDF from 550±64 to 641±57 U (19±7%), a response that was markedly different from that of sham rats when examined either at the end of the 30 min treatment period (Figure 2) or as a time course (Figure 3). Coadministration of candesartan and PD123319 resulted in responses of MAP, RBF, CLDF and MLDF that were indistinguishable from those of rats receiving candesartan alone.
Figure 3.
Time course of changes in MLDF in response to vehicle or PD123319 treatment in (a) sham and (b) 2K1C hypertensive rats. Data indicate percentage changes relative to control over 5 min intervals following commencement of antagonist infusion. Lines and error bars represent mean±s.e.m. (n=6 per group). ###P<0.001 for main effect of PD123319 treatment from analysis of variance.
Responses to infusions of Ang II
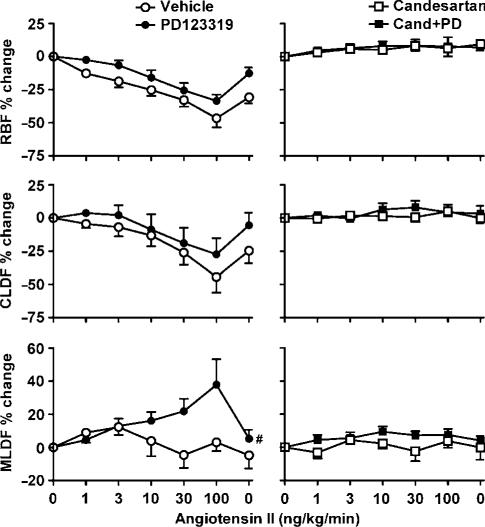
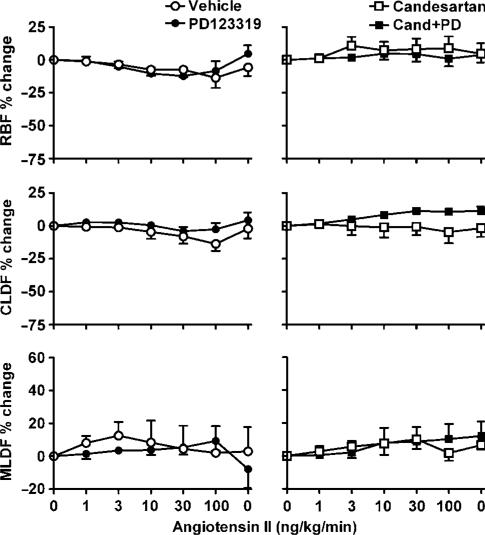
In vehicle-treated sham rats, renal arterial infusion of Ang II was accompanied by dose-dependent decreases in RBF (by 46±6% at 100 ng kg−1 min−1) and CLDF (by 44±12% at 100 ng kg−1 min−1) but not MLDF (Figure 4). These responses were virtually abolished by candesartan treatment, whether given alone or in combination with PD123319. In sham rats, PD123319 treatment had little or no effect on Ang II-induced reductions in RBF and CLDF, but uncovered a dose-dependent increase in MLDF. Thus, MLDF increased from 772±84 U (control) to 808±94, 874±99, 883±87, 932±111 and 1019±104 U, respectively, during infusion of 1, 3, 10, 30 and 100 ng kg−1 min−1 Ang II (Figure 4). Post hoc multiple comparisons, using the Ryan–Holm–Sidak procedure (Ludbrook, 1998), revealed that these changes reached statistical significance at 30 ng kg−1 min−1 (P=0.03) and 100 ng kg−1 min−1 (P<0.001). During the recovery period, MLDF returned to its control level (805±99 U). This response was virtually abolished by candesartan treatment (Figure 4). In contrast, renal arterial infusion of Ang II in vehicle-treated 2K1C rats had little effect on RBF, CLDF and MLDF (Figure 5). In 2K1C rats, responses to Ang II were not significantly altered by PD123319 (Figure 5).
Figure 4.
Responses of sham rats to renal arterial infusion of Ang II (1, 3, 10, 30 and 100 ng kg−1 min−1, followed by a recovery period). Data indicate percentage changes, during the final 10 min of each 15 min infusion, compared with baseline levels before Ang II infusion. Lines and error bars represent mean±s.e.m. (n=6). Abbreviations are the same as for Figure 2. #P⩽0.05 for main effect of PD123319 treatment from analysis of variance.
Figure 5.
Responses of 2K1C hypertensive rats to renal–arterial infusion of Ang II (1, 3, 10, 30 and 100 ng kg−1 min−1, followed by a recovery period). Abbreviations, symbols, lines and error bars are the same as for Figure 4.
Discussion
We recently obtained evidence, from studies in anaesthetised rabbits, that AT2-receptor activation counteracts AT1-receptor-mediated vasodilatation in the renal medulla (Duke et al., 2003). The role of renal medullary blood flow in the long-term control of blood pressure is now well established, based in large part on studies showing that chronic reductions in medullary perfusion can lead to salt and water retention and hypertension (Cowley, 1997; Cowley et al., 2003; Mattson, 2003). Thus, interactions between AT1 and AT2 receptors in the control of medullary blood flow could have important implications for antihypertensive therapies that target the RAS. These interactions could also contribute to the control of medullary blood flow, and thus blood pressure, in renin-dependent forms of hypertension. Therefore, the chief aim of our current study was to determine the roles of endogenous Ang II in the control of medullary blood flow in 2K1C hypertension. By using a rat model for these experiments, we were also able to validate our previous observations in rabbits, in a species more commonly used for studies of regional kidney blood flow. Our key new finding was that in 2K1C rats, the AT2-receptor antagonist PD123319 increased basal MLDF, suggesting that in this model of hypertension, AT2-receptor activation mediates tonic vasoconstriction in the medullary circulation.
To our knowledge, this is the first study to test the effects of renal arterial administration of Ang II on regional kidney blood flow in rats. Responses to renal arterial infusion of Ang II in rats were remarkably similar to those we have observed in rabbits (Rajapakse et al., 2002; Duke et al., 2003). In sham rats, renal arterial Ang II caused dose-dependent decreases in CLDF but not MLDF, and treatment with PD123319 revealed dose-dependent increases in MLDF in response to Ang II. All of these effects were blocked by treatment with candesartan. Thus, as in the rabbit kidney, AT2-receptor activation appears to blunt AT1-mediated medullary vasodilatation in the rat kidney. In both species, Ang II-induced increases in MLDF (in the presence of PD123319) were dose-dependent, and occurred at doses of Ang II that produced physiologically and pharmacologically relevant reductions in total RBF (30–70%). Also, we found that neither AT1-, AT2-, nor combined AT1- and AT2-receptor blockade, altered resting MLDF in sham rats. Therefore, as in normotensive rabbits, endogenous Ang II appears to play little role in setting basal vascular tone in the medullary circulation in normotensive rats. This conclusion is consistent with the results of previous studies, showing little effect of AT1 antagonism on medullary blood flow in rats (Ortíz et al., 1998; Badzynska et al., 2002; Sarkis et al., 2003). Earlier observations of increased medullary blood flow after blockade of Ang-converting enzyme likely reflect the effects of increased bradykinin bioavailability (Cupples et al., 1988; Mattson & Roman, 1991).
Compared to sham rats, responses of renal haemodynamics to renal arterial Ang II were greatly blunted in 2K1C rats. The most likely explanation for this is that renal vascular responses to Ang II in 2K1C rats are already near maximal, since circulating (Navar et al., 2001) and intrarenal levels of Ang II in the nonclipped kidney (Guan et al., 1992) are elevated in rats with 2K1C hypertension. We did not directly measure plasma renin activity or Ang II levels in this study, although plasma renin activity has previously been found to be elevated in 2K1C rats (15.5±4.5 ng Ang I ml−1 h−1) compared with sham rats (2.1±0.5 ng Ang I ml−1 h−1) in our laboratory (Li & Widdop, 1995). Downregulation of renal AT1A receptors (but not AT2 receptors) in the unclipped kidney of 2K1C rats (Wang et al., 1999) might also partly explain the blunted response to exogenous Ang II we observed. In rats, AT1 receptors have been detected in afferent and efferent arterioles (including those of juxtamedullary glomeruli), and outer medullary descending vasa recta, as well as tubular structures associated with these vascular elements (Miyata et al., 1999; Wang et al., 1999; Helou et al., 2003). However, the degree to which 2K1C-hypertension downregulates expression of AT1 receptors in each of these tissues (see Wang et al., 1999), and also the precise contribution of each of these tissues to the control of regional kidney blood flow (see Evans et al., 2004), remains to be determined.
In the present study, AT1-receptor blockade had similar haemodynamic effects in 2K1C rats compared with sham rats. Surprisingly, few studies have examined the effects of AT1-receptor blockade in 2K1C and sham rats simultaneously, although our results are consistent with the two previous studies of anaesthetised rats (Braam et al., 1995; Cervenka et al., 1999). Basal levels of RBF (corrected for dry kidney weight) were also similar in sham and 2K1C rats (unclipped kidney) in our study, but this does not necessarily reflect a lack of effect of RAS activation on control of renal haemodynamics. Renal arterial infusion of Ang II (0.5 ng kg−1 min−1) in conscious dogs is associated with an initial reduction in RBF, but within 48 h RBF returns to its control level (Fitzgerald et al., 1997). The recovery of RBF is presumably due to the impact of counter regulatory vasodilator mechanisms, since termination of the Ang II infusion, 28 days after it commenced, was associated with a marked increase in RBF that lasted for ∼48 h (Fitzgerald et al., 1997). Thus, multiple mechanisms act in concert to maintain renal circulatory homeostasis, even in the face of RAS activation.
Our observation that PD123319 treatment revealed increases in basal MLDF in 2K1C rats, which were abolished by candesartan, suggests that AT1 receptors mediate vasodilatation in the medullary circulation in 2K1C hypertension, but that tonic AT2-receptor-mediated vasoconstriction counterbalances this effect. These effects, on basal vascular tone in the medullary circulation, are consistent with our observation that PD123319 treatment unmasks increases in MLDF in response to exogenous Ang II in sham rats, which were also abolished by candesartan treatment in combination with PD123319. Differences between sham and 2K1C rats, in their responses to AT-receptor blockade and exogenous Ang II, are likely due to the different level of activation of circulating and intrarenal RAS in the two groups. Thus, our data indicate that in the medullary circulation, AT2-receptor activation inhibits AT1-mediated vasodilatation, and that in 2K1C hypertension, AT2-receptor activation mediates tonic vasoconstriction. There is strong evidence, from both in vitro and in vivo studies, that Ang II-induced vasodilation in the medullary circulation is mediated by release of nitric oxide (Zou et al., 1998; Walker et al., 1999; Rajapakse et al., 2002) and/or prostaglandins (Parekh et al., 1996; Oliver et al., 2002; Badzynska et al., 2003). Our data regarding the effects of PD123319 therefore suggest that AT2-receptor activation can blunt AT1-receptor-mediated release of these vasodilator factors in the medullary circulation.
However, effects of AT2-receptor activation on MLDF were not observed in the absence of concomitant AT1-receptor activation, indicating that they reflect modulation of AT1-mediated actions, rather than direct effects of activation of AT2 receptors per se. For example, renal arterial infusion of Ang II did not alter MLDF (or alter RBF or CLDF) in rats or rabbits (Duke et al., 2003) pretreated with candesartan. Furthermore, in rabbits, renal arterial infusion of the highly selective AT2-receptor agonist CGP42112A did not alter RBF, CLDF or MLDF (Duke et al., 2003). Also, PD123319 produced increases in MLDF in 2K1C rats, in which endogenous Ang II was activating AT1 receptors near maximally (see above), but not in sham rats in which renal AT1-receptor activation by endogenous Ang II was presumably only modest. These considerations also provide a likely explanation for the lack of effect of candesartan alone on basal MLDF in 2K1C rats, since AT1-mediated medullary vasodilatation under these conditions is normally inhibited by AT2-receptor activation. Collectively, these observations indicate that activation of AT2 receptors has little impact on the medullary circulation, unless AT1 receptors are also activated.
Collectively, our results suggest that AT2 receptors mediate vasoconstriction in the medullary circulation that counterbalances AT1-receptor-mediated vasodilatation. AT1-receptor-mediated vasodilatation appears to be a unique property of the medullary circulation, which relies on the close association of vascular and tubular elements within the medulla, which in turn facilitates the phenomenon of tubulovascular nitric oxide crosstalk (Dickhout et al., 2002). Given that AT2-receptor activation opposes many of the actions of Ang II mediated by AT1 receptors (Siragy & Carey, 1999; Carey et al., 2000), it seems logical that this is also the case for AT1-mediated vasodilatation in the medullary circulation, even though this is at odds with the picture that has emerged in the systemic circulation, of AT2-receptor activation opposing AT1-mediated vasoconstriction (Carey et al., 2000).
Our present results suggest that this interaction between AT1 and AT2 receptors, in the control of medullary blood flow, has considerable impact on regulation of medullary blood flow in renovascular hypertension. It remains to be determined whether it also contributes to the regulation of medullary blood flow under other conditions associated with RAS activation (e.g. sodium depletion (Gross et al., 1998) and heart failure (Schrier & Abraham, 1999)). Importantly, the role of AT2 receptors in the regulation of medullary blood flow may be enhanced under conditions of upregulation of renal AT2 receptors (e.g. sodium depletion (Ozono et al., 1997) and renal failure (Bautista et al., 2001)). It also remains to be determined whether this mechanism contributes to the control of medullary blood flow in nonrenin-dependent models of hypertension. The implications of our present findings, for the use of AT1-receptor antagonists in antihypertensive pharmacotherapy, also remain to be determined. However, the fact that candesartan seems not to alter basal medullary blood flow in rabbits (Duke et al., 2003) and rats, even when the RAS is activated (in 2K1C hypertension), does not support the notion that blockade of AT1-mediated vasodilatation in the medullary circulation can limit the long-term antihypertensive efficacy of AT1-receptor antagonists.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (143785, 143603, 143564), the Ramaciotti Foundations (A6370, RA159/98, RA032/01) and Monash University (Small Grant 2004/S62). Lisa Duke is a Monash University Graduate Scholar.
Abbreviations
- Ang II
angiotensin II
- CLDF
cortical laser Doppler flux
- DBP
diastolic blood pressure
- HR
heart rate
- 2K1C
2 kidney, 1 clip
- MAP
mean arterial pressure
- MLDF
medullary laser Doppler flux
- RAS
renin–angiotensin system
- RBF
renal blood flow
- RVR
renal vascular resistance
- SBP
systolic blood pressure
References
- BADZYNSKA B., GRZELEC-MOJZESOWICZ M., DOBROWOLSKI L., SADOWSKI J. Differential effect of angiotensin II on blood circulation in the renal medulla and cortex of anaesthetised rats. J. Physiol. 2002;538:159–166. doi: 10.1113/jphysiol.2001.012921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADZYNSKA B., GRZELEC-MOJZESOWICZ M., SADOWSKI J. Prostaglandins but not nitric oxide protect renal medullary perfusion in anaesthetised rats receiving angiotensin II. J. Physiol. 2003;548:875–880. doi: 10.1113/jphysiol.2002.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUTISTA R., SANCHEZ A., HERNANDEZ J., OYEKAN A., ESCALANTE B. Angiotensin II type AT2 receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension. 2001;38:669–673. doi: 10.1161/hy09t1.096186. [DOI] [PubMed] [Google Scholar]
- BRAAM B., NAVAR L.G., MITCHELL K.D. Modulation of tubuloglomerular feedback by angiotensin II type 1 receptors during the development of Goldblatt hypertension. Hypertension. 1995;25:1232–1237. doi: 10.1161/01.hyp.25.6.1232. [DOI] [PubMed] [Google Scholar]
- CAREY R.M., WANG Z.Q., SIRAGY H.M. Update: role of the angiotensin type-2 (AT2) receptor in blood pressure regulation. Curr. Hypertens. Rep. 2000;2:198–201. doi: 10.1007/s11906-000-0082-3. [DOI] [PubMed] [Google Scholar]
- CERVENKA L., WANG C.T., MITCHELL K.D., NAVAR L.G. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., JR Role of the renal medulla in volume and arterial pressure regulation. Am. J. Physiol. 1997;273:R1–R15. doi: 10.1152/ajpregu.1997.273.1.R1. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., JR, MORI T., MATTSON D., ZOU A.-P. Role of renal NO production in the regulation of medullary blood flow. Am. J. Physiol. 2003;284:R1355–R1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- CUNDY D., GURR P.A., KNILL A.M., TSANAKTSIDIS J. An improved synthesis of (S)-1-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid, ditrifluoroacetate, dihydrate (PD123319) Arkivoc. 2000;1:169–175. [Google Scholar]
- CUPPLES W.A., SAKAI T., MARSH D.J. Angiotensin II and prostaglandins in control of vasa recta blood flow. Am. J. Physiol. 1988;254:F417–F424. doi: 10.1152/ajprenal.1988.254.3.F417. [DOI] [PubMed] [Google Scholar]
- DICKHOUT J.G., MORI T., COWLEY A.W., JR Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ. Res. 2002;91:487–493. doi: 10.1161/01.res.0000035243.66189.92. [DOI] [PubMed] [Google Scholar]
- DUKE L.M., EPPEL G.A., WIDDOP R.E., EVANS R.G. Disparate roles of AT2 receptors in the renal cortical and medullary circulations of anesthetized rabbits. Hypertension. 2003;42:200–205. doi: 10.1161/01.HYP.0000083341.64034.00. [DOI] [PubMed] [Google Scholar]
- EDGLEY A.J., NICHOLS N.R., ANDERSON W.P. Acute intrarenal infusion of ANG II does not stimulate immediate early gene expression in the kidney. Am. J. Physiol. 2002;282:R1133–R1139. doi: 10.1152/ajpregu.00187.2001. [DOI] [PubMed] [Google Scholar]
- EVANS R.G., EPPEL G.A., ANDERSON W.P., DENTON K.M. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J. Hypertens. 2004;22:1429–1451. doi: 10.1097/01.hjh.0000133744.85490.9d. [DOI] [PubMed] [Google Scholar]
- EVANS R.G., MADDEN A.C., DENTON K.M. Diversity of responses of renal cortical and medullary blood flow to vasoconstrictors in conscious rabbits. Acta Physiol. Scand. 2000;169:297–308. doi: 10.1046/j.1365-201x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- FITZGERALD S.M., STEVENSON K.M., EVANS R.G., ANDERSON W.P. Systemic hemodynamic responses to chronic angiotensin infusion into the renal artery of dogs. Am. J. Physiol. 1997;273:R1980–R1989. doi: 10.1152/ajpregu.1997.273.6.R1980. [DOI] [PubMed] [Google Scholar]
- GROSS V., KURTH T.M., SKELTON M.M., MATTSON D.L., COWLEY A.W., JR Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am. J. Physiol. 1998;274:R1317–R1323. doi: 10.1152/ajpregu.1998.274.5.R1317. [DOI] [PubMed] [Google Scholar]
- GUAN S., FOX J., MITCHELL K.D., NAVAR L.G. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- HELOU C.M.B., IMBERT-TEBOUL M., DOUCET A., RAJERISON R., CHOLLET C., ALHENC-GELAS F., MARCHETTI J. Angiotensin receptor subtypes in thin and muscular juxtamedullary efferent arterioles of rat kidney. Am. J. Physiol. 2003;285:F507–F514. doi: 10.1152/ajprenal.00430.2002. [DOI] [PubMed] [Google Scholar]
- LAVERMAN G.D., REMUZZI G., RUGGENENTI P. ACE inhibition versus angiotensin receptor blockade: which is better for renal and cardiovascular protection. J. Am. Soc. Nephrol. 2004;15:S64–S70. doi: 10.1097/01.asn.0000093368.27046.3c. [DOI] [PubMed] [Google Scholar]
- LI X.C., WIDDOP R.E. Regional hemodynamic effects of the AT1 receptor antagonist CV-11974 in conscious renal hypertensive rats. Hypertension. 1995;26:989–997. doi: 10.1161/01.hyp.26.6.989. [DOI] [PubMed] [Google Scholar]
- LUDBROOK J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc. Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- LUDBROOK J. Multiple comparison procedures updated. Clin. Exp. Pharmacol. Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- MATTSON D.L. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am. J. Physiol. 2003;284:R13–R27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- MATTSON D.L., ROMAN R.J. Role of kinins and ANG II in the renal hemodynamic response to captopril. Am. J. Physiol. 1991;260:F670–F679. doi: 10.1152/ajprenal.1991.260.5.F670. [DOI] [PubMed] [Google Scholar]
- MIYATA N., PARK F., LI X.F., COWLEY A.W., JR Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am. J. Physiol. 1999;277:F437–F466. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- NAVAR L.G., HARRISON-BERNARD L.M., NISHIYAMA A., KOBORI H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVAR L.G., MITCHELL K.D., HARRISON-BERNARD L.M., KOBORI H., NISHIYAMA A. Intrarenal angiotensin II levels in normal and hypertensive states. J. Renin Angiotensin Aldosterone Syst. 2001;2:176–184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER J.J., RAJAPAKSE N.W., EVANS R.G. Effects of indomethacin on responses of regional kidney perfusion to vasoactive agents in rabbits. Clin. Exp. Pharmacol. Physiol. 2002;29:873–879. doi: 10.1046/j.1440-1681.2002.03742.x. [DOI] [PubMed] [Google Scholar]
- ORTÍZ M.C., FORTEPIANI L.A., RUIZ-MARCOS F.M., ATUCHA N.M., GARCÍA-ESTAÑ J. Role of AT1 receptors in the renal papillary effects of acute and chronic nitric oxide inhibition. Am. J. Physiol. 1998;274:R760–R766. doi: 10.1152/ajpregu.1998.274.3.R760. [DOI] [PubMed] [Google Scholar]
- OZONO R., WANG Z.Q., MOORE A.F., INAGAMI T., SIRAGY H.M., CAREY R.M. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- PALLONE T.L., ZHANG Z., RHINEHART K. Physiology of the renal medullary microcirculation. Am. J. Physiol. 2003;284:F253–F266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- PAREKH N., DOBROWOLSKI L., ZOU A.-P., STEINHAUSEN M. Nitric oxide modulates angiotensin II- and norepinephrine-dependent vasoconstriction in rat kidney. Am. J. Physiol. 1996;270:R630–R635. doi: 10.1152/ajpregu.1996.270.3.R630. [DOI] [PubMed] [Google Scholar]
- RAJAPAKSE N.W., OLIVER J.J., EVANS R.G. Nitric oxide in responses of regional kidney blood flow to vasoactive agents in anesthetized rabbits. J. Cardiovasc. Pharmacol. 2002;40:210–219. doi: 10.1097/00005344-200208000-00006. [DOI] [PubMed] [Google Scholar]
- SARKIS A., LIU K.L., LO M., BENZONI D. Angiotensin II and renal medullary blood flow in Lyon rats. Am. J. Physiol. 2003;284:F365–F372. doi: 10.1152/ajprenal.00248.2002. [DOI] [PubMed] [Google Scholar]
- SCHRIER R.W., ABRAHAM W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- SHIBOUTA Y., INADA Y., OJIMA M., WADA T., NODA M., SANADA T., KUBO K., KOHARA Y., NAKA T., NISHIKAWA K. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974), and its prodrug, (±)-1-cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J. Pharmacol. Exp. Ther. 1993;266:114–120. [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- TIMMERMANS P.B.M.W.M., WONG P.C., CHIN A.T., HERBLIN W.F. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol. Sci. 1991;12:55–62. doi: 10.1016/0165-6147(91)90498-h. [DOI] [PubMed] [Google Scholar]
- WALKER L.L., RAJARATNE A.A., BLAIR-WEST J.R., HARRIS P.J. The effects of angiotensin II on blood perfusion in the rat renal papilla. J. Physiol. 1999;519:273–278. doi: 10.1111/j.1469-7793.1999.0273o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z.-Q., MILLAT L.J., HEIDERSTADT N.T., SIRAGY H.M., JOHNS R.A., CAREY R.M. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- WIDDOP R.E., MATROUGUI K., LEVY B.I., HENRION D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- ZOU A.-P., WU F., COWLEY A.W., JR Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension. 1998;31:271–276. doi: 10.1161/01.hyp.31.1.271. [DOI] [PubMed] [Google Scholar]