Abstract
The pharmacology of bimatoprost, a synthetic prostaglandin-amide, was examined in prostaglandin F2α (PGF2α)-sensitive preparations. Bimatoprost potently contracted the rabbit isolated uterus (pEC50=7.92±0.16). In contrast, bimatoprost exhibited weak excitatory activity in human myometrium from pregnant and nonpregnant donors, mouse uterus, rat uterus, and endothelium-intact rabbit jugular veins, and did not stimulate DNA synthesis in mouse fibroblasts.
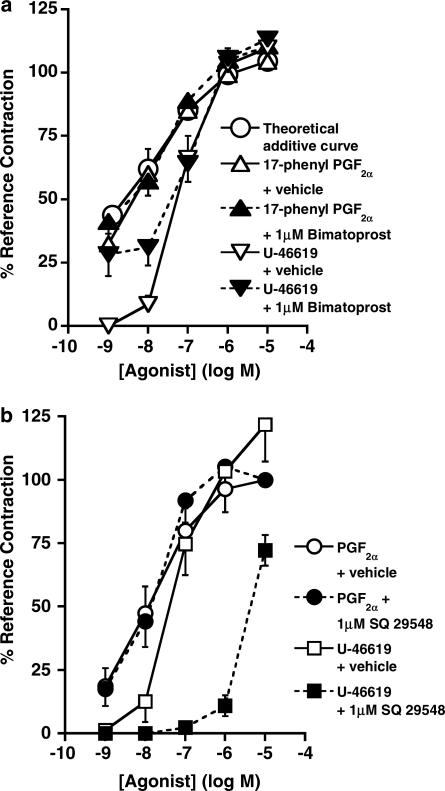
The possibility that the effects of bimatoprost may reflect partial agonism at prostanoid FP receptors was examined and the contractile effects of full agonists, 17-phenyl PGF2α (FP) and U-46619 (TP, a control), were determined in the absence and presence of 1 μM bimatoprost on the mouse uterus. Analyses of the agonist–agonist functional studies showed no antagonism, indicating that bimatoprost is not a partial agonist.
Bioassay metabolism studies of bimatoprost and latanoprost (FP receptor agonist prodrug) in the rabbit uterus were conducted using recipient mouse uterus. Results indicated that the potent responses to bimatoprost in the rabbit uterus are produced by the intact molecule and not by its putative free acid metabolite, 17-phenyl PGF2α. Some hydrolysis of latanoprost to latanoprost free acid appears to have occurred in the rabbit uterus, according to biological detection.
The pharmacology of bimatoprost could not be explained by its interaction with known prostanoid FP receptors and was independent of species-, tissue-, or preparation-related factors. The potent contractile effects of bimatoprost in the rabbit uterus provide further pharmacological evidence for the presence of a novel receptor population that preferentially recognises bimatoprost.
Keywords: Bimatoprost (AGN 192024), prostaglandin F2α (PGF2α), FP receptor, rabbit, mouse, rat, human, uterus, jugular vein, DNA synthesis
Introduction
Bimatoprost is a highly efficacious intraocular pressure (IOP) lowering drug used in glaucoma therapy (Chen & Woodward, 2002; Higginbotham et al., 2002; Noecker et al., 2003; Parrish et al., 2003). Moreover, bimatoprost effectively reduces IOP of patients who are non-responders/unresponsive to latanoprost, a potent and selective prostanoid FP receptor agonist in the form of an isopropyl ester prodrug (Williams, 2002; Gandolfi & Cimino, 2003). This clinical evidence suggests that bimatoprost and prostanoid FP receptor agonists stimulate different receptor populations.
Preclinical pharmacological evidence to date indicates that bimatoprost and prostanoid FP receptor agonists have different activity profiles (Woodward et al., 2001; 2003; Matias et al., 2004). This may be attributed to the presence of a nonacidic ethylamide moiety at position C1 instead of carboxylic acid, which is a critical structural difference between bimatoprost (17-phenyl, prostaglandin F2α (PGF2α)-1-ethylamide) and prostanoid FP receptor agonists such as 17-phenyl PGF2α (the putative metabolite of bimatoprost) and PGF2α. The pharmacology of bimatoprost has been ascribed to interaction with a novel population of receptors, termed prostamide-sensitive receptors, that is distinct from known prostanoid receptors (Woodward et al., 2001; 2003; Matias et al., 2004). However, controversy exists over this contention of novel receptors and more definitive studies involving the same in vitro assay system are needed.
In the present investigation, bimatoprost was found to produce potent contractile effects in the rabbit isolated uterus but not in the uterus from other species. The principal aims of the studies were to examine the agonist profiles of bimatoprost and 17-phenyl PGF2α and to further explore the concept of discrete prostamide-sensitive receptors as follows: (1) compare the effects of bimatoprost to those of prostanoid FP receptor agonists in PGF2α-sensitive in vitro preparations; (2) study whether bimatoprost acts as a partial agonist at prostanoid FP receptors using the mouse isolated uterus; and (3) investigate whether the potent activity of bimatoprost in the rabbit uterus is due to metabolism of bimatoprost to the potent FP agonist 17-phenyl PGF2α using bioassay detection of the putative metabolite.
Methods
Isolated tissue studies
Smooth muscle tension of isolated tissues was measured isometrically with force displacement transducers and recorded on a Grass polygraph. Each tissue was suspended in a 10 ml jacketed organ bath containing Krebs buffer for immersion bioassay (rabbit, mouse, rat) or was superfused with Krebs buffer (human) maintained at 37°C and gassed with 95% O2/5% CO2 to give a pH of 7.4. Krebs buffer had the following composition (mM): NaCl 118.0; KC1 4.7; KH2PO4 1.2; CaCl2 1.9 or 2.5; MgSO4 1.18; NaHCO3 25.0; glucose 11.7; indometacin 0.001 or 0.00279. Only one concentration- or dose–response curve was generated in each tissue. All procedures involving human tissues were approved by the local ethical committee and all donors gave informed written consent. The procedures used with animals complied with institutional, national, and international guidelines for animal care and use.
Rabbit, mouse, and rat isolated uterus
Mature, virgin, female New Zealand white rabbits (Myrtles; 4.5–5 kg), Swiss Webster mice (Bantin & Kingman, Charles River Laboratories; 21–42 g), and Sprague–Dawley rats (Harlan; 240–270 g) were used. Experimental protocols for rabbit and mouse uterine preparations have been described previously (Chen et al., 1998; 2001). Rat uterus studies were conducted as for the mouse uterus, except that the epithelial layer and portions of endometrium were removed by gently rubbing with moistened cotton Q-tips. Each uterine horn was hemi-sectioned to provide longitudinal muscle strips. Single doses (noncumulative) of test agonists (10 μl volume) were applied in concentrations increasing in log10 increments. Tissues were washed after the test compound had been applied for 10 min or after the tension returned to baseline following a contractile response. Reference contractions to 10 μM carbachol (rabbit) or 10 μM PGF2α (mouse and rat) were obtained at the end of each concentration–response curve.
In the rabbit uterus, FP agonists were not used as a reference since repetitive doses produced inconsistent responses (Chen et al., 1998). Contraction was measured as peak height (g tension), since results for carbachol using peak height and area under the curve (AUC) calculations were similar in this tissue (Chen et al., 1998). PGF2α and fluprostenol produced maximum responses that were 82±3% of the carbachol (10 μM) response, hence responses to test compounds (bimatoprost, 17-phenyl PGF2α, PGF2α, latanoprost free acid, latanoprost) were calculated as 0.82 of response to 10 μM carbachol (Chen et al., 1998). In the mouse and rat uterus, contraction was measured as the integrated AUC (g tension × min time) of the response for 5 min after administration of each dose.
Agonist–agonist functional studies in mouse isolated uterus
Bimatoprost was investigated for partial agonism at prostanoid FP receptors. Concentration–response curves were generated for 17-phenyl PGF2α, a potent FP receptor agonist (Woodward et al., 1995; Coleman et al., 1998), in the absence (vehicle-treated) and presence of a high concentration (1 μM) of bimatoprost using paired hemi-sectioned uterine horns. A TP receptor agonist U-46619 was used as a negative control and also tested in the presence of vehicle or 1 μM bimatoprost. Additional controls were provided by obtaining concentration–response curves for U-46619 and PGF2α in the absence and presence of a selective TP receptor antagonist SQ 29548 (pA2 8.1–9.1; Ogletree et al., 1985; Coleman et al., 1998). Tissues were pretreated with bimatoprost, SQ 29548, or their respective vehicles for 20 min before each dose of the test agonist. AUC of the responses to bimatoprost, SQ 29548, or vehicle were determined for the 5 min pretreatment before administration of the test agonist at each dose. PGF2α (10 μM) provided the reference contraction.
Bioassay metabolism studies in rabbit isolated uterus using recipient mouse uterus
Studies of the potential enzymatic hydrolysis of bimatoprost and latanoprost (isopropyl ester prodrug) in the rabbit uterus were conducted using bioassay detection in the mouse uterus as described below. Each single dose (10 μl volume) of the parent compound (bimatoprost or latanoprost) was administered to the organ bath medium containing the rabbit uterus. Following 10 min incubation at 37°C, 1 ml of the bimatoprost-treated or latanoprost-treated physiological solution that bathed the rabbit uterus was added to 9 ml of the organ bath medium containing the mouse isolated uterus (a 1 : 10 dilution). The presence of 17-phenyl PGF2α (potential free acid metabolite of bimatoprost) and latanoprost free acid can be detected by contractile responses in the mouse uterus. A 1 : 10 dilution of a 10 μM concentration of bimatoprost would result in a 1 μM, 100 nM, or 10 nM concentration of 17-phenyl PGF2α in the bath media following 100, 10, or 1% hydrolytic conversion of the parent compound, respectively. Reference contractions were obtained in each preparation as described in Methods section.
Human isolated myometrium
Samples of myometrium were obtained from premenopausal patients undergoing hysterectomy for benign disorders and pregnant donors (38±1 weeks, nonlabouring) during elective Caesarean section. The experimental details have been described previously (Senior et al., 1991). Tissue strips of predominantly longitudinal muscle were superfused at 2 ml min−1 with buffer. Agonists were given as a bolus dose (volume not exceeding 50 μl) into the flow of superfusate, immediately after a spontaneous contraction. Contractile responses were determined as the integrated AUC and calculated as a T/B ratio for each dose: the area of agonist-induced (T) contraction was divided by the intrinsic spontaneous background (B) contraction.
Rabbit isolated jugular vein (endothelium-intact and -denuded)
External jugular veins of New Zealand white rabbits (Myrtles; 2–4 kg) were excised and ring segments (4–5 mm length) were obtained as described previously (Chen et al., 1995). A thromboxane receptor antagonist, SQ 29548 (1 μM) or EP 092 (2 μM), was used to minimise the TP receptor-mediated contractile influence. After 30 min of pre-contraction with histamine, cumulative doses of the test compound were applied and the vasorelaxant responses were determined. Prostaglandin E2 (PGE2) at 100 nM was given at the end of each concentration–response curve. In the endothelium-denuded rings, endothelial cells were removed by gently rubbing the intimal surface with dampened cotton Q-tips for 30–60 s.
Mouse Swiss 3T3 fibroblasts: DNA synthesis
Cells were grown in six-well dishes (100,000 cells per well) in Dulbecco's modified Eagle's medium (DMEM), low glucose (1000 mg l−1 D-glucose) containing 2 mM l-glutamine, 1% antibiotic–antimycotic, and 10% foetal bovine serum (FBS) for 3 days. The cells were made quiescent by washing with Hanks' balanced salt solution (HBSS) and incubating in DMEM medium with 0.5% FBS for 24 h. The cultures were incubated, in quadruplicate for each experiment, with fresh medium containing vehicle (0.01% ethanol in medium), bimatoprost, or prostaglandins for 22 h. Cells were pulse-labelled with [methyl-3H]-thymidine ([3H]-TdR) for 5 h at 37°C (Pasquale et al., 1988). DNA extraction followed the method described by Marcelo et al. (1978). The cells were fixed and scraped into 6% trichloroacetic acid (TCA) and centrifuged at 1680 g-force (2800 r.p.m.) for 20 min at room temperature. The DNA in the pellet was resuspended in 3% perchloric acid, denatured at 95°C for 20 min, and then cooled in an ice bath for 15 min. After centrifugation as before, the supernatant containing [3H]-TdR incorporated into DNA was assayed for radioactivity by liquid-scintillation counting. The DNA concentration was determined according to the method described by Burton (1979). DNA standards and samples were mixed with the diphenylamine reagent and incubated in a water bath with shaking at 30°C for 6–24 h and absorbance was measured at 600 nm.
Materials
Bimatoprost (AGN 192024; 17-phenyl, PGF2α-1-ethylamide) was synthesised at Allergan, Inc. (Irvine, CA, U.S.A.). PGF2α, 17-phenyl PGF2α, latanoprost free acid, latanoprost, 8-epi PGF2α, prostaglandin D2 (PGD2), PGE2, 11-deoxy PGE1, BW 245C, SQ 29548 were purchased from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). Sulprostone was obtained from Burroughs Wellcome (Beckenham, U.K.) and Cayman Chemical Co. U-46619 was obtained from Berlex Labs (Cedar Knolls, NJ, U.S.A.) and Cayman Chemical Co. Fluprostenol was obtained from Pitman-Moore Ltd (Berkhampsted, U.K.) and Cayman Chemical Co. EP 092 ([1α,2β(Z),3α,4α]-,7-[3-[1-[[(phenylamino) thioxomethyl] hydrazono] ethyl] bicyclo[2.2.1] hept-2-yl]-5-heptenoic acid) was a gift from Professor Robert L. Jones (University of Edinburgh). Carbachol, acetylcholine, histamine, indometacin, TCA, perchloric acid, salmon testes DNA, and materials for the diphenylamine reagent were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Swiss mouse 3T3 fibroblasts were obtained from the American Type Culture Collection (Rockville, MD, U.S.A.). DMEM low glucose, L-glutamine, antibiotic–antimycotic, HBSS were purchased from Gibco, Life Technologies (Gaithersburg, MD, U.S.A.) and FBS from Irvine Scientific Co. (Irvine, CA, U.S.A.). Sterilised [3H]-TdR, specific activity 20–25 Ci mmol−1 (1 mCi ml−1) was purchased from Dupont NEN Research products (Boston, MA, U.S.A.) and Amersham (Arlington Heights, IL, U.S.A.). Beckman HP cocktail was purchased from Beckman (Fullerton, CA, U.S.A.).
Data and statistical analysis
In animal tissue studies, contraction is expressed as a percentage of the reference response and vasorelaxation as a percentage of the control tone elicited by histamine. For human myometrium studies, the ED1 value is used as an expression of the potency for excitatory agonists and defined as the dose of agonist required to produce a T/B ratio equal to 1 (Senior et al., 1991). For DNA synthesis studies, the data are expressed as c.p.m. ([3H]-TdR incorporated into DNA) μg−1 DNA. Values for the vehicle-treated control cultures indicated basal cell proliferation. The 50% stimulatory level is defined as midpoint between the basal level and maximal agonist effect. Data are expressed as arithmetic mean±s.e.m. Statistical comparisons consisted of testing for significance of difference between means using the Student's t-test for paired or unpaired samples as appropriate. The P-values were obtained using the COMPARE procedure of RS/1® (The Research System; Bolt Beranek and Newman Research Systems, Cambridge, MA, U.S.A.). The data were tested for normality of distribution (Wilk-Shapiro for n<50) and, in the case of unpaired samples, also the F-test for equality of variance. Parametric procedures were found to be appropriate for all samples. Differences are considered statistically significant if the P-value is less than 0.05.
Results
Effects of bimatoprost and FP receptor agonists on PGF2α-sensitive isolated uterine preparations of rabbit, mouse, and rat
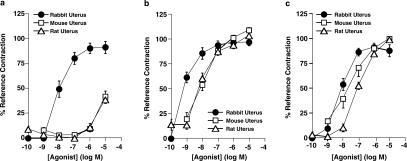
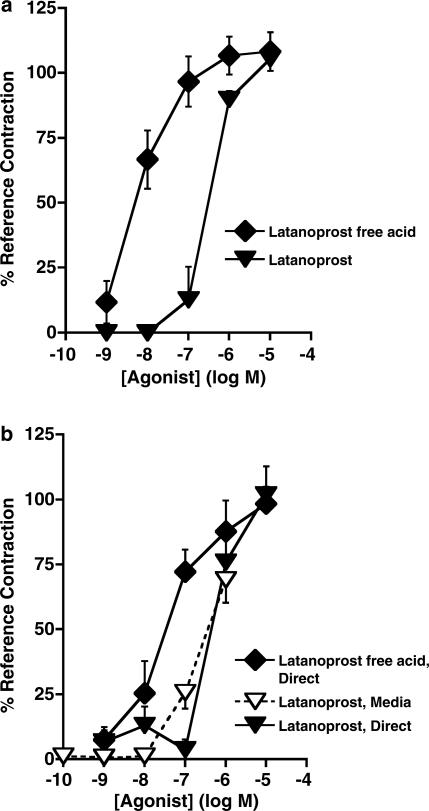
Bimatoprost produced potent contractile effects in the rabbit isolated uterus, but exhibited only weak activity in the mouse and rat uterus (Table 1, Figure 1a). 17-Phenyl PGF2α produced potent contractile effects in the rabbit, mouse, and rat uterus (Table 1, Figure 1b). PGF2α and latanoprost free acid were less active than 17-phenyl PGF2α in the tested preparations (Table 1, Figures 1c and 6a). Latanoprost was over 10 times weaker than latanoprost free acid in the rabbit and mouse uterus (Table 1, Figure 6).
Table 1.
Potencies of bimatoprost, 17-phenyl PGF2α, PGF2α, latanoprost free acid, and latanoprost
| Preparation | Bimatoprost (pEC50) | 17-phenyl PGF2α (pEC50) | PGF2α (pEC50) | Latanoprost free acid (pEC50) | Latanoprost (pEC50) |
|---|---|---|---|---|---|
| Rabbit uterus | 7.92±0.16 | 9.17±0.06 | 8.09±0.15 | 8.23±0.19 | 6.58±0.15 |
| Mouse uterus | <5 | 8.15±0.11 | 7.57±0.27 | 7.53±0.21 | 6.30±0.11 |
| Rat uterus | <5 | 8.18±0.11 | 7.06±0.07 | nt | nt |
| Rabbit intact jugular vein | 5.4* | 8.27±0.30 | 8.51±0.18 | nt | nt |
| Swiss 3T3 fibroblasts | Not active | 7.62±0.26 | 7.22±0.13 | nt | nt |
Mean pEC50 value±s.e.m. for isolated uterus of rabbit, mouse, rat (contraction), isolated endothelium-intact precontracted jugular vein of rabbit (vasorelaxation), and cultured Swiss 3T3 fibroblasts (stimulation of DNA synthesis). nt=not tested.
pEC50 value was obtained from the mean concentration–response curve because some tissues did not reach the 50% response level.
Figure 1.
Concentration–response curves for (a) bimatoprost, (b) 17-phenyl PGF2α, (c) PGF2α on contraction of rabbit, mouse, or rat isolated uterine preparations. Values are mean±s.e.m. of five to eight experiments, except on rabbit uterus where n=11 for bimatoprost and n=13 for PGF2α.
Figure 6.
Concentration–response curves for (a) latanoprost free acid and latanoprost in rabbit isolated uterus, (b) latanoprost free acid or latanoprost in the mouse isolated uterus (designated as Direct), compared to those of latanoprost and any potential active metabolites transferred from rabbit uterus media to the recipient mouse isolated uterus bioassay, graphed as final concentrations following 1 : 10 dilution (designated as Media). Results are expressed as mean±s.e.m. of five to six experiments.
Effects of bimatoprost and 17-phenyl PGF2α on PGF2α-sensitive human isolated myometrial preparations
In nonpregnant myometrium, 17-phenyl PGF2α produced dose-related contractions with a potency ED1 value of 0.06 nmol. (Table 2, Figure 2a). Bimatoprost elicited only detectable contractile responses that reached a maximum T/B ratio of 0.7±0.2 at the 100 nmol dose; this means that a response equivalent to the area of a background spontaneous contraction was not attained (Table 2, Figure 2a). In pregnant myometrium, 17-phenyl PGF2α produced dose-related contractions with a potency ED1 value of 6.0 nmol (Table 2, Figure 2b). Bimatoprost produced weak contractile responses that reached a maximum T/B ratio of 0.64±0.08 at the 500 nmol dose (Table 2, Figure 2b).
Table 2.
Potencies of bimatoprost and prostanoid FP receptor agonists on human myometrial strips taken from nonpregnant and pregnant donors
| Compound | Nonpregnant Myometrium ED1 (nmol) | Pregnant myometrium ED1 (nmol) |
|---|---|---|
| Bimatoprost | >100 | >500 |
| 17-phenyl PGF2α | 0.06 | 6.0 |
| Fluprostenol | 0.04a | 0.61b |
| PGF2α | 0.04a | 0.5b |
Mean ED1 value±s.e.m. (nmol) at a T/B ratio equal to 1.
Figure 2.
Mean stimulatory dose–effect curves for bimatoprost and 17-phenyl PGF2α in the superfused human isolated myometrium from (a) nonpregnant and (b) pregnant donors. Data are expressed as mean±s.e.m. of five experiments.
Effects of bimatoprost and FP receptor agonists on PGF2α-sensitive rabbit isolated jugular vein preparations
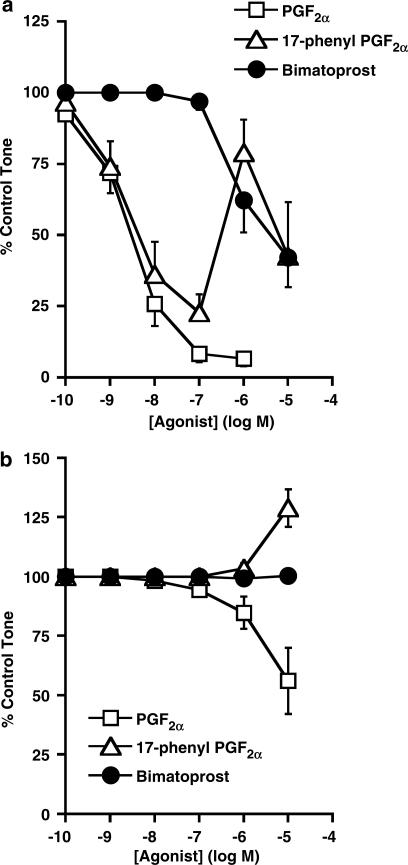
In the endothelium-intact histamine precontracted rabbit jugular vein, 17-phenyl PGF2α and PGF2α produced potent vasorelaxation (Table 1, Figure 3a). 17-Phenyl PGF2α produced maximal vasorelaxation at 100 nM, but a significant reversal of the vasorelaxant response occurred at 1 μM, P<0.05, paired t-test. Bimatoprost had weak vasorelaxant responses at high concentrations in the endothelium-intact preparation (Table 1, Figure 3a). The vasorelaxant activity of FP receptor agonists is dependent on an intact vascular endothelium (Chen et al., 1995). In the endothelium-denuded preparation, PGF2α had weak vasorelaxant effects of 15±7% (n=7) and 44±14% (n=4) at the 1 and 10 μM concentrations, respectively, which were not significantly different from the contraction elicited by histamine (Figure 3b). 17-Phenyl PGF2α produced no vasorelaxation but contraction at the high 10 μM concentration in endothelium-denuded preparations, whereas bimatoprost was inactive (Figure 3b).
Figure 3.
The activities of bimatoprost, 17-phenyl PGF2α, and PGF2α in histamine precontracted vascular endothelium-intact (a) and endothelium-denuded (b) rabbit isolated jugular vein preparations. Results are expressed as mean±s.e.m. of five to seven experiments.
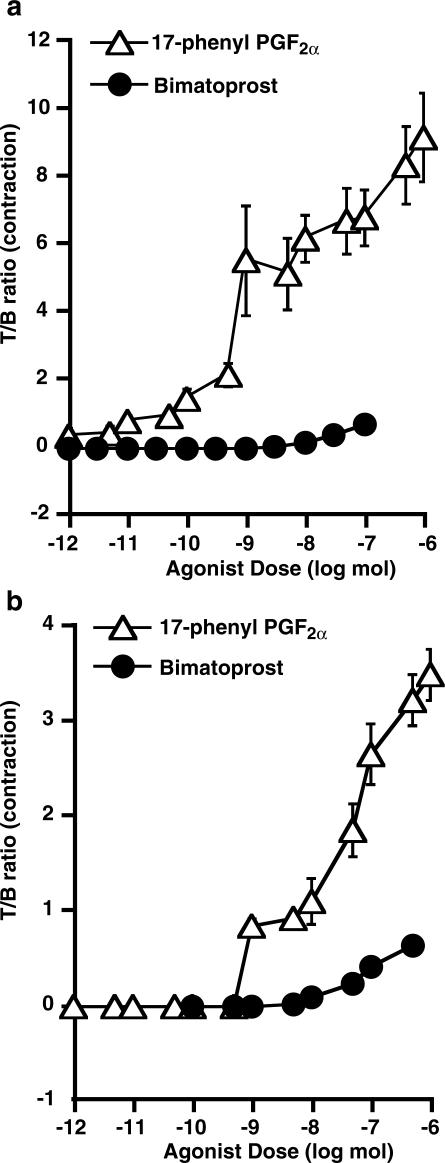
Agonist–agonist functional studies in mouse isolated uterus
The analysis of potential partial agonism by bimatoprost was performed by constructing a theoretical additive dose–response curve as described by Pöch & Holzmann (1980) and depicted in Figure 4a. Bimatoprost 1 μM pretreatment produced the same response (24%) as 17-phenyl PGF2α at a concentration of 1.3 nM (determined from curve in Figure 1b). The additive theoretical response was derived from the concentration–response curve of 17-phenyl PGF2α in Figure 4a. Comparison of the theoretical and experimental curves for the combination of bimatoprost and 17-phenyl PGF2α revealed virtually superimposed curves, with no significant difference at the maximum effects. This indicates bimatoprost is not a partial agonist at FP receptors in the mouse uterus.
Figure 4.
Concentration–response curves for (a) theoretical additive effects of 17-phenyl PGF2α and bimatoprost; 17-phenyl PGF2α and U-46619 in the presence of vehicle or 1 μM bimatoprost and (b) PGF2α and U-46619 in the presence of vehicle or 1 μM SQ 29548 in mouse isolated uterus. Results are expressed as mean±s.e.m. of five to six paired samples.
Comparisons of EC50 values show no significant difference for U-46619 in vehicle-treated and 1 μM bimatoprost-treated tissues (Figure 4a) and for PGF2α in vehicle-treated and 1 μM SQ 29548-treated tissues (Figure 4b). SQ 29548, at 1 μM, produced a parallel rightward shift in the log concentration–response curve for U-46619 and the respective EC50 values were significantly different, P<0.05, paired t-test (Figure 4b).
Bioassay metabolism studies: effects of bimatoprost and latanoprost on the rabbit isolated uterus using recipient mouse uterus
Studies to investigate the possibility that the effects of bimatoprost in the rabbit uterus could be explained by hydrolytic conversion of bimatoprost to 17-phenyl PGF2α were conducted using a bioassay detection method. Latanoprost and its free acid metabolite (latanoprost free acid) were tested for activity at 1 nM–10 μM in the rabbit uterus (Table 1, Figure 6a) and were also directly applied to the mouse uterus (Table 1, Figure 6b) to serve as comparators for potential metabolites from the rabbit uterus.
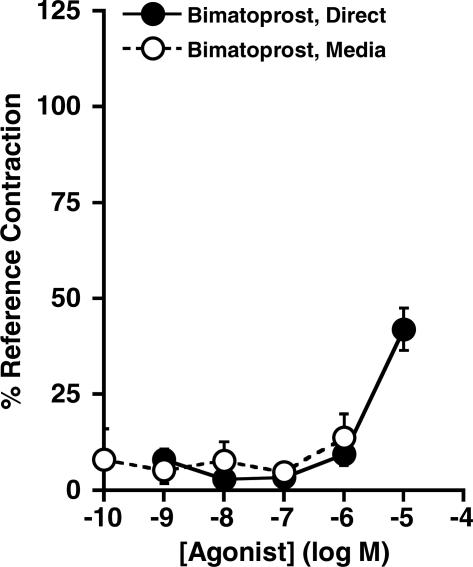
The effects of bimatoprost given by direct application to mouse uteri were not significantly different from those of bimatoprost transferred from rabbit uterus media to mouse uteri used for the bioassay detection (Figure 5). This result suggests no detectable formation of 17-phenyl PGF2α in the rabbit uterus occurred under these experimental conditions. The level of the contractile responses to latanoprost from rabbit uterus media (25.8±6.2%) at 100 nM was significantly greater than that obtained for latanoprost directly applied to the mouse uterus (3.8±3.8%), P<0.05, unpaired t-test, and similar to the effects of latanoprost free acid at the 10 nM concentration (25.5±12.4%) (Figure 6b). This finding suggests that some metabolism of latanoprost to latanoprost free acid may have occurred in the rabbit isolated uterus preparation.
Figure 5.
Effects of bimatoprost in mouse isolated uterine preparations (designated as Direct; n=8) were compared to those of bimatoprost and any potential active metabolites transferred from rabbit uterus media to the recipient mouse isolated uterus bioassay, graphed as final concentrations following 1 : 10 dilution (designated as Media; n=6). Results are expressed as mean±s.e.m.
Effects of bimatoprost and FP receptor agonists on a PGF2α-sensitive preparation of mouse Swiss 3T3 fibroblasts: stimulation of DNA synthesis
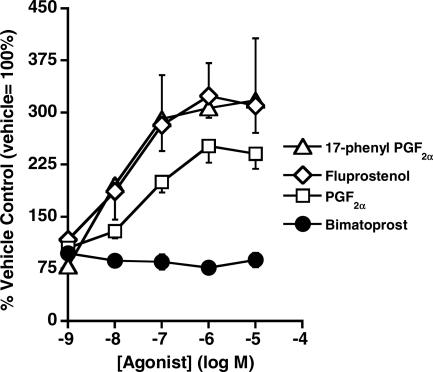
FP receptor agonists (17-phenyl PGF2α, fluprostenol, PGF2α) produced concentration-related stimulation of DNA synthesis, while bimatoprost was inactive (Table 1, Figure 7). The potency value for PGF2α (EC50: 88.7±21.5 nM) in stimulating DNA synthesis in these cells was close to previously reported values in Swiss 3T3 cells (EC50: 110–130 nM) (Jimenez de Asua et al., 1983). Vehicle-treated control values of 1895±163.6 c.p.m μg−1 DNA for [3H]-TdR incorporation into Swiss 3T3 cell DNA (n=46) were obtained in the experiments. 17-Phenyl PGF2α produced a maximal effect of 318±89% at the 10 μM concentration. PGD2 had weak stimulatory effects at 1 μM (132±5%) and 10 μM (164±6%) concentrations. The following compounds were inactive: BW 245C (DP>EP2-like), 8-epi PGF2α (F2-isoprostane; TP-like), U-46619 (TP), PGE2 (EP), 11-deoxy PGE1 (EP2/4>EP3>EP1), and sulprostone (EP3>EP1≫EP2/4). These results indicate that Swiss 3T3 fibroblasts represent a prostanoid FP receptor preparation.
Figure 7.
Concentration–response curves for bimatoprost, 17-phenyl PGF2α, fluprostenol, and PGF2α on stimulation of DNA synthesis in mouse cultured Swiss 3T3 fibroblasts (n=3–4, except n=12 for PGF2α). Values are expressed as mean±s.e.m. of (n) experiments.
Discussion
The studies described herein provide further pharmacological evidence to support the contention that bimatoprost exerts its effects by stimulating novel prostamide-sensitive receptors as opposed to prostanoid receptors. The agonist profiles of bimatoprost and 17-phenyl PGF2α were examined with respect to the following: (1) effects on discrete receptors (prostanoid FP and prostamide-sensitive); (2) partial agonism of bimatoprost at FP receptors; (3) metabolism of bimatoprost to the potent FP receptor agonist 17-phenyl PGF2α.
The contractile activity of bimatoprost was compared to that of FP receptor agonists in the rabbit isolated uterine preparation. The uterus was of particular interest since PGF2α, the natural prostanoid FP receptor agonist, and many other prostanoids are important modulators of mammalian reproductive function and dysfunction. Bimatoprost exhibited potent effects (EC50=28.1±13.7 nM) in the rabbit isolated uterus, results that are similar to those reported for the cat isolated iris sphincter (EC50=34 nM; Woodward et al., 2001) and cat isolated peripheral lung parenchyma (EC50=35–55 nM; Woodward et al., 2003). Prostanoid FP receptor agonists were also found to be highly active in rabbit uteri (Table 1; Chen et al., 1998). The potent activity of bimatoprost in the rabbit uterus suggests that its effects are mediated by prostamide-sensitive receptors in this tissue, based on previous reports that bimatoprost has no meaningful interaction (EC50 or IC50 values are inactive or >10,000 nM) with prostanoid receptors (Woodward et al., 2001; 2003) and its absent or weak effects on other FP receptor preparations described in the present investigation. A contrary conclusion that the agonist actions of bimatoprost are mediated by prostanoid FP receptors was drawn by Sharif et al. (2001; 2003). This reasoning was based on their findings of Ki and EC50 values for bimatoprost that ranged from 1,150 to 9,250 nM (Sharif et al., 2001; 2003) and which did not take into account the potent activities of bimatoprost and PGF2α-1-ethanolamide in certain preparations (Woodward et al., 2001; 2003; Matias et al., 2004). In the context of comparative potency, the established FP receptor agonist 17-phenyl PGF2α was reported to have EC50 or Ki values between 15 and 83 nM (Sharif et al., 2001; 2003). Taken together, these data suggest that the primary pharmacology of bimatoprost may be explained by its activity at putative prostamide-sensitive receptors (Matias et al., 2004) rather than by weak interaction with prostanoid FP receptors.
The use of uterine tissues under bioassay conditions in the present studies minimised differences in preparation. 17-Phenyl PGF2α elicited potent contractile effects in the mouse, rat, and rabbit uterus. In contrast, bimatoprost potently contracted the rabbit uterus, but was weakly active in the mouse and rat uterus. The large separation of effects between 17-phenyl PGF2α (potent) and bimatoprost (weakly active) in the mouse and rat uterus was also demonstrated in the superfused human isolated myometrium. 17-Phenyl PGF2α produced excitatory responses of similar potency to fluprostenol, a selective FP receptor agonist (Coleman et al., 1998), and PGF2α in human nonpregnant isolated myometrium (Table 2). The test agents were less active in pregnant myometrium compared to nonpregnant myometrium, but the differential activity between FP agonists and bimatoprost was evident in both human preparations.
In comparison to PGF2α, 17-phenyl PGF2α was more potent in the mouse and rat uterus, but less potent in the rabbit jugular vein (Table 1). The potency value for 17-phenyl PGF2α in endothelium-intact rabbit jugular vein preparations may be affected by a complex concentration–response curve that is composed of vasorelaxation and reversal of the vasorelaxant response. TP receptor stimulation does not readily explain reversal of the vasorelaxant response, since the TP antagonists used to minimise the contractile influence are highly effective, except possibly at the 10 μM agonist concentration as shown in the endothelium-denuded preparation. FP receptors in the vascular smooth muscle are not likely to interfere with the potency estimation at endothelial FP receptors, since fluprostenol is inactive in endothelium-denuded preparations (Giles et al., 1990; Chen et al., 1995). Reversal of the vasorelaxant response to 17-phenyl PGF2α may involve an endothelial FP receptor or even perhaps a previously unrecognised receptor that may cause endothelium-induced vasoconstriction.
The activity profile of an agonist may differ for species homologues of the same receptor which exhibit amino-acid sequence and binding differences (Hall et al., 1993; Watson, 1995). Hence, findings of the same agonist activity profile in different species and, in addition, a different rank order of agonist potency in preparations from within the same species may suggest that differences in activity cannot be ascribed to species homologues of the same receptor. Our results show that the activity of bimatoprost (weak) was the same in uterine preparations of human, mouse, and rat. In rabbits, bimatoprost exhibited potent activity in the uterus and weak activity in the endothelium-intact jugular vein. These results provide pharmacological evidence that species differences are not likely to account for the different activity profiles of bimatoprost. Molecular biological studies will ultimately be needed to confirm these pharmacological findings. Nevertheless, our studies yielded valuable pharmacological information since the activity of bimatoprost has not been previously compared in this manner.
Partial agonism at prostanoid FP receptors as a possible explanation for bimatoprost activity was addressed in our studies. A partial agonist is described as a compound that displays a large range of intrinsic activities at the same receptor in different functional tests (Hoyer & Boddeke, 1993; Leff, 1995). A rigorous approach using a functional interaction method (Stephenson, 1956; Pöch & Holzmann, 1980; Dougall, 2001) was used to study the effects of a full agonist 17-phenyl PGF2α at FP receptors in the presence of bimatoprost at a high concentration. The responses to 17-phenyl PGF2α and a TP agonist, U-46619, were not antagonised by bimatoprost. Additional controls were provided by pretreatment of mouse uterine tissues with 1 μM SQ 29548 (TP antagonist), which resulted in antagonism of U-46619 effects but not PGF2α effects. These results are consistent with the complete absence of bimatoprost effects on FP receptor-mediated DNA synthesis in mouse Swiss 3T3 fibroblasts (Table 1, Figure 7). The functional interaction studies described herein do not support the argument that bimatoprost behaves as a partial agonist at FP receptors.
Metabolism may be a complicating factor in the quantification of drug response. Although this factor is minimised by the use of isolated tissue bioassays (Ariëns et al., 1964), we investigated the possibility that hydrolysis of bimatoprost to its putative free acid metabolite 17-phenyl PGF2α could account for its potent effects in the rabbit uterus. The potential enzymatic hydrolysis of bimatoprost to 17-phenyl PGF2α in the rabbit isolated uterus was tested using mouse uterus as a sensitive preparation for bioassay detection. The absence of significant contractile responses in the recipient mouse uterus to bimatoprost obtained from medium bathing the rabbit uterus suggested that the effects of bimatoprost in the rabbit uterus are due to its inherent activity and not to metabolism. Low rates of hydrolysis that translated to a 0.3–1.75% formation of 17-phenyl PGF2α after 1 h of incubation with bimatoprost were previously reported in human and animal ocular tissues (Maxey et al., 2002; Davies et al., 2003). Results of a recent in vitro study further support the metabolic stability of bimatoprost in that no hydrolysis product (17-phenyl PGF2α) was found following a 4 h incubation of bimatoprost with rat brain, cat ciliary body, and cat iris homogenates (Matias et al., 2004). The isopropyl ester prodrug latanoprost was employed as a positive control in the present studies, since it is completely hydrolysed (100%) to latanoprost free acid in porcine corneas after 4 h incubation (Basu et al., 1994). The possibility was considered that bimatoprost and latanoprost are hydrolysed in the rabbit uterus, but that the generated free acid metabolites are sequestered and slowly released from tissue to bathing medium, resulting in an underestimation of free acid products under bioassay conditions. This process appears unlikely given the following: (1) bimatoprost is not a substrate for fatty acid amide hydrolase (FAAH) or the anandamide cellular uptake system (Matias et al., 2004); (2) the concentration–response curve for latanoprost from rabbit media is displaced to the left of the curve for latanoprost in the mouse uterus, which demonstrates that enzymatic hydrolysis to free acid products is indeed detectable in the medium of rabbit uterine tissues.
The present investigation, using pharmacological approaches to receptor characterisation, provided further evidence for the existence of a novel and discrete population of receptors sensitive to bimatoprost. Species differences, partial agonism, and metabolism did not provide acceptable explanations for the different activity profiles of bimatoprost and C1-acid FP receptor agonists. Results of the functional agonist–agonist interaction studies added support to the contention that bimatoprost does not act at prostanoid FP receptors. The nature of the postulated bimatoprost-sensitive receptor is not known. FP receptors and bimatoprost-sensitive receptors may co-exist in tissues such as the rabbit uterus, cat iris sphincter, and cat lung parenchyma. Alternatively, these tissues may contain a receptor that recognises both bimatoprost and FP agonists. Classification of bimatoprost-sensitive receptors as prostamide receptors or as a new subtype of known receptor awaits identification of specific antagonists or structural identification of the target protein.
Acknowledgments
We would like to thank Mr J.K. Clayton, staff, and patients of the Bradford Royal Infirmary for supplying the human tissues used in this study. We thank S. Venadas for technical assistance.
Abbreviations
- AUC
area under the curve
- DMEM
Dulbecco's modified Eagle's medium
- FAAH
fatty acid amide hydrolase
- FBS
foetal bovine serum
- HBSS
Hanks' balanced salt solution
- [3H]-TdR
[methyl-3H]-thymidine
- IOP
intraocular pressure
- PGD2
prostaglandin D2
- PGE1
prostaglandin E1
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- TCA
trichloroacetic acid
References
- ARIËNS E.J., SIMONIS A.M., VAN ROSSUM J.M.Drug–receptor interaction: interaction of one or more drugs with one receptor system Molecular Pharmacology: The Mode of Action of Biologically Active Compounds 1964New York, London: Academic Press Inc; 119–286.vol 1. ed. Ariëns, E.J. pp [Google Scholar]
- BASU S., SJÖQUIST B., STJERNSCHANTZ J., RESUL B. Corneal permeability to and ocular metabolism of phenyl substituted prostaglandin esters in vitro. Prostagland. Leukotr. Essent. Fatty Acids. 1994;50:161–168. doi: 10.1016/0952-3278(94)90139-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. Determination of DNA concentration with diphenylamine. Methods Enzymol. 1979;23:163–166. [Google Scholar]
- CHEN J., CHAMPA-RODRIGUEZ M.L., WOODWARD D.F. Identification of a prostanoid FP receptor population producing endothelium-dependent vasorelaxation in the rabbit jugular vein. Br. J. Pharmacol. 1995;116:3035–3041. doi: 10.1111/j.1476-5381.1995.tb15960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J., WOODWARD D.F., YUAN Y.-D., MARSHALL K., SENIOR J. Prostanoid-induced contraction of the rabbit isolated uterus is mediated by FP receptors. Prostaglandins Other Lipid Mediat. 1998;55:387–394. doi: 10.1016/s0090-6980(98)00034-3. [DOI] [PubMed] [Google Scholar]
- CHEN J., WOODWARD D.F., COLEMAN R.A., JONES R.L., LYDFORD S.J.Prostanoid receptor assays Unit 4.18 Current Protocols in Pharmacology 2001New York, NY, U.S.A.: John Wiley & Sons, Inc; vol 1. ed. ENNA, S.J., WILLIAMS, M., FERKANY, J.W., KENAKIN, T., PORSOLT, R.D. & SULLIVAN, J.P. pp. 4.18.1–4.18.41 [DOI] [PubMed] [Google Scholar]
- CHEN J., WOODWARD D.F. Lumigan®: a novel drug for glaucoma therapy. Optometry Pract. 2002;3:95–102. [Google Scholar]
- COLEMAN R.A., ABRAMOVITZ M., BREYER M.D., JONES R.L., NARUMIYA S., SHIMIZU T., WOODWARD D.F.Prostanoid receptors The IUPHAR Compendium of Receptor Characterization and Classification 1998London: IUPHAR Media; 229–244.First edn. ed. GIRDLESTONE, D. pp [Google Scholar]
- DAVIES S.S., JU W.-K., NEUFELD A.H., ABRAN D., CHEMTOB S., ROBERTS II L.J. Hydrolysis of bimatoprost (lumigan) to its free acid by ocular tissue in vitro. J. Ocul. Pharmacol. Ther. 2003;19:45–54. doi: 10.1089/108076803762718105. [DOI] [PubMed] [Google Scholar]
- DOUGALL I.G. Functional methods for quantifying agonists and antagonists. J. Recept. Signal Transduct. Res. 2001;21:117–137. doi: 10.1081/rrs-100107425. [DOI] [PubMed] [Google Scholar]
- GANDOLFI S.A., CIMINO L. Effect of bimatoprost on patients with primary open-angle glaucoma or ocular hypertension who are nonresponders to latanoprost. Ophthalmology. 2003;110:609–614. doi: 10.1016/S0161-6420(02)01891-2. [DOI] [PubMed] [Google Scholar]
- GILES H., BOLOFO M.L., LEFF P. Rabbit jugular vein contains three different relaxant prostaglandin receptors. Br. J. Pharmacol. 1990;99:202P. [Google Scholar]
- HALL J.M., CAULFIELD M.P., WATSON S.P., GUARD S. Receptor subtypes or species homologues: relevance to drug discovery. Trends Pharmacol. Sci. 1993;14:376–383. doi: 10.1016/0165-6147(93)90096-3. [DOI] [PubMed] [Google Scholar]
- HIGGINBOTHAM E.J., SCHUMAN J.S., GOLDBERG I., GROSS R.L., VANDENBURGH A.M., CHEN K., WHITCUP S.M. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch. Ophthalmol. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- HOYER D., BODDEKE H.W.G.M. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol. Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- JIMENEZ DE ASUA L., OTTO A.M., LINDGREN J.Å., HAMMARSTRÖM S. The stimulation of the initiation of DNA synthesis and cell division in Swiss mouse 3T3 cells by prostaglandin F2α requires specific functional groups in the molecule. J. Biol. Chem. 1983;258:8774–8780. [PubMed] [Google Scholar]
- LEFF P. The two-state model of receptor activation. Trends Pharmacol. Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- MARCELO C.L., KIM Y.G., KAINE J.L., VOORHEES J.J. Stratification, specialization, and proliferation of primary keratinocyte cultures. Evidence of a functioning in vitro epidermal cell system. J. Cell Biol. 1978;79:356–370. doi: 10.1083/jcb.79.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATIAS I., CHEN J., DE PETROCELLIS L., BISOGNO T., LIGRESTI A., FEZZA F., KRAUSS A.H.-P., SHI L., PROTZMAN C.E., LI C., LIANG Y., NIEVES A.L., KEDZIE K.M., BURK R.M., DI MARZO V., WOODWARD D. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J. Pharmacol. Exp. Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- MAXEY K.M., JOHNSON J.L., LABRECQUE J. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv. Ophthalmol. 2002;47 Suppl 1:S34–S40. doi: 10.1016/s0039-6257(02)00323-5. [DOI] [PubMed] [Google Scholar]
- NOECKER R.S., DIRKS M.S., CHOPLIN N.T., BERNSTEIN P., BATOOSINGH A.L., WHITCUP S.M. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am. J. Ophthalmol. 2003;135:55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- OGLETREE M.L., HARRIS D.N., GREENBERG R., HASLANGER M.F., NAKANE M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J. Pharmacol. Exp. Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- PARRISH R.K., PALMBERG P., SHEU W.-P. A comparison of latanoprost, bimatoprost and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am. J. Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- PASQUALE E.B., MAHER P.A., SINGER S.J. Comparative study of the tyrosine phosphorylation of proteins in Swiss 3T3 fibroblasts stimulated by a variety of mitogenic agents. J. Cell. Physiol. 1988;137:146–156. doi: 10.1002/jcp.1041370118. [DOI] [PubMed] [Google Scholar]
- PÖCH G., HOLZMANN S. Quantitative estimation of overadditive and underadditive drug effects by means of theoretical, additive dose–response curves. J. Pharmacol. Methods. 1980;4:179–188. doi: 10.1016/0160-5402(80)90036-4. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., WILLIAMS G.W., KELLY C.R. Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur. J. Pharmacol. 2001;432:211–213. doi: 10.1016/s0014-2999(01)01486-8. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., KELLY C.R., WILLIAMS G.W. Bimatoprost (Lumigan®) is an agonist at the cloned human ocular FP prostaglandin receptor: real-time FLIPR-based intracellular Ca2+ mobilization studies. Prostagland. Leukotr. Essent. Fatty Acids. 2003;68:27–33. doi: 10.1016/s0952-3278(02)00232-6. [DOI] [PubMed] [Google Scholar]
- SENIOR J., MARSHALL K., SANGHA R., BAXTER G.S., CLAYTON J.K. In vitro characterization of prostanoid EP-receptors in the non-pregnant human myometrium. Br. J. Pharmacol. 1991;102:747–753. doi: 10.1111/j.1476-5381.1991.tb12244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENIOR J., SANGHA R., BAXTER G.S., MARSHALL K., CLAYTON J.K. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br. J. Pharmacol. 1992;107:215–221. doi: 10.1111/j.1476-5381.1992.tb14489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENIOR J., MARSHALL K., SANGHA R., CLAYTON J.K. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br. J. Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON R.P. A modification of receptor theory. Br. J. Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON S.P. Factors to consider in the naming of a G protein-coupled receptor subtype. J. Recept. Signal Transduct. Res. 1995;15:5–17. doi: 10.3109/10799899509045202. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R.D. Efficacy of bimatoprost in glaucoma and ocular hypertension unresponsive to latanoprost. Adv. Ther. 2002;19:275–281. doi: 10.1007/BF02853173. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., FAIRBAIRN C.E., KRAUSS A.H.-P., LAWRENCE R.A., PROTZMAN C.E. Radioligand binding analysis of receptor subtypes in two FP receptor preparations that exhibit different functional rank orders of potency in response to prostaglandins. J. Pharmacol. Exp. Ther. 1995;273:285–291. [PubMed] [Google Scholar]
- WOODWARD D.F., KRAUSS A.H.-P., CHEN J., LAI R.K., SPADA C.S., BURK R.M., ANDREWS S.W., SHI L., LIANG Y., KEDZIE K.M., CHEN R., GIL D.W., KHARLAMB A., ACHEAMPONG A., LING J., MADHU C., NI J., RIX P., USANSKY J., USANSKY H., WEBER A., WELTY D., YANG W., TANG-LIU D.D.-S., GARST M.E., BRAR B., WHEELER L.A., KAPLAN L.J. The pharmacology of bimatoprost (Lumigan™) Surv. Ophthalmol. 2001;45 Suppl 4:S337–S345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., KRAUSS A.H.-P., CHEN J., LIANG Y., LI C., PROTZMAN C.E., BOGARDUS A., CHEN R., KEDZIE K.M., KRAUSS H.A., GIL D.W., KHARLAMB A., WHEELER L.A., BABUSIS D., WELTY D., TANG-LIU D.D.-S., CHERUKURY M., ANDREWS S.W., BURK R.M., GARST M.E. Pharmacological characterization of a novel antiglaucoma agent, bimatoprost (AGN 192024) J. Pharmacol. Exp. Ther. 2003;305:772–785. doi: 10.1124/jpet.102.047837. [DOI] [PubMed] [Google Scholar]