Abstract
Purine and pyrimidine compounds were investigated on hamster proximal urethral circular smooth muscle preparations. In situ hybridization studies were carried out to localize P2Y1, P2Y2, P2Y4 and P2Y6 mRNA. Protein expression was studied using Western blotting analysis with antibodies against P2Y1 and P2Y2 receptors.
The hamster urethra relaxed with an agonist potency order of: 2-MeSADP>β,γ-meATP=ATP=adenosine=ADP>2-MeSATP>α,β-meATP>TTP>CTP=UTP>GTP=UDP. The high potency of 2-MeSADP is suggestive of an action via P2Y1 receptors. Although the order is not characteristic for any known single P2Y receptor subtype, it may represent a combination of P2Y receptor subtypes.
The selective P2Y1 receptor antagonist MRS2179 inhibited ATP-, 2-MeSADP-, 2-MeSATP-, β,γ-meATP-, and to a lesser degree α,β-meATP-induced responses.
Adenosine, but not ATP, was inhibited by the adenosine receptor antagonist 8-phenyltheophylline, indicating that ATP was not acting via adenosine following enzymatic breakdown.
Western blotting analysis showed the expression of both P2Y1 and P2Y2 receptors, confirming the results obtained with in situ hybridization that showed the expression of both P2Y1 and P2Y2, but not P2Y4 or P2Y6 mRNA, in smooth muscle layers of the hamster proximal urethra.
It is proposed that the relaxant response of the urethra to ATP may be evoked through the activation of the combination of receptors for P2Y1 and to a lesser extent P2Y2 receptors, which may mediate a trophic effect in addition. A P2Y subtype responsive to α,β-meATP and P1 receptors may contribute to urethral smooth muscle relaxation.
Keywords: Hamster urethra, extracellular ATP, P2Y1, P2Y2receptors
Introduction
Previous studies have shown that in the hamster and other mammals, including humans, the main non-adrenergic, noncholinergic (NANC) inhibitory neurotransmitter of the urethra is nitric oxide (NO) (Garcia-Pascual et al., 1991; Andersson & Persson, 1993; Parlani et al., 1993; Lee et al., 1994; Burnstock, 2001). However, our previous studies and those of others, revealed that inhibitors of NO production do not block completely the neurogenic relaxant response induced by electrical stimulation, suggesting the involvement of at least one other inhibitory transmitter in addition to NO (Hashimoto et al., 1993; Werkstrom et al., 1995; Pinna et al., 1996; 1998; 1999; Ohnishi et al., 1997). These studies also demonstrated that relaxation of hamster and rabbit urethral strips to electrical field stimulation and to exogenous ATP were affected by ATP antagonists such as suramin and Reactive blue 2, suggesting that ATP is a cotransmitter, together with NO and possibly with vasoactive intestinal polypeptide (Hashimoto et al., 1993; Burnstock, 1999). There is already considerable evidence showing ATP as an excitatory cotransmitter with acetylcholine in the urinary bladder (Burnstock, 2001). It has been suggested that P2Y receptors mediate the relaxation of urethral smooth muscle in rat and rabbit (Callahan & Creed, 1981; Pinna et al., 1996; 1998; Ohnishi et al., 1997; Otomo et al., 1999; Andersson, 2001). However, the physiological role of ATP and related purines in this tissue is poorly understood and the localization of purinoceptor subtypes in the urethra has yet to be demonstrated.
The aim of this study was to further characterize the subtype(s) of P2 receptor mediating relaxation of the hamster proximal urethra by pharmacological means and to localize receptors by means of in situ hybridization and Western blotting analysis.
Methods
Tissue preparation
Male golden Syrian hamsters weighing (100–115 g, 1 month old) were used for the experiments. Procedures involving animals and their care conformed to Institutional Guidelines that comply with National (D.L. no. 116 G.U. suppl. 40, 18 February 1992) and International Laws and Policies (EEC Council Directive 86/609, OJL 358, 1, 12 December 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985). Animals were killed by asphyxiation with CO2 and death was confirmed by cervical dislocation, the abdomen was opened, and the bladder and the urethra were quickly removed as described previously (Pinna et al., 1996). The urethra was then separated from the bladder by a transverse cut at the level of the bladder neck.
Organ bath experiments
Urethral tissues were placed in cold, modified Krebs solution of the following composition (mM): NaCl 133, KCl 4.7, NaH2PO4 1.4, MgSO4 0.6, CaCl2 2.5, NaHCO3 16.4 and glucose 7.7, pH 7.3, when gassed with 95% O2/5% CO2.
Two circular smooth muscle strips (2 × 1 mm) were prepared from each urethra under a dissecting microscope and suspended in 5-ml organ baths containing modified Krebs solution gassed with 95% O2/5% CO2 at 37±0.5°C. Tissues were equilibrated for 1 h under a resting tension of 0.5 g (4.9 mN). Force was recorded by means of a Grass FT03C isometric force transducer coupled to a four-channel Grass 79D ink-writing oscillograph.
Preliminary experiments were performed to ensure that arginine vasopressin (AVP) at 10 nM (approximate EC50 concentration) induced a stable and long-lasting contraction of the preparations.
After the equilibration period, cumulative concentration–response curves to adenosine, ADP, ATP, 2-methylthio ADP (2-MeSADP), 2-methylthio ATP (2-MeSATP), α,β-methylene ATP (α,β-meATP), β,γ-methylene ATP (β,γ-meATP), CTP, GTP, TTP, UDP and UTP were constructed in strips precontracted with AVP (10 nM). Each preparation was challenged with one agonist only and time controls were carried out to ensure that responses were reproducible. The tissues were then washed several times with fresh Krebs solution and left (for about 1 h) until the resting tone had recovered and tonic contractions had ceased. The selective P2Y1 antagonist N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179; 1–30 μM), the adenosine antagonist 8-phenyltheophylline (8-PT; 30 μM) and the adenosine uptake inhibitor dipyridamole (10 μM) were added to the bath 30 min before the preparations were once again precontracted with AVP and subjected to the same agonists.
In situ hybridization
Probes
Antisense oligonucleotide probes, 45 nucleotides in length, for rat P2Y1, P2Y2, P2Y4 and P2Y6 receptors were obtained from either Genosys (U.K.) or MWG Biotech (Germany) and were labelled at their 3′-end with the DIG oligonucleotide tailing kit (Roche Diagnostics) according to the manufacturer's instructions. The sequences for the probes were: for P2Y1, 5′-ACG TGG CAT AAA CCC TGT CAT TGA AAG CAC ACA TTG CTG GGG TCT-3′, for P2Y2, 5′-GAT GGC GTT GAG GGT GTG GCA ACT GAG GTC AAG TGA TCG GAA GGA-3′, for P2Y4, 5′-GAC AAT GTT CAG CAC ATG ACA GTC AGC TTG CAA CAG TCT TCG CTG-3′ and for P2Y6, 5′-CGC TTC CTC TTC TAT GCC AAC CTA CAC GGC AGC ATC CTG TTC CTC-3′. The specificity of each probe was confirmed by screening the Genbank database to crossreact with mammalian P2Y1, P2Y2, P2Y4 and P2Y6 receptors, respectively.
Tissue handling
Proximal urethra were removed quickly and immediately put in ice-cold Hanks balanced salts solution, pH 7.5 (GIBCO BRL, Scotland). Unfixed tissues were embedded in Tissue-Tek (Sakura Finetek, Netherlands) and frozen in isopropanol precooled in liquid nitrogen. Cryostat sections (10 μm) were cut and placed on poly-L-lysine-coated slides. Tissues were postfixed for 6 min at room temperature in 4% formaldehyde (BDH Laboratory Supply, U.K.) in phosphate-buffered saline (PBS).
Hybridization
Tissues were dehydrated in ethanol (70, 80, 90 and 100%), air-dried and incubated in pre-hybridization buffer (50% formamide; 2 × saline sodium citrate buffer (SSC); 1 × Dennhardt's), 1 mg ml−1 denatured, sheared salmon sperm DNA (Sigma) and 1 mg ml−1 tRNA type X from bakers yeast (Sigma) for 1 h at 37°C in a humidified chamber. This was followed by incubation in hybridization buffer (1 ng labelled probe in 1 μl prehybridization buffer) at 37°C overnight. Washing of unhybridized probe was carried out as follows: 2 × 5 min in 2 × SSC at room temperature, 2 × 15 min in 2 × SSC at 37°C, 2 × 15 min in 1 × SSC at 37°C, with a final stringency wash of 2 × 30 min in 0.5 × SSC at 37°C. DIG-labelled probes were detected by anti-DIG-alkaline phosphates labelled Fab fragments (Boehringer/Mannheim). Negative controls included omission of the probe and competing labelled probe with a 75-fold excess of unlabelled probe.
Electrophoresis and Western blotting analysis
Total proteins from three hamster proximal urethras (30 mg) were extracted with 210 μl of lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 25 mM NaF, 0.5% sodium deoxycholate, 10% SDS, 1 mM EGTA, 1 mM phenylmethylsulphonyl fluoride, 1 mM orthovanadate, 10 mM sodium pyrophosphate supplemented with complete protease inhibitors cocktail 5 μl ml−1, and 2% β-mercaptoethanol. The protein extract was subjected to four cycles of ultrasonication for 30 s. The lysates were then boiled for 5 min and centrifuged for 10 min at 13,000 r.p.m. The supernatant (about 200 μl) was collected and incubated with 1800 μl of iced acetone for 30 min at −20°C. Lysates were then centrifuged at 8000 r.p.m. for 10 min at −4°C, and the pellet was collected and solubilized in 150 μl of lysis buffer. A protein determination was performed to establish the final protein concentration. Protein electrophoresis was performed on 5% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) at a constant voltage of 120 V for 120 min, using a Bio-Rad Mini-Protein II cell (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Protein loadings were 40 and 60 μg in each well. Proteins were electrophoretically transferred to nitrocellulose membranes Hybond-C extra (Amersham U.K., Amersham), using a transblotter (Mini-Protein II) at a constant voltage of 100 V for 70 min in transfer buffer containing Tris–HCl (25 mM), glycine (192 mM) and 20% methanol. After transfer, the membranes were blocked for 1 h in TBS containing 0.1% Tween 20 and 5% dried skimmed milk. The membranes were incubated with rabbit anti-P2Y1 (diluted 1 : 200), rabbit anti-P2Y2 (diluted 1 : 200) (Alomone Labs, Jerusalem, Israel) and negative control (the antibodies were preincubated with their homologous peptide antigen for 1 h at room temperature), overnight. The membranes were washed three times with washing buffer at room temperature and were incubated with an antirabbit antibody IgG peroxidase labelled (diluted 1 : 5000) (Vector Laboratories, Inc., Burlingame, CA, U.S.A.) for 45 min. After washing, a chemiluminescence system ECL Western blotting RPN 2108 (Amersham Pharmacia Biotech) was used to visualize the reaction product, and the signals were detected by autoradiography with Hyperfilm ECL (Amersham Pharmacia Biotech). Photographs were taken using an image scanner (CanoScan D1250, Tokyo, Japan) and pictures were processed using Paint Shop Pro 5 software (Jasc Software, Inc., U.K.) on an Olidata IBM-compatible PC. The procedure for electrophoresis and Western blotting analysis was repeated, using a protein extract from three different hamsters, with identical results.
Solutions and drugs
Adenosine, ADP, ATP, AVP, 2-MeSADP, 2-MeSATP, α,β-meATP, β,γ-meATP, CTP, GTP, TTP, UDP, UTP, 8-PT, dipyridamole and MRS2179 were all purchased from Sigma-Aldrich Company Ltd (Poole, U.K.). All compounds were dissolved in distilled water, with the exception of 8-PT, which was dissolved in 50% dimethyl sulfoxide/50% distilled water.
Data analysis
All the data are the means±s.e.m. of six to eight individual observations. Relaxant responses to ATP and related purines and pyrimidines are expressed as % inhibition of the AVP-induced contraction. Concentration–response curves were prepared by means of the software Prism v.3.0 (GraphPad Software, San Diego, CA, U.S.A.): for each curve, the program calculates the lower and upper plateau, the slope and the EC50 value±s.e.m. The potency of the agonist in causing relaxation was expressed as the negative log10 of the molar concentration of the agonist producing 25% of the response (p[A]25), calculated by non-linear regression analysis of the individual log concentration–response curves. A one-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to compare p[A]25 values and maximal responses to agonists. Concentration–response curves and the effect of antagonists were compared by a two-way ANOVA followed by a Tukey's post hoc test. A probability level of P<0.05 was considered significant for all tests.
Results
Effects of ATP, ADP and adenosine
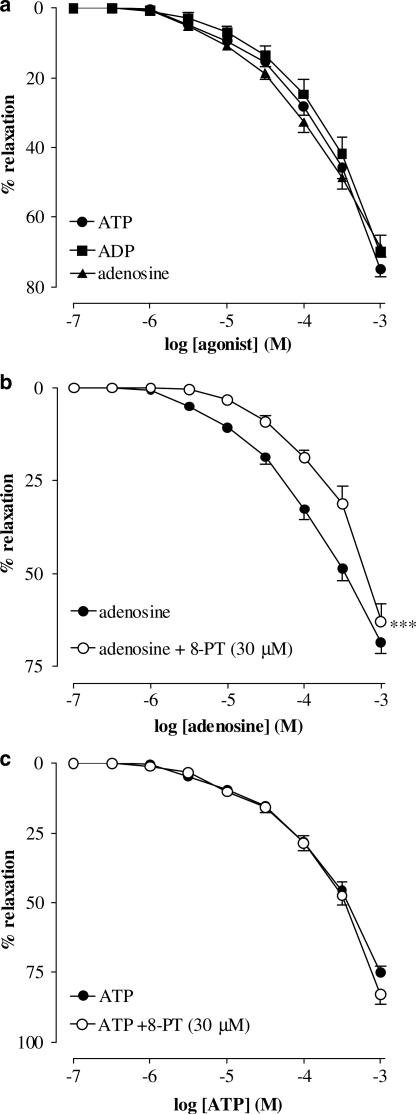
In circular smooth muscle strips of hamster proximal urethra, AVP (10 nM) induced stable, reproducible contractions (mean contraction 208.2±23.6 mg). Exogenous ATP (1 μM–1 mM), ADP (1 μM–1 mM) and adenosine (1 μM–1 mM) elicited concentration-dependent relaxations of the urethra (measured as % inhibition of the AVP-induced contraction) (Figure 1a). The p[A]25 values, threshold concentration to induce a response and the maximum relaxation at the highest concentration tested for all agonists tested are shown in Table 1.
Figure 1.
Cumulative concentration–response curves to ATP, ADP and adenosine on AVP (10 nM) precontracted urethral strips. (a) Concentration–response curves to ATP, ADP and adenosine (all from 0.1 μM to 1 mM). (b) Concentration–response curves to adenosine in the absence and presence of 8-PT (30 μM). (c) Concentration–response curves to ATP in the absence and presence of 8-PT (30 μM). All points show the mean±s.e.m. of eight experiments, unless occluded by the symbol. ***P<0.001 following a two-way ANOVA and Tukey's post hoc test.
Table 1.
Threshold concentration of agonist inducing a relaxant response, maximum relaxation (relaxant responses to all agonists were expressed as % inhibition of the AVP-induced contraction) observed at 1 mM concentration, and p[A]25 (concentration that induced 25% relaxation) values±s.e.m. of six to eight experiments in the hamster proximal urethra
| Threshold concentration (μM) | Relaxation in response to 1 mM (%) | p[A]25 | |
|---|---|---|---|
| ATP | 1.0 | 74.9±0.2 | 4.13±0.07 |
| ADP | 1.0 | 70.0±4.8 | 4.03±0.07 |
| Adenosine | 1.0 | 68.6±3.0 | 4.28±0.05 |
| α,β-meATP | 0.3 | 53.8±3.6 | 3.50±0.08a,b |
| β,γ-meATP | 0.1 | 63.7±4.4 | 4.25±0.09 |
| 2-MeSADP | 0.1 | 73.1±4.9 | 4.45±0.10 |
| 2-MeSATP | 3.0 | 59.0±3.1 | 3.89±0.07a,b |
| CTP | 100 | 44.4±3.3 | 3.31±0.17a,b |
| GTP | 30 | 32.0±5.5 | 3.16±0.16a,b |
| TTP | 10 | 48.1±3.9 | 3.43±0.12a,b |
| UDP | 3.0 | 19.2±4.5 | <3.00 |
| UTP | 1.0 | 34.0±3.8 | 3.23±0.13a,b |
Indicates significant difference of agonist p[A]25 values from p[A]25 of ATP (P<0.05 or less).
Indicates significant difference of agonist p[A]25 values from p[A]25 of 2-MeSADP (P<0.05 or less).
Effects of an adenosine receptor antagonist and uptake blocker
The adenosine receptor antagonist 8-PT (30 μM) significantly (P<0.001) inhibited adenosine-induced relaxations (Figure 1b) on AVP (10 nM) precontracted urethral strips, but failed to inhibit ATP-induced relaxations (Figure 1c). The adenosine uptake inhibitor dipyridamole (10 μM) had no effect on ATP-induced relaxations (data not shown).
Effects of ATP analogues, purines and pyrimidines
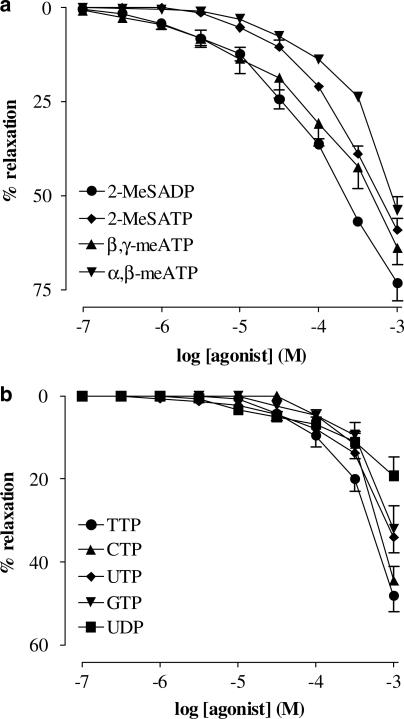
Exogenous 2-MeSADP, 2-MeSATP, α,β-meATP and β,γ-meATP (all 0.1 μM–1 mM) evoked concentration-dependent relaxations of AVP-precontracted strips of urethra (Figure 2a). In AVP-precontracted strips, CTP, GTP, UDP, UTP and TTP (Figure 2b) induced concentration-dependent relaxant responses, which were significantly smaller than responses to ATP. Based on the concentration–response curves, the agonist potency order was found to be: 2-MeSADP>β,γ-meATP=ATP=adenosine=ADP>2-MeSATP>α,β-meATP>TTP>CTP=UTP>GTP=UDP.
Figure 2.
Cumulative concentration–response curves to purine and pyrimidine agonists on AVP (10 nM) precontracted urethral strips. (a) Concentration–response curves to 2-MeSADP, 2-MeSATP, α,β-meATP and β,γ-meATP (all 0.1 μM–1 mM). (b) Concentration–response curves to TTP, CTP, UTP, GTP and UDP (all 0.1 μM–1 mM). All points show the mean±s.e.m. of six to eight experiments, unless occluded by the symbol.
Effect of the selective P2Y1 antagonist MRS2179
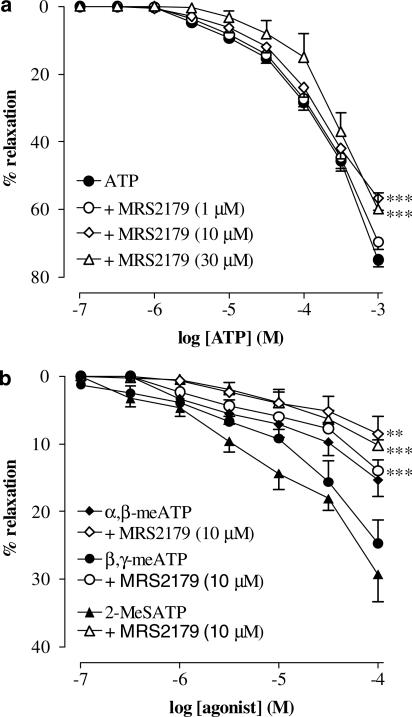
The selective P2Y1 antagonist MRS2179 (10–30 μM) significantly (P<0.001) inhibited ATP-induced responses (Figure 3a), whereas 1 μM MRS2179 had no effect. In addition, relaxant responses to 2-MeSATP, β,γ-meATP and to a lesser extent α,β-meATP were reduced by MRS2179 (10 μM) (Figure 3b) (P<0.001 for 2-MeSATP and β,γ-meATP; P<0.01 for α,β-meATP).
Figure 3.
Cumulative concentration–response curves to purine agonists on AVP (10 nM) precontracted urethral strips. (a) Concentration–response curves to ATP (0.1 μM–1 mM) in the absence and presence of MRS2170 (1–30 μM). (b) Concentration–responses curves to α,β-meATP, β,γ-meATP and 2-MeSATP (all 0.1 μM–1 mM) in the absence and presence of MRS2179 (10 μM). All points show the mean±s.e.m. of six to eight experiments, unless occluded by the symbol. **P<0.01, ***P<0.001, following a two-way ANOVA and Tukey's post hoc test.
In situ hybridization
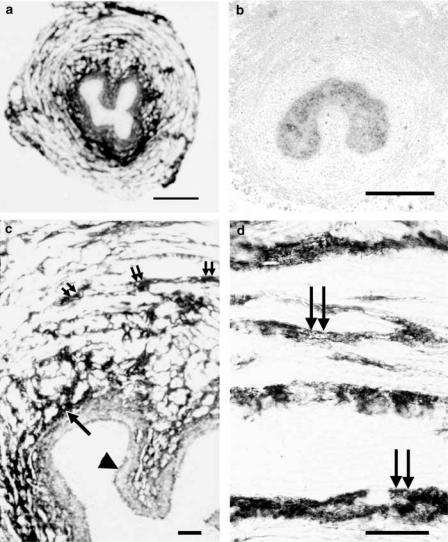
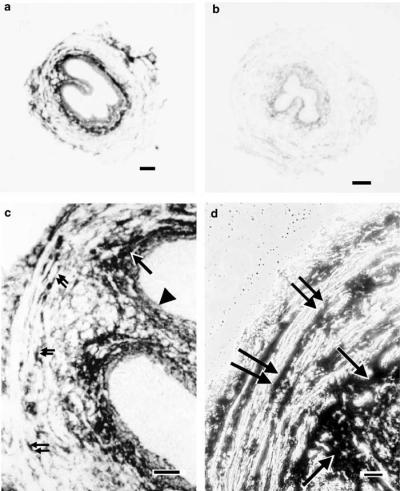
In hamster urethra, P2Y1 receptor mRNA was detected in the sub-epithelial and in the smooth muscle layer (Figure 4a, c and d). The signal appeared to be particularly strong in the circular smooth muscle layer. P2Y2 receptors were also detected in the same structures (Figure 5a, c and d), but seemed to be staining less intensely. Conversely, no P2Y4 or P2Y6 mRNA expression was detected on this tissue. Competition of labelled oligonucleotide probe for P2Y1 receptors with an excess of unlabelled probe resulted in complete absence of any staining (Figure 4b). Competition of labelled probe for P2Y2 receptors with an excess of unlabelled probe completely abolished any staining for P2Y2 receptors (Figure 5b).
Figure 4.
In situ hybridization showing localization of P2Y1 receptor mRNA in hamster proximal urethra. (a) Overview of P2Y1 receptor mRNA expression. (b) Negative control competing labelled P2Y1 oligonucleotide probe with an excess of unlabelled probe, abolishing the staining shown in (a); (c, d) show at higher magnification positive signals on the longitudinal and circular smooth muscle layer just beneath the epithelium. Scale bars: (a, b) 300 μm; (c, d) 40 μm. Double arrows: longitudinal smooth muscle; single arrows: circular smooth muscle; arrowheads: epithelium.
Figure 5.
In situ hybridization showing localization of P2Y2 receptor mRNA transcripts in hamster proximal urethra. (a) Overview of P2Y2 receptor mRNA expression. (b) Negative control competing labelled probe for P2Y2 receptors with an excess of unlabelled probe, abolishing the staining shown in (a). (c) P2Y2 receptor mRNA is expressed on the longitudinal and circular smooth muscle layer just beneath the epithelium. (d) Expression at higher magnification of P2Y2 mRNA on the circular and longitudinal smooth muscle layer in phase contrast. Scale bars: (a, b) 160 μm; (c) 40 μm and (d) 20 μm. Double arrows: longitudinal smooth muscle; single arrows: circular smooth muscle and arrowheads: epithelium.
Western blotting
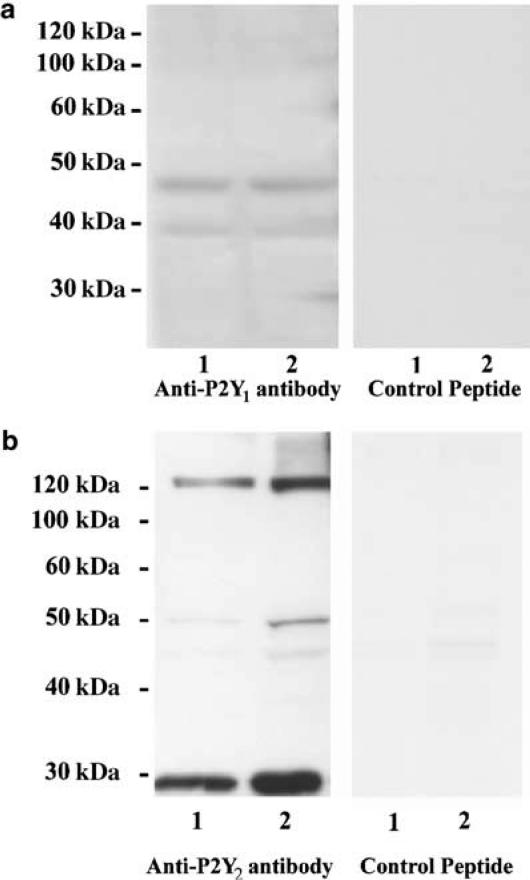
The Western blotting analysis was performed using a protein extract from three hamster urethral tissues and was repeated twice, obtaining identical results. There were two P2Y1 bands (40 and 45 kDa) (Figure 6a). In the peptide control membrane (the antibody was preincubated with its specific control peptide antigen for 1 h), no bands were found, indicating that the bands represent the P2Y1 receptor. The result for the P2Y2 receptor is shown in Figure 6b. There were three P2Y2 bands (about 30, 50 and 120 kDa), and no bands were found in the peptide control membrane, indicating that all the bands represent the P2Y2 receptor. The intensity of the P2Y2 receptor bands was higher than for the P2Y1 receptor subtype.
Figure 6.
Western blotting analysis of (a) P2Y1 and (b) P2Y2 receptor subtypes in hamster proximal urethra (protein loading, lane 1 : 40 and lane 2 : 60 μg). In the anti-P2Y1 membrane, two bands approximately at 40 and 45 kDa, have been found. In the peptide control membrane (anti-P2Y1 antibody preincubated with the control peptide antigen), no bands were found. We found three bands for P2Y2 receptors at about 30, 50 and 120 kDa. In the peptide control membrane (anti-P2Y2 antibody preincubated with the control peptide antigen), no bands were found in the specific regions.
Discussion
A role for ATP as a neurotransmitter in the lower urinary tract is well established (Burnstock, 2001) and many studies have described the activity of ATP and the localization of P2 receptors in the urinary bladder (Burnstock et al., 1978; Hegde et al., 1998; McMurray et al., 1998; Obara et al., 1998; Lee et al., 2000). The role of ATP and the localization of P2 receptors in the urethra have been less intensely studied although the possibility that ATP is a cotransmitter has been put forward (Callahan & Creed, 1981; Hills et al., 1984; Bo & Burnstock, 1992; Ohnishi et al., 1997; Deplanne et al., 1998).
Our previous studies showed that electrical field stimulation-induced relaxations of the hamster urethral smooth muscle were mediated principally by NO, as the majority of the response was inhibited by agents that attenuated NOS production, but that the residual component of the response was inhibited by suramin and Reactive blue 2, implying that ATP was mediating a proportion of the NANC response, probably via a P2Y receptor (Pinna et al., 1996; 1998). In addition, exogenous ATP was found to evoke concentration-related relaxations of the hamster urethra and removal of the epithelium did not affect these responses, suggesting localization of a receptor for ATP on the smooth muscle (Pinna et al., 1996). The aim of this study was to further characterize the P2Y receptor subtype(s) mediating the relaxation induced by ATP and other purine and pyrimidine compounds.
At present, eight P2Y receptor subtypes have been cloned and characterized in mammals (Abbracchio et al., 2003). The major P2Y receptor that has been found in smooth muscle preparations appears to be P2Y1, at which 2-MeSADP, ADP and 2-MeSATP are more potent than ATP, while the stable analogue α,β-meATP is inactive. P2Y2, P2Y4 and P2Y6 receptors have also been described on smooth muscle, where purines and pyrimidine nucleotides are both potent agonists, at least in rats (Ralevic & Burnstock, 1998).
In the absence of selective antagonists for most P2Y receptor subtypes, pharmacological characterization of P2Y receptor subtypes has relied more on their agonist profiles. In our study, the hamster proximal urethra relaxed with an agonist potency order of: 2-MeSADP>β,γ-meATP=ATP=adenosine=ADP>2-MeSATP>α,β-meATP>TTP>CTP=UTP>GTP=UDP. The greater potency of 2-MeSADP would seem to indicate that P2Y1 receptors were mediating the relaxation; however, the potency order is not characteristic for any single P2Y receptor subtype and may represent a combination of P2Y receptor subtypes. It should be noted that the highest concentration for the agonist used in this study (1 mM) might exert some non-specific effects. That ATP is acting through P2Y1 receptors is further supported since ATP is inhibited by MRS2179, an antagonist that has selectivity for P2Y1 receptors (Boyer et al., 1998; von Kügelgen & Wetter, 2000), and P2Y1 receptor mRNA was localized in the sub-epithelial and smooth muscle layers of the urethra.
MRS2179 also significantly antagonized relaxant responses to ATP, 2-MeSADP, 2-MeSATP, β,γ-meATP and α,β-meATP, suggesting that each agonist was acting at least in part through P2Y1 receptors. The concentration of antagonist that was necessary to significantly inhibit the agonists (10 μM) was found to be greater than that published in the literature. The pA2 for MRS2179 in Turkey erythrocytes is reported as being 6.99 (Boyer et al., 1998) and 6.75 at the human P2Y1 receptor (Jacobson et al., 1999). There are many instances in the literature where the concentration of MRS2179 inhibiting responses at P2Y1 receptors exceeds published pA2 values. For instance, a concentration range of 0.1–3 μM MRS2179 was used to selectively inhibit relaxations to ADP and ATP but not UTP in guinea-pig aorta (Kaiser & Buxton, 2002). In a study of rat hepatocyte function, 100 μM MRS2179 was used to inhibit P2Y1 receptors (Dixon et al., 2004). It has also been reported that MRS2179 has some activity at other P2Y and P2X1 receptors, although the concentration required (>100 μM) far exceeds that used in this study (Jacobson et al., 2000).
ATP and adenosine were found to be equipotent. ATP rapidly breaks down to adenosine by the action of ecto-nucleotidases, which sequentially dephosphorylate ATP to its corresponding nucleosides, which in turn can act at P1 receptors (Moody et al., 1984; Ralevic & Burnstock, 1998). ATP is not acting via P1 receptors in this tissue since the adenosine receptor antagonist 8-PT failed to inhibit relaxations to ATP, but did inhibit relaxations to adenosine. Also, the adenosine uptake inhibitor dipyridamole had no effect on ATP-induced relaxations.
The pyrimidine UTP activates P2Y2 receptors, reported to be either more potent than, or equipotent with, ATP (King et al., 2000). In this study, UTP was found to be considerably less potent than ATP. While the presence of P2Y2 receptor contributing to the relaxation response of the hamster urethra cannot be discounted, the very small response to UTP deems it unlikely. This is at variance with the strong signal for P2Y2 receptors seen following in situ hybridization and Western blotting analyses. P2Y2 receptors are known to mediate proliferation of cells, for instance, both UTP and ATP induce proliferation of cultured human keratinocytes and rat aortic smooth muscle cells (Erlinge et al., 1995; Dixon et al., 1999) and cultured rat mesangial cell proliferation has been shown to be mediated in part by P2Y2 receptors (Rost et al., 2002). Thus, the role of the P2Y2 receptor may be to mediate trophic effects.
The presence of a further P2Y receptor subtype in the hamster proximal urethra is suggested by the activity of α,β-meATP. An α,β-meATP-sensitive P2Y receptor has been described in the guinea-pig taenia coli, mouse intestine and in astrocytes (Brown & Burnstock, 1981; Windscheif et al., 1995; Ralevic & Burnstock, 1998; Brambilla et al., 1999; Giaroni et al., 2002). α,β-meATP is generally reported to be a potent agonist at P2X1 and P2X3 receptors, as is β,γ-meATP, both of which are thought to be generally inactive at P2Y receptors. The greater potency of β,γ-meATP in this tissue is intriguing as α,β-meATP is usually more potent than β,γ-meATP, although β,γ-meATP is reported as being more potent than α,β-meATP at inducing guinea-pig coronary vasodilatation (Vials & Burnstock, 1994). The presence of P2X1 and/or P2X3 receptors is unlikely as α,β-meATP failed to induce a contraction at resting tone (Pinna et al., 1996). The activity of MRS2179 at this receptor is unknown, although in this study MRS2179 has some inhibitory effect against α,β-meATP. This may reflect a species difference. Different species exhibit variability in agonist potency orders for P2 receptors in addition to the activity of antagonists and to date there are relatively few studies on hamster tissues compared with other rodents (see Burnstock, 1996; von Kügelgen & Wetter, 2000).
The presence of P2Y1 and P2Y2 receptors was corroborated by the in situ hybridization studies that showed that expression of mRNA for both P2Y1 and P2Y2 receptors, but not for P2Y4 and P2Y6, was localized in the smooth muscle layer in close proximity with the urothelium. These results have been confirmed by Western blotting analysis performed with antibodies raised against P2Y1 and P2Y2 receptor subtypes. The predicted molecular mass of the human P2Y1 receptor has been reported as 42 kDa (Hoffmann et al., 1999). Wang et al. (2002) found three bands for the P2Y1 receptor at 45, 90 and 180 kDa in human vascular smooth muscle and endothelial cells; they suggested that the last two bands might be due to protein association (dimers, tetramers) and/or glycosylation of the receptor. The same group also identified three bands for the P2Y2 receptor at about 33, 36 and 50 kDa (Wang et al., 2002). Christofi et al. (2004) reported the presence of a band for P2Y1 receptors at 40 kDa, and a band for P2Y2 receptors at 35 kDa in rat colon. In hamster proximal urethra, two bands for P2Y1 receptors at about 40 and 45 kDa were found, which is in agreement with results found in rat colon. Three bands for P2Y2 receptors were found at about 30, 50 and 120 kDa, the band at 30 kDa being denser than that at 50 or120 kDa and the bands for the P2Y2 receptor were generally more dense than those of the P2Y1 receptor. Pre-incubation of P2Y1 and P2Y2 receptor antibodies with the corresponding immunogenic peptides prevented the immunoreactive bands, indicating that the bands were specific for the receptors. Whether protein association (formation of dimers and/or tetramers) accounts for the presence of three bands for the P2Y2 receptor is unclear at this time.
In conclusion, the present study has shown that ATP induces relaxation of the hamster proximal urethra via P2Y1 receptors localized to the sub-epithelial and smooth muscle layers. Relaxation may also be induced via P2Y2 receptors, although the weak activity of UTP and strong expression of mRNA for P2Y2 receptors suggests a long-term trophic role. A further P2 receptor sensitive to α,β-meATP is also suggested and may contribute to relaxation together with P1 receptors.
Acknowledgments
C.P. was supported by the Wellcome Trust (London). We are grateful to Dr Brian King for his criticism on the paper, Mr Tim Robson for preparation of histological specimens, Dr Chrystalla Orphanides and to Dr Manuela Magnani for their assistance in the preparation of the manuscript.
Abbreviations
- ADP
adenosine 5′-diphosphate
- ATP
adenosine 5′-triphosphate
- AVP
arginine vasopressin
- CTP
cytidine 5′-triphosphate
- GTP
guanosine 5′-triphosphate
- α,β-meATP
α,β-methylene ATP
- β,γ-meATP
β,γ-methylene ATP
- 2-MeSADP
2-methylthio ADP
- 2-MeSATP
2-methylthio ATP
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- NANC
non-adrenergic, non-cholinergic
- NO
nitric oxide
- 8-PT
8-phenyltheophylline
- TTP
thymidine 5′-triphosphate
- UDP
uridine 5′-diphosphate
- UTP
uridine triphosphate
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSSON K.E. Neurotransmission and drug effects in urethral smooth muscle. Scand. J. Urol. Nephrol. Suppl. 2001;207:26–34. doi: 10.1080/003655901750174854. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., PERSSON K. The L-arginine/nitric oxide pathway and non-adrenergic, non-cholinergic relaxation of the lower urinary tract. Gen. Pharmacol. 1993;24:833–839. doi: 10.1016/0306-3623(93)90156-r. [DOI] [PubMed] [Google Scholar]
- BO X., BURNSTOCK G. Species differences in characteristics and distribution of [3H]α,β-methylene ATP binding sites in urinary bladder and urethra of rat, guinea-pig and rabbit. Eur. J. Pharmacol. 1992;216:59–66. doi: 10.1016/0014-2999(92)90209-m. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate. Br. J. Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAMBILLA R., BURNSTOCK G., BONAZZI A., CERUTI S., CATTABENI F., ABBRACCHIO M.-P. Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br. J. Pharmacol. 1999;126:563–567. doi: 10.1038/sj.bjp.0702333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.M., BURNSTOCK G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br. J. Pharmacol. 1981;73:617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinoceptors: ontogeny and phylogeny. Drug Dev. Res. 1996;39:204–242. [Google Scholar]
- BURNSTOCK G. Purinergic cotransmission. Bain Res. Bull. 1999;50:355–357. doi: 10.1016/s0361-9230(99)00103-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.Purinergic signalling in lower urinary tract Handbook of Experimental Pharmacology, vol 151/I. Purinergic and Pyrimidinergic Signalling I. Molecular Nervous and Urogenitary System Function 2001Berlin: Springer-Verlag; 423–515.ed. Abbracchio, M.P. & Williams, M. pp [Google Scholar]
- BURNSTOCK G., COCKS T., CROWE R., KASAKOV L. Purinergic innervation of the guinea-pig urinary bladder. Br. J. Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAHAN S.M., CREED K.E. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br. J. Pharmacol. 1981;74:353–358. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOFI F.L., WUNDERLICH J., YU J.G., WANG Y.Z., XUE J., GUZMAN J., JAVED N., COOKE H. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J. Comp. Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- DEPLANNE V., PALEA S., ANGEL I. The adrenergic, cholinergic and NANC nerve-mediated contractions of the female rabbit bladder neck and proximal, medial and distal urethra. Br. J. Pharmacol. 1998;123:1517–1524. doi: 10.1038/sj.bjp.0701757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., BOWLER W.B., LITTLEWOOD-EVANS A., DILLON J.P., BILBE G., SHARPE G.R., GALLAGHER J.A. Regulation of epidermal homeostasis through P2Y2 receptors. Br. J. Pharmacol. 1999;127:1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON J., HALL J.F., WEBB T.E., BOARDER M.Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-MeSADP J. Pharmacol. Exp. Ther. 2004. Epub ahead of print [DOI] [PubMed]
- ERLINGE D., YOU J., WAHLESTEDT C., EDVINSSON L. Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur. J. Pharmacol. 1995;289:135–149. doi: 10.1016/0922-4106(95)90178-7. [DOI] [PubMed] [Google Scholar]
- GARCIA-PASCUAL A., COSTA G., GARCIA-SACRISTAN A., ANDERSSON K.E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol. Scand. 1991;141:531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- GIARONI C., KNIGHT G.E., RUAN H.Z., GLASS R., BARDINI M., LECCHINI S., FRIGO G., BURNSTOCK G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., KIGOSHI S., MURAMATSU I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur. J. Pharmacol. 1993;231:209–214. doi: 10.1016/0014-2999(93)90451-m. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., MANDEL D.A., WILFORD M.R., BRIAUD S., FORD A.P.D.W., EGLEN R.M. Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur. J. Pharmacol. 1998;349:75–82. doi: 10.1016/s0014-2999(98)00173-3. [DOI] [PubMed] [Google Scholar]
- HILLS J., MELDRUM L.A., KLARSKOV P., BURNSTOCK G. A novel non-adrenergic, non-cholinergic nerve-mediated relaxation of the pig bladder neck: an examination of possible neurotransmitter candidates. Eur. J. Pharmacol. 1984;99:287–293. doi: 10.1016/0014-2999(84)90135-3. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C., MORO S., NICHOLAS R.A., HARDEN T.K., JACOBSON K.A. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J. Biol. Chem. 1999;274:14639–14647. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., HOFFMANN C., KIM Y.C., CAMAIONI E., NANDANAN E., JANG S.Y., GUO D.P., JI X.D., VON KÜGELGEN I., MORO S., ZIGANSHIN A.U., RYCHKOV A., KING B.F., BROWN S.G., WILDMAN S.S., BURNSTOCK G., BOYER J.L., MOHANRAM A., HARDEN T.K. Molecular recognition in P2 receptors: ligand development aided by molecular modeling and mutagenesis. Prog. Brain Res. 1999;120:119–132. doi: 10.1016/s0079-6123(08)63550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., KING B.F., BURNSOCK G. Pharmacological characterization of P2 (nucleotide) receptors. Cell Transm. 2000;16:3–23. [Google Scholar]
- KAISER R.A., BUXTON I.L.O. Nucleotide-mediated relaxation in guinea-pig aorta: selective inhibition by MRS2179. Br. J. Pharmacol. 2002;135:537–545. doi: 10.1038/sj.bjp.0704476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., BURNSTOCK G., BOYER J.L., BOEYNAEMS J.M., WEISMAN G.A., KENNEDY C., JACOBSON K.A., HUMPHRIES R.G., ABBRACCHIO M.P., MIRAS-PORTUGAL M.T.The P2Y receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000London: IUPHAR Media Ltd; 306–320.ed. Girdlestone, D. pp [Google Scholar]
- LEE H.Y., BARDINI M., BURNSTOCK G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J. Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- LEE J.G., WEIN A.J., LEVIN R.M. Comparative pharmacology of the male and female rabbit bladder neck and urethra: involvement of nitric oxide. Pharmacology. 1994;48:250–259. doi: 10.1159/000139187. [DOI] [PubMed] [Google Scholar]
- MCMURRAY G., DASS N., BRADING A.F. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br. J. Pharmacol. 1998;123:1579–1586. doi: 10.1038/sj.bjp.0701774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOODY C.J., MEGHJI P., BURNSTOCK G. Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur. J. Pharmacol. 1984;97:47–54. doi: 10.1016/0014-2999(84)90511-9. [DOI] [PubMed] [Google Scholar]
- OBARA K., LEPOR H., WALDEN P.D. Localization of P2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J. Urol. 1998;160:587–591. [PubMed] [Google Scholar]
- OHNISHI N., PARK Y.C., KURITA T., KAJIMOTO N. Role of ATP and related purine compounds on urethral relaxation in male rabbit. Int. J. Urol. 1997;4:191–197. doi: 10.1111/j.1442-2042.1997.tb00169.x. [DOI] [PubMed] [Google Scholar]
- OTOMO R., SHIMODA N., SATOH S., SATO K., OGAWA O., KATO T. The role of ATP-receptor in controlling the urinary bladder and urethral function in rats. Nippon Hinyokika Gakkai Zasshi. 1999;90:681–687. doi: 10.5980/jpnjurol1989.90.681. [DOI] [PubMed] [Google Scholar]
- PARLANI M., CONTE B., MANZINI S. Nonadrenergic, noncholinergic inhibitory control of the rat external urethral sphincter: involvement of nitric oxide. J. Pharmacol. Exp. Ther. 1993;265:713–719. [PubMed] [Google Scholar]
- PINNA C., EBERINI I., PUGLISI L., BURNSTOCK G. Presence of constitutive endothelial nitric oxide synthase immunoreactivity in urothelial cells of hamster proximal urethra. Eur. J. Pharmacol. 1999;367:85–89. doi: 10.1016/s0014-2999(98)00981-9. [DOI] [PubMed] [Google Scholar]
- PINNA C., PUGLISI L., BURNSTOCK G. ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. Br. J. Pharmacol. 1998;124:1069–1074. doi: 10.1038/sj.bjp.0701908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINNA C., VENTURA S., PUGLISI L., BURNSTOCK G. A pharmacological and histochemical study of hamster urethra and the role of urothelium. Br. J. Pharmacol. 1996;119:655–662. doi: 10.1111/j.1476-5381.1996.tb15723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROST S., DANIEL C., SCHULZE-LOHOFF E., BAUMERT H.G., LAMBRECHT G., HUGO C. P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kid. Int. 2002;62:1659–1671. doi: 10.1046/j.1523-1755.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- VIALS A.J., BURNSTOCK G. Differential effects of ATP- and 2-methylthioATP-induced relaxation in guinea pig coronary vasculature. J. Cardiovasc. Pharmacol. 1994;23:757–764. doi: 10.1097/00005344-199405000-00010. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WANG L., KARLSSON L., MOSES S., HULTGARDH-NILSSON A., ANDERSSON M., BORNA C., GUDBJARTSSON T., JERN S., ERLINGE D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- WERKSTROM V., PERSSON K., NY L., BRIDGEWATER M., BRADING A.F., ANDERSSON K.E. Factors involved in the relaxation of female pig urethra evoked by electrical field stimulation. Br. J. Pharmacol. 1995;116:1599–1604. doi: 10.1111/j.1476-5381.1995.tb16379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDSCHEIF U., PFAFF O., ZIGANSHIN A.U., HOYLE C.H., BAUMERT H.G., MUTSCHLER E., BURNSTOCK G., LAMBRECHT G. Inhibitory action of PPADS on relaxant responses to adenine nucleotides or electrical field stimulation in guinea-pig taenia coli and rat duodenum. Br. J. Pharmacol. 1995;115:1509–1517. doi: 10.1111/j.1476-5381.1995.tb16644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]