Abstract
The discovery of the endogenous cannabimimetic lipid mediators, anandamide and 2-arachidonoyl glycerol, opened the door to the discovery of other endogenous lipid mediators similar in structure and function. The majority of these compounds do not bind appreciably to known cannabinoid receptors; yet some of them produce cannabimimetic effects while others exert actions through novel mechanisms that remain to be elucidated. This review explores the growing diversity of recently discovered putative lipid mediators and their relationship to the endogenous cannabinoid system. The possibility that there remain many unidentified signalling lipids coupled with the evidence that many of these yield bioactive metabolites due to actions of known enzymes (e.g. cyclooxygenases, lipoxygenases, cytochrome P450s) suggests the existence of a large and complex family of lipid mediators about which only little is known at this time. The elucidation of the biochemistry and pharmacology of these compounds may provide therapeutic targets for a variety of conditions including sleep dysfunction, eating disorders, cardiovascular disease, as well as inflammation and pain.
Keywords: Cannabinoid, lipid mediator, acyl ethanolamide, arachidonoyl glycine, arachidonoyl dopamine, oleoyl dopamine, cyclooxygenase
Introduction
Our understanding of a physiological role of cannabinoids in memory, cardiovascular function, cognition, pain, reproduction, motor control, and immune function has grown rapidly in the last decade. These have been the subject of recent reviews in this journal (Ross, 2003; Randall et al., 2004; Walter & Stella, 2004). The landmark works that identified the endogenous compounds, arachidonoyl ethanolamide (anandamide, Devane et al., 1992) and 2-arachidonoyl glycerol (2-AG; Mechoulam et al., 1995; Sugiura et al., 1995), paved the way for the search for other endogenous lipids that are associated with cannabinoid system physiology. Historically, the complexities and limitations of lipid chemistry hampered the identification and characterization of endocannabinoids and related lipid mediators. Hence, the increased availability of sensitive techniques for the study of lipids is a primary factor in the recent advances in this field. Additional endogenous lipids discovered that show affinity for cannabinoid receptors include dihomo-γ-linolenoyl ethanolamide (Hanus et al., 1993), docosatetraenoyl ethanolamide (Hanus et al., 1993), 2-arachidonyl glycerol ether (noladin ether; Hanus et al., 2001), and N-arachidonoyl dopamine (NADA) (Huang et al., 2002). Many other endogenous lipids similar to endocannabinoids in structure and metabolism have been identified. These compounds do not bind appreciably to known cannabinoid receptors, and yet some of them demonstrate cannabimimetic effects. These novel lipid mediators will likely prove to be important to the function and regulation of cannabinoid neurophysiology or operate in parallel via overlapping signaling pathways. The following minireview provides a brief overview of these compounds and their relationship to the endogenous cannabinoid system.
Acyl glycerols
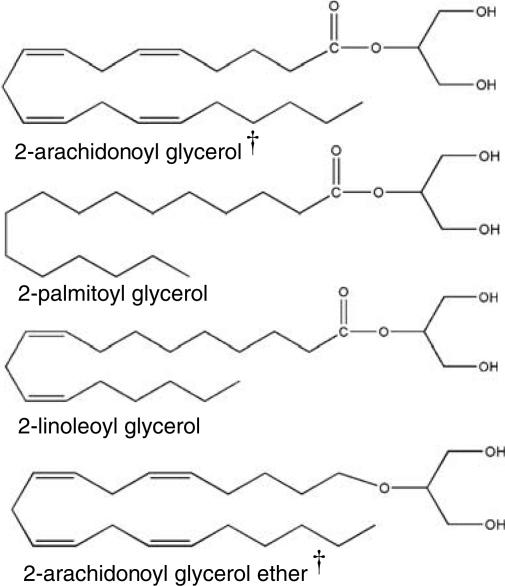
2-linoleoyl glycerol and 2-palmitoyl glycerol share structural homology with the endogenous cannabinoid 2-arachidonoyl glycerol (2-AG) in that the only difference is the fatty acid length and saturation (Figure 1). Noladin ether likewise consists of arachidonic acid and glycerol with the exception that the linkage to the glycerol moiety is an ether versus an ester as is the case for the other compounds in this class (Figure 1). Noladin ether was identified by Hanus et al. (2001) and confirmed by Fezza et al. (2002) in which it was demonstrated to occur in relatively high amounts in dissected thalamus. Oka et al. (2003), however, failed to measure noladin ether in nervous tissue. If noladin ether is in fact an endogenous compound, its location in the thalamus suggests a role in sensory processing, but its localization to sensory areas of the thalamus has not been established. Additionally, noladin ether occurs in very low levels in the spinal cord, raising a question as to any important role in spinal neurotransmission. Noladin ether possesses biological activity. Hanus et al. (2001) showed that the compound produces analgesic effects in the hot plate test following systemic administration in mice (20 mg kg−1, i.p.), binds to CB1 but not CB2 receptors, produces hypothermia, catalepsy, and decreases in locomotor activity. Additionally, noladin ether was more effective and demonstrated a more persistent response in decreasing intraocular pressure than either anandamide or 2-AG (Laine et al., 2002).
Figure 1.
Chemical structures of bioactive acyl gylcerols. †Indicates compounds with activity at either CB1 or CB2 receptors.
2-Linoleoyl glycerol and 2-palmitoyl glycerol were isolated in mouse gut (Mechoulam et al., 1995), brain (Sugiura et al., 1995), and spleen (Ben-Shabat et al., 1998). Whereas neither compound binds appreciably to CB2 receptors, when combined with 2-AG in the same percentages measured in tissue, these compounds markedly potentiated the binding of 2-AG to CB2 receptors causing a decrease in the Ki for 2-AG from 1640±260 to 273±22 nM (Ben-Shabat et al., 1998). The same synergistic effects were demonstrated in the aforementioned behavioral tests (Ben-Shabat et al., 1998).
These enhancements of cannabinoid activity by congeners that lack binding affinity have been termed entourage effects (Mechoulam et al., 1998).
Acyl ethanolamides
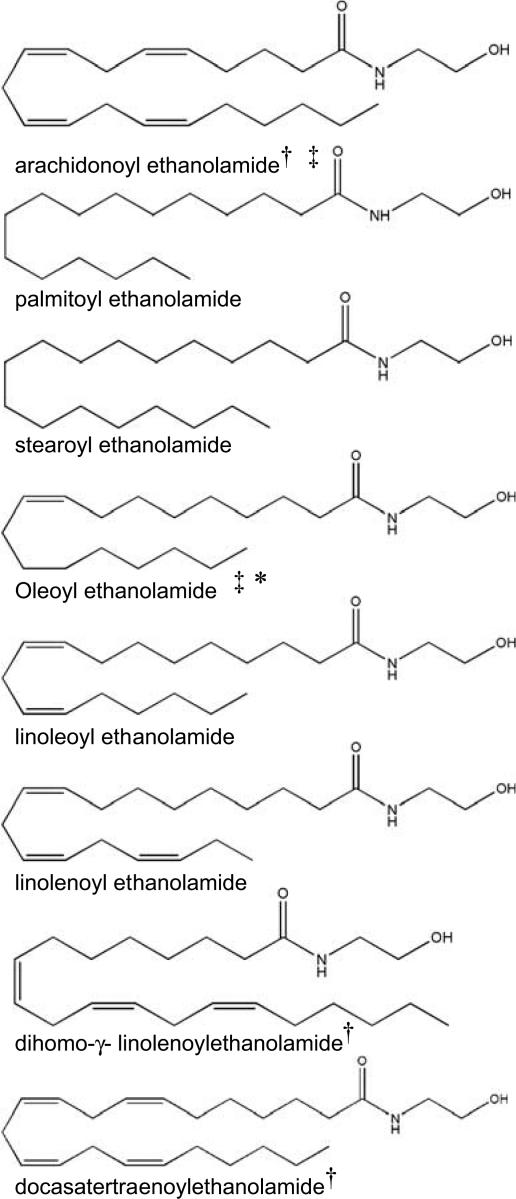
The first identified endogenous cannabinoid, arachidonoyl ethanolamide (Devane et al., 1992), was given the name, anandamide, which is derived from the Indian Sanskrit term ananda for bliss. Seven additional bioactive N-acyl ethanolamides were subsequently identified (Figure 2). Although dihomo-γ-linolenoylethanolamide, and docosatetraenoylethanolamide bind to CB1 receptors (Hanus et al., 1993), none of the others in this group show appreciable binding. Each of these compounds has been identified in various mammalian and invertebrate tissues (Di Marzo et al., 1996; Maccarrone et al., 2001; Schuel et al., 2002; Salzet & Stefano, 2002) and are hypothesized to possess cannabimimetic activity possibly due to entourage effects (Mechoulam et al., 1998).
Figure 2.
Chemical structures of bioactive acyl ethanolamides. †Indicates compounds with activity at either CB1 or CB2 receptors. ‡Indicates compounds with activity at the TRPV1 receptor. *Indicates compounds with activity at the PPARα receptor.
The oldest of these compounds, palmitoyl ethanolamide (PEA), was identified nearly 5 decades ago as the principle anti-inflammatory agent in lipid extracts of various natural products (Kuehl et al., 1957). Extensive reviews of PEA have recently been published (Lambert et al., 2002; Schmid & Berdyshev, 2002). PEA produces anti-inflammatory (Facci et al., 1995; Mazzari et al., 1996) and antinociceptive effects (Calignano et al., 1998; Jaggar et al., 1998) when administered exogenously. Synergistic effects in antinociception were observed with coadministration of anandamide and PEA, and abolished by either CB1 or CB2 antagonists, respectively (Calignano et al., 1998; 2001). PEA produced a two-fold decrease in the Ki value for anandamide binding at the transient receptor potential type vanilloid 1 receptor (TRPV1), an effect that was not due to inhibition of anandamide hydrolysis (De Petrocellis et al., 2001). Nor does it appear that this effect was caused by blocking the putative anandamide transporter (Rakhshan et al., 2000). Although PEA exhibits poor affinity for CB1 and CB2 receptors (Sheskin et al., 1997; Lambert et al., 1999), the antinociceptive effects of PEA were blocked by the CB2 antagonist SR144528, suggesting possible activation of a non-CB2 receptor of which the molecular nature, location, and signal transduction mechanisms are unknown (Calignano et al., 1998; 2001).
In contrast to PEA, oleoyl ethanolamide (OEA) inhibited anandamide uptake (Rakhshan et al., 2000) and degradation (Karava et al., 2001). Like anandamide, OEA has been implicated in the neural regulation of feeding behaviors (Rodriguez de Fonseca et al., 2001) by acting on peripheral sensory fibers. Furthermore, OEA levels were significantly decreased during starvation (Rodriguez de Fonseca et al., 2001). By contrast, anandamide levels increased during starvation (Gomez et al., 2002), suggesting a reciprocal effect of the two compounds within this system. OEA has negligible affinity for both CB1 and CB2 receptors. OEA activates the nuclear receptor, peroxisome proliferator-activated receptor α (PPAR-α; Fu et al., 2003; Guzman et al., 2004), which may explain its effects on feeding (Fu et al., 2003). OEA also activates the TRPV1 receptor in a PKC-dependent manner (Ahern, 2003).
Even though OEA does not bind to CB1 receptors, measurements of the endogenous levels of OEA revealed significant increases in cortical levels in CB1 knockout mice relative to wild-type mice at 2 months of age. At 6 months of age, there were no differences between the wild type and the knockouts (Maccarrone et al., 2001). Conversely, the levels of OEA in the hippocampus of CB1 knockout mice were significantly lower than the wild type at 2 months with a further reduction at 6 months (Maccarrone et al., 2001). PEA and stearoyl ethanolamide (SEA) showed similar changes in levels in this CB1 knockout model. These data add to the evidence that OEA, PEA, and SEA may function in concert with endocannabinoids to regulate feeding.
Linoleoyl ethanolamide, PEA, SEA, and OEA were isolated from mouse J774 macrophages and N18 neuroblastoma cells (Di Marzo et al., 1996) as well as RBL-2H3 leukocytes (Bisogno et al., 1997). The levels of these compounds were significantly increased by addition of ionomycin in each system (Di Marzo et al., 1996; Bisogno et al., 1997). Linoleoyl ethanolamide inhibits fatty acid amide hydrolase (FAAH; Maurelli et al., 1995; Maccarrone et al., 1998), and was shown to inhibit sea urchin fertilization (Berdyshev, 1999).
Acyl dopamines
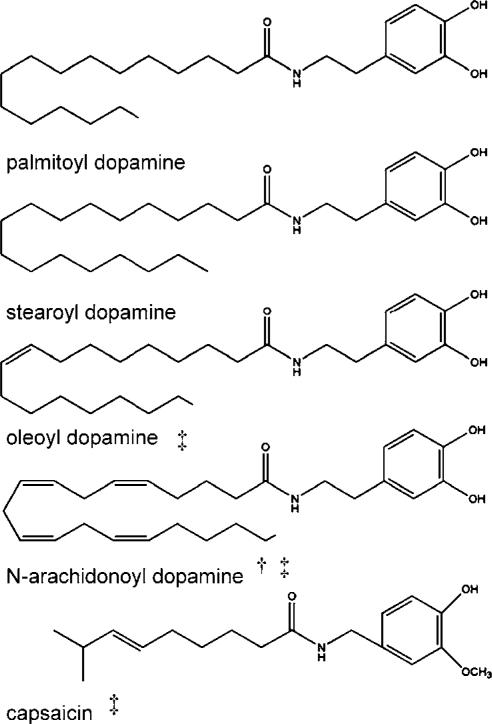
Another class of acyl amides is the acyl dopamines. These compounds (Figure 3) were recently identified in bovine and rat brain (Huang et al., 2002; Chu et al., 2003). Like the compounds discussed above, they comprised a fatty acid chain with a moiety attached at the carboxyl end via an amide linkage. The moiety in this group of compounds is dopamine, a naturally occurring aromatic amine. These compounds share a structural similarity with the potent TRPV1 agonist, capsaicin (Figure 3).
Figure 3.
Chemical structures of bioactive acyl dopamines. The TRPV1 agonist, capsaicin, is shown at the bottom to demonstrate the structural similarity to these compounds. †Indicates compounds with activity at either CB1 or CB2 receptors. ‡Indicates compounds with activity at the TRPV1 receptor.
NADA activates CB1 receptors (Ki 0.5±0.2 μM) and induces analgesia following systemic administration (but not tested with a cannabinoid antagonist) (Bisogno et al., 1997; Huang et al., 2002). Like anandamide, NADA mobilizes intracellular calcium via activation of TRPV1 receptors (Huang et al., 2002; Toth et al., 2003; Gavva et al., 2004). Premkumar et al. (2004) hypothesize that it is acting on TRPV1 in a PKC-dependent manner by demonstrating that NADA-induced currents could be blocked by the PKC inhibitor, bisindoylmaleimide. They also demonstrated that NADA-induced changes in current were increased ∼30-fold by applying NADA intracellularly, suggesting that the increased access to the TRPV1 receptor facilitated this change (Premkumar et al., 2004). The distribution of endogenous NADA in various brain areas differs from that of anandamide with the highest levels found in the striatum and hippocampus (Huang et al., 2002). It also occurs in the dorsal root ganglion, suggesting that it may serve a role in pain and sensory modulation. Patch-clamp studies of cultured DRG neurons showed that NADA elicited immediate and reversible responses, which were blocked by both the CB1 antagonist, SR141617A, and the TRPV1 antagonist, capsazepine (Sagar et al., 2004).
Electrophysiological recordings from the dorsal horn in anesthetized rats showed that neuronal responses to mechanical stimulation were inhibited by 5 μg of NADA. When low levels of mechanical pressure were applied, the effect was blocked by SR141716A. Conversely, the TRPV1 antagonist iodo-resiniferatoxin (IRTX) blocked the effects of NADA when higher levels of mechanical pressure were tested (Sagar et al., 2004). In behavioral experiments using non-anesthetized animals, a 5 μg dose of NADA caused thermal hyperalgesia when administered peripherally in rats (Huang et al., 2002) and primates (Butelman et al., 2003). Harrison et al. (2003) showed that it initiates contractions in both pig bronchi and urinary bladder in a manner similar to that of anandamide and capsaicin. Additionally, NADA was shown to inhibit T-cell activation, IL-2 and TNF-α gene activation, as well as inhibit NF-κB-dependent transcriptional activity (Sancho et al., 2004). Given that NADA is capable of eliciting analgesia upon systemic administration, hyperalgesia upon intradermal injection, inhibition of neuronal responses to mechanical stimulation, inhibition of immune responses, and initiating smooth muscle contraction, it is possible that endogenous NADA may activate TRPV1, CB1, or an additional as yet unknown receptor depending on location and circumstance.
Oleoyl dopamine (OLDA) shows only a modest affinity for the CB1 receptor (Ki 1.6±0.4 μM) and it possesses the highest potency of any putative endovanilloid identified to date in the mobilization of intracellular calcium in TRPV1-transfected HEK cells (Chu et al., 2003; Gavva et al., 2004). Additionally, OLDA appeared to be recognized by the putataive anandamide transporter (Chu et al., 2003), whereas, it did not inhibit anandamide degradation through FAAH. Like NADA, OLDA was shown to inhibit early and late events in T-cell activation; however, its effects on IL-2, TNF-α, and NF-κB were not examined (Sancho et al., 2004). Behaviorally, it induces thermal hyperalgesia that is reversed by IRTX (Chu et al., 2003). In other behavior studies, the μ-opioid receptor agonists, loperamide and fentanyl, both prevented OLDA-induced allodynia in the primate (Butelman et al., 2004).
Palmitoyldopamine (PALDA) and stearoyldopamine (STEARDA) possess very low TRPV1 activity, and failed to inhibit anandamide degradation and transport (Chu et al., 2003). However, when preincubated for 5 min with TRPV1-transfected HEK-293 cells, both compounds dose-dependently enhanced NADA's TRPV1-mediated mobilization of intracellular calcium (De Petrocellis et al., 2004). This same effect on intracellular calcium was observed for anandamide after preincubation with PALDA and STEARDA (De Petrocellis et al., 2004). Behaviorally, only STEARDA potentiated the induction of thermal hyperalgesia by NADA (De Petrocellis et al., 2004).
N-acyl amides
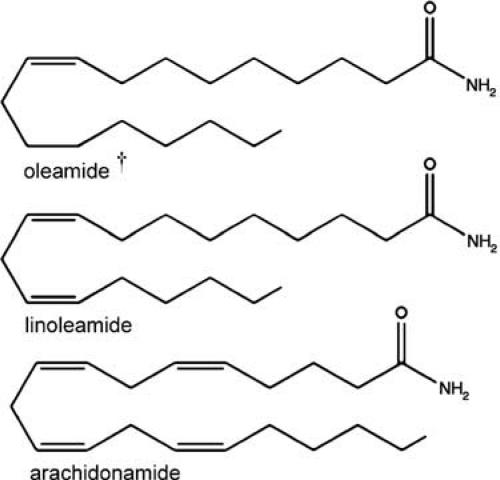
Oleamide, linoleamide, and arachidonamide are the simplest forms of fatty-acid amides, as the amide linkage is to an ammonium ion (Figure 4). These compounds produce a variety of physiological effects and they share with anandamide the same degradatory enzyme, FAAH (Maurelli et al., 1995; Cravatt et al., 1996). Oleamide and linoleamide were isolated in brain CSF, and appear to be involved in sleep regulation (Cravatt et al., 1995; Boger et al., 1998a; Huang & Jan, 2001). Using multiple reaction monitoring on a triple quadrupole mass spectrometer, we found evidence for endogenous arachidonamide in the brain (data not shown). Oleamide and arachidonamide have been reported to affect gap junctions (Boger et al., 1998b; 1999), and oleamide has been shown to affect GABAergic, dopaminergic, and serotonergic neurotransmission (Huidobro-Toro & Harris, 1996; Guan et al., 1997; Thomas et al., 1997; Yost et al., 1998; Fedorova et al., 2001). Additionally, i.p. injections of oleamide were shown to increase food intake for up to 3 h (Martinez-Gonzalez et al., 2004). It remains to be determined if linoleamide and archidonamide show similar behavioral effects.
Figure 4.
Chemical structures of bioactive acyl amides. †Indicates compounds with possible activity at the CB1 receptor. None have shown any activity at CB2 or TRPV1 receptors.
Two reports indicated that oleamide, linoleamide, and arachidonamide do not interact appreciably with cannabinoid receptors (Pinto et al., 1994; Sheskin et al., 1997). However, a recent report by Leggett et al. (2004) provided evidence that oleamide inhibits agonist and antagonist ligand binding to CB1 but not CB2 receptors, and increases GTPγS via CB1 activation. The concentrations needed for these effects (∼3–100 μM) may not be in the physiological range (Fowler, 2004). Nevertheless, cannabimimetic effects such as antinociception and hypothermia were observed after systemic administration of oleamide, and the antinociceptive effects were blocked by the CB1 antagonist SR141716A (Fedorova et al., 2001).
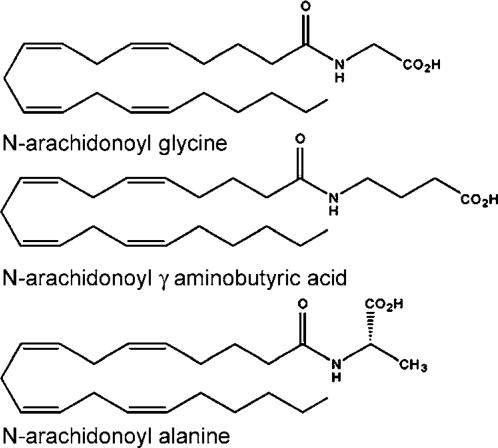
Acyl amino acids
The final class of acyl amides discussed here comprises the acyl amino acids. N-arachidonoyl glycine (NAGly; Figure 5), which differs from anandamide by a single oxygen moiety, was first synthesized by Sheskin et al. (1997) as part of a structure-activity study of anandamide-like compounds. Burstein (1999) suggested that this compound may be produced in vivo by oxidative metabolism of anandamide. We noted that the compound could be formed by conjugation of two naturally occurring molecules, arachidonic acid and glycine. This led us to search for it in the brain (Huang et al., 2001). NAGly was identified in bovine brain extracts and found to occur in rat spinal cord, intestine, testes, skin, blood, kidneys, heart (Huang et al., 2001), and more recently the reproductive tract (Bradshaw et al., 2003). This compound produces antinociceptive effects in rats, reduces edema produced by local injections of arachidonic acid, and inhibits NF-κB, a transcription factor that regulates chemokine production (Burstein et al., 2002). The effects of NAGly may be due, in part, to its ability to increase the levels of anandamide through inhibition of FAAH. However, a recent study by Cascio et al. (2004) showed that the inhibition of FAAH by NAGly is species-dependent and varies among cell and tissue types. The effects of NAGly may result, in part, from metabolites produced by cyclooxygenase-2 (COX-2). Prusakiewicz et al. (2002) showed that like the effects on anandamide and 2-AG (Yu et al., 1997; Kozak et al., 2002), COX-2 metabolizes NAGly to PGH2 glycine and hydroxyeicosatetraenoic glycine, suggesting the existence of a new class of eiscosanoids derived from NAGly.
Figure 5.
Chemical structure of bioactive acyl amino acids. None have shown any activity at CB1, CB2, or TRPV1 receptors.
In addition to NAGly, Huang et al. (2001) identified two additional arachidonoyl amino acids: arachidonoyl alanine and arachidonoyl-γ-aminobutyric acid (GABA), the latter of which produced antinociceptive effects. Recently, Mechoulam's laboratory identified N-arachidonoyl serine in a mammalian brain extract and showed that it is a potent activator of the abnormal cannabidiol receptor (Jarai et al., 1999; Milman et al., 2004). Further experiments in our laboratory using precursor ion scans and multiple reaction monitoring on a triple quadrupole mass spectrometer provided evidence for the existence of other arachidonoyl amino acids including the conjugates of arachidonic acid with valine, cysteine, and glutamine, but more work is needed to prove their existence. Similar experiments have further suggested the existence of glycine and GABA conjugates of palmitic, oleic, and stearic acid, but again, additional work is needed to verify the existence of these compounds.
Summary
In all, 20 putative lipid mediators were reviewed and their involvement in pain, immune function, reproduction, and appetite were discussed here. The majority of these compounds do not bind appreciably to CB1 or CB2 receptors but many exhibit cannabimimetic effects. These can be attributed, in part, to interference with inactivation of endocannabinoids thus enhancing their actions at cannabinoid receptors (Maccarronne et al., 1998; Di Marzo et al., 2001; Jonsson et al., 2001; Karava et al., 2001), and there remains the possibility that some of these compounds act downstream potentiating cannabinoid receptor signaling. Recent reports indicate that some of these compounds act on known signaling molecules such as PPARα, TRPV1 and probably others that remain to be identified. The diversity of the lipids identified to date, which seem to occur in a combinatorial fashion (any of many different acyl groups coupled to any of many different small polar molecules), indicates the high likelihood that there are numerous unidentified endogenous signaling lipids. This coupled with the evidence that many such compounds yield bioactive metabolites provides a hypothetical framework for a large and complex system of neural and immune signaling systems that have yet to be discovered. One may reasonably hope that as techniques for the study of lipid mediators become more sensitive, this somewhat indistinct landscape of lipid neurotransmitters and neuromodulators may resolve into a well-defined terrain.
Acknowledgments
This work was supported by Grants from the National Institute of Health (K02DA00375, DA13012, NS33247 to J. Michael Walker; F32DA016825-01 to Heather Bradshaw).
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- anandamide
N-arachidonoyl ethanolamide
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- FAAH
fatty acid amide hydrolase
- GABA
arachidonoyl-γ-aminobutyric acid
- NADA
N-arachidonoyl dopamine
- NAGly
N-arachidonoyl glycine
- noladin ether
2-arachidonoyl glycerol ether
- OEA
oleoyl ethanolamide
- OLDA
oleoyl dopamine
- PALDA
palmitoyldopamine
- PEA
palmitoyl ethanolamide
- SEA
stearoyl ethanolamide
- STEARDA
stearoyldopamine
- TRPV1
transient receptor potential type vanilloid 1 receptor
References
- AHERN G.P. Activation of TRPV1 by the satiety factor oleoylethanolamide. J. Biol. Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- BEN-SHABAT S., FRIDE E., SHESKIN T., TAMIRI T., RHEE M.H., VOGEL Z., BISOGNO T., DE PETROCELLIS L., DI MARZO V., MECHOULAM R. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- BERDYSHEV E.V. Inhibition of sea urchin fertilization by fatty acid ethanolamides and cannabinoids. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999;122:327–330. doi: 10.1016/s0742-8413(98)10136-6. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., VENTRIGLIA M., MILONE A., MOSCA M., CIMINO G., DI MARZO V. Occurrence and metabolism of anandamide and related acyl-ethanolamides in ovaries of the sea urchin Paracentrotus lividus. Biochim. Biophys. Acta. 1997;1345:338–348. doi: 10.1016/s0005-2760(97)00009-x. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., HENRIKSEN S.J., CRAVATT B.F. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr. Pharm. Des. 1998a;4:303–314. [PubMed] [Google Scholar]
- BOGER D.L., PATTERSON J.E., GUAN X., CRAVATT B.F., LERNER R.A., GILULA N.B. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc. Natl. Acad. Sci. U.S.A. 1998b;95:4810–4815. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., GUAN X., GILULA N.B. Arachidonic acid amide inhibitors of gap junction cell–cell communication. Bioorg. Med. Chem. Lett. 1999;9:1151–1154. doi: 10.1016/s0960-894x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- BRADSHAW H.B., KREY J.K., WALKER J.M.Levels of endocannabinoids and related lipid mediators in the female reproductive tract change as a function of estrous cycle Soc. Neuro. 2003. Abstracts Online Prog. Number 464.3
- BURSTEIN S.H. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol. Ther. 1999;82:87–96. doi: 10.1016/s0163-7258(98)00069-2. [DOI] [PubMed] [Google Scholar]
- BURSTEIN S.H., HUANG S.M., PETROS T.J., ROSSETTI R.G., WALKER J.M., ZURIER R.B. Regulation of anandamide tissue levels by N-arachidonylglycine. Biochem. Pharmacol. 2002;64:1147–1150. doi: 10.1016/s0006-2952(02)01301-1. [DOI] [PubMed] [Google Scholar]
- BUTELMAN E.R., BALL J.W., HARRIS T.J., KREEK M.J. Topical capsaicin-induced allodynia in unanesthetized primates: pharmacological modulation. J. Pharmacol. Exp. Ther. 2003;311:155–163. doi: 10.1124/jpet.103.052381. [DOI] [PubMed] [Google Scholar]
- BUTELMAN E.R., HARRIS T.J., KREEK M.J. Antiallodynic effects of loperamide and fentanyl against topical capsicin-induced allodyndia in unanesthetized primates. J. Pharmacol. Exp. Ther. 2004;306:1106–1114. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., PIOMELLI D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur. J. Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- CASCIO M.G., MINASSI A., LIGRESTI A., APPENDINO G., BURSTEIN S., DI MARZO V. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Comm. 2004;314:192–196. doi: 10.1016/j.bbrc.2003.12.075. [DOI] [PubMed] [Google Scholar]
- CHU C.J., HUANG S.M., DE PETROCELLIS L., BISOGNO T., EWING S.A., MILLER J.D., ZIPKIN R.E., DADDARIO N., APPENDINO G., DIMARZO V., WALKER J.M. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- CRAVATT B.F., GIANG D.K., MAYFIELD S.P., BOGER D.L., LERNER R.A., GILULA N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- CRAVATT B.F., PROSPERO-GARCIA O., SIUZDAK G., GILULA N.B., HENRIKSEN S.J., BOGER D.L., LERNER R.A. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., DAVIS J.B., DI MARZO V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., CHU C.J., MORIELLO A.S., KELLNER J.C., WALKER J.M., DI MARZO V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br. J. Pharmacol. 2004;143:251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., DE PETROCELLIS L., SEPE N., BUONO A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem. J. 1996;316 Part 3:977–984. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., ORLANDO P., BISOGNO T., ZAGOORY O., BIFULCO M., VOGEL Z., DE PETROCELLIS L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001;358:249–255. doi: 10.1042/0264-6021:3580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FACCI L., DAL TOSO R., ROMANELLO S., BURIANI A., SKAPER S.D., LEON A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDOROVA I., HASHIMOTO A., FECIK R.A., HEDRICK M.P., HANUS L.O., BOGER D.L., RICE K.C., BASILE A.S. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther. 2001;299:332–342. [PubMed] [Google Scholar]
- FEZZA F., BISOGNO T., MINASSI A., APPENDINO G., MECHOULAM R., DI MARZO V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J. Oleamide: a member of the endocannabinoids family. Br. J. Pharmacol. 2004;141:195–196. doi: 10.1038/sj.bjp.0705608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FU J., GAETANI S., OVEISI F., LO VERME J., SERRANO A., DE FONSESCA F.R., ROSENGARTH A., LUECKE H., DI GIACOMO B., TARZIA G., PIOMELLI D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- GAVVA N.R., KLIONSKY L., QU Y., SHI L., TAMIR R., EDENSON S., ZHANG T.J., VISWANDADHAN V.N., TOTH A., PEARCE L.V., VANDERAH T.W., PORRECA F., BLUMBERG M., LILE J., SUN Y., WILD K., LOUIS J.-C., TREANOR J.S. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- GOMEZ R., NAVARRO M., FERRER B., TRIGO J.M., BILBAO A., DEL ARCO I., CIPPITELLI A., NAVA F., PIOMELLI D., RODRIGUEZ D.E., FONSECA F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAN X., CRAVATT B.F., EHRING G.R., HALL J.E., BOGER D.L., LERNER R.A., GILULA N.B. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell. Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZMAN M., LO VERME J., FU J., OVEISI F., BLAZQUEZ C., PIOMELLI D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) J. Biol. Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- HANUS L., ABU-LAFI S., FRIDE E., BREUER A., VOGEL Z., SHALEV D.E., KUSTANOVICH I., MECHOULAM R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANUS L., GOPHER A., ALMOG S., MECHOULAM R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J. Med. Chem. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- HARRISON S., DE PETROCELLIS L., TREVISANI M., BENVENUTI F., BIFULCO M., GEPPETTI P., DIMARZO V. Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. Eur. J. Pharmacol. 2003;475:107–114. doi: 10.1016/s0014-2999(03)02114-9. [DOI] [PubMed] [Google Scholar]
- HUANG J.K., JAN C.R. Linoleamide, a brain lipid that induces sleep, increases cytosolic Ca2+ levels in MDCK renal tubular cells. Life Sci. 2001;68:997–1004. doi: 10.1016/s0024-3205(00)01002-x. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., PETROS T.J., CHANG S.Y., ZAVITSANOS P.A., ZIPKIN R.E., SIVAKUMAR R., COOP A., MAEDA D.Y., DE PETROCELLIS L., BURSTEIN S., DI MARZO V., WALKER J.M. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J.P., HARRIS R.A. Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGAR S.I., HASNIE F.S., SELLATURAY S., RICE A.S. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- JARAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONSSON K.O., VANDEVOORDE S., LAMBERT D.M., TIGER G., FOWLER C.J. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br. J. Pharmacol. 2001;133:1263–1275. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAVA V., FASIA L., SIAFAKA-KAPADAI A. Anandamide amidohydrolase activity, released in the medium by Tetrahymena pyriformis. Identification and partial characterization. FEBS Lett. 2001;508:327–331. doi: 10.1016/s0014-5793(01)03095-2. [DOI] [PubMed] [Google Scholar]
- KOZAK K.R., CREW B.C., MORROW J.D., WANG L.H., MA Y.H., WEINANDER R., JAKOBSSON P.J., MARNETT L.J. Metabolism of the endocannabinoids, 2-arachidonoylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- KUEHL F.A., JACOB T.A., GANLEY O.H., ORMOND R.E., MEISINGER M.A.P. The identification of N-2(hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957;79:5577–5578. [Google Scholar]
- LAINE K., JARVINEN K., MECHOULAM R., BREUER A., JARVINEN T. Comparison of the enzymatic stability and intraocular pressure effects of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest. Ophthalmol. Vis. Sci. 2002;43:3216–3222. [PubMed] [Google Scholar]
- LAMBERT D.M., DIPAOLO F.G., SONVEAUX P., KANYONYO M., GOVAERTS S.J., HERMANS E., BUEB J., DELZENNE N.M., TSCHIRHART E.J. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochim. Biophys. Acta. 1999;1440:266–274. doi: 10.1016/s1388-1981(99)00132-8. [DOI] [PubMed] [Google Scholar]
- LAMBERT D.M., VANDEVOORDE S., JONSSON K.O., FOWLER C.J. The palmitoylethanolamide family: a new class of anti-inflammatory agents. Curr. Med. Chem. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- LEGGETT J.D., ASPLEY S., BECKETT S.R., D'ANTONA A.M., KENDALL D.A. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br. J. Pharmacol. 2004;141:253–262. doi: 10.1038/sj.bjp.0705607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACCARRONE M., ATTINA M., BARI M., CARTONI A., LEDENT C., FINAZZI-AGRO A. Anandamide degradation and N-acylethanolamines level in wild-type and CB1 cannabinoid receptor knockout mice of different ages. J. Neurochem. 2001;78:339–348. doi: 10.1046/j.1471-4159.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., VANDER STELT M., ROSSI A., VELDINK G.A., VLIEGENTHART J.F., AGRO A.F. Anandamide hydrolysis by human cells in culture and brain. J. Biol. Chem. 1998;273:32332–32339. doi: 10.1074/jbc.273.48.32332. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-GONZALEZ D., BONILLA-JAIME H., MORALES-OTAL A., HENRIKSON S.J., VELAZQUEZ-MOCTEZUMA J., PROSPERO-GARCIA O. Oleamide and anandamide effects on food intake and sexual behavior of rats. Neuro. Lett. 2004;364:1–6. doi: 10.1016/j.neulet.2004.03.080. [DOI] [PubMed] [Google Scholar]
- MAURELLI S., BISOGNO T., DE PETROCELLIS L., DILUCCIA A., MARINO G., DI MARZO V. Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma ‘anandamide amidohydrolase'. FEBS Lett. 1995;377:82–86. doi: 10.1016/0014-5793(95)01311-3. [DOI] [PubMed] [Google Scholar]
- MAZZARI S., CANELLA R., PETRELLI L., MARCOLONGO G., LEON A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWBEE R.G., GRIFFIN G., BAYEWITCH M., BARG J., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., DIMARZO V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- MILMAN G., MAOR Y., HOROWITZ M., GALLILY R., HANUS L., MECHOULAM R.Arachidonoyl-serine, an endocannabinoids-like bioactive constituent of rat brain 2004. ICRS Abstracts Online 133
- OKA S., TSUCHIE A., TOKUMURA A., MURAMATSU M., SUHARA Y., TAKAYAMA H., WAKU K., SUGIURA T. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J. Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- PINTO J.C., POTIE F., RICE K.C., BORING D., JOHNSON M.R., EVANS D.M., WILKEN G.H., CANTRELL C.H., HOWLETT A.C. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol. Pharmacol. 1994;46:516–522. [PubMed] [Google Scholar]
- PREMKUMAR L.S., QI Z.-H., VAN BUREN J., RAISINGHANI M. Enhancement of potency and efficacy of NAD A by PKC-mediated phosphorylation of vanilloid receptor. J. Neurophys. 2004;91:1442–1449. doi: 10.1152/jn.00745.2003. [DOI] [PubMed] [Google Scholar]
- PRUSAKIEWICZ J.J., KINGSLEY P.J., KOZAK K.R., MARNETT L.J. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem. Biophys. Res. Commun. 2002;296:612–617. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- RAKHSHAN F., DAY T.A., BLAKELY R.D., BARKER E.L. Carrier-mediated uptake of the endogenous cannabinoid anandamide in RBL-2H3 cells. J. Pharmacol. Exp. Ther. 2000;292:960–967. [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A., O'SULLIVAN S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ DE FONSECA F., NAVARRO M., GOMEZ R., ESCUREDO L., NAVA F., FU J., MURILLO-RODRIGUEZ E., GIUFFRIDA A., LOVERME J., GAETANI S., KATHURIA S., GALL C., PIOMELLI D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- ROSS R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGAR D.R., SMITH P.A., MILLNS P.J., SMART D., KENDALL D.A., CHAPMAN V. TRPV1 and CB1 receptor-mediated effects of the endovanilloid/endocannabinoids N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur. J. Neurol. 2004;20:175–184. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- SALZET M., STEFANO G.B. The endocannabinoid system in invertebrates. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:353–361. doi: 10.1054/plef.2001.0347. [DOI] [PubMed] [Google Scholar]
- SANCHO R., MACHO A., DE LA VEGA L., CALZADO M.A., FIEBICH B.L., APPENDINO G., MUNOZ E. Immunosuppressive activity of endovanilloids N-arachidonoyl-dopamine inhibits activation of the NF-κB, NFAT, and activator protein 1 signaling pathways. J. Immunol. 2004;172:2341–2351. doi: 10.4049/jimmunol.172.4.2341. [DOI] [PubMed] [Google Scholar]
- SCHMID H.H., BERDYSHEV E.V. Cannabinoid receptor-inactive N-acylethanolamines and other fatty acid amides: metabolism and function. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:363–376. doi: 10.1054/plef.2001.0348. [DOI] [PubMed] [Google Scholar]
- SCHUEL H., BURKMAN L.J., LIPPES J., CRICKARD K., FORESTER E., PIOMELLI D., GIUFFRIDA A. N-acylethanolamines in human reproductive fluids. Chem. Phys. Lipids. 2002;121:211–227. doi: 10.1016/s0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- SHESKIN T., HANUS L., SLAGER J., VOGEL Z., MECHOULAM R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUKAGAWA A., NAKANE S., SHINODA A., ITOH K., YAMASHITA A., WAKU K. 2-arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- THOMAS E.A., CRAVATT B.F., DANIELSON P.E., GILULA N.B., SUTCLIFFE J.G. Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. J. Neurosci. Res. 1997;50:1047–1052. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- TOTH A., KEDEI N., WANG Y., BLUMBERG P.M. Arachidonyl dopamine as a ligand for the vanilloid receptor VR1 of the rat. Life Sci. 2003;73:487–498. doi: 10.1016/s0024-3205(03)00310-2. [DOI] [PubMed] [Google Scholar]
- WALTER L., STELLA N. Cannabinoids and neuroinflammation. Br. J. Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOST C.S., HAMPSON A.J., LEONOUDAKIS D., KOBLIN D.D., BORNHEIM L.M., GRAY A.T. Oleamide potentiates benzodiazepine-sensitive gamma-aminobutyric acid receptor activity but does not alter minimum alveolar anesthetic concentration. Anesth. Analg. 1998;86:1294–1300. doi: 10.1097/00000539-199806000-00031. [DOI] [PubMed] [Google Scholar]
- YU M., IVES D., RAMESHA C.S. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 1997;22:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]