Abstract
Vasomotion is the oscillation of vascular tone with frequencies in the range from 1 to 20 min−1 seen in most vascular beds. The oscillation originates in the vessel wall and is seen both in vivo and in vitro.
Recently, our ideas on the cellular mechanisms responsible for vasomotion have improved. Three different types of cellular oscillations have been suggested. One model has suggested that oscillatory release of Ca2+ from intracellular stores is important (the oscillation is based on a cytosolic oscillator). A second proposed mechanism is an oscillation originating in the sarcolemma (a membrane oscillator). A third mechanism is based on an oscillation of glycolysis (metabolic oscillator). For the two latter mechanisms, only limited experimental evidence is available.
To understand vasomotion, it is important to understand how the cells synchronize. For the cytosolic oscillators synchronization may occur via activation of Ca2+-sensitive ion channels by oscillatory Ca2+ release. The ensuing membrane potential oscillation feeds back on the intracellular Ca2+ stores and causes synchronization of the Ca2+ release. While membrane oscillators in adjacent smooth muscle cells could be synchronized through the same mechanism that sets up the oscillation in the individual cells, a mechanism to synchronize the metabolic-based oscillators has not been suggested.
The interpretation of the experimental observations is supported by theoretical modelling of smooth muscle cells behaviour, and the new insight into the mechanisms of vasomotion has the potential to provide tools to investigate the physiological role of vasomotion.
Keywords: Vasomotion, calcium, oscillations, membrane potential, endothelium, arteries, vascular smooth muscle
Introduction
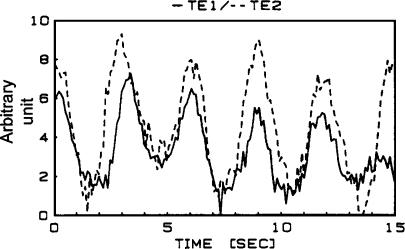
Vasomotion is the oscillation of vascular tone or vascular diameter that can be seen in many, if not all, vascular segments. It occurs both in vivo (Figure 1) and in vitro (Figure 2) and is generated from within the vascular wall, that is, it is not a consequence of the heart beat, respiration or neuronal input (although neuronal input may in some instances synchronize vasomotion in vascular beds, which are far apart; Schechner & Braverman, 1992; Porret et al., 1995). The first detailed report of vasomotion was made more than 150 years ago in the bat wing (Jones, 1852). In spite of this relatively long history, the two main questions are still incompletely understood: (1) What is the cellular background for vasomotion? and (2) what is the physiological consequence of vasomotion? One of the reasons for the lack of understanding of the cellular background is undoubtedly that there is more than one mechanism that will cause vasomotion, and that the mechanisms involved are complex and interacting in ways that are difficult to address experimentally. One of the reasons why the physiological and pathophysiological roles (if any) for vasomotion are not understood is that there is still no consistent way of inducing or inhibiting vasomotion in experimental settings.
Figure 1.
Synchronized oscillations of diameter (vasomotion) in two daughter branches in a rabbit skeletal muscle assessed under in vivo conditions. Data from Meyer et al. (1987).
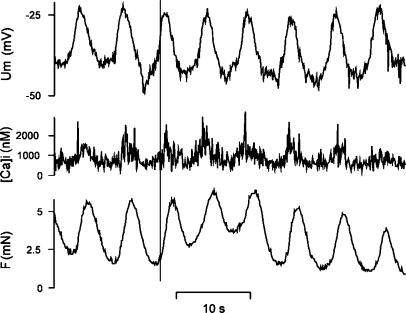
Figure 2.
Simultaneous measurements of isometric force, SMC membrane potential and SMC [Ca2+]i in an isolated rat mesenteric small artery. Note that membrane potential oscillations precede oscillations in [Ca2+]i, which again precede oscillations in tension.
This review will focus on the cellular background for vasomotion, but to enhance the reader's interest in the subject we will briefly mention the ideas on the physiological role of vasomotion, which have been suggested. The physiological importance of vasomotion is not well understood (Nilsson & Aalkjaer, 2003), although several suggestions have been made. For the same average diameter vasomotion ensures an increased flow conductance. An intriguing idea is that vasomotion and the ensuing slow oscillation of flow into a capillary bed – that is, flowmotion – provides an oscillation of oxygen tension (Misrahy et al., 1962), which ensures a better tissue oxygenation than that obtained with a steady oxygen delivery. Although theoretical evidence for this has been presented, the experimental evidence is scarce (Nilsson & Aalkjaer, 2003). There is, thus, a great need for experimental approaches that can address the relevance of vasomotion. To do this, it is important to understand the cellular background for vasomotion. Such an understanding may provide means of selectively interfering with steps in the cellular chain of events leading to vasomotion and thus specifically switch on or turn off vasomotion. When this becomes possible, the physiological relevance of the phenomenon will be much more accessible to investigation.
Cellular background for vasomotion
For vasomotion to occur, a cellular oscillator must be present, which can be modelled as a string of events forming a feedback loop, where inertia in one or more of the steps in the loop ensures oscillation. In order to get macroscopic oscillations of a blood vessel, the oscillations in individual smooth muscle cells (SMCs) must be synchronized. Therefore, some means of synchronization must be present. We will discuss these two essential elements of vasomotion separately. With respect to the oscillator, we will discuss the three different types of oscillators that have been suggested, viz. a cytosolic oscillator, a membrane oscillator and a metabolic oscillator. Of these, the cytosolic oscillator is probably the most relevant and we will consequently discuss in some detail the source of Ca2+ and the regulation that sets up the cytosolic oscillator. With respect to synchronization we will discuss the role of membrane potential and its reciprocal interaction with the sarcoplasmic reticulum (SR). In particular, we will discuss which sarcolemmal ion channels might be relevant for synchronization and also include a brief section on gap junctions. Since the role of the endothelium for vasomotion varies between different vascular beds, we will discuss the potential role of the endothelium in a separate section. In the last section, we discuss the theoretical models that have been developed for vasomotion.
The oscillator
A classification of cellular oscillators into two main types, membrane oscillators and cytosolic (or cytoplasmic) oscillators, was introduced by Berridge & Rapp (1979) (Figure 3). A membrane oscillator is considered to be one where the rhythm is generated at the membrane by oscillations in transporter activity or permeability. In contrast, a cytosolic oscillator does not depend on the cell membrane, but here oscillations arise from an intracellular instability in, for example, calcium release or energy production (the latter has also been called a metabolic oscillator).
Figure 3.
Model illustrating the differences between (a) a membrane oscillator and (b) a cytosolic oscillator.
A cytosolic oscillator in SMC
Ca2+ waves originating from intracellular Ca2+ stores
Oscillations of SMC [Ca2+]i have been reported for a long time (Weissberg et al., 1989), and, as will be discussed in some detail later, may form the basis for vasomotion even though these are often asynchronous. Blatter & Wier (1992) demonstrated that oscillations of [Ca2+]i take the form of waves of Ca2+ running parallel to the long axis of the cells in an SMC line (A7r5) and Iino et al. (1994) reported a similar behaviour of SMCs in the intact vascular wall. Following these initial observations, oscillating Ca2+ waves have been demonstrated in SMCs from many different vessels (see, e.g. Lee et al., 2002). It is very likely that vascular SMCs from all vascular beds can exhibit this behaviour. An example from rat mesenteric small arteries is shown in Figure 4. Much work has gone into defining the mechanism(s) responsible for this oscillating [Ca2+]i. Most detailed work has been carried out with regard to isolated SMCs, but information is also available from SMCs in the intact vascular wall and much of the background has come from studies of similar behaviour in many other cell systems.
Figure 4.
(a) Confocal imaging of [Ca2+]i in SMCs (upper traces) and isometric tension (lower trace) of an isolated rat mesenteric small artery. The black graph in the upper trace shows the average [Ca2+]. The different colours in the upper trace represent [Ca2+] in individual SMCs. The artery was activated with a low concentration of noradrenaline. The oscillations of SMC [Ca2+]i are first seen to be unsynchronized but then synchronize and vasomotion starts. (b) Confocal images of [Ca2+]i in a single SMC detailing the unsynchronized oscillations shown in (a). Note how the increase of [Ca2+]i runs as a wave along the axis of the SMC, which is typical for the unsynchronized activity. Data from Peng et al. (2001).
Blocking of the SR Ca2+ pump (SERCA) strongly inhibits the Ca2+ waves, suggesting that they are caused by release of Ca2+ from the SR. This is supported by the observation that the waves are present in the absence of extracellular Ca2+, although they disappear eventually (Iino et al., 1994; Ruehlmann et al., 2000; Peng et al., 2001). The reason for this is thought to be that some of the Ca2+ released from the SR during a Ca2+ wave is being pumped out of the cells, and some influx of Ca2+ is consequently necessary to refill the SR (Iino et al., 1994; Ruehlmann et al., 2000). In the rabbit portal vein both voltage-gated Ca2+ channels, nonselective cation channels and the Na+,Ca2+-exchanger have been suggested to be of importance for providing this influx maintaining the Ca2+ waves (Lee et al., 2001). A consistent characteristic of the Ca2+ waves is that they do not represent simple diffusion of released Ca2+, but involve a regenerative release of Ca2+ mediated by Ca2+-induced Ca2+ release (CICR). In vascular SMC, Ca2+ can be released via an IP3-sensitive channel via a caffeine- and ryanodine-sensitive channel and possibly via a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive channel. When agonists are used to induce Ca2+ waves IP3 is always involved. In isolated vascular and intestinal SMCs, uncaging of caged IP3 leads to generation of Ca2+ waves (Boittin et al., 1999; McCarron et al., 2003). Furthermore, blocking the IP3 receptor with heparin (Blatter & Wier, 1992; Boittin et al., 1999) and with 2-aminoethoxydiphenyl borate (Lee et al., 2001) blocks agonist-induced Ca2+ waves in A7r5 cells and in rabbit inferior vena cava, respectively. There is thus little doubt that the Ca2+ waves are caused by release from and subsequent uptake by the SR of Ca2+, and that several release channels are likely to be involved in the release (Figure 5).
Figure 5.
Model illustrating the basic elements in a cytosolic oscillator in an SMC. An IP3-producing agonist causes release of Ca2+ from the SR. This Ca2+ release is reinforced through Ca2+-induced Ca2+ release from different Ca2+ release channels. Ca2+ is taken up into the SR again or extruded from the cells via Ca2+-ATPases.
The next question is whether a primary release from the IP3 receptor leads to CICR via receptors for IP3 or ryanodine receptors. There is little doubt that acute blockade of the ryanodine receptor blocks Ca2+ waves (Blatter & Wier, 1992; Iino et al., 1994; Boittin et al., 1999; Ruehlmann et al., 2000; Peng et al., 2001), indicating a role for the ryanodine receptor. Further evidence that CICR via the ryanodine receptor is important for the IP3-initiated Ca2+ waves was obtained by Boittin et al. (1999) in the rat portal vein using IP3- and ryanodine-receptor-specific antibodies. In these experiments, care was taken to demonstrate that the inhibition of agonist-induced Ca2+ waves was not due to depletion of SR Ca2+ caused by ryanodine or ryanodine-receptor antibodies. On the other hand, chronic downregulation of the ryanodine receptors in the intact rat tail artery did not affect Ca2+ waves elicited by noradrenaline (Dreja et al., 2001) even though acute exposure to ryanodine inhibited Ca2+ release. This suggests that CICR via the IP3 receptor under some circumstances is sufficient to produce Ca2+ waves. In line with this, McCarron et al. (2003) recently showed that in isolated intestinal SMCs, IP3-induced Ca2+ waves are dependent on CICR, yet they are mediated only via the IP3 receptor and not via the ryanodine receptor (whether this also occurs in vascular smooth muscle is currently not known). It therefore seems that a primary Ca2+ release mediated via IP3 release can induce Ca2+ waves in SMCs through CICR mediated either via IP3 receptors or ryanodine receptors or a combination. Whether one or the other mechanism is important may depend on the relative concentration of the receptors (Boittin et al., 1999) or on the distribution of receptor subtypes or by luminal Ca2+ (McCarron et al., 2003) or perhaps a number of other parameters that determine the Ca2+ sensitivity of the two receptors.
The functional consequence – if any – of the different modes of Ca2+ waves is unknown, although it has been suggested that IP3-mediated Ca2+ release and ryanodine-receptor-mediated release may affect different ion channels in SMCs (Haddock & Hill, 2002). Based on this background, it is tempting to ask whether Ca2+ waves in smooth muscle can also be elicited completely independent of IP3. The answer to this is yes. Ca2+ waves elicited by high pHi in isolated VSMC from rat cerebral arteries are not inhibited by blockers of IP3 receptors (Heppner et al., 2002), but here as in the portal vein (Ruehlmann et al., 2000) and in rat mesenteric small arteries (A. Rahman, H. Nilsson, C. Aalkjær, unpublished observation), caffeine can induce Ca2+ waves. Whether pure ryanodine-receptor-mediated Ca2+ waves have functional consequences different from those seen with IP3 is unknown. It is also unclear whether cADP-ribose, which is a known endogenous agonist for the ryanodine receptor, is involved in these ryanodine-receptor-mediated Ca2+ waves in SMCs, but it has been suggested that cADP-ribose might instead lower [Ca2+]i through activation of the calcium-activated potassium channel (Boittin et al., 2003).
A third Ca2+ release channel activated by NAADP is also suggested to play a potential role. In this respect, it is of substantial interest that Boittin et al. (2002) recently showed that NAADP can induce a Ca2+ wave in VSMC from pulmonary arteries that is blocked by ryanodine but unaffected by inhibition of the IP3 receptor. The authors suggest that Ca2+ is released through an intracellular NAADP-sensitive channel, which initiates the wave through CICR from the ryanodine receptor. The importance of this mechanism in other VSMCs needs to be investigated.
Removal of Ca2+ from the cytosol
Following the increase of [Ca2+]i caused by release of Ca2+ from the SR, [Ca2+]i decreases again. This declining phase of the wave can be seen as the combination of a decrease of the Ca2+ release and the active removal of Ca2+ from the cytosol. The reason for the reduction of Ca2+ release could be a refractoriness of the Ca2+ release channels and evidence that it could be refractoriness to IP3 consequent to the high [Ca2+]i was recently reviewed (McCarron et al., 2004). The mechanisms responsible for the active removal of Ca2+ from the cytosol has been less intensely investigated compared to the release mechanism, and the likely candidates are the plasmalemmal and SR Ca2+-ATPases and the Na+, Ca2+-exchanger (Lee et al., 2001).
What causes the phase shift necessary for the oscillation?
As indicated above, inertia in one of the steps in the feedback loop ensures that the feedback signal is out of phase with the initiating event. This phase shift is essential for creating an oscillation. Several of the steps discussed above have been suggested to provide this relative ‘slowness' of the feedback loop. Possible candidates are a slow refilling of the SR after Ca2+ release, inactivation of the IP3 receptor or the ryanodine receptor either time-dependently or consequent to high [Ca2+]i or high receptor substrate concentration. In a recent detailed analysis of intestinal SMCs, a [Ca2+]i-induced inactivation of the IP3 receptor was shown to be important (McCarron et al., 2003), but the issue is not clarified in vascular smooth muscles yet.
Functional consequences of Ca2+ waves
Several functional consequences of Ca2+ waves have been suggested. From the point of view of this review, it is relevant that an oscillatory Ca2+ release has been suggested to be responsible for vasomotion (Peng et al., 2001). In general, the amplitude and frequency information contained in the Ca2+ waves has been suggested to be decoded rather acutely by transcriptional factors so that different types of oscillations lead to expression of different proteins (Dolmetsch et al., 1998; Li et al., 1998; and see recent review by Lewis, 2003). The role of this for VSM cell phenotype has not been investigated at all. Specifically for vascular smooth muscle, the force production has been shown to be graded by both recruitment of cells producing Ca2+ waves and by modulation of the frequency of Ca2+ waves in the individual cells (Iino et al., 1994; Kasai et al., 1997; Ruehlmann et al., 2000; Zang et al., 2001). This was suggested to be the background for the relation between the global intracellular calcium concentration in the vascular wall and force development. However, this may not always be the case because in mesenteric small arteries, the occurrence of Ca2+ waves seems not always to be tightly associated with force (Miriel et al., 1999; Peng et al., 2001). Interestingly, Swärd et al. (2002) have taken these observations further and suggested that even for a constant global [Ca2+]i, different combinations of frequency and amplitudes give rise to different mechanical responses. The background for this suggestion is that mitochondrial inhibition leads to higher frequency, lower amplitude Ca2+ waves and a reduced force development despite an unchanged time-averaged [Ca2+]i. These observations could be explained on the basis of a nonlinear and time-dependent relationship between [Ca2+]i myosin light-chain phosphorylation and force (Swärd et al., 2002).
Oscillations that appear independent of Ca2+ release from stores or a membrane oscillator
Another type of SMC oscillation appears to be present after blockade of the SR Ca2+-ATPase or inhibition of release of Ca2+ from ryanodine-sensitive Ca2+ channels. Such oscillations have been demonstrated by Omote and Mizusawa in a series of papers (Omote et al., 1992; 1993; Omote & Mizusawa, 1993), where they found oscillations in isometric tension of different isolated rabbit arteries after the SR Ca2+-ATPase was inhibited with cyclopiazonic acid (CPA) or Ca2+-induced Ca2+ release was inhibited with ryanodine. The oscillations were inhibited by charybdotoxin and iberiotoxin and it was suggested that the oscillations were due to an interaction between the large-conductance calcium-activated potassium channel and the voltage-dependent calcium channel. In a rabbit ear small artery, perfused in situ oscillations with a low but complex frequency were induced by low concentrations of CPA. Modelling suggested that this could represent the presence of membrane oscillator. With higher concentrations of CPA, all oscillations were inhibited (Griffith & Edwards, 1997), emphasizing the importance of the SR. Also in rat mesenteric small arteries, CPA could induce an oscillation of tone (Huang & Cheung, 1997) that similarly was abolished by charybdotoxin and by a moderate increase of extracellular potassium consistent with an important role for potassium channels. The endothelium-dependence of these oscillations differed, being endothelium-independent in the rabbit arteries but endothelium-dependent in the rat. In A7r5 cells, Ca2+ oscillations in response to low physiological concentrations of vasopressin have been demonstrated by Byron & Taylor (1993); these are present even after treatment with CPA and ryanodine, suggesting an independence from release of Ca2+ from stores sensitive to CPA and ryanodine. Furthermore, it has been suggested that this type of oscillation is dependent on activation of phospholipase D (Li et al., 2001) but the electrophysiological background has not been addressed.
Although there is some evidence for a membrane oscillator as just described, this has not been subject to as detailed an analysis as has the cytosolic-oscillator-based vasomotion. For example, it is not known to what extent the oscillations are caused by mechanisms, which are fully independent from Ca2+ release from the SR. As discussed above, the different Ca2+ stores in vascular SMCs are complex and may differ from preparation to preparation, and it is not clear from the studies with CPA and thapsigargin whether all stores have been completely blocked by the drugs (Griffith & Edwards, 1997). It therefore appears that membrane oscillators can indeed drive vasomotion under certain conditions or in certain vascular beds. However, in the majority of cases, a cytosolic oscillator seems more important. On the other hand, as discussed below, the cytosolic oscillator interacts importantly with the membrane to induce synchronization, that is, even in the cytosolic-oscillator-based oscillation, the membrane oscillates. It may therefore not be fruitful to maintain a strong distinction between the two types of oscillation.
A metabolic oscillator?
A third type of oscillator that could be responsible for vasomotion has been suggested by Siegel in a series of papers (Siegel et al., 1980; 1991; Siegel, 1983). It was suggested that oscillations in glycolysis could lead to oscillations in ATP concentrations, which then might cause oscillations in the activity of the electrogenic Na,K-pump, leading to oscillations in membrane potential. Experimental evidence for the two latter suggestions was provided. Based on modelling and experimental evidence from other tissues, Siegel and co-workers suggested that oscillations in the activity of the enzyme phosphofructokinase might be responsible. A prerequisite for this is allosteric regulation of phosphofructokinase activity by substrates, products and ATP on the regulatory control point of the glycolytic system (Siegel, 1983). Although this is an interesting possibility, nobody has followed up on this suggestion and the hypothesis is still waiting to be tested thoroughly. It should be noted that the Ca2+ oscillations discussed above could also potentially explain the oscillations of the Na,K-pump activity reported by Siegel (i.e. oscillation of the Na,K-pump may be consequent to the Ca2+ oscillation). A primary metabolic oscillation is therefore not necessary to explain this oscillation, although it is an intriguing idea. It could be added that, as pointed out by Siegel (1983), the mechanism for intercellular coupling that is necessary for vasomotion to occur is not known for this type of oscillation.
Endothelial cell oscillations
Interestingly, Ca2+ oscillations have been shown repeatedly in isolated endothelial cells (Jacob et al., 1988; Sage et al., 1989; Laskey et al., 1992; Paltauf-Doburzynska et al., 2000). Also in intact arteries, oscillations of endothelial [Ca2+]i (Schuster et al., 2001) and membrane potential (Segal & Beny, 1992; Muraki et al., 2000) have been reported in association with vasomotion. The role of these oscillations for vasomotion has not been investigated, and it is not known whether a primary endothelial oscillation can drive vasomotion. Undoubtedly, the interaction between endothelial oscillations and vascular smooth muscle oscillations is a potentially interesting area to investigate.
Synchronization
The importance of the membrane potential for synchronization
Whenever SMC membrane potential has been measured during vasomotion, a slow oscillation corresponding to the vasomotion has been reported (Mulvany et al., 1982; Hayashida et al., 1986; Garland, 1989; Segal & Beny, 1992; der Weid & Beny, 1993; Gustafsson et al., 1993; Hill et al., 1999; Peng et al., 2001; Haddock & Hill, 2002; Haddock et al., 2002; Oishi et al., 2002). The oscillation has the same frequency as the vasomotion and the oscillation in potential precedes the oscillation in smooth muscle tension (Figure 2). It should be pointed out that in human pial arteries, action potentials have been reported, which were associated with vasomotion (Gokina et al., 1996). During high-frequency oscillation of the action potentials, the associated contractions fused to a tonic contraction. Most authors have consequently suggested that vasomotion is caused by an oscillation in membrane potential. An electrical signal is also likely to be the only signal fast enough to synchronize SMC activity over several millimetres. However, it is interesting that Haddock et al. (2002) suggested that the tone of isolated irideal arterioles may oscillate independent of oscillations in membrane potential. The signal causing synchronization in this case is unknown.
Reciprocal interaction between the SR and the sarcolemma is important for synchronization in many situations
In most cases where the role of the endoplasmic reticulum has been investigated, vasomotion seems to be prevented when either release or uptake of Ca2+ into the SR is inhibited. This occurs in rabbit ear artery (Griffith & Edwards, 1993; 1997), and rat mesenteric (Gustafsson & Nilsson, 1993; Peng et al., 2001), cerebral (Haddock & Hill, 2002) and irideal (Hill et al., 1999; Haddock et al., 2002) arteries. Based on this observation, it has been suggested (Gustafsson, 1993; Griffith & Edwards, 1994; Peng et al., 2001; Haddock & Hill, 2002) that the unsynchronized Ca2+ oscillations caused by release of Ca2+ from the SR (discussed above) may entrain to initiate vasomotion (Figure 4). By entrainment, we understand that individual oscillators become phase-locked into the same phase. This is believed to occur through interaction between the oscillators (Strogatz & Stewart, 1993). In this suggestion, the Ca2+ released from the SR will activate an inward current in the membrane, leading to depolarization. As discussed above, several release channels potentially releasing Ca2+ from different stores may contribute to this. Although it has been suggested (Haddock & Hill, 2002) that release of Ca2+ from different Ca2+ stores may affect different ion channels, this problem is not easy to address experimentally. The extent to which a potentially very interesting functional coupling between specific Ca2+ stores and specific sarcolemmal ion channels is physiologically relevant therefore is still largely unknown. The vascular SMCs are electrically coupled and the current generated in one cell will run into the neighbouring SMCs. The ensuing synchronized depolarization will enhance the likelihood of Ca2+ release from the SR. This may occur either due to enhanced influx of Ca2+ through L-type Ca2+ channels leading to Ca2+-induced Ca2+ release (Peng et al., 2001) or due to depolarization-induced potentiation of IP3 production as it has been suggested for slow oscillations in the guinea-pig gastric pylorus (van Helden et al., 2000; van Helden & Imtiaz, 2003). In rat mesenteric small arteries, the former possibility seems more likely because nifedipine inhibits synchronization (Peng et al., 2001). This would not be expected if membrane-potential-induced oscillations of IP3 concentrations were causing the synchronization unless the IP3 oscillations were partly dependent on an oscillating Ca2+. An important distinction in this model (van Helden & Imtiaz, 2003) is whether the individual SMCs (or the SR in the cells) are sequentially activated following the primary activation of one cell or through entrainment of cells that all are active initially. In rat mesenteric small arteries most, possibly all, cells are active although unsynchronized and the synchronization may therefore predominantly reflect an entrainment. In the initial phase, it is possible that there is an element of sequential activation. Another implication of this model is that the release of Ca2+ from the SR is determining the Ca2+ concentration in a restricted space between the superficial SR and the sarcolemma, which, on the other hand, is not substantially affected by the Ca2+ influx through the L-type Ca2+ channels. Alternatively, the Ca2+ influx would provide a positive feedback on the depolarizing current, which would prevent the hyperpolarizing phase of the vasomotion. It is not easy to test this experimentally since it is difficult with the current techniques to detect Ca2+ in the restricted space on top of bulk Ca2+ caused by Ca2+ influx. A diagram of this model is shown in Figure 6.
Figure 6.
(a) Model indicating one proposed mechanism for initiation of vasomotion. The mechanism is based on a cytosolic oscillator, which interacts reciprocally with the membrane and which we consider an important mechanism for vasomotion (from Peng et al., 2001). (b) Diagram showing the proposed sequence of events in the model shown in (a).
Which sarcolemmal ion channel is important for vasomotion?
Several authors have implied models involving the mentioned elements, with the cytosolic oscillator interacting reciprocally with the membrane (Gustafsson, 1993; Parthimos et al., 1999; Peng et al., 2001; Haddock & Hill, 2002) to ensure entrainment of the SR. A similar mechanism has been suggested for the rhythmic contractions of the gastric pylorus (van Helden et al., 2000; van Helden & Imtiaz, 2003) and lymphatic vasomotion (Ferrusi et al., 2004) by van Helden's group. It becomes of interest to characterize the channel(s) responsible for the depolarizing current(s) induced by the Ca2+ release, which is responsible for the entrainment of the SR in this model. A likely candidate would be a Ca2+-activated Cl− current. Coupling of oscillatory Ca2+ release to activation of a Cl− channel has been shown in several smooth muscle preparations (Bakhramov et al., 1996; Liu & Farley, 1996b; Hyvelin et al., 1998), and in swine tracheal SMCs, it was shown that a tonic increase of IP3 could induce such oscillations (Liu & Farley, 1996a). The Ca2+-activated Cl− channels have not been characterized in the same detail as the voltage-dependent anion channels or the cation channels. This is partly because there are no selective blockers for these channels, and partly because the molecular identity of the channels is still controversial (Jentsch et al., 2002; Nilius & Droogmans, 2003). In a search for a Cl− channel that could be important in vasomotion, we have exploited the observation that the synchronization of oscillating smooth muscle [Ca2+]i activity as well as the inward current and depolarization induced by release of Ca2+ from intracellular Ca2+ stores is cGMP-dependent in rat mesenteric small arteries (Peng et al., 2001). The channel responsible for these electrophysiological effects is likely a Ca2+-activated, cGMP-dependent Cl− channel, with unique biophysical and pharmacological characteristics, which we have recently described (Matchkov et al., 2004a). This channel is voltage- and time-independent, has a distinct anion permeability sequence with bromide being more permeable than iodide, is relatively insensitive to classical Cl− channel blockers such as niflumic acid, IAA-94 and DIDS, but is quite sensitive to Zn2+. Single-channel recordings have shown that the channel has three conductance levels (15, 35 and 55 pS) (Piper & Large, 2004b) and is calmodulin-dependent (Piper & Large, 2004a). The channel is present in rat mesenteric arteries together with the Ca2+-activated, but cGMP-independent Cl− channel (Matchkov et al., 2004a) seen in many vascular preparations (Large & Wang, 1996). It is a distinct possibility that this novel Cl− channel provides the Ca2+-induced inward current necessary for the synchronization of the vascular SMCs in rat mesenteric small arteries. However, it remains to corroborate these suggestive observations with direct experimental evidence that this channel or another chloride channel is indeed involved in vasomotion. Obviously, other Ca2+-activated channels giving rise to an inward current could also be involved. Particularly in cGMP-independent forms of vasomotion, it is likely that another current is involved. However, little is known and this is an area that needs investigation to understand vasomotion.
Also, potassium channels may play a role as discussed above for the membrane-oscillator-based vasomotion. But also for the vasomotion based on release of Ca2+ from the SR, potassium channels may be involved since vasomotion frequency is often affected by potassium channel blockers. Although this demonstrates an influence of potassium channels, it also indicates that potassium channels are not an essential element in the feedback loop constituting the oscillation, since the blockers were incapable of inhibiting vasomotion. Potassium channels may also be involved through a different mechanism. Inhibition of potassium channels with tetraethylammonium (TEA) has consistently been shown to promote vasomotion (Kannan & Daniel, 1978; Watts et al., 1994; Wu et al., 2000; Haddock & Hill, 2002; Kamouchi et al., 2002). One possible explanation for this is a TEA-induced decrease in membrane conductance, which would promote intercellular coupling. Another suggested mechanism (Kannan & Daniel, 1978; Watts et al., 1994) is that TEA induces the formation of gap junctions (Kannan & Daniel, 1978; Sheppard & Meda, 1981; Watts et al., 1994) and in this way promotes vasomotion (see below).
The importance of gap junctions
A key element for the synchronization of the SMCs is the gap junctions, which undoubtedly mediate the electrical coupling of the SMCs in the vascular wall. The presence of gap junctions in the vascular wall has been documented with a variety of techniques (Beny & Connat, 1992; Watts et al., 1994; Little et al., 1995; Christ et al., 1996; Sandow & Hill, 2000). Most importantly in this context, it has been demonstrated that knockout of connexin 40 is associated with irregular arteriolar vasomotion (de Wit et al., 2003); however, a variety of substances that are blockers of gap junctions have been shown to inhibit vasomotion (Tsai et al., 1995; Chaytor et al., 1997; Sell et al., 2002; Matchkov et al., 2004b). Although some of these substances have nonjunctional or unspecific effects (Chaytor et al., 1997; Santicioli & Maggi, 2000; Tare et al., 2002; Matchkov et al., 2004b), the consistent inhibitory effects on vasomotion of these substances, which include peptides with specificity for the extrafacial loops of the connexins (Chaytor et al., 1997), strongly support the key role of gap junctions for vasomotion. Although so far no conclusive evidence has been presented for the role of regulation of gap junctions in vasomotion, there are interesting suggestions that this could be the case. One possibility is that TEA, as discussed in the preceding section, promotes vasomotion through induction of gap junctions, which would suggest that upregulation of gap junctions could influence the probability of getting vasomotion. Another possibility discussed in the following sections is that cGMP, through an effect on gap junctions, may modify the prevalence of vasomotion.
Role of the endothelium for vasomotion
Even though current views on vasomotion hold that vasomotion originates in the SMCs, the important modulatory role of the endothelium on smooth muscle function makes it relevant to consider the role of the endothelium in vasomotion. The influence of the endothelium seems to vary between preparations. In some arteries, removal of the endothelium or blockade of NO production with arginine analogues prevents vasomotion. This is the case in hamster aorta (Jackson, 1988), hamster cheek pouch (Jackson, 1993), rat mesenteric arteries (Gustafsson, 1993; Huang & Cheung, 1997; Mauban et al., 2001; Peng et al., 2001; Okazaki et al., 2003), rabbit mesenteric (Omote & Mizusawa, 1993; Akata et al., 1995) and coronary arteries (Akata et al., 1995) and the human cutaneous circulation (Kvandal et al., 2003). Vasomotion is promoted when the endothelium is removed (or the NO synthase inhibited) in the rabbit ear artery (Griffith & Edwards, 1993), rat aorta (Marchenko & Sage, 1994), hamster cheek pouch (Bertuglia et al., 1995) and rat mesenteric arteries (Sell et al., 2002), while in some situations the presence or absence of the endothelium is reported to be without effect on vasomotion rat aorta (Chemtob et al., 1992; Freeman et al., 1995), rabbit mesenteric arteries (Omote & Mizusawa, 1993) and pig coronary arteries (der Weid & Beny, 1993). Such a variability of results even within the same artery might suggest that one or more factors from the endothelium influence one or more of the key control variables that are important for vasomotion.
In hamster aorta (Jackson et al., 1991) and rat mesenteric arteries (Gustafsson & Nilsson, 1993; Peng et al., 2001), the role of the endothelium could be to provide a certain level of cGMP, which is necessary for coordination of the oscillators in the SMCs. This is suggested from the observation that in the absence of the endothelium, addition of cGMP will lead to a synchronization of the Ca2+ transients in the vascular SMC (Peng et al., 2001) and vasomotion (Jackson et al., 1991; Gustafsson & Nilsson, 1993; Peng et al., 2001). A constant concentration of cGMP is thus able to get the oscillation back. It has been suggested that this may be mediated by the cGMP-dependence of a Ca2+-activated, cGMP-dependent Cl− channel in the vascular SMC (Peng et al., 2001; Matchkov et al., 2004a). To explain the situations with an inhibitory effect of the endothelium and cGMP on vasomotion, it has been suggested that cGMP could inhibit vascular SMC communication via inhibition of gap junctions (Sell et al., 2002). The effect of cGMP on vascular SMC gap junctions seems complex (Hoffmann et al., 2003; Kameritsch et al., 2003), but in cardiac myocytes, conduction is decreased by cGMP (Burt & Spray, 1988; Kwak & Jongsma, 1996). On the other hand, it has been shown that conduction of a change in membrane potential in the vascular wall is inhibited after removal of the endothelium (Emerson & Segal, 2000; Segal & Jacobs, 2001; Takano et al., 2004). Although this finding has been interpreted to indicate the current runs in the endothelium, it is also possible that this may reflect inhibition of a positive effect of cGMP on SMC gap junctional conductance. If this is correct, it would support a role for gap junctions in mediating the cGMP dependence of vasomotion.
It is well documented that endothelial cells can exhibit oscillations in Ca2+ (Jacob et al., 1988; Sage et al., 1989; Laskey et al., 1992; Kasai et al., 1997; Paltauf-Doburzynska et al., 2000), which take the form of waves (Kasai et al., 1997). Oscillations of endothelial membrane potential are also reported (Laskey et al., 1992; Segal & Beny, 1992; der Weid & Beny, 1993). Furthermore, the oscillations of [Ca2+]i can be synchronized between the endothelial cells both in primary culture (Laskey et al., 1992) and in the intact artery (Schuster et al., 2001), and it is suggested that the Ca2+ oscillations drive oscillations in membrane potential (Laskey et al., 1992). The observation that the endothelial Ca2+ oscillations in the intact vascular wall are not always synchronized, for example, during activation with acetylcholine (Huang et al., 2000; Marie & Beny, 2002), suggests that endothelial membrane potential is not always important for endothelial cell [Ca2+]i. Whether under some circumstances oscillations in endothelial membrane potential can drive oscillations in endothelial [Ca2+]i is not known. In this respect, it would be very interesting to know whether synchronized oscillations in endothelial [Ca2+]i occurs in situations where the endothelial membrane potential is clamped. If this can occur, it becomes very relevant to understand which mechanism can cause the synchronization of endothelial cell Ca2+ oscillations. One possibility might be diffusion of Ca2+, IP3 (Boitano et al., 1992; Demer et al., 1993) or another second messenger through gap junctions between the endothelial cells. This, however, needs experimental testing or testing with a quantitative modelling approach. The endothelial cells may therefore have the capacity to pace directly the vascular SMCs and be responsible for vasomotion, but so far nobody has provided evidence that this might be the case.
Theoretical models of vasomotion
In the foregoing sections, we have discussed experiments that have addressed the question of which mechanisms are responsible for vasomotion and the schemes based on these experiments that have suggested an explanation for vasomotion. In this section, we will summarize those attempts that have been made to mathematically model these suggestions based on quantitative considerations.
There is a large body of literature dealing with models of oscillations of [Ca2+]i, primarily in nonexcitable cells. This work is of obvious interest in the context of vasomotion.
Membrane oscillators (Figure 3) underlie oscillation in a number of cell types, and have been extensively modelled in, for example, sinoatrial node cells (Noble & Noble, 1984; Kurata et al., 2003; Ono et al., 2003) and in pituitary cells (Li et al., 1995; Tomic et al., 1999). These generally depend on alternating activation of various depolarizing currents (such as calcium or sodium currents) and hyperpolarizing currents (typically potassium currents). In membrane oscillators, the feedback signal is often provided by voltage dependence, or by, for example, calcium feedback on ion channels; phase delay is often provided by time-dependent currents.
With respect to cytosolic oscillators, a useful overview of simple models for the generation of oscillations in [Ca2+]i is given by Goldbeter (1996). In the simple models presented by Goldbeter, the feedback is the facilitation of Ca2+ release by [Ca2+]i, and the phase shift is caused by the emptying–refilling of the SR. The facilitation of Ca2+ release, in other words CICR, could be modelled either via the ryanodine receptor or via the IP3 receptor. This is a critical component of systems producing Ca2+ oscillations. A very simple system, comprising an intracellular store with one of these receptors and a reuptake mechanism refilling the store, can theoretically suffice for oscillation. [Ca2+]i in such a system is primed by agonist-induced IP3 elevation setting [Ca2+]i to a level on top of which the CICR system oscillates. Another important conclusion from this analysis is that a system where Ca2+ simply overflows after refilling, with no CICR, is not able to sustain oscillation.
Most of these simple models incorporate the positive feedback into the Ca2+ release channel (be it either the ryanodine or the IP3 receptor). Interestingly, Meyer & Stryer (1988) found that in a model where the positive feedback was by Ca2+ on IP3 generation and not on the IP3 receptor, only a bistable [Ca2+]i was obtained: [Ca2+]i switched from low to high at a critical level of PLC activity. However, if an additional pathway for Ca2+ sequestration (via mitochondria) was included, spike-like oscillation emerged. Thus, a limit on the positive feedback may be required for oscillation.
Extensions of the models by Goldbeter are reviewed by Schuster et al. (2002), covering not only minimal models but also more involved models. As pointed out by these authors, mitochondrial Ca2+ sequestration may be important in Ca2+ oscillations for several reasons: maintaining the amplitude of Ca2+ oscillations constant over a large frequency range, and limiting the Ca2+ peaks so as not to induce apoptosis, which might be initiated by high Ca2+ levels.
The multitude of models of cytosolic Ca2+ oscillators can be regarded as a demonstration of the inherent strong tendency to oscillation of the SR calcium release mechanism. The modelling here is consistent with experimental findings.
Only a few attempts have been made to comprehensively model the mechanisms underlying vasomotion. Griffith and collaborators have modelled the cellular mechanisms underlying the complex vasomotion of the rabbit ear artery (Parthimos et al., 1999). The various patterns of oscillation that can be provoked in this artery can be modelled rather precisely by a combination of an intracellular oscillator and a membrane oscillator.
The intracellular oscillator was modelled like those described above: a cytosolic oscillator depending on ryanodine-receptor CICR to release Ca2+ from the SR in an oscillating manner. The sequestration into and release of Ca2+ from this store produce oscillation but do not affect [Ca2+]i, and thus do not influence the average contraction level. This suggests that here vasomotion may be regarded as oscillation around a mean, not as superimposed contractions or relaxations on a steady contraction.
The membrane oscillator was modelled as direct or indirect Ca2+ feedback on a multitude of transport processes in the membrane: Ca2+ channels, calcium-activated K+ channels, Cl− channels, plasma membrane Ca2+-ATPase, Na+/Ca2+ exchange and the Na+,K+-ATPase. Ca2+ entry through voltage-gated channels is the central mechanism here, coordinating other membrane events. However, in this model, the only ion channel directly sensing Ca2+ is the calcium-activated potassium channel. Depolarizing effects of intracellular Ca2+ are thus not included.
Either the cytosolic or the membrane oscillator alone can induce oscillation in this model. The membrane oscillator is responsible for fast oscillation (period less than a minute), while the cytosolic oscillator is slower. Interestingly, including the latch state of smooth muscle in the model (force being carried by slowly cycling, dephosphorylated crossbridges) dampened the oscillations even to a point where those due to the fast (membrane) oscillator were no longer visible.
The model of Parthimos et al. (1999) correlates well with available experimental data, and explains how oscillations in the individual cell may occur, but does not deal with multiple cells. The other aspect of vasomotion – synchronization – has been accommodated in three recent models (Imtiaz et al., 2002; Jacobsen, 2004; Koenigsberger et al., 2004).
Imtiaz et al. (2002) studied slow waves in gastrointestinal muscle (in the presence of L-type channel blockers) (van Helden et al., 2000), and found these to be favoured by agonists, suggesting an important contribution of IP3. They were also favoured by depolarization, which was taken as an indication of an influence of membrane potential on IP3 generation. In the model, which is based on a cytosolic oscillator model, Ca2+ release is assumed to trigger membrane channels to open and cause depolarization. This is assumed to spread between cells via gap junctions and to promote IP3 formation throughout the tissue. The positive feedback here is the indirect feedback of Ca2+ release on IP3 production, further enhancing release. However, direct evidence for IP3 oscillation is still lacking.
The model of Jacobsen (2004) is based on the experimental data of Peng et al. (2001). This model is similar to that of Imtiaz et al. (2002), although cell coupling here is mediated by the effect of depolarization on L-type Ca2+ channels, not on IP3 formation (in contrast to the model of Imtiaz et al. (2002), L-type channels were functional in this model). In both this and Imtiaz' models, spread of depolarization between cells is assumed to be via gap junctions and to be responsible for the synchronization.
The model by Koenigsberger et al. (2004) is based on the model by Parthimos et al. (1999) with respect to the cellular oscillator, but was expanded to a multicellular tissue by including cell connections. In contrast to the two models described above, this model does not include calcium-sensitive depolarizing ion channels. Interestingly, in this model electrical coupling alone was not sufficient to induce synchronization of calcium oscillations – a certain calcium permeability through gap junctions was necessary. This model also suggested that electrical communication may be a two-edged sword: a high degree of electrical coupling may accentuate the current–sink effect of neighbouring cells and therefore reduce the chances of membrane potential synchronization. Both this model and that of Jacobsen (2004) are based on data from the same tissue, but have different starting points. It would be interesting to see the influence on the Koenigsberger model of the inclusion of calcium-sensitive depolarizing channels.
Conclusion
Vasomotion has been observed for more than a 100 years, is probably present in every vascular segment and has been a nuisance to many vascular researchers who want to ascribe a well-defined tone to their preparation under a given condition; yet, the physiological and possible pathophysiological function is still not known. Based on this background, it is important to try to understand the cellular mechanisms leading to vasomotion, so as to hopefully provide instruments that can be used to interfere with vasomotion in specific ways.
Our understanding of the cellular mechanisms responsible for the synchronized oscillatory activity of the SMCs has improved substantially in recent years. In most situations, a cytosolic oscillator appears to be important because vasomotion is very sensitive to interference with release or uptake of Ca2+ from intracellular stores. Furthermore, to achieve synchronization of the cytosolic oscillators in the individual SMCs, it has been suggested that the cytosolic oscillator interacts with the membrane to establish membrane-potential changes that mediate the synchronization. Other types of oscillations, either based solely on interactions of ion currents in the sarcolemma or based on oscillations of the glycolytic pathway and consequently the Na,K-pump, have also been suggested and could play a role under some conditions, although these pathways are probably less frequent.
With tone as a relatively straightforward read-out and with well-developed techniques to study the details of excitation–contraction coupling in SMCs, we believe that the attempts to unravel the mechanism of vasomotion not only will provide information of value for vascular physiology and pharmacology but also will provide novel information on mechanisms of cell oscillation and cell synchronization in many other areas of cell biology.
Abbreviations
- CICR
Ca2+-induced Ca2+ release
- CPA
cyclopiazonic acid
- NAADP
nicotinic acid adenine dinucleotide phosphate
- SERCA
sarcoplasmic reticulum Ca2+ pump
- SMC
smooth muscle cells
- SR
sarcoplasmic reticulum
- TEA
tetraethylammonium
References
- AKATA T., KODAMA K., TAKAHASHI S. Role of endothelium in oscillatory contractile responses to various receptor agonists in isolated small mesenteric and epicardial coronary arteries. Jpn. J. Pharmacol. 1995;68:331–343. doi: 10.1254/jjp.68.331. [DOI] [PubMed] [Google Scholar]
- BAKHRAMOV A., HARTLEY S.A., SALTER K.J., KOZLOWSKI R.Z. Contractile agonists preferentially activate Cl− over K+ currents in arterial myocytes. Biochem. Biophys. Res. Commun. 1996;227:168–175. doi: 10.1006/bbrc.1996.1484. [DOI] [PubMed] [Google Scholar]
- BENY J.L., CONNAT J.L. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circ. Res. 1992;70:49–55. doi: 10.1161/01.res.70.1.49. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., RAPP P.E. A comparative survey of the function, mechanism and control of cellular oscillators. J. Exp. Biol. 1979;81:217–279. doi: 10.1242/jeb.81.1.217. [DOI] [PubMed] [Google Scholar]
- BERTUGLIA S., COLANTUONI A., INTAGLIETTA M. Capillary reperfusion after L-arginine, L-NMMA, and L-NNA treatment in cheek pouch microvasculature. Microvasc. Res. 1995;50:162–174. doi: 10.1006/mvre.1995.1050. [DOI] [PubMed] [Google Scholar]
- BLATTER L.A., WIER W.G. Agonist-induced [Ca2+]i waves and Ca2+-induced Ca2+ release in mammalian vascular smooth muscle cells. Am. J. Physiol. 1992;263:H576–H586. doi: 10.1152/ajpheart.1992.263.2.H576. [DOI] [PubMed] [Google Scholar]
- BOITANO S., DIRKSEN E.R., SANDERSON M.J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- BOITTIN F.X., DIPP M., KINNEAR N.P., GALIONE A., EVANS A.M. Vasodilation by the calcium-mobilizing messenger cyclic ADP-ribose. J. Biol. Chem. 2003;278:9602–9608. doi: 10.1074/jbc.M204891200. [DOI] [PubMed] [Google Scholar]
- BOITTIN F.X., GALIONE A., EVANS A.M. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ. Res. 2002;91:1168–1175. doi: 10.1161/01.res.0000047507.22487.85. [DOI] [PubMed] [Google Scholar]
- BOITTIN F.X., MACREZ N., HALET G., MIRONNEAU J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am. J. Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- BURT J.M., SPRAY D.C. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am. J. Physiol. 1988;254:H1206–H1210. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- BYRON K.L., TAYLOR C.W. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J. Biol. Chem. 1993;268:6945–6952. [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEMTOB S., INAYATULLA A., VARMA D.R. Eicosanoid-dependent and endothelium-independent oscillations of rat aorta. J. Vasc. Res. 1992;29:270–280. doi: 10.1159/000158942. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., SPRAY D.C., EL SABBAN M., MOORE L.K., BRINK P.R. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ. Res. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- DE WIT C., ROOS F., BOLZ S.S., POHL U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol. Genom. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- DEMER L.L., WORTHAM C.M., DIRKSEN E.R., SANDERSON M.J. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. Am. J. Physiol. 1993;264:H2094–H2102. doi: 10.1152/ajpheart.1993.264.6.H2094. [DOI] [PubMed] [Google Scholar]
- DER WEID P.Y., BENY J.L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J. Physiol. 1993;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLMETSCH R.E., XU K., LEWIS R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- DREJA K., NORDSTROM I., HELLSTRAND P. Rat arterial smooth muscle devoid of ryanodine receptor function: effects on cellular Ca2+ handling. Br. J. Pharmacol. 2001;132:1957–1966. doi: 10.1038/sj.bjp.0703986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERSON G.G., SEGAL S.S. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ. Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- FERRUSI I., ZHAO J., VAN HELDEN D.F., DER WEID P.Y. Cyclopiazonic acid decreases spontaneous transient depolarizations in guinea-pig mesenteric lymphatic vessels in an endothelium-dependent and independent manner. Am. J. Physiol. 2004;286:H2287–H2295. doi: 10.1152/ajpheart.00739.2003. [DOI] [PubMed] [Google Scholar]
- FREEMAN K.A., MAO A., NORDBERG L.O., PAK J., TALLARIDA R.J. The relationship between vessel wall tension and the magnitude and frequency of oscillation in rat aorta. Life Sci. 1995;56:L129–L134. doi: 10.1016/0024-3205(94)00912-0. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J. Influence of the endothelium and alpha-adrenoreceptor antagonists on responses to noradrenaline in the rabbit basilar artery. J. Physiol. 1989;418:205–217. doi: 10.1113/jphysiol.1989.sp017835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOKINA N.I., BEVAN R.D., WALTERS C.L., BEVAN J.A. Electrical activity underlying rhythmic contraction in human pial arteries. Circ. Res. 1996;78:148–153. doi: 10.1161/01.res.78.1.148. [DOI] [PubMed] [Google Scholar]
- GOLDBETER A.Oscillations and waves of intracellular calcium Biochemical Oscillations and Cellular Rhythms 1996Cambridge: Cambridge Unviersity Press; 351–406.Anonymous. pp [Google Scholar]
- GRIFFITH T.M., EDWARDS D.H. Modulation of chaotic pressure oscillations in isolated resistance arteries by EDRF. Eur. Heart J. 1993;14 Suppl I:60–67. [PubMed] [Google Scholar]
- GRIFFITH T.M., EDWARDS D.H. Fractal analysis of role of smooth muscle Ca2+ fluxes in genesis of chaotic arterial pressure oscillations. Am. J. Physiol. 1994;266:H1801–H1811. doi: 10.1152/ajpheart.1994.266.5.H1801. [DOI] [PubMed] [Google Scholar]
- GRIFFITH T.M., EDWARDS D.H. Ca2+ sequestration as a determinant of chaos and mixed-mode dynamics in agonist-induced vasomotion. Am. J. Physiol. 1997;272:H1696–H1709. doi: 10.1152/ajpheart.1997.272.4.H1696. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON H. Vasomotion and underlying mechanisms in small arteries. Acta Physiol. Scand. 1993;149 Suppl 614:1–44. [PubMed] [Google Scholar]
- GUSTAFSSON H., MULVANY M.J., NILSSON H. Rhythmic contractions of isolated small arteries from rat: influence of the endothelium. Acta Physiol. Scand. 1993;148:153–163. doi: 10.1111/j.1748-1716.1993.tb09545.x. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON H., NILSSON H. Rhythmic contractions of isolated small arteries from rat: role of calcium. Acta Physiol. Scand. 1993;149:283–291. doi: 10.1111/j.1748-1716.1993.tb09623.x. [DOI] [PubMed] [Google Scholar]
- HADDOCK R.E., HILL C.E. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J. Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDOCK R.E., HIRST G.D., HILL C.E. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J. Physiol. 2002;540:219–229. doi: 10.1113/jphysiol.2001.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHIDA N., OKUI K., FUKUDA Y. Mechanism of spontaneous rhythmic contraction in isolated rat large artery. Jpn. J. Physiol. 1986;36:783–794. doi: 10.2170/jjphysiol.36.783. [DOI] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., SANTANA L.F., NELSON M.T. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2169–H2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- HILL C.E., EADE J., SANDOW S.L. Mechanisms underlying spontaneous rhythmical contractions in irideal arterioles of the rat. J. Physiol. 1999;521 Part 2:507–516. doi: 10.1111/j.1469-7793.1999.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN A., GLOE T., POHL U., ZAHLER S. Nitric oxide enhances de novo formation of endothelial gap junctions. Cardiovasc. Res. 2003;60:421–430. doi: 10.1016/j.cardiores.2003.04.001. [DOI] [PubMed] [Google Scholar]
- HUANG T.Y., CHU T.F., CHEN H.I., JEN C.J. Heterogeneity of [Ca2+]i signaling in intact rat aortic endothelium. FASEB J. 2000;14:797–804. doi: 10.1096/fasebj.14.5.797. [DOI] [PubMed] [Google Scholar]
- HUANG Y., CHEUNG K.K. Endothelium-dependent rhythmic contractions induced by cyclopiazonic acid in rat mesenteric artery. Eur. J. Pharmacol. 1997;332:167–172. doi: 10.1016/s0014-2999(97)01071-6. [DOI] [PubMed] [Google Scholar]
- HYVELIN J.M., GUIBERT C., MARTHAN R., SAVINEAU J.P. Cellular mechanisms and role of endothelin-1-induced calcium oscillations in pulmonary arterial myocytes. Am. J. Physiol. 1998;275:L269–L282. doi: 10.1152/ajplung.1998.275.2.L269. [DOI] [PubMed] [Google Scholar]
- IINO M., KASAI H., YAMAZAWA T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMTIAZ M.S., SMITH D.W., VAN HELDEN D.F. A theoretical model of slow wave regulation using voltage-dependent synthesis of inositol 1,4,5-trisphosphate. Biophys. J. 2002;83:1877–1890. doi: 10.1016/S0006-3495(02)73952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON W.F. Oscillations in active tension in hamster aortas: role of the endothelium. Blood Vessels. 1988;25:144–156. doi: 10.1159/000158728. [DOI] [PubMed] [Google Scholar]
- JACKSON W.F.Role of endothelium-derived nitric oxide in vasomotion Mechanoreception by the Vascular Wall 1993Mount Kisco, NY: Futura Publishing Co; 173–196.ed. Rubanyi, G.M. pp [Google Scholar]
- JACKSON W.F., MULSCH A., BUSSE R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am. J. Physiol. 1991;260:H248–H253. doi: 10.1152/ajpheart.1991.260.1.H248. [DOI] [PubMed] [Google Scholar]
- JACOB R., MERRITT J.E., HALLAM T.J., RINK T.J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- JACOBSEN J.C.B. Ph.D. Thesis, Department of Medical Physiology, University of Copenhagen, pp. 1–114; 2004. Simulation of acute and chronic reaction patterns in the microcirculation. [Google Scholar]
- JENTSCH T.J., STEIN V., WEINREICH F., ZDEBIK A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- JONES T.W. Discovery that the veins of the bat's wing are endowed with rhythmical contractility and that onward flow of blood is accelerated by each contraction. Philos. Trans. Roy. Soc. Lond. 1852;142:131–136. [Google Scholar]
- KAMERITSCH P., HOFFMANN A., POHL U. Opposing effects of nitric oxide on different connexins expressed in the vascular system. Cell Commun. Adhes. 2003;10:305–309. doi: 10.1080/cac.10.4-6.305.309. [DOI] [PubMed] [Google Scholar]
- KAMOUCHI M., KITAZONO T., NAGAO T., FUJISHIMA M., IBAYASHI S. Role of CA(2+)-activated K+ channels in the regulation of basilar arterial tone in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2002;29:575–581. doi: 10.1046/j.1440-1681.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- KANNAN M.S., DANIEL E.E. Formation of gap junctions by treatment in vitro with potassium conductance blockers. J. Cell Biol. 1978;78:338–348. doi: 10.1083/jcb.78.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASAI Y., YAMAZAWA T., SAKURAI T., TAKETANI Y., IINO M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. J. Physiol. 1997;504:349–357. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIGSBERGER M., SAUSER R., LAMBOLEY M., BENY J.L., MEISTER J.J. Ca2+ dynamics in a population of smooth muscle cells: modeling the recruitment and synchronization. Biophys. J. 2004;87:92–104. doi: 10.1529/biophysj.103.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURATA Y., HISATOME I., IMANISHI S., SHIBAMOTO T. Roles of L-type Ca2+ and delayed-rectifier K+ currents in sinoatrial node pacemaking: insights from stability and bifurcation analyses of a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2804–H2819. doi: 10.1152/ajpheart.01050.2002. [DOI] [PubMed] [Google Scholar]
- KVANDAL P., STEFANOVSKA A., VEBER M., DESIREE K.H., ARVID K.K. Regulation of human cutaneous circulation evaluated by laser Doppler flowmetry, iontophoresis, and spectral analysis: importance of nitric oxide and prostaglandins. Microvasc. Res. 2003;65:160–171. doi: 10.1016/s0026-2862(03)00006-2. [DOI] [PubMed] [Google Scholar]
- KWAK B.R., JONGSMA H.J. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol. Cell. Biochem. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- LARGE W.A., WANG Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am. J. Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- LASKEY R.E., ADAMS D.J., CANNELL M., VAN BREEMEN C. Calcium entry-dependent oscillations of cytoplasmic calcium concentration in cultured endothelial cell monolayers. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1690–1694. doi: 10.1073/pnas.89.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE C.H., POBURKO D., KUO K.H., SEOW C.Y., VAN BREEMEN C. Ca2+ oscillations, gradients, and homeostasis in vascular smooth muscle. Am. J. Physiol. 2002;282:H1571–H1583. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
- LEE C.H., POBURKO D., SAHOTA P., SANDHU J., RUEHLMANN D.O., VAN BREEMEN C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS R.S. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem. Soc. Trans. 2003;31:925–929. doi: 10.1042/bst0310925. [DOI] [PubMed] [Google Scholar]
- LI W., LLOPIS J., WHITNEY M., ZLOKARNIK G., TSIEN R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- LI Y., SHIELS A.J., MASZAK G., BYRON K.L. Vasopressin-stimulated Ca2+ spiking in vascular smooth muscle cells involves phospholipase D. Am. J. Physiol. 2001;280:H2658–H2664. doi: 10.1152/ajpheart.2001.280.6.H2658. [DOI] [PubMed] [Google Scholar]
- LI Y.X., RINZEL J., VERGARA L., STOJILKOVIC S.S. Spontaneous electrical and calcium oscillations in unstimulated pituitary gonadotrophs. Biophys. J. 1995;69:785–795. doi: 10.1016/S0006-3495(95)79952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE T.L., BEYER E.C., DULING B.R. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am. J. Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- LIU X., FARLEY J.M. Acetylcholine-induced Ca2+-dependent chloride current oscillations are mediated by inositol 1,4,5-trisphosphate in tracheal myocytes. J. Pharmacol. Exp. Ther. 1996a;277:796–804. [PubMed] [Google Scholar]
- LIU X., FARLEY J.M. Acetylcholine-induced chloride current oscillations in swine tracheal smooth muscle cells. J. Pharmacol. Exp. Ther. 1996b;276:178–186. [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Smooth muscle cells affect endothelial membrane potential in rat aorta. Am. J. Physiol. 1994;267:H804–H811. doi: 10.1152/ajpheart.1994.267.2.H804. [DOI] [PubMed] [Google Scholar]
- MARIE I., BENY J.L. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J. Vasc. Res. 2002;39:260–267. doi: 10.1159/000063691. [DOI] [PubMed] [Google Scholar]
- MATCHKOV V.V., AALKJAER C., NILSSON H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J. Gen. Physiol. 2004a;123:121–134. doi: 10.1085/jgp.200308972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHKOV V.V., RAHMAN A., PENG H., NILSSON H., AALKJAER C. Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br. J. Pharmacol. 2004b;142:961–972. doi: 10.1038/sj.bjp.0705870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUBAN J.R., LAMONT C., BALKE C.W., WIER W.G. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- MCCARRON J.G., BRADLEY K.N., MACMILLAN D., CHALMERS S., MUIR T.C. The sarcoplasmic reticulum, Ca2+ trapping, and wave mechanisms in smooth muscle. News Physiol. Sci. 2004;19:138–147. doi: 10.1152/nips.01518.2004. [DOI] [PubMed] [Google Scholar]
- MCCARRON J.G., MACMILLAN D., BRADLEY K.N., CHALMERS S., MUIR T.C. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J. Biol. Chem. 2004;279:8417–8427. doi: 10.1074/jbc.M311797200. [DOI] [PubMed] [Google Scholar]
- MEYER J.U., LINDBOM L., INTAGLIETTA M. Coordinated diameter oscillations at arteriolar bifurcations in skeletal muscle. Am. J. Physiol. 1987;253:H568–H573. doi: 10.1152/ajpheart.1987.253.3.H568. [DOI] [PubMed] [Google Scholar]
- MEYER T., STRYER L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRIEL V.A., MAUBAN J.R., BLAUSTEIN M.P., WIER W.G. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J. Physiol. 1999;518:815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISRAHY G.A., HARDWICK D.F., BROOKS C.J., GARWOOD V.P., HALL W.P. Bone, bone marrow, and brain oxygen. Am. J. Physiol. 1962;202:225–231. doi: 10.1152/ajplegacy.1962.202.2.225. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., NILSSON H., FLATMAN J.A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J. Physiol. 1982;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAKI K., WATANABE M., IMAIZUMI Y. Nifedipine and nisoldipine modulate membrane potential of vascular endothelium via a myo-endothelial pathway. Life Sci. 2000;67:3163–3170. doi: 10.1016/s0024-3205(00)00908-5. [DOI] [PubMed] [Google Scholar]
- NILIUS B., DROOGMANS G. Amazing chloride channels: an overview. Acta Physiol. Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- NILSSON H., AALKJAER C.Vasomotion: mechanisms and physiological importance Mol. Interv. 2003379–89.51 [DOI] [PubMed] [Google Scholar]
- NOBLE D., NOBLE S.J. A model of sino-atrial node electrical activity based on a modification of the DiFrancesco-Noble (1984) equations. Proc. Roy. Soc. Lond. Ser. B. 1984;222:295–304. doi: 10.1098/rspb.1984.0065. [DOI] [PubMed] [Google Scholar]
- OISHI H., SCHUSTER A., LAMBOLEY M., STERGIOPULOS N., MEISTER J.J., BENY J.L. Role of membrane potential in vasomotion of isolated pressurized rat arteries. Life Sci. 2002;71:2239–2248. doi: 10.1016/s0024-3205(02)02014-3. [DOI] [PubMed] [Google Scholar]
- OKAZAKI K., SEKI S., KANAYA N., HATTORI J., TOHSE N., NAMIKI A. Role of endothelium-derived hyperpolarizing factor in phenylephrine-induced oscillatory vasomotion in rat small mesenteric artery. Anesthesiology. 2003;98:1164–1171. doi: 10.1097/00000542-200305000-00019. [DOI] [PubMed] [Google Scholar]
- OMOTE M., KAJIMOTO N., MIZUSAWA H. Phenylephrine induces endothelium-independent rhythmic contraction in rabbit mesenteric-arteries treated with ryanodine. Acta Physiol. Scand. 1992;145:295–296. doi: 10.1111/j.1748-1716.1992.tb09367.x. [DOI] [PubMed] [Google Scholar]
- OMOTE M., KAJIMOTO N., MIZUSAWA H. The ionic mechanism of phenylephrine-induced rhythmic contractions in rabbit mesenteric arteries treated with ryanodine. Acta Physiol. Scand. 1993;147:9–13. doi: 10.1111/j.1748-1716.1993.tb09467.x. [DOI] [PubMed] [Google Scholar]
- OMOTE M., MIZUSAWA H. The role of sarcoplasmic reticulum in endothelium-dependent and endothelium-independent rhythmic contractions in the rabbit mesenteric artery. Acta Physiol. Scand. 1993;149:15–21. doi: 10.1111/j.1748-1716.1993.tb09587.x. [DOI] [PubMed] [Google Scholar]
- ONO K., SHIBATA S., IIJIMA T. Pacemaker mechanism of porcine sino-atrial node cells. J. Smooth Muscle Res. 2003;39:195–204. doi: 10.1540/jsmr.39.195. [DOI] [PubMed] [Google Scholar]
- PALTAUF-DOBURZYNSKA J., FRIEDEN M., SPITALER M., GRAIER W.F. Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase. J. Physiol. 2000;524:701–713. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTHIMOS D., EDWARDS D.H., GRIFFITH T.M. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am. J. Physiol. 1999;277:H1119–H1144. doi: 10.1152/ajpheart.1999.277.3.H1119. [DOI] [PubMed] [Google Scholar]
- PENG H.L., MATCHKOV V., IVARSEN A., AALKJÆR C., NILSSON H. Hypothesis for the initiation of vasomotion. Circ. Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- PIPER A.S., LARGE W.A. Direct effect of Ca2+-calmodulin on cGMP-activated Ca2+-dependent Cl−channels in rat mesenteric artery myocytes. J. Physiol. 2004a;559:449–457. doi: 10.1113/jphysiol.2004.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPER A.S., LARGE W.A. Single cGMP-activated Ca2+-dependent Cl−channels in rat mesenteric artery smooth muscle cells. J. Physiol. 2004b;555:397–408. doi: 10.1113/jphysiol.2003.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRET C.A., STERGIOPULOS N., HAYOZ D., BRUNNER H.R., MEISTER J.J. Simultaneous ipsilateral and contralateral measurements of vasomotion in conduit arteries of human upper limbs. Am. J. Physiol. 1995;269:H1852–H1858. doi: 10.1152/ajpheart.1995.269.6.H1852. [DOI] [PubMed] [Google Scholar]
- RUEHLMANN D.O., LEE C.H., POBURKO D., VAN BREEMEN C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ. Res. 2000;86:E72–E79. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- SAGE S.O., ADAMS D.J., VAN BREEMEN C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J. Biol. Chem. 1989;264:6–9. [PubMed] [Google Scholar]
- SANDOW S.L., HILL C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Effect of 18beta-glycyrrhetinic acid on electromechanical coupling in the guinea-pig renal pelvis and ureter. Br. J. Pharmacol. 2000;129:163–169. doi: 10.1038/sj.bjp.0703004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHECHNER J.S., BRAVERMAN I.M. Synchronous vasomotion in the human cutaneous microvasculature provides evidence for central modulation. Microvasc. Res. 1992;44:27–32. doi: 10.1016/0026-2862(92)90099-b. [DOI] [PubMed] [Google Scholar]
- SCHUSTER S., MARHL M., HÖFER T. Modelling of simple and complex calcium oscillations. Eur. J. Biochem. 2002;269:1333–1355. doi: 10.1046/j.0014-2956.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- SCHUSTER A., OISHI H., BENY J.L., STERGIOPULOS N., MEISTER J.J. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am. J. Physiol. 2001;280:H1088–H1096. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- SEGAL S.S., BENY J.L. Intracellular recording and dye transfer in arterioles during blood flow control. Am. J. Physiol. 1992;263:H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- SEGAL S.S., JACOBS T.L. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J. Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL M., BOLDT W., MARKWARDT F. Desynchronising effect of the endothelium on intracellular Ca2+ concentration dynamics in vascular smooth muscle cells of rat mesenteric arteries. Cell Calcium. 2002;32:105–120. doi: 10.1016/s0143-4160(02)00036-2. [DOI] [PubMed] [Google Scholar]
- SHEPPARD M.S., MEDA P. Tetraethylammonium modifies gap junctions between pancreatic beta-cells. Am. J. Physiol. 1981;240:C116–C120. doi: 10.1152/ajpcell.1981.240.3.C116. [DOI] [PubMed] [Google Scholar]
- SIEGEL G. Principles of vascular rhythmogenesis. Prog. Appl. Microcirc. 1983;3:40–63. [Google Scholar]
- SIEGEL G., EBELING B.J., HOFER H.W.Foundations for vascular rhythm Ber. Bunsenges. Phys. Chem. 198084403–406.GENERIC [Google Scholar]
- SIEGEL G., HOFER H.W., WALTER A., RÜCKBORN K., SCHNALKE F., KOEPCHEN H.P.Autorhytmicity in blood vessels: its biophysical and biochemical bases Springer Ser. Synerget. 19915535–60.GENERIC [Google Scholar]
- STROGATZ S.H., STEWART I. Coupled oscillators and biological synchronization. Sci. Am. 1993;269:102–109. doi: 10.1038/scientificamerican1293-102. [DOI] [PubMed] [Google Scholar]
- SWÄRD K., DREJA K., LINDQVIST A., PERSSON E., HELLSTRAND P. Influence of mitochondrial inhibition on global and local [Ca2+]i in rat tail artery. Circ. Res. 2002;90:792–799. doi: 10.1161/01.res.0000015214.40360.84. [DOI] [PubMed] [Google Scholar]
- TAKANO H., DORA K.A., SPITALER M.M., GARLAND C.J. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J. Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARE M., COLEMAN H.A., PARKINGTON H.C. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am. J. Physiol. 2002;282:H335–H341. doi: 10.1152/ajpheart.2002.282.1.H335. [DOI] [PubMed] [Google Scholar]
- TOMIC M., KOSHIMIZU T., YUAN D., ANDRIC S.A., ZIVADINOVIC D., STOJILKOVIC S.S. Characterization of a plasma membrane calcium oscillator in rat pituitary somatotrophs. J. Biol. Chem. 1999;274:35693–35702. doi: 10.1074/jbc.274.50.35693. [DOI] [PubMed] [Google Scholar]
- TSAI M.L., WATTS S.W., LOCH-CARUSO R., WEBB R.C. The role of gap junctional communication in contractile oscillations in arteries from normotensive and hypertensive rats. J. Hypertens. 1995;13:1123–1133. doi: 10.1097/00004872-199510000-00007. [DOI] [PubMed] [Google Scholar]
- VAN HELDEN D.F., IMTIAZ M.S. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J. Physiol. 2003;548:271–296. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HELDEN D.F., IMTIAZ M.S., NURGALIYEVA K., VON DER W.P., DOSEN P.J. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J. Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS S.W., TSAI M.L., LOCH-CARUSO R., WEBB R.C. Gap junctional communication and vascular smooth muscle reactivity: use of tetraethylammonium chloride. J. Vasc. Res. 1994;31:307–313. doi: 10.1159/000159057. [DOI] [PubMed] [Google Scholar]
- WEISSBERG P.L., LITTLE P.J., BOBIK A. Spontaneous oscillations in cytoplasmic calcium concentration in vascular smooth muscle. Am. J. Physiol. 1989;256:C951–C957. doi: 10.1152/ajpcell.1989.256.5.C951. [DOI] [PubMed] [Google Scholar]
- WU L., WANG Z., WANG R. Tetraethylammonium-evoked oscillatory contractions of rat tail artery: a K–K model. Can. J. Physiol. Pharmacol. 2000;78:696–707. [PubMed] [Google Scholar]
- ZANG W.J., BALKE C.W., WIER W.G. Graded alpha1-adrenoceptor activation of arteries involves recruitment of smooth muscle cells to produce ‘all or none' Ca(2+) signals. Cell Calcium. 2001;29:327–334. doi: 10.1054/ceca.2000.0193. [DOI] [PubMed] [Google Scholar]