Abstract
Both in mammalian tissues and in transfected cells, only low levels of α1D-adrenoceptors are detected in radioligand binding studies. It has been implicated that the comparatively long N-terminal tail of the α1D-adrenoceptor is responsible for the inefficient surface expression of the receptor.
In the present study, we created gene constructs for six N-terminally truncated variants of the human α1D-adrenoceptor. These constructs were used to transfect Neuro2A cells. We show that the density of α1D-adrenoceptors, observed by [3H]-prazosin binding, gradually increased with longer truncations of the N-terminus. This seems to indicate that the long N-terminal tail nonspecifically interferes with receptor translocation to the plasma membrane.
The addition of a 16 amino acids long signal peptide to the N-terminus of the wild-type α1D-adrenoceptor increased the density of receptor binding sites 10-fold in Neuro2A and COS-7 cells. This indicates that, after the addition of a signal peptide, the long N-terminal tail of the α1D-adrenoceptor does not interfere with proper translocation of the receptor to the plasma membrane. This, in turn, indicates that the N-terminal tail of the wild-type α1D-adrenoceptor, merely by its long length, hinders the first transmembrane helix of the receptor from being a signal anchor.
Neither the wild-type α1D-adrenoceptor (for which the expression level of [3H]-prazosin binding sites is low) nor the truncated α1D-adrenoceptor variant (for which the expression level of [3H]-prazosin binding sites is high) showed any constitutive activity in stimulating inositol phosphate accumulation. This indicates that the low expression level of [3H]-prazosin binding sites, after transfection with the wild-type α1D-adrenoceptor, is not caused by constitutive activity of the receptor and subsequent receptor downregulation.
Keywords: α1D, adrenoceptor, signal peptide, truncated, N-terminal, [3H]-prazosin
Introduction
The α1-adrenoceptors are G-protein-coupled receptors (GPCRs) in the body responsive to the hormone adrenaline and the transmittor substance noradrenaline. There exist three subtypes of α1-adrenoceptors, denoted α1A-AR, α1B-AR, and α1D-AR (Zhong & Minneman, 1999; Hague et al., 2003). α1-Adrenoceptors are abundantly present in the brain, with as yet unclear functions, and they play an important role in the control of blood pressure, by influencing contraction and growth of smooth and cardiac muscle (Zhong & Minneman, 1999). Regarding the α1D-subtype, it has been shown that α1D-adrenoceptors are present in the aorta (Gisbert et al., 2003), and that they regulates arterial blood pressure via vasoconstriction (Tanoue et al., 2002). However, the functional role of the α1D-adrenoceptor is obscured by the low level of α1D-adrenoceptor binding sites in mammalian tissues (Yang et al., 1997; see Garcia-Sainz & Villalobos-Molina, 2004). Even after transfection of cultured mammalian cells with the gene for α1D-AR, only few receptors are detected by radioligand binding in membranes (Pupo et al., 2003). Instead, it seems like the α1D-adrenoceptor protein is localized mainly intracellularly (McCune et al., 2000; Chalothorn et al., 2002), in a form that apparently does not bind radioligand (Pupo et al., 2003). Recently, it has been proposed that the α1D-adrenoceptor is poorly expressed in membranes because it is not properly translocated to the plasma membrane after its synthesis (Hague et al., 2004a). The initial step in the translocation of a membrane protein to the plasma membrane is the insertion of the maturing protein into the membrane of the endoplasmatic reticulum. To accomplish this, the majority of GPCRs have short N-tails (about 40 amino acids) and use the first transmembrane domain as signal anchor, while GPCRs with longer N-tails often contain an additional cleavable signal peptide sequence (Wallin & von Heijne, 1995). Therefore, poor translocation efficieny of α1D-AR might be caused by its fairly long extracellular N-terminal tail (95 amino acids), combined with its lack of a signal peptide sequence. Indeed, truncation of the N-terminal tail of the α1D-AR increased the density of specific α1D-AR binding sites in the membranes quite dramatically compared to the wild-type receptor (Pupo et al., 2003). Studies with other GPCRs clearly show that the N-terminus of the receptor is important for their expression pattern. Thus, it has been shown that removal of the inherent signal peptide from the endothelin B receptor decreased surface expression, while further truncation of the residual tail rescued it (Köchl et al., 2002). Also, truncation of the long N-terminal tail of the cannabinoid receptor 1, or supplementing it with an N-terminal signal peptide sequence, increased expression of receptors in the plasma membrane (Andersson et al., 2003).
In the present study, we made gene constructs for six N-terminally truncated variants of the human α1D-adrenoceptor. We also added a 16 amino acids long signal peptide to the N-terminus of the wild-type α1D-adrenoceptor. These constructs were used to investigate the Bmax levels of the receptors obtained after transient transfections of Neuro2A cells.
Earlier, it has been hypothesized that the α1D-AR is constitutively active and therefore continuously becomes downregulated (McCune et al., 2000). In order to study if constitutive activity is responsible for the observed low density of α1D-AR binding sites in the plasma mambrane, we investigated basal, agonist-stimulated, and antagonist-modified levels of inositol phosphates (IPs) accumulation. This was carried out in Neuro2A cells stably expressing wild-type α1A-, α1B-, and α1D-adrenoceptors, and two truncated α1D-adrenoceptors.
Methods
Materials
[7-methoxy-3H]-prazosin (84 Ci mmol−1) and myo-[2-3H]-inositol (15 Ci mmol−1) were from Amersham Biosciences, Uppsala, Sweden. Cirazoline was a gift from Synthelabo, Paris, France. Metitepine, phenylephrine, and prazosin were from Sigma-Aldrich, Stockholm, Sweden. Primers were purchased from TAG Copenhagen A/S, Denmark, or Thermo Hybaid GmbH, Ulm, Germany. COS-7 (African green monkey kidney fibroblast) and Neuro2A (mouse neuroblastoma) cells were obtained from ATCC (Rockville, U.S.A.). All other chemicals and molecular biology reagents were purchased from the appropriate commercial sources.
Gene constructs
Truncated variants of the human α1D-adrenoceptor were obtained by PCR, using Platinum Pfx DNA polymerase, on the α1D-adrenoceptor gene inserted in pCI-neo with EcoRI and XbaI.
The forward primers used were as follows: wt α1D-AR: 5′-ga gaa ttc atg act ttc cgc gat ctc ct; Δ1–5α1D-AR: 5′-ga gaa ttc atg ctc ctg agc gtc agt ttc gag-3′; Δ1–17α1D-AR: 5′-ga gaa ttc atg agc agc gca ggg ggc tcc ag-3′; Δ1–30α1D-AR: 5′-ga gaa ttc atg agc gcg ggc ggc gcg gcc cc-3′; Δ1–58α1D-AR: 5′-ga gaa ttc atg gca ggc agc ggc gag gac aac-3′; and Δ1–89α1D-AR: 5′-ga gaa ttc atg ctg gtg gtg agc gcg cag gg-3′. The reverse primer was 5′-agg ttc acg atg aaa tag ttg gt-3′.
This reverse primer is located with its 5′-end at the position of the base 413 in the α1D-gene. These primer pairs amplified between 157 to 421 bp bands, which could be cut with EcoRI (site present in the forward primers) and BstEII (site at position 338 in the α1D-gene). The cut bands were purified by agarose gel electrophoresis, and then ligated into the EcoRI- and BstEII-cut wild-type α1D construct.
The N-terminal 95 amino acids of the human α1D-adrenoceptor can be represented as shown below. The wild-type α1D-adrenoceptor starts at the first Met, the first truncated variant (Δ1–5α1D-AR) had the first five amino acids exchanged for a Met at the first * (below), (Δ1–17α1D-AR) had 17 amino acids exchanged for a Met at the second *, (Δ1–30α1D-AR) had 30 amino acids exchanged for a Met at the third *, accidentally the Δ1–30α1D-AR primer gave rise to an extra truncated clone (Δ1S–49α1D-AR), which had 49 amino acids exchanged for Met-Ser at the **, (Δ1–58α1D-AR) had 58 amino acids exchanged for a Met at the fourth *, and (Δ1–89α1D-AR) had 89 amino acids exchanged for a Met at the fifth *.
An α1D-adrenoceptor supplemented with an N-terminal cleavable signal peptide (α1D-AR-SP) was constructed by inserting a modified Influenza hemaglutinin signal sequence (Jou et al., 1980) before the start codon of the α1D-adrenoceptor gene. The same sequence has been used previously for adding signal peptide to the hamster β2-adrenoceptor (Guan et al., 1992). The signal peptide-inducing primer was tctt (NheI>) gct agc (signal peptide>) atg aag acg atc atc gcc ctg agc tac atc ttc tgc ctg gta ttc gcc (α1D>) atg act ttc cgc gat ctc ctga. A wild-type α1D-AR construct, having the same kozak sequence (Kozak, 1991) as the α1D-AR-SP construct, was made using the corresponding forward primer, but devoid of the 48 bases encoding-the signal peptide sequence. The same reverse primer as was described previously (three sections above) (with its 5′-end at position 413 bp in the α1D-gene) was used. Inserts obtained from the PCR bands were cloned with NheI and BstEII, yielding the new wild-type α1D-AR construct, and the full-length α1D-AR-signal peptide (α1D-AR-SP) construct. The procedure described here added the peptide sequence MKTIIALSYI FCLVFA N-terminally to the wild-type α1D-adrenoceptor.
Cell culture
The COS-7 as well as Neuro2A cells were grown at 37°C, 95% air, 5% CO2, in Dulbecco's modified Eagle's medium (DMEM) (Sigma D5796), supplemented with 10% fetal calf serum and 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin, in standard tissue culture plastic material. Cells were subcultured every 3 to 4 days, after detachment with 0.5 mg ml−1 trypsin and 0.2 mg ml−1 EDTA (Sigma T3924).
Transient expression
The genes encoding the α1A-, α1B-, and α1D-adrenoceptors, as well as the truncated α1D variants, were cloned into the expression vector pCI-neo. For transfection, 15 μg of construct DNA was mixed with 200 μl of lipofectin and 600 μl of OptiMEM, and after 20 min the mixture was added to an about 70% confluent layer of COS-7 or Neuro2A cells in a 13.5 cm Petri dish, immediately followed by the addition of 20 ml of OptiMEM (in some experiments,10 μg of construct DNA was used to transfect 9 cm plates). Next day, the medium was exchanged to DMEM, with 10% fetal calf serum and 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin, and the cells were further cultured for 48 h. The transfections, membrane preparations, and determinations of Bmax values by radioligand binding were matched, so that one plate of cells was transfected with 15 μg of the corresponding wild-type α1D construct, and simultaneously other plates were transfected with the truncated α1D-AR variants or with the α1D-AR-SP construct. In matched transfections with the two wild-type α1D constructs, having Kozak sequences gaattcatga and gctagcatga, respectively, the membranes showed similar levels of [3H]-prazosin binding sites, indicating that this difference in Kozak sequence was not of critical importance for the expression levels of α1D-AR (S Uhlén, personal communication).
Establishment of stable cell lines
Clonal cell lines, expressing the truncated α1D variant Δ1-17α1D-AR, the truncated α1D variant Δ1-58α1D-AR, as well as the wild-type α1A-, α1B- and α1D-adrenoceptors, were established by transfection of Neuro2A cells with the gene constructs, as described in the section above. At 12 h after transfection, OptiMEM was exchanged for DMEM and the cells were cultured for 24 h. Then, the cells were trypsinized, diluted into medium including 800 μg ml−1 of geneticin, and seeded into a 48-well microtiter plate (about 10 cells well−1). The medium was exchanged every day or every other day, and wells containing a single attached cell were identified. After about 10 days, clonal islands had developed, and the cultures were trypsinized, transferred, and expanded in six-well plates. After 4–5 days, full monolayers had developed. These cells were then cultured in 10 ml flasks and the concentration of geneticin was sequentially decreased (first 500 μg ml−1, then 300 μg ml−1, and all subsequent 200 μg ml−1). Cell lines obtained were screened for α1-adrenoceptors with [3H]-prazosin. For each receptor variant, several clones were obtained, among themselves expressing similar levels of binding sites, which were high for the α1D variant Δ1–58α1D-AR, as well as for the α1B-adrenoceptor, but fairly low for the α1A- and α1D-adrenoceptors and for the α1D variant Δ1–17α1D-AR.
Measurement of inositol phosphate accumulation
Accumulation of [3H]-IPs was determined by a modification of the protocol described by Berridge et al. (1983). Cells stably expressing the different receptors were loaded with myo-[3H]-inositol for 18 h. Thereafter, cells were detached with Versene (137 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4, 0.54 mM EDTA, pH 7.4) at 37°C, spun at 2500 × g for 2 min, and resuspended in Na-Elliot buffer (137 mM NaCl, 5 mM KCl, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 1.2 mM MgCl2, 1 mM CaCl2, 10 mM glucose, 20 mM HEPES, pH 7.4), including 10 mM lithium. Lithium inhibits the breakdown of inositol phosphates, which are second messenger molecules for the α1-AR. The cell suspension was incubated for 10 min at 37°C, and then 100 μl of cells were added to a 96-well plate, containing 50 μl of varying concentrations of the agonists cirazoline (Horie et al., 1995) or phenylephrine (Obika et al., 1995), or the antagonist prazosin. Aliquots (100 μl) of the cell preparations were also added to scintillation vials in order to measure the total amount of [3H]-inositol taken up by the cells. The incubation in the 96-well plate then proceeded for 50 min. Cells were spun down at 2500 × g for 90 s, the supernatant discarded, the cells lyzed with 100 μl of 0.4 M perchloric acid (HClO4), and immediately frozen at −80°C. After 1 h, the suspensions were thawed, immediatelly neutralized with 50 μl of cold 0.36 M KOH+0.3 M KHCO3, and cell debris was spun down at 2000 × g for 10 min. Based on the method used by Berridge et al. (1983), the total amount of [3H]-IPs from each well was isolated on a Dowex column (BioRad AG1-X8 anion exchange column, formate form, about 1.2 ml packed suspension), equilibrated with 4 ml 0.1 M formic acid+1 M NH4-formate, followed by 4 ml dH2O. Each sample was added to a column, and then the column was washed two times with 4 ml dH2O and two times with 4 ml 5 mM Na2tetraborate+60 mM NH4-formate, and then the [3H]-IPs were eluted with 4 ml 0.1 M formic acid+1 M NH4-formate. A measure of 10 ml of scintillation cocktail (OptiPhase HiSafe 3) was added, and radioactivity was measured in the β-scintillation counter. The EC50 values for cirazoline and phenylephrine were calculated by the four-parameter logistic function, using the BindAid radioligand binding analysis package (Wan System, Umeå, Sweden).
Membrane preparations
COS-7 and Neuro2A cells transiently or stably expressing the different receptors were detached from 13.5 cm plastic culture dishes in 50 mM Tris, 5 mM EDTA. The cells were homogenized by ultraturraxing (IKA T25, 8 mm probe) for 15 s at 15,000 r.p.m. The homogenate was centrifuged at 400 × g, and the supernatant was then recentrifuged at 38,000 × g for 10 min. The final pellets were resuspended in 2.8 ml of 50 mM Tris and 1.5 mM EDTA, and was used directly in binding experiments. The protein concentrations of the membrane preparations were 0.2–1 mg ml−1, as determined by the method of Lowry et al. (1951) with the inclusion of SDS (Markwell et al., 1978).
Radioligand binding
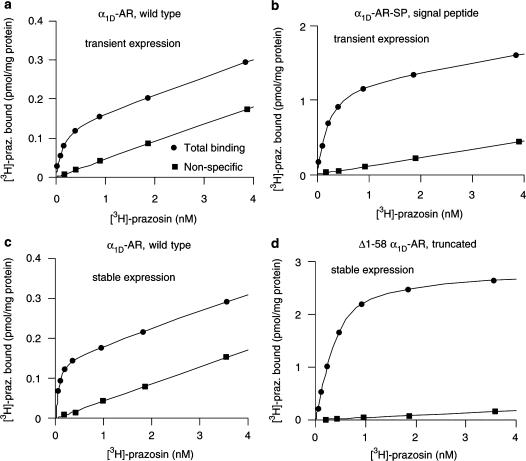
In order to determine the Bmax values of α1-adrenoceptors in the membranes, saturation experiments with [3H]-prazosin were performed. Each saturation experiment involved distributing membranes, obtained from one 13.5 cm diameter culture dish, into 24 wells of a 96-well microtiter plate at about 10–100 μg of protein well−1, in 150 μl of 33 mM Tris-HCl, 1 mM EDTA, at pH 7.5 (a four-fold dilution was made of the membranes obtained from plates transfected with constructs yielding high levels of binding sites, that is, the Δ1–58α1D-AR, α1D-AR-SP, and α1B-AR). Also present in the assays were different concentrations of [3H]-prazosin, ±2 μM metitepine (to define nonspecific binding). The incubations lasted for 1 h at room temperature, and then the suspensions were filtered and washed on Whatman GF/C filters, using a Brandel cell harvester. Radioactivity retained on the filters were counted in a Beckman β-scintillation counter. All assays were performed in duplicate. The nonspecific binding of [3H]-prazosin was lower in Neuro2A membranes as compared to COS-7 membranes. Curves were drawn using the BindAid radioligand binding analysis package (Wan System, Umeå, Sweden). Figures 1 and 2 were constructed using DeltaGraph® Pro 3.5. Figure 3 was constructed using Power Point.
Figure 1.
Saturation curves for [3H]-prazosin on membranes after transient or stable transfections of Neuro2A cells with different α1D-AR constructs. Nonspecific binding was defined by 2 μM of metitepine.
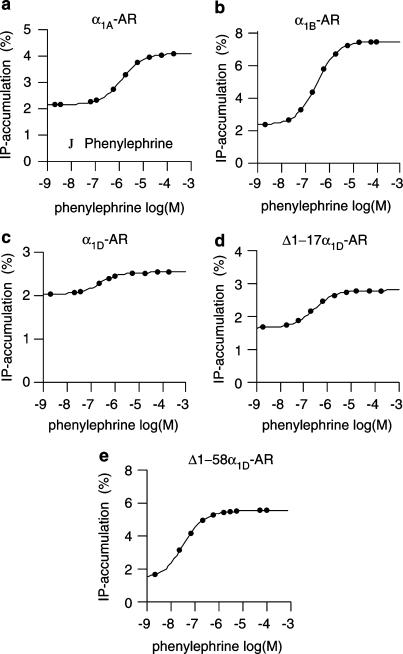
Figure 2.
Dose–response curves for the α1-agonist phenylephrine on [3H]-inositol phosphate accumulation in Neuro2A cells stably transfected with the wt α1A-, α1B-, and α1D-adrenoceptors, or with the truncated α1D-AR variants Δ1–17α1D and Δ1–58α1D.
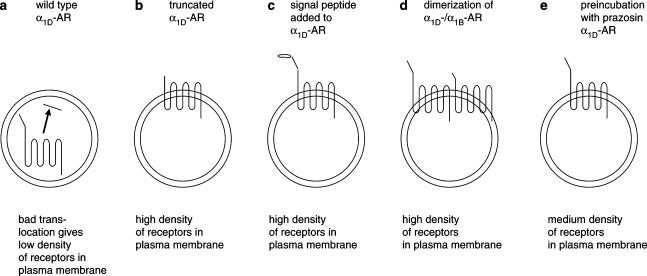
Figure 3.
Schematic illustration of α1D-adrenoceptor surface expression in transfected cells. The wt α1D-AR is poorly translocated to the plasma membrane (a). Truncated α1D-AR variant (b) and the α1D-AR supplemented with a cleavable signal peptide (c) are well expressed in the membranes. Coexpression of α1D-AR with the α1B-subtype increases surface expression of α1D-adrenoceptors (d). The well-expressed receptors (b–d) show a six to 10-fold increase in the density of binding sites in the plasma membrane compared to the wt α1D-AR. Incubation of α1D-AR-expressing cells with the α1-antagonist prazosin induces an increased density of receptors in the plasma membrane (e). The figure is based on results reported in the present study (c), and in Pupo et al. (2003) (a and b), Hague et al. (2004b) (d), and McCune et al. (2000) (e).
Results
Transient expression of the truncated α1D-AR variants
After transient expression of the α1D-AR variants in Neuro2A cells, the wild-type α1D-AR and the minutely truncated α1D variants (Δ1–5α1D-AR and Δ1–17α1D-AR) showed low levels of [3H]-prazosin binding sites. When the inherently 95 amino acids long N-terminal tail of the α1D-adrenoceptor was further shortened, there was a graded increase in the density of [3H]-prazosin binding sites (Table 1). Thus, Δ1–30α1D-AR, Δ1S–49α1D-AR, and Δ1–58α1D-AR showed increasingly higher Bmax values. However, the α1D variant with a very short N-terminal, Δ1–89α1D-AR showed a lower level than Δ1S–49α1D-AR and Δ1–58α1D-AR. The α1D variant with the highest density of [3H]-prazosin binding sites (Δ1–58α1D-AR) showed a 6.4-fold higher level compared to the wild-type α1D-adrenoceptor (Table 1). Thus, the levels of detectable α1D-adrenoceptors gradually increased as the N-terminal became shorter, at least in the span between truncation by 17, 30, 49, and 58 amino acids.
Table 1.
Density of receptors for the wild-type α1D-adrenoceptor and six gradually shorter trunctated α1D-adrenoceptor variants
| Transient expression in Neuro2A cells of wt and truncated α1D-adrenoceptors | A. Bmax receptors (fmol mg−1 protein) | B. Bmax receptors (% compared to wt) | C. pKd [3H]-prazosin | n |
|---|---|---|---|---|
| α1D-AR (wt) | 18.9±6.6 | 100 | 10.14±0.10 | 3 |
| Δ1–5α1D-AR | 10.3±3.3 | 56±12 | 10.12±0.13 | 3 |
| Δ1–17α1D-AR | 11.8±6.0 | 56±10 | 10.26±0.05 | 3 |
| Δ1–30α1D-AR | 34.7±18.3 | 156±39 | 10.40±0.00 | 3 |
| Δ1S–49α1D-AR | 106±75 | 426±198 | 10.32±0.06 | 3 |
| Δ1–58α1D-AR | 151±94 | 642±224 | 10.32±0.08 | 3 |
| Δ1–89α1D-AR | 52.6±27.2 | 238±57 | 10.32±0.02 | 3 |
The Bmax values were determined in Neuro2A cell membranes by [3H]-prazosin binding, following transient transfection of cells with 10 μg of construct DNA per 9 cm Petri dish. Column A shows the amounts of receptors (Bmax) in membrane preparations. Column B shows the Bmax in percent of the wt Bmax values, from experimentally paired plates. Column C shows the pKd values of [3H]-prazosin for the receptors.
Transient expression of the α1D-AR supplemented with a signal peptide
In a separate set of experiments, the wild-type α1D-AR and the α1D-AR supplemented with a 16 amino acids long signal sequence (α1D-AR-SP) were transiently expressed in COS-7 and Neuro2A cells. The densities of [3H]-prazosin binding sites were about 70 and 100 fmol mg−1 membrane protein for the wild-type α1D-AR in COS-7 and Neuro2A cells, respectively (Table 2). In comparison, the α1D-AR-SP receptor showed binding densities of about 800 and 1100 fmol mg−1 membrane protein in the COS-7 and Neuro2A cells. In matched experiments, this represented a 12- to 13-fold increase in the expression level of [3H]-prazosin binding sites for the α1D variant supplemented with signal peptide (α1D-AR-SP) as compared to the wild-type α1D-AR (Table 2; Figure 1). This shows that adding a signal peptide to the N-terminus of the wild-type α1D-adrenoceptor dramatically increased the density of detectable receptors in the membranes.
Table 2.
Column A shows the amounts of receptors (Bmax) in membrane preparations from COS-7 and Neuro2A cells after transient transfection with the wt α1D-adrenoceptor (wt), and with the signal peptide-supplemented α1D-adrenoceptor (SP)
| Transient expression in COS-7 or Neuro2A cells of wt and signal peptide-supplemented α1D-AR | A. Bmax receptors (fmol mg−1 protein) | B. Bmax receptors (% compared to wt) | C. pKd [3H]-prazosin | n |
|---|---|---|---|---|
| α1D-AR (wt) COS-7 | 69.3±6.4 | 100 | 9.93±0.10 | 4 |
| α1D-AR (SP) COS-7 | 838±200 | 1190±220 | 9.83±0.07 | 4 |
| α1D-AR (wt) Neuro2A | 103±31 | 100 | 10.13±0.07 | 4 |
| α1D-AR (SP) Neuro2A | 1120±80 | 1300±240 | 9.90±0.09 | 4 |
Column B shows Bmax of the signal peptide-supplemented α1D-AR (SP) in percent of that of the wild type α1D-AR (wt), from experimentally paired plates. Column C shows the pKd values of [3H]-prazosin for the receptors.
Basal level of IPs accumulation in transfected Neuro2A cells
After stabilizing the expression of the α1A-, α1B-, and α1D-AR, as well as the two truncated α1D-AR variants Δ1–17α1D-AR and Δ1–18α1D-AR with gentamicin treatment, we measured receptor-activated stimulation of second messenger production, that is, IPs accumulation. The percent conversion of [3H]-inositol to inositol phosphates (IPs accumulation) was determined by measuring how much of the [3H]-inositol, taken up by the cells, was transformed to [3H]-inositol phosphates during 1 h of incubation with lithium (which inhibits the breakdown of IPs). The basal level of IPs accumulation in one set of experiments was about 4% for untransfected Neuro2A-cells, as well as for cells transfected with the wild-type or truncated α1D-AR or with α1A-AR (Table 3A). In another set of experiments, the basal level of IPs accumulation was about 2% for the α1A-, α1B-, and α1D-AR, as well as for the two truncated α1D-AR variants (Table 3B). The equal basal levels of IPs accumulation, within the experimental sets, even within untransfected cells, show that none of the receptors was constitutively active in the Neuro2A cells.
Table 3.
IPs accumulation in Neuro2A cells, and in Neuro2A cells stably expressing wt α1A-, α1B- and α1D-adrenoceptors, and two truncated α1D-adrenoceptor variants
| (A) Cirazoline-stimulated IPs accumulation | |||||
| Receptor | IPs/total I (basal level) (% conversion) | IPs/total I (+cirazoline) (% conversion) | Stimulation (individual exp.) (fold) | pEC50 (cirazoline) | n |
| α1D-AR (wt) | 3.90±0.35 | 7.11±1.30 | 1.81±0.23 | 7.16±0.12 | 5 |
| Δ1–17α1D-AR | 4.22±0.68 | 9.05±1.16 | 2.21±0.22 | 7.18±0.20 | 5 |
| Δ1–58α1D-AR | 4.82±1.67 | 19.74±3.29 | 5.22±1.05 | 7.58±0.09 | 6 |
| α1A-AR | 3.33±0.39 | 13.68±3.09 | 4.45±1.09 | 7.43±0.16 | 6 |
| Neuro2A cells | 4.67±1.10 | no effect | N.C. | 3 | |
| (B) Phenylephrine (PE)-stimulated IPs accumulation | |||||
| Receptor | IPs/total I (basal level) (% conversion) | IPs/total I (+ PE) (% conversion) | Stimulation (individual exp.) (fold) | pEC50 (PE) | n |
| α1D-AR (wt) | 2.02±0.19 | 2.53±0.24 | 1.25±0.02 | 6.63±0.12 | 6 |
| Δ1–17α1D-AR | 1.65±0.27 | 2.82±0.68 | 1.65±0.16 | 6.72±0.10 | 4 |
| Δ1–58α1D-AR | 1.43±0.10 | 5.51±0.72 | 3.80±0.34 | 7.43±0.05 | 6 |
| α1A-AR | 2.17±0.30 | 4.12±0.44 | 1.94±0.07 | 5.80±0.08 | 6 |
| α1B-AR | 2.32±0.22 | 7.38±0.76 | 3.27±0.36 | 6.48±0.04 | 6 |
The basal-, cirazoline-, and phenylephrine-stimulated percent conversion of [3H]-inositol to [3H]-inositol phosphates, and the fold increase in IPs accumulation induced by the agonists are shown. Also shown are the pEC50 values of cirazoline and phenylephrine. In (A) the results with cirazoline, and in (B) the results with phenylephrine as shown. The incubation time with drugs was 50 min.
Agonist induced stimulation of IPs accumulation
Dose–response curves for cirazoline or phenylephrine were obtained on plain Neuro2A cells, and on cells stably expressing the wild-type α1A-, α1B-, and α1D-adrenoceptors, and the truncated Δ1–17α1D-AR and Δ1–58α1D-AR variants. The stimulated levels of IPs accumulation and the EC50 values for cirazoline and phenylephrine, at the different receptors, are reported in Table 3A and B. The α1-agonist/partial agonist cirazoline stimulated IPs accumulation in cells expressing the α1A- and α1D-AR, as well as the truncated α1D variants, but not in control Neuro2A cells. Also, the full agonist phenylephrine stimulated IPs accumulation in cells expressing the α1A-, α1B-, and α1D-adrenoceptors, as well as the truncated α1D variants (Figure 3). Altogether, these results show that all the tested receptors mediate agonist-dependent stimulation of IPs production in the cells. The pEC50 values (potencies) for both cirazoline and phenylephrine were higher at Δ1–58α1D-AR compared to, for example, the wild-type α1D-AR (Table 3A and B). This might be explained by the presence of a receptor reserve for the highly expressed Δ1–58α1D-AR, leading to the fact that only a fraction of the receptors needs to be stimulated by the agonist for inducing maximal effect.
Blockade of potential constitutively active receptors with the inverse agonist prazosin
Dose–response curves for prazosin were obtained on cells stably expressing the wild-type α1A- and α1D-adrenoceptors, and the Δ1–17α1D-AR and Δ1–58α1D-AR α1D variants. The results showed that the α1-antagonist prazosin did not influence the IPs accumulation in the transfected cells; the percent conversion was about 4% both in the presence and in the absence of prazosin (n=3). This shows that neither the wild-type α1D-AR nor the truncated variants of the α1D-AR showed any constitutive activity.
Discussion
Most GPCRs have a short N-terminal (at an average about 40 amino acids), which enables the first TM domain to function as a reverse signal anchor targeting sequence (see Andersson et al., 2003). However, the α1D-adrenoceptor has a relatively long extracellular N-terminus (95 amino acids), and lacks signal peptide sequence. While the α1A- and α1B-subtypes, which have shorter N-terminal tails, are well expressed in the plasma membrane, the α1D-adrenoceptor protein is poorly expressed in the plasma membrane, and instead seems to be localized mainly intracellularly (McCune et al., 2000; Chalothorn et al., 2002). Using radioligand ligand binding, it was shown that truncation of the N-terminal tail of α1D-AR increased the density of detectable α1D-adrenoceptors in the membranes, indicating that the N-terminal tail is responsible for the low expression level of binding competent receptors (Pupo et al., 2003). The same research group proposed that the α1D-adrenoceptor is not properly translocated to the plasma membrane after its synthesis (Hague et al., 2004a).
In the present study, we have investigated by what means the N-terminal tail of the α1D-adrenoceptor is responsible for the low receptor numbers in the plasma membrane. As working tools, we constructed 6 N-terminally truncated variants of the α1D-adrenoceptor, as well as the α1D-adrenoceptor supplemented with a signal peptide sequence. The truncated variants were made in order to investigate if there was a specific function residing in the N-terminal (mediating a decrease in the density of α1D-adrenoceptors detected by radioligand binding), which would be cut off at a certain point in the amino-acid sequence. After transient transfection of Neuro2A cells with the α1D-AR constructs, the Bmax and pKd values in the membranes were determined with the radioligand [3H]-prazosin. The results showed that the pKd values of [3H]-prazosin were almost identical for all the α1D-adrenoceptor variants (Table 1), indicating that the binding pocket of the labelled receptors were conformationally intact. However, the Bmax levels of the α1D-adrenoceptor variants gradually increased as the N-terminal was made shorter, at least in the span between truncation by 17, 30, 49, and 58 amino acids (Table 1). In a previous study, it has also been reported that a 79 amino acids truncated α1D-AR induced a high level of receptor binding sites (Pupo et al., 2003). However, our 89 amino acids truncated variant (Δ1–89α1D-AR) showed an intermediate Bmax level, probably reflecting a somewhat too short N-terminus (seven amino acids) to be well translocated to the plasma membrane. The graded response, that is, the increases in Bmax values as a result of the serial truncations, seems to exclude that certain amino acids in the N-terminus specifically induce a translocation-interfering activity. Rather, it seems as though the long N-terminal tail nonspecifically interferes with the translocation of the receptor to the plasma membrane. The graded response also indicates that the N-terminal tail does not specifically bind to the receptor itself to induce constitutive activity. Such a concept has been described for the thrombin receptor, where an N-terminal stretch of six amino acids in the cleaved N-terminal of the thrombin receptors acts as activating ligand of the receptor (MacFarlane et al., 2001). Thus, if the α1D-AR tail bound to the receptor and activated it, we believe that there would be a crucial short amino-acid stretch in the tail, important for binding, activation, and downregulation of the receptor. The model implies that such downregulation would disappear abruptly for those receptor variants for which that stretch had been truncated. However, our results seem to indicate that such a crucial stretch, that is, receptor-activating moiety, does not exist in the α1D-AR N-terminal tail.
As mentioned above, we also constructed an α1D-adrenoceptor with a signal peptide added to its N-terminal, that is, the α1D-AR-SP construct. The idea was to prolong the 95 amino acids long N-terminal with 16 extra amino acids of signal peptide character. In both COS-7 and Neuro2A cells, the addition of the signal peptide increased the levels of detectable α1D-adrenoceptors more than 10-fold (Table 2). This indicates that, after addition of a signal peptide, the long N-terminal tail of the α1D-adrenoceptor does not interfere with proper translocation of the receptor to the plasma membrane. It can be noted that there is a public database available, where one can estimate the probability of a protein being translocated to the plasma membrane (http://www.cbs.dtu.dk/services/SignalP-2.0/). In our case, this gave a hint that the N-terminal of the wild-type α1D-adrenoceptor, including the first TM domain, was not an appropriate signal anchor, while the truncated Δ1–58α1D-AR was predicted to be an excellent signal anchor. Our results seem to indicate that the long N-terminus of the α1D-adrenoceptor interferes with the translocation of the receptor to the plasma membrane, by hindering the first transmembrane helix of the receptor from being an appropriate signal anchor (see Andersson et al., 2003; Hague et al., 2004a).
It has been suggested that α1D-AR is constitutively active. This was first shown in rat-1 fibroblast cells stably expressing α1D-AR, where the inverse agonist BMY7378 decreased basal [Ca2+]i (Garcia-Sainz & Torres-Padilla, 1999). It was also suggested that constitutive activity of the α1D-AR continuously downregulates the receptor, since incubation of α1D-expressing cells with the inverse agonist prazosin induced a slight decrease in basal IPs accumulation, as well as a redistribution of immunocytochemically detected α1D-adrenoceptors from intracellular sites to the plasma membrane (McCune et al., 2000). However, in HEK 293 and SK-N-MC cells, no constitutive activity of α1D-AR was detected (Theroux et al., 1996). In the present study, we established stable expression in Neuro2A cells of the wild-type α1A-, α1B- and α1D-adrenoceptors, and the truncated α1D-adrenoceptors Δ1–17α1D-AR and Δ1–58α1D-AR. These cell lines were then used to investigate putative constitutive activity on IPs production. We measured both the basal level of IPs accumulation, the cirazoline- and phenylephrine-stimulated IPs accumulation, and the effect of prazosin (which potentially might decrease basal IPs accumulation, by blocking constitutive receptor activity). Our working hypothesis was that the wild-type α1D-AR (which shows a low density of [3H]-prazosin binding in the membranes, that is, which seems to be downregulated) would induce a high basal level of IPs accumulation. On the other hand, the truncated variant of the α1D-AR (which shows a high density of [3H]-prazosin binding in the membranes, that is, which does not seem to be downregulated) would induce a low basal level of IPs accumulation. However, we show that both wild-type and N-terminally truncated variants of the α1D-adrenoceptor stimulate IPs accumulation, but nota bene, only in the presence of agonist (Table 3A and B). We also show that α1D-AR is not constitutively active, since the inverse agonist prazosin did not decrease IPs accumulation. This seems to indicate that neither the wild-type α1D-adrenoceptor (which is present at low density in the membranes) nor the truncated α1D variant (which is present at high density in the membranes) is constitutively active in the Neuro2A cells. However, maybe the low density of the wild-type α1D-AR in the membranes made it difficult to observe constitutive activity in the Neuro2A cells.
After transient transfection of Neuro2A cells, the α1D-AR supplemented with signal peptide (α1D-AR-SP) showed a 13-fold increase in [3H]-prazosin binding as compared to the wild-type α1D-AR (Table 2). It seems unlikely that this increased level of detectable binding sites for α1D-AR-SP as compared to the wild-type α1D-AR is a reflection of constitutive activity and downregulation only of the wild-type α1D-AR. Note that α1D-AR-SP, after the presumed cleavage of its signal peptide and once present in the membranes, is supposed to be identical to the wild-type α1D-AR. In future studies, α1D-AR-SP will be an excellent tool for investigating if the α1D-AR is constitutively active, owing to its high expression level in the membranes. At present, we favor the hypothesis that the main reason the wild-type α1D-AR shows low expression of binding sites is that the translocation of the receptor protein to the plasma membrane does not work efficiently (Pupo et al., 2003; Hague et al., 2004a). Then, the best explanation for the much higher density of receptors observed after transfection with Δ1–58α1D-AR and α1D-AR-SP, compared to wild-type α1D-AR, is that these former receptors are well translocated to the plasma membrane. Whether α1D-AR is constitutively active is still an open question.
It has been shown previously that coexpression of the α1D-adrenoceptor with the α1B-adrenoceptor increased the number of functional α1D-adrenoceptors in the cells (Uberti et al., 2003; Hague et al., 2004b). This indicates that heterologuos receptor dimerization can mediate an increased translocation of the α1D-adrenoceptor to the plasma membrane. There thus seems to be at least four ways to increase the density of α1D-adrenoceptors in the plasma membrane: truncation of the N-terminal (Pupo et al., 2003), addition of a signal peptide (present study), coexpression with α1B-adrenoceptors (Hague et al., 2004b), and preincubation with antagonists (McCune et al., 2000). In Figure 3, we display a schematic presentation of these mechanisms. Whether the same mechanism of action underlies all of these four increases in the α1D-AR populations is uncertain. A new concept is that GPCRs may have inherent instability and therefore become downregulated, and that antagonists may stabilize receptor conformations that are less prone to downregulation (Prinster et al., 2003; Zeng et al., 2003). Thus, not only blocking of constitutively active receptors but also stabilization of unstable receptors, could explain the previously observed antagonist-mediated increment in membrane-localized α1D-adrenoceptors (McCune et al., 2000; see Garcia-Sainz & Villalobos-Molina, 2004).
It seems like the long N-terminal tail of the α1D-AR is responsible for less efficient translocation of the receptor to the plasma membrane, while this can be overcome by truncation of the tail or by the addition of a signal peptide sequence to the α1D-AR gene. A fundamental question is why nature has provided signal peptides to some GPCRs with long N-terminal tails (i.e. the endothelin B receptor), but not to others (i.e. the α1D-adrenoceptor and the cannabinoid receptor 1). Further studies may show whether the inefficient translocation of α1D-adrenoceptors to the plasma membrane has functional importance, for example, if the α1D-adrenoceptors under physiological circumstances are activated by interactions with some regulatory proteins (see Pupo & Minneman, 2003; Hague et al., 2004b). The present study highlights the importance of the N-terminus for the proper expression of binding competent receptors in the plasma membrane.
Acknowledgments
We thank Kenneth P. Minneman at Emory University for providing the human α1D-adrenoceptor gene, Gozoh Tsujimoto at Tokyo Children's Hospital for the human α1A-adrenoceptor gene, and Dianne Perez at Cleveland Clinic for the human α1B-adrenoceptor gene. This study was supported by the Swedish Research Council (Grant No. 04X-12606 (S.U.) and the Åke Wiberg Foundation (S.U.). I.K. was supported by the Swedish Insitute, J.K. was supported by the Wenner-Gren foundation and the European Union Marie Curie Fellowship programme and H.B.S. was supported by the Swedish Research Council (VR, medicine). Portions of this work have been reported previously as a meeting abstract (Eur. J. Biochem., 270 (Suppl 1), PS01-0104, 2003).
Abbreviations
- α1D-AR
α1D-adrenoceptor
- DMEM
Dulbecco's modified Eagle's medium
- GPCR
G-protein-coupled receptor
- IPs
inositol phosphates
- PE
phenylephrine
References
- ANDERSSON H., D'ANTONA A.M., KENDALL D.A., VON HEIJNE G., CHIN C.N. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol. Pharmacol. 2003;64:570–577. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., DAWSON R.M., DOWNES C.P., HESLOP J.P., IRVINE R.F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem. J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHALOTHORN D., MCCUNE D.F., EDELMANN S.E., GARCIA-CAZARIN M.L., TSUJIMOTO G., PIASCIK M.T. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol. Pharmacol. 2002;6:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- GARCIA-SAINZ J.A., TORRES-PADILLA M.E. Modulation of basal intracellular calcium by inverse agonists and phorbol myristate acetate in rat-1 fibroblasts stably expressing α1d-adrenoceptors. FEBS Lett. 1999;443:277–281. doi: 10.1016/s0014-5793(98)01738-4. [DOI] [PubMed] [Google Scholar]
- GARCIA-SAINZ J.A., VILLALOBOS-MOLINA R. The elusive α1D-adrenoceptor: molecular and cellular characteristics and integrative roles. Eur. J. Pharmacol. 2004;500:113–120. doi: 10.1016/j.ejphar.2004.07.016. [DOI] [PubMed] [Google Scholar]
- GISBERT R., PEREZ-VIZCAINO F., COGOLLUDO A.L., NOGUERA M.A., IVORRA M.D., TAMARGO J., D'OCON P. Cytosolic Ca2+ and phosphoinositide hydrolysis linked to constitutively active α1D-adrenoceptors in vascular smooth muscle. J. Pharmacol. Exp. Ther. 2003;305:1006–10014. doi: 10.1124/jpet.102.046169. [DOI] [PubMed] [Google Scholar]
- GUAN X.M., KOBILKA T.S., KOBILKA B.K. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J. Biol. Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- HAGUE C., CHEN Z., UBERTI M., MINNEMAN K.P. α1-adrenergic receptor subtypes: non-identical triplets with different dancing partners. Life Sci. 2003;74:411–418. doi: 10.1016/j.lfs.2003.07.008. [DOI] [PubMed] [Google Scholar]
- HAGUE C., CHEN Z., PUPO A.S., SCHULTE N., TOEWS M.L., MINNEMAN K.P. The N-terminus of the human α1D-adrenergic receptor prevents cell surface expression. J. Pharmacol. Exp. Ther. 2004a;309:388–397. doi: 10.1124/jpet.103.060509. [DOI] [PubMed] [Google Scholar]
- HAGUE C., UBERTI M.A., CHEN Z., HALL R.A., MINNEMAN K.P. Cell surface expression of α1D-adrenergic receptors is controlled by heterodimerization with α1B-adrenergic receptors. J. Biol. Chem. 2004b;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- HORIE K., OBIKA K., FOGLAR R., TSUJIMOTO G. Selectivity of the imidazoline α-adrenoceptor agonists (oxymetazoline and cirazoline) for human cloned α1-adrenoceptor subtypes. Br. J. Pharmacol. 1995;116:1611–1618. doi: 10.1111/j.1476-5381.1995.tb16381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOU W.M., VERHOEYEN M., DEVOS R., SAMAN E., FANG R., HUYLEBROECK D., FIERS W., THRELFALL G., BARBER C., CAREY N., EMTAGE S. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980;19:683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- KOZAK M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- KÖCHL R., ALKEN M., RUTZ C., KRAUSE G., OKSCHE A., ROSENTHAL W., SCHULEIN R. The signal peptide of the G protein-coupled human endothelin B receptor is necessary for translocation of the N-terminal tail across the endoplasmic reticulum membrane. J. Biol. Chem. 2002;277:16131–16138. doi: 10.1074/jbc.M111674200. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARKWELL M.A., HAAS S.M., BIEBER L.L., TOLBERT N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G.D., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MCCUNE D.F., EDELMANN S.E., OLGES J.R., POST G.R., WALDROP B.A., WAUGH D.J., PEREZ D.M., PIASCIK M.T. Regulation of the cellular localization and signaling properties of the α1B- and α1D-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 2000;57:659–966. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- OBIKA K., SHIBATA K., HORIE K., FOGLAR R., KIMURA K., TSUJIMOTO G. NS-49, a novel α1A-adrenoceptor-selective agonist characterization using recombinant human α1-adrenoceptors. Eur. J. Pharmacol. 1995;291:327–334. doi: 10.1016/0922-4106(95)90073-x. [DOI] [PubMed] [Google Scholar]
- PRINSTER S.C., SCHULTE N.A., COLLINS M.R., TOEWS M.L. Up-regulation of α1B-adrenergic receptors with defects in G protein coupling: ligand-induced protection from receptor instability. Mol. Pharmacol. 2003;64:1126–1135. doi: 10.1124/mol.64.5.1126. [DOI] [PubMed] [Google Scholar]
- PUPO A.S., UBERTI M.A., MINNEMAN K.P. N-terminal truncation of human α1D-adrenoceptors increases expression of binding sites but not protein. Eur. J. Pharmacol. 2003;462:1–8. doi: 10.1016/s0014-2999(03)01292-5. [DOI] [PubMed] [Google Scholar]
- PUPO A.S., MINNEMAN K.P. Specific interactions between gC1qR and alpha1-adrenoceptor subtypes. J. Recept. Signal Transduct. Res. 2003;23:185–195. doi: 10.1081/rrs-120025200. [DOI] [PubMed] [Google Scholar]
- TANOUE A., NASA Y., KOSHIMIZU T., SHINOURA H., OSHIKAWA S., KAWAI T., SUNADA S., TAKEO S., TSUJIMOTO G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEROUX T.L., ESBENSHADE T.A., PEAVY R.D., MINNEMAN K.P. Coupling efficiencies of human alpha 1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol. Pharmacol. 1996;50:1376–1387. [PubMed] [Google Scholar]
- UBERTI M.A., HALL R.A., MINNEMAN K.P. Subtype-specific dimerization of α1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol. Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- WALLIN E., VON HEIJNE G. Properties of N-terminal tails in G-protein coupled receptors: a statistical study. Protein Eng. 1995;8:693–698. doi: 10.1093/protein/8.7.693. [DOI] [PubMed] [Google Scholar]
- YANG M., VERFURTH F., BUSCHER R., MICHEL M.C. Is α1D-adrenoceptor protein detectable in rat tissues? Naunyn-Schmiedeberg's. Pharmacol. 1997;355:438–446. doi: 10.1007/pl00004966. [DOI] [PubMed] [Google Scholar]
- ZENG F.Y., MCLEAN A.J., MILLIGAN G., LERNER M., CHALMERS D.T., BEHAN D.P. Ligand specific up-regulation of a Renilla reniformis luciferase-tagged, structurally unstable muscarinic M3 chimeric G protein-coupled receptor. Mol. Pharmacol. 2003;64:1474–1484. doi: 10.1124/mol.64.6.1474. [DOI] [PubMed] [Google Scholar]
- ZHONG H., MINNEMAN K.P. α1-Adrenoceptor subtypes. Eur. J. Pharmacol. 1999;375:261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]