Abstract
We have investigated the effects of connexin-mimetic peptides homologous to the Gap 26 and Gap 27 domains of Cxs 37, 40 and 43 against gap junctional communication and connexin expression in rat aortic endothelial cells (RAECs) and A7r5 myocytes.
Immunostaining and Western blot analysis confirmed the presence of gap junction plaques containing Cx43, but not Cx40, in RAECs, whereas plaques containing Cxs 40 and 43 were evident in A7r5 cells. Expression of Cx37 was limited in RAECs and absent from A7r5 cells.
Under control conditions calcein-loaded RAECs transferred dye to ∼70% of subjacent A7r5 cells after coculture for 4–5 h. Dye transfer was inhibited by a peptide targeted to Cxs 37 and 43 (37,43Gap 27), but minimally affected by peptides targeted to Cxs 37 and 40 (37,40Gap 26 and 40Gap 27). These findings suggest that the myoendothelial gap junctions that couple RAECs and A7r5 cells are constructed principally from Cx43.
Inhibition of dye transfer from RAECs to A7r5 cells cocultured in the presence of 37,43Gap 27 plus 37,40Gap 26 for 5 h was fully reversible.
In A7r5 cells, endogenous expression of Cx40 and Cx43 was unaffected by incubation with 37,43Gap 27, 37,40Gap 26, either individually or in combination, and the peptide combination did not impair connexin trafficking or the de novo formation of gap plaques in A7r5 cells transfected to express Cx43-GFP.
Treatment of A7r5 cells with 37,43Gap 27 plus 37,40Gap 26 abolished synchronized oscillations in intracellular [Ca2+] induced by the α1-adrenoceptor agonist phenylephrine.
The reversibility and lack of effect of the peptides on plaque formation suggests that they may be considered ideal probes for functional studies of connexin-mediated communication in the vascular wall.
Keywords: Gap junctions, connexin43-green fluorescent protein, connexin-mimetic peptides, intercellular communication
Introduction
Gap junctions are channels in the plasma membrane that allow direct communication between coupled cells and thereby ensure coordination of cellular activity in multicellular organisms (Evans & Martin, 2002). Their assembly is a dynamic process that involves oligomerization of six connexin protein subunits to form hemichannels (called connexons) that traffic to the plasma membrane and align and dock at points of cell to cell contact with hemichannels from neighbouring cells (Evans & Martin, 2002). Connexins consist of a family of highly conserved proteins of which at least 20 different subtypes have been identified in mammals and can be classified according to their molecular mass in kDa (Willecke et al., 2002). Gap junctions may sometimes be constructed from mixtures of different connexin protein subtypes and may therefore be homotypic (both connexons constructed from the same connexin subtype) heterotypic (each connexon constructed from a different connexin subtype) or heteromeric (each connexon constructed from mixtures of connexin subtypes). Their connexin subunits span the cell membrane four times, and consist of two highly conserved extracellular loops and one variable intracellular loop. The amino and carboxy termini are located intracellularly with the major sequence divergence being in the length of the carboxyl tail, which in longer tailed connexins, such as Cx43, can be extensively phosphorylated (Evans & Martin, 2002). Each connexin has unique functional properties suggesting that the connexin composition of a tissue may reflect specific physiological requirements (Saez et al., 2003).
Gap junctions cluster in the cell membrane to form plaque-like structures that can contain many hundreds of individual units, and there is evidence to suggest that the extent of functional coupling between adjacent cells correlates with plaque size as a result of cooperative interactions between the component channels (Bukauskas et al., 2000). Compounds that inhibit intercellular communication pharmacologically have been widely employed to probe the role of gap junctional communication in different systems. These include aliphatic alcohols such as heptanol and lipophilic compounds such as oleamide and 18α-glycyrrhetinic acid (Guan et al., 1997; Guo et al., 1999) whose actions are nonspecific, so that they cannot be used to target specific connexin subtypes. By contrast, short synthetic peptides that possess sequence homology with conserved domains of the extracellular connexin loops have emerged as connexin-specific inhibitors of gap junction communication in a wide range of cell types. For example, such agents have clarified the role of homocellular gap junctional communication via specific connexin subtypes in cardiac myocytes (Warner et al., 1995), vascular smooth muscle cells (Chaytor et al., 1997), fibroblasts (Chaytor et al., 1999), liver cells (Kwak & Jongsma, 1999), pulmonary epithelial cells (Boitano & Evans, 2000), osteoclasts (Ilvesaro et al., 2001) and lymphocytes (Oviedo-Orta et al., 2002). Connexin-mimetic peptides have also been extensively used to study heterocellular communication between endothelial and smooth muscle cells in the vascular wall (see Griffith, 2004 for a review) and between endothelial cells and macrophages (Zahler et al., 2003).
Three principal connexin subtypes, Cxs 37, 40 and 43, are found in the vasculature with expression of connexin protein in gap junction plaques generally being more abundant in the endothelium than in smooth muscle, and the location of these different connexin subtypes varying with vessel type (reviewed in Griffith, 2004). In certain artery types, there is also evidence for limited expression of Cx45 (Ko et al., 2001). Direct coupling between the endothelium and smooth muscle cells in the vascular media occurs via myoendothelial gap junction plaques that can be visualized by electron microscopy (Spagnoli et al., 1982; Sandow & Hill, 2000). In many arteries, an electrotonic spread of agonist-induced endothelial hyperpolarization, rather than extracellular transfer of a freely diffusible endothelium-derived hyperpolarizing factor or EDHF, appears to account for endothelium-dependent vascular relaxations that are independent of nitric oxide and prostanoid synthesis (Griffith, 2004). By interrupting coupling between the endothelium and smooth muscle cells, synthetic peptides homologous to the Gap 26 and Gap 27 domains of the extracellular loops of Cxs 37, 40 and 43 may thus attenuate the transmission of endothelium-dependent hyperpolarization into the vascular media and the associated mechanical relaxation (Griffith, 2004). Such peptides also uncouple vascular smooth muscle cells, so that a component of their action against the EDHF phenomenon might reflect an ability to attenuate the electrotonic relay of endothelial hyperpolarization through successive layers of the media (Chaytor et al., 1997; Yamazaki & Kitamura, 2003). Functional studies have shown that heterogeneity in the patterns of endothelial and medial connexin expression present in rabbit ear, middle cerebral and iliac arteries and rat hepatic arteries lead to wide differences in the ability of individual peptides and peptide combinations to inhibit EDHF-type responses (Chaytor et al., 2001, 2003; Berman et al., 2002; Griffith et al., 2002; Ujiie et al., 2003). Individual connexin-mimetic peptides have nevertheless been shown to be effective inhibitors of NO-independent vasodilation in vivo (De Vriese et al., 2002).
Despite growing evidence that connexin-mimetic peptides interrupt gap junctional communication, the mechanisms underlying their mode of action remain unresolved. In the present study, we therefore examined the effects of such agents on the integrity and dynamic assembly of gap junctions by immunocytochemical analysis and transfection of smooth muscle cells with Cx43-GFP to monitor connexin trafficking. We also assessed the effects of the peptides on dye coupling via myoendothelial gap junctions in a model endothelial/smooth muscle coculture cell system and their ability to affect coordinated intracellular calcium signalling events in coupled smooth muscle cells.
Methods
Cells and cell culture
The rat aortic A7r5 smooth muscle cell line was maintained in DMEM supplemented with 10% foetal calf serum, penicillin–streptomycin (100 μg ml−1) amphotericin (100 μg ml−1 and L-glutamine (2 mM). For immunocytochemical analysis and Ca2+ measurements, cells (∼3 × 105) were cultured in 24 mm2 coverglass chambers (Labtek). In some of these studies, A7r5 cells were transfected with 0.25 μg Cx43-GFP cDNA using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Glasgow, U.K.) (Paemeleire et al., 2000).
Preparation and culture of primary rat aortic endothelial cells (RAECs)
Male Sprague–Dawley rats (250–300 g) were killed by intravenous administration of sodium pentobarbital (120 mg kg−1) and the aorta was surgically removed. To obtain RAECs, these freshly isolated vessels were cut into 2–3 mm rings and incubated in complete M199 medium (supplemented as above and with 2.5 mM HEPES buffer, pH 7.4) for 48 h prior to centrifugation at 500 r.p.m. for 5 min and careful removal of supernatant. The rings were then washed twice in phosphate-buffered saline (PBS) and incubated with trypsin (0.5 mg ml−1) for 15 min at 37°C. Five volumes of complete M199 were then added to the cells followed by centrifugation at 289 × g for 5 min. This step was repeated twice. The cells were then incubated in complete M199 medium for 24 h and washed gently in prewarmed PBS and supplemented with additional complete M199. Cells were then incubated in complete M199 for 5–7 days without medium change. Complete monolayers were formed in 10–15 days. Cells were used for up to four passages.
Immuncytochemistry and image analysis
The integrity of endogenous Cx43 and Cx40 gap junction plaques in the plasma membrane of A7r5 cells was analysed before and after incubation with connexin-mimetic peptides for periods of 1–4 h by immunocytochemical staining with a monoclonal antibody against the carboxyl tail of Cx43 (1 : 250 dilution, Chemicon, Chandlers Ford, U.K.) or a polyclonal antibody to Cx40 (1 : 250 dilution, Alpha Diagnostics, San Antanio U.S.A.). RAECs were also stained with a polyclonal antibody to Cx37 (1 : 250 dilution, Alpha Diagnostics) and FITC-conjugated von Willebrand Factor (Sigma, Poole U.K.). The secondary antibody was goat anti-mouse conjugated to Alexa 488 (1 : 700 dilution, Molecular Probes, Leiden, Netherlands) or goat anti-rabbit conjugated to Alexa 567 as appropriate (Chaytor et al., 2001). Slides were viewed under an Axiovert 100 microscope linked to a BIORAD MRC 1024MP laser scanning system. Images were acquired under similar conditions and magnification (typically × 40). The Biorad Lasersharp software enabled quantification of total immunofluorescence following peptide treatment to be assessed. The mean fluorescent signal from selected areas of equal area along the plasma membrane were recorded from up to 10 different areas per field of view from 4–5 images for each peptide treatment. The average pixel intensity was recorded and subtracted from the background pixel intensity, recorded from a similarly sized region of intracellular fluorescence to extract a relative fluorescent index for each set of treatments (Martin et al., 2004).
Western blot analysis
A7r5 cells and RAECs were grown on 60 mm dishes (1 × 106 cells) and harvested in 100 μl of ice-cold lysis buffer (1% w v−1 SDS, 1 mM DTT, 1 mM NaVO4, 4 ng ml−1 leupeptin, 4 ng ml−1 apoprotinine and 1 mM phenylmethylsulphonyl fluoride in PBS), followed by freeze thawing and sonication for 2 × 15 s at 15 mHz. Protein content was measured using the BIORAD protein assay kit and equal amounts of protein (50 μg) were analysed on SDS–PAGE (10%) followed by transfer to nitrocellulose in 100 mM Na2CO3 buffer for 3 h at 300 mA. The efficiency of transfer was assessed by Ponceau S (Sigma) staining of the blots prior to further processing (Martin et al., 2004) and probing with polyclonal antibodies to Cxs 37 and 40 (both from Alpha Diagnostics, 1 : 1000 dilution) and Cx43 (Zymed, Cambridge, U.K., 1 : 4000 dilution), and a secondary goat anti-rabbit horseradish peroxidase antibody (Biorad, Hemel Hempsted, U.K.). Blots were developed by enhanced chemiluminescence (ECL).
Coculture
To study direct communication between endothelial and smooth muscle cells, a coculture system was established in which donor RAECs were loaded with the fluorescent polar tracer calcein (MW 623 Da, charge – 4) and acceptor A7r5 cells were stained with PKH26, a fluorescent lipohilic dye (excitation 595 nm, emission 640 nm) that irreversibly binds to cell membranes and does not leak to surrounding medium or transfer dye to other cells (Slezak & Horan, 1989). Briefly, freshly trypsinized A7r5 cells (1 × 107 cells) were resuspended in cDMEM and centrifuged at 289 × g for 8 min, followed by washing in serum-free medium and labelling with PKH26 according to the manufacturer's details. The resulting labelled cells were reseeded into a T25 cm2 flask and allowed to recover overnight prior to seeding onto coverglass chambers for functional experiments (∼2.5 × 105 cells). The next day a freshly prepared stock solution of 2.5 μM calcein AM was added to confluent RAEC cell monolayers in 60 mm dishes (∼1 × 106 cells) and incubated at 37°C for 30 min with occasional rocking. Calcein AM was removed, cells were washed 2–3 times in PBS and incubated for a further 30 min in cDMEM at 37°C. The cells were then trypsinized, washed twice in cDMEM and resuspended in cDMEM (∼6 × 105 cells ml−1). The incubation medium was supplemented with gap junction inhibitors as required and 500 μl of cell suspension was added to the labelled A7r5 cell cultures. Dye transfer was observed by dual wavelength confocal microscopy at intervals between 1 and 5 h following overlay.
Gap junction inhibitors
Peptides possessing sequence homology with the Gap 26 or Gap 27 domains (amino-acid residues 63–75 and 204–214, respectively) of rat Cx43, Cx40 or Cx37 were used at a concentration of 600 μM when used in isolation or 300 μM each when used in combination. Using a previously proposed nomenclature, these peptides were designated 37,43Gap 27, 40Gap 27 and 37,40Gap 26 (Chaytor et al., 2001). Previous studies have confirmed that their action is connexin-specific. For example, in confluent COS fibroblasts expressing Cx43, intercellular dye transfer of Lucifer yellow is impaired by 37,43Gap 27, but not 40Gap 27, despite the sequences of these peptides differing by just three amino acids (Chaytor et al., 1999). In some experiments, an alternative nonspecific inhibitor of gap junctional communication 18α-GA was used at a concentration of 25 μM.
Dye transfer
Cocultures were viewed under a × 40 oil immersion lens on a confocal laser scanning microscope MRC 1024 MP. Single channel images had excitation spectra of 488 nm laser line (LL) and emission 515 nm long pass (LP), while two channel images had excitation wavelengths of 488 and 567 nm and emissions of 530/40 nm and 580 nm LP, respectively. Images were captured using Biorad Lasersharp Software and analysed using Confocal Assistant. The efficiency of calcein transfer at different time points following overlay of A7r5 cells with RAECs was quantified by determining the percentage of calcein labelled RAECs that donated dye to 0, 1–2 or ⩾3 A7r5 cells, and the effects of gap junction inhibitors quantified by determining the percentage of RAECs that donated dye to 0 or ⩾1 A7r5 cells. Dye transfer by >50 RAECs was assessed per experiment and each experiment was repeated three times.
Calcium measurements
For intracellular Ca2+ measurements, A7r5 or RAEC cells were grown on coverglass chambers (∼3 × 105 cells) and allowed to settle out overnight. Cells were loaded with Fura2 AM (2 μg ml−1) for 20 min at 37°C in HEPES buffered Tris (HBS) (1.3 mM NaCl, 4.2 mM KCl, 1 mM MgCl2, 5.8 mM glucose, 10 mM Hepes, 0.1% w v−1 BSA, pH 7.5) supplemented with 1.3 mM CaCl2 followed by 5 min rest in HBS containing 1.3 mM Ca2+ and viewed on an Axiovert fluorescence microscope (Zeiss, U.K.) linked to a Hamamatsu ORCA digital camera with images being collected at 340 and 380 nm excitation in rapid succession and image pairs collected every 5 s on a heated stage maintained at 37oC. The fluorescence intensity resulting from excitation at 340 and 380 nm was recorded from 8–10 adjacent cells per field of view using Kinetic Imaging software following stimulation by phenylephrine (500 nM). In some experiments, the cells were preincubated with 37,43Gap 27+37,40Gap 26 at 300 μM each or 25 μM 18α-GA for 1 h as required. Data were exported to a spreadsheet and the 340:380 nm ratio calculated to estimate changes in [Ca2+]i using the Kinetic Imaging software and plotted in arbitrary units that reflect the 340:380 ratio. Experiments were repeated three times.
Materials
A7r5 cells were obtained from ECACC, Wiltshire, U.K. DMEM, M199, supplements for cell growth and Lipafectamine 200 were supplied by Invitrogen (Glasgow, U.K.). Calcein AM, Fura2 AM, and Alexa secondary antibodies were obtained from Molecular Probes, (Leiden, Netherlands). The Cx43 monoclonal antibody used for immunofluorescence was obtained from Chemicon (Chandlers Ford, U.K.) and other antibodies were obtained from Zymed (Cambridge, U.K.) and Alpha Diagnostics (San Antonio, U.S.A.), respectively. The goat antimouse horseradish peroxidase was supplied by BioRad (Hemel Hempsted, U.K.) and the ECL system from Pierce (Tattenhall, U.K.). All other reagents were purchased from Sigma (Poole, U.K.). Connexin-mimetic peptides were dissolved directly in buffer and 18α-GA in DMSO. Preliminary experiments confirmed that DMSO was inactive at the final concentrations employed.
Statistics
Data were evaluated by ANOVA followed by Dunnett's multiple comparison test, with P<0.05 being considered significant.
Results
Connexin profiles in smooth muscle and endothelial cells
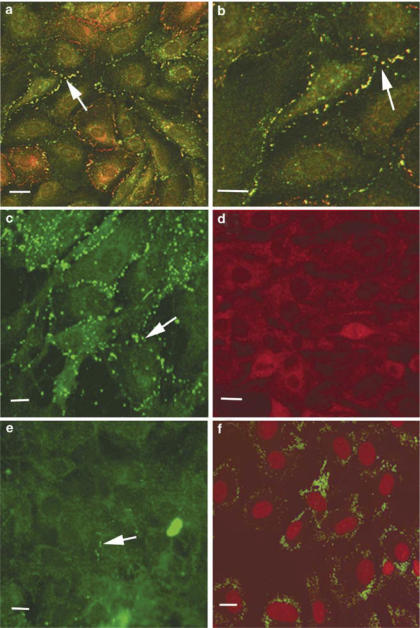
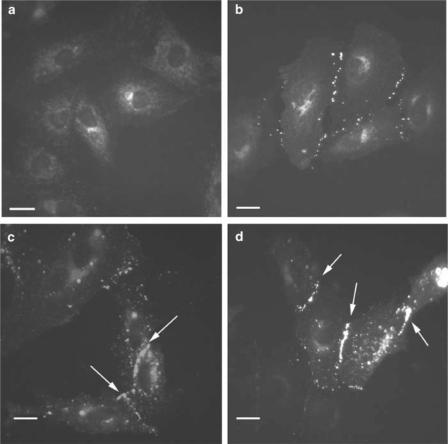
The connexin profiles of the different cell types under investigation were analysed by immunocytochemistry and Western blot analysis. A7r5 cells were confirmed to express Cx43 and Cx40 as previously reported (He et al., 1999; Chaytor et al., 2001) (Figure 1a and b). In some regions, Cx43 and Cx40 were found to colocalize at points of cell-to-cell contact whereas in other regions plaques containing only Cx43 or Cx40 were evident. By contrast, RAECs predominantly expressed Cx43, which was found in high amounts at regions of cell to cell contact (Figure 1c). Cx40 was not detected in these cells (Figure 1d), and low levels of Cx37 were localized to limited regions of the cell membrane (Figure 1e). The endothelial origin of the RAECs was confirmed by staining for von Willebrand factor (Figure 1f). These cells were used for only up to four passages, after which there was generally loss of Cx37 staining (data not shown).
Figure 1.
Connexin profiles in A7r5 cells and RAECs. (a, b) Costaining of A7r5 cells for Cx43 (green) and Cx40 (red) at two magnifications, with gap junction plaques containing both connexin subtypes identified in yellow. (c) Cx43 staining of RAECs. (d) Cx40 staining of RAECs. (e) Cx37 staining of RAECs. (f) von Willebrand factor staining of RAECs (green) with nuclei (red) identified by propidium iodide. Cells were viewed at a magnification of × 40 and in some cases a zoom of up to 1.4 was applied. The image size was calculated by the Biorad Lasersharp software. Bars=10 μM. Arrows indicate gap junction plaques at points of cell-to-cell contact.
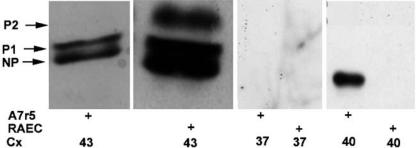
Western blots were performed to compare the phosphorylation status of Cx43 in the two cell types. In A7r5 cells, Cx43 was found in nonphosphorylated (NP) and P1 isoforms whereas in RAECs it was found in three isoforms, NP, P1 and P2 (Figure 2). Western blot analysis also confirmed that Cx40 was expressed in A7r5 cells but not RAECs, consistent with the immunofluorescence findings (Figure 2). Cx37 was not identified in either cell type, indicating that the levels observed by immunofluorescence in the RAECs were too low to be detected by Western analysis.
Figure 2.
Western blot analysis of connexin expression profiles in A7r5 and RAECs. NP, P1 and P2 denote nonphosphorylated and phosphorylated isoforms of Cx43, respectively.
Effects of connexin-mimetic peptides and 18α-GA on gap junction functionality
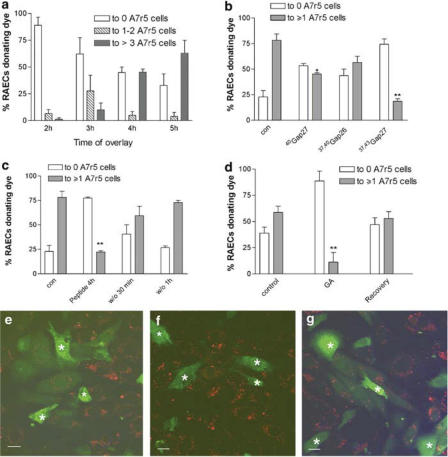
Initial experiments followed the time taken for donor RAEC cells to settle on A7r5 cell monolayers, form functional gap junctions and subsequently transfer dye. At 2 h following overlay, dye transfer from individual RAECs to 1–2 A7r5 cells was 6.6±3.6% and this increased to 27.7±14.6% after 3 h with transfer to third- and fourth-order A7r5 cells remaining limited. At 4 h following overlay, dye transfer to ⩾3 neighbouring A7r5 cells was 45.2±2.9% and after 5 h this increased to 60.0±3.5% (Figure 3a). Further incubation did not significantly increase the number of smooth muscle cells accepting dye so that in subsequent experiments dye transfer was recorded 5 h following overlay.
Figure 3.
Effects of gap junction blockade on dye transfer from endothelial to smooth muscle cells. A7r5 cells were labelled with PHK26 (red) and overlaid with calcein-loaded RAECs (green). (a) Percentage of RAECs donating dye to different numbers of A7r5 cells after 2, 3, 4 and 5 h under control conditions. (b) Effect of connexin-mimetic peptides on dye transfer at 600 μM each. (c) Recovery of dye transfer following washout of a combination of 37,43Gap 27+37,40Gap 26 peptides at 300 μM each. (d) Effect of 18α-GA (25 μM) on dye transfer followed by recovery. (e) Typical field of view of highly coupled cells under control conditions. (f) Typical field of view of cells cocultured for 5 h in the presence of 37,43Gap 27+37,40Gap 26 at 300 μM each. (g) Typical field of view following washout of this peptide combination for 1 h. Asterisks identify RAECs loaded with calcein. Bars=10 μM. Magnification × 40. *P<0.05, **P<0.001 compared to control.
To examine the effects and specificity of connexin-mimetic peptides on gap junction functionality, RAECs were overlayed onto A7r5 cells and cocultures were incubated for 5 h with peptides differentially targeted to the extracellular loops of Cxs 37, 40 and/or Cx43. In this series of experiments, 78.2±6.0% of endothelial cells donated dye to subjacent A7r5 cells under control conditions. Preincubation with peptides targeted either to Cx40 or Cx40 plus Cx37, that is, 40Gap 27 or 37,40Gap 26 (at 600 μM each) caused slight attenuation of dye transfer to A7r5 cells, although dye coupling was significantly reduced compared to control only for 40Gap 27 (P<0.05) (Figure 3b). By contrast, when the cocultures were incubated with a peptide targeted to Cx43, that is, 37,43Gap 27 (600 μM) there was a marked >80% reduction in the number of loaded endothelial cells donating calcein to A7r5 cells (P<0.001) (Figure 3b and f). Preincubation with peptides corresponding to both Cx40 and Cx43, that is, 43,37Gap 27 + 37,40Gap 26 (at 300 μM each) had no greater effect than 37,43Gap 27 alone. Washout of the 43,37Gap 27+37,40Gap 26 combination with peptide-free incubation medium resulted in recovery of dye transfer to control levels after 30–60 min (Figure 3c and g).
Incubation of the cells with 25 μM 18α-GA, a well-established but nonspecific inhibitor of gap junction intercellular communication, resulted in marked attenuation of dye coupling, with >90% of RAECs then being unable to donate dye to A7r5 cells (Figure 3d). Removal of 18α-GA from the cells 4–5 h after overlay resulted in recovery of endothelial to smooth muscle dye transfer within 30 min. Although control cells in this batch of experiments were apparently not as efficiently coupled as those in panels b and c, statistical analysis confirmed that there was no significant difference between the corresponding data sets.
Effects of connexin-mimetic peptides on gap junction integrity
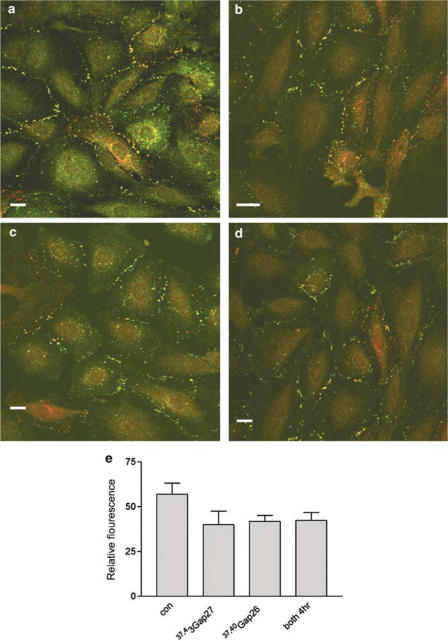
To gain insights into the mechanisms that underlie the action of connexin-mimetic peptides against gap junctional communication their effects on connexin-specific staining were determined in A7r5 cells 4–5 h after incubation, that is, at times when calcein transfer was monitored. Cells were stained for both Cx43 and Cx40 following treatment with 37,43Gap27, 37,40Gap26 or both peptides in combination (Figure 4). After peptide treatment, plaques in the plasma membrane remained intact with levels of fluorescence not differing significantly from control, indicating that the peptides did not disrupt gap junction plaques.
Figure 4.
Integrity of gap junction plaques in A7r5 cells fixed and stained for Cx43 (green) and Cx40 (red) following 4 h incubation with connexin-mimetic peptides. (a) Control, (b) 37,43Gap 27 (600 μM), (c) 37,40Gap 26 (600 μM), (d) 37,43Gap 27+37,40Gap 26 (300 μM each). (e) Histogram showing plaque integrity quantified by analysis of Cx43 and Cx40 fluorescence at the plasma membrane subtracted from background fluorescence following the various treatments. Results are given as mean relative fluorescence±s.e.m. Bars=10 μm. Magnification × 40.
In a further series of experiments, we investigated the effects of 37,43Gap 27 + 37,40Gap 26 on the dynamic formation of gap junction plaques in A7r5 cells transfected to express Cx43-GFP. In these experiments, the peptides were added to the cells 6 h following transfection,that is, at a time before gap junction plaques were formed and expression of the protein was evident only in areas of the cell corresponding to the Golgi apparatus (Figure 5). At 18 h post-transfection, when Cx43-GFP had trafficked to the plasma membrane and punctate staining was apparent, plaque formation was compared to that in cells not exposed to the peptides. There was no difference in the level of plaque formation in the different treatment groups compared to control, thus indicating that connexin-mimetic peptides do not interfere with connexin trafficking or the ability of hemichannels to form plaques at the plasma membrane (Figure 5c and d).
Figure 5.
Effect of 37,43Gap 27 + 37,40Gap 26 (300 μM each) on the assembly of gap junction plaques in A7r5 cells transfected with Cx43-GFP: (a) 6 h post-transfection, (b) 9 h post-transfection, (c) 18 h post-transfection, (d) 18 h post-transfection in the presence of 37,43Gap 27+37,40Gap 26 added 6 h after transfection, that is, the time point corresponding to (a). Bars=10 μm. Magnfication × 40. Arrows indicate gap junction plaques at points of cell-to-cell contact.
Effects of connexin-mimetic peptides on synchronized intracellular signalling events
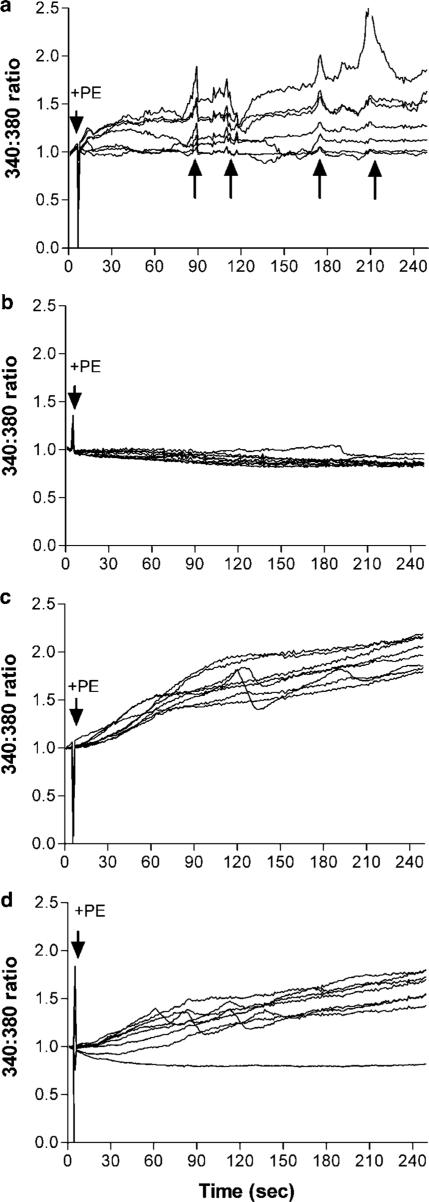
We also investigated the effects of connexin-mimetic peptides on the coordination of intracellular Ca2+ signalling events in A7r5 cells. Under control conditions, stimulation of confluent monolayers of these myocytes with phenylephrine (500 nM) resulted in an increase in intracellular [Ca2+] in adjacent cells, superimposed on which were repetitive synchronous intracellular calcium transients (Figure 6a). By contrast, phenylephrine had no effect on [Ca2+]i in RAECs (Figure 6b). Incubation of A7r5 cells either with 43,37Gap 27 + 37,40Gap 26 (at 300 μM each) or with 18α-GA (25 μM) resulted in suppression of synchronized oscillatory transients and most cells then exhibited a monotonic increase in intracellular [Ca2+] to a plateau, with only a few cells demonstrating asynchronous oscillatory activity (Figure 6c and d). Representative traces of one set of experiments are shown. These were performed in triplicate to confirm the findings.
Figure 6.
Effects of connexin-mimetic peptides on synchronized intracellular Ca2+ oscillations. Cells were loaded with Fura2 AM under control conditions or following incubation with connexin-mimetic peptides or 18α-GA for 90 min. They were then treated with phenylephrine and Fura2 fluorescence at 340 : 380 nm recorded for 5 min. (a) A7r5 cells, (b) RAECs, (c) A7r5 cells following treatment with 37,43Gap 27+37,40Gap 26 (300 μM each), (d) A7r5 cells following treatment with 25 μM 18α-GA.
Discussion
The present study has employed endothelial/smooth muscle and homocellular smooth muscle culture systems to investigate the mechanisms that contribute to the ability of connexin-mimetic peptides to interrupt gap junctional communication in vascular cells. The major new findings are that synthetic peptides homologous to the Gap 26 and 27 domains of the dominant vascular connexins (Cxs 37, 40 and 43) do not impair the synthesis and expression of connexin protein or de novo formation of gap junction plaques. The study also provides evidence that the action of such peptides is sustained but reversible on washout, and that they are capable of suppressing synchronized oscillations in intracellular [Ca2+] in coupled smooth muscle cell monolayers.
We first defined the expression of Cxs 37, 40 and 43 in the two cell types. Gap junction plaques containing Cx43 were abundant in RAECs, whereas Cx40 was completely absent from the plasmalemma of these cells. Although isolated plaques containing Cx37 could be visualized in some RAECs, this connexin subtype was present in low amounts and could not be detected by Western blot analysis. By contrast, A7r5 cell monolayers abundantly expressed both Cx43 and Cx40, with these connexins often colocalizing in the same gap junction plaque, as previously described (Chaytor et al., 2001), whereas Cx37 was not detected either by immunostaining or Western blot analysis in this smooth muscle cell line. Functional studies quantifying the effects of individual connexin-mimetic peptides (at concentrations of 600 μM) against endothelial to smooth muscle dye transfer were consistent with these expression profiles. The peptides 40Gap 27 and 37,40Gap 26, which respectively target Cx40 and Cxs 37/40, attenuated dye transfer from RAECs to A7r5 cells to only a minor extent, and in the case of 37,40Gap 26 this attenuation failed to achieve statistical significance, thus suggesting that the limited expression of Cx37 in RAECs did not contribute significantly to dye transfer. By contrast, 37,43Gap27, a peptide targeted to Cx43 (and Cx37), markedly attenuated myoendothelial dye transfer, with more than 70% of RAECs then being unable to donate calcein to A7r5 cells. Taken together, these observations suggest that the myoendothelial junctions that mediate communication from RAECs to A7r5 cells in coculture are homotypic and constructed principally from Cx43, with a possible, but nevertheless limited, contribution from heterotypic or heteromeric gap junctions constructed from Cx43 in the endothelium and Cx40/Cx43 in smooth muscle cells. These conclusions are supported by previous findings that unlike 37,43Gap 27, the 40Gap 27 peptide does not reduce dye coupling between cultured COS fibroblasts (a cell line that expresses only Cx43), and that the degree of inhibition of endothelial to smooth muscle dye transfer observed with 37,43Gap 27 in the present study is comparable with that observed with 500 μM 37,43Gap 27 in HeLa cells transfected and selected to express Cx43-GFP protein to render them communication competent (Chaytor et al., 1999; Berman et al., 2002). Other reports have confirmed the ability of Cxs 40 and 43 to form heterotypic and heteromeric gap junctions (He et al., 1999; Valiunas et al., 2000). It remains to be established whether differences in the phosphorylation status of Cx43 in RAECs and A7r5 cells affect dye transfer via myoendothelial gap junctions, since Cx43 was shown to exist in a highly phosphorylated state in the endothelial cell line.
The stability of the peptides in aqueous solution was evident from observations that the inhibitory properties of the individual connexin-mimetic peptides and a peptide combination (37,40Gap 26+37,43Gap 27) against diffusion of calcein through myoendothelial gap junctions were maintained for at least 5 h following overlay of calcein-loaded RAECs on A7r5 cells. Notably, however, experiments with the peptide combination demonstrated that normal dye transfer could be readily ‘restored' by peptide washout after 30–60 min, even after prolonged incubation. The action of 25 μM 18α-GA against coupling in A7r5 and RAEC cocultures was similarly found to be reversible even though other studies indicate that reversibility may not always be evident with this compound, particularly at high concentrations. In alveolar epithelial cells expressing Cx43, for example, the initial interruption of intercellular communication by 100 μM 18α-GA occurs within 15–30 min, is reversible and not associated with changes in the integrity of gap junction plaques (Guo et al., 1999). However, following prolonged incubation at this concentration there is plaque disassembly and internalization, with a progressive reduction in the expression of Cx43 becoming evident as exposure times are extended beyond 30 min (Guo et al., 1999). This observation could explain why the inhibition of EDHF-mediated relaxations by 100 μM 18α-GA becomes irreversible following 1 h incubation in rabbit arteries (Chaytor et al., 1998, 2000). The glycoside ouabain, which is structurally related to glycyrrhetinic acid, also promotes a time- and concentration-dependent removal of gap junctions from the cell membrane (Martin et al., 2004). The stable action of connexin-mimetic peptides and their rapid reversibility consequently make such agents particularly attractive tools for manipulating direct intercellular communication in the experimental situation.
We have previously used a direct microinjection technique to investigate the effects of connexin-mimetic peptides on dye transfer in coupled A7r5 myocytes (Chaytor et al., 2001). Intercellular transfer of Lucifer yellow in monolayers of these cells was shown to be almost unaffected by peptides targeted to Cx40 or Cx43 when administered individually, but markedly attenuated when such peptides were applied in combination, which is consistent with the abundant dual expression of Cxs 40 and 43 in A7r5 cells (Chaytor et al., 2001). To test the possibility that connexin-mimetic peptides affect the formation and stability of gap junction plaques, A7r5 cell were cultured for periods of up to 5 h in the presence of 37,40Gap 26, 37,43Gap 27 or their combination, followed by immunocytochemical analysis of Cx40 and Cx43. The findings confirmed that such peptides, even in combination, do not reduce the number or size of gap junction plaques present at the cell membrane. This is consistent with previous observations in HeLa cells transfected to express Cx43-GFP in which connexin-mimetic peptides did not interfere with the structural stability of preformed gap junction plaques as assessed by Cx43-GFP membrane fluorescence (Berman et al., 2002). The present study has extended these findings by showing that the combination of 37,40Gap 26+37,43Gap 27, applied to A7r5 cells 6 h after transfection with Cx43-GFP, did not impair their ability to synthesize Cx43 protein, interfere with the trafficking of this protein to the cell membrane or perturb the ability of Cx43 to form gap junction plaques over the following 12 h. During the life cycle of a gap junction plaque, new hemichannels are continuously recruited to the plasma membrane where they add to the edge of the existing aggregate, while older paired connexons are removed from the centre of the plaque as annular gap junctions and internalised (Gaietta et al., 2002; Lauf et al., 2002). This natural history suggests that the inhibitory activity of connexin-mimetic peptides is unlikely to reflect an ability to prevent connexon docking. Indeed, a gap junction plaque typically consists of a highly-ordered hexagonal array of up to several hundred docked hemichannels, and it is difficult to see how such structures could assemble if hemichannels were forced to remain mobile in the cell membrane because normal alignment was prevented by connexin-mimetic peptides. As noted in the Introduction, cooperative interactions between the individual channels that aggregate to form gap junction plaques in the cell membrane may be essential for efficient coupling between adjacent cells (Bukauskas et al., 2000). Speculatively, this cooperativity might be impaired by the presence of connexin-mimetic peptides as a result of electrostatic or steric interactions with the Gap 26 and 27 domains of the connexin proteins present within plaques and associated effects on channel gating. Effects on channel gating are also suggested by observations that connexin-mimetic peptides can also block the functional activity of free hemichannels, whose active physiological role can be monitored by dye uptake assays and by the release of ATP and NAD+ from the intracellular compartment (Bennett et al., 2003; Braet et al., 2003).
We also investigated the effect of connexin-mimetic peptides on the coordination of intracellular signalling events following stimulation of smooth cells by phenylephrine, an α1-adrenoceptor agonist that induces smooth muscle contraction and was found to induce synchronized oscillations in intracellular calcium levels in neighbouring A7r5 cells, but not in RAECs, presumably because endothelial cells do not possess α1-adrenoceptors (Dora et al., 2000). Blockade of gap junctional communication by connexin-mimetic peptides or 18α-GA abolished synchronized smooth muscle transients and most cells then exhibited monotonic increases in intracellular [Ca2+] following treatment with phenylephrine, rather than oscillatory responses. These observations confirm that intercellular signalling via gap junctions plays a central role in the coordination of intracellular Ca2+ events in smooth muscle cells, and provides an explanation for their ability to inhibit rhythmic contractile activity in endothelium-denuded arterial segments (Chaytor et al., 1997). Intercellular Ca2+ signalling, presumably involving an InsP3-mediated pathway has been reported in a variety of cell types, including HeLa cell transfected to express Cx43-GFP (Paemeleire et al., 2000) and alveolar epithelial cells in which connexin-mimetic peptides block intercellular Ca2+ wave propagation (Isakson et al., 2003).
In conclusion, the present data provide new insights into the mechanisms underlying the action of connexin-mimetic peptides by demonstrating that they do not impair connexin trafficking and the subsequent formation of gap junction plaques. Their specificity and reversibility, even after prolonged incubation times, opens new avenues to permit the controlled regulation of intercellular communication via gap junctions and therefore provide a versatile way to investigate the role of direct intercellular communication in integrated cellular activity.
Acknowledgments
The study was supported by the MRC. We thank Dr RJ Errington for helpful discussions on image analysis and Dr E Oviedo-Orta for preparation of RAECs.
Abbreviations
- Cx
connexin
- GFP
green fluorescent protein
- 18α-GA
18α-glycyrrhetinic acid
- InsP3
inositol 1,4,5-trisphosphate
References
- BENNETT M.V., CONTRERAS J.E., BUKAUSKAS F.F., SAEZ J.C. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN R.S., MARTIN P.E., EVANS W.H., GRIFFITH T.M. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc. Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- BOITANO S., EVANS W.H. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am. J. Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- BRAET K., VANDAMME W., MARTIN P.E., EVANS W.H., LEYBAERT L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin-mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- BUKAUSKAS F.F., JORDAN K., BUKAUSKIENE A., BENNETT M.V., LAMPE P.D., LAIRD D.W., VERSELIS V.K. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EDWARDS D.H., BAKKER L.M., GRIFFITH T.M. Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15212–15217. doi: 10.1073/pnas.2435030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARSH W.L., HUTCHESON I.R., GRIFFITH T.M. Comparison of glycyrrhetinic acid isoforms and carbenoxolone as inhibitors of EDHF-type relaxations mediated viagap junctions. Endothelium. 2000;7:265–278. doi: 10.3109/10623320009072213. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E., EDWARDS D.H., GRIFFITH T.M. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am. J. Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E.M., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIESE A.S., VAN DE VOORDE J., LAMEIRE N.H. Effects of connexin-mimetic peptides on nitric oxide synthase – and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61:177–185. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- DORA K.A., HINTON J.M., WALKER S.D., GARLAND C.J. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br. J. Pharmacol. 2000;129:381–387. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W.H., MARTIN P.E. Gap junctions: structure and function. Mol. Membr. Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- GAIETTA G., DEERINCK T.J., ADAMS S.R., BOUWER J., TOUR O., LAIRD D.W., SOSINSKY G.E., TSIEN R.Y., ELLISMAN M.H. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- GRIFFITH T.M. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis. Br. J. Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITH T.M., CHAYTOR A.T., TAYLOR H.J., GIDDINGS B.D., EDWARDS D.H. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction viagap junctions. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAN X.J., CRAVATT B.F., EHRING C.R., HALL J.E., BOGER D.L., LERNER R.A., GILULA N.B. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO Y.H., MARTINEZ WILLIAMS C., GILBERT K.A., RANNELS D.E. Inhibition of gap junction communication in alveolar epithelial cells by 18α-glycyrrhetinic acid. Am. J. Physiol. 1999;20:L1018–L1026. doi: 10.1152/ajplung.1999.276.6.L1018. [DOI] [PubMed] [Google Scholar]
- HE D.S., JIANG J.X., TAFFET S.M., BURT J.M. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6495–6500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILVESARO J., TAVI P., TUUKKANEN J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC Musculoskelet. Disord. 2001;2:10. doi: 10.1186/1471-2474-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAKSON B.E., SEEDORF G.J., LUBMAN R.L., EVANS W.H., BOITANO S. Cell–cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;29:552–561. doi: 10.1165/rcmb.2002-0281OC. [DOI] [PubMed] [Google Scholar]
- KO Y.S., COPPEN S.R., DUPONT E., ROTHERY S., SEVERS N.J. Regional differentiation of desmin, connexin43, and connexin45 expression patterns in rat aortic smooth muscle. Arterioscler. Thromb. Vasc. Biol. 2001;21:355–364. doi: 10.1161/01.atv.21.3.355. [DOI] [PubMed] [Google Scholar]
- KWAK B.R., JONGSMA H.J. Selective inhibition of gap junction channel activity by synthetic peptides. J. Physiol. 1999;516:679–685. doi: 10.1111/j.1469-7793.1999.0679u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUF U., GIEPMANS B.N., LOPEZ P., BRACONNOT S., CHEN S.C., FALK M.M. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN P.E., HILL N.S., KRISTENSEN B., ERRINGTON R.J., GRIFFITH T.M. Ouabain exerts biphasic effects on connexin functionality and expression in vascular smooth muscle cells. Br. J. Pharmacol. 2004;141:374–384. doi: 10.1038/sj.bjp.0705671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVIEDO-ORTA E., ERRINGTON R.J., EVANS W.H. Gap Junction intercellular communication during lymphocyte transendothelial migration. Cell Biol. Int. 2002;2002:253–263. doi: 10.1006/cbir.2001.0840. [DOI] [PubMed] [Google Scholar]
- PAEMELEIRE K., MARTIN P.E., COLEMAN S.L., FOGARTY K.E., CARRINGTON W.A., LEYBAERT L., TUFT R.A., EVANS W.H., SANDERSON M.J. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol. Biol. Cell. 2000;11:1815–1827. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAEZ J.C., BERTHOUD V.M., BRANES M.C., MARTINEZ A.D., BEYER E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., HILL C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- SLEZAK S.E., HORAN P.K. Fluorescent in vivo tracking of hematopoietic cells. Part I. Tech. Considerations Blood. 1989;74:2172–2177. [PubMed] [Google Scholar]
- SPAGNOLI L.G., VILLASCHI S., NERI L., PALMIERI G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982;38:124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- UJIIE H., CHAYTOR A.T., BAKKER L.M., GRIFFITH T.M. Essential role of gap junctions in NO- and prostanoid-independent relaxations evoked by acetylcholine in rabbit intracerebral arteries. Stroke. 2003;34:544–550. doi: 10.1161/01.str.0000054158.72610.ec. [DOI] [PubMed] [Google Scholar]
- VALIUNAS V., WEINGART R., BRINK P.R. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ. Res. 2000;86:E42–E49. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- WARNER A., CLEMENTS D.K., PARIKH S., EVANS W.H., DEHAAN R.L. Specific motifs in the external loops at connexin proteins can determine gap junction formation between chick heart myocytes. J. Physiol. 1995;488:721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLECKE K., EIBERGER J., DEGEN J., ECKARDT D., ROMUALDI A., GULDENAGEL M., DEUTSCH U., SOHL G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI J., KITAMURA K. Intercellular electrical coupling in vascular cells present in rat intact cerebral arterioles. J. Vasc. Res. 2003;40:11–27. doi: 10.1159/000068934. [DOI] [PubMed] [Google Scholar]
- ZAHLER S., HOFFMANN A., GLOE T., POHL U. Gap-junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J. Leukoc. Biol. 2003;73:118–126. doi: 10.1189/jlb.0402184. [DOI] [PubMed] [Google Scholar]