Abstract
Although statins have been reported to inhibit the prepro-endothelin-1 (ET-1) gene transcription in endothelial cells, their effects on the vascular function of ET-1 have not been explored. We, therefore, examined the effects of statins on contraction and DNA synthesis mediated by ET-1 in vascular smooth muscle. The effects of statins on contraction induced by ET-1 were compared to those mediated by noradrenaline (NA) and KCl.
Simvastatin (SV) induced a concentration-dependent relaxation of tonic contraction mediated by ET-1 (10 nM) (IC50 value of 1.3 μM). The relaxation was also observed in rings precontracted with NA (0.1 μM) and KCl (60 mM). In contrast, pravastatin did not have any effect on the contractions.
Endothelial denudation or pretreatment with L-NAME did not prevent the relaxation, but did reduce the relaxant activity of SV.
SV prevented Rho activation caused by ET-1 and KCl in aortic homogenates, as assessed by a Rho pulldown assay.
The Rho kinase inhibitor HA-1077 mimicked the effects of SV on tonic contractions induced by ET-1, NA and KCl.
Pretreatment with the Kv channels inhibitor, 4-aminopyridine, attenuated the ability of SV to relax contractions mediated by ET-1 and NA.
In quiescent VSM cells, SV significantly inhibited DNA synthesis and Rho translocation stimulated by ET-1, as assessed by [3H]thymidine incorporation and Western blot, respectively.
Inhibition of Rho geranylgeranylation by GGTI-297, or treatment with HA-1077, mimicked the effects of SV on DNA synthesis stimulated by ET-1.
The results show that the statin potently inhibits both ET-1-mediated contraction and DNA synthesis via multiple mechanisms. Clinical benefits of statins may result, in part, from their effects on vascular function of ET-1.
Keywords: Endothelin-1, simvastatin, vascular smooth muscle, calcium sensitization, Rho kinase, HA-1077, voltage-dependent calcium channels, voltage-dependent potassium channels, DNA synthesis, GGTI-297
Introduction
Inhibitors of HMG-CoA reductase (statins) are potent hypolipidemic agents used as first-line therapy for prevention of cardiovascular disease (Treasure et al., 1995; Endres et al., 1998; Brown, 2001; Lefer et al., 2001). Statins block the conversion of HMG-CoA to mevalonic acid and, consequently, attenuate the biosynthesis of cholesterol. Recent clinical trials and experimental studies with statins, however, have demonstrated remarkable beneficial cardiovascular effects beyond those predicted by lowering plasma cholesterol (Treasure et al., 1995; Lefer et al., 2001). Among the cholesterol-independent effects of statins, those on endothelial function and vascular proliferation are thought to be clinically relevant (Laufs et al., 1999; Lefer et al., 2001). Statin therapy favorably modifies the endothelial dysfunction in cardiovascular disease states, including coronary artery disease, myocardial ischemia and cerebral vasospasm (Endres et al., 1998; Lefer et al., 1999; McGirt et al., 2002). Treatment with statins also improves clinical outcome in patients with vascular proliferative disease states such as post-angioplasty restenosis and transplant vasculopathy, and reduces growth factor-induced vascular smooth muscle cell proliferation in a manner independent of cholesterol synthesis (Shepherd et al., 1995; Laufs et al., 1999; Sawada et al., 2000). The statin-induced improvement of endothelial function is likely to occur by both enhancement of nitric oxide (NO) generation and attenuation of vasoconstrictor activity of endogenous agonists generated in endothelial cells. Statins directly enhance endothelial nitric oxide synthase (eNOS) ability to generate NO in endothelial cells, and upregulate eNOS by inhibition of geranylgeranylation of a monomeric G protein Rho, which acts as a negative regulator of the enzyme mRNA stability (Laufs & Liao, 1998; Kaesemeyer et al., 1999).
Statins also cause a downregulation of the prepro-endothelin-1 (ET-1) mRNA level in endothelial cells and thus reduce the ET-1 synthesis (Hernández-Perera et al., 1998, 2000). The potential functional significance of this action is demonstrated by the fact that ET-1 is a powerful vasoconstrictor and growth factor, which plays an essential role in regulation of vascular tone and vascular smooth muscle mitogenesis (Bobik et al., 1990; Schiffrin & Touyz, 1998). These effects of ET-1 are thought to play a role in the pathogenesis of atherosclerosis, pulmonary hypertension and cerebral vasospasm (Lerman et al., 1995; Seifert et al., 1995; Chen et al., 1997; Ihling et al., 2001; Iglarz & Schiffrin, 2003). Vascular contraction evoked by ET-1 is mediated by the ETA and ETB receptors coupled to the heterotrimeric G proteins, Gq/G11 and G12/G13. The Gq activation leads to the initiation of contraction via phospholipase C (PLC)-dependent intracellular Ca2+ mobilization and activation of protein kinase C (PKC), whereas stimulation of G12 or G13 activates the monomeric G protein Rho and its downstream effector Rho kinase (Göhla et al., 2000; Somlyo & Somlyo, 2003). In vascular smooth muscle, activation of the Rho/Rho kinase pathway promotes Ca2+ sensitization of contraction, and cell migration and proliferation, which are central events in a variety of vascular disorders including hypertension, vasospasm and atherosclerosis (Uechata et al., 1997; Laufs et al., 1999; Sato et al., 2000). Rho is also involved in basal expression of prepro-ET-1 in endothelial cells, and statins inhibit this process by blocking Rho geranylgeranylation, a post-translational modification that is essential for the membrane anchoring and activation of Rho (Hernández-Perera et al., 2000). There is evidence to indicate that the ability of statins to prevent the Rho geranylgeranylation arises from the inhibition of the synthesis of isoprenoid intermediates of the HMG–CoA reductase–mevalonate pathway, such as geranylgeranyl pyrophosphate, and, thus, is independent of the cholesterol synthesis (Goldstein & Brown, 1990; Hall, 1998). Although statins were shown to inhibit the Rho-dependent activation of the prepro-ET-1 gene expression in endothelial cells, their direct effect on the ET-1-regulated vascular function has not been demonstrated. Therefore, the aim of the present studies was to examine whether statins can inhibit the ET-1-dependent vascular contraction and mitogenesis, and, if so, to identify the intracellular mechanisms involved.
Methods
Materials
Simvastatin (SV) was generously provided by Merck & Co., Inc., Rathway, NJ, U.S.A. ET-1, noradrenaline (NA), NG-nitro-L-arginine methyl ester (L-NAME), nimodipine, 4-aminopyridine (4-AP), tetraethylammonium (TEA) and apamin were from Sigma, St Louis, MO, U.S.A. Pravastatin (PV), HA-1077 and GGTI-297 were from Calbiochem, San Diego, CA, U.S.A. Rho Activation Assay Kit, including an agarose-conjugated rhotekin-binding domain (RBD), was from Upstate (Lake Placid, NY, U.S.A.). Anti-RhoA monoclonal antibody was purchased from Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A. All other reagents were of analytical grade.
Isometric tension measurements
Protocols for using animals were approved by the Animal Care Committee at the University of Alberta and were in accordance with the guidelines of the Canadian Council for Animal Care in Research. Male Sprague–Dawley rats (∼200 g) were killed by decapitation. The thoracic aorta was quickly removed to a dish filled with oxygenated Krebs–Henseleit buffer of the following composition (in mM): 120 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgSO4, 1.2 KH2PO4, 25 NaHCO3 and 10 dextrose. The endothelium was removed mechanically. Each vessel was cut into ∼3 mm-long sections and suspended in tissue baths containing Krebs–Henseleit buffer at 37°C, gassed with 95% O2 and 5% CO2, and attached to a force–displacement transducer (model FT.03, Grass). Ring preparations were equilibrated for 1 h under a resting tension of 1 g. After an equilibration period, during which time the Krebs–Henseleit buffer was changed every 20 min, the responses to KCl (60 mM) were recorded to determine viability, and ring preparations were washed until resting tension was again obtained. Isometric tension was recorded via Power Lab 4/20 Quad bridge amplifier (AD Instruments) connected to a computer hard drive. Viability of preparations was determined by maximal force response to a high concentration (60 mM) of KCl. Contractions mediated by ET-1 (10 nM), NA (0.1 μM) and KCl (60 mM) reached a maximum defined as 100% at times which varied from 4 to 10 min after the agents were first administered. Cumulative concentrations of SV (0.1–10 μM), PV (0.1–15 μM), HA-1077 (0.1–1 μM) or nimodipine (0.001–1 μM) were administered to organ baths after sustained tonic responses to the vasoconstrictors had developed. In the experiments in which the contractile responses to ET-1, NA or KCl were studied in the presence of L-NAME, mevalonate or 4-AP, tissues were exposed to the inhibitors for 30 min or 2 h (mevalonate) prior to the exposure to the vasoconstrictors. The presence of an intact endothelium was examined by evaluating the relaxant responses to bradykinin (0.1–100 nM) in preparations, which had been precontracted with NA (0.1 μM). Data analysis was performed using Chart version 4.12 software (AD Instruments).
Cell culture
Cells were prepared as previously published (Wickman et al., 2001, 2003). Briefly, canine basilar arteries were isolated under sterile conditions and placed in a Petri dish containing Dulbecco's modified Eagle's medium (DMEM). The vessels were cut into segments and the endothelium was removed mechanically. The explants were transferred to culture flasks containing DMEM supplemented with 10% calf serum, penicillin (100 U ml−1), and streptomycin (100 μg ml−1). The confluent vascular smooth muscle (VSM) cells were subcultured at a split ratio of 1 : 3. The cells were test positive for smooth muscle α actin and demonstrated the typical hill-and-valley pattern.
Rho activation assay
Rho activity was determined using the rhotekin affinity precipitation assay (a pull-down assay) incorporating a glutathione S-transferase (GST)-tagged fusion protein corresponding to residues 7–89 of mouse rhotekin Rho-binding domain (RBD), which specifically binds to and precipitates the active GTP-bound form of Rho (Ren et al, 1999). Briefly, endothelium-denuded aortic rings were exposed to either SV (2 μM) or nimodipine for 10 min in Krebs–Henseleit buffer before addition of ET-1 (10 nM) or KCl (60 mM) for a further 5 or 30 min and then snap frozen in liquid nitrogen. The effects of the statin on KCl-induced Rho activation were examined in the presence of 10 μM of an α adrenergic receptor antagonist phentolamine to prevent the potential receptor activation caused by NE released from perivascular nerves by depolarizing concentration of KCl. Frozen tissues were homogenized in a Mg2+ lysis-wash buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA630, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 1 μg ml−1 aprotinin, 10 μg ml−1 leupeptin and 1 mM sodium orthovanadate), and supernatants were obtained by centrifugation (5 min, 14,000 × g, 4°C). Protein concentration was determined using micro-Bradford assay (Bio-Rad). The supernatants were incubated for 45 min at 4°C with agarose-conjugated rhotekin RBD, and then washed three times with a Mg2+ lysis-wash buffer. Agarose beads were boiled for 5 min in SDS–PAGE sample buffer to release active Rho, and protein-matched samples were resolved on 15% gels followed by immunoblotting with a monoclonal RhoA antibody (3 μg ml−1). To detect bound primary antibodies, blots were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody. The bands were detected on film using an Epson Perfection 636 scanner with Epson Twain scanning software. The density of bands was measured using Sigma-Gel software (Jandel).
Western immunoblotting
RhoA expression and translocation in cultured CVSM cells were determined using quiescent monolayers of the cells exposed to ET-1 (10 nM) in the presence or absence of SV administered 24 h before ET-1. After stimulation, the cells were harvested, homogenized and fractionated in a sample buffer as described previously (Wickman et al., 2003; Lan et al., 2004). The homogenates were centrifuged at 15,000 × g for 1 h at 4°C to obtain membrane and cytosolic fractions. Protein-matched samples were separated by SDS–PAGE, transferred to nitrocellulose membranes, and blocked with nonfat milk. Equal line loading was confirmed by inspection of membranes after reversible Ponceau staining. Blots were then incubated with a monoclonal RhoA antibody and analyzed as described above for Rho affinity precipitation assay.
[3H]thymidine incorporation
Cerebrovascular smooth muscle cells (SMC) were grown to 80% confluence and growth-arrested by incubation for 24 h in serum-free media. The cells were then treated with statins (0.1–10 μM) for 24 h before administration of ET-1 (10 nM) for 48 h. In the experiments, in which the effects of GGTI-297 (200 nM) or HA-1077 (1 μM) were investigated, cells were exposed to these agents for 30 min prior to administration of ET-1 for 48 h. The effects of the drugs were compared to the effects of vehicle (0.3% DMSO). As assessed by [3H]thymidine incorporation, DMSO in control cells did not have any effect on DNA synthesis. [3H]thymidine (1 μCi ml−1) was added to the cells for a period of 24 h before the cells were harvested. At the end of the incubation, the medium was removed, cells were washed twice with PBS, and then fixed in 5% trichloroacetic acid. Cells were then washed several times in ice-cold PBS and lysed in 0.5 ml of 1.0 M NaOH. Incorporated radioactivity was determined using a liquid scintillation counter (Beckman LS6500). Cell viability was determined by trypan blue exclusion and cell counting.
Statistical analyses
The unpaired two-tailed Student's t-test and one-way ANOVA were used in the statistical analysis when appropriate. Post hoc comparisons of individual groups were performed using the Tukey–Kramer test. The IC50 values for the vasorelaxants were calculated using nonlinear regression analyses (Slidewrite 3.0). A P-value less than 0.05 was considered significant. All results are expressed as mean±s.e.m. for the stated number of observations.
Results
SV acutely inhibits sustained vascular contraction mediated by ET-1, NA and KCl in rat aortic rings
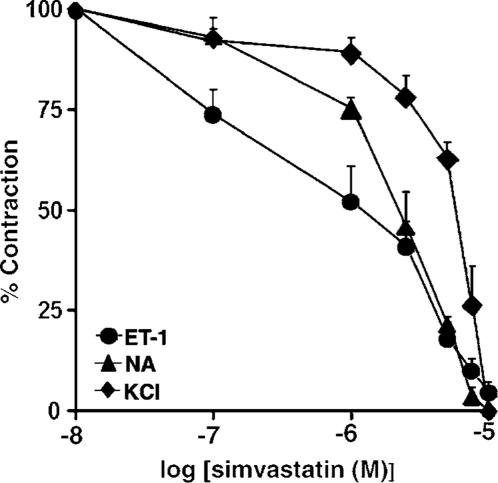
The effects of statins were studied in rings precontracted with ET-1 (10 nM) and compared to those observed in preparations contracted with NA (0.1 μM) and KCl (60 mM). NA and KCl were used as a positive control in all our tension study because most of the knowledge concerning the acute vasorelaxant effects of statins has been derived from studies in vascular smooth muscle precontracted with these vasoconstrictors (Álvarez de Sotomayor et al., 2000; Bergdahl et al., 2003; Mukai et al., 2003). The concentrations of ET-1 and NA were equally effective in producing tonic contractions of the muscle (0.930±0.14 and 0.94±0.26 g tension for ET-1 and NA, respectively). The average value of precontraction with 60 mM KCl corresponded to 0.323±0.03 g tension. SV, administered in increasing cumulative concentrations (0.1–10 μM) to aortic rings in which a plateau tension in response to either ET-1 or NA had developed, produced rapid, concentration-dependent relaxation with the calculated IC50 values of 1.3±0.5 and 2.5±0.1 μM, respectively. As shown in Figure 1, the statin also evoked relaxation of rings precontracted with high depolarizing concentrations of KCl, which produces contraction through activation of Ca2+ influx via L-type VDCC (the IC50 value of 6.1±0.6 μM). The IC50 value for SV-mediated relaxation of contraction induced by KCl was significantly greater compared to those for relaxation of contractions mediated by either ET-1 or NA (P<0.001 and <0.01, respectively). In contrast, pravastatin (0.1–15 μM) did not produce relaxation (data not shown).
Figure 1.
Effects of SV on tonic contractions induced by ET-1, NA and KCl. Aortic rings were precontracted with either ET-1 (10 nM), NA (0.1 μM), or KCl (60 mM) prior to obtaining cumulative concentration–response curves to SV. Results are expressed as a percentage of maximum tonic contraction produced by the vasoconstrictors, and are expressed as means±s.e.m. for experiments with 10 or more rings.
The vasorelaxant effects of SV are partially dependent on the presence of endothelium
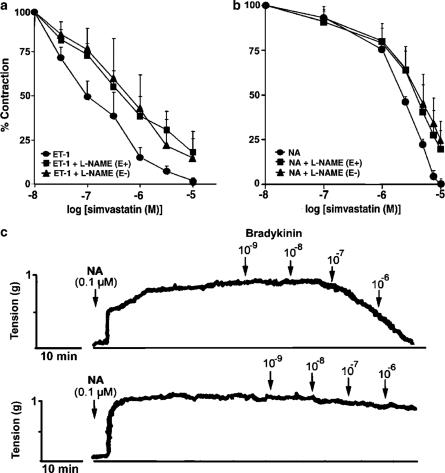
To determine whether the relaxation to SV was dependent on the presence of endothelium, the effects of SV were assessed in the endothelium-denuded preparations precontracted with either ET-1 (10 nM) or NA (0.1 μM). The absence of a functional endothelium increased smooth muscle tension evoked by either ET-1 or NA by approximately 15% (1.07±0.12 g tension) and 20% (1.13±0.16 g tension), respectively. The relaxation was observed in both endothelium-intact and -denuded preparations precontracted with ET-1, indicating that the effects of SV were, at least in part, independent of endothelium; however, the potency of SV in the endothelium-denuded preparations was significantly reduced (IC50 of 5.7±1 μM; P<0.05) (Figure 2a). Similar effects were observed in preparations precontracted with NA (0.1 μM) (IC50 of 5.2±2 μM) (Figure 2b).
Figure 2.
Effects of endothelial denudation and L-NAME on relaxation of rat aortic rings caused by SV. Aortic rings were pretreated for 30 min with L-NAME (200 μM) in the presence (E+) or absence (E−) of endothelium, and then precontracted with either ET-1 (10 nM) (panel a) or NA (0.1 μM) (panel b), prior to obtaining cumulative concentration–effect curves for SV. Exposure to L-NAME resulted in an increase in tension to ET-1 and NA by about 20%. Results in panels a and b are expressed as a percentage of maximum contraction induced by either ET-1 or NA, and are presented as means±s.e.m. for experiments with 10 rings. Panel c shows representative traces of the effects of bradykinin (0.001–1 μM) on rings of rat aorta with preserved and denuded endothelium. The rings were precontracted with NA (0.1 μM). Bradykinin produced concentration-dependent relaxations in preparations, which had been precontracted with NA, in the presence (upper trace) but not in the absence (lower trace) of endothelium. Each trace illustrates the response of an individual ring preparation and is representative of eight or more independent experiments.
Bradykinin (0.001–1 μM) produced concentration-dependent relaxations in rat aortic rings, which had been precontracted with NA (0.1 μM), in the presence but not in the absence of endothelium (Figure 2c).
To assess the contribution of eNOS to the effects of SV, vascular rings were pretreated for 30 min with the competitive, nonselective inhibitor of NOS, L-NAME (200 μM). L-NAME increased smooth muscle tension raised by either ET-1 or NA (by approximately 15%), and modestly decreased relaxation induced by SV (Figure 2a and b). The extent of the relaxation in the presence of L-NAME was not significantly different from that observed in the endothelium-denuded preparations.
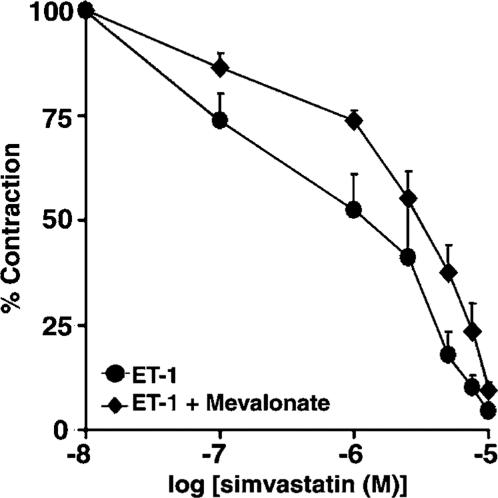
Effects of mevalonate on SV-mediated relaxation in rings contracted with ET-1
To determine whether SV produces vascular relaxation through the inhibition of HMG-CoA reductase, vascular rings were pretreated for 2 h with either vehicle or mevalonate (200 μM), a precursor of cholesterol and isoprenoids, and SV was administered after plateau tension to ET-1 had developed. As shown in Figure 3, mevalonate only partially restored contraction inhibited by SV (IC50 of 3.4±0.5 μM), indicating that the effects of SV are largely independent of cholesterol or isoprenoid metabolism.
Figure 3.
Effects of mevalonate on relaxation mediated by SV in rat aortic rings precontracted with ET-1. Aortic rings were pretreated with either mevalonate (200 μM) or vehicle for 2 h, and then precontracted with ET-1 (10 nM) prior to obtaining cumulative concentration–effect curves for SV. Results are expressed as a percentage of the maximum tonic contraction to ET-1, and are presented as means±s.e.m. for experiments with six rings.
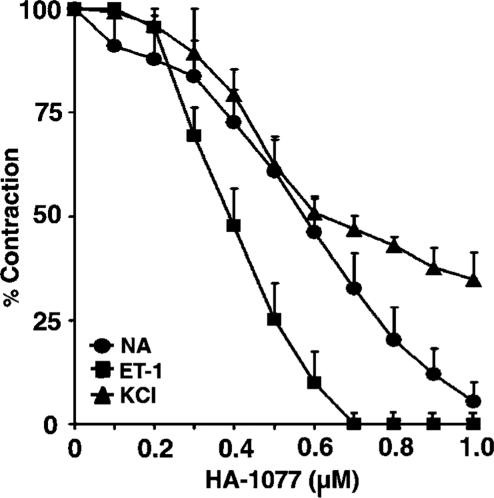
Vascular effects of the Rho kinase inhibitor HA-1077
Previous studies have shown that Ca2+ sensitization of contraction is mediated via receptor-dependent activation of the Rho/Rho kinase pathway (Somlyo & Somlyo, 2003). We, therefore, determined the effects of the selective Rho kinase inhibitor, HA-1077, on tonic contractions induced by ET-1 and NA. As shown in Figure 4, HA-1077 (0.1–1 μM) produced concentration-dependent decreases in tone raised by ET-1 and NA (the IC50 values against ET-1 and NA of 0.4±0.045 and 0.56±0.09 μM, respectively), consistent with the inhibition of Rho kinase activity. HA-1077 also produced a concentration-dependent relaxation of the contractile responses induced by 60 mM KCl (IC50 value of 0.64±0.03 μM; Figure 4), indicating that Rho kinase is activated during depolarization and that its activation increases vascular smooth muscle tension.
Figure 4.
Effects of HA-1077 on tonic contractions developed in response to ET-1, NA and KCl. HA-1077 was administered in increasing cumulative concentrations (0.1–1 μM) to preparations, in which tonic contractions to the vasoconstrictors had developed. Results are expressed as a percentage of maximum tonic contraction produced by either ET-1 (10 nM), NA (0.1 μM) or KCl (60 mM), and are presented as means±s.e.m. for experiments with six rings.
Effects of SV on Rho activation induced by ET-1 and KCl
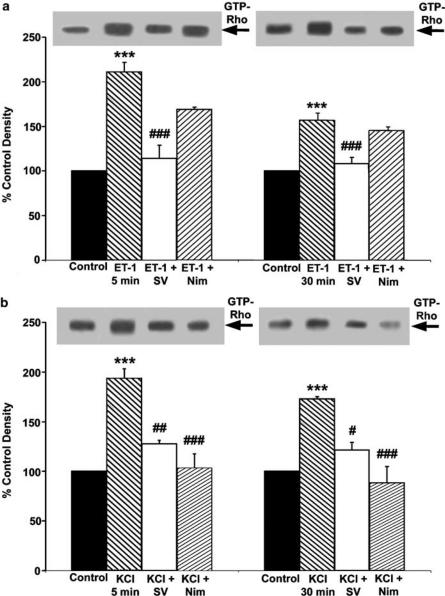
Our observations with HA-1077 have suggested that inhibition of the Rho/Rho kinase pathway was the likely mechanism to explain the vasorelaxant effects of SV. Therefore, to determine whether the effects of SV could be due to an inhibition of the pathway, we examined the ability of the drug to influence the Rho activation mediated by ET-1 and KCl. In these experiments, endothelium-denuded aortic smooth muscle preparations exposed to either ET-1 (10 nM) or KCl (60 mM) were rapidly frozen after 5 and 30 min of stimulation and Rho activation was determined in aortic homogenates by affinity precipitation with the recombinant Rho effector rhotekin (Ren et al., 1999). As shown in Figures 5a and b, the amount of active Rho significantly increased within 5 min of stimulation with either ET-1 (211±10.5% of control; P<0.001) or KCl (193±9%; P<0.001). The level of activated Rho remained significantly elevated after 30 min of stimulation (156±8%; P<0.001 and 173±2%; P<0.001 for ET-1 and KCl, respectively). The increases in Rho activation observed after 5 and 30 min of the stimulation corresponded to the increases in the isolated aortic ring tension developed in response to ET-1 and KCl, indicating a temporal relation between these two processes. Treatment with SV (2 μM) abolished Rho activation mediated by ET-1 (114±15 and 108±7% of control after 5 and 30 min of stimulation, respectively) (Figure 5a). SV also significantly inhibited Rho activation mediated by KCl (128±3 and 122±8% of control after 5 and 30 min of stimulation, respectively) (Figure 5b).
Figure 5.
Effects of SV and nimodipine on Rho activation in rat aorta stimulated with either ET-1 or KCl. The endothelium-denuded rat aorta preparations were stimulated with either ET-1 (10 nM) or KCl (60 mM), and the muscle was snap frozen after 5 and 30 min of stimulation. SV (2 μM) or nimodipine (0.1 μM) were administered 10 min before the administration of ET or KCl. Rho activation was determined by using an affinity precipitation assay with GST-fusion protein of the RBD of the Rho effector rhotekin, as described in Methods. Panel a shows the effects of SV (2 μM) and nimodipine (0.1 μM) on Rho activation in rat aorta stimulated for 5 min (left) and 30 min (right) with ET-1 (10 nM). Panel b shows the effects of SV and nimodipine on Rho activation in rat aorta stimulated with KCl (60 mM) for 5 min (left) and 30 min (right). The position of GTP-Rho is indicated on the right of the representative immunoblots. Quantification of intensities of GTP-Rho bands was performed by scanning densitometry. Results are expressed as a percentage of respective controls, and are means±s.e.m. of duplicate determinations from five experiments.
Effects of the L-type Ca 2+ channel blockade on Rho activation and contractions mediated by KCl and ET-1
Previous reports suggested that depolarization- and receptor agonist-induced Rho activation is a Ca2+-dependent process (Sakurada et al., 2003). Thus, we examined whether inhibition of L-type voltage-dependent Ca2+ channels (VDCC) with the selective antagonist, nimodipine, would affect Rho activation and contraction mediated by KCl and ET-1 in rat aorta preparations. As shown in Figure 5b, nimodipine (0.1 μM) abolished the increase in the amount of active Rho at 5 and 30 min of stimulation with 60 mM KCl (103±14 and 88±16% of control, respectively), supporting the role for Ca 2+ influx in Rho activation. In contrast, nimodipine was not very effective at reversing the ET-1-induced Rho activation, implying that Ca2+ influx through L-type VDCC does not significantly contribute to this response (Figure 5a).
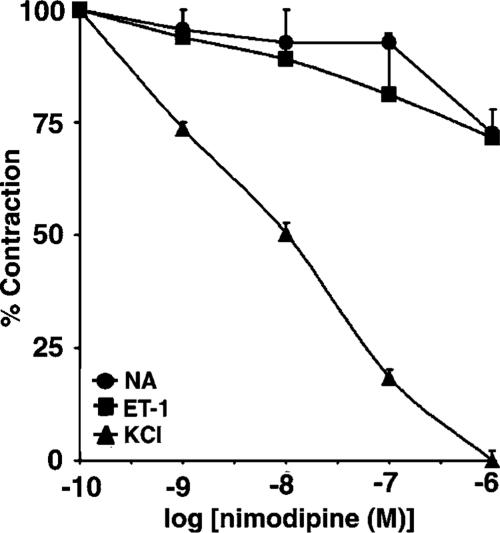
To assess the contribution of L-type VDCC to the agonist-and KCl-induced contraction, nimodipine was administered in increasing cumulative concentrations (0.1 nM–1 μM) to vascular rings, in which tonic contractions in response to the vasoconstrictors had developed. As shown in Figure 6, nimodipine modestly attenuated tonic contractions developed in response to both ET-1 and NA (the IC50 values of 40 and 28 μM, respectively), and abolished contraction mediated by KCl (IC50 of 0.01 μM), consistent with the contribution of L-type VDCC to KCl- but not the agonist-induced contraction.
Figure 6.
Effects of nimodipine on tonic contraction developed in response to ET-1, NA and KCl. Aortic rings were precontracted with either ET-1 (10 nM), NA (0.1 μM) or KCl (60 mM) and nimodipine was administered in increasing cumulative concentrations (0.1 nM–1 μM) to preparations in which tonic contractions to the vasoconstrictors had developed. Results are expressed as a percentage of maximum tonic contraction produced by ET-1, NA or KCl, and are presented as means±s.e.m. for experiments with six rings.
Involvement of voltage-dependent potassium (Kv) channels in the vasorelaxant effects of SV
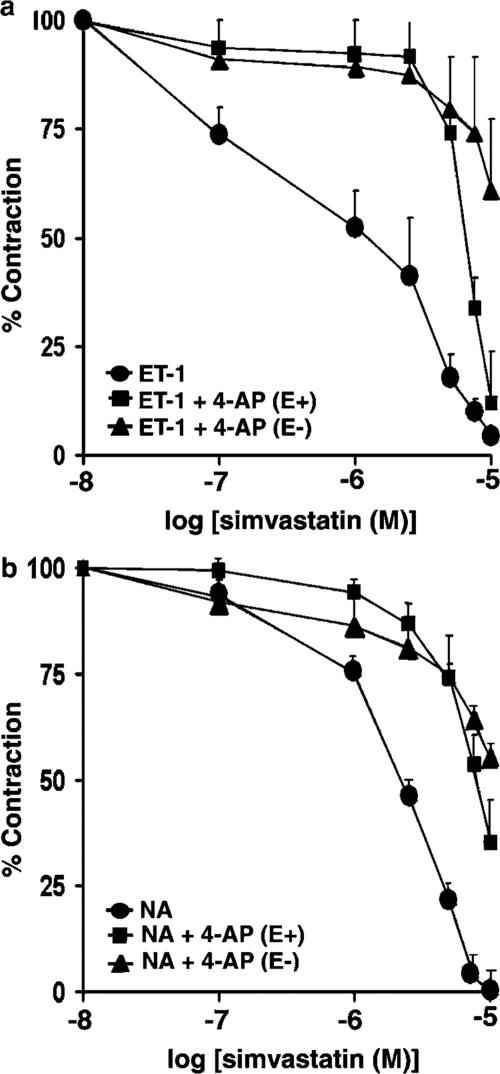
Based on our observation that depolarization with KCl attenuated the potency of SV, we attempted to determine the contribution of K+ channels to the SV-mediated relaxation. Pretreatment with 1 mM 4-AP, a concentration that selectively inhibits voltage-dependent potassium channels (Kv) channels, significantly attenuated the potency of SV for relaxation of contraction mediated by ET-1 (IC50 of 6.7±0.26 μM; P< 0.001) (Figure 7a), indicating a role of Kv channels in this response. Endothelial denudation further increased the ability of 4-AP to reduce the relaxation (IC50 of 12±2.5 μM; P<0.001). Pretreatment with 4-AP also suppressed the effects of SV in preparations precontracted with NA (0.1 μM) (Figure 7b). The IC50 values for SV in preparations pretreated with 4-AP prior to administration of NA were 8.8±1.4 μM; P<0.01 and 12±0.8 μM; P<0.001, in the presence and absence of endothelium, respectively. Neither 1 mM tetraethylammonium (TEA), a concentration that inhibits Ca2+-dependent K+ channels, nor 1 μM apamin, a concentration that inhibits inward rectifying K+ channels, had any effect on relaxation mediated by SV in preparations precontracted with either ET-1 or NA (results not shown).
Figure 7.
Effects of the inhibitor of Kv channels, 4-AP, on SV-induced relaxation of rat aortic rings. Preparations were pretreated for 30 min with 4-AP (1 mM) in the presence (E+) or absence (E−) of endothelium, and then precontracted with either ET-1(10 nM) (panel a) or NA (0.1 μM) (panel b), prior to obtaining cumulative concentration–response curves for SV. Results are expressed as a percentage of maximum tonic contraction induced by either ET-1 or NA and are presented as means±s.e.m. for experiments with six rings.
SV inhibits DNA synthesis stimulated by ET-1 in quiescent cerebrovascular smooth muscle cells
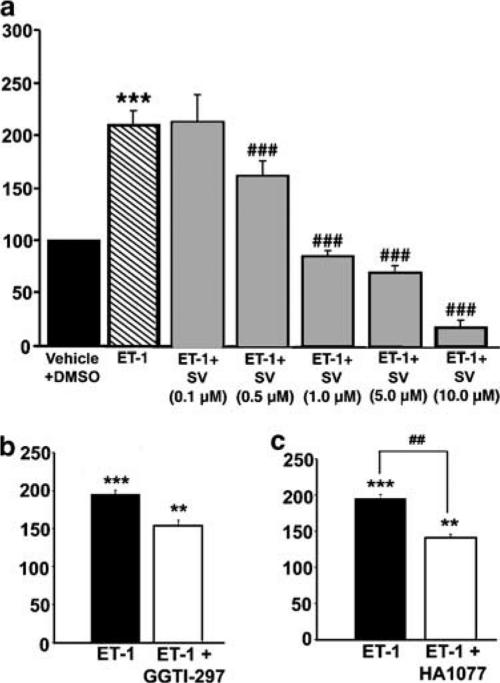
Although previous reports have demonstrated that statins inhibit cell proliferation induced with growth factors and serum in a variety of cell types (Laufs et al., 1999; Seasholtz et al., 1999; Sawada et al., 2000), the effects of these drugs on ET-1-induced DNA synthesis in VSM cells have not been investigated. Therefore, to explore the effects of SV on this process, we used cultured VSM cells, as an in vitro model. [3H]thymidine incorporation was used as an index of DNA synthesis and cell proliferation. VSM cells were growth arrested for 24 h, and then subjected to ET-1 (10 nM) in the absence or presence of SV (0.1–10 μM) for 48 h. ET-1 induced a significant increase in [3H]thymidine incorporation (211±13% of control; P<0.001). As shown in Figure 8a, SV induced a significant, concentration-dependent inhibition of [3H]thymidine incorporation stimulated by ET-1 with an IC50 value of 0.6±0.05 μM. SV (0.1–10 μM) had no effect on cell viability for up to 48 h. However, higher concentration of SV (12–20 μM) caused cell detachment from the culture flasks after 48 h. Detached cells constituted about 20% of nontreated or DMSO-treated cells. Of this population approximately 15% were trypan blue permeable, indicating that the higher concentrations of the drug were cytotoxic. Therefore, these concentrations of SV were not used in the experiments. PV (0.1–15 μM) did not have any significant effect on [3H]thymidine incorporation mediated by ET-1 (data not shown).
Figure 8.
Effects of SV, GGTI-297 and HA-1077 on [3H]thymidine incorporation stimulated by ET-1 in VSM cells. [3H]thymidine incorporation was used to assess DNA synthesis. Subconfluent, quiescent cells were incubated with either vehicle (0.3% DMSO) or ET-1 (10 nM) for 48 h. SV (0.1–10 μM) (panel a), was added to quiescent cells 24 h before administration of ET-1.GGTI-297 (200 nM) (panel b) and HA-1077 (1 μM) (panel c) were administered 30 min prior to addition of ET-1 (10 nM) for 48 h. Data represent the means±s.e.m. of six experiments performed in triplicate. ***P<0.001 versus control; ##P<0.01 and ###P<0.001 versus ET-1.
Inhibitors of geranylgeranyl transferase and Rho kinase mimic the effects of SV on DNA synthesis mediated by ET-1
To determine whether geranylgeranylation was involved in ET-1-induced DNA synthesis, we examined the effects of GGTI-297, a selective inhibitor of geranylgeranyl transferase I, the enzyme that regulates isoprenylation of Rho. In these experiments, quiescent VSM cells were exposed to GGTI-297 (200 nM) 30 min prior to administration of ET-1 (10 nM) for 48 h. As shown in Figure 8b, GGTI-297 attenuated the 3[H]thymidine incorporation, consistent with the involvement of Rho isoprenylation in the effects of ET-1.
Previous studies have shown that a number of the downstream effects of Rho activation are mediated via activation of Rho kinase (Seasholtz et al., 1999; Sawada et al., 2000). To determine whether Rho kinase plays a role in the ET-1- mediated DNA synthesis, quiescent cells were exposed to HA-1077 (1 μM) 30 min prior to stimulation with ET-1 (10 nM) for 48 h. As shown in Figure 8c, HA-1077 inhibited the thymidine incorporation, suggesting that the effects of ET were due, in part, to activation of Rho kinase. Under these experimental conditions, there were no observable adverse effects of GGTI-297 or HA-1077 on cell viability.
SV prevents Rho translocation stimulated by ET-1 in quiescent vascular smooth muscle cells
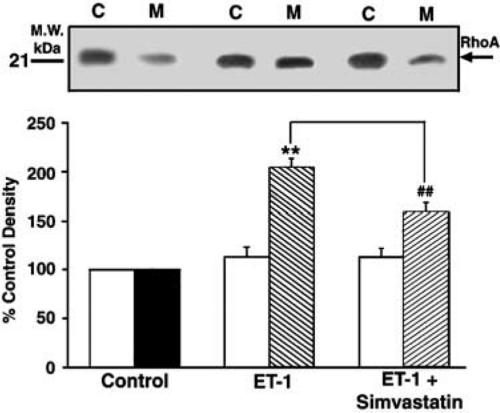
The results with GGTI-297 suggested that inhibition of Rho geranylgeranylation was the likely mechanism responsible for the effects of SV on DNA synthesis. We therefore examined the effects of SV on Rho translocation, a hallmark of the enzyme activation. In these experiments, quiescent VSM cells were stimulated with ET-1 (10 nM) in the presence or absence of SV, and then subjected to SDS–PAGE, followed by Western blotting with anti-Rho monoclonal antibody. The expression of Rho was measured in both the cytosolic and membrane fractions. In untreated cells, approximately 30% of Rho were membrane-bound and 70% was cytosolic (data not shown). ET-1 significantly stimulated Rho association with the plasma membrane (205±9% of untreated control; P<0.01). As shown in Figure 9, SV (5 μM) significantly reduced Rho translocation mediated by ET-1 (P<0.01).
Figure 9.
Effects of SV on Rho translocation mediated by ET in VSM cells. Subconfluent, quiescent cells were stimulated with ET-1 (10 nM) for 10 min. SV was administered for 24 h before treatment with ET-1. Protein-matched samples from cytosolic (C) and membrane (M) fractions were resolved by SDS–PAGE and immunoblotted with a monoclonal Rho antibody as described in Methods. Upper panel shows a representative Western blot illustrating Rho translocation induced with ET-1 in the absence or in the presence of SV. Quantification of intensities of Rho bands was performed by densitometry (arbitrary units). Bars (lower panel) represent Rho distribution in the cytosol and membrane fractions, expressed as a percentage of respective controls. Results are expressed as means±s.e.m. from three experiments performed in duplicate. **P<0.01 versus control; ##P<0.01 versus ET-1.
Discussion
Although previous studies have demonstrated a role for statins in the expression of the prepro-ET-1 gene in endothelial cells (Hernández-Perera et al., 1998, 2000), direct effects of these agents on vascular function of ET-1 have not been defined. The present studies show, for the first time, that SV potently inhibits vasoconstriction in response to ET-1 and suppresses DNA synthesis induced by this peptide in VSM cells through multiple mechanisms.
Our findings that vascular relaxation in response to the statin is dependent, in part, on the presence of the endothelium, are consistent with those of previous studies demonstrating the ability of statins to increase the eNOS activity and, consequently, NO generation (Kaesemeyer et al., 1999; Álvarez de Sotomayor et al., 2000; Mukai et al., 2003). Previous studies demonstrated that the production of ET-1 and vascular contraction evoked by this peptide are inhibited by NO (Boulanger & Luscher, 1990; Schini et al., 1991). Thus, it is conceivable that SV attenuates the contractile effects of ET-1 in part by enhancing eNOS activity and NO release from endothelial cells. Although the mechanism by which statins may increase eNOS activity is not clear, recent reports suggest that the effects of these agents are due to modulation of the serine/threonine protein kinase Akt (Fulton et al., 1999; Kureishi et al., 2000). Statins activate the phosphoinositide 3-kinase (PI 3-kinase)/Akt pathway in endothelial cells, thus leading to phosphorylation of eNOS and, consequently, enhanced NO generation (Kureishi et al., 2000). An other recent study supports this finding, demonstrating that blockade of PI-3-kinase with the selective inhibitors, wortmannin and LY-294002, attenuates the enhancement of acetylcholine-induced vascular relaxation and Akt phosphorylation mediated by statins (Mukai et al., 2003). In addition to these effects, statins directly enhance eNOS expression by inhibiting the isoprenylation of the small G-protein Rho, which negatively regulates eNOS synthesis (Endres et al., 1998; Laufs & Liao, 1998). It is rather unlikely that the rapid vasorelaxant effects of the statin observed in the present studies resulted from an enhancement of eNOS synthesis. However, the involvement of Akt in vasorelaxation mediated by SV cannot be discounted, and it remains to be investigated.
As shown in the present studies, the vasorelaxation mediated by SV possesses a significant component that is independent of endothelium. The endothelium-independent component of the relaxation is likely to arise from the ability of the statin to inhibit the Rho/Rho kinase pathway. Consistent with this suggestion is the observation that SV inhibited ET-1-induced Rho activation and contraction in the endothelium-denuded vascular preparations. Furthermore, inhibition of Rho kinase with HA-1077 mimicked the effects of the statin on tonic contraction to ET-1, implying that the statin-mediated vasorelaxation is the consequence of its ability to inhibit Rho kinase activity. Previous reports have demonstrated that activation of the Rho/Rho kinase pathway by agonists acting on Gq and G12/G13-coupled receptors, including ET-1 and NA, leads to an inhibition of myosin light chain phosphatase (MLCP) and subsequent MLC20 phosphorylation, resulting in Ca2+ sensitization of VSM (Somlyo & Somlyo, 2003). Although it is well established that Rho/Rho kinase-dependent contraction is mediated by agonists acting on the G-protein-coupled receptors, recent evidence indicates that receptor-independent mechanisms, including KCl-dependent depolarisation of vascular smooth muscle cell membrane, also play a role in this phenomenon (Mita et al., 2002; Ayman et al., 2003; Sakurada et al., 2003; Urban et al., 2003). The data reported in the present studies support these observations, demonstrating that the increase in Rho activity occurs in intact vascular smooth muscle depolarized with KCl, with a time-course corresponding to KCl-induced tonic contraction.
The involvement of Rho kinase in contraction mediated by KCl is suggested based on findings with HA-1077, which was equally effective at inhibiting both the agonist- and KCl-induced tonic contractions. The concentrations of HA-1077 required for 50% inhibition of the contractions corresponded to the Ki value (0.4 μM) reported for purified Rho kinase (Uechata et al., 1997) and, therefore, the observed effects of this agent are consistent with the involvement of the enzyme in the contraction.
SV inhibited both Rho activation and tonic contraction mediated by KCl, suggesting that the effects of the drug are due to the inhibition of the Rho/Rho kinase pathway.
Although the mechanism by which the statin inhibits the Rho/Rho kinase pathway is not clear, there is a possibility that the inhibition may arise from the ability of the drug to attenuate Ca2+ influx. Consistent with this suggestion is the observation that the selective blocker of L-type VDCC, nimodipine, which inhibits Ca2+ influx via the channel, mimicked the effects of the statin on Rho activation and contraction mediated by depolarization with KCl. Support for this mechanism includes evidence that lipophilic statins inhibit L-type current and attenuate contraction in cerebral vascular smooth muscle (Bergdahl et al., 2003). The effects of statins on L-type current also have been demonstrated in other cell types (Yada et al., 1999). Further support is provided by the observation demonstrating that Rho activation and Rho kinase-mediated contraction induced by depolarization with KCl are both Ca2+-dependent (Sakurada et al., 2003). Collectively, these findings suggest that, in addition to the activation of MLCK, KCl-mediated Ca2+ influx activates the Rho/Rho kinase pathway that is responsible for Ca2+ sensitization of VSM and the development of tonic contraction.
On the other hand, failure of nimodipine to inhibit Rho activation and contraction induced by ET-1 would point to the existence of a mechanism other than that involving inhibition of Ca2+ influx via L-type VDCC. ET-1 has been shown to activate Rho via the G12/G13-dependent mechanism involving the guanine–nucleotide exchange factors (GEFs) (Kozasa et al., 1999; Göhla et al., 2000). Thus, it is conceivable that the statin could produce its effects via the G-protein-dependent mechanisms. It is also likely that, in addition to the G12/G13-dependent regulation of Rho activation, receptor-dependent Ca2+ elevation plays a role in this process, as suggested for NA-induced Rho activation in VSM (Sakurada et al., 2003). Consistent with this notion, previous studies demonstrated that statins were able to influence both Ca2+ mobilization and influx in response to receptor agonists, angiotensin II and vasopressin (Escobales et al., 1996; Tesfamariam et al., 1999).
The effectiveness of SV is probably due to its lipophilicity because hydrophilic pravastatin did not have any major effect on vascular function of ET-1. This notion is consistent with previous data demonstrating that lipophilic statins were effective at inhibiting Ca2+ influx and contraction in cerebrovascular smooth muscle, whereas pravastatin failed to produce these effects (Bergdahl et al., 2003).
Although the vasorelaxant effects of SV were partially reversed by a prolonged treatment with mevalonate, the rapid time-course of the response renders unlikely the possibility that significant alterations in isoprenoid or cholesterol synthesis play a role in the effects of this agent on Rho activation. This contention is consistent with recent reports showing that mevalonate failed to restore the acute vasodilatory effects of statins (Bergdahl et al., 2003; Mukai et al., 2003).
The present studies also have shown that vasorelaxation mediated by SV in preparations contracted with the agonists is due, in part, to the action of this agent on voltage-dependent potassium (Kv) channels. The involvement of the channels in the effects of the statin is suggested based on findings with the selective inhibitor of the channel, 4-AP, which suppressed the ability of SV to produce the relaxations. This observation suggests that the statin caused vasorelaxation, in part, via activation of Kv channels and VSM cell membrane hyperpolarization. Consistent with this suggestion are recent studies demonstrating that other statins, cerivastatin and fluvastatin, produced vascular relaxation by activating Kv channels (Mukai et al., 2003). Although endothelial denudation enhanced the ability of 4-AP to reduce relaxation mediated by SV, it did not abolish the relaxation, suggesting that vascular smooth muscle hyperpolarization, conceivably induced by the statin, is independent of the endothelium. Previous studies have shown that, in vascular smooth muscle, ET-1 inhibits 4-AP-sensitive K+ currents of Kv1.4 and Kv4.3 channels through the PKC-dependent phosphorylation of the channels (Shimoda et al., 2001; Hagiwara et al., 2003). The ability of NA to modulate Kv channel activity in vascular smooth muscle also has been demonstrated (Berg, 2002). Thus, it is conceivable that the statin inhibits the ET-1- and NA-mediated contractions, at least in part, via activation of Kv channels. Whether SV does so directly, at the level of the channels, or indirectly, via modulating of cellular events, remains to be established.
Another significant observation to emerge from the present studies is that SV was capable of attenuating ET-1-stimulated DNA synthesis at concentrations much lower than those used in previous studies (Hernández-Perera et al., 1998) to inhibit ET-1 synthesis in endothelial cells. The ability of the statin to prevent DNA synthesis most likely arises from the inhibition of Rho geranylgeranylation, a post-translational modification that targets this protein to the plasma membrane and thus promotes its activation through GDP/GTP exchange (Hall, 1998). This notion is supported by the observations that the statin mimicked the effects of a selective inhibitor of geranylgeranyl transferase, GGTI-297, and prevented Rho translocation mediated by ET-1 in quiescent cells. Furthermore, HA-1077 mimicked the effects of the statin on ET-1-induced DNA synthesis, suggesting that Rho kinase is also required for this process. Together, these findings indicate an important role of Rho and Rho kinase in ET-1-stimulated mitogenesis, and are consistent with previous studies showing that statins attenuated DNA synthesis in human aortic smooth muscle cells stimulated with serum and platelet-derived growth factor (PDGF) via inhibition of Rho geranylgeranylation (Laufs et al., 1999). There is evidence to indicate that Rho brings about changes in DNA synthesis by upregulation of G1 phase cyclins and degradation of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1, a potent inducer of the G1 checkpoint (Laufs et al., 1999). The subsequent activation of Cdks induces phosphorylation of retinoblastoma (Rb) protein, leading to increased transcription of S-phase cell cycle genes. Treatment with statins decreases Rho geranylgeranylation, inhibits Cdk activity and Rb phosphorylation, and blocks PDGF- or serum-induced smooth muscle cell DNA synthesis by preventing the Rho-dependent downregulation of p27Kip1 (Laufs et al., 1999). Statins, therefore, prevent membrane association of Rho and disrupt its signaling to the downstream effectors including Rho kinase. The involvement of Rho kinase in smooth muscle mitogenesis was previously suggested based on findings with the selective inhibitor of Rho kinase, Y-27632 (Seasholtz et al., 1999; Sawada et al., 2000). Inhibition of Rho kinase with Y-27632 reversed the decrease in levels of p27Kip1, thereby suppressing DNA synthesis and, consequently, reducing cell proliferation (Sawada et al., 2000). Furthermore, the overexpression of dominant-negative Rho kinase suppressed the PDGF and lysophosphatidic acid-induced DNA synthesis in human aortic smooth muscle cells (Sawada et al., 2000), indicating the role of the enzyme in the control of cell proliferation. It also has been demonstrated that inhibition of Rho or Rho kinase prevents thrombin-stimulated DNA synthesis in aortic smooth muscle cells, providing evidence that this process is dependent on the Rho/Rho kinase pathway (Seasholtz et al., 1999). Together, these findings indicate that Rho and Rho kinase negatively regulate p27Kip1 expression and suggest that statins prevent this effect by blocking Rho geranylgeranylation. Further studies are needed to determine whether the effect of the statin on DNA synthesis stimulated by ET-1 is due to a blockade of the Rho/Rho kinase-dependent downregulation of p27Kip1.
The potential clinical importance of our studies is underscored by the finding that SV inhibited the effects of ET-1 at considerably lower concentrations than those previously reported to inhibit the prepro-ET-1 expression and ET-1 synthesis, and close to what can be achieved clinically. This suggests that lipophilic statins may be more effective in attenuating vascular events mediated by ET-1 than in modulating its synthesis. Although the reported therapeutic plasma concentrations of statins (0.01–0.1 μM) (Neuvonen et al., 1998) are lower than those shown to inhibit the vasorelaxation and DNA synthesis in the present studies, it is likely that these effects may play a role in clinical settings because human vascular smooth muscle cells are characterized by higher sensitivity to statins than those of experimental animals (Corsini et al., 1993). In addition, statins interact with other drugs, which can elevate their bioavailability. The major mechanism for this interaction includes the inhibition of cytochrome P-450 enzyme CYP3A4, resulting in a reduction of drug metabolism and a subsequent elevation of drug plasma levels. The concomitant administration of statins with CYP3A4 inhibitors, which include immunosuppressants, antifungal agents, macrolide antibiotics, antidepressants and agents such as grapefruit juice, can alter the pharmacokinetic parameters and thus produce a large increase in the plasma concentration of statins.
In conclusion, the present studies show that SV inhibits both vasoconstriction and DNA synthesis mediated by ET-1. The vasorelaxation is mediated by multiple mechanisms, whereas the inhibition of DNA synthesis is exerted by the statin via a mechanism involving inhibition of the Rho/Rho kinase pathway. We suggest that clinical benefits of statins may result, in part, from the effects of these agents on vascular function of ET-1.
Acknowledgments
These studies were supported by a grant-in-aid from the Heart and Stroke Foundation of Canada (to B. Vollrath).
Abbreviations
- 4-AP
4-aminopyridine
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- GGTI-297
geranylgeranyl transferase I inhibitor
- HA-1077
(5-isoquinolinesulfonyl) homopiperazine
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl-CoA reductase
- Kv
voltage-dependent potassium channel
- L-NAME
L-NG nitroarginine methyl ester
- MLC20
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- NA
noradrenaline
- NO
nitric oxide
- SV
simvastatin
- VDCC
voltage-dependent calcium channel
References
- ÁLVAREZ DE SOTOMAYOR M., HERRERA M.D., MARHUENDA E., ANDRIANTSITOHAINA R. Characterization of endothelial factors involved in the vasodilatory effects of simvastatin in aorta and small mesenteric artery of the rat. Br. J. Pharmacol. 2000;131:1179–1187. doi: 10.1038/sj.bjp.0703668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYMAN S., WALLACE P., WAYMAN C.P., GIBSON A., MCFADZEAN I. Receptor-independent activation of Rho-kinase-mediated calcium sensitisation in smooth muscle. Br. J. Pharmacol. 2003;139:1532–1538. doi: 10.1038/sj.bjp.0705394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG T. Analysis of the pressor response to the K+ channel inhibitor 4- aminopyridine. Eur. J. Pharmacol. 2002;452:325–337. doi: 10.1016/s0014-2999(02)02306-3. [DOI] [PubMed] [Google Scholar]
- BERGDAHL A., PERSSON E., HELLSTRAND P., SWÄRD K. Lovastatin induces relaxation and inhibits L-type Ca2+ current in the rat basilar artery. Pharmacol. Toxicol. 2003;93:128–134. doi: 10.1034/j.1600-0773.2003.930304.x. [DOI] [PubMed] [Google Scholar]
- BOBIK A., GROOMS A., MILLAR J.A., MITCHELL A., GRINPUKEL S. Growth factor activity of endothelin in vascular smooth muscle. Am J Physiol. 1990;258:C408–C415. doi: 10.1152/ajpcell.1990.258.3.C408. [DOI] [PubMed] [Google Scholar]
- BOULANGER C., LUSCHER T.F. Release of endothelin from the porcine aorta: inhibition by endothelium-derived nitric oxide. J. Clin. Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN W.V. Novel approaches to lipid lowering: what is on the horizon. Am. J. Cardiol. 2001;87:23B–27B. doi: 10.1016/s0002-9149(01)01452-7. [DOI] [PubMed] [Google Scholar]
- CHEN S.J., CHEN Y.F., OPGENORTH T.J., WESSALE J.L., MENG Q.C., DURAND J., DICARLO V.S., OPARIL S. The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague–Dawley rats. J. Cardiovasc. Pharmacol. 1997;29:713–725. doi: 10.1097/00005344-199706000-00003. [DOI] [PubMed] [Google Scholar]
- CORSINI A., MAZZOTTI M., RAITERI M., SOMA M.R., GABBIANI G., FUMAGALLI R., PAOLETTI R. Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. Atherosclerosis. 1993;101:117–125. doi: 10.1016/0021-9150(93)90107-6. [DOI] [PubMed] [Google Scholar]
- ENDRES M., LAUFS U., HUANG Z., NAKAMURA T., HUANG O., MOSCOWITZ M.A., LIAO J.K. Stroke protection by 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCOBALES N., CASTRO M., ALTIERI P.I., SANABRIA P. Simvastatin releases Ca2+ from a thapsigargin-sensitive pool and inhibits InsP3-dependent Ca2+ mobilisation in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 1996;27:383–391. doi: 10.1097/00005344-199603000-00011. [DOI] [PubMed] [Google Scholar]
- FULTON D., GRATTON J.P., MCCABE T.J. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÖHLA A., SCHULTZ G., OFFERMANNS S. Role for G12G13 in agonist-induced vascular smooth muscle contraction. Circ. Res. 2000;87:221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN J.L., BROWN M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA K., NUNOKI K., ISHII K., ABE T., YANAGISAWA T. Differential inhibition of transient outward currents of Kv1.4 and Kv4.3 by endothelin. Biochem. Biophys. Res. Commun. 2003;310:634–640. doi: 10.1016/j.bbrc.2003.09.062. [DOI] [PubMed] [Google Scholar]
- HALL A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- HERNÁNDEZ-PERERA O., PÉREZ-SALA D., NAVARRO-ANTOLIN J., SANCHEZ-PASCUALA R., HERNÁNDEZ G., DIAZ C., LAMAS S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J. Clin. Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ-PERERA O., PÉREZ-SALA D., SORIA E., LAMAS S. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ. Res. 2000;87:616–622. doi: 10.1161/01.res.87.7.616. [DOI] [PubMed] [Google Scholar]
- IGLARZ M., SCHIFFRIN E.T. Role of endothelin-1 in hypertension. Curr. Hypertens. Rep. 2003;5:144–148. doi: 10.1007/s11906-003-0071-4. [DOI] [PubMed] [Google Scholar]
- IHLING C., SZOMBATHY T., BOHRMANN B., BROCKHAUS M., SCHAEFER H.E., LOEFFLER B.M. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation. 2001;104:864–869. doi: 10.1161/hc3301.094742. [DOI] [PubMed] [Google Scholar]
- KAESEMEYER W.H., CALDWELL R.B., HUANG J., CALDWELL R.W. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J. Am. Coll. Cardiol. 1999;33:234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- KOZASA T., JIANG X., HART M.J., STERNWEIS P.M., SINGER W.D., GILMAN A.G., BOLLAG G., STERNWEIS P.C. P115RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1999;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- KUREISHI Y., LUO Z., SHIOJIMA I., BIALIK A., FULTON D., LEFER D.J., SESSA W.C., WALSH K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAN C., DAS D., WLOSKOWICZ A., VOLLRATH B. Endothelin-1 modulates hemoglobin-mediated signaling in cerebrovascular smooth muscle via RhoA/Rho kinase and protein kinase C. Am. J. Physiol. Heart. Circ. Physiol. 2004;286:H165–H173. doi: 10.1152/ajpheart.00664.2003. [DOI] [PubMed] [Google Scholar]
- LAUFS U., LIAO J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- LAUFS U., MARRA D., NODE K., LIAO J.K. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced downregulation of p27 (Kip1) J. Biol. Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., CAMPBELL B., SHIN Y-K, SCALIA R., HAYWARD R., LEFER D.J. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–184. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., SCALIA R., LEFER D.J. Vascular effects of HMG CoA-reductase inhibitors (statins) unrelated to cholesterol lowering: new concepts for cardiovascular disease. Cardiovasc. Res. 2001;49:281–287. doi: 10.1016/s0008-6363(00)00247-9. [DOI] [PubMed] [Google Scholar]
- LERMAN A., HOLMES D.R., BELL M.R., GARRATT K.N., NISHIMURA R.A., BURNETT J.C. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- MCGIRT M.J., LYNCH J.R., PARRA A., SHENG H., PEARLSTEIN R.D., LASKOWITZ D.T., PELLIGRINO D.A., WARNER D.S. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2957. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- MITA M., YANAGIHARA H., HISHINUMA S., SAITO M., WALSH M.P. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem. J. 2002;364:431–440. doi: 10.1042/BJ20020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKAI Y., SHIMOKAWA H., MATOBA T., HIROKI J., KUNIHIRO I., FUJIKI T., TAKESHITA A. Acute vasodilator effects of HMG-CoA reductase inhibitors. J. Cardiovasc. Pharmacol. 2003;42:118–124. doi: 10.1097/00005344-200307000-00018. [DOI] [PubMed] [Google Scholar]
- NEUVONEN P.J., KANTOLA T., KIVISTO K.T. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin. Pharmacol. Ther. 1998;63:332–341. doi: 10.1016/S0009-9236(98)90165-5. [DOI] [PubMed] [Google Scholar]
- REN X.-D., KIOSSES W.B., SCHWARTZ M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURADA S., TAKUWA N., SUGIMOTO N., WANG Y., SETO M., SASAKI Y., TAKUWA Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ. Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- SATO M., TANI E., FUJIKAWA H., KAIBUCHI K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ. Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- SAWADA N., ITOH H., UEYAMA K., YAMASHITA J., DOI K., CHUN T.H., INOUE M., MASATSUGU K., SAITO T., FUKUNAGA Y., SAKAGUCHI S., ARAI H., OHNO N., KOM M., NAKAO K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L., TOUYZ R.M. Vascular biology of endothelin. J. Cardiovasc. Pharmacol. 1998;32:S2–S13. [PubMed] [Google Scholar]
- SCHINI V.B., KIM N.D., VANHOUTTE P.M. The basal and stimulated release of EDRF inhibits the contractions evoked by endothelin-1 and endothelin-3 in aortae of normotensive and spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1991;17 Suppl 7:S267–S271. doi: 10.1097/00005344-199100177-00076. [DOI] [PubMed] [Google Scholar]
- SEASHOLTZ T.M., MAJUMDAR M., KAPLAN D.D., BROWN J.H. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ. Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- SEIFERT V., LÖFFLER B., ZIMMERMANN M., ROUX S., STOLKE D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage. Correlation with cerebral vasospasm, delayed ischemia, neurological deficit and volume of hematoma. J. Neurosurg. 1995;82:55–62. doi: 10.3171/jns.1995.82.1.0055. [DOI] [PubMed] [Google Scholar]
- SHEPHERD J., COBBE S.M., FORD I., ISLES C.G., LORIMER A.R., MACFARLANE P.W., McKILLOP J.H., PACKARD C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- SHIMODA L.A., SYLVESTER J.T., BOOTH G.M., SHIMODA T.H., MEEKER S., UNDEM SHAM J.S. Inhibition of voltage-gated K(+) currents by endothelin-1 in human pulmonary arterial myocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1115–L1122. doi: 10.1152/ajplung.2001.281.5.L1115. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAM B., FROHLICH B.H., GREGG R.E. Differential effects of pravastatin, simvastatin, and atorvastatin on Ca2+ release and vascular reactivity. J. Cardiovasc. Pharmacol. 1999;34:95–101. doi: 10.1097/00005344-199907000-00016. [DOI] [PubMed] [Google Scholar]
- TREASURE C.B., KLEIN J.L, WEINTRAUB W.S., TALLEY J.D., STILLABOWER M.E., KOSINSKI A.S., ZHANG J., BOCCUZZI S.J., CEDARHOLM J.C., ALEXANDER R.W. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary disease. N. Engl. J. Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- UECHATA M., ISCHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MACKAWA M., NARUMIYA S. Calcium sensitisation of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- URBAN N.H., BERG K.M., RATZ P.H. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitisation of arterial muscle. Am. J. Physiol. 2003;285:C1377–C1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- WICKMAN G., LAN C., VOLLRATH B. Functional roles of the Rho/Rho kinase pathway and protein kinase C in the regulation of cerebrovascular constriction mediated by hemoglobin. Circ. Res. 2003;92:809–816. doi: 10.1161/01.RES.0000066663.12256.B2. [DOI] [PubMed] [Google Scholar]
- WICKMAN G., NESSIM M.A., COOK D.A., VOLLRATH B. The polycationic aminoglycosides modulate the vasoconstrictive effects of endothelin: relevance to cerebral vasospasm. Br. J. Pharmacol. 2001;133:5–12. doi: 10.1038/sj.bjp.0704025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YADA T., NAKATA M., SHIRAISHI T., KAKEI M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insuline secretion due to blockade of L-type Ca 2+ channels in rat islet beta-cells. Br. J. Pharmacol. 1999;126:1205–1213. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]