Abstract
Synaptotagmin has been reported to function in clathrin-mediated endocytosis. Here, we investigated its involvement in agonist-stimulated internalization of M4 muscarinic acetylcholine receptors exogenously expressed in human embryonic kidney (HEK-293 tsA201) cells.
Synaptotagmin I was present at low levels in these cells, and when overexpressed resided at the plasma membrane.
Synaptotagmin overexpression alone did not affect receptor internalization, but ‘rescued' internalization that had been inhibited by either dominant-negative dynamin-1 or dominant-negative arrestin-2. Both normal and ‘rescued' internalization were sensitive to inhibitors of clathrin-mediated endocytosis, but not to inhibitors of the function of caveolae.
There was no increase in AP-2 recruitment to the plasma membrane in cells overexpressing synaptotagmin. However, a mutant form of the receptor lacking a potential AP-2 recruitment motif, while being internalized normally in response to agonist stimulation, was not rescued by synaptotagmin in cells expressing dominant-negative dynamin or arrestin.
A mutant form of synaptotagmin (K326,327A), which binds phosphatidylinositol-4,5-bisphosphate (PIP2) much more weakly than the wild-type protein, did not rescue internalization. Furthermore, internalization was inhibited by the PH domain of phospholipase C-δ1, which sequesters PIP2, and synaptotagmin was now unable to rescue.
We propose that AP-2 binding to the C-terminal tail of the receptor is not normally required for its endocytosis, but that the synaptotagmin-mediated rescue involves the formation of a ternary complex with the receptor and AP-2. PIP2 might play a role as an intermediary in the formation of this complex.
Keywords: Muscarinic receptor, internalization, synaptotagmin, arrestin, dynamin, AP-2, PIP2
Introduction
In unstimulated cells, G protein-coupled receptors are found predominantly at the plasma membrane (Koenig & Edwardson, 1997). Within seconds of agonist stimulation, the receptors are phosphorylated by G protein-coupled receptor kinases (Benovic et al., 1986), which increases the affinity of the receptor for cytosolic arrestins (Miller & Lefkowitz, 2001). Binding of arrestin to the receptor causes rapid uncoupling from its G protein, which results in homologous desensitization (Hausdorff et al., 1990). Over a longer time course, the receptor is usually endocytosed by clathrin-coated pits/vesicles, a process that modulates the responsiveness of the cell to agonists (Koenig & Edwardson, 1997). The trigger for endocytosis is again the binding of arrestin, which is able to bind to clathrin (Miller & Lefkowitz, 2001). Arrestin is also able to interact with the adaptor protein AP-2 (Laporte et al., 1999; 2000), which itself binds to clathrin (Schmid, 1997). In the case of the β2-adrenergic receptor, the binding of the receptor–arrestin complex to AP-2, rather than directly to clathrin, appears to be the crucial interaction (Laporte et al., 2000). The pinching off of the clathrin-coated pit to form a coated vesicle requires an additional protein, the GTPase dynamin (Schmid, 1997). Hence, the endocytosis of a G protein-coupled receptor in response to agonist stimulation involves the co-ordinated operation of a number of proteins. Furthermore, the phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) interacts with both AP-2 and dynamin (as well as other components of the endocytotic machinery), and is also likely to play a key role, perhaps by initiation of coated pit formation at the plasma membrane (Ford et al., 2001; Takei & Haucke, 2001).
In all, 13 synaptotagmin genes are known to be expressed in mammals, and six further sequences may represent additional isoforms (Craxton, 2001). These different isoforms are expressed at significant levels in many tissues (Li et al., 1995) and at various subcellular locations (Butz et al., 1999; Martinez et al., 2000). The best characterized isoform is the neuronal-specific synaptotagmin I (Augustine, 2001). Synaptotagmin I is present in synaptic vesicle membranes, where it is likely to function as the Ca2+ sensor for exocytosis at the nerve terminal (Augustine, 2001; Fernández-Chacón et al., 2001; Littleton et al., 2001). Synaptotagmin I spans the vesicle membrane once, and its cytoplasmic domain contains two C2 domains, both of which bind Ca2+ (Davletov & Südhof, 1993; Sutton et al., 1995; Desai et al., 2000; Ubach et al., 2001). The first C2 domain (C2A) binds phospholipids in a Ca2+-dependent manner (Davletov & Südhof, 1993; Chapman & Jahn, 1994), and is also required for the Ca2+-dependent binding of synaptotagmin to syntaxin (Li et al., 1995; Chapman et al., 1996; Davis et al., 1999). In contrast, the C2B domain mediates binding to the phosphoinositides PIP2 and phosphatidylinositol-3,4,5-trisphosphate (Schiavo et al., 1996; Bai et al., 2004), and also to AP-2 (Zhang et al., 1994). Synaptotagmin also exhibits two oligomerization properties – a Ca2+-dependent oligomerization reported to be mediated by the C2B domain (Chapman et al., 1996; Sugita et al., 1996) and a Ca2+-independent oligomerization mediated by the transmembrane region and the membrane-proximal region of the cytoplasmic domain (Brose et al., 1992; Bai et al., 2000; von Poser et al., 2000; Fukuda et al., 2001).
It has been reported that synaptotagmin I functions in endocytosis at the nerve terminal through the recruitment of AP-2 (Zhang et al., 1994). It has also been shown that tyrosine-based endocytotic motifs of membrane proteins can enhance the binding of AP-2 to the C2B domain of synaptotagmin (Haucke & De Camilli, 1999), suggesting a way in which coated pit formation may be coupled to the selection of ‘cargo' proteins. The significance of the C2B domain with respect to endocytosis is highlighted by the finding that its disruption appears to inhibit synaptic vesicle recycling at the nerve terminal in both Caenorhabditis elegans (Jorgensen et al., 1995) and Drosophila (Littleton et al., 2001).
In this study, we examined the effect of synaptotagmin on the agonist-stimulated internalization of a typical G protein-coupled receptor, the M4 muscarinic receptor for acetylcholine, which couples to adenylyl cyclase via Gi/Go (Koenig & Edwardson, 1994). We used both wild-type synaptotagmin I and a form containing a mutation (K326,327A) in its C2B domain, which has been reported almost to abolish AP-2 binding (Chapman et al., 1998; Desai et al., 2000). Both forms of synaptotagmin were tagged at their C-termini with green fluorescent protein (GFP) and expressed in human embryonic kidney (HEK)-293 tsA201 cells. We report that neither form of synaptotagmin has any significant effect on normal receptor internalization. However, internalization inhibited by either dominant-negative dynamin-1 or dominant-negative arrestin-2 can be rescued by wild-type synaptotagmin but not by the K326,327A mutant. This rescue appears to involve both a putative tyrosine-based endocytotic motif in the C-terminal tail of the receptor and the participation of PIP2.
Methods
Constructs
The sequence for the mouse M4 muscarinic acetylcholine receptor (originally provided by C.J. van Koppen, Essen, Germany), tagged at its N-terminus with the haemagglutinin epitope, was ligated into the BamHI site of the vector pCIN4 (Rees et al., 1996). The residue Y472 was mutated to A using the polymerase chain reaction, with the mutagenizing primers: 5′-CCTTTTGCTGTGCCAGGCTCGGAACATCGGCACAG-3′ (forward) and 5′-CTGTGCCGATGTTCCGAGCCTGGCACAGCAAAAGG-3′ (reverse). The introduction of the mutation was confirmed by DNA sequencing. A rat synaptotagmin I clone (Perin et al., 1990) was originally obtained from T.C. Südhof (Dallas, TX, U.S.A.), and the D374 mutation was restored to G374 (Desai et al., 2000). Wild-type and mutant (K326,327A) full-length synaptotagmin I, fused at their C-termini to GFP (De Angelis et al., 1998), were subcloned into the NheI and EcoRI sites of the vector pCINeo (Promega). Wild-type and mutant (K326,327A) full-length synaptotagmin I were also subcloned into the EcoRI and BamHI sites of the vector pECFP-C1 (Clontech). Residues 1–265 of synaptotagmin I were expressed in the vector pGEX-4T (Pharmacia) to produce a glutathione-S-transferase- (GST-) tagged protein. Wild-type and dominant-negative (amino acids 319–418) bovine arrestin-2 in the vector pcDNA3 (InVitrogen; Matharu et al., 2001), and wild-type and dominant-negative (K44A) human dynamin-1, both tagged at their N-termini with an HA epitope, also in pcDNA3 (Matharu et al., 2001) were obtained from Dr E. Kelly (Bristol, U.K.). The PH domain of phospholipase C-δ1 in the vector pEGFP-N1 (Clontech) was obtained from Dr R.D. Murrell-Lagnado (Cambridge, U.K.).
Cell culture
HEK-293 tsA201 cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were detached from the culture flasks for passaging every 2–4 days by brief (<2 min) incubation with trypsin (0.5 mg ml−1) and EDTA (0.2 mg ml−1) in phosphate-buffered saline. For experiments, cells were grown on either poly-D-lysine-treated (100 μg per well) 24-well plates or poly-D-lysine-treated glass coverslips.
Transient transfection of cells
HEK-293 tsA201 cells were transiently transfected with purified plasmids using a calcium phosphate transfection kit (Clontech). For mock transfections, plasmid DNA was omitted, but the procedure used was otherwise identical. Cells were seeded into 25 cm2 tissue culture flasks at approximately 50% confluence 24 h before transfection. DNA (9 μg) was mixed with the calcium phosphate reagents and incubated with the cells. After 8 h the cells were washed with warm phosphate-buffered saline and fresh medium was added to the flasks. After a further 16 h, the transfected cells were transferred either to 24-well plates (for radioligand binding) or to six-well plates containing poly-D-lysine-treated glass coverslips (for immunofluorescence). Experiments were then carried out after a further 24 h.
Immunoblot analysis of synaptotagmin and AP-2 in plasma membranes
Mock-transfected cells or cells transfected with either wild-type or K326,327A synaptotagmin I-GFP were lysed by incubation in hypotonic buffer (10 mM Tris–HCl, pH 7.0, containing 2 mM EDTA and a protease inhibitor cocktail) for 20 min at 4°C, followed by homogenization. A post-nuclear supernatant was prepared by centrifugation at 400 × g for 2 min. Membranes were collected by centrifugation at 21,000 × g for 15 min, and then boiled in SDS-sample buffer. Samples were analysed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting. Synaptotagmin I was detected by using mouse monoclonal antibody 41.1 (Tucker et al., 2003). Synaptotagmin I expression in the cells was estimated by quantitative immunoblotting, using GST-tagged recombinant synaptotagmin I (residues 1–265) as standard. AP-2 was detected using mouse monoclonal anti-α-adaptin antibody 100/2 (Sigma, Poole, U.K.). Immunoreactive bands were visualized using a horseradish peroxidase-conjugated goat anti-mouse secondary antibody, with enhanced chemiluminescence.
Determination of muscarinic receptor internalization
Cell-surface receptor number was quantitated through the binding of the membrane-impermeant radioligand [3H] N-methylscopolamine ([3H]NMS, specific activity 80 Ci mmol−1, concentration 0.4 nM; Amersham Pharmacia Biotech) to cells growing on 24-well plates, as described previously (Koenig & Edwardson, 1994; Madziva & Edwardson, 2001). All experiments were performed on cells that had been pretreated for 30 min at 37°C with cycloheximide (20 μg ml−1) to block protein synthesis. To measure receptor internalization, cells were incubated at 37°C with carbachol (1 mM) for 1 h. Nonspecific binding was determined in the presence of N-methylatropine (1 μM). Data are means±s.e. The statistical significance of differences between means was assessed using Student's t-test for unpaired data.
In additional experiments, the binding characteristics of the wild-type and Y472A receptor under various circumstances were determined through the displacement of the specific binding of [3H]NMS (concentration 0.4 nM; temperature 4°C) by either unlabelled NMS or carbachol.
Confocal laser-scanning microscopy
Cells were processed for microscopy as described previously (Madziva & Edwardson, 2001). Dilutions of antibodies were as follows: rabbit polyclonal anti-C2A domain, 1 : 100; mouse monoclonal anti-haemagglutinin, 1 : 100; goat polyclonal anti-arrestin-2, 1 : 100; TRITC-conjugated anti-rabbit IgG, 1 : 20; FITC-conjugated anti-mouse IgG, 1 : 250; and Alexa 488-conjugated anti-goat IgG, 1 : 200. Cells were visualized using a Leica NT-TCS confocal laser-scanning microscope, using a × 100 objective lens with 1.0 numeric aperture. Images were collected with appropriate filters: GFP, FITC and Alexa 488 were excited using the 488 nm line of a krypton/argon laser and imaged with a 510–540 nm band-pass filter; TRITC was excited with the 568 nm line and imaged with a long-pass 590 nm filter.
Binding of AP-2 to bead-attached plasma membranes
Bead-attached inside-out plasma membranes were prepared essentially as described previously (Haucke & De Camilli, 1999). Briefly, HEK-293 tsA201 cells in suspension were incubated with poly-D-lysine-coated (10 mg ml−1) Affi-prep 10 acrylamide beads (BioRad) for 30 min at room temperature. The bead-attached cells were disrupted by sonication for 15 s, washed with 0.5 M Tris–HCl, pH 7.0 containing DTT (1 mM), and suspended in cytosolic buffer (25 mM HEPES, pH 7.4, 25 mM KCl, 2.5 mM magnesium acetate, 150 mM potassium glutamate). Membrane orientation was assessed through the accessibility of synaptotagmin-GFP to trypsin (0.1 mg ml−1; 30 min on ice) in the absence and presence of Triton X-100 (0.2%). AP-2 binding to the membranes was determined by incubation of the bead-attached membranes with crude rat brain cytosol, prepared as described previously (Haucke & De Camilli, 1999). Membranes were washed, and AP-2 association was determined by immunoblotting, as described above. In some cases, the membranes were treated with trypsin before incubation with cytosol; trypsin was inactivated with soybean trypsin inhibitor (0.2 mg ml−1) before the incubation.
Results
We first established which isoforms of synaptotagmin are endogenously expressed in HEK-293 tsA201 cells by immunoblotting crude membrane fractions obtained from the cells with antibodies specific for various synaptotagmin isoforms. The specificity of the antibodies has been confirmed by immunoblotting against recombinant synaptotagmin isoforms I–XI (Tucker et al., 2003). Of the antibodies tested, only those against synaptotagmins I (Figure 1a) and VII (data not shown) gave positive signals. Quantitative immunoblotting, using known amounts of recombinant synaptotagmin I (residues 1–265), revealed that 100 μg of membrane protein contains approximately 0.5 ng of synaptotagmin I (i.e. 5 ng synaptotagmin per mg protein).
Figure 1.
Expression and targeting of wild-type and K326,327A synaptotagmin I-GFP in HEK-293 tsA201 cells. (a) Quantitative immunoblot analysis of the expression level of endogenous synaptotagmin I (syt). GST-tagged recombinant synaptotagmin I (residues 1–265), and membrane preparations from mock-transfected cells, were analysed by SDS–PAGE and immunoblotting, using mouse monoclonal anti-synaptotagmin I antibody 41.1. Immunoreactive bands were visualized using a horseradish peroxidase-conjugated goat anti-mouse secondary antibody and enhanced chemiluminescence. The positions of molecular mass markers (kDa) are indicated on the right. (b, c) Quantitative immunoblot analysis of expression levels of synaptotagmin I in cells transfected with either wild-type (b) or K326,327A (c) synaptotagmin I-GFP. (d–g) Immunofluorescence detection of the intracellular localization of GFP-tagged synaptotagmin. Cells were transiently transfected with either wild-type (d, e) or K326,327A (f, g) synaptotagmin I-GFP, and fixed 24 h later. Synaptotagmin was visualized either through the fluorescence of GFP (d, f), or by immunofluorescence using a rabbit polyclonal anti-C2A domain antibody and a TRITC-conjugated secondary antibody (e, g). Scale bar: 10 μm.
Cells were transiently transfected with wild-type or K326,327A mutant synaptotagmin I-GFP, and expression of the proteins was assessed by quantitative immunoblotting of membrane fractions with an anti-synaptotagmin I antibody (Figure 1b, c). In transfected cells, both wild-type and mutant GFP-tagged proteins were detected as 90-kDa bands. At the exposure shown, the weak signal given by the endogenous protein cannot be seen. For both wild-type (Figure 1b) and K326,327A mutant protein (Figure 1c), 30 μg of membrane protein contains approximately 5 ng synaptotagmin I-GFP (i.e. 165 ng synaptotagmin per mg protein, or 33 times the amount of endogenous synaptotagmin I). Typical transfection efficiency was approximately 50% (Madziva & Edwardson, 2001); we therefore estimate that the overexpression of the transfected proteins was 60–70-fold.
The intracellular targeting of the transfected proteins was determined both through the fluorescence of GFP and by immunofluorescence using a rabbit polyclonal anti-C2A antibody. As shown in Figure 1d–g, the distribution of the two signals was identical, and both wild-type and mutant synaptotagmin were expressed almost exclusively at the plasma membrane. This intracellular sorting contrasts sharply with that normally seen in neurons and neuroendocrine cells, where synaptotagmin is delivered to synaptic vesicles and secretory granules, respectively (Matthew et al., 1981), but is in accord with the observed targeting of synaptotagmin to the plasma membrane in Chinese hamster ovary (CHO) cells (Jarousse & Kelly, 2001). We observed similar targeting using wild-type and mutant synaptotagmins tagged at their N-termini with cyan fluorescent protein (CFP; data not shown), indicating that plasma membrane targeting in HEK-293 tsA201 cells does not depend on the location of the tag. Similarly, a C-terminal GFP tag has previously been found not to interfere with the targeting of synaptotagmin VII to lysosomes in normal rat kidney cells (Martinez et al., 2000).
In mock-transfected cells, there was no specific binding of the radioligand [3H]NMS (data not shown), indicating the absence of endogenous muscarinic acetylcholine receptors. In contrast, after transfection of the cells with the M4 muscarinic receptor construct, a clear radioligand-binding signal (typically about 10,000 d.p.m. for a well containing about 1.5 × 105 cells) was detected. The affinity constant for NMS binding to the receptor, determined through the ability of unlabelled NMS to displace the specific binding of [3H]NMS, was 6.2 × 109 M−1, close to the value of 9.1 × 109 M−1 reported previously for M4 receptors endogenously expressed in NG108-15 cells (Koenig & Edwardson, 1994). Hence, [3H]NMS at a concentration of 0.4 nM, will occupy 71% of the receptors. Assuming a 50% transfection efficiency (above), it can be calculated that each transfected cell has about 600,000 receptors at its surface.
Incubation of the cells with the muscarinic agonist carbachol for 1 h caused a 37±2% (n=13) reduction of [3H]NMS binding, due to receptor internalization (Koenig & Edwardson, 1994). The extent of agonist-stimulated receptor internalization was not significantly affected by overexpression of either wild-type (43±3%, n=8) or K326,327A mutant (34±4%, n=8) synaptotagmin I-GFP (Figure 2). The affinity constant for the binding of carbachol to the receptor, determined through its ability to displace the specific binding of [3H]NMS, was 0.9 × 105 M−1 in the absence and 1.4 × 105 M−1 in the presence of wild-type synaptotagmin I-GFP. Hence, co-expression of synaptotagmin I-GFP with the receptor had only a minor effect on carbachol binding. The levels of receptor expression, as judged by [3H]NMS binding, varied somewhat between the different transfection conditions within each experiment; however, there were no systematic differences between experiments, indicating that expression of either wild-type or K326,327A mutant synaptotagmin I-GFP did not significantly affect receptor expression.
Figure 2.
Neither wild-type nor K326,327A synaptotagmin affects agonist-stimulated internalization of M4 muscarinic acetylcholine receptors. Cells were transiently transfected with M4 muscarinic receptor alone (n=13), or with receptor plus either wild-type (n=8) or K326,327A (n=8) synaptotagmin I-GFP (syt), and incubated with carbachol (1 mM) for 1 h at 37°C. The number of receptors remaining at the cell surface was determined through the binding of the membrane-impermeant radioligand [3H]NMS. The internalization of receptors was calculated from the reduction in radioligand binding relative to the binding in unstimulated cells. Note that the unstimulated cells were also transfected with receptor, or with receptor plus wild-type or K326,327A mutant synaptotagmin, as appropriate. Internalization was expressed as a percentage of the receptors originally present at the cell surface.
We next looked for an effect of synaptotagmin on receptor endocytosis that had been inhibited by manipulation of known components of the endocytotic machinery. Dynamin is essential for vesicle budding from the plasma membrane, probably because it acts as a ‘pinchase' that severs the neck of the bud (Schmid, 1997; Stowell et al., 1999). Agonist-stimulated endocytosis of several G protein-coupled receptors, including the muscarinic receptor, has been shown to be inhibited by overexpression of dominant-negative forms of dynamin, that fail to mediate the pinching reaction (Vögler et al., 1998; Werbonat et al., 2000). We therefore expressed both wild-type and a dominant-negative mutant form of dynamin-1 in our model system. The mutation (K44A) is in the first of three putative GTP-binding motifs, and appears to result in greatly reduced GTP-binding affinity and impaired GTPase activity (van der Bliek et al., 1993). As shown in Figure 3a, b, both forms of dynamin were delivered predominantly to the plasma membrane, where dominant-negative dynamin reduced carbachol-triggered internalization of the M4 receptor over 1 h of stimulation from 37±2% (n=13) to 3±4% (n=8; P<0.001; Figure 3c). Interestingly, expression of wild-type dynamin also produced a small but significant inhibition of receptor internalization (to 28±4, n=8; P<0.05), as reported previously (Lee et al., 1998), perhaps by titrating out other proteins essential for endocytosis. In cells expressing both dominant-negative dynamin and wild-type synaptotagmin I-GFP, internalization was not significantly different from internalization in cells expressing receptor alone (39±7%, n=8). In contrast, in cells expressing dominant-negative dynamin and K326,327A synaptotagmin I-GFP, internalization remained inhibited (2±5%, n=6; P<0.001 relative to cells expressing receptor alone). Hence, agonist-stimulated receptor internalization can be rescued by wild-type synaptotagmin, but not by the K326,327A mutant. This finding, of course, raises the question of what essential molecular property the mutant lacks.
Figure 3.
Wild-type but not K326,327A synaptotagmin rescues muscarinic acetylcholine receptor endocytosis inhibited by dominant-negative dynamin-1. (a, b) Intracellular localization of dynamin. Cells were transiently transfected with either wild-type (a) or dominant-negative (K44A; b) dynamin, and fixed 24 h later. Dynamin was visualized by immunofluorescence using a mouse monoclonal anti-HA antibody and an FITC-conjugated secondary antibody. Scale bars: 10 μm. (c) Effects of dynamin and synaptotagmin on receptor internalization. Cells were transiently transfected with either M4 muscarinic receptor alone (n=13), with receptor plus wild-type (n=8) or dominant-negative (K44A; n=8) dynamin, or with dominant-negative dynamin plus either wild-type (n=8) or K326,327A (n=6) synaptotagmin I-GFP (syt). Cells were incubated with carbachol (1 mM) for 1 h at 37°C, and receptor internalization was determined through the binding of [3H]NMS. *Significantly different from cells expressing receptor alone (P<0.05); ***P<0.001.
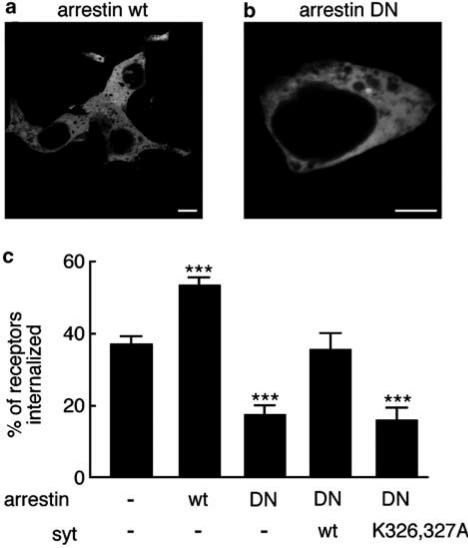
Another class of proteins that are widely involved in modulating the activity of G protein-coupled receptors are the arrestins. For instance, there is strong evidence that arrestins 2 and 3 are responsible for the recruitment of the β2-adrenergic receptor into clathrin-coated pits (Laporte et al., 1999; 2000). When we transfected cells with either wild-type arrestin-2 or a truncated form (residues 319–418) that binds to clathrin but interacts only weakly with phosphorylated receptors, we found that both forms of the protein were distributed evenly throughout the cytoplasm (Figure 4a, b). Arrestin-2 (319–418) significantly reduced carbachol-stimulated internalization of the M4 receptor over 1 h (from 37±2%, n=13, to 17±3%, n=4; P<0.001; Figure 4c), whereas wild-type arrestin enhanced receptor internalization (to 53±2, n=5; P<0.001). Co-expression of wild-type synaptotagmin I-GFP with dominant-negative arrestin again rescued receptor internalization (to 35±5%, n=8). The K326,327A mutant form of synaptotagmin, on the other hand, had no effect on internalization, which remained inhibited (15±4%, n=6; P<0.001 relative to cells expressing receptor alone).
Figure 4.
Wild-type but not K326,327A synaptotagmin rescues muscarinic acetylcholine receptor endocytosis inhibited by dominant-negative arrestin-2. (a, b) Intracellular localization of arrestin. Cells were transiently transfected with either wild-type (a) or dominant-negative (319–418; b) arrestin-2, and fixed 24 h later. Arrestin was visualized by immunofluorescence using a goat polyclonal antibody and an Alexa 488-conjugated secondary antibody. Scale bars: 10 μm. (c) Effects of arrestin and synaptotagmin on receptor internalization. Cells were transiently transfected with M4 muscarinic receptor alone (n=13), with receptor plus either wild-type (n=5) or dominant-negative (319–418; n=4) arrestin-2, or with dominant-negative arrestin plus either wild-type (n=8) or K326,327A (n=6) synaptotagmin I-GFP (syt). Cells were incubated with carbachol (1 mM) for 1 h at 37°C, and receptor internalization was determined through the binding of [3H]NMS. ***Significantly different from cells expressing receptor alone (P<0.001).
In order to establish whether receptor internalization under conditions of synaptotagmin rescue was occurring via the same route as that followed under normal conditions, we first compared the time courses of control and rescued internalization. As shown in Figure 5a, the time courses of internalization, at least up to 30 min, were very similar. To obtain further information about the internalization process under the different conditions, we tested the effects of agents that had been shown previously to delineate routes used by receptors for entry into the cell. Both methyl-β-cyclodextrin and sucrose have been reported to inhibit endocytosis via clathrin-coated pits (Hansen et al., 1993; Holroyd et al., 1999; Subtil et al., 1999). Methyl-β-cyclodextrin acts by depleting the plasma membrane of cholesterol, a lipid that is required for the formation of clathrin-coated pits. In contrast, sucrose ‘freezes' the pits at the membrane and inhibits their budding. The polyene antibiotic nystatin is a specific blocker of caveolae-mediated trafficking, and inhibits the endocytosis of agents such as cholera toxin and SV40 viral antigen (Shin & Abraham, 2001). The effects of these three agents on M4 muscarinic receptor internalization under both control and ‘synaptotagmin rescue' conditions were tested. As shown in Figure 5b, sucrose (0.6 M) and methyl-β-cyclodextrin (10 mM) inhibited receptor internalization by approximately the same extent (50–75%) under all conditions. In contrast, nystatin (5 μg ml−1) had no inhibitory effect. These results indicate that both control and rescued receptor internalization occur via clathrin-coated pits, and do not involve caveolae. We found no effect of sucrose, methyl-β-cyclodextrin or nystatin on the extent of [3H]NMS binding to cells expressing the M4 receptor (data not shown). Furthermore, the affinity constants for the binding of carbachol to the receptor, as determined through its ability to displace the specific binding of [3H]NMS, were similar under the four conditions (control, 1.0 × 105 M−1; sucrose, 1.6 × 105 M−1; methyl-β-cyclodextrin, 0.4 × 105 M−1; nystatin, 1.0 × 105 M−1).
Figure 5.
Normal and ‘rescued' internalization of the M4 muscarinic receptor have similar time courses, and are sensitive to inhibitors of clathrin-mediated endocytosis, but not to inhibitors of the function of caveolae. (a) Time course of internalization. Cells were transiently transfected with either M4 muscarinic receptor alone, or with receptor plus dominant-negative (K44A) dynamin or dominant-negative arrestin and wild-type synaptotagmin I-GFP (n=3). Cells were incubated with carbachol (1 mM) for various times at 37°C. Receptor internalization was determined through the binding of [3H]NMS. (b) Effects of various agents on receptor internalization. Cells were transiently transfected with either M4 muscarinic receptor alone (n=13), with receptor plus wild-type synaptotagmin I-GFP (syt; n=3), or with receptor plus either dominant-negative (K44A) dynamin or dominant-negative arrestin and wild-type synaptotagmin I-GFP (n=3). Cells were incubated with carbachol (1 mM) for 1 h at 37°C in the presence of the indicated agents. Sucrose (0.6 M) was added at the same time as carbachol. Methyl-β-cyclodextrin (MβCD; 10 mM) was present for 30 min before the addition of carbachol. Nystatin (5 μg ml−1) was present for 3 h before addition of carbachol. Receptor internalization was determined through the binding of [3H]NMS. *Significantly different from cells expressing receptor alone P<0.05; **P<0.01; ***P<0.001.
Since synaptotagmin has been reported to act as a membrane receptor for AP-2, we speculated that the synaptotagmin rescue of internalization of the M4 receptor might be mediated through an increased recruitment of AP-2 to the plasma membrane. In support of this idea, it had already been reported that synaptotagmin binds AP-2 via its C2B domain (Zhang et al., 1994), and also that the K326,327A mutation almost abolishes this binding (Chapman et al., 1998; Desai et al., 2000). We tested this possibility in two ways: by measuring the amount of AP-2 bound to membranes isolated from the cells, and by comparing the abilities of bead-attached, AP-2-free membranes prepared from the cells to bind AP-2 from a cytosolic extract. The results of these experiments are shown in Figure 6. First, we found that there was no difference in the amount of AP-2 bound to membranes prepared from control cells and from cells overexpressing either wild-type or K326,327A mutant synaptotagmin I-GFP (Figure 6a). We then prepared bead-attached inside-out plasma membranes as described by Haucke & De Camilli (1999). To confirm the orientation of the membranes, the accessibility of synaptotagmin I-GFP to added protease was tested. As shown in Figure 6b, the majority of the protein was accessible to trypsin, even in the absence of Triton X-100; in the presence of detergent, all of the protein was cleaved. We next showed that the membranes from control cells bound AP-2 from rat brain cytosol, and that binding was sensitive to prior treatment of the membranes with trypsin (Figure 6c), as reported previously (Zhang et al., 1994; Haucke & De Camilli, 1999). Finally, we found that the bead-attached membranes from control cells and from cells overexpressing either wild-type or K326,327A mutant synaptotagmin I-GFP bound approximately equal amounts of AP-2 (Figure 5d). Hence, AP-2 binding to the membranes requires a protein receptor, but the presence of exogenous synaptotagmin does not affect the extent of binding. Taken together, these results argue strongly against the idea that synaptotagmin exerts its effects on receptor internalization by increasing the bulk recruitment of AP-2 to the plasma membrane.
Figure 6.
Synaptotagmin overexpression does not affect AP-2 binding to the plasma membrane. (a) Effect of synaptotagmin (syt) overexpression on AP-2 association with the plasma membrane. Membrane preparations from either untransfected cells, or cells that had been transfected with wild-type or K326,327A synaptotagmin I-GFP, were immunoblotted using a monoclonal anti-α-adaptin antibody. Immunoreactive bands were visualized by enhanced chemiluminescence. (b) Orientation of bead-attached membranes. Bead-attached membranes prepared from cells overexpressing wild-type synaptotagmin I-GFP were treated with either pre-inhibited or active trypsin, either in the absence or presence of Triton X-100. Synaptotagmin was detected by immunoblotting, using a rabbit polyclonal anti-C2A antibody. (c) Trypsin sensitivity of AP-2 binding to membranes. Bead-attached membranes from untransfected cells were washed, treated with either pre-inhibited or active trypsin, and incubated with either rat brain cytosol or cytosolic buffer. The amount of AP-2 bound to the membranes was determined by immunoblotting, using a rabbit polyclonal anti-α-adaptin antibody. (d) Effect of synaptotagmin overexpression on AP-2 binding to membranes. Bead-attached membranes from either untransfected cells, or from cells overexpressing either wild-type or K326,327A mutant synaptotagmin I-GFP, were washed and incubated with either rat brain cytosol or cytosolic buffer. AP-2 recruitment to the membranes was determined as in (c).
It has been shown previously that the presence of peptides carrying the endocytotic motif YXXØ, where X is any amino acid and Ø is a bulky hydrophobic residue, stimulate the in vitro interaction between synaptotagmin and AP-2, indicating that the three molecules are able to form a ternary complex, which has a role in the endocytosis of proteins carrying this motif (Haucke & De Camilli, 1999). Interestingly, the carboxy-terminal tail of the M4 receptor has such a motif (YRNI), suggesting that the receptor might interact directly with AP-2 in the presence of synaptotagmin. To test the functional significance of this motif, we mutated Y472 to A, and examined the behaviour of the mutant receptor in response to agonist stimulation. The affinity constant for the binding of carbachol to the Y472A receptor, as determined through its ability to displace the specific binding of [3H]NMS, was 0.7 × 105 M−1, very similar to that for the wild-type receptor (0.9 × 105 M−1), and there was no discernible effect of the mutation on the level of receptor expression. As shown in Figure 7, the mutant receptor was internalized to the same extent (42±3%, n=3) as the wild-type receptor (43±3, n=8) in response to carbachol stimulation. This result indicates that direct binding of AP-2 to the receptor, at least at this site, is unlikely to be involved in normal agonist-stimulated internalization. Dominant-negative dynamin and dominant-negative arrestin both inhibited internalization of the mutant receptor, to 12±1% (n=3) and 19±4% (n=3), respectively. However, internalization of the Y472A receptor was no longer rescued when wild-type synaptotagmin I-GFP was co-expressed. Only 16±9% (n=3) of the receptors were internalized in the presence of synaptotagmin and dominant-negative dynamin, and 16±8% (n=3) were internalized in the presence of synaptotagmin and dominant-negative arrestin. It appears then that AP-2 recruitment to the receptor might in fact be involved in synaptotagmin-mediated rescue, even though bulk recruitment of AP-2 to the plasma membrane via synaptotagmin does not occur.
Figure 7.
Mutation of a putative AP-2 recruitment motif in the tail of the M4 muscarinic receptor abrogates the ability of synaptotagmin to rescue internalization. Cells were transiently transfected with either Y472A M4 muscarinic receptor alone, with receptor plus dominant-negative (K44A) dynamin or dominant-negative arrestin, or with dominant-negative dynamin/arrestin plus wild-type synaptotagmin I-GFP (syt). Cells were incubated with carbachol (1 mM) for 1 h at 37°C, and receptor internalization was determined through the binding of [3H]NMS (n=3). *Significantly different from cells expressing receptor alone (P<0.05); **P<0.01; ***P<0.001.
The lack of effect of the K326,327A synaptotagmin mutant, which binds AP-2 only very poorly (Chapman et al., 1998; Desai et al., 2000), lends further support to the idea that an interaction between AP-2 and synaptotagmin is involved in the rescue of receptor internalization. Since synaptotagmin binds PIP2 via its C2B domain (Schiavo et al., 1996; Bai et al., 2004), and the K326,327A mutation dramatically reduces this PIP2 binding (Supplementary Information in Bai et al., 2004), we speculated that PIP2 might be involved in the effects of synaptotagmin on the trafficking of the M4 receptor, and that the K326,327A mutation might render it unable to interact with AP-2 (and consequently nonfunctional) by blocking its ability to bind PIP2. We therefore investigated the involvement of PIP2 in the internalization of the M4 receptor. A standard means of sequestering PIP2 in the plasma membrane is through expression of the PH domain of phospholipase C (PLC). When we expressed the PH domain of PLC-δ1 (fused to GFP at its C-terminus) in our model system, the protein was almost exclusively localized to the plasma membrane (Figure 8a). When agonist-stimulated internalization of the M4 receptor was measured in cells expressing the PH domain, it was found to be reduced from 47±3% (n=6) over 30 min to 28±3% (n=6; P<0.01; Figure 8b). Furthermore, internalization was no longer rescued by either wild-type (29±3%, n=6) or K326,327A (29±3%, n=6) synaptotagmin I-GFP. Over a 60-min internalization period, the effect of the PH domain on internalization was smaller (52±1%–36±4%, n=6) but still highly significant. Over this time period, too, neither wild-type (41±3%, n=6) nor K326,327A (37±2%, n=6) synaptotagmin I-GFP rescued internalization. These results suggest that synaptotagmin was unable to recruit PIP2 in the presence of the PH domain.
Figure 8.
Involvement of PIP2 in the functional effects of synaptotagmin. (a) Intracellular localization of PLC-δ1 PH domain-GFP. Cells were transiently transfected with PH domain-GFP, and fixed 24 h later. Scale bar: 10 μm. (b) Effects of the PH domain and synaptotagmin on M4 receptor internalization. Cells were transiently transfected with M4 muscarinic receptor alone, with receptor plus PH domain, or with PH domain plus either wild-type or K326,327A synaptotagmin I-GFP (syt). Cells were incubated with carbachol (1 mM) for either 30 or 60 min at 37°C, and receptor internalization was determined through the binding of [3H]NMS (n=6). **Significantly different from cells expressing receptor alone (P<0.01; ***P<0.001).
Discussion
We have shown that neither wild-type nor K326,327A synaptotagmin I-GFP has any significant effect on the normal internalization of the M4 muscarinic receptor, but that internalization inhibited by either dominant-negative dynamin-1 or dominant-negative arrestin-2 can be rescued by wild-type synaptotagmin I-GFP but not by the K326,327A mutant. The role of arrestin in M4 receptor internalization has been controversial. For example, it was reported that arrestin-2 (319–418), the truncated form used in the present study, had no effect on the internalization of M1, M3 or M4 muscarinic receptors (Lee et al., 1998). In contrast, Vögler et al. (1999) found that both arrestin-2 (319–418) and another arrestin-2 mutant (V53D) had a dominant-negative effect on M4 receptor internalization. Our results agree with those of Vögler et al. The discrepancy between our results and those of Lee et al. might be explained by differences in receptor expression levels. We have found that under conditions of high receptor expression, such as those occurring in our experiments, receptor internalization is extremely sensitive to the changes in the availability of functional arrestin. For instance, concomitant stimulation of another G protein-coupled receptor co-expressed in the cells (the thyrotropin releasing hormone receptor) reduces the carbachol-stimulated internalization of the M4 muscarinic receptor through competition for the endogenous pool of arrestin (M.T. Madziva & J.M. Edwardson, unpublished observations). It is possible that the cells used by Lee et al. (1998) expressed fewer receptors, so that the reduction in function of the endogenous arrestin-2 caused by overexpression of the 319–418 form was not great enough to compromise receptor internalization.
The observation that the Y472A mutation in the carboxy-terminal tail of the M4 muscarinic receptor does not affect its normal internalization but completely abrogates the synaptotagmin-mediated rescue indicates that the mechanisms underlying the two forms of internalization are distinct, and also argues strongly against a significant role for endogenous synaptotagmin in the internalization of this receptor. Our results are consistent with the possibility that under normal circumstances internalization depends on an interaction between a receptor–arrestin complex and AP-2, which in turn mediates the binding of the complex to clathrin. The fact that the Y472A mutation will ablate a potential AP-2-binding site on the receptor further suggests that the synaptotagmin-mediated rescue of internalization might involve the binding of AP-2 to the receptor, perhaps in a ternary complex with synaptotagmin. We must emphasize, however, that we have no direct evidence for the formation of such a complex, and it is possible that the effect of synaptotagmin occurs through another, AP-2-independent, pathway. The involvement of direct AP-2 binding in the internalization of a G protein-coupled receptor (the α1b-adrenergic receptor) was in fact demonstrated for the first time recently (Diviani et al., 2003). However, in this case, the AP-2-binding site on the receptor consisted of a polyarginine sequence in the C-terminal tail, rather than a canonical sequence such as YXXØ.
The involvement of synaptotagmin in the constitutive internalization of the LDL receptor has been investigated previously (von Poser et al., 2000). This receptor is internalized through a mechanism that depends on the presence of an NPXY motif (NPVY in the human receptor) in the intracellular domain of the receptor. There is still considerable controversy as to the mechanism underlying LDL receptor internalization, although there is evidence that the NPXY sequence binds clathrin (Boll et al., 2002). Unlike the M4 muscarinic receptor used in the present study, there is no evidence that LDL receptor internalization involves arrestin. It was found that expression of C-terminally truncated forms of synaptotagmins I and VII in HeLa cells potently inhibited LDL uptake. In contrast, expression of the corresponding wild-type proteins was without effect, as we found in the present study. The inhibitory effect of truncated synaptotagmin VII was dependent on two intramembrane cysteine residues that were found to be required for synaptotagmin oligomerization (von Poser et al., 2000). It was proposed that the exogenous truncated constructs were interacting with endogenous synaptotagmin isoforms and blocking AP-2 recruitment. We cannot exclude the possibility that the lack of effect of the K326,327A mutant synaptotagmin I-GFP on the normal internalization of the M4 muscarinic receptor seen in our experiments might be caused by the failure of the GFP-tagged full-length protein to interact with the endogenous synaptotagmin isoforms, although it is not clear why the GFP-tagged protein would be deficient in this respect. It is perhaps more likely that under normal circumstances the endogenous synaptotagmin isoforms, present at low levels in the HEK-293 tsA201 cells, do not play a significant role in agonist-stimulated internalization of the M4 receptor, and that the effect of synaptotagmin is seen only when the protein is expressed at high levels and receptor internalization is compromised. Synaptotagmin I might operate in a similar way in the nerve terminal. It is expressed here at high levels (Brose et al., 1992) and there is a requirement for the rapid and efficient recycling of synaptic vesicle proteins that are not known to interact with arrestin (Zhang et al., 1994).
The effects of methyl-β-cyclodextrin and sucrose clearly indicate that both normal and rescued internalization of the M4 receptor proceed via clathrin-coated pits. How then is the overexpression of synaptotagmin able to overcome the block in internalization caused by overexpression of dominant-negative arrestin and dynamin? It is straightforward to envisage a situation in which the presence of dominant-negative arrestin interferes with the ability of the receptor to form a complex with endogenous wild-type arrestin, and thereby prevents the entry of the receptor into clathrin-coated pits. The presence of overexpressed synaptotagmin might then increase AP-2 recruitment to the receptor, restoring the ability of the receptor to bind to clathrin. It is more difficult to account for the ability of overexpressed synaptotagmin to rescue receptor internalization in the presence of dominant-negative dynamin, since the operation of dynamin is believed to be crucial to the scission of clathrin-coated pits. We can only speculate that the effect of synaptotagmin is to enhance the reduced level of internalization occurring in the presence of the dominant-negative dynamin in a manner analogous to the behaviour of high-copy suppressors in yeast, where, for example, overexpression of the SNAP-25 homologue Sec9 rescues the phenotype of a Sec4 GTPase effector domain mutant (Brennwald et al., 1994). In this case, the defect caused by the reduced activity of one protein is overcome by the presence of an excess of another protein acting at a different stage in the same process (i.e. membrane fusion).
Both synaptotagmin and the μ2 subunit of AP-2 have been shown to bind PIP2 (Schiavo et al., 1996; Rohde et al., 2002; Bai et al., 2004). Furthermore, the ability of α-adaptin to co-immunoprecipitate synaptotagmin from a brain detergent extract has been found to be enhanced in the presence of ATP and GTPγS (conditions that favour PIP2 synthesis), and dramatically reduced by the PIP2-binding reagent neomycin (Haucke & De Camilli, 1999). These results suggest that PIP2 might mediate the interaction between the two proteins. Our observations – that the K326,327A synaptotagmin mutant is unable to rescue receptor internalization in the presence of dominant-negative dynamin or arrestin, and that wild-type synaptotagmin cannot rescue internalization that has been inhibited by the PH domain of PLC-δ1 – are consistent with this idea. Of course, the fact that the PH domain reduces receptor internalization indicates that the normal mode of internalization also requires the availability of PIP2. Since it is known that several proteins crucial to the process of clathrin-mediated endocytosis, such as AP-2, AP-180 and dynamin, are recruited to the plasma membrane at least in part by binding to PIP2 (Ford et al., 2001; Takei & Haucke, 2001), this is perhaps unsurprising.
The results presented here beg the questions of whether the form of internalization revealed under conditions of synaptotagmin rescue is peculiar to the M4 muscarinic receptor, and whether it can be physiologically relevant. It is certainly the case that putative AP-2-binding motifs of the YXXØ type are present in the C-terminal tails of a number of other G protein-coupled receptors, such as the thyrotropin-releasing hormone receptor and the AT1 angiotensin receptor. It is also well established that the internalization characteristics of a particular G protein-coupled receptor vary significantly between cell types, raising the possibility that synaptotagmin might play a role in receptor internalization under some circumstances, particularly in cells with high synaptotagmin expression levels. Within the family of muscarinic receptors, the YXXØ motif is found in subtypes M2 and M4, which couple to Gi/Go, but not in M1, M3 and M5, which couple to Gq, and there are significant differences in the internalization characteristics of these two subtype groups. For instance, M2 and M4 receptors are internalized more efficiently than M1 and M3 receptors in CHO cells (Koenig & Edwardson, 1996). Intriguingly, when we expressed M1 receptors in HEK-293 tsA201 cells, we found that, in accordance with the prediction of the model developed above, internalization was inhibited by overexpression of either dominant-negative dynamin or arrestin but was not rescued by synaptotagmin (data not shown). In light of our findings, we suggest that the possibility that AP-2 binding to G protein-coupled receptors might be physiologically relevant warrants further investigation.
Acknowledgments
This work was supported by Project Grant 063920 from the Wellcome Trust and a Parke-Davis Exchange Fellowship (to J.M.E.), and grants from the National Institutes of Health (NIGMS GM 56827 and NIMH MH 61876), American Heart Association (9750326N), and the Milwaukee Foundation (to E.R.C.). M.T.M. was supported by an Overseas Research Studentship and a Cambridge Commonwealth Trust Studentship. J.B. was supported by an American Heart Association Pre-Doctoral Fellowship. We thank Dr R. Jahn (Göttingen, Germany) for providing the mouse monoclonal anti-synaptotagmin antibody 41.1, and the rabbit polyclonal anti-C2A domain antibody. We are grateful to Dr J.E. Rothman (New York, U.S.A.) for the GFP-tagging vector, to Dr E. Kelly (Bristol, U.K.) for the arrestin and dynamin constructs, and to Dr R. Murrell-Lagnado (Cambridge, U.K.) for the PH-domain of PLC-δ1, tagged with GFP. We also thank Dr V. Haucke (Göttingen, Germany), for advice on the preparation and use of the bead-attached membranes, and Mr Barney Leeke for excellent technical assistance.
Abbreviations
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- HBS
HEPES-buffered saline
- HEK
human embryonic kidney
- NMS
N-methylscopolamine
- PAGE
polyacrylamide gel electrophoresis
- PIP2
phosphatidylinositol-4,5-bisphosphate
References
- AUGUSTINE G.J. How does calcium trigger neurotransmitter release. Curr. Opin. Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- BAI J., EARLES C., LEWIS J., CHAPMAN E.R. Membrane-embedded synaptotagmin interacts with cis and trans target membranes and assembles into oligomers via a novel mechanism. J. Biol. Chem. 2000;275:25427–25435. doi: 10.1074/jbc.M906729199. [DOI] [PubMed] [Google Scholar]
- BAI J., TUCKER W.C., CHAPMAN E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- BENOVIC J.L., STRASSER R.H., CARON M.G., LEFKOWITZ R.J. Adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLL W., RAPOPORT I., BRUNNER C., MODIS Y., PREHN S., KIRCHHAUSEN T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppØ sorting signals at distinct sites. Traffic. 2002;3:590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- BRENNWALD P., KEARNS B., CHAMPION K., KERANEN S., BANKAITIS V., NOVICK P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- BROSE N., PETRENKO A.G., SÜDHOF T.C., JAHN R. Synaptotagmin: a Ca2+ sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- BUTZ S., FERNÁNDEZ-CHACÓN R., SCHMITZ F., JAHN R., SÜDHOF T.C. The subcellular localizations of atypical synaptotagmins III and IV. J. Biol. Chem. 1999;274:18290–18296. doi: 10.1074/jbc.274.26.18290. [DOI] [PubMed] [Google Scholar]
- CHAPMAN E.R., AN S., EDWARDSON J.M., JAHN R. A novel function for the second C2 domain of synaptotagmin. Ca2+-triggered dimerization. J. Biol. Chem. 1996;271:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- CHAPMAN E.R., DESAI R.C., DAVIS A.F., TORNEHL C.K. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J. Biol. Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- CHAPMAN E.R., JAHN R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- CRAXTON M. Genomic analysis of synaptotagmin genes. Genomics. 2001;77:43–49. doi: 10.1006/geno.2001.6619. [DOI] [PubMed] [Google Scholar]
- DAVIS A.F., BAI J., FASSHAUER D., WOLOWICK M.J., LEWIS J.L., CHAPMAN E.R. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- DAVLETOV B.A., SÜDHOF T.C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- DE ANGELIS D.A., MIESENBÖCK G., ZEMELMAN B.V., ROTHMAN J.E. PRIM: proximity imaging of green fluorescent protein-tagged polypeptides. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12312–12316. doi: 10.1073/pnas.95.21.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESAI R.C., VYAS B., EARLES C.A., LITTLETON J.T., KOWALCHYCK J.A., MARTIN T.F.J., CHAPMAN E.R. The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J. Cell Biol. 2000;150:1125–1135. doi: 10.1083/jcb.150.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIVIANI D., LATTION A.-L., ABUIN L., STAUB O., COTECCHIA S. The adaptor complex 2 directly interacts with the α1b-adrenergic receptor and plays a role in receptor endocytosis. J. Biol. Chem. 2003;278:19331–19340. doi: 10.1074/jbc.M302110200. [DOI] [PubMed] [Google Scholar]
- FERNÁNDEZ-CHACÓN R., KÖNIGSTORFER A., GERBER S.H., GARCÍA J., MATOS M.F., STEVENS C.F., BROSE N., RIZO J., ROSENMUND C., SÜDHOF T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- FORD M.G.J., PEARSE B.M.F., HIGGINS M.K., VALLIS Y., OWEN D.J., GIBSON A., HOPKINS C.R., EVANS P.R., MCMAHON H.T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- FUKUDA M., KANNO E., OGATA Y., MIKOSHIBA K. Mechanism of the SDS-resistant synaptotagmin clustering mediated by the cysteine cluster at the interface between the transmembrane and spacer domains. J. Biol. Chem. 2001;276:40319–40325. doi: 10.1074/jbc.M105356200. [DOI] [PubMed] [Google Scholar]
- HAUCKE V., DE CAMILLI P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 1999;285:1268–1271. doi: 10.1126/science.285.5431.1268. [DOI] [PubMed] [Google Scholar]
- HANSEN S.H., SANDVIG K., VAN DEURS B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSDORFF W.P., CARON M.G., LEFKOWITZ R.J. Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- HOLROYD E.W., SZEKERES P.G., WHITTAKER R.D., KELLY E., EDWARDSON J.M. Effect of G protein-coupled receptor kinase 2 on the sensitivity of M4 muscarinic acetylcholine receptors to agonist-induced internalization and desensitization in NG108-15 cells. J. Neurochem. 1999;73:1236–1245. doi: 10.1046/j.1471-4159.1999.0731236.x. [DOI] [PubMed] [Google Scholar]
- JAROUSSE N., KELLY R.B. The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis. J. Cell Biol. 2001;154:1–10. doi: 10.1083/jcb.200103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORGENSEN E.M., HARTWIEG E., SCHUSKE K., NONET M.L., JIN Y., HORVITZ H.R. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M. Kinetic analysis of the trafficking of muscarinic acetylcholine receptors between the plasma membrane and intracellular compartments. J. Biol. Chem. 1994;269:17174–17182. [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M. Intracellular trafficking of the muscarinic acetylcholine receptor: importance of subtype and cell type. Mol. Pharmacol. 1996;49:351–359. [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol. Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- LAPORTE S.A., OAKLEY R.H., HOLT J.A., BARAK L.S., CARON M.G. The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- LAPORTE S.A., OAKLEY R.H., ZHANG J., HOLT J.A., FERGUSON S.S.G., CARON M.G., BARAK L.S. The β2-adrenergic receptor/βarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl Acad. Sci. U.S.A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K.B., PALS-RYLAARSDAM R., BENOVIC J.L., HOSEY M. Arrestin-independent internalization of the m1, m3, and m4 subtypes of muscarinic cholinergic receptors. J. Biol. Chem. 1998;273:12967–12972. doi: 10.1074/jbc.273.21.12967. [DOI] [PubMed] [Google Scholar]
- LI C., ULLRICH B., ZHANG J.Z., ANDERSON R.G.W., BROSE N., SÜDHOF T.C. Ca2+-dependent and Ca2+-independent activities of neural and nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- LITTLETON J.T., BAI J., VYAS B., DESAI R., BALTUS A.E., GARMENT M.B., CARLSON S.D., GANETZKY B., CHAPMAN E.R. Synaptotagmin mutants reveal essential functions for the C2B-domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADZIVA M.T., EDWARDSON J.M. Trafficking of green fluorescent protein-tagged muscarinic M4 receptors in NG108-115 cells. Eur. J. Pharmacol. 2001;428:9–18. doi: 10.1016/s0014-2999(01)01266-3. [DOI] [PubMed] [Google Scholar]
- MARTINEZ I., CHAKRABARTI S., HELLEVIK T., MOREHEAD J., FOWLER K., ANDREWS N. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHARU A.-L., MUNDELL S.J., BENOVIC J.L., KELLY E. Rapid agonist-induced desensitization and internalization of the A2B adenosine receptor is mediated by a serine residue close to the COOH terminus. J. Biol. Chem. 2001;276:30199–30207. doi: 10.1074/jbc.M010650200. [DOI] [PubMed] [Google Scholar]
- MATTHEW W.D., TSAVALER L., REICHARDT L.F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER W.E., LEFKOWITZ R.J. Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- PERIN M.S., FRIED V.A., MIGNERY G.A., JAHN R., SÜDHOF T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory domain of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- REES S., COOTE J., STABLES J., GOODSON S., HARRIS S., LEE M.G. Bicistronic vector for the creation of stable mammalian cell lines that prediposes all antibiotic-resistant cells to express recombinant protein. Biotechniques. 1996;20:102–110. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- ROHDE G., WENZEL D., HAUCKE V. A phosphatidylinositol (4,5)-bisphosphate binding site within μ2-adaptin regulates clathrin-mediated endocytosis. J. Cell Biol. 2002;158:209–214. doi: 10.1083/jcb.200203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIAVO G., GU Q.-M., PRESTWICH G.D., SÖLLNER T.H., ROTHMAN J.E. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID S.L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- SHIN J.-S., ABRAHAM S.N. Caveolae as portals of entry for microbes. Microbes Infect. 2001;3:755–761. doi: 10.1016/s1286-4579(01)01423-x. [DOI] [PubMed] [Google Scholar]
- STOWELL M.H.B., MARKS B., WIGGE P., MCMAHON H.T. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat. Cell Biol. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- SUBTIL A., GAIDAROV I., KOBYLARZ K., LAMPSON M.A., KEEN J.H., MCGRAW T.E. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGITA S., HATA Y., SÜDHOF T.C. Distinct Ca2+-dependent properties of the first and second C2-domains of synaptotagmin I. J. Biol. Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- SUTTON R.B., DAVLETOV B.A., BERGHUIS A.M., SÜDHOF T.C., SPRANG S.R. Structure of the first C2 domain of synaptotagmin 1: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- TAKEI K., HAUCKE V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- TUCKER W.C, EDWARDSON J.M., BAI J., KIM H.-J., MARTIN T.F.J., CHAPMAN E.R. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell. Biol. 2003;162:199–209. doi: 10.1083/jcb.200302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UBACH J., LAO Y., FERNANDEZ I., ARAC D., SÜDHOF T.C., RIZO J. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry. 2001;40:5854–5860. doi: 10.1021/bi010340c. [DOI] [PubMed] [Google Scholar]
- VAN DER BLIEK A.M., REDELMEIER T.E., DAMKE H., TISDALE E.J., MEYEROWITZ E.M., SCHMID S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VÖGLER O., BOGATKEWITSCH G.S., WRISKE C., KRUMMENERL P., JAKOBS K.H., VAN KOPPEN C.J. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. J. Biol. Chem. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- VÖGLER O., NOLTE B., VOSS M., SCHMIDT M., JAKOBS K.H., VAN KOPPEN C.J. Regulation of muscarinic acetylcholine receptor sequestration and function by β-arrestin. J. Biol. Chem. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- VON POSER C., ZHANG J.Z., MINEO C., DING Y., YING Y., SÜDHOF T.C., ANDERSON R.G.W. Synaptotagmin regulation of coated pit assembly. J. Biol. Chem. 2000;275:30916–30924. doi: 10.1074/jbc.M005559200. [DOI] [PubMed] [Google Scholar]
- WERBONAT Y., KLEUTGES N., JAKOBS K.H., VAN KOPPEN C.J. Essential role of dynamin in internalization of M2 muscarinic acetylcholine and angiotensin AT1A receptors. J. Biol. Chem. 2000;275:21969–21974. doi: 10.1074/jbc.M001736200. [DOI] [PubMed] [Google Scholar]
- ZHANG J.Z., DAVLETOV B.A., SÜDHOF T.C., ANDERSON R.G.W. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]