Abstract
We have characterised the effects of piperine, a pungent alkaloid found in black pepper, on the human vanilloid receptor TRPV1 using whole-cell patch-clamp electrophysiology.
Piperine produced a clear agonist activity at the human TRPV1 receptor yielding rapidly activating whole-cell currents that were antagonised by the competitive TRPV1 antagonist capsazepine and the non-competitive TRPV1 blocker ruthenium red.
The current–voltage relationship of piperine-activated currents showed pronounced outward rectification (25±4-fold between −70 and +70 mV) and a reversal potential of 0.0±0.4 mV, which was indistinguishable from that of the prototypical TRPV1 agonist capsaicin.
Although piperine was a less potent agonist (EC50=37.9±1.9 μM) than capsaicin (EC50=0.29±0.05 μM), it demonstrated a much greater efficacy (approximately two-fold) at TRPV1.
This difference in efficacy did not appear to be related to the proton-mediated regulation of the receptor since a similar degree of potentiation was observed for responses evoked by piperine (230±20%, n=11) or capsaicin (284±32%, n=8) upon acidification to pH 6.5.
The effects of piperine upon receptor desensitisation were also unable to explain this effect since piperine resulted in more pronounced macroscopic desensitisation (t1/2=9.9±0.7 s) than capsaicin (t1/2>20 s) and also caused greater tachyphylaxis in response to repetitive agonist applications.
Overall, our data suggest that the effects of piperine at human TRPV1 are similar to those of capsaicin except for its propensity to induce greater receptor desensitisation and, rather remarkably, exhibit a greater efficacy than capsaicin itself. These results may provide insight into the TRPV1-mediated effects of piperine on gastrointestinal function.
Keywords: Piperine, vanilloid, TRPV, TRPV1, VR1, ion channel, electrophysiology, capsaicin, pain
Introduction
Piperine (1-peperoylpiperidine), the primary pungent alkaloid in black peppercorns derived from the fruit bodies of Piper nigrum, is commonly ingested in many diets throughout the world. In addition to its common utility as a gustatory enhancer, it has found many rather diverse applications ranging from brandy flavouring to its use as an insecticide (Budavari et al., 1989). It is also reported to have antioxidant activity yielding potentially beneficial results versus the damaging effects of a high-fat diet (Naidu & Thippeswamy, 2002; Vijayakumar et al., 2004) and is reported to exhibit antidiarrhoeal and gastroprotective properties in rodents (Bajad et al., 2001; Szolcsanyi & Bartho, 2001).
Immediately following ingestion, piperine is often described as tasteless; however, this initial impression is subsequently surpassed by a recognisable sharp, peppery, ‘burning' aftertaste. This burning sensation is thought to occur via the activation of the heat and capsaicin receptor TRPV1. Such claims are based on pioneering studies that demonstrated that piperine shares a common binding site with other natural plant products such as capsaicin and resiniferatoxin on the ‘vanilloid receptor' expressed in native tissue (Szolcsanyi, 1983; Patacchini et al., 1990; Szallasi & Blumberg, 1991). Subsequent supporting studies further defined a common site of action for piperine and capsaicin (Green, 1996) and demonstrated the ability of piperine to activate whole-cell currents in rat sensory neurones isolated from trigeminal ganglia (Liu & Simon, 1996a; reviewed by Szallasi & Blumberg, 1999).
Vanilloid receptor-1, which is now referred to as TRPV1, was the first member of the vanilloid (V) subgroup of transient receptor potential (TRP) channels to be identified at the molecular level (Caterina et al., 1997; reviewed by Gunthorpe et al., 2002). Many naturally occurring pungent compounds have now been demonstrated to be bona fide activators of TRPV1. These include the true vanilloid compounds (those bearing a 4-hydroxy-3-methoxybenzyl or ‘vanillyl' group) such as the cactus extract resiniferatoxin, the ginger extracts zingerone and gingerol, and the nutmeg and clove oil constituent eugenol (Szallasi & Blumberg, 1999; Dedov et al., 2002). Many nonvanilloids are also agonists at TRPV1 and include the triprenyl phenols such as the fungal extract scutigeral, many unsaturated dialdehyde sesquiterpenes (found in a diverse array of plants, fungi, insects and other animals) and piperine (Sterner & Szallasi, 1999). More recently other ligands, which may act as endogenous activators of TRPV1 (also referred to as ‘endovanilloids'), have been identified (see Van Der Stelt & Di Marzo (2004) for review). These include anandamide, N-arachidonyl dopamine, and eicosanoids such as 12-(S)-hydroperoxyeicosatetraenoic acid (Zygmunt et al., 1999; Hwang et al., 2000; Smart et al., 2000; Huang et al., 2002).
These discoveries point towards a broad agonist pharmacology for TRPV1 which, due to its heat and proton sensitivity (Caterina et al., 1997; Tominaga et al., 1998), is an exquisite molecular integrator of multiple chemical and physical stimuli to which the receptor is exposed. This, combined with the documented expression of TRPV1 in human sensory neurones involved in pain pathways and gastrointestinal (GI) function (Hayes et al., 2000; Ward et al., 2003), means that TRPV1 represents a good target for pharmaceutical intervention. At present, both agonist and antagonist strategies are thought to have merit for the treatment of a whole range of conditions ranging from inflammatory and neuropathic pain, to bladder dysfunction and irritable bowel syndrome (see Szallasi & Appendino (2004) for a review). Indeed with particular respect to the latter, it is noteworthy that recent reports also suggest that TRPV1 may actually be upregulated in such chronic conditions further contributing to the pathophysiology of such debilitating diseases (Chan et al., 2003; Schicho et al., 2004).
Given the interesting effects of piperine reported above, with the noted absence of experimental data on its activity at recombinant TRPV1, we have characterised the pharmacological and biophysical properties of piperine at human recombinant TRPV1 expressed in HEK293 cells using the patch-clamp technique and compared its activity to the prototypical agonist capsaicin. We find that the effects of piperine at human TRPV1 are consistent with it acting as an agonist at the receptor but that it shows a clear propensity to induce receptor desensitisation and, rather remarkably, a greater efficacy than capsaicin itself. Some of this work has previously been published in abstract form (McNamara et al., 2004).
Methods
Cloning and expression of human TRPV1
A human embryonic kidney cell line stably expressing human TRPV1 (hTRPV1.HEK293 cells) was generated as described previously (Hayes et al., 2000). Cells were cultured on plastic tissue culture dishes in modified Eagles's medium with Earle's salts and supplemented with 10% fetal bovine serum, nonessential amino acids and 0.2 mM L-glutamine while being maintained under 5% CO2 at 37°C. For electrophysiological experiments, cells were plated at a 30,000 cells cm−2 density onto 19 mm glass coverslips coated with poly-L-lysine with experiments being performed 24–48 h thereafter.
Electrophysiological techniques
Whole-cell patch-clamp experiments were performed according to standard methods, using an Axopatch 200B amplifier, as described previously (Hayes et al., 2000). Thick-walled borosilicate glass electrodes (GC120F10; Harvard Apparatus) having 1.5–4 MΩ resistance were used to record currents following drug application using an automated three-barrelled solution switching device (Warner Instruments SF-77B). The extracellular solution consisted of (mM): NaCl, 130; KCl, 5; BaCl2, 2; MgCl2, 1; glucose, 30; HEPES-NaOH, 25; pH 7.3 and electrodes were filled with intracellular solution as follows (mM): CsCl, 140; MgCl2, 4; EGTA, 10; HEPES-CsOH, 10; pH 7.3. Concentration–response curves were generated by comparing the peak response evoked by a test concentration of agonist to that evoked by a previous control current recorded in response to 1 μM capsaicin. Current–voltage relationships were established by measuring the net agonist-evoked current response during a voltage ramp (−70 to +70 mV). A baseline, obtained from the mean of two or three voltage-ramps in control solution prior to drug addition, was subtracted from the mean of three to five voltage-ramps at peak current in presence of drug (see Figure 2c). In these experiments, all data were normalised to the initial current obtained at the holding potential of −70 mV.
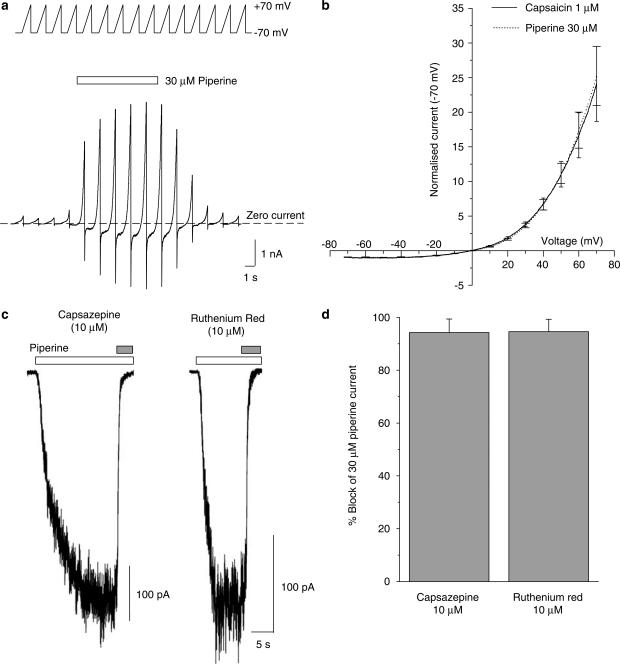
Figure 2.
Rectification and pharmacological properties of piperine-gated currents. (a) The current–voltage relationship for piperine- and capsaicin-activated currents was established using a voltage-ramp protocol (see top inset) ranging from −70 to +70 mV. A series of voltage-ramps (15 × −70 mV to +70 mV at 0.14 mV ms−1) was used to capture data prior to, during and following the recovery of the agonist-induced response. The net agonist-evoked current was calculated by subtracting the mean background current from the agonist-evoked current obtained from ramps, which coincided with the steady-state phase of the response. The example shown is for a piperine-evoked response where the agonist was applied for the duration indicated by the bar. Similar experiments were conducted for capsaicin. (b) A plot of the current–voltage relationship obtained for piperine (30 μM dotted line; n=5) and capsaicin (1 μM, solid line; n=5) from pooled data generated from experiments similar to those in (a). Currents were normalised to the steady-state current observed at −70 mV and then averaged across cells. Occasional error bars (±1s.e.m.) are shown at 10 mV intervals. Piperine responses show clear outward rectification (I+70 mV/I−70 mV=25±4 compared to 24±5 for capsaicin; P=0.87, unpaired Student's t-test) and exhibit a reversal potential close to zero (Erev=0.0±0.4 mV compared to −1.0±0.8 mV for capsaicin; P=0.29, unpaired Student's t-test). (c) Piperine-evoked currents (clear bar; 30 μM) were inhibited by coapplication of capsazepine 10 μM or ruthenium red 10 μM (grey bars). (d) Pooled data showing the % block of the piperine-evoked current expressed by capsazepine, (n=3) and ruthenium red, (n=4).
Drugs and reagents
Piperine (97%) was obtained from Sigma (U.K.); capsaicin and capsazepine were from Tocris (Bristol, U.K.) and ruthenium red from RBI. All cell culture media were obtained from Life Technologies (Paisley, U.K.). Stock solutions of piperine (100 mM), capsaicin (10 mM) and capsazepine (10 mM) were prepared in dimethylsulphoxide (DMSO), while ruthenium red (10 mM) was dissolved in distilled water. All drugs were diluted to working concentrations from frozen aliquots using the extracellular solution defined above. The maximum DMSO concentration used was 0.1% and, in control experiments, this was without effect on the cells used (data not shown).
Data analysis
Data were acquired and analysed using the pClamp 9.0 software suite (Axon Instruments) and Origin (Microcal Software Inc., MA, U.S.A.). Data given are typically mean±s.e.m. Statistical significance was assessed using two-tailed paired or unpaired Student's t-test, ANOVA or Planned Comparisons as indicated.
Results
Piperine is an agonist at the human vanilloid receptor TRPV1
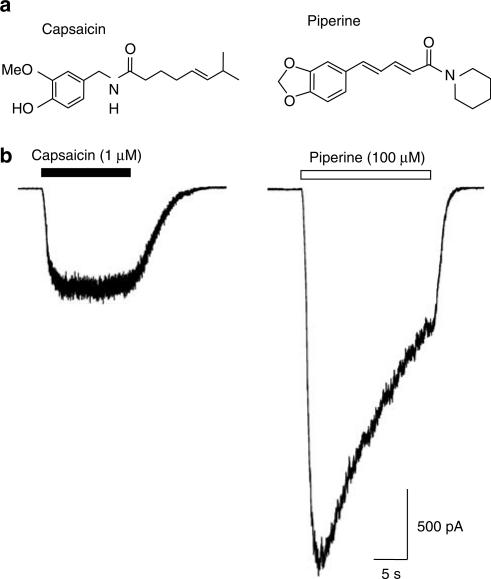
We studied the effects of piperine and capsaicin (Figure 1a) on HEK293 cells stably expressing the human TRPV1 receptor (hTRPV1.HEK293 cells). At a holding potential of −70 mV, application of piperine (100 μM) led to the appearance of large inward currents similar, but not identical, in appearance to those gated by 1 μM capsaicin (Figure 1). Piperine-mediated currents activated more rapidly (the 30–70% growth time for the piperine-gated current was 620±60 ms compared to 1690±370 ms for capsaicin; P<0.05, n=6; paired Student's t-test) and were typically larger than those gated by capsaicin (Ipiperine=1600±360 pA and Icapsaicin=610±140 pA, n=6; P<0.01, Student's paired test). Piperine-gated currents also showed a greater degree of agonist-induced desensitisation reaching 50% of their initial peak response (t1/2) in 9.9±0.7 s (n=6). In contrast, capsaicin evidenced little ability to induce macroscopic desensitisation during applications lasting 20 s (Figure 1b). Neither agent had any effect on parental wild-type HEK293 cells (data not shown).
Figure 1.
Piperine activates human TRPV1. (a) The chemical structures of the vanilloid capsaicin and the related nonvanilloid congener piperine, which lacks the 4-hydroxy-3-methoxybenzyl or ‘vanillyl' group of capsaicin, are shown. (b) Capsaicin (1 μM; a concentration close to Emax, see Figure 3) activated robust inward currents in hTRPV1.HEK293 cells demonstrating the clear expression of human TRPV1 in these cells. In the same cells, piperine (100 μM) typically activated larger currents which had a distinct kinetic profile due to the effects of desensitisation. The traces shown are from one experiment and are typical of five others. Mean data for these experiments were: Ipiperine=1600±360 pA and Icapsaicin=610±140 pA; P<0.01, n=6, Student's paired test). Capsaicin and piperine were without effect on parental wild-type HEK293 cells (data not shown).
To confirm that the piperine-activated currents were indeed mediated via TRPV1, we characterised their current–voltage relationship and pharmacology. Current–voltage relationships for piperine- and capsaicin-gated currents were calculated using a voltage-ramp protocol (−70 to +70 mV at 0.14 mV ms−1) applied prior to, during and following the recovery of the agonist induced response (Figure 2a). This protocol allowed the net agonist-induced current responses to be generated by subtracting the mean background current from the agonist-evoked current obtained from voltage-ramps, which coincided with the steady-state phase of the response. Using this protocol, piperine-gated currents were found to be indistinguishable from those of capsaicin. The current–voltage relationship obtained for piperine was outwardly rectifying (I+70 mV/I−70 mV=25±4 compared to 24±5 for capsaicin, n=5; P=0.87, unpaired Student's t-test) and exhibited a reversal potential close to zero mV (Erev=0.0±0.4 mV compared to −1.0±0.8 mV for capsaicin, n=5; P=0.29, unpaired Student's t-test) as expected for a non-selective cation channel such as TRPV1 (Figure 2b).
Consistent with an action at TRPV1, the currents gated by piperine in hTRPV1.HEK293cells were also antagonised by coapplication of the competitive TRPV1 antagonist capsazepine (10 μM) or the noncompetitive antagonist ruthenium red (10 μM), resulting in rapid inhibition of 94±5.1% (n=3) or 95±4.7% (n=4) compared to control, respectively (Figure 2c and d).
Piperine shows greater efficacy at TRPV1 than capsaicin
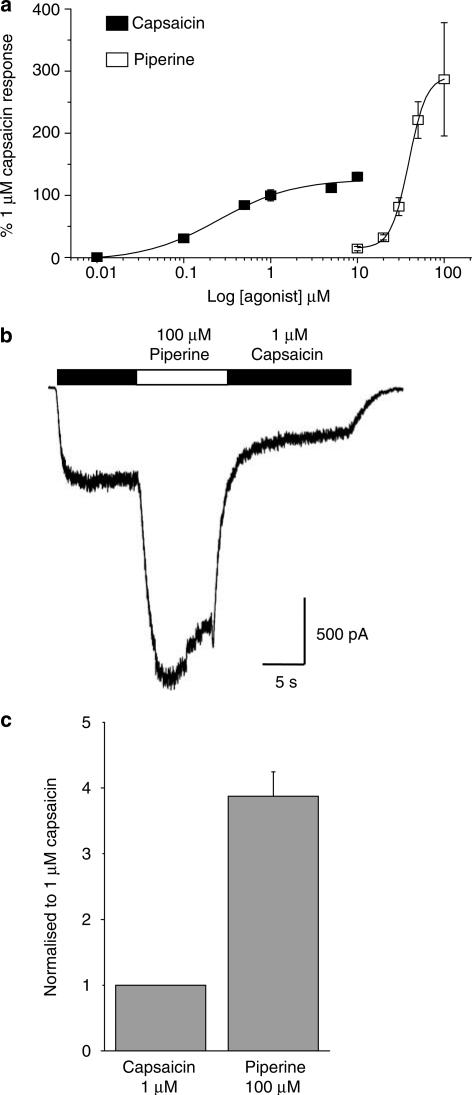
Given our initial data above which showed that 100 μM piperine-gated currents were nearly three-fold larger than those of 1 μM capsaicin, we sought to profile the concentration-response relationships for these agonists in detail to determine if a difference in efficacy rather than potency was responsible for these observations. To minimise any contribution of receptor desensitisation to our concentration–response profiles for capsaicin and piperine, all data were generated by normalising the response to a test concentration of the agonist under study to that of a control response evoked by 1 μM capsaicin immediately beforehand. Using this protocol, the concentration–response curve generated for capsaicin yielded an EC50 of 292±54 nM and Hill coefficient of 1.2±0.2 (n=3–10 per concentration; Figure 3a), similar to values reported for capsaicin activation of the rat recombinant receptor by electrophysiological methods (Caterina et al., 1997; Gunthorpe et al., 2000) and to EC50s reported for human TRPV1 determined by oocyte electrophysiology and calcium imaging (Hayes et al., 2000; Smart et al., 2001).
Figure 3.
Piperine exhibits greater efficacy than capsaicin at the human TRPV1. (a) The concentration-response relationship for capsaicin (0.01–10 μM) and piperine (10–100 μM) are shown. These normalised data were generated by measuring the net currents evoked in response to a test concentration of agonist and are expressed as a percentage of a preceding 1 μM capsaicin control response recorded in the same cell. Data are expressed as the mean±s.e.m., where n=3–10 individual cells. The EC50 for capsaicin was 292±54 nM (Hill coefficient of 1.2±0.2; n=3–10 per concentration). The concentration–response profile for piperine clearly indicates the less potent nature of this compound with an EC50 of 37.9 μM±1.9 (Hill coefficient 3.7±0.5; n=5–9 per concentration) indicating a greater efficacy and degree of cooperativity for piperine than for capsaicin. (b) A representative trace from an experiment designed to quantify the relative difference in efficacy between a 1 μM capsaicin and 100 μM piperine activated response (n=4). A TRPV1 response was first-evoked by capsaicin (black bar) before subsequent addition of piperine. Upon switching to the solution containing piperine the peak response was significantly increased and a greater degree of receptor desensitisation was evident. (c) Mean data from the experiments described in (b) showing a 3.9±0.4 fold increase in TRPV1 current by addition of piperine. Control experiments in which the solution was switched from 1 μM capsaicin to a second identical 1 μM capsaicin solution yielded no change in peak current.
The concentration–response profile for piperine clearly indicates the less potent nature of this compound with an EC50 of 37.9±1.9 μM and associated Hill coefficient of 3.7±0.5 (n=5–9 per concentration) indicating an apparently greater degree of cooperativity involved in the activation of TRPV1 by piperine (Figure 3a). In these experiments, piperine again clearly exhibited a significantly higher efficacy than capsaicin with a two-fold larger response at 100 μM (286±91%) compared to 10 μM capsaicin (130±7%). Unfortunately, higher concentrations of piperine could not be examined in these experiments due to the limited solubility of piperine in the extracellular solution used. This should not, however, have confounded our results regarding the comparative efficacy of the compounds in question since any reduction in the piperine response due to poor solubility would only serve to reinforce the opposite conclusion. Nevertheless, we decided to pursue a second approach to accurately measure the comparative efficacy of these compounds. In these further experiments (Figure 3b), we first activated TRPV1 receptors by application of 1 μM capsaicin. Once a steady-state response was achieved, we then switched into a solution bearing 100 μM piperine before once again returning to the original agonist solution containing 1 μM capsaicin (n=4). In these experiments, it is clear that 100 μM piperine produces a much larger current than capsaicin (Figure 3b and c) confirming the greater efficacy of this agonist.
Acid potentiates the piperine-activated current
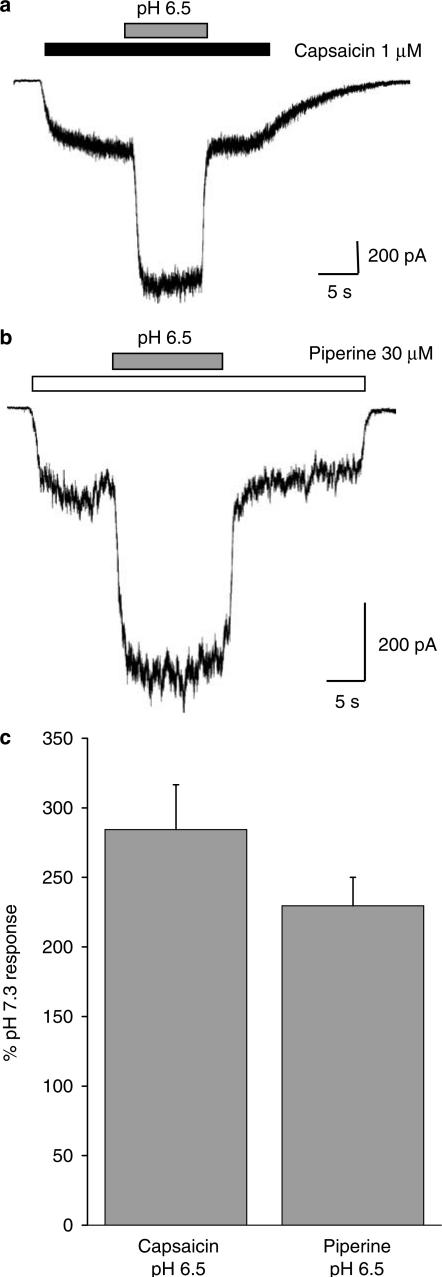
TRPV1 is extremely sensitive to the effect of extracellular protons (acidification) which can potentiate, gate and even inhibit the receptor at progressively lower pHs (Tominaga et al., 1998; Jordt et al., 2000; Gunthorpe et al., 2004). We therefore sought to examine if the greater efficacy demonstrated by piperine may be linked to this mechanism. To do this, we quantified the effects of acidification to pH 6.5 on both piperine and capsaicin responses (Figure 4). The effects of pH 6.5 were studied by first activating the currents at pH 7.3 (standard extracellular pH) before switching to an equivalent agonist solution at pH 6.5. In both cases, the inward current size increased rapidly in response to acidification, an effect that was rapidly reversible on return to pH 7.3 (Figure 4a and b). The degree of acid-induced augmentation was similar for piperine 230±20% (n=11) and capsaicin 284±32% (n=8; P>0.05, unpaired Student's t-test; Figure 4c) and was similar to the level of potentiation reported previously for other TRPV1 agonists such as olvanil and resiniferatoxin at pH 6.5 (Gunthorpe et al., 2004).
Figure 4.
Modulation of the piperine-activated TRPV1 current by acid. (a) A typical recording trace showing the dramatic potentiation of a capsaicin-(1 μM, black bar)-mediated TRPV1 response by extracellular acidification from pH 7.3 to pH 6.5 (grey bar). In these experiments, pH 6.5 was selected for study as it is a subagonist pH versus TRPV1 (data not shown). pH 6.5 potentiated the capsaicin response by 284±32% (n=8). (b) A similar protocol was employed to study the effects of acidification on the piperine response. Switching from pH 7.30 to pH 6.5 potentiated the piperine-(30 μM gated response, white bar) by 230±20% (n=11) (c) Pooled data showing that capsaicin and piperine are similarly modulated by acid (pH 6.5). The data are expressed as a percentage of the mean current recorded in response to the agonist at pH 7.3.
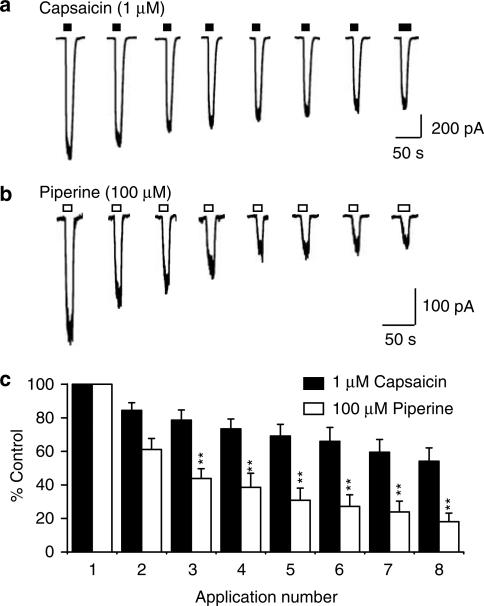
Desensitisation of hTRPV1 in response to piperine
The temporal effects of TRPV1 agonists are often largely shaped by their ability to elicit receptor desensitisation. Following on from our initial observation, that piperine produced a greater degree of macroscopic desensitisation than capsaicin, we were interested to examine the effects of piperine on desensitisation and tachyphylaxis (defined as the diminution of response upon repeated application of agonist) in more detail. Repeated applications of a high concentration of either piperine (n=8) or capsaicin (n=7) to TRPV1 expressing cells resulted in clear tachyphylaxis (Figure 5). Piperine produced a progressively greater reduction in response magnitude with each repeated application compared to capsaicin (P<0.05 for second application; P<0.01 for third to eighth application, using Planned Comparisons) resulting in a diminution of the response after eight applications to 18±5.1% of the original current, compared with 54±7.9% for capsaicin. Our results therefore highlight a clear propensity for piperine to cause greater TRPV1 receptor desensitisation and tachyphylaxis than capsaicin.
Figure 5.
Piperine produces a greater degree of desensitisation than capsaicin. The effects of piperine and capsaicin on TRPV1 desensitisation and tachyphylaxis were compared using a repeated applications protocol. Representative traces showing the typical profile of responses obtained during eight repeated applications of (a) 1 μM capsaicin (closed bar) or (b) 100 μM piperine (open bar) are shown. (b) Agonists were applied for 20 s with intervening washout periods of 1 min (indicated by the gaps). (c) The pooled data from the experiments described in (a and b) are given. Agonist responses are expressed as a percentage of the initial current for each of eight repeated applications of capsaicin (n=7) or piperine (n=8). *P<0.05; **P<0.01 versus the corresponding capsaicin-mediated response.
Discussion
We have shown that piperine, like capsaicin and a range of other natural plant products utilised as gustatory enhancers, is an effective agonist at the human TRPV1 receptor. These vanilloid and nonvanilloid agents alike clearly have the ability to evoke a painful burning sensation sufficient to be a deterrent to most animals and yet, somewhat paradoxically, these agents are still ingested by humans (Szallasi & Blumberg, 1999). Such agents also have the potential to offer therapeutic benefit by desensitisation of the TRPV1 receptor directly and/or by a rather more nonselective ‘defunctionalisation' of the sensory neurones bearing such receptors (Szallasi & Blumberg, 1999; Geppetti & Trevisani, 2004). Again, the latter is well known to us all and is easily appreciated from ones experience regarding the ability to eat ‘hotter' and ‘hotter' food, albeit with continued practice.
Piperine is less potent but more efficacious at human TRPV1
Our detailed characterisation of the effects of piperine on TRPV1 clearly defines that this compound is not only a full agonist at the receptor but that it actually shows greater efficacy than the prototypical vanilloid receptor ligand capsaicin. Clearly, piperine shows a lesser potency than capsaicin at TRPV1 consistent with its relative pungency-taste threshold of 10.5 μM vis-à-vis 0.6 μM for capsaicin (Szolcsanyi & Jancso-Gabor, 1975); however, the greater efficacy versus the receptor becomes apparent when sufficient concentrations are reached to define the maximum obtainable current in the concentration–response profile.
Although the efficacy of many TRPV1 agonists such as resiniferatoxin, capsiate and gingerols have been characterised in detail in direct functional assays employing TRPV1 (Caterina et al., 1997; Dedov et al., 2002; Iida et al., 2003), none have been shown to have maximal efficacy greater than that of capsaicin. Similar findings have also been reported for anandamide and related analogues, PPAHV, and N-arachidonoyldopamine as determined by calcium imaging or calcium uptake (Jerman et al., 2000; Ross et al., 2001; Huang et al., 2002). There are some reports, however, that RTX can produce a greater increase in intracellular [Ca2+] than capsaicin although this may be due to either an interaction with internal Ca2+ stores (Marshall et al., 2003) or the ability of this compound to interact with protein kinase C pathways as a result of its phorbol ester activity (Harvey et al., 1995).
Given the above, our results with piperine could be considered to be somewhat puzzling, however, few studies have ever characterised the effects of piperine at native receptors and this is the first to examine the effects of piperine at the recombinant TRPV1. We are confident that a species difference, or indeed a difference between the native and recombinant receptor, does not underlie these findings since we have observed qualitatively similar effects of piperine in studies on rat dorsal root ganglion neurones (McNamara & Gunthorpe, unpublished observations). Nevertheless, the previous reports on the effects of piperine on rat trigeminal ganglion cells by Liu & Simon (1996a) suggested that, rather than a full or more efficacious agonist than capsaicin, piperine was in fact a partial agonist of the receptor. We noted with interest, however, that these studies compared pooled normalised data from independent experiments on a large number of TG neurones in culture and it is therefore possible that any differences in efficacy would not be highlighted in these data. This, combined with the effects of pronounced desensitisation exhibited by the TG neurone preparation (Liu & Simon, 1996a), may have confounded the ability to identify this interesting aspect of piperine pharmacology. We must note, however, that it is also equally plausible that TRPV1 receptors in the trigeminal ganglia may differ from those in recombinant and DRG preparations as has been previously suggested to explain differences in the pharmacology of capsazepine and indeed capsaicin (Liu & Simon, 1996b).
We cannot currently explain why piperine, a structurally similar molecule to capsaicin (Figure 1a), which is thought to act at the same site on the receptor (as defined by displacement of [3H]Resiniferatoxin, Szallasi & Blumberg, 1991), should show this greater efficacy. Piperine-gated currents were clearly similar to those of capsaicin in many respects including their rectification properties, regulation by protons (see below) and sensitivity to capsazepine and ruthenium red. These properties are typical of those reported for other TRPV1 agonists (Caterina et al., 1997; Tominaga et al., 1998), suggesting that piperine activates the receptor by an essentially similar mechanism. Piperine did, however, display a greater degree of cooperativity than capsaicin, as indicated by the larger Hill coefficient of 3.7±0.5 (cf. 1.2±0.2 for capsaicin). This could be interpreted as an indication that piperine-mediated activation of TRPV1 involves a greater number of interacting sites than are required for capsaicin-gating of the receptor. As TRPV1 is likely to be a tetrameric complex based on biochemical evidence and analogy to the structurally similar K+ channels (Kedei et al., 2001), multiple agonist binding sites are probable. Although many studies have found positive cooperativity for other TRPV1 ligands (typically ∼2), none reach the value obtained here for piperine. It remains possible that piperine may therefore recruit more TRPV1 subunits in the gating mechanism or have additional binding sites on the TRPV1 receptor.
Possible sites of action of piperine on TRPV1
A number of elegant studies have recently begun to shed light on the key structural elements of the TRPV1 receptor which may contribute key amino acids to the vanilloid binding site (Jordt & Julius, 2002; Gavva et al., 2004). Through the study of natural species variations in vanilloid pharmacology such as the relative insensitivity of birds (Jordt & Julius, 2002) and lesser sensitivity of rabbits (Gavva et al., 2004) to capsaicin it is now known that amino acids such as Y511 and T550 in the region of transmembrane 3 and 4 of the receptor are key determinants of the vanilloid binding site. Whether or not these and/or additional residues are also required for the activity of piperine will therefore be an interesting piece of work for the future. The prevalence of a large number of natural and synthetic piperine analogues (Ribeiro et al., 2004) should also be of use in progressing such studies and may shed light on whether the 1,3-benzodioxole group or the increased rigidity or planarity of the piperine structure (cf. capsaicin) may underlie its pharmacological properties.
Regulation of responses to piperine by protons
The polymodal nature of TRPV1 encompasses its sensitivity to ambient temperature and surrounding pH. At core body temperature, acidification to pH 6.4 is sufficient to activate TRPV1 by reducing its heat threshold from >42°C to below 37°C. Even at room temperature TRPV1 can be directly activated by protons, but acidification to a more extreme pH of ⩽6.0 is required (Tominaga et al., 1998; Jordt & Julius, 2002; Gunthorpe et al., 2004). Lowering the pH in the acidic range no doubt results in protonation of key extracellular sites (Jordt et al., 2000) and stabilisation of an open state of the channel (Ryu et al., 2003) such that TRPV1 receptor activity is increased, however, further acidification can actually lead to blockade of the receptor (Gunthorpe et al., 2004). In this study, we have demonstrated a similar level of potentiation by pH 6.5 on the current activated by piperine and capsaicin. This suggests that these ligands activate the receptor by a similar mechanism, which is then subject to modulation by protons. Presumably, this also reflects the fact that the protonatable sites on TRPV1, which contribute to this effect (Jordt et al., 2000), are likely to be different to those mediating capsaicin (Welch et al., 2000) and by inference, piperine binding. Our data serve to highlight the regulation of piperine responses by acid, which is of key importance given the range of pHs to which TRPV1 is exposed in the GI tract (Ward et al., 2003), and suggests that differences in the pH regulation of the receptor are unlikely to explain the higher efficacy of piperine observed.
Piperine effects on TRPV1 desensitisation
As noted above, the temporal effects of TRPV1 agonists are often largely shaped by their ability to elicit receptor desensitisation. Indeed, the therapeutic potential of agonists such as resiniferatoxin has been extrapolated from their ability to induce lasting desensitisation of sensory neurones following initial receptor activation (Szallasi & Blumberg, 1999). Previous reports utilising native tissue have documented a clear ability of piperine and capsaicin responses to cross desensitize (Szolcsanyi, 1983), while electrophysiological studies have documented agonist-evoked TRPV1 desensitisation and tachyphylaxis in DRG neurones, TG neurones and recombinant preparations in response to for example capsaicin and zingerone (Liu & Simon, 1996a; Liu et al., 2000).
Our data demonstrate that piperine induces greater receptor desensitisation and tachyphylaxis than capsaicin. Although there are few reports in the literature regarding this aspect of piperine action, it is clear that our finding contrasts those of Liu & Simon (1996a), who compared capsaicin and piperine responses in TG neurones and concluded that piperine induces a lesser degree of tachyphylaxis than capsaicin. As discussed above, it is possible that differences in the native TRPV1 receptor of TG neurones may underlie these differences, although they may also reflect differences in the recording conditions employed. We chose to replace extracellular Ca2+ with Ba2+ so as to reduce the Ca2+-dependent component of desensitisation (Koplas et al., 1997), and gain a more accurate insight into the agonist-induced desensitisation profile obtained. In terms of its physiology, it is interesting to note that the greater degree of desensitisation produced by piperine may help shape its apparent pungency and will contribute to its long lasting effects versus TRPV1 in the body. In terms of an agonist-based therapeutic desensitisation strategy, molecules exhibiting this property could conceivably offer therapeutic potential and the search for such molecules with little or no pungency may yet pay dividends.
Although few papers have explored the physiology and pharmacology of piperine in detail, those that have pertain to the effects of piperine on GI function. This aspect of piperine action is of particular relevance given the documented expression of TRPV1 on both intrinsic and extrinsic (spinal and vagal) neurons, which innervate the musculature, enteric nerve plexuses and mucosa of the gut (Patterson et al., 2003; Ward et al., 2003; see Holzer, 2004, for review). Piperine has been shown to cause a reduction in GI transit (Izzo et al., 2001), inhibit vagally evoked contractions of oesophageal striated muscle (Izumi et al., 2003) and reduce castor oil-induced intestinal fluid accumulation (Capasso et al., 2002). The majority of these studies also suggest, however, that the actions of piperine, although mediated by capsaicin-sensitive sensory neurones, are not mediated by TRPV1 receptors since the effects are often not replicated by capsaicin and are insensitive to the competitive TRPV1 antagonist capsazepine (Izzo et al., 2001; Capasso et al., 2002). We have shown that capsazepine can clearly inhibit the response to piperine and so a trivial difference in antagonist pharmacology seems unable to bridge the apparent discrepancy between the in vitro data demonstrating the activity of piperine versus TRPV1 and the conclusions of Izzo et al. (2001) and Capasso et al. (2002) based on their work on GI secretion and transit in mice. It therefore remains a distinct possibility that TRPV1 activation may not be the main action of piperine responsible for its effects on GI function. However, one must also note that capsazepine has only moderate potency versus TRPV1 and is reported to have a number of nonselective actions at other receptors which may complicate the interpretation of the GI studies conducted to date (see Nocerino et al., 2002). Further studies with more potent and selective TRPV1 antagonists will therefore be required to examine this further to better understand the precise role of TRPV1 in these processes in both physiological and perhaps, more importantly, pathophysiological conditions where TRPV1 may be upregulated (Yiangou et al., 2001; Chan et al., 2003; Schicho et al., 2004; Szallasi & Appendino, 2004).
In conclusion, we have clearly demonstrated that piperine activates human TRPV1 with an efficacy superior to that of the prototypical reference agonist capsaicin. Furthermore, clear differences exist in the desensitisation profiles for capsaicin and piperine with the latter eliciting greater desensitisation upon single or repeated application. It remains to be determined if TRPV1 agonists bearing such properties may contribute to the structure–activity relationship and design of new and improved therapeutic agents.
Acknowledgments
We thank Dr Harshad Rami for insightful discussions concerning the structure of compounds of the vanilloid class. F.N.M. is a recipient of a European Union framework V grant.
Abbreviations
- CZP
capsazepine
- DMSO
dimethylsulphoxide
- GI
gastrointestinal
- HEK293
human embryonic kidney 293 (cells)
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulphonic acid
- hTRPV1
human TRP vanilloid subtype 1
- TRP
transient receptor potential
References
- BAJAD S., BEDI K.L., SINGLA A.K., JOHRI R.K. Antidiarrhoeal activity of piperine in mice. Planta Med. 2001;67:284–287. doi: 10.1055/s-2001-11999. [DOI] [PubMed] [Google Scholar]
- BUDAVARI S., O'NEIL M.J., SMITH A., HECKELMAN P.E. Merck Index 1989NJ, U.S.A.: Merck & Co., Inc; 1186–1187.(ed.)11th edn., pp [Google Scholar]
- CAPASSO R., IZZO A.A., BORRELLI F., RUSSO A., SAUTEBIN L., PINTO A., CAPASSO F., MASCOLO N. Effect of piperine, the active ingredient of black pepper, on intestinal secretion in mice. Life Sci. 2002;71:2311–2317. doi: 10.1016/s0024-3205(02)02019-2. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHAN C.L., FACER P., DAVIS J.B., SMITH G.D., EGERTON J., BOUNTRA C., WILLIAMS N.S., ANAND P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- DEDOV V.N., TRAN V.H., DUKE C.C., CONNOR M., CHRISTIE M.J., MANDADI S., ROUFOGALIS B.D. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br. J. Pharmacol. 2002;137:793–798. doi: 10.1038/sj.bjp.0704925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVVA N.R., KLIONSKY L., QU Y., SHI L., TAMIR R., EDENSON S., ZHANG T.J., VISWANADHAN V.N., TOTH A., PEARCE L.V., VANDERAH T.W., PORRECA F., BLUMBERG P.M., LILE J., SUN Y., WILD K., LOUIS J.C., TREANOR J.J. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- GEPPETTI P., TREVISANI M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br. J. Pharmacol. 2004;8:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN B.G. Rapid recovery from capsaicin desensitization during recurrent stimulation. Pain. 1996;68:245–253. doi: 10.1016/s0304-3959(96)03211-3. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., DAVIS J.B., RANDALL A.D.Activation and modulation of human vanilloid receptor-1 (hTRPV1) by protons J. Physiol. 2004. 555P, C2
- GUNTHORPE M.J., HARRIES M.H., PRINJHA R.K., DAVIS J.B., RANDALL A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J. Physiol. 2000;525:747–749. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J.S., DAVIS C., JAMES I.F., BURGESS G.M. Activation of protein kinase C by the capsaicin analogue resiniferatoxin in sensory neurones. J. Neurochem. 1995;65:1309–1317. doi: 10.1046/j.1471-4159.1995.65031309.x. [DOI] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARRIES M.H., DUCKWORTH D.M., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin. Ther. Targets. 2004;8:107–123. doi: 10.1517/14728222.8.2.107. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIDA T., MORIYAMA T., KOBATA K., MORITA A., MURAYAMA N., HASHIZUME S., FUSHIKI T., YAZAWA S., WATANABE T., TOMINAGA M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology. 2003;44:958–967. doi: 10.1016/s0028-3908(03)00100-x. [DOI] [PubMed] [Google Scholar]
- IZUMI N., MATSUYAMA H., KO M., SHIMIZU Y., TAKEWAKI T. Role of intrinsic nitrergic neurones on vagally mediated striated muscle contractions in the hamster oesophagus. J. Physiol. 2003;551:287–294. doi: 10.1113/jphysiol.2003.044669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., CAPASSO R., PINTO L., DI CARLO G., MASCOLO N., CAPASSO F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br. J. Pharmacol. 2001;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., PRINJHA R., HARRIES M.H., DAVIS J.B., SMART D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- JORDT S.E., TOMINAGA M., JULIUS D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEDEI N., SZABO T., LILE J.D., TREANOR J.J., OLAH Z., IADAROLA M.J., BLUMBERG P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- KOPLAS P.A., ROSENBERG R.L., OXFORD G.S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J.Neurophysiol. 1996a;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J. Neurophysiol. 1996b;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- LIU L., WELCH J.M., ERICKSON R.P., REINHART P.H., SIMON S.A. Different responses to repeated applications of zingerone in behavioral studies, recordings from intact and cultured TG neurons, and from VR1 receptors. Physiol. Behav. 2000;69:177–186. doi: 10.1016/s0031-9384(00)00200-6. [DOI] [PubMed] [Google Scholar]
- MARSHALL I.C., OWEN D.E., CRIPPS T.V., DAVIS J.B., MCNULTY S., SMART D. Activation of vanilloid receptor 1 by resiniferatoxin mobilizes calcium from inositol 1,4,5-trisphosphate-sensitive stores. Br. J. Pharmacol. 2003;138:172–176. doi: 10.1038/sj.bjp.0705003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMARA F., RANDALL A.D., GUNTHORPE M.J.The effects of Piperine, the pungent ingredient of black pepper, at the human vanilloid receptor (hTRPV1) J. Physiol. 2004. 555P, C1 [DOI] [PMC free article] [PubMed]
- NAIDU K.A., THIPPESWAMY N.B. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol. Cell Biochem. 2002;229:19–23. doi: 10.1023/a:1017930708099. [DOI] [PubMed] [Google Scholar]
- NOCERINO E, IZZO AA, BORRELLI F, CAPASSO F, CAPASSO R, PINTO A, SAUTEBIN L, MASCOLO N. Relaxant effect of capsazepine in the isolated rat ileum. Naunyn. Schmiedebergs Arch. Pharmacol. 2002;365:187–192. doi: 10.1007/s00210-001-0522-x. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., MAGGI C.A., MELI A. Capsaicin-like activity of some natural pungent substances on peripheral endings of visceral primary afferents. Naunyn. Schmiedebergs Arch. Pharmacol. 1990;342:72–77. doi: 10.1007/BF00178975. [DOI] [PubMed] [Google Scholar]
- PATTERSON L.M., ZHENG H., WARD S.M., BERTHOUD H.R. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- RIBEIRO T.S., FREIRE-DE-LIMA L., PREVIATO J.O., MENDONCA-PREVIATO L., HEISE N., DE LIMA M.E. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2004;14:3555–3558. doi: 10.1016/j.bmcl.2004.04.019. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI M.V., PERTWEE R.G. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYU S., LIU B., QIN F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J. Gen. Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHICHO R., FLORIAN W., LIEBMANN I., HOLZER P., LIPPE I.T. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur. J. Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., GUNTHORPE M.J., BROUGH S.J., RANSON J., CAIRNS W., HAYES P.D., RANDALL A.D., DAVIS J.B. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur. J. Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- STERNER O., SZALLASI A. Novel natural vanilloid receptor agonists: new therapeutic targets for drug development. Trends Pharmacol. Sci. 1999;20:459–465. doi: 10.1016/s0165-6147(99)01393-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., APPENDINO G. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse. J. Med. Chem. 2004;47:2717–2723. doi: 10.1021/jm030560j. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Characterization of vanilloid receptors in the dorsal horn of pig spinal cord. Brain Res. 1991;547:335–338. doi: 10.1016/0006-8993(91)90982-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- SZOLCSANYI J. Tetrodotoxin-resistant non-cholinergic neurogenic contraction evoked by capsaicinoids and piperine on the guinea-pig trachea. Neurosci. Lett. 1983;42:83–88. doi: 10.1016/0304-3940(83)90426-3. [DOI] [PubMed] [Google Scholar]
- SZOLCSANYI J., BARTHO L. Capsaicin-sensitive afferents and their role in gastroprotection: an update. J. Physiol. Paris. 2001;95:181–188. doi: 10.1016/s0928-4257(01)00023-7. [DOI] [PubMed] [Google Scholar]
- SZOLCSANYI J., JANCSO-GABOR A. Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents. Arzneimittelforschung. 1975;25:1877–1881. [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- VAN DER STELT M., DI MARZO V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- VIJAYAKUMAR R.S., SURYA D., NALINI N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox. Rep. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- WARD S.M., BAYGUINOV J., WON K.J., GRUNDY D., BERTHOUD H.R. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J. Comp. Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- WELCH J.M., SIMON S.A., REINHART P.H. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIANGOU Y., FACER P., DYER N.H., CHAN C.L., KNOWLES C., WILLIAMS N.S., ANAND P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]