Abstract
Cannabinoid receptor agonists elicit analgesic effects in acute and chronic pain states via spinal and supraspinal pathways. We investigated whether the combination of a cannabinoid agonist with other classes of antinociceptive drugs exerted supra-additive (synergistic) or additive effects in acute pain models in mice.
The interactions between the cannabinoid agonist CP55,940, α2-adrenoceptor agonist dexmedetomidine and μ-opioid receptor agonist morphine were evaluated by isobolographic analysis of antinociception in hot plate (55°C) and tail flick assays in conscious male Swiss mice. Drug interactions were examined by administering fixed-ratio combinations of agonists (s.c.) in 1 : 1, 3 : 1 and 1 : 3 ratios of their respective ED50 fractions.
CP55,940, dexmedetomidine and morphine all caused dose-dependent antinociception. In the hot plate and tail flick assays, ED50 values (mg kg−1) were CP55,940 1.13 and 0.51, dexmedetomidine 0.066 and 0.023, and morphine 29.4 and 11.3, respectively. Synergistic interactions existed between CP55,940 and dexmedetomidine in the hot plate assay, and CP55,940 and morphine in both assays. Additive interactions were found for CP55,940 and dexmedetomidine in the tail flick assay, and dexmedetomidine and morphine in both assays.
Thus, an α2-adrenoceptor agonist or μ opioid receptor agonist when combined with a cannabinoid receptor agonist showed significant synergy in antinociception in the hot plate test. However, for the tail flick nociceptive response to heat, only cannabinoid and μ opioid receptor antinociceptive synergy was demonstrated. If these results translate to humans, then prudent selection of dose and receptor-specific agonists may allow an improved therapeutic separation from unwanted side effects.
Keywords: α2-Adrenoceptor; antinociception; cannabinoid receptor; CP55,940; dexmedetomidine; hot plate; isobolographic analysis; morphine; synergy; tail flick
Introduction
Marijuana (Cannabis sativa) has been used for medicinal purposes through history. Its main psychoactive compound Δ9-tetrahydrocannabinol (Δ9-THC) causes a variety of effects in different animal species, such as antinociception, hypoactivity, catalepsy, hypothermia and cardiovascular changes (Chaperon & Thiebot, 1999; Randall et al., 2004). There has been much interest in Δ9-THC and its synthetic derivatives (e.g. CP55,940) due to their antinociceptive effects and ability to increase the potency of other analgesic drugs, such as μ opioid and α2-adrenoceptor agonists. The identification and cloning of the two cannabinoid receptors, CB1 and CB2, in the 1990s and the subsequent synthesis of selective receptor ligands have greatly elucidated many aspects of cannabinoid pharmacology and the endocannabinoid system (Pertwee, 2000; 2001). CB1 receptors are present only in low levels in the hypothalamus and almost absent in the respiratory region of the brainstem (Howlett et al., 2002). This correlates with the absence of respiratory depression and low mortality rates associated with cannabis overdose. Thus, the antinociceptive effects of cannabinoid receptor agonists may offer clinical therapeutic advantages. CP55,940, a bicyclic nonclassical cannabinoid, is a full agonist at both CB1 and CB2 receptors and 10–50 times more potent than Δ9-THC (Pertwee, 2000; Howlett et al., 2002).
The CB1, α2-adrenoceptor and μ opioid receptors are all seven-transmembrane Gi/o protein-coupled receptors sharing signal transduction pathways, such as inhibiting adenyl cyclase and modulating K+ and Ca2+ channel activity (Welch et al., 1995a; Khan et al., 1999; Manzanares et al., 1999; Morisset et al., 2001; Przewlocki & Przewlocka, 2001; Howlett et al., 2002). These receptors are also similarly distributed in the periaqueductal gray and substantia gelatinosa, areas in the CNS highly implicated in antinociception (Yaksh, 1985; Behbehani, 1995; Lichtman et al., 1996; Martin & Lichtman, 1998; Furst, 1999; Khan et al., 1999; Pertwee, 2001). Both supraspinal and spinal pathways are involved in α2-adrenoceptor-mediated analgesia. The spinal antinociceptive actions of α2-adrenoceptors are mediated mainly by the α2A-subtype (Jones et al., 1982; Khan et al., 1999; Kamibayashi & Maze, 2000; Malmberg et al., 2001), although α2C-adrenoceptors also contribute (Fairbanks et al., 2002). Dexmedetomidine is a selective α2-adrenoceptor agonist currently approved for clinical use under intensive care conditions (Khan et al., 1999; Coursin & Maccioli, 2001).
Due to these similarities between the cannabinoid, opioid and adrenergic systems, it is likely that one or more combinations of selective agonists for each system will interact and possibly elicit supra-additive (or synergistic) antinociceptive effects. The aim of this work was to investigate the antinociceptive activity of combinations of doses (based on equieffective ED50 fractions) of CP55,940 with morphine or with dexmedetomidine using acute pain models in conscious mice. In addition, the combination of dexmedetomidine and morphine was tested. The effects of the combined drug treatments were tested by isobolographic analysis to determine if interactions were synergistic or merely additive, as described by Tallarida (2001). An abstract of some of this work has been published (Tham et al., 2002).
Methods
Animals
This study was approved by the University of Melbourne Animal Ethics Experimentation Committee in accordance with the guidelines of the National Health and Medical Research Council of Australia. Swiss male mice weighing 22–25 g at the start of the study were housed in groups of four with ad libitum access to food and water, a 12 h light–dark cycle and temperature of 21±1°C. Prior to the start of each experiment, the animals were given at least 15 min to acclimatise to the laboratory conditions (temperature 21±1°C). Each animal was used for a maximum of four experiments, with each experiment using a different dosing regimen. A recovery period of at least 7 days was allowed between experiments. The animals were euthanised upon the completion of the last experiment with an overdose of pentobarbitone (approx. 160 mg kg−1 i.p.).
Agonists and protocols
CP55,940 ((−)-cis 3-(2-hydroxy-4-(1,1-dimethylheptyl)phenyl)-trans-4-(3-hydroxypropyl) cyclohexanol; Tocris Cookson Ltd, Bristol, U.K.) dissolved in 1 part ethanol, 1 part cremaphor and 18 parts 0.9% saline (vehicle 1 : 1 : 18) was administered s.c. with doses ranging from 0.3 to 3.0 mg kg−1. The α2-adrenoceptor agonist dexmedetomidine hydrochloride (IDC Abbott Laboratories Ltd, Queenborough, Kent, U.K.) and the μ opioid agonist morphine sulphate (David Bull Laboratories, Melbourne, Australia) dissolved in saline were administered s.c. with doses in the range of 3–100 μg kg−1 and 3–30 mg kg−1, respectively. In separate groups of mice, either saline or 1 : 1 : 18 vehicle was administered (2.5 ml kg−1 s.c.).
Each drug was administered separately generating dose–response curves with their respective ED50. Combinations of CP55,940 with dexmedetomidine, CP55,940 with morphine and dexmedetomidine with morphine were then simultaneously administered s.c. in a fixed 1 : 1 ED50 ratio of fractions (1.0, 0.5, 0.25, 0.125 and 0.0625) of their respective agonist ED50 values. The combination of CP55,940 and dexmedetomidine was analysed further by administering the drugs in 1 : 3 and 3 : 1 ED50 ratios, and doses were calculated such that they added to the same effect level as expected above. The resulting data were used to construct combination dose–response curves, which were then subject to ‘fixed ratio design' isobolographic analysis. This was to determine whether an interaction was additive or otherwise, assuming the agonists worked through ‘similar independent action' (Tallarida, 2000; 2001). Theoretical values for a purely additive interaction were compared to the actual experimental dose-pairs required for the same effect. A simple additive interaction occurred when a known combination of agonist doses caused a mathematically predictable effect, with the knowledge of the individual agonist potencies. Synergism or supra-additivity described the result when the same agonist combinations caused an exaggerated effect, that is, doses less than those predicted by additivity were needed to cause a normal effect (Tallarida, 2000; 2001).
Nociceptive testing
Hot plate assay
Each mouse was placed unrestrained on a hot plate (Ugo Basile model 7280, Comerio, Italy; 55±0.5°C) for the baseline measurement just prior to drug administration. Measurements were then taken 15, 30, 60, 90 and 120 min after drug administration. Two tests were performed 2 min apart and averaged for a measurement at each time. The end point for each test occurred when the animal displayed the characteristic physical reactions to noxious thermal stimuli, that is, paw-licking or jumping (Le Bars et al., 2001), upon which the mouse was instantly removed and returned to its home cage. Baseline latencies were usually within the range of 7–10 s. A maximum cutoff time of 50 s was set to prevent tissue damage.
Tail flick assay
A modified version of the rat tail flick assay (D'amour & Smith, 1941) was used in mice to elicit a spinal tail flick response to noxious thermal stimuli. Each mouse was restrained in a 50 ml plastic tube generously ventilated with large holes. This unit was placed on a plantar test apparatus (Ugo Basile model 7371; infrared setting 90) modified for tail flick experiments. Measurements were taken as for the hot plate assay. Baseline latencies were between 4 and 6 s, and a 12 s cutoff time was set to prevent tissue damage (Le Bars et al., 2001). The movable heat source was placed directly under the tail and when activated, it caused a distinctive tail flick reflex, which stopped an automated timer (based on infrared reflection). Different points on the tail were used to prevent localised tissue damage, and results were averaged. Each mouse was acclimatised to the restraining tube for approximately 30 min on the day prior to its first experiment. Subsequently, it was restrained for approximately 10 min for acclimatisation prior to each measurement, and for no more than 30 min at a time.
Statistical analyses
For each test time, the percentage maximum possible effect (%MPE) was calculated as (test latency−baseline latency) × (cutoff time−baseline latency)−1 × 100% (Harris & Pierson, 1964). %MPE values at the time point at which the greatest antinociceptive responses were observed for each respective agonist (60 min for CP55,940 and morphine; 30 min for dexmedetomidine; see Figure 1) were used to construct dose–response curves, which included 4–5 doses. The ED50 (i.e. the dose that caused 50% of maximum antinociception) and associated 95% confidence intervals were generated from standard nonlinear regression analysis of the log dose–response curve (Prism 4.0, Graphpad Software, San Diego, CA, U.S.A.).
Figure 1.
Dose- and time-dependent antinociceptive effects of single s.c. bolus administration of CP55,940 (0.1–3 mg kg−1; top panels), dexmedetomidine (3–100 μg kg−1; middle panels) and morphine (3–50 mg kg−1; bottom panels) in the hot plate (panels a–c) and tail flick (d–f) assays in conscious mice. Responses are expressed as %MPE (see Methods) from 0 min (bolus injection) to 120 min postadministration; symbols are mean±s.e.m. For each agonist dose, n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 n=8–16 and 7–11; dexmedetomidine n=10–12 and 6–12; and morphine n=6–8 and 6–12. %MPE values at the time point at which the greatest antinociceptive responses were observed for each respective agonist (60 min for CP55,940 and morphine; 30 min for dexmedetomidine) were used to plot the dose–response curves shown in Figure 2.
Agonist combinations were analysed for additive interactions using ‘fixed ratio design' isobolograms whereby combinations of two drugs in known ratios were administered as fractions of their respective ED50, as outlined above (Horvath et al., 1992; Pöch, 1993; Tallarida, 2000; 2001; Miranda et al., 2002). The isobologram consists of an additivity line that connects ED50, Drug A on the vertical axis to ED50, Drug B on the horizontal axis. The theoretical dose required for a purely additive interaction (Zadd=(f)ED50, Drug A+(1−f)ED50, Drug B, where f is the fraction of drug A used) was calculated and compared via a modified version of the Student's t-test to the actual dose (Zmix, determined from the ED50 of the combination dose–response curve) required to achieve the same effect experimentally (Tallarida, 2000). The variance for Zadd was calculated as Var(Zadd)=(f)2Var(ED50, Drug A)+(1−f)2Var(ED50, Drug B) and 95% confidence intervals were derived from these variances based on the proportions of the individual drugs. Confidence intervals for Zmix were generated with Prism.
Simple additivity was termed when the combination of drugs led to a mathematically predictable effect (i.e. Zmix not significantly different from Zadd) with the knowledge of the individual drug potencies. Synergism or supra-additivity described observations in which combinations elicited an enhanced effect, which suggested that drug quantities less than those predicted by additivity (i.e. Zmix significantly less than Zadd) were needed for the same effect (Tallarida, 2000; 2001). Values of P<0.05 were considered statistically significant.
Results
Antinociceptive activity of CP55,940, dexmedetomidine and morphine
Single drug administration of CP55,940, dexmedetomidine and morphine (s.c.) to conscious male Swiss mice caused dose- and time-dependent antinociceptive effects in the hot plate and tail flick assays, with different individual potencies. The greatest antinociceptive responses for doses of CP55,940 or morphine occurred 60 min after drug administration, and at 30 min after doses of dexmedetomidine, in the hot plate (Figure 1a–c) and tail flick (Figure 1d–f) assays. The agonist log dose–response curves are displayed in Figure 2. The corresponding ED50 values with their associated 95% confidence intervals derived from the dose–response curves are shown in Table 1. In separate groups of mice treated with vehicle (saline or 1 : 1 : 18 s.c.), hot plate or tail flick response latencies were consistent over the 0–120 min postadministration test period, as well as between four experimental days (data not shown).
Figure 2.
Agonist dose–antinociceptive response curves (single agonist administration s.c.) in the (a) hot plate and (b) tail flick assays in conscious mice. Responses are expressed as %MPE (see Methods). %MPE values at the time point at which peak antinociceptive responses were observed for each respective agonist (see Figure 1) were used to plot the curves. The total n values for each agonist curve in the hot plate and tail flick assays, respectively, were as follows: CP55,940 n=50 and 33; dexmedetomidine n=57 and 46; and morphine n=34 and 40. For each agonist dose, n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 n=8–16 and 7–11; dexmedetomidine n=10–12 and 6–12; and morphine n=6–8 and 6–12. Symbols are mean±s.e.m.
Table 1.
Potency of CP55,940, dexmedetomidine and morphine as single s.c. drug treatments in the hot plate and tail flick assays
| Hot plate assay | Tail flick assay | |||
|---|---|---|---|---|
| Agonist | ED50 (mg kg−1) | n | ED50 (mg kg−1) | n |
| CP55,940 | 1.13 (0.83–1.33) | 50 | 0.51 (0.31–0.83) | 33 |
| Dexmedetomidine | 0.066 (0.060–0.072) | 57 | 0.023 (0.016–0.030) | 46 |
| Morphine | 29.4 (27.3–31.6) | 34 | 11.3 (9.6–13.4) | 40 |
Values represent ED50 (mg kg−1) (with 95% confidence intervals). n: number of mice.
Interactions between CP55,940, dexmedetomidine and morphine
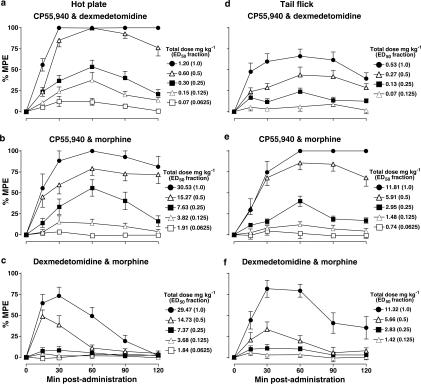
Dose- and time-dependent antinociceptive effects of the drug combinations (s.c.; 1 : 1 fixed ratio of ED50 of agonist A : ED50 of agonist B) in the hot plate and tail flick assays are shown in Figure 3. The time points at which the greatest antinociceptive effects were observed after coadministration of each combination were 60 min for CP55,940 with either dexmedetomidine or morphine (Figure 3a, b, d and e) and 30 min for dexmedetomidine with morphine (Figure 3c and f). Dose–response curves for drug combinations were then determined for the hot plate (Figure 4a) and tail flick (Figure 4b) assays. Dose fraction (an arbitrary value) ED50 values were determined and converted to absolute dose values for isobolographic analysis. The theoretical and experimental values of Zadd and Zmix, respectively, in the hot plate and tail flick assays (Table 2) were tested for significance with a modified Student's t-test. These values and their corresponding 95% confidence intervals are displayed as isobolograms in Figure 5.
Figure 3.
Dose- and time-dependent antinociceptive effects of paired combinations of CP55,940, dexmedetomidine and morphine in the hot plate (panels a–c) and tail flick (d–f) assays in conscious mice. Responses are expressed as %MPE (see Methods) from 0 min (bolus coadministration) to 120 min postadministration; symbols are mean±s.e.m. The total dose indicated is the sum of the doses of the two drugs being used in combination (mg kg−1). The combinations were of equal fractions (1.0, 0.5, 0.25, 0.125 or 0.0625) of each paired agonist's respective ED50 value (see Table 1) coadministered in a fixed 1 : 1 ratio of the ED50 of agonist A : ED50 of agonist B. For each curve, total n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 and dexmedetomidine n=52 and 38; CP55,940 and morphine n=57 and 45; and dexmedetomidine and morphine n=65 and 41. For each agonist dose combination, n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 and dexmedetomidine n=7–13 and 7–11; CP55,940 and morphine n=8–13 and 3–12; and dexmedetomidine and morphine n=12–16 and 10–11. %MPE values at the time point at which the greatest antinociceptive responses were observed for each respective combination (a, b, d, e: 60 min; c, f: 30 min) were used to plot the combination dose–response curves shown in Figure 4.
Figure 4.
Agonist dose–antinociceptive response curves for paired combinations of CP55,940, dexmedetomidine and morphine (s.c.) in the (a) hot plate and (b) tail flick assays in conscious mice. Responses are expressed as %MPE (see Methods). %MPE values at the time point at which peak antinociceptive responses were observed for each respective agonist (see Figure 3) were used to plot the curves. The total dose for each agonist combination is shown on the x-axis. Combinations were of equal fractions (1.0, 0.5, 0.25, 0.125 or 0.0625) of each paired agonist's respective ED50 value (see Table 1) coadministered in a fixed 1 : 1 ratio of the ED50 of agonist A : ED50 of agonist B. For each curve, total n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 and dexmedetomidine n=52 and 38; CP55,940 and morphine n=57 and 45; and dexmedetomidine and morphine n=65 and 41. For each agonist dose, n values in the hot plate and tail flick assays, respectively, were as follows: CP55,940 and dexmedetomidine n=7–13 and 7–11; CP55,940 and morphine n=8–13 and 3–12; and dexmedetomidine and morphine n=12–16 and 10–11. Symbols are mean±s.e.m.
Table 2.
Theoretical Zadd and experimental Zmix ED50 antinociceptive values obtained from the three drug combinations (s.c.) in the hot plate and tail flick assays
| Hot plate assay | Tail flick assay | |||||
|---|---|---|---|---|---|---|
| Drug combination | Zadd | Zmix | n | Zadd | Zmix | n |
| CP55,940+dexmedetomidine | 0.598 | 0.223* | 52 | 0.266 | 0.324 | 38 |
| CP55,940+morphine | 15.3 | 7.54* | 57 | 5.91 | 3.31* | 45 |
| Dexmedetomidine+morphine | 14.7 | 16.7 | 65 | 5.66 | 6.97 | 41 |
n: number of mice. Each combination was a 1 : 1 fixed ratio of the respective ED50 of drug A : ED50 of drug B. Zadd: theoretical dose required for a purely additive interaction; Zmix: experimental value determined from the combination dose–response curve (see Methods).
Statistically significant synergistic or supra-additive interaction (P<0.05).
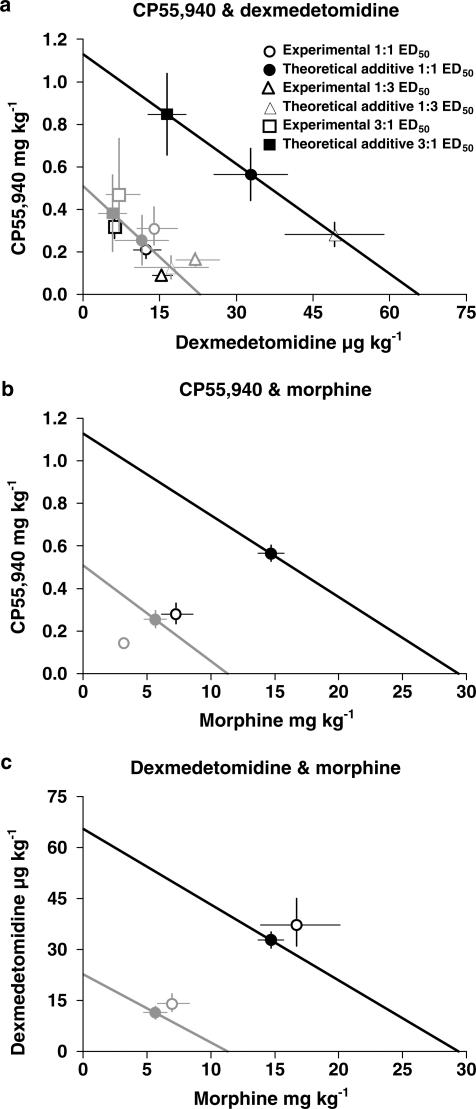
Figure 5.
Isobolograms for the effects of simultaneous s.c. administration of drug combinations (in 1 : 1 fixed ratio of ED50 of drug A : ED50 of drug B) in the hot plate (panels a–c) and tail flick (d–f) assays in conscious mice. The line in each panel connects the ED50 of drug A on the x-axis and the ED50 of drug B on the y-axis and represents the locus of points of dose combinations for purely additive interactions. Symbols represent theoretical additive and experimental ED50 values, with their associated 95% confidence intervals. The drug combinations (and interactions) were as follows: (a, d) CP55,940 and dexmedetomidine (synergy – hot plate; additive – tail flick); (b, e) CP55,940 and morphine (synergy – both assays); and (c, f) dexmedetomidine and morphine (additive – both assays).
Using 1 : 1 fixed ED50 ratios, the combination of CP55,940 and dexmedetomidine (s.c.) resulted in a significant synergistic or supra-additive interaction (Zmix<Zadd; P<0.05) in the hot plate assay (Figure 5a), but not in the tail flick assay (Figure 5d). CP55,940 and morphine caused significantly synergistic interactions in both the hot plate (Figure 5b) and tail flick (Figure 5e) assays. However, the dexmedetomidine and morphine combination only showed simple additive interactions in both hot plate (Figure 5c) and tail flick (Figure 5f) assays, with Zadd and Zmix not significantly different (Table 2).
The combination of CP55,940 and dexmedetomidine administered in a 1 : 3 and 3 : 1 ED50 ratio gave similar results as for the 1 : 1 ratio combination. Zadd and Zmix values were tested for significance (Table 3). Significantly synergistic interactions were observed for the hot plate assay (Figure 6a), whereas additive results were obtained with the tail flick assay (Figure 6b). In Figure 7, isobolograms for both the hot plate and tail flick experiments are shown plotted on the same scale for each drug combination to allow a direct comparison of assay sensitivity.
Table 3.
Theoretical Zadd and experimental Zmix ED50 antinociceptive values for CP55,940 and dexmedetomidine in 1 : 1, 3 : 1 and 1 : 3 ratios (s.c.) in the hot plate and tail flick assays
| CP55,940 : dexmedetomidine | Hot plate assay | Tail flick assay | ||||
|---|---|---|---|---|---|---|
| Fixed ED50 ratio | Zadd | Zmix | n | Zadd | Zmix | n |
| 1 : 1 | 0.598 | 0.223* | 52 | 0.266 | 0.324 | 38 |
| 3 : 1 | 0.86 | 0.33* | 47 | 0.39 | 0.48 | 48 |
| 1 : 3 | 0.33 | 0.11* | 48 | 0.15 | 0.19 | 53 |
n: number of mice. Each combination was a fixed ratio (1 : 1, 3 : 1 or 1 : 3) of the ED50 of CP55,940:ED50 of dexmedetomidine. Zadd: theoretical dose required for a purely additive interaction; Zmix: experimental value determined from the combination dose–response curve (see Methods).
Statistically significant synergistic or supra-additive interaction (P<0.05).
Figure 6.
Isobolograms for the effects of simultaneous s.c. administration of CP55,940 with dexmedetomidine in 1 : 1, 3 : 1 and 1 : 3 fixed ratios of ED50 of drug A (dexmedetomidine) : ED50 drug of B (CP55,940) in the (a) hot plate and (b) tail flick assays in conscious mice. The line in each panel connects the ED50 of drug A on the x-axis and the ED50 of drug B on the y-axis and represents the locus of points of dose combinations for purely additive interactions. Symbols represent theoretical additive and experimental ED50 values, with their associated 95% confidence intervals. Synergistic interactions were found for all dose ratios in the hot plate assay, while additive interactions occurred in the tail flick assay.
Figure 7.
Isobolograms for the effects of simultaneous s.c. administration of drug combinations in hot plate and tail flick assays in conscious mice. The lines (black: hot plate; grey: tail flick) in each panel connect the ED50 of drug A on the x-axis and the ED50 of drug B on the y-axis and represent the locus of points of dose combinations for purely additive interactions. Symbols represent theoretical additive and experimental ED50 values, with their associated 95% confidence intervals. The drug combinations were (a) CP55,940 and dexmedetomidine; (b) CP55,940 and morphine; and (c) dexmedetomidine and morphine. Fixed ratios of ED50 of drug A : ED50 of drug B were 1 : 1 (a–c), 3 : 1 (a) and 1 : 3 (a) for the hot plate (black) and tail flick (grey) assays, respectively. Note that for each agonist, the ED50 values for antinociception in the hot plate assay were generally three-fold greater than in the tail flick assay.
Discussion
We found that single s.c. administration of a cannabinoid, α2-adrenoceptor or μ opioid receptor agonist was antinociceptive when tested in either the hot plate or tail flick assays in mice. Of great interest were the findings from combinations of two drugs to test for synergy or simple additivity. Our results showed firstly that a cannabinoid agonist combined with a μ opioid agonist displayed synergy in the tail flick and hot plate assays, and secondly that a cannabinoid agonist combined with an α2-adrenoceptor agonist showed simple additivity in the tail flick assay, but synergy in the hot plate assay. In both assays, combined α2-adrenoceptor and μ opioid receptor activation was simply additive. These findings indicate that only some combined receptor activations are capable of synergy and that they may be specific to the spinal and supraspinal pathways involved in each type of acute pain stimulus.
The isobolographic analysis we used is a relatively robust method for testing drug combination in vivo. This technique is vulnerable to agonists with different slopes in their individual dose–response curves (Pöch, 1993). To test this possibility, we applied 1 : 1, 3 : 1 and 1 : 3 drug ratios and found consistent results of synergy for α2-adrenoceptor and cannabinoid receptor activation in hot plate, and additivity for tail flick, assays (Figure 6). Our results therefore support the conclusion of synergy for α2-adrenoceptor and cannabinoid agonists.
This work shows that the three drugs were consistently more potent (with lower ED50 values) as antinociceptive agents in the tail flick assay by three-fold for dexmedetomidine and morphine and two-fold for CP55,940. This apparent increase in sensitivity may be explained by stress-induced analgesia (Lewis et al., 1980) caused by the brief restraint of the conscious mice for this test. It is well documented that even brief restraint, or simply frequent handling, of rats and mice elicits a stress response typified by increased levels of endogenous opioid agonists, as well as catecholamines (Yamada & Nabeshima, 1995; Dunn, 1999; D'Arbe et al., 2002) and endocannabinoids (Hohmann, 2002). Further, both supraspinal and spinal mechanisms have been shown to be involved in restraint stress-induced enhancement of opioid analgesia (reviewed in Yamada & Nabeshima, 1995). Another possibility is that the choice of a 12 s cutoff time for the tail flick assay, compared to the 50 s of the hot plate test, may have led to an ‘apparent' increased sensitivity due to this ‘arbitrary' maximum, designed to avoid tissue injury (Le Bars et al., 2001). It is most unlikely, however, that the increased sensitivity to the agonists in the tail flick assay (compared with the hot plate test) contributed to the type of interactions found in this study as the cannabinoid and μ opioid agonist combination displayed synergy in both tail flick and hot plate assays, while α2-adrenoceptor and μ opioid agonist combination consistently displayed additivity (Figure 7).
The mechanism(s) of the synergistic interactions cannot be established by this study. The possible mechanisms include (i) receptor colocation with amplified signal transduction; (ii) pre- and postjunctional stimulation or inhibition; or (iii) receptor stimulation by endogenous opioid, α2-adrenoceptor or cannabinoid ligands. The synergistic interaction between opioid and cannabinoid agonists has not been completely elucidated although two possible mechanisms have been postulated (Welch et al., 1995b; Manzanares et al., 1999). The first proposed mechanism is that of signal transduction interactions. Opioid and cannabinoid receptors are both Gi/o protein-coupled receptors, with similar intracellular signalling mechanisms (Welch et al., 1995b; Manzanares et al., 1999; Pertwee, 2001; Przewlocki & Przewlocka, 2001). The assumption is that both opioid and cannabinoid receptors coexist on neurones and therefore share a common pool of G proteins. The coupling of these receptors to a common G protein family may lead to inter-receptor signalling whereby activation of one receptor causes redistribution of its G proteins, which increase the sensitivity of the other receptor (Djellas et al., 2000). Another possibility is that cannabinoid agonists induce synthesis and release of endogenous opioid agonists leading to potentiation of opioid antinociception (Cichewicz & McCarthy, 2003); the spinal dynorphin system and other opioid peptides have been implicated (Manzanares et al., 1999; Houser et al., 2000). In the rat, CP55,940 (i.t.) has been shown to cause release of dynorphin B, which contributes to its antinociceptive actions (Pugh et al., 1997). However, the same study found that neither CP55,940 nor dynorphin B itself potentiated the effects of coadministered morphine in the spinal cord of mice (Pugh et al., 1997).
CP55,940 and dexmedetomidine interact in a synergistic fashion in the more complex (compared with the tail flick response) supraspinally controlled behavioural response to the hot plate noxious stimulus. Such an interaction may be due to the similarities between the two systems. For instance, both CB1 receptors and α2-adrenoceptors are primarily found on presynaptic membranes and have similar signal transduction, including presynaptic modulation of primary afferent neurones, inhibition of postsynaptic membrane excitability and sensory neurone Ca2+ conductance and related neurotransmitter release; other interactions may also exist (Yaksh, 1985; Furst, 1999; Howlett et al., 2002). Both receptors are found in areas of the CNS that process and modulate nociceptive information, involving both spinal and supraspinal components (Behbehani, 1995; Lichtman et al., 1996; Martin & Lichtman, 1998). Cannabinoid receptors are found in the substantia gelatinosa of the spinal cord, suggesting modulation of nociceptive input from primary afferent neurones, and both cannabinoid receptors and α2-adrenoceptors are found in the peripheral nervous system on primary afferent neurones (Khan et al., 1999; Pertwee, 2001; Howlett et al., 2002). However, some studies suggest that the antinociceptive actions of α2-adrenoceptors are purely mediated at the spinal level, mainly via the α2A-subtype present in the substantia gelatinosa (Jones et al., 1982; Takano & Yaksh, 1992; Hamalainen & Pertovaara, 1995; Stone et al., 1998; Khan et al., 1999; Kamibayashi & Maze, 2000; Malmberg et al., 2001). Stimulation of α2-adrenoceptors in the spinal cord causes inhibition of afferent nociceptive impulses from Aδ and C fibres, as well as modulation of substance P and calcitonin gene-related peptide release in the dorsal horn (Yaksh, 1985; Takano et al., 1993; Furst, 1999; Khan et al., 1999).
Results for the combination of CP55,940 and dexmedetomidine in the mouse tail flick assay were simply additive, implying that cannabinoid receptors and α2-adrenoceptors cause antinociception in this spinal reflex by independent mechanisms or sites of action. However, in conflict with the latter, Lichtman & Martin (1991) found that Δ9-THC (i.v.) activated descending noradrenergic pathways in the rat spinal cord, which caused antinociception (tail flick assay) via activation of spinal α2-adrenoceptors. It may be that Δ9-THC activates or modulates different receptors or mechanisms compared to CP55,940 given the complexity of the descending control pathways (Millan, 2002). Further, in the rodent spinal cord, i.t. Δ9-THC elicits release of dynorphin A, whereas CP55,940 causes release of dynorphin B (Pugh et al., 1997; Houser et al., 2000). Opioid agonists may also act supraspinally on spinal function through the activation of descending noradrenergic inhibitory pathways. So, considering the similarities in CNS locations and cell signalling mechanisms of α2-adrenoceptors and opioid receptors, the two systems may be anticipated to elicit synergistic antinociceptive actions. In rats, Ossipov et al. (1990) found that the racemate medetomidine (dexmedetomidine is the dextro-isomer) had a synergistic interaction with morphine in the tail flick, but not hot plate, assay when the drugs were administered to the spinal cord. However, the interaction was only additive in both assays when systemic administration was used (Ossipov et al., 1990), which supports our results in mice. Thus, both the route of drug administration and the particular (spinal vs supraspinal) nociceptive pathways involved in responses to types of acute pain appear to determine whether drug combinations will have additive or synergistic effects.
The fact that very low doses of antinociceptive agonists may be used in combination to cause effective pain relief has great potential clinical benefit. Single administration of dexmedetomidine is associated with side effects such as hypotension, dry mouth, impairment of memory and psychomotor performance, as well as sedation (Hall et al., 2000). Opioid use may cause constipation, respiratory depression and the development of tolerance and dependence, while cannabinoid drugs elicit central side effects including psychomotor impairment and sedation (Ashton, 2001). Synergistic drug combinations may improve effective pharmacotherapy of pain as the lower clinical dose requirements for each agent will minimise drug-specific adverse effects (Raffa et al., 2003).
In conclusion, this study highlights the potential advantage of combining two antinociceptive drugs acting through specific receptor systems to lower the dose of each required for antinociception. That the dose of each can be decreased to less than that for a simple additive effect suggests that a major theoretical advantage can be ascribed to some but not all combinations. Thus, this study in mice demonstrated that an α2-adrenoceptor agonist or μ opioid receptor agonist when combined with a cannabinoid receptor agonist showed significant potentiation or synergy in hot plate antinociception. For tail flick antinociception, only cannabinoid and μ opioid receptor synergy was demonstrated. If these findings translate to the human clinical situation, then judicial selection of dose and receptor-specific agonists may allow a better therapeutic window from unwanted side effects.
Acknowledgments
We thank Mrs Kirsten A. Messick for expert research assistance and Dr Arthur Christopoulos for helpful advice on isobolographic and statistical analyses.
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- MPE
maximum possible effect
- Zadd
theoretical doses of a purely additive interaction for an ED50 effect
- Zmix
experimental doses of drug combination for an ED50 effect
References
- ASHTON C.H. Pharmacology and effects of cannabis: a brief review. Br. J. Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- BEHBEHANI M.M. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- CHAPERON F., THIEBOT M.H. Behavioral effects of cannabinoid agents in animals. Crit. Rev. Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- CICHEWICZ D.L., MCCARTHY E.A. Antinociceptive synergy between Δ9-tetrahydrocannabinol and opioids after oral administration. J. Pharmacol. Exp. Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- COURSIN D.B., MACCIOLI G.A. Dexmedetomidine. Curr. Opin. Crit. Care. 2001;7:221–226. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- D'AMOUR F.E., SMITH D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- D'ARBE M., EINSTEIN R., LAVIDIS N.A. Stressful animal housing conditions and their potential effect on sympathetic neurotransmission in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1422–R1428. doi: 10.1152/ajpregu.00805.2000. [DOI] [PubMed] [Google Scholar]
- DJELLAS Y., ANTONAKIS K., LE BRETON G.C. Shifts in the affinity distribution of one class of seven-transmembrane receptors by activation of a separate class of seven-transmembrane receptors. Biochem. Pharmacol. 2000;59:1521–1529. doi: 10.1016/s0006-2952(00)00296-3. [DOI] [PubMed] [Google Scholar]
- DUNN A.J. Brain catecholaminergic and tryptophan responses to restraint are attenuated by nitric oxide synthase inhibition. Neurochem. Int. 1999;33:551–557. doi: 10.1016/s0197-0186(98)00064-3. [DOI] [PubMed] [Google Scholar]
- FAIRBANKS C.A., STONE L.S., KITTO K.F., NGUYEN H.O., POSTHUMUS I.J., WILCOX G.L. α2C-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J. Pharmacol. Exp. Ther. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- FURST S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- HALL J.E., UHRICH T.D., BARNEY J.A., ARAIN S.R., EBERT T.J. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth. Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- HAMALAINEN M.M., PERTOVAARA A. The antinociceptive action of an α2-adrenoceptor agonist in the spinal dorsal horn is due to a direct spinal action and not to activation of descending inhibition. Brain Res. Bull. 1995;37:581–587. doi: 10.1016/0361-9230(95)00044-f. [DOI] [PubMed] [Google Scholar]
- HARRIS L.S., PIERSON A.K. Some narcotic antagonists in the benzomorphan series. J. Pharmacol. Exp. Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- HOHMANN A.G. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem. Phys. Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- HORVATH G., SZIKSZAY M., RUBICSEK G., BENEDEK G. An isobolographic analysis of the hypnotic effects of combinations of dexmedetomidine with fentanyl or diazepam in rats. Life Sci. 1992;50:L215–L220. doi: 10.1016/0024-3205(92)90071-v. [DOI] [PubMed] [Google Scholar]
- HOUSER S.J., EADS M., EMBREY J.P., WELCH S.P. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Δ9-THC and anandamide. Brain Res. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- JONES D.J., KENDALL D.E., ENNA S.J. Adrenergic receptors in rat spinal cord. Neuropharmacology. 1982;21:191–195. doi: 10.1016/0028-3908(82)90162-9. [DOI] [PubMed] [Google Scholar]
- KAMIBAYASHI T., MAZE M. Clinical uses of α2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- KHAN Z.P., FERGUSON C.N., JONES R.M. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- LE BARS D., GOZARIU M., CADDEN S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- LEWIS J.W., CANNON J.T., LIEBESKIND J.C. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., COOK S.A., MARTIN B.R. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- LICHTMAN A.H., MARTIN B.R. Cannabinoid-induced antinociception is mediated by a spinal α2-noradrenergic mechanism. Brain Res. 1991;559:309–314. doi: 10.1016/0006-8993(91)90017-p. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., HEDLEY L.R., JASPER J.R., HUNTER J.C., BASBAUM A.I. Contribution of α2 receptor subtypes to nerve injury-induced pain and its regulation by dexmedetomidine. Br. J. Pharmacol. 2001;132:1827–1836. doi: 10.1038/sj.bjp.0704032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANZANARES J., CORCHERO J., ROMERO J., FERNANDEZ-RUIZ J.J., RAMOS J.A., FUENTES J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- MARTIN B.R., LICHTMAN A.H. Cannabinoid transmission and pain perception. Neurobiol. Dis. 1998;5:447–461. doi: 10.1006/nbdi.1998.0218. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., SIERRALTA F., PINARDI G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br. J. Pharmacol. 2002;135:1591–1597. doi: 10.1038/sj.bjp.0704599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORISSET V., AHLUWALIA J., NAGY I., URBAN L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. Eur. J. Pharmacol. 2001;429:93–100. doi: 10.1016/s0014-2999(01)01309-7. [DOI] [PubMed] [Google Scholar]
- OSSIPOV M.H., HARRIS S., LLOYD P., MESSINEO E., LIN B.S., BAGLEY J. Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology. 1990;73:1227–1235. doi: 10.1097/00000542-199012000-00022. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptor ligands: clinical and neuropharmacological considerations, relevant to future drug discovery and development. Expert Opin. Investig. Drugs. 2000;9:1553–1571. doi: 10.1517/13543784.9.7.1553. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- PÖCH G. Combined Effects of Drugs and Toxic Agents: Modern Evaluation in Theory and Practice. 1–51. Wien: Springer-Verlag; 1993. pp. 117–121. [Google Scholar]
- PRZEWLOCKI R., PRZEWLOCKA B. Opioids in chronic pain. Eur. J. Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- PUGH G., JR, MASON D.J., JR, COMBS V., WELCH S.P. Involvement of dynorphin B in the antinociceptive effects of the cannabinoid CP55,940 in the spinal cord. J. Pharmacol. Exp. Ther. 1997;281:730–737. [PubMed] [Google Scholar]
- RAFFA R.B., CLARK-VETRI R., TALLARIDA R.J., WERTHEIMER A.I. Combination strategies for pain management. Expert Opin. Pharmacother. 2003;4:1697–1708. doi: 10.1517/14656566.4.10.1697. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A., O'SULLIVAN S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE L.S., BROBERGER C., VULCHANOVA L., WILCOX G.L., HÖKFELT T., RIEDL M.S., ELDE R. Differential distribution of α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. J. Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO M., TAKANO Y., YAKSH T.L. Release of calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal polypeptide (VIP) from rat spinal cord: modulation by α2 agonists. Peptides. 1993;14:371–378. doi: 10.1016/0196-9781(93)90055-l. [DOI] [PubMed] [Google Scholar]
- TAKANO Y., YAKSH T.L. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J. Pharmacol. Exp. Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- TALLARIDA R.J. Drug Synergism and Dose Effect Data Analysis. New York: Chapman & Hall–CRC; 2000. [Google Scholar]
- TALLARIDA R.J. Drug synergism: its detection and applications. J. Pharmacol. Exp. Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- THAM S.M., ANGUS J.A., TUDOR E., WRIGHT C.E.Interactions between CP55,940, dexmedetomidine and morphine in a mouse acute pain model Proceedings of the Australian Health and Medical Research Congress 2002 (ISSN 1447–6010) 20022027pMelbourne
- WELCH S.P., DUNLOW L.D., PATRICK G.S., RAZDAN R.K. Characterization of anandamide- and fluoroanandamide-induced antinociception and cross-tolerance to Δ9-THC after intrathecal administration to mice: blockade of Δ9-THC-induced antinociception. J. Pharmacol. Exp. Ther. 1995a;273:1235–1244. [PubMed] [Google Scholar]
- WELCH S.P., THOMAS C., PATRICK G.S. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J. Pharmacol. Exp. Ther. 1995b;272:310–321. [PubMed] [Google Scholar]
- YAKSH T.L. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol. Biochem. Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- YAMADA K., NABESHIMA T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav. Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]