Abstract
Nociceptin/orphanin FQ (N/OFQ) is the endogenous peptide ligand for a specific G-protein coupled receptor, the N/OFQ peptide receptor (NOP). The N/OFQ-NOP receptor system has been reported to play an important role in pain, anxiety and appetite regulation. In airways, N/OFQ was found to inhibit the release of tachykinins and the bronchoconstriction and cough provoked by capsaicin.
Here we evaluated the effects of NOP receptor activation in bronchoconstriction and airway microvascular leakage induced by intraesophageal (i.oe.) hydrochloric acid (HCl) instillation in rabbits. We also tested the effects of NOP receptor activation in SP-induced plasma extravasation and bronchoconstriction.
In anesthetized New Zealand rabbits bronchopulmonary function (total lung resistance (RL) and dynamic compliance (Cdyn)) and airway microvascular leakage (extravasation of Evans blue dye) were evaluated.
Infusion of i.oe. HCl (1 N) led to a significant increase in bronchoconstriction and plasma extravasation in the main bronchi and trachea of rabbits pretreated with propranolol, atropine and phosphoramidon.
Bronchoconstriction and airway microvascular leakage were inhibited by N/OFQ (3–30 μg kg−1 i.v.) in a dose-dependent manner. The NOP receptor agonist [Arg14,Lys15]N/OFQ mimicked the inhibitory effect of N/OFQ, being 10-fold more potent, UFP-101, a peptide selective NOP receptor antagonist, blocked the inhibitory effects of both agonists.
Under the same experimental conditions, N/OFQ and [Arg14,Lys15]N/OFQ did not counteract the bronchoconstriction and airway microvascular leakage induced by substance P.
These results suggest that bronchoconstriction and airway plasma extravasation induced by i.oe. HCl instillation are inhibited by activation of prejunctional NOP receptors.
Keywords: N/OFQ; [Arg14,Lys15]N/OFQ; NOP receptor; gastroesophageal reflux; inflammation; bronchoconstriction
Introduction
Nociceptin/orphanin FQ (N/OFQ) (Meunier et al., 1995; Reinscheid et al., 1995) is the endogenous peptide ligand for a specific opioid like G-protein coupled receptor recently named N/OFQ peptide receptor (NOP) (Cox et al., 2000). The N/OFQ-NOP receptor system has been reported to play an important role in various central functions such as pain, anxiety, memory, locomotion and appetite regulation as well as in the periphery on the cardiovascular, renal, gastrointestinal and airway systems (Calò et al., 2000; Mogil & Pasternak, 2001). NOP receptors are located both in the CNS and in the periphery, and mRNA is expressed in upper vagal sensory ganglia, where cell bodies of the tachykinin-containing sensory neurons are located (Peiser et al., 2000). In addition, N/OFQ-immunoreactive nerve fibers in the airway wall, distinct from the tachykinin-containing fibers, were identified as a pulmonary source of N/OFQ (Fischer et al., 1998; Peiser et al., 2000).
Recent data demonstrated that in the CNS, activation of NOP receptors by N/OFQ inhibits the release of several neurotransmitters such as noradrenaline, serotonin, acetylcholine and glutamate (Schlicker & Morari, 2000). In airways, N/OFQ was found to inhibit the contractions of the guinea-pig isolated bronchus induced by electrical field stimulation (Fischer et al., 1998; Shah et al., 1998; Rizzi et al., 1999), an effect that is mediated by a prejunctional mechanism and not involving the classical opioid receptors. Other studies showed that N/OFQ inhibits acetylcholine release in the trachea (Patel et al., 1997) and capsaicin-induced bronchoconstriction in isolated lungs (Corboz et al., 2000). In vivo studies have shown that N/OFQ inhibits bronchoconstriction and cough induced by capsaicin in guinea-pigs (McLeod et al., 2001; Jia et al., 2002) or by mechanical stimulation of intrathoracic airways in the cat (Bolser et al., 2001). Finally, N/OFQ inhibits guinea-pig airway inflammation induced by esophageal hydrochloric acid (HCl) instillation (Rouget et al., 2004). Collectively, these studies demonstrate that N/OFQ may influence airway physiology by modulating cholinergic and/or tachykininergic neurotransmission.
Gastroesophageal reflux (GER) disease is a common condition contributing to cough, bronchoconstriction and airway inflammation (Theodoropoulos et al., 2002), and is frequent in asthmatic patients (Harding et al., 2000). Several lines of evidence suggest that GER may induce moderate bronchoconstriction in dogs (Mansfield et al., 1981; Ishikawa et al., 1999), rabbits (Gallelli et al., 2003), and in asthmatic patients (Spaulding et al., 1982; Hervè et al., 1986). Esophageal HCl perfusion also induced airway plasma extravasation in guinea-pigs (Hamamoto et al., 1997; Daoui et al., 2002). The mechanism by which GER might aggravate asthmatic symptoms remains unclear. Two major mechanisms have been proposed: (i) microaspiration of acid contents into the airways (reflux theory) (Ishikawa et al., 1999; Ricciardolo, 2001); (ii) involvement of vagal and sensory nerve terminals in the lower esophagus (reflex theory) (Mansfield & Stein, 1978; Kjellen et al., 1981; Hervè et al., 1986).
Although instillation of acid into airways could induce bronchoconstriction in animal models, pulmonary regurgitation of refluxed stomach contents, has not been clearly demonstrated in asthmatic patients with GER (Ghaed & Stein, 1979; Berquist et al., 1981). In contrast, the involvement of the vagus nerve and cholinergic neurotransmission in GER induced bronchoconstriction has been demonstrated in both animal and human studies (Mansfield et al., 1981; Hervè et al., 1986; Colson et al., 1990; Advenier et al., 2002).
The role of sensory nerves and of tachykinins in the airway effects of intraesophageal (i.oe.) HCl infusion has been demonstrated in animals. Indeed, airway plasma extravasation or bronchoconstriction (increase in total lung resistance (RL) and decrease in dynamic compliance (Cdyn)) induced by i.oe. HCl infusion in guinea-pigs (Hamamoto et al., 1997; Daoui et al., 2002) or rabbits (Daoui et al., 2002; Gallelli et al., 2003) is abolished by pretreatment with capsaicin, a drug that induces a depletion of tachykinins from sensory nerves or by treatment with neurokinin receptor antagonists (Hamamoto et al., 1997; Daoui et al., 2002; Gallelli et al., 2003). Tachykinin involvement in bronchoconstriction and airway microvascular leakage has been demonstrated with tachykinin receptor antagonists in our previous paper using this experimental model of GER (Gallelli et al., 2003).
Therefore, the aim of the present study was to evaluate in the rabbit the effects of N/OFQ in airway microvascular leakage and bronchoconstriction induced by i.oe. HCl instillation. We also tested the effects of recently described selective and potent NOP receptor agonist, [Arg14,Lys15]N/OFQ (Okada et al., 2000; Rizzi et al., 2002), and antagonist UFP-101 (Calò et al., 2002), in this experimental model of GER.
Methods
Animals
Rabbits (2.5–2.8 kg) of either sex were used throughout the study. Animals were housed at constant temperature (21±1°C), relative humidity (55±5%), under a regular light–dark schedule (light 0700–1900). Food and water were freely available. The experimental procedures were in accordance with Italian DL. 116/92.
Bronchopulmonary function measurement
Animals were subjected to neuroleptoanalgesia (hypnorm 0.4 ml kg−1; a mixture of fentanyl citrate 0.315 mg ml−1 and fluanisone 10 mg ml−1, i.m.) and intubated with endotracheal and endoesophageal tubes for the measurement of flow and pleural pressure. Total lung resistance RL and Cdyn values were evaluated through an on-line respiratory analyzer (PMS version 8.4, Mumed Ltd, London).
Esophageal stimulation with HCl
Following neuroleptoanalgesia, the thoracic wall was partly sectioned at the level of the 3rd–5th tracheal cartilage ring and a catheter placed in the midesophagus for HCl infusion. The upper portion of the esophagus was ligated to inhibit HCl leakage, while a latex balloon attached at the end of endoesophageal tube for pleural pressure, blocked all communication between esophagus and stomach.
Measurement of airway microvascular leakage
Vascular permeability was quantified by the extravasation of Evans blue dye (Rogers et al., 1988). Evans blue dye (30 mg kg−1) was injected into the rabbit's ear marginal vein followed 1-min later by i.oe. HCl 1 N or saline (0.4 ml). After the induction of leakage (10 min after completion of the i.oe. HCl infusion), the thorax was opened and a blunt ended 13-14-gauge needle was passed through a left ventriculotomy into the aorta. The ventricule was cross-clamped and blood was expelled through an incision into the right atrium at 80 mmHg pressure with 700 ml of phosphate buffer. The lung was then removed and dye concentration was evaluated as previously described (Daoui et al., 2002).
Experimental protocol
All animals were pretreated 30 min before experimentation with atropine (1 mg kg−1, i.p.) and propranolol (1 mg kg−1, i.p.) to block muscarinic and beta-adrenergic receptors, and with phospharamidon (1 mg kg−1, i.p.) to inhibit tachykinin degradation. To evaluate the effect of NOP receptor activation on bronchoconstriction and airway plasma extravasation, N/OFQ (3, 10 and 30 μg kg−1) or [Arg14,Lys15]N/OFQ (0.3 and 3 μg kg−1), an NOP receptor agonist, or their saline vehicle were injected 1 min before Evans blue.
In a complementary series of experiments, UFP-101, an NOP receptor antagonist, or its vehicle was injected at the dose of 100 μg kg−1 10 min before NOP receptor agonist injection. In a separate set of experiments, NOP receptor activation on SP-induced bronchoconstriction and airway plasma extravasation was evaluated. SP (0.3 μg kg−1) was injected intravenously (i.v.) 5 min before Evans blue dye injection. Bronchoconstriction and leakage were evaluated 10 min later. N/OFQ was used at a dose of 30 μg kg−1 and [Arg14,Lys15]N/OFQ at a dose of 3 μg kg−1.
Drugs
Atropine sulfate, Evans blue dye, formamide, phosphoramidon, propranolol, HCl and SP were obtained from Sigma, Italy. Hypnorm was obtained from Janssen Pharmaceutical Ltd, Grove, Oxfordshire, U.K. [Arg14,Lys15]N/OFQ, UFP-101 and N/OFQ were synthesized according to Guerrini et al. (1997). All the drugs used were dissolved in 0.9% NaCl.
Statistical analysis
All data are expressed as mean±standard error (s.e.m.). Statistical evaluation was performed by analysis of variance (ANOVA), followed by the Student–Newman–Keuls post-test. The threshold of statistical significance was set at P<0.05.
Results
Effect of NOP receptor activation on HCl-induced bronchoconstriction
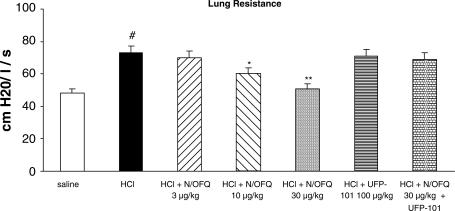
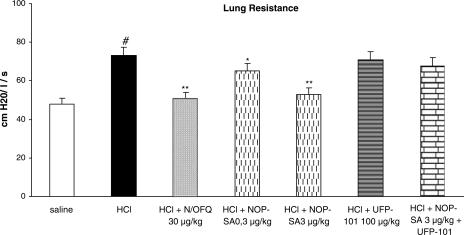
i.oe. HCl (1 N) infusion induced significant bronchoconstriction (P<0.01), in terms of RL increase (Figure 1) and Cdyn decrease (data not shown). This effect was dose-dependently decreased by N/OFQ treatment (P<0.01) (Figure 1). Similar results were obtained using [Arg14,Lys15]N/OFQ, an NOP receptor agonist (Figure 2); however, the N/OFQ analog was found to be approx 10-fold more potent than the natural peptide. Pretreatment with the selective NOP receptor antagonist (UFP-101; 100 μg kg−1, i.v.) alone did not affect HCl induced bronchoconstriction, but blocked the effects of both N/OFQ and [Arg14,Lys15]N/OFQ (Figures 1 and 2).
Figure 1.
Effects of N/OFQ (3, 10 and 30 μg kg−1) on bronchoconstriction induced by i.oe. HCl infusion (1 N) in anesthetized rabbits with or without NOP receptor antagonist (UFP-101) pretreatment. Rabbits were pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). UFP-101 was administered 10 min before agonists. All values are expressed as mean±s.e.m., n=5 per group; #P<0.01 vs saline; *P<0.05 vs HCl; **P<0.01 vs HCl.
Figure 2.
Comparison of the effects of NOP-SA (selective agonist of NOP receptor: [Arg14,Lys15]N/OFQ) with respect to N/OFQ (30 μg kg−1) on bronchoconstriction induced by i.oe. HCl infusion (1 N) in anesthetized rabbits with or without NOP receptor antagonist (UFP-101) pretreatment. Rabbits were pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). UFP-101 was administered 10 min before agonists. All values are expressed as mean±s.e.m., n=5 per group. #P<0.01 vs saline; *P<0.05 vs HCl; **P<0.01 vs HCl.
Effect of NOP receptor activation on HCl-induced plasma extravasation
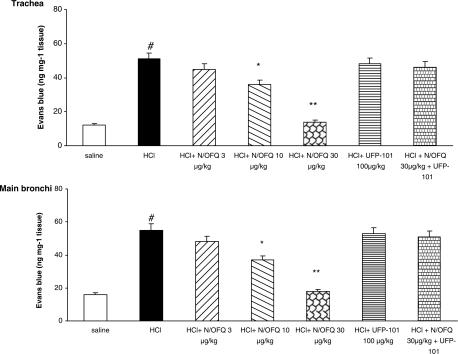
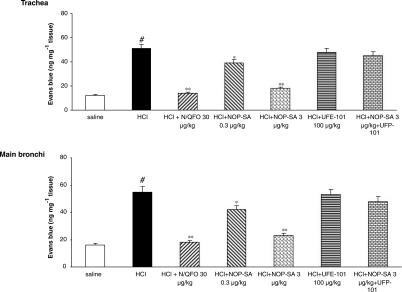
i.oe. HCl (1 N) infusion significantly increased plasma extravasation in the trachea and main bronchi. N/OFQ produced a significant, dose-dependent decrease of HCl on microvascular leakage in both trachea and main bronchi (Figure 3). Treatment with [Arg14,Lys15]N/OFQ (3 μg kg−1, i.v.) produced a similar inhibitory effect to that of 30 μg kg−1, i.v. N/OFQ (Figure 4). The NOP receptor antagonist UFP-101 (100 μg kg−1, i.v.) alone had no effect on HCl-induced microvascular leakage, but blocked the inhibitory effects of both NOP agonists (Figures 3 and 4).
Figure 3.
Effects of N/OFQ (3, 10 and 30 μg kg−1) on the microvascular leakage induced by i.oe. HCl infusion (1 N) in anesthetized rabbits with or without NOP receptor antagonist (UFP-101) pretreatment. Rabbits were pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). UFP-101 was administered 10 min before agonists. All values are expressed as mean±s.e.m., n=5 per group. #P<0.01 vs saline; *P<0.05 vs HCl; **P<0.01 vs HCl.
Figure 4.
Comparison of the effects of NOP-SA (selective agonist of NOP receptor: [Arg14,Lys15]N/OFQ), with respect to N/OFQ (30 μg kg−1) on the microvascular leakage induced by i.oe. HCl infusion (1 N) in anesthetized rabbits with or without NOP receptor (UFP-101) pretreatment. Rabbits were pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). UFP-101 was administered 10 min before agonists. All values are expressed as mean±s.e.m., n=5 per group. #P<0.01 vs saline; *P<0.05 vs HCl; **P<0.01 vs HCl.
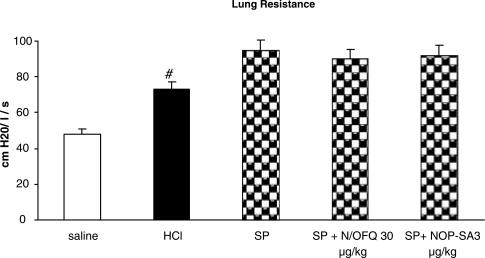
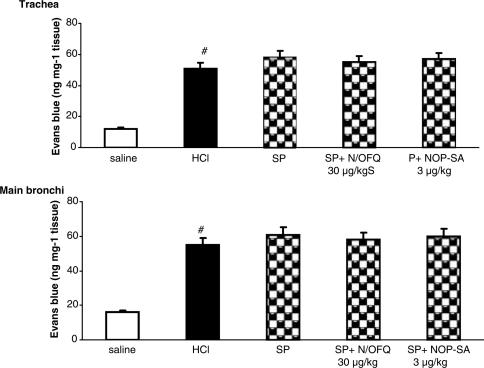
Effect of NOP receptor activation on SP-induced bronchoconstriction and airway microvascular leakage
SP (0.3 μg kg−1 i.v.) administration induced a significant bronchoconstriction (P<0.01), in terms of RL increase (Figure 5) and Cdyn decrease (data not shown), and significantly increased plasma extravasation in the trachea and main bronchi (Figure 6). Both N/OFQ and [Arg14,Lys15]N/OFQ, at doses active against i.oe. HCl infusion, did not produce any effect on SP-induced bronchoconstriction or plasma extravasation (Figures 5 and 6).
Figure 5.
Effects of N/OFQ and NOP-SA (selective agonist of NOP receptor: [Arg14,Lys15]N/OFQ) on SP-induced bronchoconstriction in anestethized rabbits pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). SP (0.3 μg kg−1, i.v.) was injected 5 min before dye infusion. Data are expressed as mean±s.e.m., n=5 per group; #P<0.01 vs saline.
Figure 6.
Effects of N/OFQ and NOP-SA (selective agonist of NOP receptor: [Arg14,Lys15]N/OFQ) on SP-induced microvascular leakage of anestethized rabbits pretreated with atropine (1 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). SP (0.3 μg kg−1, i.v.) was injected 5 min before dye infusion. Data are expressed as mean±s.e.m., n=5 per group. #P<0.01 vs saline.
Discussion
In the present study we have documented the effects of NOP receptor activation on lung responses in the rabbit induced by i.oe. HCl infusion. In addition to our previous findings in the guinea-pig (Rouget et al., 2004), we have shown that HCl induced plasma extravasation but also bronchoconstriction were significantly reduced by a pretreatment with N/OFQ (10–30 μg kg−1) and the highly potent NOP receptor agonist [Arg14,Lys15]N/OFQ (0.3–3 μg kg−1) (Okada et al., 2000; Rizzi et al., 2002). We have also shown that the effects of both agonists were abolished by the selective NOP receptor antagonist UFP-101 (Calò et al., 2002), suggesting that their preventive actions on vascular leakage and bronchoconstriction are exclusively due to NOP receptor activation and share common mechanisms.
In our experimental model, we observed that the NOP receptor selective agonist [Arg14,Lys15]N/OFQ was able to mimic N/OFQ effects being approx 10-fold more potent than the natural peptide. These results are in line with previous data demonstrating that [Arg14,Lys15]N/OFQ binds and fully activates the recombinant human NOP receptor with affinity/potency higher than N/OFQ while maintains high selectivity over classical opioid receptors (Okada et al., 2000). This pharmacological profile was later confirmed at native NOP receptors; in fact, [Arg14,Lys15]N/OFQ behaved in various N/OFQ sensitive tissues as full agonist, displayed higher potency than N/OFQ (by three to more than 10 folds), and, more importantly, its effects were antagonized by NOP antagonists ([Nphe1]N/OFQ(1-13)NH2 and J-113397) with pA2 values similar to those obtained against N/OFQ (Rizzi et al., 2002).
More importantly, the actions of both NOP agonists were prevented by the peptide antagonist UFP-101 (Calò et al., 2000). The NOP selective antagonist properties of this molecule have been demonstrated in a variety of in vitro preparations ranging from cells expressing recombinant receptors (Calò et al., 2000; McDonald et al., 2003), to isolated tissues (Calò et al., 2000), and to brain preparations investigated with neurochemical (Marti et al., 2004; Mela et al., 2004) or electrophysiological (Gavioli et al., 2004; Marti et al., 2004) techniques. The pharmacological activity of UFP-101 has been also confirmed in vivo in rodents after central and peripheral administration where the peptide antagonized N/OFQ actions on pain transmission (Calò et al., 2000), locomotor activity (Calò et al., 2000; Marti et al., 2004), neurotransmitter release (Koizumi et al., 2004; Marti et al., 2004) and cardiovascular parameters (Hashiba et al., 2003). Moreover, similar to other NOP selective antagonists (i.e. [Nphe1]N/OFQ(1-13)NH2 and J-113397) (Redrobe et al., 2002) UFP-101 produced antidepressant like effects in mice and rats (Gavioli et al., 2003; 2004). Interestingly enough, the antagonist/agonist dose ratio used in the present experiments for blocking N/OFQ effects is similar to that used in the above-mentioned studies (range 1–10) further suggesting that these drugs interact with the same functional sites, namely the NOP receptor.
As sensory nerves activation and endogenous tachykinins release seem to be involved in our experimental model of GER in the rabbit (Gallelli et al., 2003), our results suggest that NOP receptor activation inhibits sensory nerve activity. These results are in agreement with several lines of evidence indicating inhibitory actions of N/OFQ on sensory nerves in the guinea-pig, such as inhibition of the contraction of the isolated bronchus induced by EFS (Fischer et al., 1998), of capsaicin-induced bronchoconstriction of isolated lung (Corboz et al., 2000), of airway microvascular leakage induced by i.oe. HCl (Rouget et al., 2004), or in human isolated bronchi, inhibition of airway hyperresponsiveness induced by beta2-adrenoceptor agonists (Faisy et al., 2003). In addition, in this study, we also showed that N/OFQ and [Arg14,Lys15]N/OFQ did not inhibit SP-induced bronchoconstriction and microvascular leakage in airways, suggesting that NOP agonists do not interact with postsynaptic neurokinin receptors, but exert their inhibitory effects on sensory nerves at presynaptic sites.
Since endogenous N/OFQ has been localized on nerve fibers within guinea-pig bronchus, and NOP receptor mRNA is expressed in jugular ganglion neuron cells, a direct inhibitory effect of N/OFQ on the release of tachykinins can be suggested (Fischer et al., 1996; 1998). Moreover, N/OFQ inhibits the micturition reflex in rats (Lecci et al., 2000) and in patients with neurogenic incontinence (Lazzeri et al., 2001; 2003) through its ability to activate inhibitory NOP receptor expressed on bladder C-fibers.
However, a vagally mediated autonomic pathway may also be involved in the experimental models described here, as Hamamoto et al. (1997) have shown that airway leakage induced by HCl was abolished in guinea-pigs by bilateral vagotomy. However, in our study, the effects of N/OFQ do not involve a cholinergic component of vagal activity since animals were pretreated by atropine. Our data cannot rule out a central action of N/OFQ. Indeed, several reports have documented the involvement of tachykinins (Mutoh et al., 2000, Mazzone & Canning, 2002) in sensory nerve-mediated reflexes (Mazzone & Geraghty, 1999) related to the central control of cardiovascular and respiratory function by nucleus of the solitary tract (NTS). Since activation of NOP receptors, largely expressed in the NTS (Bunzow et al., 1994, Anton et al., 1996; Mao & Wang, 2000a), is able to reduce the NTS neuronal responsiveness to baroreceptor input (Mao & Wang, 2000a, 2000b), we may thus suggest that N/OFQ is able to reduce sensory nerve excitability in this area (Dong et al., 2002) and consequently to inhibit the bronchoconstriction and airway microvascular leakage evoked following esophageal acidification.
However, as N/OFQ is a long (17 aa) peptide and contains several positively charge residues, it is likely that the nerve endings and/or nerve connections between the esophagus and airways in the autonomic ganglia (Myers & Undem, 1993; Myers et al., 1996), where anatomical studies have demonstrated the presence of axon collaterals from visceral afferent fibers (gastrointestinal tract, bladder, airways) (Coleridge et al., 1989; Mawe, 1995; Canning et al., 1996; Myers et al., 1996), represent the site of action of N/OFQ.
Interestingly, Canning et al. (1998) have reported in an in vitro preparation of guinea-pig trachea and esophagus with their respective nerves that capsaicin elicited a concentration-dependent relaxation of the trachea only when the adjacent esophagus was intact. On the basis of the ability of tetrodotoxin to abolish the effect of capsaicin and of additional immunochemistry studies, it was concluded that the responses of the guinea-pig trachea induced by esophagus stimulation were mediated by nerve fibers from parasympathetic ganglia that are intrinsic to the adjacent esophagus (Canning et al., 1998). Similarly, in the present study, bronchoconstriction and airway microvascular leakage following esophageal acidification may occur as a result of the activation of the intermediate neurons including those from parasympathetic ganglia, by HCl stimulation of capsaicin-sensitive fibers in the esophagus.
Moreover, acid in the distal esophagus precipitates cough, and there is evidence for an esophageal–tracheobronchial cough reflex mechanism in patients with chronic cough associated with GER (Irwin et al., 1993). As sensory C-fibers also play a role in the generation of cough, this study may contribute to a better understanding of the pathophysiological mechanisms responsible for cough related to GER, thus improving pharmacotherapy.
In conclusion, our results suggest that bronchoconstriction and airway plasma extravasation induced by i.oe. HCl instillation are inhibited by NOP receptor activation, suggesting a possible use of N/OFQ and NOP receptor agonists for future investigations of the mechanisms involved in gastroesophageal-reflux induced bronchoconstriction and inflammation in asthmatic patients, and may represent a novel approach to the treatment of airway diseases relate to GER.
Abbreviations
- Cdyn
dynamic compliance
- CNS
central nervous system
- GER
gastroesophageal reflux
- HCl
hydrochloric acid
- N/OFQ
nociceptin/orphanin FQ
- NOP
N/OFQ peptide receptor
- NOP-SA
selective agonist of NOP receptor: [Arg14,Lys15]N/OFQ
- NTS
nucleus of the solitary tract
- RL
total lung resistance
- SP
substance P
References
- ADVENIER C., D'AGOSTINO B., CUI Y.Y., EUTAMENE H., ROUGET C., BARDOU M., BUENO L.Animal models for investigating gastroesophageal reflux disease-induced asthma Experimental and Clinical Pharmacology of Gastroesophageal Reflux-induced Asthma 2002Pisa: Pacini Editore; 51–54.ed. Dal Negro, R.W., Geppetti, P. & Morice, A.H., pp [Google Scholar]
- ANTON B., FEIN J., TO T., LI X., SILBERSTEIN L., EVANS C.J. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- BERQUIST W.E., RACHELEFSKY G.S., KADDEN M., SIEGEL S.C., KATZ R.M., FONKALSRUD E.W. Ament ME. Gastroesophageal reflux-associated recurrent pneumonia and chronic asthma in children. Pediatrics. 1981;68:29–35. [PubMed] [Google Scholar]
- BOLSER D.C., MCLEOD R.L., TULSHIAN D.B., HEY J.A. Antitussive action of nociceptin in the cat. Eur. J. Pharmacol. 2001;430:107–111. doi: 10.1016/s0014-2999(01)01244-4. [DOI] [PubMed] [Google Scholar]
- BUNZOW W.J.R., SAEZ C., MORTRUD M., BOUVIER C., WILLIAMS J.T., LOW M., GRANDY D.K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- CALÒ G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALÒ G., RIZZI A., RIZZI D., BIGONI R., GUERRINI R., MARZOLA G., MARTI M., MCDONALD J., MORARI M., LAMBERT D.G., SALVADORI S., REGOLI D. [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002;136:303–311. doi: 10.1038/sj.bjp.0704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANNING B.J., FISCHER A., UNDEM B.J. Pharmacological analysis of the tachykinin receptors that mediate activation of nonadrenergic, noncholinergic relaxant nerves that innervate guinea pig trachealis. J. Pharmacol. Exp. Ther. 1998;284:370–377. [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J., KARAKOUSIS P.C., DEY R.D. Effects of organotypic culture on parasympathetic innervation of guinea pig trachealis. Am. J. Physiol. 1996;271:L698–L706. doi: 10.1152/ajplung.1996.271.5.L698. [DOI] [PubMed] [Google Scholar]
- COLERIDGE H.M., COLERIDGE J.C., SCHULTZ H.D. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol. Ther. 1989;42:1–63. doi: 10.1016/0163-7258(89)90021-1. [DOI] [PubMed] [Google Scholar]
- COLSON D.J., CAMPBELL C.A., WRIGHT V.A., WATSON B.W. Predictive value of oesophageal pH variables in children with gastro-oesophageal reflux. Gut. 1990;31:370–373. doi: 10.1136/gut.31.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBOZ M.R., RIVELLI M.A., EGAN R.W., TULSHIAN D., MATASI J., FAWZI A.B., BENBOW L., SMITH-TORHAN A., ZHANG H., HEY J.A. Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur. J. Pharmacol. 2000;402:171–179. doi: 10.1016/s0014-2999(00)00505-7. [DOI] [PubMed] [Google Scholar]
- COX B.M., CHAVKIN C., CHRISTIE M.J., CIVELLI O., EVANS C., HAMON M.D., HOELLT V., KIEFFER B., KITCHEN I., MCKNIGHT A.T., MEUNIER J.C., PORTOGHESE P.S.Opioid receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000London: IUPHAR Media Ltd; 321–333.ed. Girdlestone, D., pp [Google Scholar]
- DAOUI S., D'AGOSTINO B., GALLELLI L., ALT X.E., ROSSI F., ADVENIER C. Tachykinins and airway microvascular leakage induced by HCl intra-oesophageal instillation. Eur. Respir. J. 2002;20:268–273. doi: 10.1183/09031936.02.00250902. [DOI] [PubMed] [Google Scholar]
- DONG X.W., WILLIAMS P.A., JIA Y.P., PRIESTLEY T. Activation of spinal ORL-1 receptors prevents acute cutaneous neurogenic inflammation: role of nociceptin-induced suppression of primary afferent depolarization. Pain. 2002;96:309–318. doi: 10.1016/S0304-3959(01)00460-2. [DOI] [PubMed] [Google Scholar]
- FAISY C., NALINE E., ROUGET C., GUEROT E., FAGON J.Y., CHINET T., ADVENIER C. Nociceptin inhibits the in vitro fenoterol-induced neurosensitization of human bronchi. Am. J. Respir. Crit. Care Med. 2003;167:A148. doi: 10.1007/s00210-004-0974-x. [DOI] [PubMed] [Google Scholar]
- FISCHER A., FORSSMANN W.G., UNDEM B.J. Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea pig bronchus. J. Pharmacol. Exp. Ther. 1998;285:902–907. [PubMed] [Google Scholar]
- FISCHER A., MCGREGOR G.P., SARIA A., PHILIPPIN B., KUMMER W. Induction of tachykinin gene and peptide expression in guinea pig no dose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLELLI L., D'AGOSTINO B., MARROCCO G., DE ROSA G., FILIPPELLI W., ROSSI F., ADVENIER C. Role of tachykinins in the bronchoconstriction induced by HCl intraesophageal instillation in the rabbit. Life Sci. 2003;72:1135–1142. doi: 10.1016/s0024-3205(02)02372-x. [DOI] [PubMed] [Google Scholar]
- GAVIOLI E.C., MARZOLA G., GUERRINI R., BERTORELLI R., ZUCCHINI S., DE LIMA T.C., RAE G.A., SALVADORI S., REGOLI D., CALO G. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur. J. Neurosci. 2003;17:1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- GAVIOLI E.C., VAUGHAN C.W., MARZOLA G., GUERRINI R., MITCHELL V.A., ZUCCHINI S., DE LIMA T.C., RAE G.A., SALVADORI S., REGOLI D., CALO G. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:547–553. doi: 10.1007/s00210-004-0939-0. [DOI] [PubMed] [Google Scholar]
- GHAED N., STEIN M.R. Assessment of a technique for scintigraphic monitoring of pulmonary aspiration of gastric contents in asthmatics with gastroesophageal reflux. Ann. Allergy. 1979;42:306–308. [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIANCHI C., LAZARUS L.H., SALVADORI S., TEMUSSI P.A., REGOLI D. Address and message sequences for the nociceptin receptor: a structure–activity study of nociceptin-(1-13)-peptide amide. J. Med. Chem. 1997;40:1789–1793. doi: 10.1021/jm970011b. [DOI] [PubMed] [Google Scholar]
- HAMAMOTO J., KOHROGI H., KAWANO O., IWAGOE H., FUJII K., HIRATA N., ANDO M. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J. Appl. Physiol. 1997;82:738–745. doi: 10.1152/jappl.1997.82.3.738. [DOI] [PubMed] [Google Scholar]
- HARDING S.M., GUZZO M.R., RICHTER J.E. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am. J. Respir. Crit. Care Med. 2000;162:34–39. doi: 10.1164/ajrccm.162.1.9907072. [DOI] [PubMed] [Google Scholar]
- HASHIBA E., HIROTA K., KUDO T., CALO G., GUERRINI R., MATSUKI A. Effects of nociceptin/orphanin FQ receptor ligands on blood pressure, heart rate, and plasma catecholamine concentrations in guinea pigs. Naunyn Schmiedebergs Arch. Pharmacol. 2003;367:342–347. doi: 10.1007/s00210-003-0704-9. [DOI] [PubMed] [Google Scholar]
- HERVÉ P., DENJEAN A., JIAN R., SIMONNEAU G., DUROUX P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am. Rev. Respir. Dis. 1986;134:986–989. doi: 10.1164/arrd.1986.134.5.986. [DOI] [PubMed] [Google Scholar]
- IRWIN R.S., FRENCH C.L., CURLEY F.J., ZAWACKI J.K., BENNETT F.M. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104:1511–1517. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA T., SEKIZAWA T.I., SANT'AMBROGIO F.B., SANT'AMBROGIO G. Larynx vs esophagus as reflexogenic sites for acid induced bronchoconstriction in dogs. J. Appl. Physiol. 1999;86:1226–1230. doi: 10.1152/jappl.1999.86.4.1226. [DOI] [PubMed] [Google Scholar]
- JIA Y., WANG X., APONTE S.I., RIVELLI M.A., YANG R., RIZZO C.A., CORBOZ M.R., PRIESTLEY T., HEY J.A. Nociceptin/orphanin FQ inhibits capsaicin-induced guinea-pig airway contraction through an inward-rectifier potassium channel. Br. J. Pharmacol. 2002;135:764–770. doi: 10.1038/sj.bjp.0704515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJELLEN G., BRUNDIN A., TIBBLIG L., WRANNE B. Oesophageal function in asthmatics. Eur. J. Respir. Dis. 1981;62:87–94. [PubMed] [Google Scholar]
- KOIZUMI M., MIDORIKAWA N., TAKESHIMA H., MURPHY N.P. Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. J. Neurochem. 2004;89:257–263. doi: 10.1111/j.1471-4159.2003.02322.x. [DOI] [PubMed] [Google Scholar]
- LAZZERI M., CALO G., SPINELLI M., GUERRINI R., BENEFORTI P., SANDRI S., ZANOLLO A., REGOLI D., TURINI D. Urodynamic and clinical evidence of acute inhibitory effects of intravesical nociceptin/orphanin FQ on detrusor overactivity in humans: a pilot study. J. Urol. 2001;166:2237–2240. [PubMed] [Google Scholar]
- LAZZERI M., CALO G., SPINELLI M., GUERRINI R., SALVADORI S., BENEFORTI P., SANDRI S., REGOLI D., TURINI D. Urodynamic effects of intravesical nociceptin/orphanin FQ in neurogenic detrusor overactivity: a randomized, placebo-controlled, double-blind study. Urology. 2003;61:946–950. doi: 10.1016/s0090-4295(02)02587-6. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., MEINI S., MAGGI C.A. Nociceptin and the micturition reflex. Peptides. 2000;21:1007–1021. doi: 10.1016/s0196-9781(00)00241-2. [DOI] [PubMed] [Google Scholar]
- MANSFIELD L.E., HAMEISTER H.H., SPAULDING H.S., SMITH N.J., GLAB N. The role of the vague nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Ann. Allergy. 1981;47:431–434. [PubMed] [Google Scholar]
- MANSFIELD L.E., STEIN M.R. Gastroesophageal reflux and asthma: a possible reflex mechanism. Ann. Allergy. 1978;41:224–226. [PubMed] [Google Scholar]
- MAO L., WANG J.Q. Microinjection of nociceptin (Orphanin FQ) into nucleus tractus solitarii elevates blood pressure and heart rate in both anesthetized and conscious rats. J. Pharmacol. Exp. Ther. 2000a;294:255–262. [PubMed] [Google Scholar]
- MAO L., WANG J.Q. Pharmacological activation of nociceptin receptors in the nucleus tractus solitarius inhibits baroreceptor reflex in pentobarbital-anesthetized rats. Neuroscience. 2000b;101:435–440. doi: 10.1016/s0306-4522(00)00370-5. [DOI] [PubMed] [Google Scholar]
- MARTI M., MELA F., VERONESI C., GUERRINI R., SALVADORI S., FEDERICI M., MERCURI N.B., RIZZI A., FRANCHI G., BEANI L., BIANCHI C., MORARI M. Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J. Neurosci. 2004;24:6659–6666. doi: 10.1523/JNEUROSCI.0987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAWE G.M. Tachykinins as mediators of slow EPSPs in guinea-pig gall-bladder ganglia: involvement of neurokinin-3 receptors. J. Physiol. 1995;485:513–524. doi: 10.1113/jphysiol.1995.sp020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., CANNING B.J. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br. J. Pharmacol. 1999;127:473–481. doi: 10.1038/sj.bjp.0702522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD J., CALO G., GUERRINI R., LAMBERT D.G. UFP-101, a high affinity antagonist for the nociceptin/orphanin FQ receptor: radioligand and GTPgamma(35)S binding studies. Naunyn Schmiedebergs Arch. Pharmacol. 2003;367:183–187. doi: 10.1007/s00210-002-0661-8. [DOI] [PubMed] [Google Scholar]
- MCLEOD R.L., PARRA L.E., MUTTER J.C., ERICKSON C.H., CAREY G.J., TULSHIAN D.B., FAWZI A.B., SMITH-TORHAN A., EGAN R.W., CUSS F.M., HEY J.A. Nociceptin inhibits cough in the guinea-pig by activation of ORL(1) receptors. Br. J. Pharmacol. 2001;132:1175–1178. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELA F., MARTI M., ULAZZI L., VACCARI E., ZUCCHINI S., TRAPELLA C., SALVADORI S., BEANI L., BIANCHI C., MORARI M. Pharmacological profile of nociceptin/orphanin FQ receptors regulating 5-hydroxytryptamine release in the mouse neocortex. Eur. J. Neurosci. 2004;19:1317–1324. doi: 10.1111/j.1460-9568.2004.03220.x. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER L., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- MUTOH T., BONHAM A.C., JOAD J.P. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1215–R1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- MYERS A.C., UNDEM B.J. Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J. Physiol. 1993;470:665–679. doi: 10.1113/jphysiol.1993.sp019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS A., UNDEM B., KUMMER W. Anatomical and electrophysiological comparison of the sensory innervation of bronchial and tracheal parasympathetic ganglion neurons. J. Auton. Nerv. System. 1996;61:162–168. doi: 10.1016/s0165-1838(96)00081-1. [DOI] [PubMed] [Google Scholar]
- OKADA K., SUJAKU T., CHUMAN Y., NAKASHIMA R., NOSE T., COSTA T., YAMADA Y., YOKOYAMA M., NAGAHISA A., SHIMOHIGASHI Y. Highly potent nociceptin analog containing the Arg-Lys triple repeat. Biochem. Biophys. Res. Commun. 2000;278:493–498. doi: 10.1006/bbrc.2000.3822. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., GIEMBYCZ M.A., SPICUZZA L., BARNES P.J., BELVISI M.G. Naloxone-insensitive inhibition of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by the novel opioid, nociceptin. Br. J. Pharmacol. 1997;120:735–736. doi: 10.1038/sj.bjp.0701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEISER C., UNDEM B.J., FISCHER A. Nociceptin effects in the airways. Peptides. 2000;21:995–998. doi: 10.1016/s0196-9781(00)00239-4. [DOI] [PubMed] [Google Scholar]
- REDROBE J.P., CALO G., REGOLI D., QUIRION R. Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedeberg's Arch. Pharmacol. 2002;365:164–167. doi: 10.1007/s00210-001-0511-0. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L. Mechanisms of citric acid-induced bronchoconstriction. Am. J. Med. 2001;111:18S–24S. doi: 10.1016/s0002-9343(01)00816-6. [DOI] [PubMed] [Google Scholar]
- RIZZI A., CALO G., TREVISANI M., TOGNETTO M., FABBRI L., MAPP C., GUERRINI R., SALVADORI S., REGOLI D., GEPPETTI P. Nociceptin receptor activation inhibits tachykinergic non adrenergic non cholinergic contraction of guinea pig isolated bronchus. Life Sci. 1999;64:PL157–PL163. doi: 10.1016/s0024-3205(99)00045-4. [DOI] [PubMed] [Google Scholar]
- RIZZI A., RIZZI D., MARZOLA G., REGOLI D., LARSEN B.D., PETERSEN J.S., CALO G. Pharmacological characterization of the novel nociceptin/orphanin FQ receptor ligand, ZP120: in vitro and in vivo studies in mice. Br. J. Pharmacol. 2002;137:369–374. doi: 10.1038/sj.bjp.0704894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D.F., BELVISI M.G., AURSUDKIJ B., EVANS T.W., BARNES P.J. Effects and interactions of sensory neuropeptides on airway microvascular leakage in guinea-pigs. Br. J. Pharmacol. 1988;95:1109–1116. doi: 10.1111/j.1476-5381.1988.tb11745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUGET C., CUI Y.Y., D'AGOSTINO B., FAISY C., NALINE E., BARDOU M., ADVENIER C. Nociceptin inhibits airway microvascular leakage induced by HCl intra-oesophageal instillation. Br. J. Pharmacol. 2004;141:1077–1083. doi: 10.1038/sj.bjp.0705704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICKER E., MORARI M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- SHAH S., PAGE C.P., SPINA D. Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br. J. Pharmacol. 1998;125:510–516. doi: 10.1038/sj.bjp.0702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAULDING H.S., JR, MANSFIELD L.E., STEIN M.R., SELLNER J.C., GREMILLION D.E. Further investigation of the association between gastroesophageal reflux and bronchoconstriction. J. Allergy Clin. Immunol. 1982;69:516–521. doi: 10.1016/0091-6749(82)90176-2. [DOI] [PubMed] [Google Scholar]
- THEODOROPOULOS D.S., PECORARO D.L., EFSTRATIADIS S.E. The association of gastroesophageal reflux disease with asthma and chronic cough in the adult. Am. J. Respir. Med. 2002;1:133–146. doi: 10.1007/BF03256602. [DOI] [PubMed] [Google Scholar]