Abstract
The object of the present study was to clarify the neurotransmitters controlling membrane responses to electrical field stimulation (EFS) in the longitudinal smooth muscle cells of the chicken anterior mesenteric artery.
EFS (5 pulses at 20 Hz) evoked a depolarization of amplitude 19.7±2.1 mV, total duration 29.6±3.1 s and latency 413.0±67.8 ms. This depolarization was tetrodotoxin (TTX)-sensitive and its amplitude was partially decreased by atropine (0.5 μM); however, its duration was shortened by further addition of prazosin (10 μM).
Atropine/prazosin-resistant component was blocked by the nonspecific purinergic antagonist, suramin, in a dose-dependent manner, indicating that this component is mediated by the neurotransmitter adenosine 5′-triphosphate (ATP).
Neither desensitization nor blocking of P2X receptor with its putative receptor agonist α,β-methylene ATP (α,β-MeATP, 1 μM) and its antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic (PPADS, up to 50 μM), had significant effect on the purinergic depolarization. In contrast, either desensitization or blocking of P2Y receptor with its putative agonist 2-methylthioATP (2-MeSATP, 1 μM) and its antagonist Cibacron blue F3GA (CBF3GA, 10 μM) abolished the purinergic depolarization, indicating that this response is mediated through P2Y but not P2X receptor.
The purinergic depolarization was inhibited by pertussis toxin (PTX, 600 ng ml−1). Furthermore, it was significantly inhibited by a phospholipase C (PLC) inhibitor, U-73122 (10 μM), indicating that the receptors involved in mediating the purinergic depolarization are linked to a PTX-sensitive G-protein, which is involved in a PLC-mediated signaling pathway.
Data of the present study suggest that the EFS-induced excitatory membrane response occurring in the longitudinal smooth muscle of the chicken anterior mesenteric artery is mainly purinergic in nature and is mediated via P2Y purinoceptors.
Keywords: ATP, chicken, mesenteric artery, NANC
Introduction
Avian vasculature is of less interest than those of other species. Since about 4 decades, few morphological and functional studies have been conducted on chicken anterior mesenteric artery. Ball et al. (1963) found that the pattern of the anterior mesenteric artery of chicken differs structurally from the basic pattern observed in mammalian arteries, in which arterial smooth muscle is oriented circularly and helically (Furchgott, 1955). The difference in structure lies in the fact that the chicken anterior mesenteric artery contains two well-developed muscular components: an inner circular and an outer longitudinal layer (Ball et al., 1963; Bell, 1969a; Bolton, 1969; Bennett & Malfmors, 1970), analogous to the arrangement of the muscularis externa of the intestinal wall and venous wall (Yamamoto et al., 1984; Kennedy & Burnstock, 1985; Orre et al., 1996).
The anterior mesenteric artery of chicken has not only a specialized wall structure but also unusual innervation patterns, possibly reflecting enhanced capability for regional control of blood flow. Most mammalian arterial muscle is innervated by excitatory adrenergic nerves (Keatinge, 1966). In the anterior mesenteric artery of chicken, adrenergic fibers and varicose terminals are found at the adventitial-medial border in this as in other arteries, but these elements also extend into the longitudinal muscle itself. Longitudinal muscle of this vessel also receives cholinergic innervation (Bolton, 1967; Bell, 1969a; Bennett & Malfmors, 1970). Adrenergic nerve stimulation produces contraction of the circular muscle, which is mimicked and blocked by α-adrenergic agonists and antagonists, respectively (Bell, 1969a; Bolton, 1969; Gooden, 1980). In contrast, the longitudinal muscle is relaxed by adrenergic nerve stimulation, acting through β-adrenergic receptors; however, stimulation of cholinergic nerve fibers caused the longitudinal muscle to contract (Bolton, 1969; Bell, 1969a). Since then, there have been no further studies into the innervation of the chicken anterior mesenteric artery.
There is now a substantial body of evidence to show that norepinephrine (NE) and adenosine 5′-triphosphate (ATP) act as cotransmitters, being released from sympathetic nerves in variable proportions depending on the tissue and the species (Burnstock, 1986). In addition, it has been reported that ATP is colocalized with CGRP in capsaicin-sensitive sensory C-fibers innervating the mesenteric small artery of rabbit producing nonadrenergic, noncholinergic (NANC) relaxation (Kakuyama et al., 1998). It is also reported that in the longitudinal muscle of the rabbit portal vein (Kennedy & Burnstock, 1985) and in the longitudinal muscle of the portal vein of the guinea pig (Orre et al., 1996), ATP is mediating relaxation and contraction, respectively. The receptors mediating responses to ATP have been characterized as P2 purinoceptors (Burnstock, 1987). P2 purinoceptors have been subdivided into two major classes: P2X and P2Y purinoceptors (Abbracchio & Burnstock, 1994). P2X and P2Y receptors are ligand-gated ion channels (Brake et al., 1994; Valera et al., 1994) and G-protein-coupled receptors (Boarder et al., 1995), respectively. P2X purinoceptors were proposed to mediate contractile effects of ATP on smooth muscle. P2Y purinoceptors were proposed to mediate the relaxant effects of ATP on smooth muscle and/or endothelium (Ralevic et al., 1988).
In the present study, we investigate: (1) the possible contribution of NANC transmitters, like ATP, to the functional activity of the longitudinal muscle layer of the chicken mesenteric artery using microelectrode technique, (2) the subtype of P2 purinoceptors and the signal transduction mechanism involved in the membrane responses to electrical field stimulation (EFS).
Methods
Tissue preparation
Male white leghorn chickens aged 10–14 weeks were killed by dislocation of the neck. The anterior mesenteric artery was severed at its origin from the aorta and at the region of the sub-branches supplying the intestine, and placed in a physiological salt solution (PSS; see below), at room temperature. The distal portions of the anterior mesenteric artery were cleaned from connective tissue and the vessels were cannulated at the proximal end with a glass micropipette (200 μm tip diameter) attached to the gravity-driven perfusion apparatus to perfuse the vessels with warmed (29°C) PSS to remove blood from the vessels. Ethics and experimental procedures were approved by the Gifu University Animal Care and Use Committee and were in accordance with the Japanese Department of Agriculture guidelines, and all efforts were made to minimize animal suffering and to reduce the number of animals used.
Electrophysiological recordings
Arteries were placed in the partition chamber in which large extracellular silver–silver chloride plates were used to elicit nerve stimulation, as described previously (Bolton et al., 1984). The preparation was perfused at constant flow rate (3 ml min−1) with pre-warmed (29°C) PSS. This temperature was adopted in this experiment, since spontaneous activity, which makes recording from the cells difficult, was frequently produced at 35°C (see Results). Tissue preparations were allowed to equilibrate for approximately 1 h before experiments were undertaken. Membrane potentials were recorded with a conventional microelectrode technique, using glass capillary microelectrodes filled with 3 M KCl with tip resistances ranging from 50 to 80 MΩ (Takewaki & Ohashi, 1977). The microelectrode insertions were made into the longitudinal muscle cells through the adventitial side, within 2 mm of the stimulation electrode. Electrical activity was monitored on an oscilloscope (CS 4026, Kenwood, Tokyo, Japan) and recorded on a thermal-array recorder (RTA-1100 M, Nihon Kohden, Tokyo, Japan). EFS was applied by 15 V pulses of 1 ms duration using variable pulse numbers.
Focal application system
A pneumatic pump was used for the application of small quantities of ATP to localized regions and at a particular time, drugs were pressure-ejected from a micropipette with a pressure of 10 psi and pulse duration of 10 ms. Fiber-filled glass micropipettes (outside diameter=1 mm, inside diameter=0.5 mm) were drawn with a microelectrode puller (Narishige, Japan, type PP- 83), yielding an outside diameter of the tip of 20–30 μm. The pipette was then filled with 10–100 μM solution of ATP. To avoid possible desensitization due to leakage of drugs, the pipette was maintained at a distance from the preparation and positioned next to the electrode only after a stable impalement was obtained; drug application was initiated thereafter. After application of drug, the pipette was withdrawn.
Physiological solution
The physiological solutions used in this study had the following composition in mM: NaCl 118, KCl 4.6, CaCl2 2.7, MgCl2 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11. The solution in the supply reservoir was gassed continuously with 95% O2 : 5% CO2 gas mixture creating a pH of 7.2 and was warmed to 29°C.
Drugs
The following drugs have been used in the present study: atropine, prazosin, suramin, cibacron blue F3GA (CBF3GA), pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic (PPADS), ATP, 2-methylthio ATP (2-MeSATP), α,β-methylene ATP (α,β MeATP), 5-nitro-2(3-phenylpropylamino)benzoic acid (NPPB), PTX, 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122), 1-[6-[[17β)-3-methoxyestra-1,3,5(10)-trien-17yl]amino]hexyl]-2,5-pyrollidinedione (U73343), TTX and propranolol. All drugs have been purchased from Sigma (St Louis, MO, U.S.A.) and were serially diluted in the PSS solution to the required final concentration just before the experiments.
Statistics
Data are expressed as a mean±s.e.m.; (n) indicates the number of separate arteries in which electrical events were recorded. Statistical analysis was performed with Student's unpaired t-test, and P-values<0.05 were considered to be statistically significant.
Results
General observations
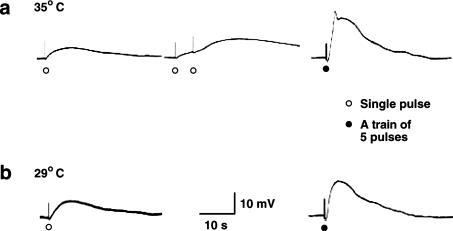
The mean resting membrane potential from longitudinal smooth muscle cells of chicken mesenteric artery was −34.6±3.1 mV (n=15). At a bath temperature of 35°C, many cells showed spontaneous activity, which made recording from the cells difficult. When recordings were successful, single pulse stimulation evoked slow depolarization (Figure 1a; left panel), which was graded in amplitude with stimulus strength. Application of single pulses at intervals of 4 s (0.25 Hz) resulted in facilitation and/or summation of depolarization. Incomplete action potential was usually initiated when 5 pulse stimulation at 20 Hz was used (Figure 1a; right panel), and with this stimulus strength the EFS-evoked depolarization may reach certain threshold depolarization at which the increased frequency of opening of voltage-gated calcium channels becomes so great as to produce a positive feedback and trigger action potentials that were associated with contractions, which often dislodged the microelectrodes. As the temperature was reduced to 29°C, EFS evoked slow depolarization; however, action potentials were often not observed (Figure 1b; right panel) even with stimulus strength over 5 pulse at 20 Hz; accordingly, contraction was also not observed and continuous recording from the cells could be achieved without dislodging the microelectrodes. Therefore, to establish a continuous recording from the cells and maintain microelectrode impalements when electrical stimulation was used, the bath temperature was lowered from 35 to 29°C. In such condition, EFS-evoked slow depolarization was constantly recorded. Table 1 shows parameters of depolarization evoked by single pulse of 1 ms duration and by 5 pulses at 20 Hz for the same duration of time. EFS-evoked depolarization elicited by 5 pulses at 20 Hz was selected for all experiments in this piece of research. The excitatory membrane responses were blocked by TTX, (0.3 μM), indicating that they are neuronal in origin.
Figure 1.
Depolarization produced by single and multiple nerve stimulation at 35 and 29°C bath temperature. (a) Depolarization produced by a single pulse (1 ms) (left panel), repetitive nerve stimulation by single pulses at 0.25 Hz resulted in facilitation and/or summation (middle panel) and 5 pulse at 20 Hz evoked depolarization with an initial spike (left panel) at 35°C bath temperature. (b) Depolarization produced by a single pulse (1 ms) (left panel) and 5 pulse at 20 Hz evoked depolarization without an initial spike (left panel) at 29°C bath temperature. Membrane potential values for (a) and (b) were −38 and −36 mV, respectively.
Table 1.
Parameters of depolarization evoked by single pulse and by 5 pulses at 20 Hz
| Amplitude (mV) | Latency (ms) | Time to peak (s) | Total duration (s) | |
|---|---|---|---|---|
| Single pulse (n=11) | 8.9±1.7 | 380.0±53.5 | 4.5±0.3 | 24.0±2.5 |
| 5 pulses (20 Hz) (n=28) | 19.7±2.1 | 413.0±67.8 | 4.0±0.4 | 29.6±3.1 |
Effects of atropine and prazosin on EFS-evoked depolarization
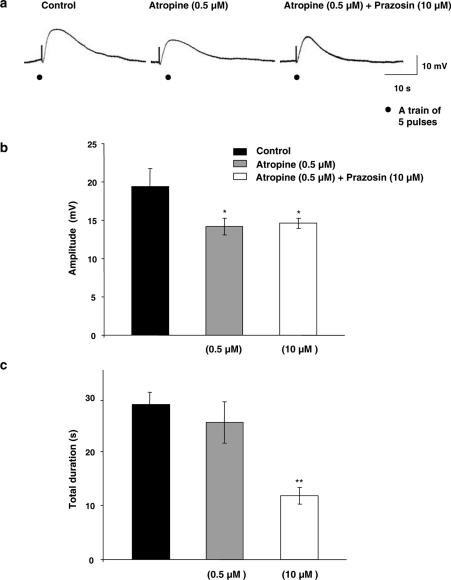
As shown in Figure 2a and b, atropine (0.5 μM), a muscarinic blocker, had significant (P<0.05) inhibitory effect on the amplitude of EFS-evoked depolarization, without affecting the resting membrane potential (−35.1±2.2 mV, n=7). Prazosin (10 μM), an α-adrenergic blocker, when added in the presence of atropine, significantly (P<0.01) decreased duration of EFS-evoked depolarization (Figure 2a and c), although it had no effect on its amplitude (Figure 2a and b), without affecting the resting membrane potential (−34.9±1.7 mV, n=12). The resistant component to the effects of atropine and prazosin has been taken as control in the following experiments for investigation of the contribution of NANC excitatory transmitter ATP.
Figure 2.
Effects of atropine and prazosin on the EFS-evoked depolarization. (a) Typical recordings showing the effects of atropine (0.5 μM) alone; n=7, and in combination with prazosin (10 μM); n=12, on the amplitude and duration of the depolarization evoked by EFS (1 ms, 5 pulses and 20 Hz). (b, c) Summary graphs showing the effects of atropine alone or combined with prazosin on the amplitude (b) and duration (c) of EFS-evoked depolarization. Membrane potential for (a) was −35 mV.
Effect of purinergic blockers on EFS-evoked depolarization
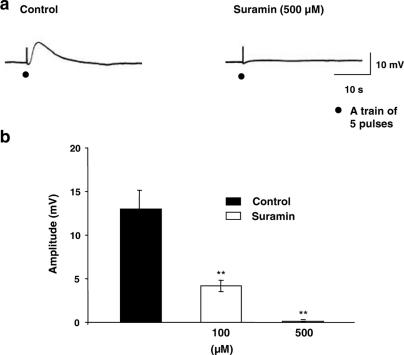
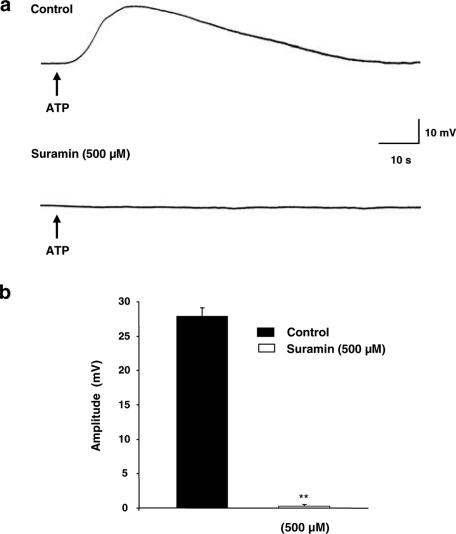
In the presence of atropine and prazosin, the nonspecific purinergic antagonist, suramin, showed dose-dependent inhibition of the amplitude of EFS-evoked depolarization (Figure 3b), with complete and significant (P<0.01) abolition upon application of 500 μM suramin (Figure 3a and b), without affecting the resting membrane potential (−36.1±1.4 mV, n=6).
Figure 3.
Effect of suramin on the EFS-evoked depolarization. (a) Typical recordings showing the effect of suramin (500 μM); n=6. (b) Summary graph showing concentration-dependent inhibition of suramin on the amplitude of the EFS-evoked depolarization. Membrane potential for (a) was −37 mV.
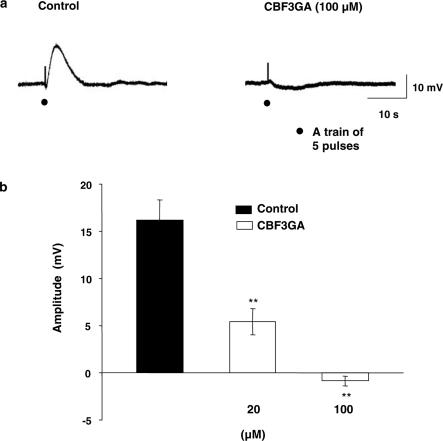
It is well established that ATP mediates its action through two types of purinergic receptors, namely P2X (mainly excitatory) and P2Y (mainly inhibitory) receptors. Therefore, the following experiments have been carried out to elucidate the receptor subtype through which ATP produces its EFS-evoked depolarization. The specific blocker for P2X receptor, PPADS (50 μM) had no effect on the recorded EFS-evoked depolarization (n=5). In contrast, P2Y-specific blocker CBF3GA exhibited dose-dependent inhibition of EFS-evoked depolarization (Figure 4a) with complete and significant (P<0.01) abolition at 100 μM (Figure 4a and b), without affecting the resting membrane potential (−33.7±3.5 mV, n=8). Moreover, the EFS-evoked depolarization was converted to a small inhibitory junction potential (IJP; −0.85±0.3 mV, n=8) (Figure 4a and b).
Figure 4.
Effect of CBF3GA on the EFS-evoked depolarization. (a) Typical recordings showing the effect of CBF3GA (100 μM); n=8, on the EFS-evoked depolarization. Note that EFS-evoked depolarization was converted to a small IJP. (b) Summary graph showing concentration-dependent inhibition of CBF3GA on the amplitude of EFS-evoked depolarization. Membrane potential for (a) was −34 mV.
Exogenous application of ATP and UTP
For confirmation of the contribution of ATP to the EFS-evoked depolarization exhibited by chicken mesenteric artery longitudinal smooth muscle cells upon EFS, ATP was applied exogenously using the pressure-ejection method. ATP (100 μM) produced slow depolarization (27.8±2.1 mV; n=6) (Figure 5a). This depolarization was not affected by PPADS. However, it was significantly (P<0.01) abolished by application of either suramin (500 μM) (Figure 5a and b) or CBF3GA (100 μM) (n=3 for each).
Figure 5.
Effect of ATP on membrane potential. (a) Typical recordings showing the effect of exogenous ATP (100 μM); n=6, on the resting membrane potential under control conditions (top) and after suramin (500 μM); n=3. (b) Summary graph showing the inhibitory effect of suramin (500 μM) on the depolarizing effect of ATP. Membrane potential for (a) was −39 mV.
UTP (100 μM) also upon its exogenous application produced slow depolarization (25.8±1.7 mV; n=3).
Effects of P2X and P2Y receptor agonists' desensitization on NANC EFS-evoked depolarization
To confirm subtype of P2 receptors, which mediated the inhibitory effects of suramin and CBF3GA on the NANC EFS-evoked depolarization, the putative P2X and P2Y receptor agonists, α,β-MeATP (1 μM) and 2-MeSATP (1 μM), respectively, have been used. Application of α,β-MeATP resulted in rapidly developing depolarization (37.8±3.1 mV; n=4) after which the membrane potential repolarized back to the baseline value. Application of P2Y receptor agonist 2-MeSATP resulted also in depolarization (31.2±3.3; n=4), although it was slow developing and long-lasting (the membrane potential repolarized back to the baseline value after 10.4±2.6 min) compared application at to α,β-MeATP.
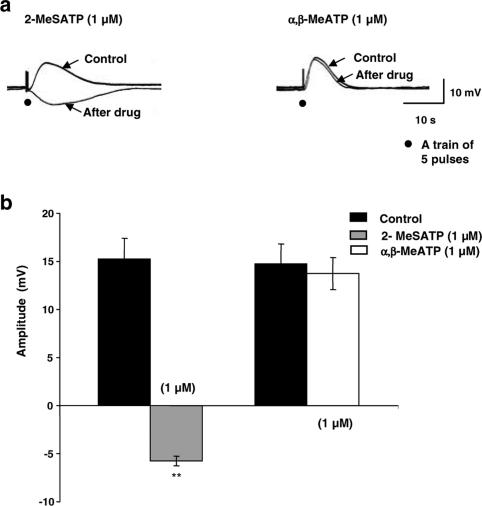
To desensitize P2 receptor subtypes, specimens were incubated for 30 min with the respective agonist and then the amplitude of EFS-evoked depolarization was recorded. By such pretreatment, the putative P2X receptor agonist α,β-MeATP had no effect on the EFS-evoked depolarization (Figure 6a right panel and b). In contrast, the putative P2Y receptor agonist 2-MeSATP significantly (P<0.01) inhibited the EFS-evoked depolarization and converted it to an inhibitory junction potential (IJP) (−5.75±1.6 mV; n=4) (Figure 6a left panel and b), which was propranolol-resistant (data not shown).
Figure 6.
Effect of desensitization of P2 receptors by their agonists on the EFS-evoked depolarization. (a) Typical recordings showing the effect of P2Y receptor agonist; 2-MeSATP (1μM); n=4, note that EFS-evoked depolarization was converted to a small IJP (left panel) and P2X receptor agonist; α,β-MeATP (1μM); n=4 (right panel) desensitization on EFS-evoked depolarization. (b) Summary graph showing the inhibitory effect of 2-MeSATP; and the noneffect of α,β-MeATP on the recorded EFS-evoked depolarization. Membrane potential for (a) was −36 and −34 mV for the left and right panel, respectively.
Effects of G-protein and PLC inhibitor on NANC EFS-evoked depolarization
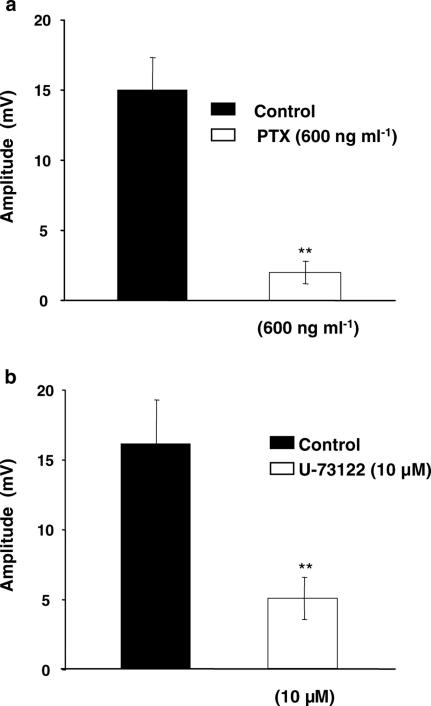
The results shown earlier suggested that the NANC EFS-evoked depolarization is mediated by P2Y receptors. P2Y receptors are variously coupled via PTX-sensitive and -insensitive G-proteins to PLC, stimulating inositol triphosphate formation and intracellular Ca2+ release. To obtain insight into the sensitivity of the G-protein involved to PTX, artery preparations were preincubated with PTX (600 ng ml−1) for 2 h and then the amplitude of EFS-evoked depolarization was recorded. By such pretreatment, the EFS-evoked depolarization was significantly (P<0.01) inhibited (Figure 7a), without affecting the resting membrane potential (−35.6±2.4 mV, n=5).
Figure 7.
Effect of pertussis toxin (PTX) and the phospholipase C inhibitor U-73122 on the EFS-evoked depolarization. (a) Histogram showing the sensitivity of the EFS-evoked depolarization to PTX (600 ng ml−1); n=5. (b) Histogram showing the inhibitory effect of U-73122 on the recorded EFS-evoked depolarization; n=5.
The application of the PLC inhibitor, U-73122, at 10 μM for 30 min significantly (P<0.01) inhibited (70%) the EFS-evoked depolarization (Figure 7b), without affecting the resting membrane potential (−34.3±2.7 mV, n=5). To exclude the possibility that U-73122 had inhibited the EFS-evoked depolarization by some nonspecific action, an isomer, U-73343, which lacks the inhibitory effect on PLC (Jin et al., 1994), was tested. This agent failed to inhibit the EFS-evoked depolarization; n=3 (data not shown).
Effect of Cl− channel blocker on NANC EFS-evoked depolarization
The application of the potent Cl− channel blocker 5-nitro-2(3-phenylpropylamino)benzoic acid (NPPB) at 20 μM for 15 min significantly (P<0.01) inhibited both the EFS-evoked depolarization (n=3) and the exogenous application of ATP and 2-MeSATP (n=3 for each) (data not shown).
Discussion
The present study provides evidence that ATP mediates the NANC EFS-evoked depolarization by acting on P2Y receptors in longitudinal smooth muscle of chicken anterior mesenteric artery. Supportive lines of evidence for this conclusion are: (i) sensitivity of the EFS-evoked depolarization to TTX, (ii) blockade by the nonspecific purinergic antagonist, suramin, as well as by the specific P2Y purinergic receptor antagonist, CBF3GA, which inhibited the depolarization evoked by EFS, (iii) exogenous application of ATP and 2-MeSATP produced slow developing depolarization, mimicking EFS-evoked depolarization, (iv) desensitization of P2Y purinergic receptor with 2-MeSATP-abolished EFS response or even exposed an underlying slow IJP, and (v) inhibition of the EFS-evoked depolarization to the G-protein inhibitor, PTX, and the PLC inhibitor U-73122.
To our knowledge, this is the first report shedding some light on the membrane potential properties of the longitudinal smooth muscle of chicken anterior mesenteric artery. The first characteristic property was that such smooth muscle cells have resting membrane potential of −34.6±3.1 mV, which is less negative than that of other circular vascular smooth muscle cells that always have a resting potential of −60 mV or lower (Levick, 1995). The resting membrane potential of longitudinal smooth muscle cells is nearer to that of intestinal smooth muscle. In addition, unlike most circular vascular smooth muscle cells, which are electrically quiescent when unstimulated, the longitudinal muscle cells of anterior mesenteric artery of chicken frequently exhibited spontaneous electrical activity that was inhibited by decreasing the bath temperature. In spite of lack of previous intracellular recordings from arterial longitudinal smooth muscle cells, these data may be consistent with those reported by Bolton (1968b), who recorded spontaneous activity of longitudinal muscle using sucrose-gap method. The activity consisted of large contractions, which occurred at about 30 s intervals, and these large contractions consisted of several smaller contractions each of which was accompanied by an action potential. It seems that the longitudinal smooth muscle activity together with the circular one would be well adapted to perform a peristaltic-like action or autonomous pulsation, which would help in increasing the rate of blood flow in distal branches supplying all viscera.
Unlike mammalian circular arterial smooth muscle cells in which EFS evoked fast excitatory junctional potentials (EJPs) with short latencies, 10–20 ms and total durations of 0.3–1 s (Burnstock & Holman, 1961; Bell, 1969b; Bennett, 1972; Hirst, 1977; Itoh et al., 1983; Hottenstein & Kreulen, 1987), EFS to mesenteric arterial longitudinal smooth muscle cells did not produce fast EJPs but depolarizations that were relatively slowly developing (380 ms latency; 4.5 s time to peak) and long lasting (24 s total duration), similar to those recorded from the guinea-pig mesenteric vein (Hirst & Jobling, 1989; Hottenstein & Kreulen, 1987; Suzuki, 1981). Such long latency of EFS-evoked depolarization recorded from chicken mesenteric arterial longitudinal smooth muscle cells might relate to the EFS-evoked depolarization being mediated via metabotropic receptor rather than the ionotropic one. This seems more or less similar to EJPs evoked at muscarinic (G-protein coupled) junctions, which, unlike ligand-gated junctions, have latencies ranging from 100 to 500 ms (Furness, 1969; Bauer et al., 1991; Cousins et al., 1993). In addition, the extraordinary long-lasting EFS-evoked depolarization suggests that the neurotransmitter(s) would stay for a long time at junctional receptor sites or that the neuromuscular transmission in the chicken anterior mesenteric artery may involve the activation of α-adrenoceptors, since the EJPs evoked at α-adrenoceptor junctions last for several seconds with prolonged rising and falling phases that reflect the activation of Ca2+-activated Cl− channels (Suzuki, 1981; 1983; van Helden, 1988a; Hill et al., 1993). Although further studies are needed to clarify the underlying mechanism of slowly developing and long-lasting EFS-evoked depolarization, these properties may be related to specific neuromuscular transmission in the chicken anterior mesenteric artery.
Application of atropine at its maximum inhibitory concentration (0.5 μM) partially inhibited the amplitude of EFS-evoked depolarization, indicating the small contribution of acetylcholine (ACh). This seems apparently different from what is reported by Bolton (1969) and Bell (1969a) that the excitatory mechanical response of chicken anterior longitudinal smooth muscle is cholinergic in nature. This discrepancy may give an indication that the cholinergic mechanical response is independent of changes in membrane potential and is mainly due to pharmacomechanical coupling. In presence of atropine, prazosin significantly decreased the total duration of EFS-evoked depolarization. Considering the substantial separation of the inhibitory effect of these blockers, it is rational to assume that the EFS-evoked response may comprise two components, in spite of the fact that there is no clear distinction between them: first and second slow depolarization. In support of this, it has been reported that EFS-evoked excitatory responses recoded from circular smooth muscle cells of rat tail artery (Sneddon & Burnstock 1984; McLaren et al., 1995) and guinea-pig ear artery (Morris et al., 1998) exhibit two clear components on their EJP; the first fast component was mainly purinergic in nature and mediated via P2X receptor and the second slow one was mainly adrenergic in nature and mediated via α-adrenoceptor. Similar to these reports where the contribution of norepinephrene (NE) via α-adreoceptor to the second slow component has been pointed out, the second slow depolarization recorded from longitudinal muscle cells of the anterior mesenteric artery of chicken is mediated at least, in part, by NE via α-adrenoceptor. This may be consistent with what has been reported by Bolton (1968a; 1969), who suggested a role of NE via α-adrenoceptor in mediating the excitatory response of the longitudinal muscle of the chicken mesenteric artery.
Many studies concerning ATP as an NANC neurotransmitter have been carried out on the circular vascular smooth muscle of different species including rat tail and hamster mesenteric artery (Sneddon & Burnstock, 1984; Bao et al., 1989; Thapaliya et al., 1999). In this study, we determined whether ATP is the mediator responsible for the first or atropine/prazosin-resistant component of EFS-evoked depolarization in the longitudinal smooth muscle cells of the anterior mesenteric artery of chicken. Our results showing that suramin completely abolished the NANC EFS-evoked depolarization provide further evidence that longitudinal smooth muscle, in addition to circular smooth muscle, is controlled by purinergic neurons. It should be noted, however, that different types of purinergic receptors are involved in the effects of ATP on circular and longitudinal smooth muscle. There are two main families through which ATP produces its action termed P2X receptor, which is ligand-gated ion channels, and P2Y receptor, which is G-protein-coupled. In most mammalian arterial smooth muscle, vasoconstriction or EJP evoked following ATP release from perivascular nerves is mediated predominantly by P2X receptors, while vasodilatation is mediated by smooth muscle P2Y receptors (P2Y1- like) (Ralevic & Burnstock, 1998). In the present study, the NANC EFS-evoked depolarization was resistant to either PPADS, the specific P2X receptor antagonist, or α,β-MeATP desensitization, indicating a minor contribution of P2X receptor to the NANC EFS-evoked depolarization. In contrast, the NANC EFS-evoked depolarization was completely abolished by both CBF3GA, a specific P2Y receptor antagonist, and 2-MeSATP desensitization. It is thus most probable that in the chicken mesenteric arterial longitudinal smooth muscle, unlike most mammalian arterial smooth muscle, ATP mediates its excitatory response via P2Y receptor rather than P2X. Interestingly, this is quite consistent with veins in which slow depolarization induced by nerve stimulation is resistant to purinoceptor desensitization with α,β-MeATP (Hirst & Jobling, 1989). If it is taken into consideration that analogous electrophysiological properties (i.e., slow onset and long-lasting depolarization) are also found in the longitudinal smooth muscle of the chicken mesenteric artery along with that of the vein, it can be expected that the arterial longitudinal smooth muscle plays a similar role to that of venous smooth muscle; for example, in enhancing blood flow to distal branches supplying the intestine.
Exogenous application of ATP and 2-MeSATP produced membrane depolarization. Exogenous application of UTP also produced membrane depolarization, which was approximately equal to that of ATP, providing further supportive evidence for the excitatory role of P2Y receptors upon their stimulation. P2Y receptors are also present on some vascular smooth muscle and mediate vasoconstriction to exogenous application of purines and pyrimidines (Mutafova-Yambolieva et al., 2000; Malmsjö et al., 2003; Miyagi et al., 2004). On vascular smooth muscle, all of the so far cloned P2Y receptors where the second messenger pathway has been determined are coupled via Gq protein to PLC, stimulating the synthesis of the diacylglycerol, inositol triphosphate formation and intracellular Ca2+ release (Erlinge, 1998). It is apparent that this pathway is relatively time consuming and thus the response time of P2Y receptors is longer than that of the rapid responses mediated by the ligand-gated ion channels P2X receptors. This may be consistent with our finding of slow development and slow decay of recorded EFS-evoked depolarization. In case of desensitization of P2Y receptor using 2-MeSATP, the EFS-evoked depolarization has been completely abolished and converted into IJP. This IJP seems to be NANC in nature as it was resistant to the β-adrenergic receptor blocker, propranolol. It is clearly of further interest to elucidate the mediator(s) of this IJP.
Not only the P2Y purinergic agonist 2-MeSATP but also the P2X purinergic agonist α,β-MeATP produced membrane depolarization upon its exogenous application, indicating that both types of P2-purinergic receptors are present in the longitudinal smooth muscle cells. The discrepancy between EFS-evoked depolarization and agonist-induced depolarization would be explained on the basis of purinergic receptors distribution throughout the cell membrane; P2X receptor may be distributed only in the extrajunctional space and responsible for depolarization produced by exogenously applied α,β-MeATP as well as ATP. On the other hand, P2Y receptor may be distributed both intra- and extrajunctionally and responsible for depolarization produced by EFS and exogenously applied 2-MeSATP as well as ATP. This may be parallel with what has been recorded by Hirst & Jobling (1989), where their observations indicate that P2 purinoceptors are present on guinea-pig mesenteric veins; however, they are not involved in the generation of the venous EJPs.
Currently, eight mammalian P2Y receptor subtypes have been cloned and functionally characterized, namely, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000; Communi et al., 2001; Nicholas, 2001; Sak & Webb, 2002). P2Y receptors are variously coupled to PTX-sensitive and -insensitive G proteins, which activate or inhibit various effector enzymes including PLC-β (Chang et al., 1995; Akbar et al., 1996), phospholipase D (Irving & Exton, 1987), phospholipase A2 (Lazarowski et al., 1994) and adenylyl cyclase (Dubyak & El- Motassim, 1993; Harden et al., 1995). The inositol phosphate response mediated by P2Y1, P2Y6 and P2Y11 receptors was not inhibited by PTX, whereas the P2Y2 and P2Y4 receptors exhibited a partial sensitivity (Communi et al., 2000). Data presented in this study showed that the EFS-evoked depolarization was sensitive to PTX indicating that it may be mediated via P2Y2/P2Y4 class receptor subtype. The marked inhibition of EFS-evoked depolarization with the PLC inhibitor U-73122 also provided evidence that the responsible receptor subtype may be coupled to Gq/11-protein, like P2Y2/P2Y4 class, which activates PLC. P2Y receptors exhibit variable affinity for purine and pyrimidine nucleotides. P2Y1 are purinoceptors and adenine nucleotide-specific (Webb et al., 1993; Schachter et al., 1996), whereas P2Y2 receptors have equal affinity for adenine and uridine nucleotide triphosphate (Lusting et al., 1993; Nicholas et al., 1996). P2Y3, P2Y4 and P2Y6 are pyrimidinoceptors (Nicholas et al., 1996; Webb et al., 1996). Data presented in this study showed that ATP (adenine nucleotide) and UTP (uridine nucleotide) produced approximately equal membrane depolarization upon their exogenous application, indicating the expression of P2Y2 receptors on the longitudinal smooth muscle of chicken anterior mesenteric artery. Secondary to activation of PLC and mobilization of Ca2+, the P2Y2-like receptor mediates the opening of Ca2+-sensitive Cl− channels in airway epithelia (Clarke & Boucher, 1992; Stutts et al., 1992) and avian exocrine salt gland cells (Martin & Shuttleworth, 1995). Similarly, our data showed that the Cl− channel blocker NPPB significantly inhibited the EFS-evoked depolarization as well as the exogenous application of ATP and 2-MeSATP. Taking all these concepts together, the recorded EFS-evoked depolarization in our study may be mediated via P2Y2-like receptor and it involves second messenger system (activation of PLC) and ionic conductance (opining of Cl− channels) mediated by G-protein (PTX-sensitive) coupling.
In conclusion, the results of the present study indicate that, in the longitudinal smooth muscle of chicken anterior mesenteric artery, ATP or ATP-like endogenous P2Y purinoceptor ligand is the NANC excitatory mediator involved in the generation of EFS-evoked depolarization, and this excitatory action may be mediated via P2Y2-like receptor subtype. A role of NE as well as of ACh in maintaining the excitatory pathway is also indicated.
Abbreviations
- 2-MeSATP
2-methylthioATP
- α,β-MeATP
α,β-methylene ATP
- ACh
acetylcholine
- ATP
adenosine 5′-triphosphate
- CBF3GA
Cibacron blue F3GA
- EFS
electrical field stimulation
- EJP
excitatory junction potential
- IJP
inhibitory junction potential
- NANC
nonadrenergic, noncholinergic
- NE
norepinephrine
- NPPB
5-nitro-2(3-phenylpropylamino)benzoic acid
- PLC
phospholipase C
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic
- PSS
physiological salt solution
- PTX
pertussis toxin
- TTX
tetrodotoxin
- U-73122
1-[6-[[(17 β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- AKBAR G.K.M., DASARI V.R., WEBB T.E., AYYANATHAN K., PILLARISETTI K., SANDHU A.K., ATHWAL R.S., DANIEL J.L., ASHBY B., BARNARD E.A., KUNAPULI S.P. Molecular cloning of a novel P2 purinoceptor from human erythroleukemia cells. J. Biol. Chem. 1996;271:18363–18367. doi: 10.1074/jbc.271.31.18363. [DOI] [PubMed] [Google Scholar]
- BALL R.A., SAUTTER J.H., KATTER M.S. Morphological characteristics of the anterior mesenteric artery of fowl. Anat. Rec. 1963;146:251–255. doi: 10.1002/ar.1091460311. [DOI] [PubMed] [Google Scholar]
- BAO J.X., ERIKSSON I.E., STJRANE I. On pre- and/or postjunctional roles of ATP and unknown ‘Substance X' as sympathetic co-transmitters in rat tail artery. Acta Physiol. Scand. 1989;135:65–66. doi: 10.1111/j.1748-1716.1989.tb08551.x. [DOI] [PubMed] [Google Scholar]
- BAUER V., HOLZER P., ITO Y. Role of extra- and intracellular calcium in the contractile action of agonists in the guinea-pig ileum. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:58–64. doi: 10.1007/BF00180677. [DOI] [PubMed] [Google Scholar]
- BELL C. Indirect cholinergic vasomotor control of intestinal blood flow in the domestic chicken. J. Physiol. 1969a;205:317–327. doi: 10.1113/jphysiol.1969.sp008967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J. Physiol. 1969b;205:695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT M.R. Autonomic Neuromuscular Transmission 1972Cambridge: Cambridge University Press; Monographs of the physiological Society No. 30 [PubMed] [Google Scholar]
- BENNETT T., MALFMORS T. The adrenergic nervous system of the domestic fowl (Gallus domesticus (L.)) Z. Zellforsch. 1970;106:22–50. doi: 10.1007/BF01027715. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., WEISMAN G.A., TURNER J.T., WILKINSON G.F. G-protein-coupled P2 purinoceptors: from molecular biology to functional responses. Trends Pharmacol. Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B. Intramural nerves in the ventricular myocardium of the domestic fowl and other animals. Br. J. Pharmacol. Chemother. 1967;31:253–268. doi: 10.1111/j.1476-5381.1967.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Studies on the longitudinal muscle of the anterior mesenteric artery of the domestic fowl. J. Physiol. 1968a;196:273–281. doi: 10.1113/jphysiol.1968.sp008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Electrical and mechanical activity of the longitudinal muscle of the anterior mesenteric artery of the domestic fowl. J. Physiol. 1968b;196:283–292. doi: 10.1113/jphysiol.1968.sp008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Spontaneous and evoked release of neurotransmitter substances in the longitudinal muscle of the anterior mesenteric artery of the domestic fowl. Br. J. Pharmacol. 1969;35:112–120. doi: 10.1111/j.1476-5381.1969.tb07971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B., LANG R.J., TAKEWAKI T. Mechanism of action of noradrenalin and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J. Physiol. 1984;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAKE A.J., WAGENBACH M.J., JULIUS D. New structural motif for ligand-gated ion channels defined by an inotropic ATP receptor. Nature. 1994;371:519–522. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.Purines as co-transmitters in adrenergic and cholinergic neurons Coexistance of Neuronal Messengers: a New Principle in Chemical transmission 1986Amsterdam: Elsevier; 193–203.(eds) Hokfelt, T., Fuxe, K. & Pernow, B. pp [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptors Cell Membrane Receptors for Drugs and Hormones, A Multidisciplinary Approach 1987New York, NY: Raven Press; 107–118.ed. Straub, R.W. & Bolis, L. pp [Google Scholar]
- BURNSTOCK G., HOLMAN M.E. The transmission of excitation from autonomic nerve to smooth muscle. J. Physiol. 1961;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG K., HANAOKA K., KUMADA M., TAKAWA Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J. Biol. Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- CLARKE L.L., BOUCHER R.C. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. Am. J. Physiol. 1992;263:C348–C356. doi: 10.1152/ajpcell.1992.263.2.C348. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., GONZALES N.S., DETHEUX M., BREZILLON S., LANNOY V., PARMENTIER M., BOEYNAEMS J.M. Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., JANSSENS R., SUAREZ-HUERTA N., ROBAYE B., BOEYNAEMS J.M. Advances in signaling by extracellular nucleotides: the role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351–360. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- COUSINS H.M., EDWARDS F.R., HIRST G.D.S., WENDT I.R. Cholinergic neuromuscular transmission in the longitudinal muscle of the guinea-pig ileum. J. Physiol. 1993;471:61–86. doi: 10.1113/jphysiol.1993.sp019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBYAK G.R., EL- MOTASSIM C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- ERLINGE D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen. Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F. Pharmacology of vascular smooth muscle. Pharmacol. Rev. 1955;7:183–265. [PubMed] [Google Scholar]
- FURNESS J.B. An electrophysiological study of the innervation of the smooth muscle of the colon. J. Physiol. 1969;205:549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODEN B.A. The effect of hypoxia on vasoconstrictor responses of isolated mesenteric arterial vasculature from chicken and duckling. Comp. Biochem. Physiol. C. 1980;67:219–222. doi: 10.1016/0306-4492(80)90022-2. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K., BOYER J.S., NICHOLAS R.A. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu. Rev. Pharmacol. Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- HILL C.E., KLEMM M., EDWARDS F.R., HIRST G.D.S. Sympathetic transmission to the dilator muscle of the rat iris. J. Auton. Nerv. Syst. 1993;45:107–123. doi: 10.1016/0165-1838(93)90123-c. [DOI] [PubMed] [Google Scholar]
- HIRST G.D.S. Neuromuscular transmission in arterioles of guinea pig submucosa. J. Physiol. 1977;273:263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G.D.S., JOBLING P. The distribution of γ-adrenoceptor and P2 purinoceptors in mesenteric arteries and veins of the guinea pig. Br. J. Pharmacol. 1989;96:993–999. doi: 10.1111/j.1476-5381.1989.tb11912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTTENSTEIN O.D., KREULEN D.L. Comparison of the frequency dependence of the venous and arterial responses to sympathetic nerve stimulation in guinea-pigs. J. Physiol. 1987;384:153–167. doi: 10.1113/jphysiol.1987.sp016448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVING H.R., EXTON J.H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J. Biol. Chem. 1987;262:3440–3443. [PubMed] [Google Scholar]
- ITOH T., KITAMURA K., KURIYAMA H. Roles of extra-junctional receptors of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J. Physiol. 1983;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN W., LO T.M., LO H.H., THAYER S.A. U-73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- KAKUYAMA M., VALLANCE P., AHLUWALIA A. Endothelium-dependent sensory NANC vasodilatation: involvement of ATP, CGRP and a possible NO store. Br. J. Pharmacol. 1998;123:310–316. doi: 10.1038/sj.bjp.0701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEATINGE W.R. Electrical and mechanical response of arteries to stimulation of sympathetic nerves. J. Physiol. 1966;185:701–715. doi: 10.1113/jphysiol.1966.sp008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY C., BURNSTOCK G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur. J. Pharmacol. 1985;111:49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C., HARDEN T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Am. J. Physiol. 1994;266:C406–C415. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- LEVICK J.R. An Introduction to Cardiovascular Physiology. Oxford: Butterworth-Heinemann Ltd; 1995. [Google Scholar]
- LUSTING K.D., SHIAU A.K., BRAKE A.J., JULIUS D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSJÖ M., HOU M., PENDERGAST W., ERLINGE D., EDVINSSON L. The stable pyrimidines UDPβS and UTPγS discriminate between contractile cerebrovascular P2 receptors. Eur. J. Pharmacol. 2003;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- MARTIN S.C., SHUTTLEWORTH T.J. Activation of P2U ‘nucleotide' receptor in an axocrine cell. Br. J. Pharmacol. 1995;115:321–329. doi: 10.1111/j.1476-5381.1995.tb15880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAREN G.J., KENNEDY C., SNEDDON P. The effects of suramin on purinergic and noradrenergic neurotransmission in the rat isolated tail artery. Eur. J. Pharmacol. 1995;277:57–61. doi: 10.1016/0014-2999(95)00065-s. [DOI] [PubMed] [Google Scholar]
- MIYAGI Y., KIMURA H., CARPENTER R.C., PARENT A.D., ZHANG J. α,β-MeATP augments the UTP contraction of rabbit basilar artery. Eur. J. Pharmacol. 2004;488:117–125. doi: 10.1016/j.ejphar.2004.02.002. [DOI] [PubMed] [Google Scholar]
- MORRIS J.L., CUNNANE T.C., HIRST G.D.S. Regional difference in sympathetic neurotransmission to cutaneous arteries in the guinea-pig isolated ear. J. Auton. Nerv. Syst. 1998;73:115–124. doi: 10.1016/s0165-1838(98)00122-2. [DOI] [PubMed] [Google Scholar]
- MUTAFOVA-YAMBOLIEVA V.N., CAROLAN B.M., HARDEN K., KEEF K.D. Multiple P2Y receptors mediate contraction in guinea pig mesenteric vein. Gen. Pharmacol. 2000;34:127–136. doi: 10.1016/s0306-3623(00)00054-9. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A. Identification of the P2Y12 receptor: a novel member of P2Y family of receptors activated by extracellular nucleotides. Mol. Pharmacol. 2001;60:416–420. [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- ORRE M., PENNEFATHER J.N., STORY M.E., HAYNES J.M. The effects of P2 purinoceptor agonists on the isolated portal vein of the guinea pig. Eur. J. Pharmacol. 1996;316:229–236. doi: 10.1016/s0014-2999(96)00687-5. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., MILNER P., HUDLICKÁ O., KRISTEK F., BURNSTOCK G. Shear stress causes release of substance P from rat hind limb vascular endothelial. Reg. Pep. 1988;22:427. [Google Scholar]
- SAK K., WEBB T.E. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch. Biochem. Biophys. 2002;397:131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON P., BURNSTOCK G. ATP as a co-transmitter in rat tail artery. Eur. J. Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- STUTTS M.J., CHINET T.C., MASON S.J., FULLTON J.M., CLARKE L.L., BOUCHER R.C. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI H. Effects of endogenous and exogenous noradrinaline on the smooth muscle of guinea-pig mesenteric vein. J. Physiol. 1981;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI H. An electrophysiological study of excitatory neuromuscular transmission in guinea-pig main pulmonary artery. J. Physiol. 1983;336:47–59. doi: 10.1113/jphysiol.1983.sp014565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEWAKI T., OHASHI O. Non- cholinergic excitatory transmission to intestinal smooth muscle cells. Nature. 1977;268:749–750. doi: 10.1038/268749a0. [DOI] [PubMed] [Google Scholar]
- THAPALIYA S., MATSUYAMA H., TAKEWAKI T. ATP released from perivascular nerves hyperpolarizes smooth muscle cells by releasing an endothelium-derived factor in hamster mesenteric arteries. J. Physiol. 1999;521:192–199. doi: 10.1111/j.1469-7793.1999.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VAN HELDEN D.F. Electrophysiology of neuromuscular transmission in guinea-pig mesenteric veins. J. Physiol. 1988a;401:469–488. doi: 10.1113/jphysiol.1988.sp017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KUGELGEN I., WETTER A. Molecular pharmacology of P2Y receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., HENDERSON D., KING B.J., WANG S., SIMON J., BATESON A.N., BURNSTOCK G., BARNARD E.A. A novel G protein-coupled P2 purinoceptor (P2Y3) activated preferentially by nucleoside diphosphates. Mol. Pharmacol. 1996;50:258–265. [PubMed] [Google Scholar]
- WEBB T.E., SIMON J., KRISHEK B J., BATESON A.N., SMART T.G., KING B.J., BURNSTOCK G., BARNARD E.A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., HOTTA K., MATSUDA T. Effect of methionine-enkaphalin on the spontaneous electrical and mechanical activity of the smooth muscle of the rat portal vein. Life Sci. 1984;34:993–999. doi: 10.1016/0024-3205(84)90304-7. [DOI] [PubMed] [Google Scholar]