Abstract
The aim of this study was to investigate the mechanisms by which histamine causes nasal blockage. Histamine, 40–800 μg, intranasally into each nostril, induced significant blockage of the nasal airway in normal human subjects, as measured by acoustic rhinometry.
Oral pretreatment with cetirizine, 5–30 mg, the H1 antagonist, failed to reverse completely the nasal blockage induced by histamine, 400 μg.
Dimaprit, 50–200 μg, the H2 agonist, intranasally, caused nasal blockage, which was reversed by oral pretreatment with ranitidine, 75 mg, the H2 antagonist.
A combination of cetirizine, 20 mg, and ranitidine, 75 mg, caused greater inhibition of the nasal blockage caused by histamine, 400 μg, than cetirizine alone. In the presence of both antagonists, there was residual histamine-induced nasal blockage.
R-α-methylhistamine (R-α-MeH), 100–600 μg, the H3 agonist, intranasally, caused nasal blockage, which was not inhibited by either cetirizine or ranitidine.
Thioperamide, 700 μg, the H3 antagonist, intranasally, reversed the R-α-MeH-induced nasal blockage. Thioperamide alone had no significant action on the nasal blockage induced by histamine, 400 and 1000 μg, but, in the presence of cetirizine, 20 mg, thioperamide further reduced the histamine-induced nasal blockage.
Corynanthine, 2 mg, the α1-adrenoceptor antagonist, administered intranasally, caused nasal blockage.
Corynanthine produced a greater increase in nasal blockage when in combination with bradykinin compared to its combination with R-α-MeH.
There appears to be a contribution of H1, H2 and H3 receptors to histamine-induced nasal blockage in normal human subjects. The sympathetic nervous system actively maintains nasal patency and we suggest that activation of nasal H3 receptors may downregulate sympathetic activity.
Keywords: Histamine, nasal airway, human, H3 receptor, sympathetic nerves
Introduction
In allergic rhinitis, it is believed that the interaction of antigen with antigen-specific IgE bound to IgE receptors on the surface of nasal mast cells causes the release of mediators that generate the symptoms of the disease. The symptoms include nasal congestion, pruritus, sneezing and rhinorrhea. Histamine is one of the mediators released from mast cells that may be responsible for the production of symptoms, and it is known that application of histamine to the nasal mucosa of nonallergic subjects mimics some of the symptoms of allergic rhinitis (Doyle et al., 1990; Rajakulasingam et al., 1993; Howarth et al., 2000). The actions of histamine on the nasal mucosa have been shown to be mediated largely by H1 receptors (Kirkegaard et al., 1983; Hilberg et al., 1995). It has also been reported that H2 receptors mediate a proportion of the nasal blockage caused by histamine (Secher et al., 1982; Mygind et al., 1983; Wood-Baker et al., 1996), although the evidence for this in no way parallels the strength of evidence supporting a role for H1 receptors. More recently, the role of the H3 receptor in the nasal mucosa of cat, pig and man has been investigated (McLeod et al., 1999; 2003; Varty & Hey, 2002; Varty et al., 2004). Only a combination of H1 and H3 antagonists decreased the nasal blockage caused by compound 48/80 (a histamine releasing agent) in the cat. Using electrical field stimulation of isolated human and porcine nasal mucosa, it has been shown that activation of the H3 receptor reduces sympathetic activity. In vivo, activation of α-adrenoceptors causes vasoconstriction that leads to nasal decongestion (Johnson & Hricik, 1993). It is proposed that activation of the H3 receptor on the prejunctional terminals of sympathetic neurones reduces noradrenaline release and this may contribute, together with the activation of the postjunctional H1 receptors, to the nasal blockage caused by histamine. It follows that H3 antagonists together with H1 antagonists may reduce nasal blockage in allergic rhinitis to a greater extent than H1 antagonists alone. The aim of this study was to test the hypothesis that there are, besides H1 receptors, functional H2 and H3 receptors in the human nasal airway that, when activated, cause nasal blockage.
Methods
Materials
Histamine diphosphate and corynanthine hydrochloride were obtained from Sigma (Poole, U.K.). Dimaprit dihydrochloride, R-α-methylhistamine (R-α-MeH) dihydrobromide, S-α-methylhistamine (S-α-MeH) dihydrobromide and thioperamide maleate were obtained from Tocris (Bristol, U.K.). Bradykinin (BK) was obtained from Merck Biosciences (Nottingham, U.K.). Ranitidine hydrochloride and cetirizine hydrochloride were obtained from University College Hospital (U.K.) pharmacy.
Subjects
The subjects used in these experiments were normal healthy volunteers in the age range 19–54 years. No subject had any clinical history of allergic disease or any nasal pathology. The subjects took no medication at the time of, or in the 4 weeks preceding the experiments. The protocols were approved by the local Ethics Committee.
Measurement of nasal patency
Acoustic rhinometry is an established research technique for objectively measuring nasal blockage (Austin & Foreman, 1994; Fisher et al., 1994). The acoustic rhinometer, supplied by GM instruments (Kilwinning, U.K.), produces a sound pulse that travels up a hollow tube, through a 6 cm sterile plastic nose piece, and into the subject's nasal cavity. The acoustic rhinometer was clamped in the same position throughout each protocol, and each subject maintained the same posture for each recording so as to minimise variation in recordings. The sound is reflected from the internal structures of the nasal cavity and back down the tube to the internal microphone of the acoustic rhinometer. The signal is amplified and sent to a computer. The Nasal Area Distance Acquisition Program calculates the internal cross-sectional area along the length of the subject's nasal airways. The minimum cross-sectional area (Amin) between 1.5 and 7 cm from the nasal orifice (the location of the inferior and middle turbinates) was recorded as the objective measurement of nasal congestion. Both nostrils were measured separately three times at each time point of the protocols. For each time point an overall mean nasal Amin was then calculated.
Nasal challenge
Nasal challenge with histamine, dimaprit, R-α-MeH, S-α-MeH, thioperamide, corynanthine or BK was via a nasal pump delivering 100 μl of aerosol (Perfect-Valois, U.K. Ltd) into each nostril. The dose administered was controlled by the concentration of the solution. Solutions were made up in sterile saline (NaCl 154 mM) in a class II microbiological safety cabinet, aliquoted into 7 ml sterile containers and stored at −20°C.
Experimental design
All experiments followed the same basic double-blind design: baseline Amin recording, followed by nasal challenge followed by Amin recordings 5, 10 and 15 min later. For each experiment, subjects received all treatments in a random order with only one treatment allowed per day. Oral histamine antagonists, cetirizine and ranitidine, and oral placebos were administered 2 h before nasal challenge. Cetirizine and ranitidine doses and time courses were based on previously published reports (McNeil et al., 1981; Dubuske, 1995). Thioperamide, the H3 antagonist, was administered in a 100 μl aerosol in each nostril, prior to nasal challenge. The control for thioperamide was a saline aerosol. The dose and time-course for thioperamide studies were based on animal experiments (McLeod et al., 1999; Varty & Hey, 2002).
The protocols of the experiments were:
Nasal challenge with saline, 40, 100, 400 or 800 μg histamine.
Pretreatment with oral placebo, 5, 20 or 30 mg cetirizine 2 h before nasal challenge with saline or 400 μg histamine.
Pretreatment with oral placebo or 150 mg ranitidine 2 h before nasal challenge with saline, 50 or 200 μg dimaprit.
Pretreatment with oral placebo, 75 mg ranitidine, 20 mg cetirizine, or 75 mg ranitidine plus 20 mg cetirizine combination 2 h before nasal challenge with saline or 400 μg histamine.
Pretreatment with oral placebo, 32.5, 75 or 150 mg ranitidine 2 h before nasal challenge with saline or 400 μg histamine.
Pretreatment with oral placebo, 75 mg ranitidine or 20 mg cetirizine 2 h before nasal challenge with saline, 100, 300 or 600 μg R-α-MeH or 600 μg S-α-MeH.
Pretreatment with oral placebo or 20 mg cetirizine 75 min before nasal challenge with saline or 700 μg thioperamide, 45 min before nasal challenge with saline, 400 μg histamine or 600 μg R-α-MeH.
Pretreatment with oral placebo or 20 mg cetirizine 60 min before nasal challenge with saline or 700 μg thioperamide, 60, 40 and 20 min before nasal challenge with saline or 1000 μg histamine.
Nasal challenge with saline, 2 mg corynanthine, 200 μg BK, 200 μg BK plus 2 mg corynanthine combination, 600 μg R-α-MeH, or 600 μg R-α-MeH plus 2 mg corynanthine combination.
Data analysis
The response to nasal challenge was assessed by measuring Amin. For each subject in each experiment, the Amin recorded at 5, 10 and 15 min after nasal challenge were normalised to the Amin recorded just prior to nasal challenge. The normalised Amin response was then plotted against time after challenge with a drug, and the area under the curve (AUC) calculated (for example see Figure 1a). Thus, for each subject in each experiment, the nasal response to challenge was quantified by a single AUC value. This was done to take account of the response to treatment over the entire time-course of the experiment. The mean AUC value (±standard error) was calculated for each treatment group for graphical representation. AUCs of different treatment groups were analysed for statistical significance using the nonparametric Wilcoxon matched pairs test. A P-value less than 0.05 was taken as significant.
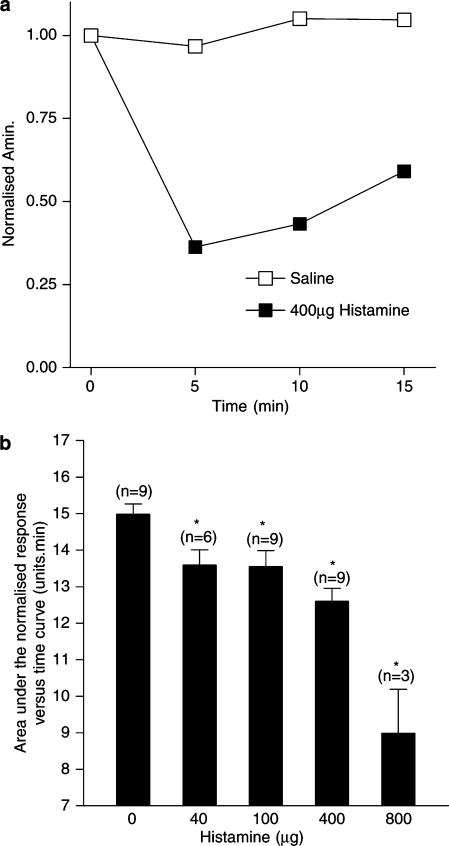
Figure 1.
(a) The effect of 400 μg histamine nasal challenge (dark squares) and saline nasal challenge (hollow squares) on the normalised Amin recorded from the same individual over a period of 15 min following nasal challenge. Amin was measured immediately before, and 5, 10 and 15 min later. Amin values were normalised to the prechallenge value. The area under the normalised Amin versus time curve measured over a period of 15 min for 400 μg histamine and saline nasal challenge, for example, was calculated as being 7.96 U min and 15.21 U min, respectively. (b) Dose–response curve for the action of histamine on the area under the normalised Amin versus time curve measured over a period of 15 min following the administration of histamine as an aerosol, at the dose shown, into each nostril. The number of subjects contributing to each data point is shown in parentheses. The vertical bars represent the s.e.m. *Significant decrease in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test).
Results
Application, by aerosol, of histamine, 400 μg, to each nostril of a normal human volunteer caused a decrease in normalised Amin over a period of 15 min (Figure 1a), indicating nasal blockage. In the same individual, application, by aerosol, of saline, had no effect on the normalised Amin over a 15 min period, indicating no change in nasal patency. This experiment was repeated for different doses of histamine in the number of subjects shown in parenthesis in Figure 1b. The response is shown as the mean area under the normalised Amin over a 15 min time period (AUC) and indicates that histamine, in the dose range 40–800 μg, produced a significant reduction in nasal patency throughout the entire 15 min and thus caused nasal blockage in normal human volunteers.
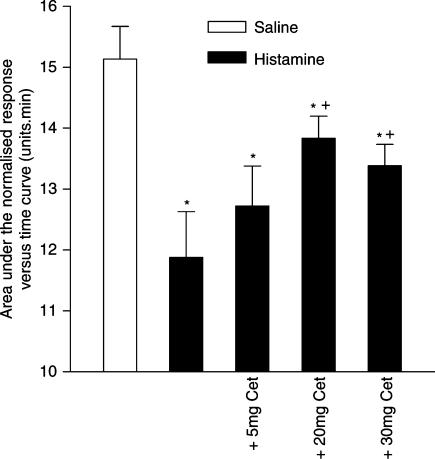
The response to histamine was significantly inhibited following oral pretreatment of the subjects with the H1 antagonist, cetirizine, 20 and 30 mg, given 2 h prior to challenge with histamine (Figure 2). The effect of cetirizine did not increase when the dose was increased from 20 to 30 mg. There was still a significant response to histamine, 400 μg, in the presence of the highest dose of cetirizine compared to the saline control (Figure 2), indicating residual nasal blockage. Cetirizine alone had no effect on nasal patency or on the response to saline (data not shown).
Figure 2.
Dose–response curve for the inhibition by orally administered cetirizine (Cet), given 2 h prior to challenge with histamine, of nasal blockage caused by histamine, 400 μg, administered as an aerosol to each nostril. The data are the means from eight subjects and represent the area on the normalised Amin versus time curve (AUC) measured over a 15 min period following the administration of histamine. Vertical bars represent the s.e.m. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test). +Significant increase in AUC as compared to histamine challenge without cetirizine pretreatment (P<0.05, Wilcoxon matched pairs test).
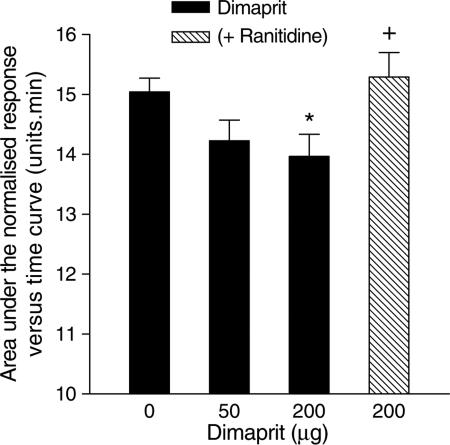
The H2 agonist dimaprit, 200 μg, given by intranasal aerosol, caused significant nasal blockage, which was completely reversed when the subjects were pretreated with the H2 antagonist, ranitidine, 150 mg, given orally 2 h prior to the intranasal challenge with dimaprit (Figure 3).
Figure 3.
Dose–response curve for the effect of dimaprit on the area under the normalised Amin versus time curve (AUC) measured over a 15 min period after the administration of the dimaprit. The data are means from eight subjects and the vertical bars represent the s.e.m. Dark column – dimaprit alone; hatched column – dimaprit in the presence of ranitidine, 75 mg given orally 2 h prior to dimaprit challenge. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test). +Significant increase in AUC as compared to dimaprit challenge without ranitidine pretreatment (P<0.05, Wilcoxon matched pairs test).
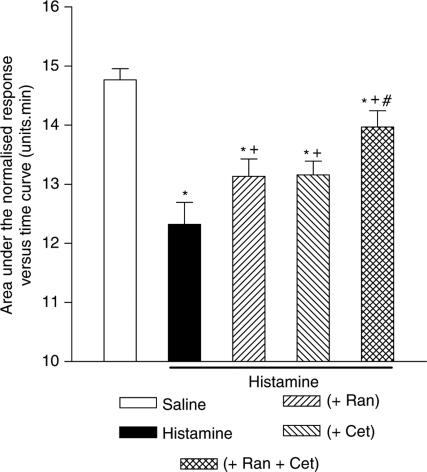
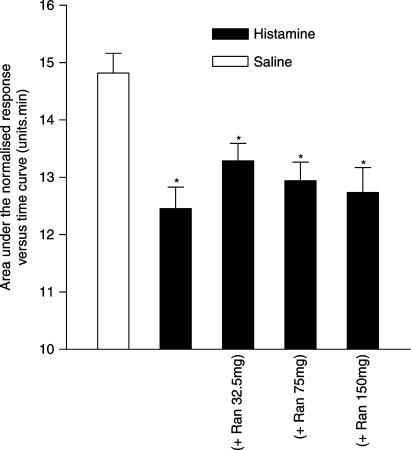
The histamine-induced nasal blockage was also significantly inhibited by ranitidine, 75 mg, given orally 2 h prior to challenge with histamine (Figure 4). In addition, the response to histamine was inhibited to a greater degree by a combination of ranitidine, 75 mg, and cetirizine, 20 mg, given orally 2 h prior to challenge with histamine, than by oral pretreatment with cetirizine alone. However, this combination of ranitidine and cetirizine failed to abolish the response to histamine (Figure 4), which indicates residual nasal blockage. Interestingly, further investigation showed that the response to histamine was not inhibited by ranitidine at doses between 32.5 and 150 mg, given orally 2 h prior to challenge with histamine (Figure 5).
Figure 4.
The effect of ranitidine (Ran), 75 mg and cetirizine (Cet), 20 mg alone and in combination, on the nasal blockage caused by histamine, 400 μg, given as an aerosol into each nostril. Both drugs were given orally 2 h prior to histamine challenge. The data are the means from 16 subjects and represent the area under the normalised Amin versus time curve (AUC) measured over a 15 min period following histamine challenge. The vertical bars represent the s.e.m. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test). +Significant increase in AUC as compared to histamine challenge without antagonist pretreatment (P<0.05, Wilcoxon matched pairs test). #Significant increase in AUC as compared to histamine challenge after cetirizine pretreatment (P<0.05, Wilcoxon matched pairs test).
Figure 5.
Dose–response curve for ranitidine (Ran), administered orally 2 h prior to challenge with histamine, and nasal blockage caused by histamine. The data are the means from 12 subjects and represent the area under the normalised Amin versus time curve (AUC) measured over a 15 min period following the administration of histamine. Vertical bars represent the s.e.m. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test).
Given that the combined antagonism of H1 and H2 receptors failed to abolish the nasal response to histamine, 400 μg, the role of the H3 receptor was investigated to ascertain whether or not activation of this receptor might be responsible for the residual nasal blockage.
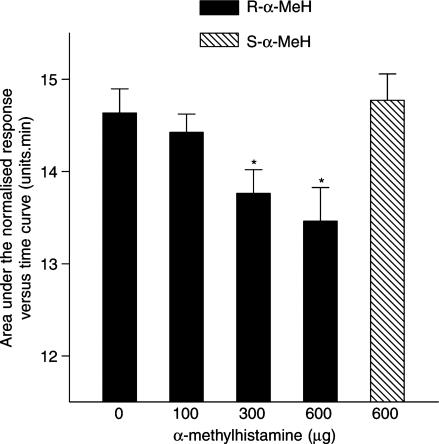
The H3 receptor agonist R-α-MeH, 300 and 600 μg, given intranasally by aerosol, caused significant nasal blockage. The less potent H3 receptor agonist S-α-MeH, 600 μg, given intranasally by aerosol, had no effect on nasal patency (Figure 6). The effect of the highest dose of R-α-MeH, 600 μg (resulting in a mean AUC of 13.66 U min±0.43), was not affected by oral pretreatment of the subjects, 2 h prior to the administration of R-α-MeH, with either cetirizine, 20 mg (resulting in a mean AUC of 13.24 U min±0.57), or ranitidine, 75 mg (resulting in a mean AUC of 13.62 U min±0.56).
Figure 6.
Dose–response curve for the action of R-α-methylhistamine (R-α-MeH) and S-α-methylhistamine (S-α-MeH) on the area under the normalised Amin versus time curve (AUC) measured over a 15 min period following the administration of R-α-MeH or S-α-MeH as an aerosol into each nostril. The data are the means from 10 subjects and the vertical bars represent the s.e.m. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test).
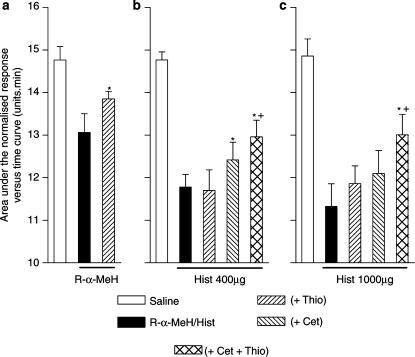
The response to R-α-MeH 600 μg, given intranasally, was partly inhibited following the intranasal administration of the H3 receptor antagonist, thioperamide, 700 μg, given 45 min prior to the challenge with the R-α-MeH (Figure 7a). Thioperamide at this dose had no effect on unstimulated nasal patency (data not shown). The same dose of thioperamide given alone, failed to affect the response to histamine, 400 μg (Figure 7b). In addition, repeated dosing of thioperamide, 700 μg, also failed to reverse the response to histamine, 1000 μg (Figure 7c). However, when thioperamide, 700 μg, (administered intranasally 45 min prior to challenge with histamine) was given in combination with an oral dose of cetirizine, 20 mg, 2 h prior to histamine challenge, the thioperamide+cetirizine combination caused greater inhibition of the response to 400 μg histamine than cetirizine alone (Figure 7b). In addition, only when thioperamide, (administered intranasally 60, 40 and 20 min prior to challenge with histamine) was given in combination with an oral dose of cetirizine, 20 mg, 2 h prior to histamine challenge, was the response to 1000 μg histamine inhibited (Figure 7c).
Figure 7.
The effect of thioperamide (Thio) on the nasal blockage caused by R-α-methylhistamine (R-α-MeH) (a) or two doses of histamine (Hist) (b and c) in the presence or absence of cetirizine (Cet). Nasal blockage is measured as the area under the normalised Amin versus time curve (AUC) over a period of 15 min following the intranasal administration of R-α-MeH or histamine, as an aerosol. (a) Thioperamide, 700 μg, was given intranasally as an aerosol 45 min prior to the administration of R-α-MeH, 600 μg. The data are the means from 10 subjects and vertical bars represent the s.e.m. (b) Cetirizine, 20 mg was given orally 2 h prior to challenge with histamine, 400 μg. Thioperamide, 700 μg, was given intranasally as an aerosol 45 min prior to the administration of histamine, 400 μg. The data are the means from 15 subjects and vertical bars represent the s.e.m. (c) Cetirizine, 20 mg was given orally 2 h prior to challenge with histamine, 1000 μg. Thioperamide, 700 μg, was given intranasally as an aerosol 60, 40 and 20 min prior to the administration of histamine, 1000 μg. The data are the means from 10 subjects and vertical bars represent the s.e.m. *Significant increase in AUC following antagonist pretreatment as compared to nasal challenge without antagonist (P<0.05, Wilcoxon matched pairs test). +Significant increase in AUC following pretreatment with the combination of thioperamide and cetirizine as compared to nasal challenge following pretreatment with cetirizine alone (P<0.05, Wilcoxon matched pairs test).
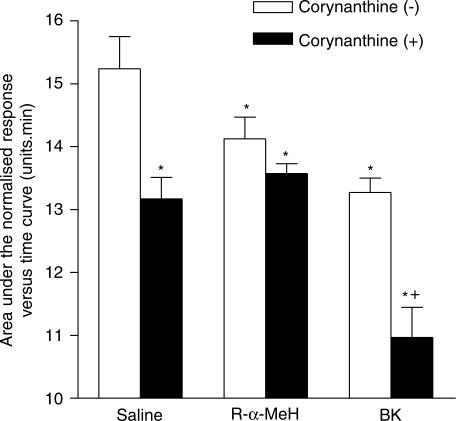
In animal studies, there is evidence that H3 receptor activation increases nasal blockage by inhibiting, prejunctionally, the release of noradrenaline from sympathetic neurones. Thus, if an effect of H3 receptor activation is to be observed, there must be an existing level of sympathetic activity. To demonstrate whether or not the sympathetic neurones in the nasal airway were active, we determined the effect of intranasal application of the selective α1-adrenoceptor antagonist, corynanthine. Figure 8 shows that intranasal administration of corynanthine, 2 mg, caused significant nasal blockage, indicating the presence of basal sympathetic control of nasal patency in the resting nasal airway.
Figure 8.
The effect of corynanthine, 2 mg, given as an aerosol into each nostril, on the patency of the nasal airway and the nasal blockage caused by either R-α-methylhistamine (R-α-MeH) or bradykinin (BK). Nasal blockage is measured as the area under the normalised Amin versus time curve over a 15 min period following the intranasal administration of corynanthine, 2 mg, R-α-MeH, 600 μg or BK, 200 μg as aerosols into each nostril. The data are the means from 13 subjects and the vertical bars represent the s.e.m. *Significant difference in AUC as compared to saline control (P<0.05, Wilcoxon matched pairs test). +Significant decrease in AUC after challenge with corynanthine combination as compared to challenge without corynanthine (P<0.05, Wilcoxon matched pairs test).
The question then arose as to whether modulation of the sympathetic nervous system is likely to have any significant effect on nasal blockage caused by inflammatory mediators. Figure 8 shows that R-α-MeH, 600 μg, caused significant nasal blockage, but this effect of R-α-MeH was not affected by corynanthine 2 mg. Thus, the response to R-α-MeH was not additive to the response to corynanthine. BK also caused nasal blockage but, in this case, corynanthine produced a marked increase in the nasal blockage induced by BK. The response to BK was additive with the response to corynanthine.
Discussion and conclusions
We have confirmed, using acoustic rhinometry, that histamine induces nasal blockage in the human nasal airway, as illustrated by the decrease in the area under the normalised Amin versus time curve over a 15 min period following nasal challenge. In agreement with Kirkegaard et al. (1983) and Hilberg et al. (1995), the effect of histamine on the nasal airway was inhibited by the H1 antagonist cetirizine but, interestingly, the inhibition by cetirizine did not achieve a complete reversal of the effect of histamine even at three times the normal clinical dose, indicating that there is an action of histamine in causing nasal blockage in human subjects other than that mediated through the H1 receptor. The potential for H2 receptor-mediated nasal blockage caused by histamine is confirmed by the action of the H2 agonist, dimaprit. Dimaprit caused a small but significant amount of nasal blockage, which was completely reversed by the H2 antagonist, ranitidine. However, H2 antagonism produced more varied effects on histamine-induced nasal blockage. In one experiment, ranitidine had no dose-related effect on histamine-induced nasal blockage, whereas in another experiment, ranitidine caused a reduction in histamine-induced nasal blockage as well as causing further inhibition of the histamine-induced nasal blockage when in combination with cetirizine. The role of H2 receptors in histamine-induced nasal blockage is controversial. Some studies have shown only an effect of H2 antagonism when in combination with H1 antagonists (Wood-Baker et al., 1996), while other studies have shown H2 antagonists reducing histamine-induced nasal blockage without concomitant H1 antagonism (Secher et al., 1982; Mygind et al., 1983). Nevertheless, even in the presence of both cetirizine and ranitidine, histamine was able to cause some residual nasal blockage, indicating that non-H1 and non-H2 receptor mechanisms are operating. This is consistent with the reports from animal studies that H3 receptors have a role in mediating nasal blockage in response to histamine (McLeod et al., 1999; 2003).
R-α-MeH, a full agonist at H3 receptors, caused a dose-related nasal blockage that was reversed by thioperamide, the H3 antagonist, but not by cetirizine or ranitidine, suggesting that activation of H3 receptors is capable of mediating nasal blockage in human subjects. In addition, S-α-MeH, an H3 agonist 120 times less potent than R-α-MeH, failed to cause nasal blockage at similar doses. Interestingly, the dose of thioperamide (700 μg) failed to abolish the nasal blockage caused by R-α-MeH. As neither H1 nor H2 antagonism reduced R-α-MeH-induced nasal blockage, it is quite possible that 700 μg thioperamide is a submaximal dose. However, for ethical considerations, it was not possible to investigate the effect of higher doses of thioperamide, nor the effect of oral or intravenous administration.
Thioperamide, although not active against histamine by itself, increased the inhibition of histamine-induced nasal blockage in the presence of cetirizine. Interestingly, although cetirizine, 20 mg, was unable to reduce the nasal blockage caused by histamine, 1000 μg, it was sufficient, when combined with thioperamide, to reverse the nasal blockage caused by histamine, 1000 μg. We have no explanation as to why thioperamide is inactive against histamine when administered alone. As mentioned above, it was not possible to investigate the effect of higher doses of thioperamide. However, McLeod et al. (1999) showed that only a combination of H1 and H3 antagonists is able to reduce the nasal blockage caused by compound 48/80 in the cat; neither antagonist on its own has any significant effect. This observation suggests that there may be some interaction between histamine receptors in the nasal mucosa. Taken together, our data suggest that in addition to H1 receptor-mediated and, possibly, H2 receptor-mediated blockage of the human nasal airway, that the H3 receptor may also contribute to histamine-induced nasal blockage. It is important to point out that with the pharmacological tools that we have been able to employ, in particular thioperamide which is active at both H3 and H4 receptors, it is not possible to exclude a role for the H4 receptor. However, although R-α-MeH is active at H4 receptors, its potency at these receptors is several hundred times lower than at H3 receptors (Schneider et al., 2002). In addition, in the cat, there is little doubt that the nasal blockage is influenced by H3 receptors rather than by H4 receptors as clobenpropit, which is an antagonist at H3 receptors and an agonist at H4 receptors, reduced the nasal blockage caused by compound 48/80.
Recently, it has been shown that the H3 effect in human and pig nasal mucosa may be attributable to a prejunctional inhibition of noradrenaline release from sympathetic neurones (Varty & Hey, 2002; Varty et al., 2004). Such a presynaptic effect of the H3 receptor on sympathetic neurones has been reported many times in recent years (Molderings et al., 1992; Danko et al., 1994; Ishikawa & Sperelakis, 1999; Mazenot et al., 1999; Valentine et al., 1999; Blandizzi et al., 2000; Yamasaki et al., 2001; Silver et al., 2002; Varty & Hey, 2002). Increased blood flow to the nasal mucosa is largely responsible for nasal blockage and so reducing nasal mucosal blood flow will reduce nasal blockage: hence the use of sympathomimetic drugs as nasal decongestants. If the mechanism by which H3 receptor activation causes nasal blockage is through the reduction in noradrenaline release, it follows that for the effect of an H3 agonist to be detectable, there must be ongoing sympathetic neuronal activity in the nasal airway. We have shown that the selective α1-adrenoceptor antagonist, corynanthine, caused nasal blockage in normal human subjects in the absence of any other challenge to the nasal airway, which reinforces previous reports of nasal blockage as a side effect of α1-adrenoceptor antagonists used to treat prostatic obstruction (Moser, 1958; Caine et al., 1981; Kirby, 1999). This observation is consistent with there being resting sympathetic neuronal activity that is maintaining the patency of the nasal airway.
The combination of corynanthine and R-α-MeH failed to produce greater nasal blockage than R-α-MeH alone. Thus, if the R-α-MeH is reducing the release of noradrenaline in order to produce its nasal blocking effect, corynanthine produces no further blockage because there is no noradrenaline to antagonise. In contrast, the combination of corynanthine and BK produced significantly greater nasal blockage than BK alone. BK causes nasal blockage by acting as a vasodilator and it probably does not have any neuronally mediated effects since local anaesthesia does not affect the nasal response to BK (Dear et al., 1996). Thus, when corynanthine is given together with BK, the nasal blockage observed may result from two mechanisms: a direct vasodilator effect of BK and an inhibition of sympathetic activity by corynanthine. We cannot, however, exclude an effect of BK on noradrenaline release. There are reports that BK increases noradrenaline release from sympathetic neurones, although these studies were investigating rat knee joints (Basbaum & Levine, 1991) and rat vas deferens (Llona et al., 1991), not nasal mucosa.
In conclusion, the data suggest that in humans, H1, H2 and H3 receptors may all have a role in the control of histamine-induced nasal blockage. Our data support the previously documented H1-mediated mechanism. An H2 agonist caused nasal blockage but, as previously reported, the effect of H2 antagonism was variable. We are presenting evidence that is compatible with a role for H3 receptors, but we are unable to exclude a role for H4 receptors. We suggest the H3 receptor reduces the release of noradrenaline, which normally maintains nasal patency. It is conceivable that H3 receptor antagonists, possibly in combination with H1 antagonists, may have a role in the alleviation of the symptoms of allergic rhinitis.
Acknowledgments
T.T.-C. acknowledges the provision by the Medical Research Council of a postgraduate studentship.
Abbreviations
- Amin
minimum cross-sectional area
- AUC
area under the curve
- BK
bradykinin
- Cet
cetirizine
- Hist
histamine
- R-α-MeH
R-α-methylhistamine
- Ran
ranitidine
- S-α-MeH
S-α-methylhistamine
- Thio
thioperamide
References
- AUSTIN C.E., FOREMAN J.C. Acoustic rhinometry compared with posterior rhinomanometry in the measurement of histamine- and bradykinin-induced changes in nasal airway patency. Br. J. Clin. Pharmacol. 1994;37:33–37. doi: 10.1111/j.1365-2125.1994.tb04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASBAUM A.I., LEVINE J.D. The contribution of the nervous system to inflammation and inflammatory disease. Can. J. Physiol. Pharmacol. 1991;69:647–651. doi: 10.1139/y91-096. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., TOGNETTI M., COLUCCI R., DEL TACCA M. Histamine H3 receptors mediate inhibition of noradrenaline release from intestinal sympathetic nerves. Br. J. Pharmacol. 2000;129:1387–1396. doi: 10.1038/sj.bjp.0703194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAINE M., PERLBERG S., SHAPIRO A. Phenoxybenzamine for benign prostatic obstruction. Review of 200 cases. Urology. 1981;17:542–546. doi: 10.1016/0090-4295(81)90071-6. [DOI] [PubMed] [Google Scholar]
- DANKO G., HEY J.A., EGAN R.W., KREUTNER W., CHAPMAN R.W. Histamine H3 receptors inhibit sympathetic modulation of airway microvascular leakage in allergic guinea pigs. Eur. J. Pharmacol. 1994;254:283–286. doi: 10.1016/0014-2999(94)90466-9. [DOI] [PubMed] [Google Scholar]
- DEAR J.W., GHALI S., FOREMAN J.C. Attenuation of human nasal airway responses to bradykinin and histamine by inhibitors of nitric oxide synthase. Br. J. Pharmacol. 1996;118:1177–1182. doi: 10.1111/j.1476-5381.1996.tb15521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOYLE W.J., BOEHM S., SKONER D.P. Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J. Allergy Clin. Immunol. 1990;86:924–935. doi: 10.1016/s0091-6749(05)80156-3. [DOI] [PubMed] [Google Scholar]
- DUBUSKE L. Dose-ranging comparative evaluation of cetirizine in patients with seasonal allergic rhinitis. Ann. Allergy Asthma Immunol. 1995;74:345–354. [PubMed] [Google Scholar]
- FISHER E.W., LUND V.J., SCADDING G.K. Acoustic rhinometry in rhinological practice: discussion paper. J. Roy. Soc. Med. 1994;87:411–413. doi: 10.1177/014107689408700713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILBERG O., GRYMER L.F., PEDERSEN O.F. Nasal histamine challenge in nonallergic and allergic subjects evaluated by acoustic rhinometry. Allergy. 1995;50:166–173. doi: 10.1111/j.1398-9995.1995.tb05075.x. [DOI] [PubMed] [Google Scholar]
- HOWARTH P.H., SALAGEAN M., DOKIC D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55 Suppl 64:7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA S., SPERELAKIS N. A novel class (H3) of histamine receptors on perivascular nerve terminals. Nature. 1999;327:158–160. doi: 10.1038/327158a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON D.A., HRICIK J.G.The pharmacology of alpha-adrenergic decongestants Pharmacotherapy 199313110S–115S.discussion 143S–146S [PubMed] [Google Scholar]
- KIRBY R.S.Clinical pharmacology of alpha1-adrenoceptor antagonists Eur. Urol. 199936Suppl 148–53.Discussion 65 [DOI] [PubMed] [Google Scholar]
- KIRKEGAARD J., SECHER C., BORUM P., MYGIND N. Inhibition of histamine-induced nasal symptoms by the H1 antihistamine chlorpheniramine maleate: demonstration of topical effect. Br. J. Dis. Chest. 1983;77:113–122. doi: 10.1016/0007-0971(83)90017-7. [DOI] [PubMed] [Google Scholar]
- LLONA I., GALLEGUILLOS X., BELMAR J., HUIDOBRO-TORO J.P. Bradykinin modulates the release of noradrenaline from vas deferens nerve terminals. Life Sci. 1991;48:2585–2592. doi: 10.1016/0024-3205(91)90616-j. [DOI] [PubMed] [Google Scholar]
- MAZENOT C., RIBUOT C., DURAND A., JOULIN Y., DEMENGE P., GODIN-RIBUOT D. In vivo demonstration of H3-histaminergic inhibition of cardiac sympathetic stimulation by R-alpha-methyl-histamine and its prodrug BP 2.94 in the dog. Br. J. Pharmacol. 1999;126:264–268. doi: 10.1038/sj.bjp.0702257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEOD R.L., MINGO G.G., HERCZKU C., DEGENNARO-CULVER F., KREUTNER W., EGAN R.W., HEY J.A. Combined histamine H1 and H3 receptor blockade produces nasal decongestion in an experimental model of nasal congestion. Am. J. Rhinol. 1999;13:391–399. doi: 10.2500/105065899781367483. [DOI] [PubMed] [Google Scholar]
- MCLEOD R.L., RIZZO C.A., WEST R.E., ASLANIAN R., MCCORMICK K., BRYANT M., HSIEH Y., KORFMACHER W., MINGO G.G., VARTY L., WILLIAMS S.M., SHIH N.Y., EGAN R.W., HEY J.A. Pharmacological characterization of the novel histamine H3-receptor antagonist N-(3,5-dichlorophenyl)-N′-[[4-(1H-imidazol-4-ylmethyl)phenyl]-methyl]-urea (SCH 79687) J. Pharmacol. Exp. Ther. 2003;305:1037–1044. doi: 10.1124/jpet.103.049254. [DOI] [PubMed] [Google Scholar]
- MCNEIL J.J., MIHALY G.W., ANDERSON A., MARSHALL A.W., SMALLWOOD R.A., LOUIS W.J. Pharmacokinetics of the H2 receptor antagonist ranitidine in man. Br. J. Clin. Pharmacol. 1981;12:411–415. doi: 10.1111/j.1365-2125.1981.tb01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., WEISSENBORN G., SCHLICKER E., LIKUNGU J., GOTHERT M. Inhibition of noradrenaline release from the sympathetic nerves of the human saphenous vein by presynaptic histamine H3 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346:46–50. doi: 10.1007/BF00167569. [DOI] [PubMed] [Google Scholar]
- MOSER M. Phenoxybenzamine. Practitioner. 1958;180:609–616. [PubMed] [Google Scholar]
- MYGIND N., SECHER C., KIRKEGAARD J. Role of histamine and antihistamines in the nose. Eur. J. Respir. Dis. Suppl. 1983;128:16–20. [PubMed] [Google Scholar]
- RAJAKULASINGAM K., POLOSA R., LAU L.C., CHURCH M.K., HOLGATE S.T., HOWARTH P.H. Comparative nasal effects of bradykinin and histamine: influence on nasal airways resistance and plasma protein exudation. Thorax. 1993;48:324–329. doi: 10.1136/thx.48.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER E., ROLLI-DERKINDEREN M., AROCK M., DY M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–263. doi: 10.1016/s1471-4906(02)02215-9. [DOI] [PubMed] [Google Scholar]
- SECHER C., KIRKEGAARD J., BORUM P., MAANSSON A., OSTERHAMMEL P., MYGIND N. Significance of H1 and H2 receptors in the human nose: rationale for topical use of combined antihistamine preparations. J. Allergy Clin. Immunol. 1982;70:211–218. doi: 10.1016/0091-6749(82)90044-6. [DOI] [PubMed] [Google Scholar]
- SILVER R.B., POONWASI K.S., SEYEDI N., WILSON S.J., LOVENBERG T.W., LEVI R. Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc. Natl. Acad. Sci. U.S.A. 2002;99:501–506. doi: 10.1073/pnas.012506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE A.F., RIZZO C.A., RIVELLI M.A., HEY J.A. Pharmacological characterization of histamine H3 receptors in human saphenous vein and guinea pig ileum. Eur. J. Pharmacol. 1999;366:73–78. doi: 10.1016/s0014-2999(98)00904-2. [DOI] [PubMed] [Google Scholar]
- VARTY L., HEY J. Histamine H3 receptor activation inhibits neurogenic sympathetic vasoconstriction in porcine nasal mucosa. Eur. J. Pharmacol. 2002;452:339–345. doi: 10.1016/s0014-2999(02)02336-1. [DOI] [PubMed] [Google Scholar]
- VARTY L.M., GUSTAFSON E., LAVERTY M., HEY J.A. Activation of histamine H3 receptors in human nasal mucosa inhibits sympathetic vasoconstriction. Eur. J. Pharmacol. 2004;484:83–89. doi: 10.1016/j.ejphar.2003.10.051. [DOI] [PubMed] [Google Scholar]
- WOOD-BAKER R., LAU L., HOWARTH P.H. Histamine and the nasal vasculature: the influence of H1 and H2-histamine receptor antagonism. Clin. Otolaryngol. 1996;21:348–352. doi: 10.1111/j.1365-2273.1996.tb01085.x. [DOI] [PubMed] [Google Scholar]
- YAMASAKI T., TAMAI I., MATSUMURA Y. Activation of histamine H3 receptors inhibits renal noradrenergic neurotransmission in anesthetized dogs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1450–R1456. doi: 10.1152/ajpregu.2001.280.5.R1450. [DOI] [PubMed] [Google Scholar]