Abstract
In a previous study, we demonstrated that a dextromethorphan analog, dimemorfan, has neuroprotective effects.
Dextromethorphan and dimemorfan are high-affinity ligands at σ1 receptors. Dextromethorphan has moderate affinities for phencyclidine sites, while dimemorfan has very low affinities for such sites, suggesting that these sites are not essential for the anticonvulsant actions of dimemorfan.
Kainate (KA) administration (10 mg kg−1, i.p.) produced robust convulsions lasting 4–6 h in rats. Pre-treatment with dimemorfan (12 or 24 mg kg−1) reduced seizures in a dose-dependent manner. Dimemorfan pre-treatment also attenuated the KA-induced increases in c-fos/c-jun expression, activator protein (AP)-1 DNA-binding activity, and loss of cells in the CA1 and CA3 fields of the hippocampus. These effects of dimemorfan were comparable to those of dextromethorphan.
The anticonvulsant action of dextromethorphan or dimemorfan was significantly counteracted by a selective σ1 receptor antagonist BD 1047, suggesting that the anticonvulsant action of dextromethorphan or dimemorfan is, at least in part, related to σ1 receptor-activated modulation of AP-1 transcription factors.
We asked whether dimemorfan produces the behavioral side effects seen with dextromethorphan or dextrorphan (a phencyclidine-like metabolite of dextromethorphan). Conditioned place preference and circling behaviors were significantly increased in mice treated with phencyclidine, dextrorphan or dextromethorphan, while mice treated with dimemorfan showed no behavioral side effects.
Our results suggest that dimemorfan is equipotent to dextromethorphan in preventing KA-induced seizures, while it may lack behavioral effects, such as psychotomimetic reactions.
Keywords: Anticonvulsants, dimemorfan, dextromethorphan, phencyclidine, conditioned place preference, σ1 receptor, kainate, hippocampus, AP-1 DNA-binding activity, neuronal cell loss
Introduction
Dextromethorphan (DM; 3-methoxy-17-methylmorphinan) is a non-narcotic morphinan derivative widely used as an antitussive for almost 40 years. It has attracted attention due to its anticonvulsant and neuroprotective properties (Tortella & Musacchio, 1986; Choi, 1987; Ferkany et al., 1988; Loscher & Honack, 1993; Tortella et al., 1994; Kim et al., 1996; 2001a; 2003a; Yin & Sun, 1999; Liu et al., 2003; Shin et al., 2004b). However, case reports of toxicity in children (Rammer et al., 1988) and phencyclidine (PCP)-like psychotomimetic reactions (Holtzman, 1994; Cranston & Yoast, 1999; Price & Lebel, 2000) associated with high-dose DM ingestion are likely attributable to dextrorphan (DX; a major metabolite of DM), as is the reported abuse potential in adolescent youths (Noonan et al., 2000). Therefore, a DM analog that retained its anticonvulsant and neuroprotective activities without being converted into DX in vivo would be highly useful (Tortella et al., 1989; 1994; Chou et al., 1999; Kim et al., 2001b; 2003a, 2003b).
The DM analog dimemorfan (DF; 3-methyl-17-methylmorphinan) has been recognized as an antitussive since 1976 (Kasé et al., 1976), with an efficacy that is about equal to that of DM (Chou et al., 1999; Shin et al., 2004b). Like DM, the potent anticonvulsant activity of DF has been observed in the maximal electroshock tests (Chou et al., 1999) and in seizures induced by L-type calcium channel activator BAY k-8644 (Shin et al., 2004b). Since DF has an established safety record in humans at antitussive doses, and it is not metabolized to the PCP-like compound DX (Ida, 1997), the anticonvulsant and neuroprotective properties of this promising compound deserve further study.
DM-binding sites may represent the functional receptors responsible for mediating the anticonvulsant effects and those of other nonopioid antitussives (Tortella et al., 1989). The sites in the brain to which radiolabelled DM binds with high affinity show striking similarities in binding characteristics and regional distribution to σ1 receptors (Tortella et al., 1989).
Kainate (KA)-induced neuronal brain damage is similar to that seen in temporal lobe epilepsy of humans (Ben-Ari, 1985). It is well known that various stimuli to neuronal circuits, including epileptic seizures, lead to a specific pattern of increase in the expression in the limbic system of immediate-early genes such as c-fos, c-jun, c-myc or zif/268 (Morgan & Curran, 1991; Pennypacker et al., 1995). c-fos and c-jun are a family of inducible transcription factors (Morgan & Curran, 1991; Pennypacker et al., 1995). This complex recognizes activator protein (AP)-1 DNA response elements in the promotor regions of target genes to regulate gene transcription. Lesions that produce seizure activity can also induce the expression of AP-1 transcription factors; systemic administration of KA also induces c-fos and c-jun in the brain (Morgan & Curran, 1991; Pennypacker et al., 1995).
In the present study, we evaluated whether PCP-like behavioral effects (Noda et al., 1995; Kim et al., 2001b; 2003b) can occur after treatment with morphinans (DM, DX and DF), and examined the effects of DM and DF on seizures induced by KA. Simultaneously, we examined the effects of DM and DF on KA-induced increases in c-fos and c-jun mRNA levels, c-Fos and c-Jun protein levels, and AP-1 DNA-binding activity in the rat hippocampus. Since the anticonvulsant/neuroprotective action of the morphinans may be related to σ receptor modulation (Tortella et al., 1989; Chou et al., 1999; Kim et al., 2003b; Wang et al., 2003), we assessed the role of σ receptors in the pharmacological action of DM and DF.
Methods
Treatment of animals and preparation of samples
All animals were treated in strict accordance with the NIH Guide for the Humane Care and Use of Laboratory Animals (NIH Guide for the Humane Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985). In our preliminary study, we observed that mice are more sensitive to the behavioral effects induced by morphinans than are rats, while rats are more sensitive to the seizure behaviors induced by KA than are mice (Kim et al., 1998; 2003b; Park et al., 2002; Shin et al., 2004b). Both species were therefore used in the present study.
Male C57 BL/6 mice (in total, 135 mice were used for evaluating locomotor activity and conditioned place preference (CPP); Bio Genomics, Inc., Charles River Technology, Gapyung-Gun, Gyeonggi-Do, Korea) weighing about 27±3 g and male Sprague–Dawley rats (in total, 600 rats were used for KA study; Bio Genomics, Inc., Charles River Technology, Gapyung-Gun, Gyeonggi-Do, Korea) weighing about 250 g were maintained on a 12 : 12 h light : dark cycle and fed ad libitum. They were adapted for 2 weeks to the above conditions before experiment. Rats that did not express seizures after KA treatment, with or without any additional drug treatment, were excluded from biochemical and histological measurements. Therefore, 290 rats were used for biochemical and histological measurements.
Injection of DF phosphate (Park et al., 2002; Shin et al., 2004b) or DM hydrobromide (12 or 24 mg kg−1, s.c.) (Kim et al., 1996; 2001b; 2003b) occurred 30 min prior to KA administration. A DM or DF concentration of 12 mg kg−1 was chosen as the minimal anticonvulsant dose against KA-induced seizures in rats (Kim et al., 1996; Park et al., 2002; Shin et al., 2004b). Rats were killed at 12 h (for biochemical study) or 72 h (for histological evaluation) after KA (10 mg kg−1, i.p.) injection. Control rats received the same volume of saline.
BD 1047 is an antagonist of both σ1 and σ2 receptor (Bowen et al., 1989; 1993; Matsumoto et al., 1995; McCracken et al., 1999), although BD 1047 has a 51-fold greater affinity for σ1-binding-sites than for σ2 sites (Matsumoto et al., 1995). Ifenprodil is selective for σ-binding sites at 37°C (Hashimoto & London, 1993; Hashimoto et al., 1994), while it is selective for N-methyl-D-aspartate (NMDA)-polyamine site at 4°C in the rat brain (Hashimoto et al., 1994), although ifenprodil binds to several other sites, such as the dopamine transporter (Witkin & Acri, 1995), alpha-adrenergic receptors (Kurihara et al., 1993) and delta-8,7-isomerase protein (Moebius et al., 1998). As no highly selective σ1 and σ2 receptor antagonists are available, we used BD 1047 as a selective σ1 receptor antagonist (Matsumoto et al., 1995; McCracken et al., 1999; Kim et al., 2001c) and ifenprodil as a nonspecific σ2 receptor antagonist (Hashimoto & London, 1993; 1995; Hashimoto et al., 1994) in this study. BD 1047 dihydrobromide (1 or 2 mg kg−1, i.p.) (McCracken et al., 1999; Kim et al., 2001c) or ifenprodil (5 or 10 mg kg−1, i.p.) (Gotti et al., 1990; Bath et al., 1996) was administered 15 min before KA.
Radioligand binding
σ and PCP receptor-binding experiments were performed on male Sprague–Dawley rat brain membranes and were modified from those employed by Calderon et al. (1994). The dissociation constant (Kd, nM) and the density of receptor sites (Bmax, fmol mg−1 protein−1) values for the radio ligands used in this study were as follows: σ1 receptor ([3H](+)-N-allylnormetazocine ((+)-SKF10047), 49.2 Ci mmol−1), Kd=20±2, Bmax=315±9); σ2 receptor (([3H]1,3-di(2-tolyl)guanidine (DTG), 35 Ci mmol−1), Kd=25±1, Bmax=792±45); PCP receptor (([3H]1-[1-(2-thienyl)-cyclohexyl]piperidine (TCP), 57.6 Ci mmol−1), Kd=21±1, Bmax=1071±52). The Kd and Bmax values of these radioligands were comparable with previous reports (Calderon et al., 1994; McCann et al., 1994). In competition experiments, various concentrations of test drugs were added with a fixed concentration of the radioligand for receptor binding (5 nM [3H](+)-SKF10047, 7 nM [3H]DTG, 5 nM [3H]TCP). Binding data were analyzed using the GraFit program (Erithacus Software Ltd) (Chou et al., 1999).
Seizure activity
Using the automated video-tracking system (Noldus Information Technology, Wageningen, Netherlands), seizure activity was evaluated after KA administration. The seizure activity was rated during a 6-h period following the KA challenge in rats by investigators blind to the treatment the animals received, according to the scale devised by Racine (1972): stage 1, facial clonus; stage 2, nodding; stage 3, forelimb clonus; stage 4, forelimb clonus with rearing; stage 5, rearing, jumping and falling. Seizure stages were scored only when rats showed more than three consecutive seizures at a given stage.
Isolation of RNA and proteins
Total cellular RNAs and proteins were extracted from rat hippocampi using a rapid guanidine thiocyanate-water saturated phenol/chloroform extraction and subsequent precipitation with acidic sodium acetate (Chomcznski & Sacchi, 1987). Total cellular RNAs in the aqueous phase were precipitated with cold isopropyl alcohol. Isolated RNA samples were subjected to spectrophotometric analysis at 260 and 280 nm. The separated organic layer was extracted 2 × with an equal volume of sterile Millipore water and proteins were precipitated by adding 2 v of absolute ethanol to the water-extracted organic phase. The protein pellets were washed 2 × with cold absolute ethanol and dried. The dried pellets were dissolved in a denaturing buffer (6 M guanidium chloride, 20 mM Tris–HCl (pH 8.0) and 1 mM EDTA). The protein samples were dialyzed against a renaturing buffer (20 mM Tris–HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 5 mM dithiothreitol, 5 mM MgCl2, 0.4 mM phenylmethylsulfonyl fluoride and 20% glycerol) at 4°C. The concentration of protein was determined with the Coomasie blue protein assay reagent using bovine serum albumin as a standard.
Non-isotope Northern blot hybridization analysis
Total RNA was (10 μg) were denatured and electrophoresed on 1% agarose-formaldehyde gels (Kopchik et al., 1981) and transferred to nylon hybond-N hybridization membrane sheets. After baking for 1–2 h at 80°C, the membranes were pre-hybridized at 68°C for at least 1 h in prehybridization buffer (5 × SSC, 50% formamide, 0.02% SDS, 0.1% sodium N-lauroyl sarcosine, 2% blocking agent). The DIG-labelled c-fos and c-jun probes were added to pre-hybridization buffer containing 50% formamide. The membranes were incubated overnight at 68°C in shaking water bath and washed 2 × for 10 min per wash in 2 × wash solution (0.1 × SSC, 0.1% SDS) at room temperature. Then, the membranes were washed 2 × for 15 min per wash in 0.1 × wash solution (0.1 × SSC, 0.1% SDS). After equilibrating in buffer I (100 mM maleic acid, 150 mM NaCl (pH 7.5)) for 1 min, the membranes were gently agitated in buffer II (1% blocking agent in buffer I) for 30–60 min. The membranes were hybridized with the diluted anti-DIG-alkaline phosphatase (1 : 10,000 (75 mU ml−1)) in a buffer II for 30 min. After washing 2 × for 15 min per wash in 0.3% Tween-20 (in buffer I), the membranes were equilibrated in buffer III (100 mM Tris–HCl (pH 9.5), 100 mM NaCl, 50 mM MgCl2) for 2 min. Lumi-phos TM 530 (≈0.5 ml 100 cm2) was spread over the surface of membrane. After incubation at 37°C for 15–20 min, the membranes were exposed to Hyperfilm-MP for detection of the chemi-luminiscent signal. For re-hybridization, blots were washed for 20 min at room temperature in sterile Millipore water, then further washed overnight at 65°C in 50 mM Tris–HCl (pH 8.0), 50% dimethylsulfonamide and 1% SDS to remove the hybridized probe and re-hybridized to Dig-labelled rat glyceraldehyde phosphate dehydrogenase (GAPDH) cRNA probe. The cRNA probes for c-fos (Curran et al., 1987), c-jun (Boham et al., 1987) and GAPDH (Fort et al., 1985) were synthesized in vitro from linearized expression vector using DIG-UTP, as suggested by the manufacturer (Boehringer Mannheim, Germany).
Western immunoblot analysis
Total cellular proteins (50 μg) were separated on 12% sodium dodecyl sulfate–polyacrylamide gel, transferred onto nitrocellulose membranes and the resulting blot was blocked in PBS containing 3% skim milk for 30 min. Each blot was incubated overnight at 4°C with the antibody against c-Fos or c-Jun at a 1 : 1000 dilution, respectively. After washing in PBS, membranes were incubated with a biotinylated goat anti-rabbit antibody for 1 h, followed by incubation in avidin-biotin conjugated with horseradish peroxidase at room temperature. The blots were then incubated in an enhanced chemiluminescence (ECL) reagent, placed against Amersham hyperfilm and developed after a 2-min exposure. Relative intensities of the bands were quantitated by laser densitometry.
Nonisotope electrophoretic mobility shift assay
Annealing was achieved by incubating an equal molar concentration of each single-stranded oligo in 10 mM Tris–HCl (pH 8.0), 1 mM EDTA and 200 mM NaCl for 10 min at 95°C for 10 min, and then the mixture was allowed to cool to room temperature gradually. The AP-1 oligomer (22-mer; 5′-CTAGTGATGAGTCAGCCGGATC-3′) contained the consensus sequence (5′-TGAGTCA-3′). Nuclear extracts for the AP-1 DNA-binding assay were prepared from hippocampi from each animal. Binding reactions were performed at room temperature for 15 min and reaction mixtures contained 50 μg of nuclear protein, 20 mM HEPES (pH 7.6), 30 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 0.2% Tween-20, 50 ng ml−1 of poly(DI-dC) and ≈0.3 pmol of specified probe labelled with DIG-ddUTP by terminal transferase. Protein DNA complexes were separated on a 5% nondenaturing polyacrylamide gel. Gels were run at room temperature in 89 mM Tris (pH 8.3), 89 mM boric acid, 2 mM EDTA at a constant voltage (8 V cm−1) and electroblotted onto positive charged nylon membrane. The membranes were baked in 80°C for 15 min, washed with 0.3% Tween-20 in buffer I and hybridized with the diluted anti-DIG-alkaline phosphatase (1 : 10,000 (75 mU ml−1)) in buffer II for 30 min after washing 2 × for 15 min with Tween-20 (in buffer I), the membranes were equilibrated in a buffer III (100 mM Tris–HCl (pH 9.5), 100 mM NaCl, 50 mM MgCl2) for 2 min. The method of chemiluminescent detection was identical with the method used for nonisotope Northern hybridization analysis (Won et al., 1997). The relative intensities of the bands were quantified by laser densitometry (Kim et al., 1999; 2001b; 2003a).
Histology
The rats from each group were anesthetized with 50 mg kg−1 of pentobarbital and perfused transcardially with a 140 ml syringe containing saline (40 ml 100 g−1, b.w.) followed by 4% paraformaldehyde (70 ml 100 g−1, b.w). The brains were removed and then cut at 40 μm in the horizontal plane with a sliding microtome. The cresyl-violet stain was used to examine the degenerating neurons. The sections containing the dorsal hippocampus were stained with cresyl-violet in order to examine the degenerating neurons in the stratum pyramidale of the CA1 and CA3 regions. The neuronal density was evaluated by an image analysis system with polaroid microscopic camera (Optima version 6.2) (Kim et al., 1999; 2001a).
Conditioned place preference (CPP)
As a control, C57 BL/6 mice received an i.p. injection of saline just before entering the white or black compartment. Each compound (24 or 36 mg kg−1, i.p.) dissolved in saline (0.1 ml 10 g−1) was administered immediately before the mice were placed in the white compartment. On day 1, the mice were pre-exposed to the test apparatus for 5 min. The guillotine doors were raised and the mice were allowed to move freely between the two compartments. On day 2, the time each mouse spent in each compartment was recorded for 15 min. On days 3, 5, 7, 9, 11 and 13, the mice were injected with each drug before being confined to the white compartment, the nonpreferred side, for 40 min. On days 4, 6, 8, 10 and 12, the mice were injected with saline before being confined to the black compartment, the preferred side, for 40 min. On day 14, the guillotine doors were raised. The mice were initially placed in the tunnel and the time spent by the mice in the two compartments was recorded for 15 min. The scores were calculated from the differences in the time spent in the white compartment in the testing and pre-testing phases (Jhoo et al., 2000; Kim et al., 2003b). Data were analyzed between 09:00 and 17:00 h.
Circling behaviors
C57 BL/6 mice received each compound (seventh drug challenge) 1 day after CPP paradigm (sixth drug challenge). At 10 min after the last treatment with each drug, the ‘absolute turn angular (the sum of absolute angles between the movement vectors of two consecutive sample intervals)' was analyzed in a 30 min monitoring period using an automated video-tracking system (Noldus Information Technology, Wagenin, The Netherlands) to examine circling behaviors (i.e. marginal activities). Locomotor facilitation at the borders (margins) of the test box was defined as circling behaviors (or marginal activities) (Jhoo et al., 2000; Kim et al., 2001b; 2003b). Eight test boxes (40 × 40 × 30 cm3 high) were operated simultaneously by an IBM computer. Animals were studied individually during locomotion in each test box, where they were adapted for 10 min before starting the experiment. A printout for each session showed the pattern of the ambulatory movements of the test box. The distance travelled in cm by the animals in horizontal locomotor activity was analyzed (Jhoo et al., 2000; Kim et al., 2001b; 2003b). Data were collected and analyzed between 09:00 and 17:00 h.
Drugs, solutions and reagents
All solutions were freshly made using distilled deionized water or saline. KA and DM hydrobromide were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). DF phosphate was synthesized (Park et al., 2002; Shin et al., 2004b) according to the method of Murakami et al. (1972). BD 1047 dihydrobromide and ifenprodil were purchased from Tocris Cookson Ltd, (Bristol, U.K.). KA was injected by i.p. in a dosage of 10 mg ml−1 kg−1. The other drugs were administered in a volume of 0.1 ml 10 g−1. The chemical structures of the morphinans are shown in Figure 1.
Figure 1.
Chemical structures of dextrorotatory morphinan analogs.
Coomasie blue protein assay reagent and ECL reagent were purchased from Pierce Chemical (Rockford, IL, U.S.A.). Antibodies against c-Fos and c-Jun were supplied by Santa Cruz Biotech. Inc. (Santa Cruz, CA, U.S.A.). AP-1 oligomer was obtained from Stratagene (La Jolla, CA, U.S.A.).
Statistics
Statistical analyses were performed using the χ2 test for convulsing or mortality, and a nonparametric Wilcoxon signed rank test for seizure latency or seizure score (as the seizure latency or seizure score did not satisfy the assumption of the parametric approach, an ordered nonparametric approach was utilized) (Shin et al., 2004a). The significance of differences in the CPP or circling behavior was assessed using the ANOVA with Duncan's new multiple (DMR) test. Differences in the biochemical and histological studies were compared by Student's t-test. A significant level of less than 0.05 was accepted for comparisons.
Results
Dimemorfan is a selective σ1 ligand with very low affinity to phencyclidine sites
The affinities of different compounds to σ1, σ2 and PCP receptors are shown in Table 1. The Ki values of reference compounds concurred with previous investigations (Calderon et al., 1994; McCann et al., 1994). DF, DM and DX exhibited high affinity and selectivity at σ1 over σ2 receptors and showed approximately equipotent potency at the σ1 receptor (Ki range 0.1–0.2 μM). However, among these three drugs, DX has the highest affinity (Ki=0.9 μM), followed by DM (Ki=7.3 μM), and DF was with the lowest affinity (Ki=17.0 μM) to PCP sites. DF, DM and DX showed 113-, 35- and six-fold affinity for σ1 vs PCP sites, respectively. These results revealed that DF is a selective σ1 ligand with very low affinity to PCP sites. As the method used to determine the σ2-binding data was carried out without a (+)-benzomorphan to mask σ1 binding, it reflects binding to σ1 and σ2 receptors combined. Therefore, the σ2 Ki values given in Table 1 are actually composite σ1/σ2 values. This means that the σ1/σ2 selectivity of the compounds is underestimated.
Table 1.
The Ki values of drugs for σ1, σ2 and phencyclidine (PCP) receptors in rat brain membranes
| Compound | Receptor | ||
|---|---|---|---|
| σ1 (nM) | σ2 (nM) | PCP (nM) | |
| DF | 151±17 | 4421±277 | 16,978±488 |
| DM | 205±42 | 11,060±1320 | 7253±302 |
| DX | 144±37 | 11,325±1395 | 906±77 |
| (+)-SKF10047 | 45±7 | 13,694±4989 | 587±108 |
| Haloperidol | 13±4 | 148±31 | 63,951±3858 |
| MK801 | 33,756±1999 | 51111±7630 | 8.2±1.6 |
| TCP | ND | ND | 36.3±0.2 |
The data are shown as mean±s.e.m. of Ki values from three to four.experiments with duplicate determinations. ND, not determined (reproduced from Chou et al. (1999) with permission).
The attenuation by dextromethorphan or dimemorfan of KA-induced seizures is mediated via σ1 receptor activation in rats
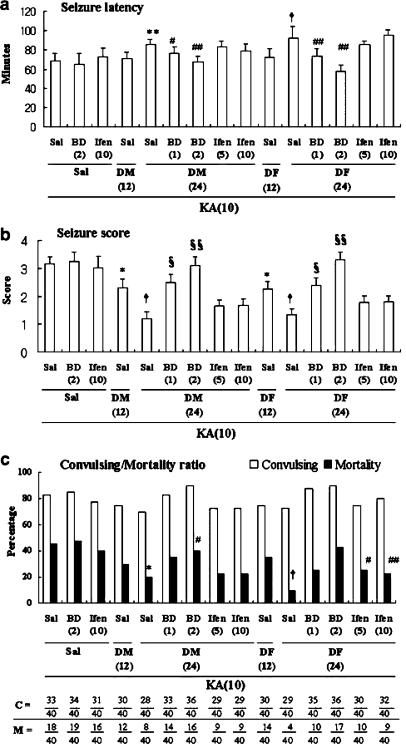
Rats pretreated with saline and given 10 mg kg−1 of KA showed robust behavioral seizures (seizure score: 3.1±0.3) lasting 5–6 h; clonic seizures were seen 68.8±7.3 min after KA administration. In all, 33 of the 40 rats (82.5%) exhibited seizures, and 18 of the 40 rats (45%) died within 72 h of KA injection. Neither BD 1047 nor ifenprodil alone significantly affected KA-induced seizures and mortality. By contrast, DM or DF pre-treatment significantly protected against the convulsive behavior. Seizure latency was increased in the presence of a high dose of DM or DF (KA vs 24 mg kg−1 DM+KA, P<0.02; KA vs 24 mg kg−1 DF+KA, P<0.005; nonparametric Wilcoxon signed rank test), as was mortality induced by KA (KA vs 24 mg kg−1 DM+KA, P<0.05; KA vs 24 mg kg−1 DF+KA, P<0.002; χ2 test). Seizure severity was decreased in a dose-dependent manner by treatment with DM or DX (KA vs 12 or 24 mg kg−1 DM+KA, P<0.05 or P<0.005; KA vs 12 or 24 mg kg−1 DF+KA, P<0.05 or P<0.005; nonparametric Wilcoxon signed rank test). The anticonvulsant effect of DM is equipotent to that of DF. Pre-treatment with BD 1047 (1 or 2 mg kg−1), but not ifenprodil (5 or 10 mg kg−1), dose-dependently counteracted neuroprotection by DM (24 mg kg−1) or DF (24 mg kg−1) against KA insult (Figure 2).
Figure 2.
Effects of BD 1047 (BD) or ifenprodil (Ifen) on the attenuation of DM and DF against KA-induced toxic behavioral signs in rats. The convulsions were induced with KA (10 mg kg−1, i.p.). C=number convulsing (the number of convulsing animals/total numbers of animals receiving each treatment). M=mortality (the number of dead animals/total number of animals receiving each treatment). Sal=saline treatment. Each dose (mg kg−1) in each group is in parenthesis. Either seizure behavior or number of convulsing animals was scored for 4 h after KA administration. Mortality was observed for 72 h after KA injection. Animals without KA exposure did not express any toxic behavior. Each value is the mean±s.e.m. of 40 animals. The statistical significance of the seizure latency (a) or seizure score (b) was calculated using the nonparametric Wilcoxon signed rank test. The statistical analysis of the number convulsing or mortality (c) was calculated using the χ2 test. *P<0.05, **P<0.02, †P<0.005 vs Sal+Sal+KA, #P<0.05, ##P<0.01, §P<0.01, §§P<0.0001 vs either DM or DF+KA.
The attenuation by dextromethorphan or dimemorfan of KA-induced hippocampal increases in c-fos and c-jun mRNA levels and protein levels is mediated via σ1 receptors
c-fos and c-jun mRNA levels were significantly increased (P<0.01 and P<0.01, respectively) 12 h after KA treatment. Treatment with saline, DM, or DF showed very little inductions of c-fos and c-jun mRNA levels. Neither BD 1047 nor ifenprodil alone was effective on the hippocampal increases in c-fos and c-jun mRNA induced by KA. DM or DF caused significant, dose-dependent attenuation of KA-induced increases in c-fos (KA vs 12 or 24 mg kg−1 DM+KA, P<0.01 or P<0.01; KA vs 12 or 24 mg kg−1 DF+KA, P<0.01 or P<0.01) and c-jun (KA vs 12 or 24 mg kg−1 DM+KA, P<0.05 or P<0.01; KA vs 12 or 24 mg kg−1 DF+KA, P<0.05 or P<0.01). Again, DF appeared to be equipotent to DM. Pre-treatment with BD 1047 (1 or 2 mg kg−1), but not ifenprodil (5 or 10 mg kg−1), dose-dependently counteracted the attenuation due to 24 mg kg−1 DM or DF (c-fos: DM+KA vs 1 or 2 mg kg−1 BD 1047+DM+KA, P<0.01 or P<0.01; DF+KA vs 1 or 2 mg kg−1 BD 1047+DF+KA, P<0.05 or P<0.01; c-jun: DM+KA vs 1 or 2 mg kg−1 BD 1047+DM+KA, P<0.05 or P<0.05; DF+KA vs 1 or 2 mg kg−1 BD 1047+DF+KA, P<0.05 or P<0.05; Student's t-test). c-fos mRNA expression in the presence of a selective σ1 receptor antagonist appeared to be more pronounced than did c-jun expression (Figure 3a).
Figure 3.
Effects of BD 1074 (BD) or ifenprodil (Ifen) on the attenuation by DM and DF of KA-induced increases in the expression of c-fos and c-jun mRNA (a) and c-Fos and c-Jun protein (b) in the rat hippocampus. In all, 10 μg of total RNA was used for determination of c-fos and c-jun mRNA levels (a). Total cellular proteins (50 μg) in each group were used for determination of proto-onco-protein levels using Western blot analysis. Polyclonal antibodies against c-Fos and c-Jun were used at a 1 : 1000 dilution. The 62- and 39-kDa bands indicate the c-Fos and c-Jun proteins (b). Specific signals were measured with laser densitometry. Each value is the mean±five animals. *P<0.01 vs Saline+Saline+Saline, #P<0.05, ##P<0.01 vs Saline+Saline+KA, †P<0.05, ‡P<0.01 vs either DM 24 or DF 24+KA (Student's t-test).
Fos and Jun protein levels were also increased at 12 h after KA treatment (P<0.01 and P<0.01, respectively). Negligible induction was observed in the absence of KA. Neither BD 1047 nor ifenprodil altered the increases in the c-Fos and c-Jun protein expression following KA administration. DM or DF attenuation of KA-induced increases in protein expression were significant and dose-dependent (c-Fos: KA vs 12 or 24 mg kg−1 DM+KA, P<0.05 or P<0.01; KA vs 12 or 24 mg kg−1 DF+KA, P<0.05 or P<0.01; c-Jun: KA vs 12 or 24 mg kg−1 DM+KA, P<0.05 or P<0.01; KA vs 12 or 24 mg kg−1 DF+KA, P<0.05 or P<0.01). The attenuating effects of DF were comparable to those of DM. Pretreatment with BD 1047 (1 or 2 mg kg−1), but not ifenprodil (5 or 10 mg kg−1), dose-dependently reversed attenuation (c-Fos: DM+KA vs 1 or 2 mg kg−1 BD 1047+DM+KA, P<0.05 or P<0.01; DF+KA vs 1 or 2 mg kg−1 BD 1047+DF+KA, P<0.05 or P<0.01; c-Jun: DM+KA vs 1 or 2 mg kg−1 BD 1047+DM+KA, P<0.05 or P<0.01; DF+KA vs 1 or 2 mg kg−1 BD 1047+DF+KA, P<0.05 or P<0.01; Student's t-test) (Figure 3b).
The attenuation by dextromethorphan or dimemorfan of KA-induced hippocampal increases in AP-1 DNA-binding activity is mediated via σ1 receptors
Treatment with saline, DM or DF produced very little induction of AP-1 DNA-binding activity in the rat hippocampus. Although binding activity was significantly induced (P<0.01) at 12 h after KA, this increase did not change in the presence of BD 1047 or ifenprodil. DF and DM were equipotent attenuators of this enhancement in AP-1 DNA-binding activity (KA vs 12 or 24 mg kg−1 DM+KA, P<0.05 or P<0.01; KA vs 12 or 24 mg kg−1 DF+KA, P<0.05 or P<0.01). Pretreatment with BD 1047 (1 or 2 mg kg−1) dose-dependently reversed attenuation by 24 mg kg−1 DM or DF (DM+KA vs 1 or 2 mg kg−1 BD 1047+DM+KA, P<0.05 or P<0.01; DF+KA vs 1 or 2 mg kg−1 BD 1047+DF+KA, P<0.05 or P<0.01; Student's t-test). Ifenprodil did not affect the attenuation provided by DM or DF (Figure 4).
Figure 4.
Effects of BD 1074 (BD) or ifenprodil (Ifen) on the attenuation by DM and DF of KA-induced increases in the AP-1 DNA-binding activity in rat hippocampus. Each value is the mean±s.e.m. of five animals. AP-1 oligomer (22-mer; 5′-CTAGTGATGAGTCAGCCGGATC-3′) contains the consensus sequence (5′-TGAGTCA-3′). *P<0.01 vs Saline+Saline+Saline, #P<0.05, ##P<0.01 vs Saline+Saline+KA, †P<0.05, ‡P<0.01 vs either DM 24 or DF 24+KA (Student's t-test).
Attenuation by dextromethorphan or dimemorfan of KA-induced neuronal loss is mediated via σ1 receptors
The neuronal layers of the hippocampus in the saline, DM, DF, BD 1047 and ifenprodil groups were clearly visible with cresyl-violet staining, indicating that the pyramidal neurons and the granule cells of the dentate gyrus remained intact. Significant neuronal loss in regions CA1 and CA3 was observed at 72 h after KA injection. This neuronal loss was not altered by the treatment with BD 1047 or ifenprodil. However, pre-treatment with DM or DF significantly blocked this loss. BD 1047 (2 mg kg−1) reversed attenuation afforded by DM or DF (both CA1 and CA3: Saline+Saline+Saline vs Saline+Saline+KA, P<0.01; Saline+Saline+KA vs Saline+DM or DF+KA, P<0.01 or P<0.01; Saline+DM or DF+KA vs BD 1047 (2 mg kg−1)+DM or DF+KA, P<0.01 or P<0.01; Student's t-test). Again, ifenprodil had no effect on the attenuation mediated by DM or DF (Figure 5).
Figure 5.
Representative photomicrographs of cresyl-violet-stained sections at 3 days after KA treatment. Rats treated with saline (a), DM, DF, BD 1047 (BD), ifenprodil (Ifen), DM+BD, DM+Ifen, DF+BD or DF+Ifen had clearly visible neuronal layers in the hippocampus after cresyl-violet staining. KA-induced neuronal damage was very severe; cresyl-violet did not clearly stain areas of coagulation necrosis in regions CA1 and CA3 (b). Neither BD (c) nor Ifen (d) did affect KA-induced neuronal degeneration. Pre-treatment with DM significantly reduced KA-induced neuronal loss (e). BD blocked neuroprotection seen with DM (f). By contrast, Ifen did not affect the protective effects of DM against the KA-induced loss of neurons (g). DF significantly attenuated the loss of pyramidal cells induced by KA (h). As seen with DM, BD reversed the DF-mediated neuroprotection (i). This counteraction was not observed in the presence of Ifen (j). Each value is the mean±s.e.m. of five animals. *P<0.01 vs Saline+Saline+Saline, #P<0.01 vs Saline+Saline+KA, †P<0.01 vs either Saline+DM+KA or Saline+DF+KA. Scale bar=500 μm.
Dimemorfan does not produce selectively CPP and circling behaviors in mice
No CPP effects were observed in saline-treated animals. DM treatment produced CPP (DM 24 or 36 mg kg−1 vs Saline, F(1,28)=9.30, P<0.01 or F (1,28)=11.42, P<0.001), as did treatment with DX (DX 24 or 36 mg kg−1 vs Saline, F (1,28)=12.92, P<0.001 or F (1,28)=14.07, P<0.001). The most significant CPP followed treatment with PCP (PCP 2.5 or 5 mg kg−1 vs Saline, F (1,28)=14.22, P<0.001 or F (1,28)=16.30, P<0.001). In contrast, DF-treated animals did not show selective CPP effects. DF-treated animals exhibited significantly fewer CPP effects than seen in DM- or DX-treated animals (DF 24 mg kg−1 vs DM 24 mg kg−1 or DX 24 mg kg−1, F (1,28)=15.15, P<0.001 or F (1,28)=16.18, P<0.001; DF 36 mg kg−1 vs DM or DX 36 mg kg−1, F (1,28)=8.79, P<0.01 or F (1,28)=7.84, P<0.01; ANOVA with DMR test)- (Figure 6a).
Figure 6.
Changes in CPP profile (a) and circling behavior (b) following repeated administration of PCP or morphinans (DM, DX and DF). DM=dextromethorphan, DX=dextrorphan, DF=dimemorfan. Each value is the mean±s.e.m. of 15 animals. *P<0.01, **P<0.001, #P<0.0001 vs Saline, ##P<0.01 vs corresponding DX or DM, †P<0.01 vs DX 36 mg kg−1 or either dose (2.5 or 5 mg kg−1) of PCP (ANOVA with DMR test).
Saline-treated animals exhibited basal circling activity as we demonstrated previously (Kim et al., 2003b; Kwon et al., 2004). The repeated administration of DX or DM significantly increased circling behavior (DX 24 or 36 mg kg−1 vs Saline, F (1,28)=6.27, P<0.01 or F (1,28)=19.54, P<0.001; DM 36 mg kg−1 vs Saline, F (1,28)=8.59, P<0.01). This effect appeared to be more pronounced in the animals treated with DX than in those treated with DM (DX 36 mg kg−1 vs DM 36 mg kg−1, F (1,28)=6.35, P<0.01). The circling behavior induced by DX was comparable to that of PCP [2.5 or 5.0 mg kg−1 vs Saline, F (1,28)=30.20, P<0.0001 or F (1,28)=45.27, P<0.0001). Circling behavior following treatment with DF was similar to that seen with saline. In addition, there was less circling behavior after DF treatment than after DX treatment (DF 36 mg kg−1 vs DX 36 mg kg−1, F (1,28)=10.80, P<0.01; ANOVA with DMR test) (Figure 6b).
Discussion
In this study, we showed that DF and DM are selective σ1 ligands, with very low affinities for NMDA-linked PCP sites. This also suggests that PCP sites are not required for the anticonvulsant effects of this morphinan (Chou et al., 1999). Indeed, at least one of the high-affinity DM-binding sites is identical to the σ1 site (Walker et al., 1990; Rothman et al., 1991; Zhou & Musacchio, 1991). We speculate that the anticonvulsant activity of DM or DF is related to the ability to bind to such sites.
Fos-like proteins dimerize with Jun-like proteins and the resultant complex recognizes AP-1 DNA-binding elements to regulate gene transcription (Morgan & Curran, 1991; Pennypacker et al., 1995). AP-1 DNA-binding activity generally increases in parallel with the levels of AP-1 transcription factors and increases are correlated with generalized seizure activity (Morgan & Curran, 1991; Pennypacker et al., 1995; Bing et al., 1997; Kim et al., 1999). DM and DF dose-dependently attenuated KA-induced increases in AP-1 transcription factors at the level of mRNA and protein, and reduced pyramidal cell loss in the hippocampus. These effects were blocked by the selective σ1 receptor antagonist, BD 1047, but not by ifenprodil. Therefore, DM or DF administration might influence, via σ1 receptors, target gene expression (via AP-1) and neurotoxicity.
Interestingly, other high-affinity anticonvulsant dextromethorphan ligands such as carbetapentane also exhibit relatively high affinities for σ1 sites (Tortella & Musacchio, 1986; Leander, 1989; Walker et al., 1990). Recently, we demonstrated that carbetapentane's anticonvulsant action is at least partly mediated by σ1 receptor modulation in the KA model (Kim et al., 2001c). Carbetapentane, like DM, might be influenced by voltage-sensitive ion channels (Tortella et al., 1989). Therefore, a functional relationship may exist between anticonvulsant activity and high-affinity DM-site binding/voltage-sensitive ion channels in the brain.
A variety of calcium channel antagonists also compete for the high-affinity DM (presumably σ1) site (Tortella et al., 1989), raising the possibility of a relationship between DM and the voltage-gated ion channel (Netzer et al., 1993; Kim et al., 2001a). Recently, we demonstrated that DM (12.5 mg kg−1, s.c.) attenuated the seizures induced in rats by KA (10 mg kg−1, i.p.) plus an L-type calcium channel agonist of the dihydropyridine class (BAY k-8644 (Brown et al., 1984), 2 mg kg−1, s.c.) more effectively than did DX (Kim et al., 2001a). We also showed that DM or DF attenuated BAY k-8644-evoked seizures in mice more strongly than did DX (Kim et al., 2001a; Shin et al., 2004b). These results suggest that the anticonvulsant effects of the morphinans involve, in part, an L-type calcium channel and that σ1 ligands may have particular therapeutic utility in combating convulsions/neurotoxicity induced via L-type calcium channel. Owing to the complex pharmacology of σ1 ligands, however, their interaction with the L-type calcium channel remains to be characterized further, possibly by using more specific tools in the future.
In this study, we observed significant anticonvulsant effects of DF and DM. In addition, CPP and circling behaviors were induced by DM or DX. The action of DX was qualitatively similar to that of PCP, in line with previous investigations (Jhoo et al., 2000; Kim et al., 2001a; 2003b; Shin et al., 2004b). Although the DM-induced behavioral changes appeared to be less pronounced than those of its metabolite DX, psychotropic effects were observed. To reduce PCP-like behavioral side effects, while retaining the anticonvulsant effects, position 3 in the morphinan ring system was substituted with a methyl group to yield DF, which cannot be metabolized into DX (Murakami et al., 1972; Kasé et al., 1976; Ida, 1997; Chou et al., 1999). Significant circling behaviors were not observed following treatment with DF. Moreover, the anticonvulsant effect of DF was equipotent to that of DM, suggesting that metabolism to DX is not a prerequisite for morphinan anticonvulsant activity (Kim et al., 2003a).
The localization of [3H]DM binding in the hippocampal formation suggests that area CA1 is one of the primary sites for the anticonvulsant actions of DM (Tortella et al., 1989). High-affinity KA-binding sites are preferentially located in the stratum lucidum of area CA3, the commissural/associational layer of the dentate gyrus and the pyramidal cell layer of area CA3. This may lead to the secondary release of endogenous glutamate in area CA1, and NMDA-sensitive glutamate-binding sites are predominantly located in the stratum radiatum of CA1 (Tortella et al., 1989). Although KA shows a greater affinity for the KA receptors, some of its epileptic effects may be mediated by activation of NMDA receptors, for which it shows moderate affinity (Sperk, 1994). Thus, the facilitative action of NMDA toward KA receptors might, in part, play a role in seizure generation. We observed that both DM and DF attenuate neuronal losses in the CA1 and CA3 regions following KA administration, suggesting that administered DM or DF is an important attenuator of the neuroexcitotoxicity induced by NMDA and KA in vivo, although precise mechanisms remain to be elucidated.
Together, the results of this study suggest that both DM and DF have prominent protective effects in response to KA-induced seizures, while only DM has behavioral side effects. DF represents a novel class of σ1 ligands that are potential candidates for the treatment of epilepsy (Chou et al., 1999; Shin et al., 2004b). The precise mechanisms by which DF attenuates seizures in various models remain to be determined.
Acknowledgments
This study was supported by a grant (R01-2003-000-10435-0) from the Korea Science & Engineering Foundation (KOSEF) and by a grant (#M103KV01000803K2201 00820) from the Brain Research Center from the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea.
Abbreviations
- AP-1
activator protein-1
- BAY k-8644
1,4-dihydro-2,6-dimethyl-3-nitro-4-[2-(trifluoromethyl)-phenyl]-3-pyridinecarboxylic acid methyl ester
- BD-1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine
- CPP
conditioned place preference
- ifenprodil
2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol
- KA
kainate
- σ1
sigma-1
References
- BATH C.P., FARRELL L.N., GILMORE J., WARD M.A., HICKS C.A., O'NEILL M.J., BLEAKMAN D. The effects of ifenprodil and eliprodil on voltage dependent Ca2+ channels and in gerbil global cerebral ischemia. Eur. J. Pharmacol. 1996;299:103–112. doi: 10.1016/0014-2999(95)00846-2. [DOI] [PubMed] [Google Scholar]
- BEN-ARI Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- BING G., WILSON B., HUDSON P., JIN L., FENG Z., ZHANG W., BING R., HONG J.S. A single dose of kainic acid elevates the levels of enkephalins and activator protein-1 transcription factors in the hippocampus for up to 1 year. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9422–9427. doi: 10.1073/pnas.94.17.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHAM D., BOS T.J., ADMON A., NISHIMURA T., VOGT P.K., TJIAN R. Human proto-oncogene c-jun encodes a DNA-binding protein with structural and functional properties of transcription factor AP-1. Science. 1987;238:1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- BOWEN W.D., HELLEWELL S.B., MCGARRY K. Evidence for a multi-site model of the rat brain σ receptor. Eur. J. Pharmacol. 1989;163:309–318. doi: 10.1016/0014-2999(89)90200-8. [DOI] [PubMed] [Google Scholar]
- BOWEN W.D., DE COSTA B.R., FELLEWELL S.B., WALKER J.M., RICE K.C. 3H]-(+)-pentazocine: a potent and highly selective benzomorphan-based probe for sigma-1 receptors. Mol. Neuropsychopharmacol. 1993;3:117–126. [Google Scholar]
- BROWN A.M., KUNZE D.L., YATANI A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984;311:570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- CALDERON S.N., IZENWASSER S., HELLER B., GUTKIND J.S., MATTSON M.V., SU T.P., NEWMAN A.H. Novel 1-phenylcycloalkanecarboxylic acid derivatives are potent and selective σ1 ligand. J. Med. Chem. 1994;37:2285–2291. doi: 10.1021/jm00041a006. [DOI] [PubMed] [Google Scholar]
- CHOI D.W. Dextrorphan and dextromethorphan attenuate glutamate neurotoxicity. Brain Res. 1987;403:333–336. doi: 10.1016/0006-8993(87)90070-9. [DOI] [PubMed] [Google Scholar]
- CHOMCZNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHOU Y.C., LIAO J.F., CHANG W.Y., LIN M.F., CHEN C.F. Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan. Brain Res. 1999;821:516–519. doi: 10.1016/s0006-8993(99)01125-7. [DOI] [PubMed] [Google Scholar]
- CRANSTON J.W., YOAST R. Abuse of dextromethorphan. Arch. Fam. Med. 1999;8:99–100. doi: 10.1001/archfami.8.2.99. [DOI] [PubMed] [Google Scholar]
- CURRAN T., GORDON M.B., RUBINO K.L., SAMBUCETTI L.C. Isolation and characterization of c-fos cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- FERKANY J.W., BOROSKY S.A., CLISSOLD D.B., PONTECORVO M.J. Dextromethorphan inhibits NMDA-induced convulsions. Eur. J. Pharmacol. 1988;151:151–154. doi: 10.1016/0014-2999(88)90707-8. [DOI] [PubMed] [Google Scholar]
- FORT P., MARTY L., PIECHACZYK M., SABROUTY E., DANI C., JEANTEUR P., BLANCHARD J.M. Various rat adult tissues express only one major mRNA species from glyceraldehydes-3-phosphate dehydrogenase mutagen family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTI B., BENAVIDES J., MACKENZIE E.T., SCATTON B. The pharmacotherapy of focal cortical ischaemia in the mouse. Brain Res. 1990;522:290–307. doi: 10.1016/0006-8993(90)91473-t. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., LONDON E.D. Further characterization of [3H]ifenprodil binding to sigma receptors in rat brain. Eur. J. Pharmacol. 1993;236:159–163. doi: 10.1016/0014-2999(93)90241-9. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., LONDON E.D. Interaction of erythro-ifenprodil, threo-ifenprodil, erythro-iodoifenprodil, and eliprodil with subtypes of σ receptors. Eur. J. Pharmacol. 1995;273:307–310. doi: 10.1016/0014-2999(94)00763-w. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., MANTIONE C.R., SPADA M.R., NEUMEYER J.L., LONDON E.D. Further characterization of [3H]ifenprodil binding in rat brain. Eur. J. Pharmacol. 1994;266:67–77. doi: 10.1016/0922-4106(94)90211-9. [DOI] [PubMed] [Google Scholar]
- HOLTZMAN S.G. Discriminative stimulus effects of dextromethorphan in the rat. Psychopharmacology. 1994;116:249–254. doi: 10.1007/BF02245325. [DOI] [PubMed] [Google Scholar]
- IDA H. The nonnarcotic antitussive drug dimemorfan: a review. Clin. Ther. 1997;19:215–231. doi: 10.1016/s0149-2918(97)80111-7. [DOI] [PubMed] [Google Scholar]
- JHOO W.K., SHIN E.J., LEE Y.H., CHEON M.A., OH K.W., KANG S.Y., LEE C., YI B.C., KIM H.C. Dual effects of dextromethorphan on cocaine-induced conditioned place preference in mice. Neurosci. Lett. 2000;288:76–80. doi: 10.1016/s0304-3940(00)01188-5. [DOI] [PubMed] [Google Scholar]
- KASÉ Y., KITO G., MIYATA T., UNO T., TAKAHAMA K., IDA H. Antitussive activity and other related pharmaceutical properties of D-3-methyl-N-methylmorphinan (AT-17) Arzneim-Forsch. (Drug Res.) 1976;26:353–360. [PubMed] [Google Scholar]
- KIM H.C., BING G., JHOO W.K., KIM W.K., SHIN E.J., IM D.H., KANG K.S., KO K.H. Metabolism to dextrorphan is not essential for dextromethorphan's anticonvulsant activity against kainate in mice. Life Sci. 2003a;72:769–783. doi: 10.1016/s0024-3205(02)02309-3. [DOI] [PubMed] [Google Scholar]
- KIM H.C., BING G., JHOO W.K., KO K.H., KIM W.K., LEE D.C., SHIN E.J., HONG J.S. Dextromethorphan modulates the AP-1 DNA binding activity induced by kainic acid. Brain Res. 1999;824:125–132. doi: 10.1016/s0006-8993(99)01155-5. [DOI] [PubMed] [Google Scholar]
- KIM H.C., JHOO W.K., KIM W.K., SHIN E.J., CHEON M.A., SHIN C.Y., KO K.H. Carbetapentane attenuates kainate-induced seizures viaσ-1 receptor modulation. Life Sci. 2001c;69:915–922. doi: 10.1016/s0024-3205(01)01181-x. [DOI] [PubMed] [Google Scholar]
- KIM H.C., KO K.H., KIM W.K., SHIN E.J., KANG K.S., SHIN C.Y., JHOO W.K. Effects of dextromethorphan on the seizures induced by kainate and the calcium channel angonist BAY k-8644: comparison with the effects of dextrorphan. Behav. Brain Res. 2001a;120:169–175. doi: 10.1016/s0166-4328(00)00372-7. [DOI] [PubMed] [Google Scholar]
- KIM H.C., LEE P.H., JHOO W.K.The complex pharmacological action of dextromethorphan; requirement of development of neuroprotective dextromethorphan analogs with negligible psychotomimetic effects International Symposium on the Molecular Monitoring in the Neuroscience Field 1998Nagoya, Japan; 1–2.Vol 3 [Google Scholar]
- KIM H.C., NABESHIMA T., JHOO W.K., KO K.H., KIM W.K., SHIN E.J., CHO M., LEE P.H. Anticonvulsant effects of new morphinan derivatives. Bioorg. Med. Chem. Lett. 2001b;11:1651–1654. doi: 10.1016/s0960-894x(01)00262-1. [DOI] [PubMed] [Google Scholar]
- KIM H.C., PENNYPACKER K., BING G., BRONSTEIN D., MCMILLIAN M., HONG J.S. The effect of dextromethorphan on kainic acid-induced seizures in the rat. Neurotoxicology. 1996;17:375–386. [PubMed] [Google Scholar]
- KIM H.C., SHIN C.Y., SEO D.O., JHOO J.H., JHOO W.K., KIM W.K., SHIN E.J., LEE Y.H., LEE P.H., KO K.H. New morphinan derivatives with negligible psychotropic effects attenuate convulsions induced by maximal electroshock in mice. Life Sci. 2003b;72:1883–1895. doi: 10.1016/s0024-3205(02)02505-5. [DOI] [PubMed] [Google Scholar]
- KOPCHIK T.T., CULLEN R., STACEY D.W. Rapid analysis of small nucleic acid samples by gel electrophoresis. Anal. Biochem. 1981;115:419–423. doi: 10.1016/0003-2697(81)90027-0. [DOI] [PubMed] [Google Scholar]
- KURIHARA J., TAMAOKI S., KATO H. Blockade of alpha 2-adrenoreceptors protects the vagal baroreflex system from transient global cerebral ischemia in dogs. Eur. J. Pharmacol. 1993;240:73–76. doi: 10.1016/0014-2999(93)90547-u. [DOI] [PubMed] [Google Scholar]
- KWON Y.S., ANN H.S., NABESHIMA T., SHIN E.J., KIM W.K., JHOO J.H., JHOO W.K., WIE M.B., KIM Y.S., JANG K.J., KIM H.C. Selegiline potentiates the effects of EGb 761 in response to ischemic brain injury. Neurochem. Int. 2004;45:157–170. doi: 10.1016/j.neuint.2003.10.005. [DOI] [PubMed] [Google Scholar]
- LEANDER J.D. Evaluation of dextromethorphan and carbetapentane as anticonvulsants and N-methyl-D-asparatic acid antagonists in mice. Epilepsy Res. 1989;4:28–33. doi: 10.1016/0920-1211(89)90055-7. [DOI] [PubMed] [Google Scholar]
- LIU Y., QIN L., LI G., ZHANG W., AN L., LIU B., HONG J.S. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J. Pharmacol. Exp. Ther. 2003;305:212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- LOSCHER W, HONACK D. Differences in anticonvulsant potency and adverse effects between dextromethorphan and dextrorphan in amygdala-kindled and non-kindled rats. Eur. J. Pharmacol. 1993;238:191–200. doi: 10.1016/0014-2999(93)90847-b. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO R.R., BOWEN W.D., TOM M.A., VO V.N., TRUONG D.D., DE COSTA B.R. Characterization of two novel σ receptor ligands: antidystonic effects in rats suggest σ receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- MCCANN D.J., WEISSMAN A.D., SU T.P. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- MCCRACKEN K.A., BOWEN W.K., DE COSTA B.R., MATSUMOTO R.R. Two novel sigma receptor ligands, BD 1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur. J. Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- MOEBIUS F.F., REITER R.J., BERMOSER K., GLOSSMANN H., CHO S.Y., PAIK Y.K. Pharmacological analysis of sterol Δ8-Δ7 isomerase. Proteins with [3H]ifenprodil. Mol. Pharmacol. 1998;54:591–598. doi: 10.1124/mol.54.3.591. [DOI] [PubMed] [Google Scholar]
- MORGAN J.L., CURRAN T. Proto-oncogene transcription factors and epilepsy. Trends Pharmacol. Sci. 1991;12:343–349. doi: 10.1016/0165-6147(91)90594-i. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., INUKAI N., NAGANO N. Studies on morphinan derivatives I. The synthesis of several new 3-substituted derivatives of N-methylmorphinan ring having antitussive activities. Chem. Pharm. Bull. 1972;20:1699–1705. doi: 10.1248/cpb.20.1699. [DOI] [PubMed] [Google Scholar]
- NETZER R., PFLIMLIN P., TRUBE G. Dextromethorphan blocks N-methyl-D-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur. J. Pharmacol. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- NODA Y., YAMADA K., FURUKAWA H., NABESHIMA T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br. J. Pharmacol. 1995;116:2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOONAN W.C., MILLER W.R., FEENEY D.M. Dextromethorphan abuse among youth. Arch. Fam. Med. 2000;9:791–792. doi: 10.1001/archfami.9.9.791-a. [DOI] [PubMed] [Google Scholar]
- PARK S.Y., SHIN E.J., JHOO W.K., KO K.H., KIM W.K., KIM H.C.Dimemorfan provides neuroprotection via activation of sigma-1 receptor and blocking L-type calcium channels; models of kainate and BAY k-8644 Soc. Neurosci. (abstract) 200232program no 798.5 [Google Scholar]
- PENNYPACKER K., HONG J.S., MCMILLIAN M. Implications of prolonged expression of Fos-related antigens. Trends Pharmacol. Sci. 1995;16:317–321. doi: 10.1016/s0165-6147(00)89061-6. [DOI] [PubMed] [Google Scholar]
- PRICE L.H., LEBEL J. Dextromethorphan-induced psychosis. Am. J. Psychiatry. 2000;157:304. doi: 10.1176/appi.ajp.157.2.304. [DOI] [PubMed] [Google Scholar]
- RACINE R.J. Modification of seizure activity by eletrical stimulation: motor seizure. EEG Clin. Nuerophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- RAMMER L., HOLMGREN P., SANDLER H. Fatal intoxication by dextromethorphan: a report on two cases. Forensic Sci. Int. 1988;37:766–768. doi: 10.1016/0379-0738(88)90230-7. [DOI] [PubMed] [Google Scholar]
- ROTHMAN R.B., MAHBOUBI A., REID A.A., KIM C.H., DECOSTA B.R., JACOBSON A.E., RICE K.C. [3H]1,3-D1(2-tolyl)guanidine labels two high affinity binding sites in guinea pig brain: evidence for allosteric regulation by calcium channel blockers and sigma ligands. NIDA Res. Mongr. 1991;105:335–336. [PubMed] [Google Scholar]
- SHIN E.J., JHOO J.H., KIM W.K., JHOO W.K., LEE C., JUNG B.D., KIM H.C. Protection against kainate neurotoxicity by pyrrolidine dithiocarbamate. Clin. Exp. Pharmacol. Physiol. 2004a;31:320–326. doi: 10.1111/j.1440-1681.2004.03990.x. [DOI] [PubMed] [Google Scholar]
- SHIN E.J., NABESHIMA T., LEE P.H., KIM W.K., KO K.H., JHOO J.H., JHOO W.K., CHA J.Y., KIM H.C. Dimemorfan prevents seizures induced by the L-type calcium channel activator BAY k-8644 in mice. Behav. Brain Res. 2004b;151:267–276. doi: 10.1016/j.bbr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- SPERK G. Kainic acid seizures in the rat. Prog. Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- TORTELLA F.C., MUSACCHIO J.M. Dextromethorphan and carbetapentane: centrally acting non-opioid antitussive agents with novel anticonvulsant properties. Brain Res. 1986;383:314–318. doi: 10.1016/0006-8993(86)90031-4. [DOI] [PubMed] [Google Scholar]
- TORTELLA F.C., PELLICANO M., BOWERY N.G. Dextromethorphan and neuromodulation: old drug coughs up new activities. Trends Pharmacol. Sci. 1989;10:501–507. doi: 10.1016/0165-6147(89)90050-3. [DOI] [PubMed] [Google Scholar]
- TORTELLA F.C., ROBLES L., WITKEN J.M., NEWMAN A.H. Novel anticonvulsant analogs of dextromethorphan: improved efficacy, potency, duration and side-effect profile. J. Pharmacol. Exp. Ther. 1994;268:727–733. [PubMed] [Google Scholar]
- WALKER J.M., BOWEN W.D., WALKER F.O., MATSUMOTO R.R., DECOSTA B., RICE K.C. Sigma receptors: biology and function. Pharmacol. Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- WANG H.H., CHIEN J.W., CHOU Y.C., LIAO J.F., CHEN C.F. Anti-amnesic effect of dimemorfan in mice. Br. J. Pharmacol. 2003;138:941–949. doi: 10.1038/sj.bjp.0705117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKIN J.M., ACRI J.B. Effects of ifenprodil on stimulatory, discriminative stimulus, and convulsant effects of cocaine. Behav. Pharmacol. 1995;6:245–253. [PubMed] [Google Scholar]
- WON J.S., KIM Y.H., SONG D.K., SUH H.W. The effects of cycloheximide on the regulation of proenkephalin and prodynorphan gene expressions induced by kainic acid in rat hippocampus. Mol. Bain Res. 1997;47:303–310. doi: 10.1016/s0169-328x(97)00067-3. [DOI] [PubMed] [Google Scholar]
- YIN K.J., SUN F.Y. Effect of dextromethorphan, a NMDA antagonist, on DNA repair in rat photochemical thrombotic cerebral ischemia. Brain Res. 1999;815:29–35. doi: 10.1016/s0006-8993(98)01071-3. [DOI] [PubMed] [Google Scholar]
- ZHOU G.Z., MUSACCHIO J.M. Computer-assisted modeling of multiple dextromethorphan and sigma binding sites in guinea pig brain. Eur. J. Pharmacol. 1991;206:261–269. doi: 10.1016/0922-4106(91)90108-t. [DOI] [PubMed] [Google Scholar]