Abstract
This study aims to investigate whether or not long-term statin treatment causes upregulation of D1 and D2 receptor gene expression with concomitant increase in endothelial nitric oxide synthase (eNOS) expression in Sprague–Dawley rats.
Serum triglyceride levels were dose dependently reduced in the simvastatin-treated rats reaching statistical significance at the highest dose (49% reduction), while pravastatin caused similar effects (52%) at the same dose. Cholesterol levels remained unchanged in both groups at all doses.
Simvastatin, 10 or 30 mg kg−1 day−1, increased D1 and D2 receptor expressions in the prefrontal cortex. Similar upregulation was observed neither with simvastatin in the striatum nor with pravastatin in both brain regions.
Simvastatin (10 mg kg−1 day−1) also increased eNOS expression in the prefrontal cortex but not neuronal NOS or inducible NOS.
D1 receptor activation by chloro-APB (5 μM) increased cAMP levels in synaptosomes prepared from the prefrontal cortex of control and simvastatin-treated rats by 88 and 285%, respectively. This effect was markedly attenuated by the selective D1 antagonist SCH-23390 (25 μM).
D2 receptor activation by quinpirole (5 μM) had no effect on the basal cAMP levels in synaptosomes prepared from the prefrontal cortex of control and simvastatin-treated rats, while the same concentration of quinpirole completely abolished the D1 receptor-mediated increase.
These results suggest that lipophilic statins can alter dopaminergic functions in the prefrontal cortex possibly via a central mechanism. The possibility of a nitric oxide mechanism involving eNOS requires further investigation.
Keywords: Hydroxymethylglutaryl-coenzyme reductase inhibitors, dopamine D1 and D2 receptors, upregulation, endothelial nitric oxide synthase, rat prefrontal cortex, statins
Introduction
Statins reduce serum low-density lipoprotein (LDL) cholesterol by inhibiting the rate-limiting enzyme, hydroxymethylglutaryl-coenzyme reductase, in cholesterol synthesis. These drugs are now widely used clinically for the prevention of atheromatous disease. Meta-analyses of data from major clinical trials have shown that the risk of ischaemic heart disease events is reduced by 60% and stroke by 17% (Law et al., 2003). The statins are also being recognised to have potential application in peripheral arterial disease, end-stage renal disease, diabetes mellitus, and Alzheimer's disease (AD) (McKenney, 2003; Vega et al., 2003). In animal studies, statins also appear to be beneficial in traumatic brain injury (Lu et al., 2004) and neuroinflammation (Adamson & Greenwood, 2003). Despite growing evidence for a role of statins in CNS diseases such as stroke and AD, there is relatively little knowledge of their effects in the brain.
Current evidence indicates that statins may provide neuroprotection against ischaemic damage through endothelial nitric oxide synthase (eNOS)-dependent mechanisms (Endres et al., 2004). Statins can increase the production of NO directly by increasing eNOS activity or indirectly by decreasing factors such as oxidised LDL, oxidised LDL receptor 1, and Rho, which negatively regulate eNOS expression (see Laufs, 2003 for a review). In the kidney, the natriuretic and diuretic responses mediated by D1 receptors were attenuated by the NOS inhibitor NG-nitro-L-arginine (L-NAME) in anaesthetised rats (Venkatakrishnan et al., 2000), while D2 receptor blockade potentiated the renal effects of L-NAME in humans (Montanari et al., 1998). It has also been reported that chemical hypoxia increased dopamine D1A receptor mRNA expression in renal epithelial cells (LLC-PK1 cell line), which is also blocked by L-NAME, indicating that the expression of this dopamine receptor is positively regulated by the NO system (Healy et al., 2000). In the brain, NO mediates release of dopamine (Hirsch et al., 1993; Dominguez et al., 2004). It has also been reported that NO is involved in the nicotine-induced burst firing of dopaminergic neurons in the ventral tegmental area of the rat brain (Schilstrom et al., 2004), and that these dopaminergic cells also express NOS (Klejbor et al., 2004). Therefore, it is interesting to investigate any possible effects of statins on the central dopaminergic system. Specifically, we studied as the first step whether or not long-term statin treatment causes upregulation of D1 and D2 receptor gene expression with concomitant increase in eNOS expression in Sprague–Dawley rats.
Methods
Animals and statin pretreatment
Male Sprague–Dawley rats (280–300 g) were obtained from the University Laboratory Animal Centre and housed four or five per cage with food and water available ad libitum under natural light-dark cycle (approx. 12–12 h). All experiments were carried out in accordance with the guidelines set by the National University of Singapore (adapted from Howard-Jones, 1985). Either simvastatin (1, 10, or 30 mg kg−1 day−1) (Ranbaxy Laboratories, Dewas, India) or pravastatin (30 mg kg−1 day−1) (Bristol-Myers Squibb, Noble Park, Australia) suspended in 1.5 ml saline was administered by gavage daily for a period of 4 weeks. Control rats received only saline.
Serum cholesterol and triglyceride
At the end of the 4-week treatment period, blood samples were collected for the determination of fasting (10–12 h) serum cholesterol and triglyceride levels. Triglyceride and cholesterol assay kits were obtained from ThermoTrace (Noble Park, Australia).
Reverse transcriptase–polymerase chain reaction (RT–PCR)
Rats were killed with CO2 and the prefrontal cortices and striata were removed on ice. Tissues were homogenised in ice-cold Trizol reagent (1 : 10 (w : v)) using a Polytron homogeniser. RT–PCR was carried out using a standard protocol. Extracted RNA (5 μg) was incubated with reverse transcriptase to synthesise single-stranded cDNA using oligo-dT primers (GIBCO-BRL, Life Technologies, Rockville, MD, U.S.A.). PCR was carried out in a total volume of 100 μl containing 20 mM Tris–HCl, 50 mM KCl, 1.25 mM MgCl2, 0.2 mM dNTPs, 0.2 mM of each primer, and 2.5 U of Taq DNA polymerase (GIBCO-BRL Life Technologies, Rockville, MD, U.S.A.). The expression of β-actin mRNA was used as an internal standard. Primer sequences are: D1 sense 5′-GATTCCATCACCTTCGATGTG-3′; D1 antisense 5′-GGTGTCATAGTCCAATATGACCG-3′ (359 bp); D2 sense 5′-CCCTCCTCATCTTTATCATCG-3′; D2 antisense 5′-GGTCTGTATTGTTGAGTCCGAA-3′ (410 bp) (Reuss & Unsicker, 2001); eNOS sense 5′-TGCACCCTTCCGGGGATTCT-3′; eNOS antisense 5′-GGATCCCTGGAAAAGGCGGT-3′ (189 bp) (De Gennaro Colonna et al., 2002); nNOS sense 5′-GGCACTGGCATCGCACCCTT-3′; nNOS antisense 5′-CTTTGGCCTGTCCGGTTCCC-3′ (213 bp); iNOS sense 5′-AGCATCACCCCTGTGTTCCACCC-3′; iNOS antisense 5′-TGGGGCAGTCTCCATTGCCA-3′ (388 bp) (Wu et al., 1999); and β-actin sense 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′; β-actin antisense 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ (870 bp) (Loo et al., 2002). PCR denaturing, annealing, and extension reactions proceeded, respectively, at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min (β-actin 30 cycles); 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min (D1 35 cycles); 94°C for 30 s, 59°C for 30 s, and 72°C for 45 s (D2 35 cycles); 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s (eNOS 35 cycles); 94°C for 1 min, 66°C for 1 min, and 72°C for 1 min (nNOS 35 cycles); or 94°C for 1 min, 64°C for 1 min, and 72°C for 1 min (iNOS 35 cycles). The PCR amplifications were linear. PCR product bands were analysed and quantified by the Viber Lourmat Imaging system and expressed as band intensity relative to the β-actin band.
Western blot analysis
Brain tissue was homogenised in a buffer (1 : 20 (w : v)) containing 0.32 M sucrose, 10 mM Tris–HCl (pH 7.4), 2 mM EDTA, and 10 μl ml−1 Protease-Arrest (protease inhibitor, Genotech, St Louis, MI, U.S.A.). The homogenate was centrifuged at 900 × g for 10 min, and the resultant supernatant was centrifuged at 100,000 × g for 1 h. The final pellet was resuspended in buffer containing 50 mM Tris–HCl, 10 mM EDTA, 100 mM NaCl, and 8 mM MgCl2 (pH 7.4) (0.2 g original tissue in 0.5 ml), and then mixed with an equal volume of a 2 × loading buffer containing 125 mM Tris–HCl, 20% (w : v) glycerol, 4% (w : v) SDS, 0.02% (w : v) bromophenol blue and 4% (w : v) β-mercaptoethanol. Membrane proteins were denatured at 95°C for 3 min, and then separated using 10% discontinuous SDS–polyacryamide gel electrophoresis (PAGE) (150 μg protein/lane). The resolved membrane proteins were transferred onto a 0.2-μm Hybond-P nitrocellulose sheet by semidry electroblotting for 1 h, and then soaked overnight at 4°C in 4% goat serum or evaporated goat milk for dopamine receptors; or 5% (w : v) nonfat dry milk in Tris-buffered saline containing Tween-20 (TBS-T; 10 mM Tris–HCl (pH 7.2), 250 mM NaCl, 0.05% (w : v) Tween-20) for eNOS and β-actin. The membranes were incubated at room temperature for 1 h with a primary antibody (rabbit anti-rat D1 or D2 receptor polyclonal antibody (Calbiochem, San Diego, CA, U.S.A.), 1 : 5000 dilution; rabbit anti-rat eNOS polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), 1 : 800; mouse anti-rat β-actin monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), 1 : 5000 in TBS-T, washed, and then incubated for 1 h with an appropriate secondary antibody, 1 : 5000 dilution; biotinylated anti-rabbit for D1 or D2 receptor, goat anti-rabbit for eNOS and goat anti-mouse for β-actin (Amersham Biosciences, Little Chalfont, U.K.). For D1 or D2 receptor only, this was followed by reacting with peroxidase-conjugated streptavidin at room temperature for 1 h. Protein bands were visualised by chemiluminescence (ECL Western Blotting kit, Amersham), analysed by Scan-gel-it software (Syngene, Cambridge, U.K.), and expressed as band intensity relative to the β-actin (the internal standard) band.
Determination of cAMP in rat synaptosomal preparation
Prefrontal cortices were homogenised in ice-cold 0.32 M. sucrose (10% (w : v)) using a Teflon-glass homogeniser with 0.25 mm clearance. The homogenate was centrifuged at 1000 × g for 15 min and the supernatant was centrifuged at 20,000 × g for 20 min. The resultant P2 pellet was resuspended in the same initial volume of sucrose solution. This synaptosomal preparation was kept at 2–4°C until use within 2 h.
Synaptosomal preparation (0.1 ml) was added to phosphate-buffered Krebs medium containing NaCl (128 mM), Na2HPO4 (10), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), D-glucose (11), ascorbic acid (1.1), and Na2EDTA (0.16) at pH 7.0, aerated with 95% O2 and 5% CO2 for 1 h. R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH-23390, 25 μM) was also added if appropriate. The mixture was warmed at 37°C for 10 min. Agonist drugs, 6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (chloro-APB), quinpirole hydrochloride, or both at 5 μM each were then added and incubation continued for another 25 min. The final incubation volume was 1 ml. All drugs and chemicals were of analytical grade and obtained from Sigma-Aldrich (St Louis, MI, U.S.A.).
After incubation, tissue was recovered by centrifugation (20,000 × g for 20 min, 4°C) and resuspended in water to give a protein concentration of 0.25–0.35 mg ml−1. cAMP was measured using a direct enzyme immunoassay kit (Sigma-Aldrich, St Louis, MI, U.S.A.).
Protein measurement
Protein contents of samples used in Western blot and cAMP determination were determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, U.S.A.).
Statistical analysis
All statistical procedures including independent sample t-test, one-, and two-way analysis of variance (ANOVA) (General Linear Model multivariate analysis) were performed by SPSS for Windows v12.
Results
Effects on serum cholesterol and triglyceride
Data presented in Table 1 show that simvastatin treatment dose dependently reduced serum triglyceride levels by 19, 29, and 49% in rats treated with 1, 10 or 30 mg kg−1 day−1, respectively, reaching statistical significance at the highest dose. Similarly, pravastatin (30 mg kg−1 day−1) reduced serum triglyceride levels by 52% when compared to the saline-treated control. Serum cholesterol levels, however, remained unchanged.
Table 1.
Serum triglyceride and cholesterol levels in rats after a 4-week treatment period with simvastatin (sim) or pravastatin (pra)
| Triglyceride (mg dl−1) | Cholesterol (mg dl−1) | |
|---|---|---|
| Saline treated | 63±4 | 69±3 |
| Sim treated (1 mg kg−1 day−1) | 51±14 | 67±6 |
| Sim treated (10 mg kg−1 day−1) | 45±9 | 82±2 |
| Sim treated (30 mg kg−1 day−1) | 32±5* | 66±1 |
| Pra treated (30 mg kg−1 day−1) | 30±4* | 72±4 |
Statistics were performed by general linear model multivariate analysis: triglyceride F(4,31)=5.259, P<0.005; cholesterol F(4,31)=1.523, P>0.2, n=5–17 per group, total n=37.
Significantly different from the saline-treated group, P<0.02, by post hoc analysis with Bonferroni correction.
Upregulation of dopamine receptor expression
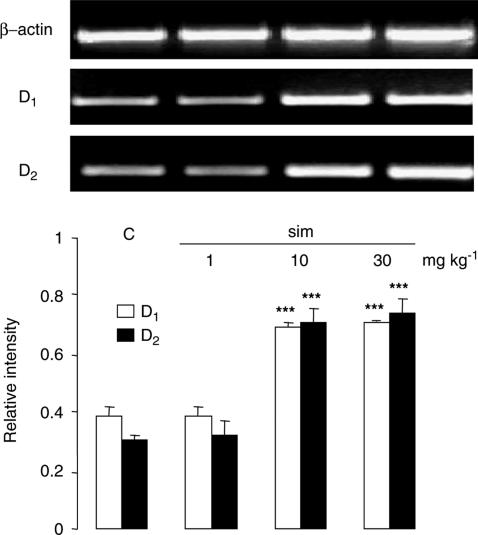
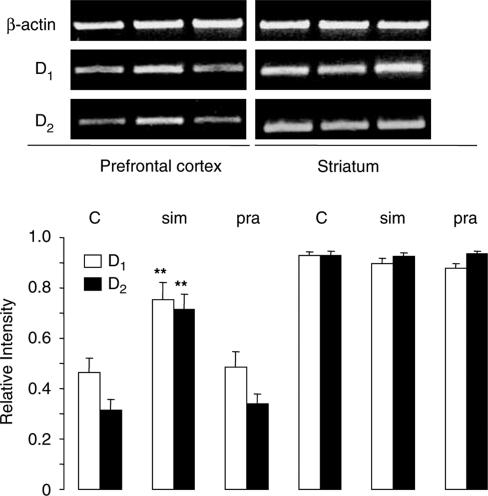
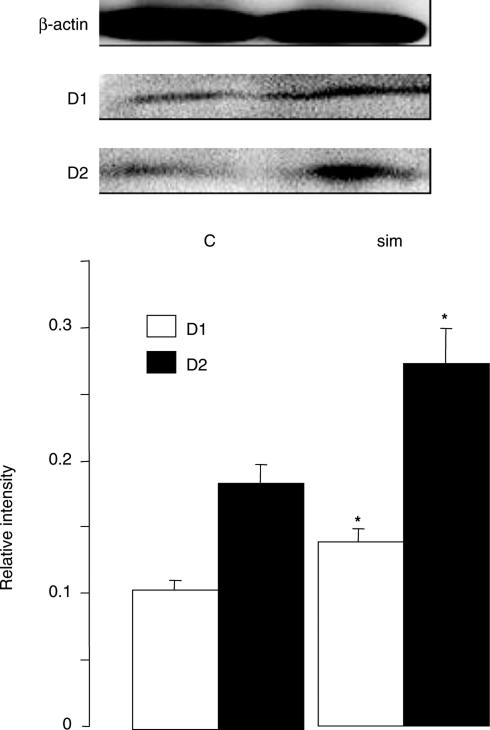
Simvastatin treatment at 10 or 30 mg kg−1 day−1 increased the mRNA expression of both D1 and D2 receptors in the prefrontal cortex increased by 1.8- and 2.4-fold, respectively, while a low dose of 1 mg kg−1 day−1 was without effect (Figure 1). In contrast, pravastatin, at 30 mg kg−1 day−1, failed to give similar effects (Figure 2). Both statins were without effect in the striatum (Figure 2). The upregulation of D1 and D2 receptors was further confirmed at the protein level, demonstrating a 1.3- and 1.5-fold increase, respectively, in the prefrontal cortex after simvastatin treatment (30 mg kg−1 day−1, Figure 3). Similar results were obtained also with 10 mg kg−1 day−1 (data not shown).
Figure 1.
Expression of mRNA of D1 and D2 receptors in the prefrontal cortex of saline- and simvastatin-treated rats. RT–PCR showed that simvastatin (sim, 10 or 30 mg kg−1 day−1) increased D1 and D2 receptor mRNA expression by about 1.8- and 2.4-fold, respectively, in the prefrontal cortex when compared to the saline-treated control (C) group. No changes were observed with sim treatment at 1 mg kg−1 day−1. Graphs showed band intensity relative to the corresponding β-actin band. Error bars represent s.e.m., n=6 per group. One-way ANOVA: F(3,16)=85.607, P<0.001 for D1 and F(3,16)=30.793, P<0.001 for D2. ***P<0.001 by post hoc analysis with Bonferroni correction. Top panel shows typical bands of β-actin, D1 and D2 receptors in the same order (left to right) as presented in the graph.
Figure 2.
Expression of mRNA of D1 and D2 receptors in the prefrontal cortex and striatum of saline-, simvastatin- and pravastatin-treated rats. RT–PCR showed that simvastatin (sim, 30 mg kg−1 day−1), but not pravastatin (pra, 30 mg kg−1 day−1), increased D1 and D2 receptor mRNA expression by 1.6- and 2.2-fold, respectively, in the prefrontal cortex when compared to the saline-treated control (C) group. No changes were observed in the striatum. Graphs showed band intensity relative to the corresponding β-actin band. Error bars represent s.e.m., n=6 per group. One-way ANOVA: F(2,27)=6.641, P<0.005 for D1 and F(2,15)=21.460, P<0.001 for D2 in the prefrontal cortex. **P<0.01 by post hoc analysis with Bonferroni correction. Top panel shows typical bands of β-actin, D1 and D2 receptors in the same order (left to right) as presented in the graph.
Figure 3.
Expression of D1 and D2 receptor proteins in the prefrontal cortex of saline- and simvastatin-treated rats. Simvastatin (30 mg kg−1 day−1) increased D1 and D2 receptor expression by 1.3- and 1.5-fold, respectively, in the prefrontal cortex when compared to the saline-treated control group. Graphs showed band intensity relative to the corresponding β-actin band. Error bars represent s.e.m. Top panel shows typical bands of β-actin, D1 and D2 receptors. *P<0.05 by independent sample t-test: t=2.524 for D1 and 2.984 for D2, n=6 per group.
Upregulation of eNOS expression
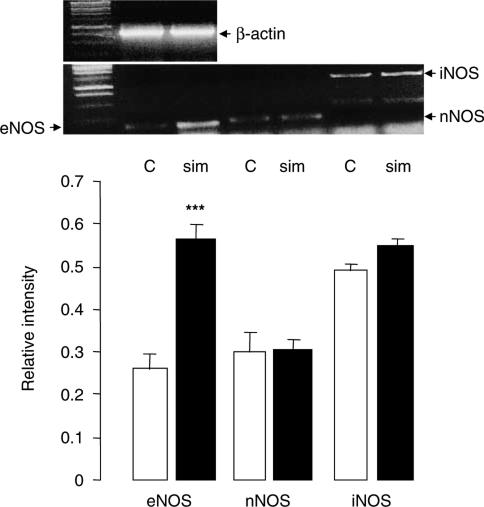
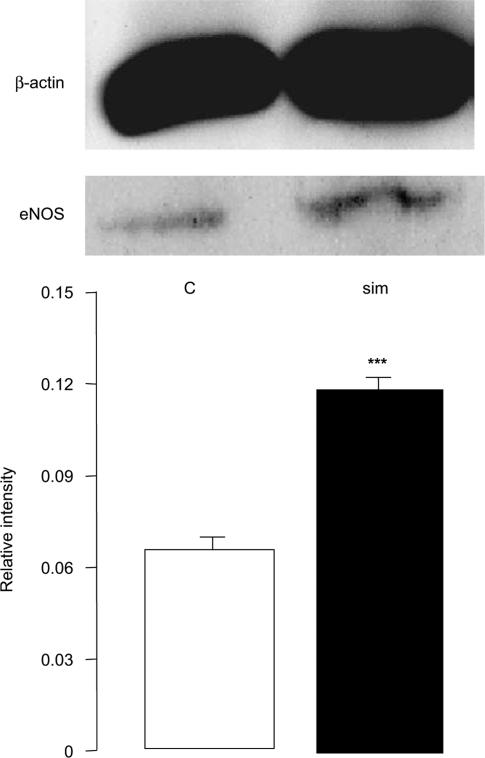
Since maximum upregulation of dopamine receptors was observed at 10 mg kg−1 day−1 dose of simvastatin treatment, this dose was used for the determination of eNOS expression. It was found that mRNA expression of eNOS increased by 2.1-fold, in the prefrontal cortex, but neither nNOS nor iNOS was affected by the simvastatin treatment (Figure 4). The upregulation of eNOS was confirmed at the protein levels (1.8-fold, Figure 5). No changes in eNOS expression were observed in the striatum and pravastatin (30 mg kg−1 day−1) also had no effects in either brain regions (data not shown).
Figure 4.
Expression of mRNA of eNOS, nNOS and iNOS in the prefrontal cortex of saline- and simvastatin-treated rats. RT–PCR showed that simvastatin (sim, 10 mg kg−1 day−1) increased eNOS mRNA expression by 2.2-fold in the prefrontal cortex when compared to the saline-treated control (C) group. No changes in expression were observed for nNOS and iNOS. Graphs showed band intensity relative to the corresponding β-actin band. Error bars represent s.e.m. Top panel shows typical bands of β-actin, eNOS, nNOS and iNOS as indicated. ***P<0.001 by independent sample t-test: t=4.460 for eNOS, n=7 per group.
Figure 5.
Expression of eNOS protein in the prefrontal cortex of saline- and simvastatin-treated rats. Simvastatin (10 mg kg−1 day−1) increased eNOS protein expression by 1.8-fold in the prefrontal cortex when compared to the saline-treated control group. Graphs showed band intensity relative to the corresponding β-actin band. Error bars represent s.e.m. Top panel shows typical bands of β-actin and eNOS. ***P<0.001 by independent sample t-test: t=19.694, n=6 per group.
Effects of dopamine agonists on synaptosomal cAMP levels
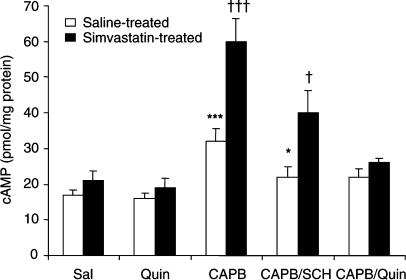
Figure 6 shows that D2 receptor activation by quinpirole (5 μM) did not significantly alter the synaptosomal cAMP levels. In contrast, D1 receptor activation by chloro-APB (5 μM) significantly increased cAMP levels by 88% in synaptosomes from control rats and 285% in synaptosomes from simvastatin-treated rats. These increases are markedly attenuated by the D1 antagonist SCH23390 (25 μM). Moreover, the chloro-APB-induced increases were completely abolished by quinpirole (5 μM).
Figure 6.
Effects of D1 and D2 receptor activation on cAMP levels in synaptosomes prepared from the prefrontal cortex of saline- and simvastatin-treated rats. Synaptosomal preparations were obtained from the prefrontal cortex of rats treated with saline or simvastatin (10 mg kg−1 day−1) and then incubated with various drugs as indicated. cAMP levels were determined as described in text. Sal=saline, Quin=quinpirole (5 μM), CAPB=chloro-APB (5 μM), SCH=SCH-23390 (25 μM), n=4–14 per group, total n=65. Statistical analysis was performed using general linear model multivariate analysis: pretreatment (control vs simvastatin-treated rats): F(1,55)=22.202, P<0.001; drug treatment: F(4,55)=23.502, P<0.001; interaction: F(4,55)=5.234, P<0.002. *** or †††P<0.001 against corresponding Sal, Quin and CAPB/Quin groups, and * or †P<0.05 against corresponding Sal, Quin and CAPB groups by post hoc analysis with Bonferroni correction.
Discussion
Both simvastatin and pravastatin, at 30 mg kg−1 day−1 dose, significantly decreased serum triglyceride level by 50% in rats after 4 weeks of treatment, while serum cholesterol levels remained unchanged. Both statins thus produced a significant alteration in the lipid metabolism at the highest dose used. These results are consistent with previous reports (Schoonjans et al., 1999; Roglans et al., 2002), for example, Schoonjans et al. reported that an extremely high dose of simvastatin (120 mg kg−1 day−1) for 4 days reduced triglyceride by 43% without affecting the total cholesterol in the serum.
Upregulation of eNOS expression in vascular tissues by statins has been reported in several species (Kano et al., 1999; Mital et al., 2000; Yamada et al., 2000; Wassmann et al., 2001). To the best of our knowledge, only Endres et al. (1998) demonstrated simultaneous upregulation of eNOS in the aorta and brain (cerebral hemisphere) of 129/SV mice treated for 14 days with simvastatin (20 mg kg−1 day−1). Our present data extended this finding by showing upregulation of eNOS expression in the rat brain and regional differences between the prefrontal cortex and striatum. Endres et al. further demonstrated that the neuroprotective effects of simvastatin against focal cerebral ischaemia observed in these mice were completely absent in eNOS-knockout mice, indicating that increased eNOS activity is the underlying mechanism of the neuroprotection. Our observation that nNOS and iNOS were not upregulated together with eNOS is consistent with this conclusion.
In contrast to simvastatin, pravastatin did not increase the expression of eNOS, as well as the dopamine receptors. We attribute this to the hydrophilicity of pravastatin, which is thus unable to cross the blood–brain barrier readily. The lipophilicity, expressed as log D at pH 7.4, for simvastatin and pravastatin are 1.6 and −0.84, respectively (McTaggart et al., 2001). Consistently, Botti et al. (1991) reported that pravastatin was undetectable in the CSF of healthy human subjects when their serum drug concentration was >40 ng ml−1. On the contrary, the CSF concentration of lovastatin, a lipophilic statin similar to simvastatin, was found to be about 11% of that in the serum (1.3 vs 11.5 ng ml−1). This, together with the fact that pravastatin treatment altered serum triglyceride levels to the same extent as simvastatin treatment, strongly indicated that the lack of effects of pravastatin on the expression of eNOS and dopaminergic receptors in the prefrontal cortex is due to its absence (or presence in insufficient concentration) in the brain. Thus, simvastatin is acting through a central mechanism to cause the observed upregulation of eNOS and both D1 and D2 receptors.
Rosuvastatin (up to 20 mg kg−1 day−1) was also reported to provide neuroprotection similar to those of simvastatin and atorvastatin while upregulating eNOS in mice (Laufs et al., 2002). However, in this study, upregulation of eNOS in the brain was presumed based on the upregulation of eNOS in the aorta. Since rosuvastatin has low lipophilicity (log D at pH 7.4 is −0.33, McTaggart et al., 2001), it is uncertain whether eNOS was indeed upegulated in the brain by rosuvastatin in view of our present finding with pravastatin.
Concomitant to the increased eNOS expression, we have observed upregulation of D1 and D2 receptor expression in the prefrontal cortex. It has been reported that inhibition of NO synthesis by L-NAME reduced significantly haloperidol-induced supersensitivity resulting from dopamine (D2) receptor upregulation (Pudiak & Bozarth, 1997). Similarly, rats chronically treated with haloperidol showed elevated nNOS expression (Lau et al., 2003). Chemical hypoxia-induced increase in D1A receptor mRNA expression in renal epithelial cells was also blocked by L-NAME (Healy et al., 2000). It is therefore tempting to speculate that the upregulation of D1 and D2 receptors is secondary to that of eNOS in the prefrontal cortex after simvastatin treatment. However, present data do not provide any evidence for a cause-effect relationship between eNOS and dopamine receptor upregulation. Work is in progress in our laboratory using eNOS knockout mice to provide firm evidence for a possible crosstalk between the NO and dopaminergic systems in this brain region.
Dopamine receptor function was studied by measuring cAMP levels in synaptosomes since D1 and D2 receptors are linked positively and negatively, respectively, to adenylyl cyclase. The basal level of cAMP in the synaptosomal preparation was found to be about 20 pmol mg−1 protein, consistent with previously reported values (Heuschneider & Schwartz, 1989). The observation that activation of D2 receptors by quinpirole did not cause any significant reduction in cAMP levels indicates a very slow cAMP turnover rate in the synaptosomal preparations. On the other hand, activation of D1 receptors raised cAMP levels by 88% in synaptosomes prepared from untreated control rats. In synaptosomes prepared from simvastatin-treated rats, D1 receptor activation caused a markedly enhanced effect (a 285% increase). Thus, the observed 1.3-fold increase by simvastatin in D1 receptor protein expression (Figure 3) was translated to a 2.6-fold increase in function based on the chloro-APB-induced increase in cAMP levels. Chloro-APB-mediated increase in cAMP levels was markedly attenuated by SCH-23390 at five times the concentration of the agonist, further confirming the involvement of D1 receptors. Moreover, D2 receptor function was demonstrated by the complete abolition of the D1-mediated increase in cAMP. It is interesting to note that simultaneous activation of D1 and D2 receptors in these synaptosomes prepared from both control and simvastatin-treated rat prefrontal cortex resulted in a zero net change in cAMP levels. However, this may not reflect the in vivo situation given the differential potency of dopamine at the D1 and D2 receptors and differential distribution of these receptors in pre- and postsynaptic locations.
The dopamine-mediated mesocorticolimbic pathways are linked to the brain reward and reinforcing circuits, and thus relevant to drug abuse and addiction (Bonci et al., 2003). Dopaminergic abnormality is also associated with the pathogenesis of schizophrenia (Harrison, 2000). However, it should be noted that significant adverse events relating to the CNS has not been reported after long-term use of statins in humans (Pedersen et al., 1996). Thus, it appears probable that upregulation of dopamine receptors requires exposure to higher dosages than those currently used clinically.
Acknowledgments
This work was supported by a grant (R-184-000-039-112) from the National University of Singapore. We are grateful to P.H. Wang and Y.Z. Zhu for their excellent technical advice.
Abbreviations
- AD
Alzheimer's disease
- chloro-APB
6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide
- eNOS
endothelial nitric oxide synthase
- LDL
low-density lipoprotein
- L-NAME
NG-nitro-L-arginine
- NO
nitric oxide
- SCH-23390
R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
References
- ADAMSON P., GREENWOOD J. How do statins control neuroinflammation. Inflamm. Res. 2003;52:399–403. doi: 10.1007/s00011-003-1201-9. [DOI] [PubMed] [Google Scholar]
- BONCI A., BERNARDI G., GRILLNER P., MERCURI N.B. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction. Trends Pharmacol. Sci. 2003;24:172–177. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- BOTTI R.E., TRISCARI J., PAN H.Y., ZAYAT J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin. Neuropharmacol. 1991;14:256–261. doi: 10.1097/00002826-199106000-00010. [DOI] [PubMed] [Google Scholar]
- DE GENNARO COLONNA V., ROSSONI G., RIGAMONTI A., BONOMO S., MANFREDI B., BERTI F., MULLER E. Enalapril and quinapril improve endothelial vasodilator function and aortic eNOS gene expression in L-NAME-treated rats. Eur. J. Pharmacol. 2002;450:61–66. doi: 10.1016/s0014-2999(02)02046-0. [DOI] [PubMed] [Google Scholar]
- DOMINGUEZ J.M., MUSCHAMP J.W., SCHMICH J.M., HULL E.M. Nitric oxide mediates glutamate-evoked dopamine release in the medial preoptic area. Neuroscience. 2004;125:203–210. doi: 10.1016/j.neuroscience.2004.01.022. [DOI] [PubMed] [Google Scholar]
- ENDRES M., LAUFS U., HUANG Z., NAKAMURA T., HUANG P., MOSKOWITZ M.A., LIAO J.K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDRES M., LAUFS U., LIAO J.K., MOSKOWITZ M.A. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- HARRISON P.J. Dopamine and schizophrenia – proof at last. Lancet. 2000;356:958–959. doi: 10.1016/S0140-6736(00)02710-0. [DOI] [PubMed] [Google Scholar]
- HEALY D.P., JAYARAMAN G., ASHIROVA O. Chemical hypoxia-induced increases in dopamine D1A receptor mRNA in renal epithelial cells are mediated by nitric oxide. Acta Physiol. Scand. 2000;168:233–238. doi: 10.1046/j.1365-201x.2000.00666.x. [DOI] [PubMed] [Google Scholar]
- HEUSCHNEIDER G., SCHWARTZ R.D. cAMP and forskolin decrease γ-aminobutyric acid-gated chloride flux in rat brain synaptoneurosomes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2938–2942. doi: 10.1073/pnas.86.8.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH D.B., STEINER J.P., DAWSON T.M., MAMMEN A., HAYEK E., SNYDER S.H. Neurotransmitter release regulated by nitric oxide in PC-12 cells and brain synaptosomes. Curr. Biol. 1993;3:749–754. doi: 10.1016/0960-9822(93)90022-g. [DOI] [PubMed] [Google Scholar]
- HOWARD-JONES N. A CIOMS ethical code for animal experimentation. WHO Chron. 1985;39:51–56. [PubMed] [Google Scholar]
- KANO H., HAYASHI T., SUMI D., ESAKI T., ASAI Y., THAKUR N.K., JAYACHANDRAN M., IGUCHI A. A HMG-CoA reductase inhibitor improved regression of atherosclerosis in the rabbit aorta without affecting serum lipid levels: possible relevance of up-regulation of endothelial NO synthase mRNA. Biochem. Biophys. Res. Commun. 1999;259:414–419. doi: 10.1006/bbrc.1999.0799. [DOI] [PubMed] [Google Scholar]
- KLEJBOR I., DOMARADZKA-PYTEL B., LUDKIEWICZ B., WOJCIK S., MORYS J. The relationship between neurons containing dopamine and nitric oxide synthase in the ventral tegmental area. Folia Histochem. Cytobiol. 2004;42:83–87. [PubMed] [Google Scholar]
- LAUFS U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. Eur. J. Clin. Pharmacol. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- LAUFS U., GERTZ K., DIRNAGL U., BOHM M., NICKENIG G., ENDRES M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischaemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- LAU Y.S., PETROSKE E., MEREDITH G.E., WANG J.Q. Elevated neuronal nitric oxide synthase expression in chronic haloperidol-treated rats. Neuropharmacology. 2003;45:986–994. doi: 10.1016/s0028-3908(03)00314-9. [DOI] [PubMed] [Google Scholar]
- LAW M.R., WALD N.J., RUDNICKA A.R. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–1429. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOO L.S., NG Y.K., ZHU Y.Z., LEE H.S., WONG P.T.H. Cortical expression of endothelin receptor subtypes A and B following middle cerebral artery occlusion in the rats. Neuroscience. 2002;112:993–1000. doi: 10.1016/s0306-4522(02)00043-x. [DOI] [PubMed] [Google Scholar]
- LU D., GOUSSEV A., CHEN J., PANNU P., LI Y., MAHMOOD A., CHOPP M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- MCKENNEY J.M. Potential nontraditional applications of statins. Ann. Pharmacother. 2003;37:1063–1071. doi: 10.1345/aph.1C499. [DOI] [PubMed] [Google Scholar]
- MCTAGGART F., BUCKETT L., DAVIDSON R., HOLDGATE G., MCCORMICK A., SCHNECK D., SMITH G., WARWICK M. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am. J. Cardiol. 2001;87:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- MITAL S., ZHANG X., ZHAO G., BERNSTEIN R.D., SMITH C.J., FULTON D.L., SESSA W.C., LIAO J.K., HINTZE T.H. Simvastatin upregulates coronary vascular endothelial nitric oxide production in conscious dogs. Am. J. Physiol. 2000;279:H2649–H2657. doi: 10.1152/ajpheart.2000.279.6.H2649. [DOI] [PubMed] [Google Scholar]
- MONTANARI A., TATEO E., FASOLI E., DONATINI A., CIMOLATO B., PERINOTTO P., DALL'AGLIO P. Dopamine-2 receptor blockade potentiates the renal effects of nitric oxide inhibition in humans. Hypertension. 1998;31:277–282. doi: 10.1161/01.hyp.31.1.277. [DOI] [PubMed] [Google Scholar]
- PEDERSEN T.R., BERG K., COOK T.J., FAERGEMAN O., HAGHFELT T., KJEKSHUS J., MIETTINEN T., MUSLINER T.A., OLSSON A.G., PYORALA K., THORGEIRSSON G., TOBERT J.A., WEDEL H., WILHELMSEN L. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch. Intern. Med. 1996;156:2085–2092. [PubMed] [Google Scholar]
- PUDIAK C.M., BOZARTH M.A. Nitric oxide synthesis inhibition attenuates haloperidol-induced supersensitivity. J. Psychiatr. Neurosci. 1997;22:61–64. [PMC free article] [PubMed] [Google Scholar]
- REUSS B., UNSICKER K. Atypical neuroleptic drugs downregulate dopamine sensitivity in rat cortical and striatal astrocytes. Mol. Cell. Neurosci. 2001;18:197–209. doi: 10.1006/mcne.2001.1017. [DOI] [PubMed] [Google Scholar]
- ROGLANS N., VERD J.C., PERIS C., ALEGRET M., VAZQUEZ M., ADZET T., DIAZ C., HERNANDEZ G., LAGUNA J.C., SANCHEZ R.M. High doses of atorvastatin and simvastatin induce key enzymes involved in VLDL production. Lipids. 2002;37:445–454. doi: 10.1007/s11745-002-0916-0. [DOI] [PubMed] [Google Scholar]
- SCHILSTROM B., MAMELI-ENGVALL M., RAWAL N., GRILLNER P., JARDEMARK K., SVENSSON T.H. Nitric oxide is involved in nicotine-induced burst firing of rat ventral tegmental area dopamine neurons. Neuroscience. 2004;125:957–964. doi: 10.1016/j.neuroscience.2003.12.021. [DOI] [PubMed] [Google Scholar]
- SCHOONJANS K., PEINADO-ONSURBE J., FRUCHART J.C., TAILLEUX A., FIEVET C., AUWERX J. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors reduce serum triglyceride levels through modulation of apolipoprotein C-III and lipoprotein lipase. FEBS Lett. 1999;452:160–164. doi: 10.1016/s0014-5793(99)00632-8. [DOI] [PubMed] [Google Scholar]
- VEGA G.L., WEINER M.F., LIPTON A.M., VON BERGMANN K., LUTJOHANN D., MOORE C., SVETLIK D. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch. Neurol. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- VENKATAKRISHNAN U., CHEN C., LOKHANDWALA M.F. The role of intrarenal nitric oxide in the natriuretic response to dopamine-receptor activation. Clin. Exp. Hypertens. 2000;22:309–324. doi: 10.1081/ceh-100100080. [DOI] [PubMed] [Google Scholar]
- WASSMANN S., LAUFS U., BAUMER A.T., MULLER K., AHLBORY K., LINZ W., ITTER G., ROSEN R., BOHM M., NICKENIG G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- WU F., PARK F., COWLEY A.W., JR, MATTSON D.L. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am. J. Physiol. 1999;276:F874–F881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- YAMADA M., HUANG Z., DALKARA T., ENDRES M., LAUFS U., WAEBER C., HUANG P.L., LIAO J.K., MOSKOWITZ M.A. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J. Cereb. Blood Flow Metab. 2000;20:709–717. doi: 10.1097/00004647-200004000-00008. [DOI] [PubMed] [Google Scholar]