Abstract
Vascular reactivity to the alpha-adrenoceptor agonist phenylephrine (PE) was enhanced in small mesenteric arteries (SMA) from diabetic (db/db) mice under both high and low in vitro oxygen conditions.
Mechanical removal of the endothelium significantly attenuated the enhanced vascular reactivity of SMA from db/db mice.
Acute incubation of the SMA with sepiapterin, a precursor of tetrahydrobiopterin, and Nω-nitro L-arginine (L-NA), an inhibitor of nitric oxide (NO) synthase (NOS), resulted in no significant change in the enhanced vascular reactivity to PE in db/db mice. Endothelial nitric oxide synthase (eNOS) mRNA and protein levels in SMA were not different between db/+ and db/db mice.
Acute incubation of SMA with a combination of polyethylene glycol superoxide dismutase and catalase significantly reduced the enhanced contraction to PE in db/db mice. There were higher levels of malondialdehyde, a marker of lipid peroxidation and basal superoxide as measured by dihydroethidium staining, in SMA from db/db mice compared to db/+ mice.
Acute incubation with indomethacin, a nonselective inhibitor of cyclooxygenase, SQ 29548, a selective thromboxane receptor antagonist and furegrelate, a thromboxane synthesis inhibitor, significantly attenuated the enhanced contraction to PE in SMA from db/db mice.
This study demonstrates that the enhanced contractility of SMA from db/db mice to PE was endothelium dependent and involves elevated reactive oxygen species, cyclooxygenase activity and thromboxane synthesis, but not changes in the eNOS/NO pathway.
Keywords: Phenylephrine, oxidative stress, db/db, small mesenteric arteries, cyclooxygenase, thromboxane, endothelial nitric oxide synthase
Introduction
Macro- and microvascular diseases are the principal contributors to the increased morbidity and mortality associated with type I and type II diabetes and the importance of this fact is illustrated by the finding that type II diabetic patients suffer from a mortality rate 3–4 times that of the general population (Haffner et al., 1998). Endothelial dysfunction is considered the primary cause of vascular dysfunction in type I and type II diabetes. Impaired endothelium-dependent relaxation to agonist such as acetylcholine (Ach) is a common feature in both conduit and resistance arteries from experimental models of type I and type II diabetes and the literature has been extensively reviewed (Cosentino & Lüscher, 1998; De Vriese et al., 2000; Pannirselvam et al., 2003a; Triggle et al., 2003).
Diabetes induced hyper-reactivity to α-adrenergic stimulation has been widely reported in both conduit and resistance arteries from type I and type II diabetes (Piercy & Taylor, 1998; Kanie & Kamata, 2000; Pannirselvam et al., 2002; Okon et al., 2003). In most cases, hyper-reactivity is related to decreased nitric oxide (NO) bioavailability in the diabetic state, as even in control vessels, inhibition of nitric oxide synthase (NOS) has been shown to increase the vascular reactivity to α-adrenoceptor agonists. However, other mechanisms have been reported to contribute to enhanced vascular reactivity. For instance, Kanie & Kamata (2000) reported enhanced vascular reactivity to norepinephrine (NE) in aorta from a mouse model of type II diabetes (db/db), which was inhibited by the addition of superoxide dismutase (SOD) as well as indomethacin. These data suggest that increased superoxide levels generated through the prostanoid pathway contribute to the enhanced vascular reactivity (Kanie & Kamata, 2000). Increased levels of superoxide anion have been shown in vessels from diabetic mice as well as diabetic patients (Guzik et al., 2002; Yun et al., 2004). In our previous study, we reported enhanced vascular reactivity to phenylephrine (PE) of small mesenteric arteries (SMA) from db/db mice (Pannirselvam et al., 2002). Interestingly, chronic oral supplementation of mice with sepiapterin, a precursor of tetrahydrobiopterin (BH4), completely normalized both the hyper-reactivity to PE as well as the elevated levels of malondialdehyde in db/db mice (Pannirselvam et al., 2003b). Thus, the objective of the present study is to further characterize the mechanisms involved in the enhanced vascular reactivity of SMA from db/db mice to PE with the hypothesis that increased oxidative stress mediated the hyper-reactivity to PE.
Methods
Animals
Male C57BL/KsJ db/db mice (db/db), 16-week old, age-matched littermates (db/+), male C57BL/6J-Lepob mice (ob/ob), 24-week old, and respective age-matched controls from the Jackson Laboratory (Bar Harbour, ME, U.S.A.) were used in this study. Mice were killed by cervical dislocation in accordance with a protocol approved by the University of Calgary Animal Care Committee. Blood samples were collected by cardiac puncture under halothane anesthesia in the morning. Nonfasting plasma glucose, total cholesterol and triglyceride were assayed using commercial kits (Sigma Diagnostics, St Louis, MO, U.S.A.) The mesenteric arcades were removed and first-order branches of the mesenteric artery (≈150–200 μm in diameter) were dissected out into cold Krebs solution of the following composition (in mM): NaCl 120, NaHCO3 25, KCl 4.8, NaH2PO4 1.2, MgSO4 1.2, dextrose 11.0, CaCl2 1.8 aerated with 95% O2 and 5% CO2. The remaining mesenteric arcade, not including the superior mesenteric artery, was immediately stored at −70°C for biochemical measurements.
Wire myograph studies
Isometric tension studies using wire myograph were performed as described previously (Mulvany & Halpern, 1977). Briefly, SMA were cut into 2 mm long rings and mounted on a Mulvany–Halpern myograph. The passive tension-internal circumference was determined by stretching to achieve an internal circumference equivalent to 90% of that of the blood vessel under a transmural pressure of 100 mmHg. All experiments were performed at 37°C. Tissues were maximally contracted with 120 mM KCl. The first set of experiments was carried out to compare the vascular reactivity of SMA from db/+ and db/db mice under low oxygen (95% air and 5% CO2) and high oxygen (95% oxygen and 5% CO2) conditions. In the next set of experiments, we studied the concentration–response curves to PE in the presence or absence of various drugs and normalized the data relative to the force of contraction induced by 120 mM KCl.
Determination of eNOS mRNA and protein expression levels
Real-time–polymerase chain reaction (RT–PCR)
Total RNA was extracted from db/+ and db/db mesenteric artery using the RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized using the Superscript RT Kit (Qiagen). PCR employed the following forward and reverse primers specific to mouse eNOS and β-actin were used: eNOS (F3) 5′-CAACGCTACCACGAGGACA TT-3′, eNOS (R5) 5′-CTCCTGCAAAGAAAAGCTCTGG-3′, β-actin (F3) 5′-ACGGCCAGGTCATCACTATTG-3′, β-actin (R5) 5′-CCAAGAAGGAAGGCTGGAAAAGA-3′. RT-PCR reactions were set up using 0.25 μl of first-strand cDNA in a total reaction volume of 25 μl containing 1 × SYBR Green Supermix (Bio-Rad Laboratories) and 0.25 μM forward and reverse primers. PCR reactions were hot started (95°C for 3 min) and then exposed to 40 cycles of 95°C for 0.5 min, 62°C for 0.5 min and 72°C for 0.5 min. The amplicons were visualized using a DNA 500 LabChip Kit with the Agilent Technologies 2100 Bioanalyzer to ensure product purity. PCR efficiencies were measured by performing RT-PCR on serial dilutions of mouse brain cDNA (94.6% for β-actin, 96.7% for eNOS). PCR reaction products were excised from a 1.5% (w/v) agarose gel and the identity was confirmed using automated DNA sequencing. The relative quantity of eNOS message was determined following the 2−CT method that, in this case, employed β-actin as an internal control (Livak & Schmittgen, 2001).
Western blot analysis
Western blot analysis of the mesenteric homogenate was performed with a commercially available monoclonal eNOS antibody (1 : 500, BD Transduction Laboratories), IRDyeTM 800-conjugated affinity-purified goat anti-mouse IgG secondary antibody (1 : 10,000, Rockland incorporation, PA, U.S.A.) and detected using Odyssey infrared imaging system (LI-COR biosciences).
Measurements of lipid peroxidation
Malondialdehyde, a marker for lipid peroxidation, was measured in plasma and mesenteric arteries using OXI-TEK commercial kit (ZeptoMetrix Co. U.S.A.) according to the manufacturer's instructions. The level of malondialdehyde was normalized to milligram of protein.
Oxidative fluorescence microtopography
Dihydroethidium (DHE), an oxidative fluorescent dye, was used to evaluate levels of superoxide in situ frozen unfixed sections of SMA (Guzik et al., 2002). DHE (3 μM) was applied to each tissue section and a cover slip applied. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. In a separate group of experiments, tissues were incubated with polyethylene glycol superoxide dismutase (PEG-SOD, 600 U ml−1) for 30 min prior to addition of the dye. Images were obtained using Leica DM RXA2 fluorescent microscope and analyzed using Adobe photoshop software (Version 5.5). Settings were identical for acquisition of images from db/+ and db/db sections with or without PEG-SOD.
Protein assay
Protein content was determined by the method of bicinchoninic acid using a commercial kit from Pierce Co. with bovine serum albumin as a standard.
Drugs
All drugs were obtained from Sigma Chemicals (St Louis, MO, U.S.A.) except furegrelate (BIOMOL, Plymouth Meeting, PA, U.S.A). All drugs were dissolved in distilled water except for indomethacin and furegrelate, which was dissolved in 95% ethanol and sepiapterin, which was dissolved in dimethyl sulfoxide.
Statistics
All values are expressed as mean±s.e.m. (shown as vertical bars in the figures). In all experiments, n equals the number of animals used in the protocol. Sensitivity (pD2) and maximum response was calculated from the concentration–response curves to PE in the presence or absence of the inhibitors. pD2 is defined as the negative logarithm to base 10 of the EC50 values and maximum response (Emax) is defined as the maximal contraction to PE at the highest concentration tried and expressed as percentage relative contractions to 120 mM KCl. The statistical differences in the pD2 and maximum response between groups were compared by Student's t-test or one-way ANOVA and Student–Newman–Keuls multiple comparison post hoc test. Biochemical parameters were compared using Student's unpaired t-test. A P-values less than 0.05 was considered statistically significant.
Results
Characteristics of db/db and ob/ob mice
The db/db mice showed significantly higher body weight compared to their age-matched litter mates (db/+; 47±1 vs 29±1 g, P<0.01, n=8) with higher levels of plasma glucose (537±23 vs 163±15 mg dl−1, P<0.01, n=8), triglyceride (117±9 vs 73±9 mg dl−1, P<0.01, n=8) and cholesterol (138±6 vs 73±6 mg dl−1, P<0.01, n=8) levels. The ob/ob mice were severely obese (76±6 vs 30±0.3 g, P<0.01, n=6) with high levels of triglyceride (128±28 vs 69±16 mg dl−1, P<0.01, n=6) and cholesterol (262±43 vs 71±11 mg dl−1, P<0.01, n=6) compared to their age-matched wild-type controls. However, plasma glucose levels were similar to age-matched wild-type controls (192±43 vs 168±19 mg dl−1, n=6).
Influence of oxygen tension on vascular reactivity of SMA from diabetic mice
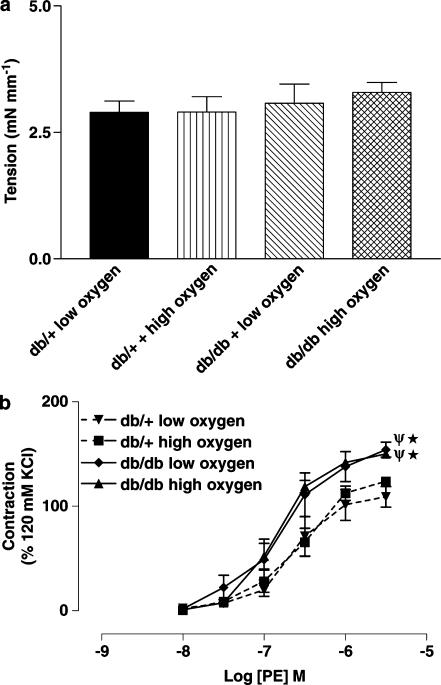
PE-induced contractions of SMA from ob/ob mice were not different compared to contractions observed in age-matched wild-type controls. The pD2 value and Emax (% KCl-induced contraction) for PE-induced contraction of SMA from control C57BL/6J and ob/ob mice were 6.7±0.1 and 140±6 and 6.8±0.1 and 127±5 (P>0.05, n=6), respectively. A depolarizing concentration of KCl induced a maximum contraction of SMA from both db/+ and db/db mice under low and high oxygen tension, which were not statistically different (Figure 1a). PE induced a concentration-dependent contraction of SMA from db/+ and db/db mice; however, the maximal contraction to PE was significantly higher in db/db mice compared to db/+ mice. Moreover, PE-induced contractions of SMA from db/db mice were not significantly different when obtained under either low or high oxygen tension protocols (Figure 1b).
Figure 1.
(a) Potassium chloride (120 mM)-induced contraction of SMA (n=6–8) from db/+ and db/db mice under low and high oxygen tension. (b) Concentration–response curves to PE of SMA from db/+ and db/db mice (n=7–8) under low and high oxygen tension. *P<0.01 compared to db/+ mice under low oxygen tension and Ψ, P<0.01 compared to db/+ mice under high oxygen tension.
Contribution of the endothelium and eNOS to the enhanced vascular reactivity of SMA from db/db mice
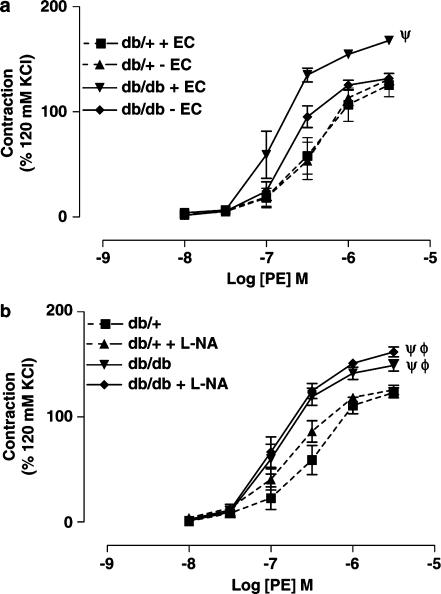
Mechanical removal of the endothelium had no effect on vascular reactivity of SMA from db/+ mice, but significantly reduced the maximum contraction of SMA from db/db mice (Figure 2a). Further, acute incubation of SMA with Nωnitro L-arginine (L-NA, 100 μM) for 30 min produced no significant change in vascular reactivity either in db/+ or db/db mice (Figure 2b). L-NA incubation significantly reduced the Ach-induced endothelium-dependent relaxation of SMA from db/+ mice, but not from db/db mice (data not shown).
Figure 2.
(a) Concentration–response curves to PE of SMA from db/+ mice (n=6) and db/db mice (n=6) with or without endothelium. Ψ, P<0.01 compared to db/+ mice with endothelium, db/+ mice without endothelium and db/db mice without endothelium. (b) Concentration–response curves to PE of SMA from db/+ mice (n=6) and db/db mice (n=6) in the presence or absence of L-NA (100 μM) for 30 min. Φ, P<0.01 compared to the db/+ group in the absence of L-NA and Ψ, P<0.01 compared to the db/+ group in the presence of L-NA.
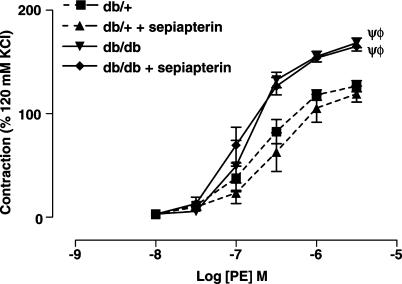
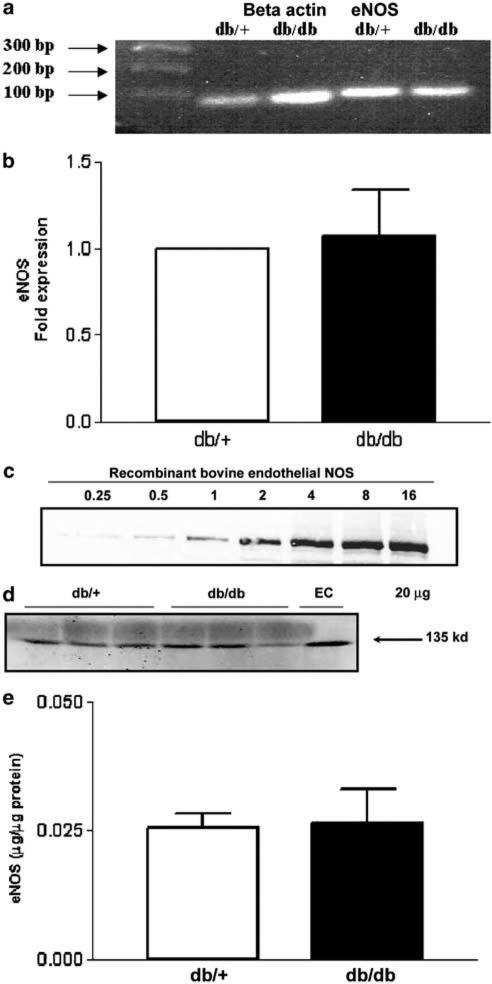
Acute incubation of SMA with sepiapterin (100 μM), a precursor of BH4, did not significantly affect the vascular reactivity to PE of SMA from db/+ and db/db mice (Figure 3). To investigate a possible change in eNOS gene expression, we quantified eNOS mRNA in the mesenteric artery. As shown in the Figure 4b, there was no significant change in the expression of eNOS mRNA levels in db/db mice compared to db/+ mice. Further, we measured eNOS protein levels by Western blot analysis. As illustrated in Figure 4e, there were no significant differences changes in eNOS protein levels in db/db mice compared to db/+ mice. This was further confirmed by immunohistochemistry of eNOS distribution in SMA (data not shown).
Figure 3.
Concentration–response curves to PE of SMA from db/+ (n=5) and db/db (n=5) with or without sepiapterin (100 μM) incubation. Ψ, P<0.01 compared to the db/+ group without sepiapterin and Φ, P<0.01 compared to the db/+ group plus sepiapterin.
Figure 4.
eNOS gene and protein expression of SMA from db/+ and db/db mice. (a) Representative agarose gel demonstrating eNOS and β-actin expression in SMA from db/+ and db/db mice using RT-PCR. (b) Quantitative analysis of eNOS expression in db/+ and db/db mice following real-time PCR. Normalized to β-actin expression, results are mean±s.e.m. (n=7 animals). (c) Western blot showing various concentrations of recombinant bovine eNOS. (d) Western blot showing eNOS protein levels in SMA from db/+ and db/db mice. (e) Densitometric analysis of eNOS protein expression calculated using recombinant bovine eNOS as standard. Results are mean±s.e.m. (n=5).
Role of oxidative stress on enhanced vascular reactivity of SMA from db/db mice
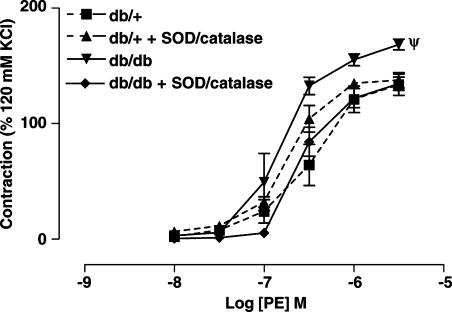
The acute incubation of SMA with a combination of PEG-SOD (200 U ml−1) and catalase (80 U ml−1) for 30 min significantly reduced the enhanced contractions to PE of SMA from db/db mice, but produced no significant change in contractions to PE in SMA from db/+ mice (Figure 5).
Figure 5.
Concentration–response curves to PE of SMA from db/+ (n=6) and db/db (n=5) with or without a combination of PEG-SOD (200 U ml−1) and catalase (80 U ml−1). Ψ, P<0.01 compared to db/+, db/++ SOD/catalase and db/db+ SOD/catalase group.
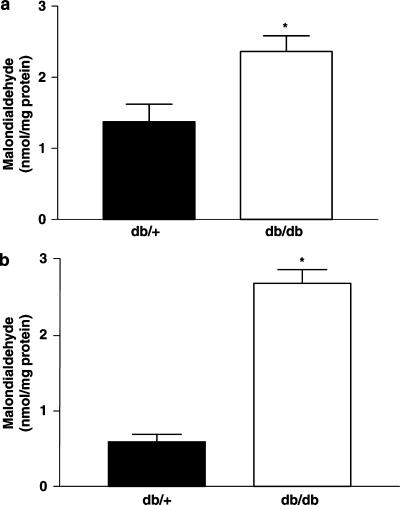
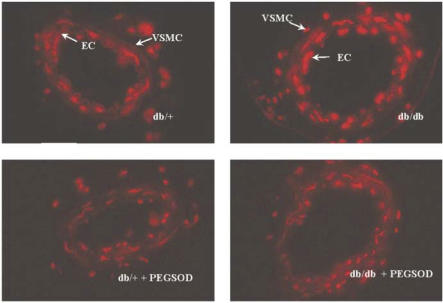
Malondialdehyde, a marker for lipid peroxidation, was significantly elevated in plasma and SMA from db/db mice compared to db/+ mice (Figure 6a and b). Mesenteric artery sections from db/db mice had increased basal superoxide levels as measured by DHE fluorescence when compared to db/+ vessels (Figure 7). The increase in basal superoxide levels was observed notably in the endothelial cells in db/db mice compared to db/+ mice. Incubation with PEG-SOD (600 U ml−1) reduced the superoxide levels in mesenteric arteries from db/db mice (Figure 7), but still a significant amount of DHE staining could be noticed in db/+ and db/db section in the presence of PEG-SOD which could be accounted for nonspecific reaction of DHE with other biological molecules such as cytochrome c.
Figure 6.
Malondialdehyde levels, as marker of lipid peroxidation, in plasma (a) and SMA (b) from db/+ and db/db mice (n=5 animals). *P<0.05 compared to the db/+ group.
Figure 7.
Fluorescent photomicrograph of SMA from db/+ and db/db mice. Vessels were labeled with DHE dye, which gives red fluorescence when oxidized by superoxide. Adjacent sections were preincubated with PEG-SOD (600 U ml−1). Data are representative for n=4 animals.
Contribution of the cyclooxygenase and thromboxane pathway to enhanced vascular reactivity of SMA from db/db mice
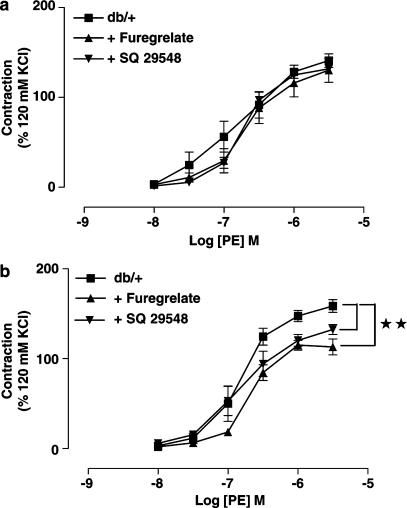
Incubation of SMA with indomethacin (10 μM), a nonselective inhibitor of cyclooxygenase, significantly reduced the maximum contraction as well as sensitivity to PE of SMA from db/db mice, but had no significant effect on the PE-induced contractions of SMA from db/+ mice (data not shown). SQ 29548 (10 μM), a selective thromboxane receptor antagonist, and furegrelate (100 μM), a thromboxane synthesis inhibitor, also significantly inhibited the maximum contraction to PE in SMA from db/db mice but not in db/+ mice (Figure 8a and b).
Figure 8.
Concentration–response curves to PE of SMA from db/+ (a, n=4–6) and db/db mice (b, n=5–7) before or after incubation with either furegrelate (100 μM) or SQ 29548 (10 μM) for 30 min. **P<0.01 contraction compared to the db/db group.
Discussion
The present study demonstrates that removing the endothelium or incubating the SMA with PEG-SOD and catalase can abolish the enhanced vascular reactivity to PE in SMA from db/db mice. Furthermore, the cyclooxygenase inhibitor indomethacin, thromboxane receptor antagonist SQ 29548 and thromboxane synthesis inhibitor furegrelate all reversed the enhanced vascular reactivity to PE, whereas neither sepiapterin, a precursor of BH4, nor L-NA, inhibitor of NOS, had a significant effect on the PE-induced contraction. RT-PCR and Western blotting demonstrated no changes in eNOS gene or protein expression. Basal levels of superoxide in the endothelial cells were higher in db/db mice with higher tissue levels of malondialdehyde.
The ob/ob mice at 24 weeks of age were, compared to wild-type controls, obese and exhibited higher levels of plasma cholesterol and triglyceride levels, whereas nonfasting plasma glucose was not different. Okon et al. (2003) reported hyperglycemia in ob/ob mice at 8 weeks of age where aorta and mesenteric artery showed both enhanced vascular reactivity to PE and impaired relaxation to Ach. In contrast, we did not observe a significant change in either the PE-induced contraction or the Ach-induced relaxation (data not shown) in ob/ob mice at 24 weeks of age compared to wild-type controls. Such differences, may relate to the differences in the age groups studied (8–16 vs 24 weeks) and further investigation is warranted in order to determine whether changes in the vascular reactivity to PE and endothelium-dependent relaxation to Ach correlates to the glycemic state of the mouse. However, the ob/ob mouse phenotype on the C57BL/6J background is only mildly hyperglycemic and considered a model of obesity and insulin resistance (Haluzik et al., 2004), whereas the db/db mice, on the C57BL/KsJ background, have considerably higher plasma glucose levels and are a model of type 2 diabetes (Coleman, 1982). Consistent with our previous study, SMA from db/db mice showed enhanced vascular reactivity to PE (Pannirselvam et al., 2002). The present study suggests that hyperglycemia is likely the major contributor to the enhanced vascular reactivity to PE in db/db mice. However, additional contributions from both high lipid and insulin levels cannot be totally ruled out in the pathogenesis of vascular dysfunction. Furthermore, additional studies of vascular reactivity and oxidative stress in the ob/ob mouse model of diabetes would be of value in order to better understand the link between plasma glucose, oxidative stress and endothelial-vascular dysfunction.
Enhanced vascular reactivity to PE in SMA from db/db mice is endothelium-dependent as removal of endothelium significantly attenuated the PE-induced hyper reactivity in SMA from db/db mice, but not in db/+ mice. Several reports have shown that enhanced vascular reactivity to α-adrenoceptor agonists could be due to a decrease in the bioavailability of NO in the diabetic state. Thus, acute inhibition of NOS with L-NA has been reported to reduce PE-induced contractions in conduit as well as resistance arteries in both control and diabetic conditions (Jones et al., 1993; Piercy & Taylor, 1998; Okon et al., 2003). In contrast, we did not see any significant change in the PE-induced contraction following inhibition of NOS either in SMA from db/+ or db/db mice. However, Lagaud et al. (2001) studied the myogenic properties of third- and fourth-order mesenteric arteries from 8–16-week-old db/db and db/+ mice under pressurized myograph conditions and reported that L-nitro-arginine methyl ester significantly potentiated myogenic tone in vessels from 12-week-old db/+ but not db/db mice. These data indicate that basal NO release is a factor in the regulation of myogenic tone in SMA from nondiabetic mice, but this contribution is impaired in diabetes. The failure to observe a role for NO release in the regulation of PE-induced tone in the present study, at least in SMA from the db/+ mice, likely reflects experimental differences resulting from the use of wire, in the current study, vs pressurized myography in the Lagaud et al. (2001) study. Further, we did not see any change in the expression of eNOS RNA or protein. Kobayashi & Kamata (2001) have provided comparable data from the aortae of streptozotocin-treated type I diabetic rats, whereas Hink et al. (2001) have reported an increase in eNOS expression in the aortae of streptozotocin-induced diabetic rats. Inconsistent results have been reported for the effects of hyperglycemia on eNOS expression (see Li et al., 2002) and although the reason for such discrepancies is unclear this may reflect differences in the choice of the house-keeping gene used for comparison as expression of this gene product may also change in diabetes. Further evidence for a lack of involvement of NO release in the regulation of vascular reactivity is provided by the failure to observe a significant change in the PE-induced contraction following the acute administration of sepiapterin to SMA from either db/+ or db/db mice. Although there is no detectable change in the eNOS gene or protein levels, it is possible that changes may have occurred in the ability of eNOS to generate NO; however, we have not measured eNOS activity in SMA from db/db mice. Nonetheless, we believe that the decreased bioavailability of NO may not be the cause for the observed hyper-reactivity to PE for two reasons: Firstly, the acute incubation of SMA with sepiapterin did not affect the hyper-reactivity to PE, although it did significantly improve the impaired endothelium-dependent relaxation to Ach. Secondly, incubation of SMA with L-NA did not affect the vascular reactivity to PE of SMA from db/+ mice but significantly reduced the endothelium-dependent relaxation to Ach. Chronic oral supplementation with sepiapterin in the diet to db/db mice completely normalized the enhanced vascular reactivity to PE and this reduction in reactivity correlated with the reduction in the malondialdehyde levels, a marker of oxidative stress (Pannirselvam et al., 2003b). These results together suggest that the enhanced vascular reactivity to PE in the SMA is probably not due to a decrease in the bioavailability to NO in db/db mice, but rather to other mechanism(s) such as an increase in the generation of reactive oxygen species. Indeed, acute incubation of SMA with PEG-SOD and catalase inhibited the hyper-reactivity to PE in db/db mice suggesting that increased oxidative stress is responsible for the hyper-reactivity to PE. Further, we confirmed an increased superoxide levels and oxidative stress in mesenteric tissue with DHE staining and the lipid peroxidation assay. To rule out the possibility of the nonenzymatic source of ROS resulting from the high in vitro oxygen tension conditions used in this study, we compared PE-induced contraction under high and low oxygen protocols. Enhanced vascular reactivity of SMA from db/db mice to PE was observed under both conditions and was equally sensitive to antioxidant treatment, ruling out the possibility of artefacts from high oxygen tension.
Increased oxidative stress mediates enhanced vascular reactivity through multiple pathways viz., activation of protein kinase C, stress kinases, activation of cyclooxygenase/thromboxane pathway. We investigated the possible link between oxidative stress and the cyclooxygenase/thromboxane pathway to enhanced vascular reactivity. Incubation of the SMA from the db/db mouse with the nonselective inhibitor of cyclooxygenase, indomethacin, a thromboxane synthesis inhibitor, furegrelate or a thromboxane receptor blocker, SQ 29548, all reduced the enhanced vascular reactivity to PE thus inferring a role for the thromboxane pathway. Of importance is that Lagaud et al. (2001) also reported that indomethacin and SQ29548 produced a significant inhibition of myogenic tone in SMA from db/db, but not db/+, mice; however, in the Lagaud et al. (2001) study, endothelium denudation did not alter myogenic tone in the db/db mouse. Koltai et al. (1990) reported a greater decrease in the conductance of the femoral arterial bed in diabetic dogs compared to healthy dogs and this effect was abolished by intra-arterial injection of indomethacin and acetyl salicylic acid. They also reported an increase in the synthesis of thromboxane in femoral arteries in diabetic dogs, which was blocked by phentolamine, suggesting that the increased synthesis of thromboxane is mediated through α-adrenoceptor activation. It has been reported that aortae from db/db mice showed enhanced vascular reactivity to NE, which was inhibited with SOD and indomethacin pretreatment suggesting that the generation of superoxide via the cyclooxygenase pathway may enhance the contraction induced by NE (Kanie & Kamata, 2000). Cosentino et al. (2003) have also reported that high glucose upregulates RNA and protein expression for cyclooxygenase-2 and also eNOS protein (RNA was not assessed) in human aortic endothelial cells exposed to 22.2 mmol l−1 glucose. Yang et al. (2002) have reported that a diffusible, endothelium-derived substance caused contraction in response to Ach in aortae from the spontaneously hypertensive rat. The contraction was inhibited by vareryl salicylate, an irreversible cyclooxygenase inhibitor and S18886, a selective TP receptor antagonist, suggesting the contribution of an endothelium-derived contracting factor that is linked to cyclooxygenase-mediated thromboxane synthesis (Yang et al., 2002; 2003). Furthermore, the hyperglycemia associated with diabetes has been hypothesized to lead to the production of constrictor prostanoids, which may further exacerbate the production of flow-mediated NO production and lead to changes in vascular reactivity (Bohlen, 2004). Thus, there is an accumulation of evidence that supports the conclusion that the enhanced production of constrictor prostanoids may play a key role in the pathogenesis of vascular dysfunction associated with diabetes and hypertension.
In summary, this study demonstrates that the enhanced contractility of first-order branches of mesenteric artery from db/db mice to PE involves the cyclooxygenase pathway and thromboxane synthesis, but not alterations of eNOS gene or protein expression or NO per se. However, this study did not explore the involvement of other endothelium-derived contracting factors candidates such as hydroxyeicosatetraenoic acids or isoprostanes, which are also known to be generated by the cyclooxygenase pathway as well as mediate their effects via thromboxane receptor activation. In addition, and as stressed by De Vriese et al. (2000), the heterogeneity of the vasculature and the diabetic state studied should be taken into consideration before extrapolating the results of this study to our understanding of the pathogenesis of vascular dysfunction in diabetes.
Acknowledgments
We acknowledge the financial support of an operating grant from Norman D. MacDougall Canadian Diabetes Association (to TJA & CRT) and a graduate fellowship award from Pfizer/CHS/CIHR and AHFMR (to MP).
Abbreviations
- Ach
acetylcholine
- BH4
tetrahydrobiopterin
- DHE
dihydroethidium
- eNOS
endothelial nitric oxide synthase
- L-NA
Nω-nitro L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- NE
norepinephrine
- PE
phenylephrine
- PEG-SOD
polyethylene glycol-superoxide dismutase
- RT–PCR
real-time–polymerase chain reaction
- SMA
small mesenteric arteries
- SOD
superoxide dismutase
References
- BOHLEN H.G. Mechanisms for early microvascular injury in obesity and type II diabetes. Curr. Hypertens. Rep. 2004;6:60–65. doi: 10.1007/s11906-004-0013-9. [DOI] [PubMed] [Google Scholar]
- COLEMAN D.L. Diabetes-obesity syndromes in mice. Diabetes. 1982;31:1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., ETO M., DE PAOLIS P., VAN DER LOO B., BACHSCHMID M., ULLRICH V., KOUROEDOV A., DELLI GATTI C., JOCH H., VOLPE M., LÜSCHER T.F. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., LÜSCHER T.F. Endothelial dysfunction in diabetes mellitus. J. Cardiovasc. Pharmacol. 1998;32:S54–S61. [PubMed] [Google Scholar]
- DE VRIESE A.S., VERBEUREN T.J., VAN DE VOORDE J., LAMEIRE N.H., VANHOUTTE P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZIK T.J., WEST N.E., PILLAI R., TAGGART D.P., CHANNON K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- HAFFNER S.M., LEHTO S., RONNEMAA T., PYORALA K., LAAKSO M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- HALUZIK M., COLOMBO C., GAVRILOVA O., CHUA S., WOLF N., CHEN M., STANNARD B., DIETZ K.R., LE ROITH D., REITMAN M.L. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- HINK U., LI H., MOLLNAU H., OELZE M., MATHEIS E., HARTMANN M., SKATCHKOV M., THAISS F., STAHL R.A., WARNHOLTZ A., MEINERTZ T., GRIENDLING K., HARRISON D.G., FORSTERMANN U., MUNZEL T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- JONES C.J., DEFILY D.V., PATTERSON J.L., CHILIAN W.M. Endothelium-dependent relaxation competes with alpha 1- and alpha 2-adrenergic constriction in the canine epicardial coronary microcirculation. Circulation. 1993;87:1264–1274. doi: 10.1161/01.cir.87.4.1264. [DOI] [PubMed] [Google Scholar]
- KANIE N., KAMATA K. Contractile responses in spontaneously diabetic mice. I. Involvement of superoxide anion in enhanced contractile response of aorta to norepinephrine in C57BL/KsJ(db/db) mice. Gen. Pharmacol. 2000;35:311–318. doi: 10.1016/s0306-3623(02)00115-5. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., KAMATA K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis. 2001;155:313–320. doi: 10.1016/s0021-9150(00)00583-9. [DOI] [PubMed] [Google Scholar]
- KOLTAI M.Z., ROSEN P., BALLAGI-PORDANY G., HADHAZY P., POGATSA G. Increased vasoconstrictor response to noradrenaline in femoral vascular bed of diabetic dogs. Is thromboxane A2 involved. Cardiovasc. Res. 1990;24:707–710. doi: 10.1093/cvr/24.9.707. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J.L., MASH-KHAN E., KAI S., VAN BREEMEN C., DUBE G.P. Influence of type II diabetes on arterial tone and endothelium function in murine resistance arteries. J Vasc. Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- LI H., WALLERATH T., MUNZEL T., FORSTERMANN U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149–164. doi: 10.1016/s1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- LIVAK K.J., SCHMITTGEN T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- OKON E.B., SZADO T., LAHER I., McMANUS B., VAN BREEMEN C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J. Vasc. Res. 2003;40:520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- PANNIRSELVAM M., ANDERSON T., TRIGGLE C.R. Endothelial cell dysfunction in type I and II diabetes – the cellular basis for dysfunction. Drug Dev. Res. 2003a;58:28–41. [Google Scholar]
- PANNIRSELVAM M., SIMON V., VERMA S., ANDERSON T., TRIGGLE C.R. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br. J. Pharmacol. 2003b;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNIRSELVAM M., VERMA S., ANDERSON T.J., TRIGGLE C.R. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db−/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br. J. Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCY V., TAYLOR S.G. A comparison of spasmogenic and relaxant responses in aortae from C57/BL/KsJ diabetic mice with those from their non-diabetic littermates. Pharmacology. 1998;56:267–275. doi: 10.1159/000028208. [DOI] [PubMed] [Google Scholar]
- TRIGGLE C.R., HOLLENBERG M., ANDERSON T.J., DING H., JIANG Y., CERONI L., WIEHLER W.B., NG E.S., ELLIS A., ANDREWS K., MCGUIRE J.J., PANNIRSELVAM M. The endothelium in health and disease-a target for therapeutic intervention. J. Smooth Muscle Res. 2003;39:249–267. doi: 10.1540/jsmr.39.249. [DOI] [PubMed] [Google Scholar]
- YANG D., FELETOU M., BOULANGER C.M., WU H.F., LEVENS N., ZHANG J.N., VANHOUTTE P.M. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br. J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG D., FELETOU M., LEVENS N., ZHANG J.N., VANHOUTTE P.M. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- YUN M.R., KIN J.J., IM D.S., YANG S.D., KIM C.D. Involvement of NAD(P)H oxidase in the enhanced expression of cell adhesion molecules in the aorta of diabetic mice. Life Sci. 2004;75:2463–2472. doi: 10.1016/j.lfs.2004.04.041. [DOI] [PubMed] [Google Scholar]