Abstract
We investigated whether withanolide A (WL-A), isolated from the Indian herbal drug Ashwagandha (root of Withania somnifera), could regenerate neurites and reconstruct synapses in severely damaged neurons. We also investigated the effect of WL-A on memory-deficient mice showing neuronal atrophy and synaptic loss in the brain. Axons, dendrites, presynapses, and postsynapses were visualized by immunostaining for phosphorylated neurofilament-H (NF-H), microtubule-associated protein 2 (MAP2), synaptophysin, and postsynaptic density-95 (PSD-95), respectively.
Treatment with Aβ(25–35) (10 μM) induced axonal and dendritic atrophy, and pre- and postsynaptic loss in cultured rat cortical neurons. Subsequent treatment with WL-A (1 μM) induced significant regeneration of both axons and dendrites, in addition to the reconstruction of pre- and postsynapses in the neurons.
WL-A (10 μmol kg−1 day−1, for 13 days, p.o.) recovered Aβ(25–35)-induced memory deficit in mice. At that time, the decline of axons, dendrites, and synapses in the cerebral cortex and hippocampus was almost recovered.
WL-A is therefore an important candidate for the therapeutic treatment of neurodegenerative diseases, as it is able to reconstruct neuronal networks.
Keywords: Axon, dendrite, presynapse, postsynapse, Morris water maze, Aβ(25–35), Withania somnifera, Ashwagandha, withanolide
Introduction
Despite a number of ongoing investigations, neurodegenerative diseases remain incurable. Although patients suffering from neurodegenerative diseases can benefit from medication with numerous drugs, the progression of these diseases cannot yet be halted. In patients with Alzheimer's disease, neuritic atrophy and synaptic loss are considered the major causes of cognitive impairment, as based on the results of neuropathological post-mortem studies of the brain (De Kosky & Scheff, 1990; Terry et al., 1991; Dickson & Vickers, 2001). In the brains of patients suffering from other neurodegenerative diseases such as Parkinson's disease, Huntington's disease, and Creutzfeldt–Jakob disease, the atrophy of neurites has also been observed (Jackson et al., 1995; Liberski & Budka, 1999; Mattila et al., 1999). Such atrophy leads to the destruction of neuronal networks, and subsequently to fatal dysfunction of the brain systems in these patients. The prevention of, or at least a decrease in the magnitude of the cause of each disease, may prevent the progression of symptoms, but such inhibition is not associated with the repair of already severely damaged brain function. We hypothesized that reconstructing neuronal networks in the injured brain would be the most necessary step in the fundamental recovery of brain function. In order to reconstruct neuronal networks, neuritic regeneration and synaptic reconstruction must take place in the damaged brain. Therefore, we aimed to explore compounds that would facilitate the regeneration of neurites and the reconstruction of synapses, even in severely damaged neurons, and to show evidence of the effects in vivo as well as in vitro.
Ashwagandha (root of Withania somnifera Dunal) is the most popular herbal drug in Ayurvedic medicine, and has been used traditionally and commonly as a tonic and nootropic agent. It has also been reported to be associated with improvements in scopolamine-induced memory deficits in mice (Dhuley, 2001). We previously demonstrated that a methanol extract of Ashwagandha was associated with neurite extension and, in particular, that of dendrites (Tohda et al., 2000). In addition, we identified that six constituents isolated from the methanol extract induced neurite outgrowth in human neuroblastoma SH-SY5Y cells (Zhao et al., 2002). In normal cortical neurons, predominant axonal outgrowth was observed in the treatment with withanolide A (WL-A), which was one of the major active constituents isolated from Ashwagandha (Kuboyama et al., 2002).
Amyloid β is a major pathological cause of Alzheimer's disease due to the formation of a β-sheet structure (Simmons et al., 1994); amyloid β forms deposits in the brain, and subsequently induces neuronal cell death (Bobinski et al., 1997), neuritic atrophy (Canning et al., 1993; Knowles et al., 1999), and synaptic loss (Terry et al., 1991). Aβ(25–35) is an active partial fragment of amyloid β. This fragment also forms a β-sheet structure (Pike et al., 1995) and induces neuronal cell death (Yankner et al., 1990; Pike et al., 1995), neuritic atrophy (Grace et al., 2002; Tohda et al., 2004), synaptic loss (Grace et al., 2002; Tohda et al., 2003; 2004), and memory impairment (Maurice et al., 1996; Tohda et al., 2003; 2004). In this study, we investigated the effects of WL-A on neuritic regeneration and synaptic reconstruction in cultured neurons damaged by Aβ(25–35) and cognitive-deficient mice by Aβ(25–35)-injection. In particular, synaptic formations at both the presynaptic region (axon and presynapse) and the postsynaptic region (dendrite and postsynapse) were observed discriminatively.
Methods
Materials
WL-A was isolated from the methanol extract of Ashwagandha (root of Withania somnifera Dunal), as previously described (Zhao et al., 2002). Aβ(25–35) (Sigma, Saint Louis, MO, U.S.A.) and Aβ(1–42) (Sigma) were dissolved in sterile distilled water at a concentration of 5 and 1 mM, respectively, and were incubated at 37°C for 4 days to allow fibril formation. Neurobasal media and B-27 supplement were purchased from Gibco BRL (Rockville, MD, U.S.A.). Mouse β-NGF was purchased from Astral Biologicals (San Ramon, CA, U.S.A.). A monoclonal antibody to phosphorylated neurofilament-H (NF-H) was purchased from Sternberger Monoclonals Incorporated (Lutherville, MD, U.S.A.). A monoclonal antibody to microtubule-associated protein 2 (MAP2), an antiserum to MAP2, a monoclonal antibody to synaptophysin were purchased from Chemicon (Temecula, CA, U.S.A.). A monoclonal antibody to postsynaptic density-95 (PSD-95) was purchased from Affiniti BioReagents (Golden, CO, U.S.A.) Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 546-conjugated goat anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR, U.S.A.).
Primary culture
Embryos were removed from pregnant Sprague–Dawley rats (Japan SLC, Shizuoka, Japan) at 17–18 days of gestation. The animals were handled in accordance with the Guide for Animal Experiments, Toyama Medical and Pharmaceutical University. The cortices were dissected and the dura mater was removed. The cells were minced and dissociated, and were then grown in cultures with Neurobasal media including 12% horse serum on 4- or 8-well chamber slides (Falcon, Franklin Lakes, NJ, U.S.A.) coated with poly-D-lysine at 37°C in a humidified incubation with 10% CO2. When Aβ(25–35) or other compounds were added, half of the medium in each well was replaced with fresh medium containing serum (Figure 2) or fresh medium containing 2% B-27 supplement without serum (Figures 1, 3, 4 and 5). In cases of long-term culture (Figures 4 and 5), half of the medium in each well was replaced with serum-free medium containing the 2% B-27 supplement at 3–4 days after initiation of the culture period. At every 4–6 days of culture, half of the medium was replaced with fresh serum-free medium. The time schedules of the experiments are shown below the figures.
Figure 2.
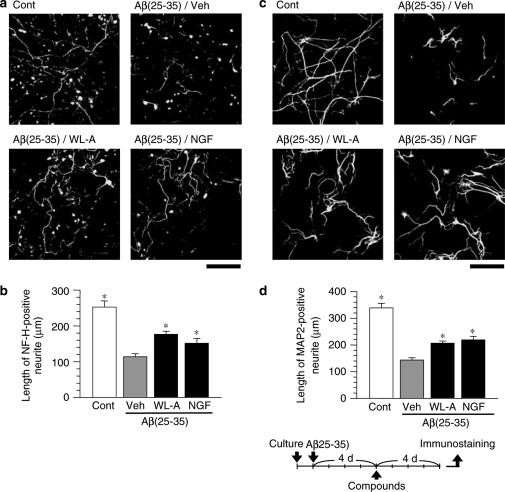
The effects of withanolide A (WL-A) on axonal and dendritic regeneration after Aβ(25–35)-induced atrophy. Cortical neurons were cultured for 24 h, and were then treated with or without (Cont) 10 μM Aβ(25–35). At 4 days after the administration of Aβ(25–35), the cells were treated with WL-A at a concentration of 1 μM; or NGF at a concentration of 100 ng ml−1; or the vehicle (Veh). At 4 days after treatment, the cells were fixed and immunostained for phosphorylated NF-H (a) or MAP2 (c). The lengths of NF-H-positive (b) or MAP2-positive (d) neurites were measured in each treatment. The values represent the means and s.e.m. of 30 cells. *P<0.05 when compared with Veh. Scale=100 μm.
Figure 1.
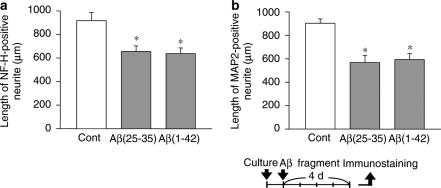
Axonal and dendritic atrophy induced by Aβ(25–35) and Aβ(1–42). Cortical neurons were cultured for 24 h, and then the cells were treated with 10 μM Aβ(25–35), 10 μM Aβ(1–42), or the vehicle (Cont). At 4 days after treatment, the cells were fixed and immunostained for phosphorylated NF-H or MAP2. Lengths of NF-H-positive (a) or MAP2-positive (b) neurites per cell were measured in each treatment. The values represent the means and s.e.m. of four images. *P<0.05 when compared with Cont.
Figure 3.
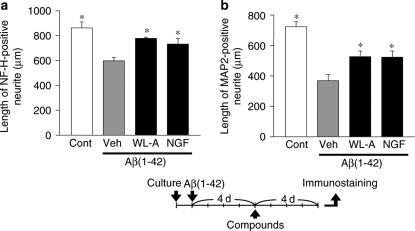
The effects of withanolide A (WL-A) on axonal and dendritic regeneration after Aβ(1–42)-induced atrophy. Cortical neurons were cultured for 24 h, and were then treated with or without (Cont) 10 μM Aβ(1–42). At 4 days after the administration of Aβ(1–42), the cells were treated with WL-A at a concentration of 1 μM; or NGF at a concentration of 100 ng ml−1; or the vehicle (Veh). At 4 days after treatment, the cells were fixed and immunostained for phosphorylated NF-H or MAP2. The lengths of NF-H-positive (a) or MAP2-positive (b) neurites per cell were measured in each treatment. The values represent the means and s.e.m. of four images. *P<0.05 when compared with Veh.
Figure 4.
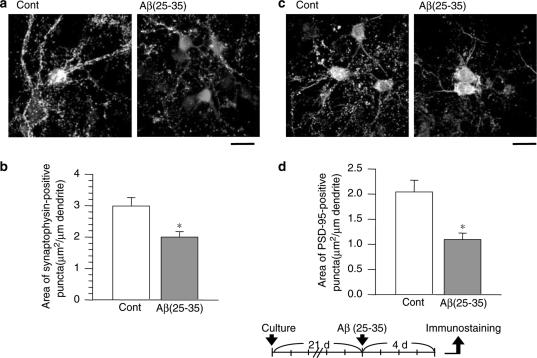
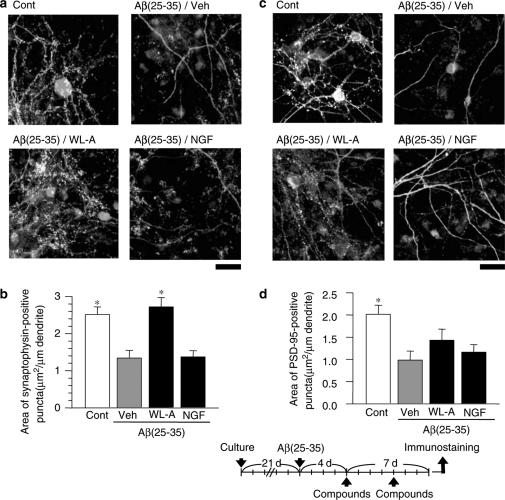
Pre- and postsynaptic loss induced by Aβ(25–35). After culture for 21 days, the cortical neurons were treated with or without (Cont) 10 μM Aβ(25–35) for 4 days. The cells were then double-immunostained for synaptophysin (a, green color in online version) or PSD-95 (b, green color in online version), plus MAP2 (red color in online version). Areas of synaptophysin- (b) or PSD-95- (d) positive puncta per 1 μm of dendrite were measured. The values represent the means and s.e.m. of 24–28 dendrites. *P<0.05 when compared with Cont. Scale=50 μm.
Figure 5.
The effects of withanolide A (WL-A) on pre- and postsynaptic reconstruction after Aβ(25–35)-induced synaptic loss. After culture for 21 days, the cortical neurons were treated with or without (Cont) Aβ(25–35). The cells were then treated with WL-A at a concentration of 1 μM; or NGF at a concentration of 100 ng ml−1; or with the vehicle (Veh). At 7 days after treatment, the cells were double-immunostained for synaptophysin (a, green in online version) or PSD-95 (c, green in online version), plus MAP2 (red in online version). Areas of synaptophysin- (b) PSD-95- (d) positive puncta per 1 μm of dendrites were measured. The values represent the means and s.e.m. of 20–35 dendrites. *P<0.05 when compared with Veh. Scale=20 μm.
Analysis of neurite outgrowth
Rat cortical neurons were cultured in four or eight-well chamber slides at a density of 0.86 × 105−1.43 × 105 cells cm−2 for 24 h. The cells were then treated with 10 μM Aβ(25–35) or 10 μM Aβ(1–42) for 4 days, and were then fixed by 4% paraformaldehyde (Figure 1). In Figures 2 and 3, 1 μM WL-A, 100 ng ml−1 mouse β-NGF or vehicle (0.1% DMSO) was administered to the cells after treatment with Aβ(25–35) or Aβ(1–42). After 4 days, the cells were fixed. The fixed cells were immunostained with a monoclonal antibody to phosphorylated NF-H (1 : 1000) as an axonal marker, or with a monoclonal antibody to MAP2 (1 : 200) as a dendritic marker. Alexa Fluor 488-conjugated goat anti-mouse IgG (1 : 200) was used as a second antibody. In Figures 1 and 3, the fluorescence images were captured by a fluorescence microscope (AX-80, Olympus, Tokyo, Japan) at 1709.4 μm × 2273.5 μm (Figures 1 and 3), and four images were captured per treatment. The total lengths of neurites positive for phosphorylated NF-H or MAP2 were automatically measured by an image analyzer (Neurocyte, Kurabo, Osaka, Japan) for each image, and then the lengths were averaged by the number of neurons in each image (56–161 cells per image). In Figure 2, the fluorescence images were captured by a confocal laser scanning microscope (LSM-GB200-IMT-2, Olympus, Tokyo, Japan) at 470 μm × 630 μm, and four images were captured per treatment. The lengths of neurites testing positive for phosphorylated NF-H or MAP2 were measured using an image analyzer (Scion Image, Scion, Frederick, MD, U.S.A.) for each cell.
Analysis of synaptic formation
Rat cortical neurons were cultured in eight-well chamber slides at a density of 8.57 × 104 cells cm−2. (Figure 4) or 1.14 × 105 cells cm−2 (Figure 5) for 21 days. The cells were treated with 10 μM Aβ(25–35) for 4 days, and were then fixed (Figure 4). In Figure 5, test compounds were administered to the cells after treatment with Aβ(25–35). At 4 days after administration, half of the medium in each well was replaced with fresh medium containing each test compound. Then, 3 days after replacement of the medium (i.e. 7 days of incubation with the compound), the cells were fixed and double-immunostained with a combination of a monoclonal antibody to synaptophysin (1 : 500) as a presynaptic marker and an antiserum to MAP2 (1 : 1000), or with a combination of a monoclonal antibody to PSD-95 (1 : 200) as a postsynaptic marker and an antiserum to MAP2. Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 546-conjugated goat anti-rabbit IgG (1 : 200) were used as secondary antibodies. The fluorescence images were captured using a confocal laser scanning microscope (Radiance 2100, Bio-Rad, Hercules, CA, U.S.A.) at 205 μm × 205 μm, and four images were captured per treatment. The area of positive puncta to synaptophysin or PSD-95 on each dendrite was measured using an image analyzer (ATTO densitograph, ATTO, Tokyo, Japan). The length of the dendrites was measured with Scion Image software (Scion).
Water maze test
Male ddY mice (6 weeks old, SLC, Shizuoka, Japan) were housed with free access to food and water, and were kept in a constant environment (22±2°C, 50±5% humidity, 12-h light cycle starting at 0700). The animals were handled in accordance with the Guide for Animal Experiments, Toyama Medical and Pharmaceutical University. Aβ(25–35) were dissolved in saline at a concentration of 5 mM and incubated at 37°C for 4 days to allow for fibril formation. The mice were anesthetized, and Aβ(25–35) (25 nmol) or the vehicle (saline) was injected into the right ventricle, with stereotaxic coordinates from the bregma being, in mm, A −0.22, L −1.0, and V 2.5. At 7 days after an i.c.v. injection of Aβ(25–35), WL-A (10 μmol kg−1 day−1) or the vehicle (0.5% gum arabic solution) was administered orally once daily for 13 days.
White-colored water was poured into a circular pool (diameter, 120 cm; height, 28 cm), and a white platform (diameter, 12 cm) was placed 1.2 cm below the water level in the middle of a fixed quadrant. The water temperature was adjusted to 21–23°C. Memory-acquisition trials (training) were performed four times daily for 6 days to reach a steady escape latency. At 1.5 h after p.o. administration of the drug, the mice were allowed to swim freely for 60 s and were left for an additional 30 s on the platform. The interval during four trials was 90 min. The pattern of the start positions in each trial was changed every day. Mice failing to find the platform were manually placed on the platform.
Memory-retention tests were performed 7 days after the last training session, that is, 7 days after the discontinuation of p.o. administration of the drugs. The platform was removed, and each mouse was allowed a free 60-s swim. The number of crossings over the point where the platform had been located was counted by video recorder replay.
Immunohistochemistry
At 2 days after the retention test, the mice were killed by decapitation. The brains were quickly removed from the skull, and frozen in powdered dry ice. Among them, we randomly selected three brains from each group. The brains were cut in 12-μm coronal sections using a cryostat (CM3050S, Leica, Heidelberg, Germany). The slices were fixed by 4% paraformaldehyde and stained with a monoclonal antibody to phosphorylated NF-H, MAP2, or synaptophysin. Alexa Fluor 488-conjugated goat anti-mouse IgG was used as the secondary antibody. The fluorescence images were captured using a fluorescence microscope (AX-80) at 332 μm × 423 μm. One brain slice per mouse was captured, and data of three mice were averaged for each treatment. In the same treatment, no clear differences of expression levels were seen in serial slices of each brain region. The area positive for phosphorylated NF-H, MAP2, synaptophysin, or PSD-95 was measured in five brain regions (ATTO densitograph, ATTO, Tokyo, Japan). In each region, the measuring points were selected from 20 squares of 41.5 μm × 41.5 μm.
Statistical analysis
Statistical comparisons were carried out using Student's t-test or one-way analysis of variance followed by Dunnett's post hoc test. Values of P<0.05 were considered significant. The means of the data are presented together with the s.e.m.
Results
Aβ(25–35) induced axonal and dendritic atrophy in cultured neurons
The cortical neurons were treated with Aβ(25–35), an active partial fragment of amyloid β(1–40,42) or full-length Aβ(1–42). At 24 h after the culture was initiated, Aβ(25–35) or Aβ(1–42) was added to the culture medium. After 4 days, the cells were fixed and immunostained for phosphorylated NF-H as an axonal marker, or for MAP2 as a dendritic marker. Aβ(25–35) treatment for 4 days significantly inhibited the outgrowth of both NF-H-positive neurites (71.4% of the control, Figure 1a) and MAP2-positive neurites (62.8% of the control, Figure 1b), showing that Aβ(25–35) induced both axonal and dendritic atrophy in rat cortical neurons. Aβ(1–42) treatment for 4 days also significantly induced atrophy of both axons (69.5% of the control, Figure 1a) and dendrites (65.6% of the control, Figure 1b). There was no significant difference between the Aβ(25–35) treatment and the Aβ(1–42) treatment in the induction of axonal and dendritic atrophy.
Next, we determined the ratio of neurons to grail cells in this culture condition. At 5 days after the neuronal culture started, the cells were double-immunostained with a combination of a monoclonal antibody to MAP2 (neuronal marker) and an antiserum to glial fibrillary acidic protein (GFAP, astrocytic marker), or with another combination of a monoclonal antibody to CD11b (microglial marker) and an antiserum to MAP2. The cells were observed using a confocal laser scanning microscope, and the ratios of neurons to astrocytes, and neurons to microglias were estimated. The percentage of neurons was about 80%, and that of astrocytes was 20%. Microglias were hardly observed.
WL-A induced axonal and dendritic regeneration in damaged neurons
We examined the effects of WL-A on neurite regeneration after neuritic atrophy had occurred. Rat cortical neurons were cultured only with Aβ(25–35) for 4 days, after which WL-A (1 μM), NGF (100 ng ml−1), or the vehicle (0.1% DMSO) was added. The dose of WL-A was consistent with the previously described optimal dose for neurite extension in normal neurons (Kuboyama et al., 2002). After drug treatment for 4 days, the cells were fixed and immunostained for phosphorylated NF-H or MAP2. Lengths of axons and dendrites in the neurons treated with the vehicle were shorter than the control at 8 days after treatment with Aβ(25–35) (Figure 2), whereas treatment with WL-A significantly increased the lengths of both axons (Figure 2a and b) and dendrites (Figure 2c and d), as compared with treatment with the vehicle. Treatment with NGF, which is known to induce neurite outgrowth in normal neurons (Studer et al., 1994), also induced the extension of both axons and dendrites as long as WL-A.
We then tested the effect of WL-A in damaged neurons by Aβ(1–42). After 4 days' treatment with Aβ(1–42), the cells were treated with the vehicle, WL-A, or NGF. Treatment with WL-A and NGF significantly extended both axons and dendrites compared with vehicle treatment (Figure 3), that is, WL-A could regenerate axons and dendrites in damaged neurons by not only Aβ(25–35) but also Aβ(1–42).
Pre- and postsynaptic reconstruction in damaged neurons
It is crucial to determine whether regenerated neurites are also able to reconstruct synapses. Since WL-A was shown to regenerate axons and dendrites, we tested the effects of WL-A on pre- and postsynaptic maturation. Rat cortical neurons needed to be cultured for 21 days to construct mature synapses in vitro, as also shown in another report (Zhang & Benson, 2001); after the culture period, Aβ(25–35) was added to the samples. At 4 days after the addition of Aβ(25–35), the cells were fixed and immunostained with an antibody for synaptophysin or PSD-95. Dendritic shafts were visualized by double-immunostaining with an MAP2 antibody. Synaptophysin- and PSD-95-positive puncta were observed at the edge of the dendritic shafts (Figures 4a, c and 5a, c). When a new axodendritic contact was formed by electric stimulation, PSD-95, glutamate receptors, and the presynaptic active-zone protein Bassoon were expressed at new synaptic sites within 1–2 h in cultured hippocampal neurons (Friedman et al., 2000). The expression of synaptophysin- and PSD-95-expressed puncta was mostly co-localized on dendritic shafts in cultured hippocampal pyramidal neurons (Okabe et al., 2001). These results suggest that synaptophysin- and PSD-95-positive puncta on dendritic shafts may show the synaptic active sites of pre- and those of post-synapses, respectively.
The number and fluorescence intensity of synaptophysin- (Figure 4a) and PSD-95-(Figure 4c) positive puncta on dendrites were obviously decreased by treatment with Aβ(25–35). Quantified areas of synaptophysin- and PSD-95-positive puncta were significantly decreased by treatment with Aβ(25–35) for 4 days (53.4 and 66.9% of the control, respectively, Figures 4b and d). These results indicate that Aβ(25–35) induced the loss of both pre- and postsynaptic structures in long-term-cultured cortical neurons.
WL-A, NGF, and the vehicle were added to the culture medium after treatment with Aβ(25–35) for 4 days when synaptic loss had already occurred, as shown in Figure 4. At 7 days after addition of the drug, the cells were fixed and immunostained for synaptophysin or PSD-95. Synaptophysin- and PSD95-positive areas in the neurons treated with the vehicle continued to decrease 11 days after treatment with Aβ(25–35) compared with control neurons (Figure 5). On the other hand, treatment with WL-A significantly increased in synaptophysin (Figure 5a and b) and PSD-95 expressions (Figure 5c and d), as compared with treatment with the vehicle. These results indicate that WL-A facilitated the reconstruction of pre- and postsynaptic regions in neurons in which severe synaptic loss had already occurred. Treatment with NGF, however, did not lead to an increase in the development of either the presynapses (54.4% of the control, Figure 5b) or the postsynapses (57.7% of the control, Figure 5d). It was previously reported that NGF induced both axonal and dendritic growth in cultured striatal neurons (Studer et al., 1994) and it increased dendritic arbors in cultured visual cortical slices (Mc Allister et al., 1995). However, evidence of NGF-induced synaptogenesis has rarely been observed. Although we repeatedly tested the NGF effects on synaptogenesis according to various application schedules, neither pre- nor postsynaptic regions were enhanced by NGF treatment.
Aβ(25–35) is known to induce neuronal cell death, especially in vitro (Yankner et al., 1990). Although WL-A promoted neuritic regeneration (Figure 2) and synaptic reconstruction (Figure 5), these effects might have been due to protection from Aβ(25–35)-induced cell death. To investigate the specific effect of WL-A on cell survival, trypan blue staining was performed. Treatment with 10 μM Aβ(25–35) and 10 μM Aβ(1–42) for 3 days similarly induced remarkable cell death (Aβ(25–35): 61.1% of control, Aβ(1–42): 61.6% of control) in cortical neurons. Simultaneously administered 1 μM WL-A with Aβ(25–35) did not protect the neurons from cell death (64.5% of control). A measure of 100 ng/ml NGF-treatment also did not protect from cell death (57.1% of control).
WL-A ameliorated the impairment of spatial memory by neurite outgrowth and synaptic construction
We had previously confirmed that neuritic and synaptic losses occurred in the hippocampus and cerebral cortex of mice 7 days after the i.c.v. administration of Aβ(25–35), and these losses continued for at least 14 days after the i.c.v. administration of Aβ(25–35). Furthermore, we also confirmed that spatial memory deficit occurred 14 days after the i.c.v. administration of Aβ(25–35) (data not shown). We therefore started p.o. administration of WL-A from 7 days after the i.c.v. administration of Aβ(25–35), when neuronal and synaptic loss had already occurred.
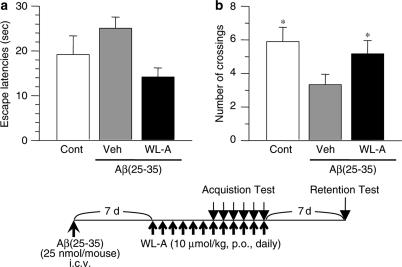
Mice were trained in the water maze for 6 days, starting 7 days after the p.o. administration of WL-A, that is, 14 days after the i.c.v. administration of Aβ(25–35). All the mice reduced the time to reach the platform (escape latencies) training-day dependently (data not shown). At training day 5, Aβ(25–35)-injected mice tended to increase the escape latencies compared with control mice (Figure 6a), while the administration of WL-A decreased the escape latencies compared with administration of the vehicle.
Figure 6.
The effect of withanolide A on spatial memory deficit induced by Aβ(25–35). (a) Escape latencies of four trials are shown on training day 5 in a Morris water maze. (b) Crossing numbers over the position where the platform had been located were measured for 60 s 7 days after the last training day. This was also 7 days after the discontinuance of drug treatment. The time schedule of the experiment is shown below the figure. The values represent the means and s.e.m. of 6–9 mice. *P<0.05 when compared with Veh.
From the day after the last training day, serial drug administration was discontinued, and then 7 days after the last training day, the retention test was performed. In the retention test, the number of crossings over the platform position significantly decreased in the Aβ(25–35)-injected mice compared with the control mice (Figure 6b), while the administration of WL-A significantly increased the crossing numbers compared with administration of the vehicle. Locomotion activities of the mice were not different among any groups (data not shown).
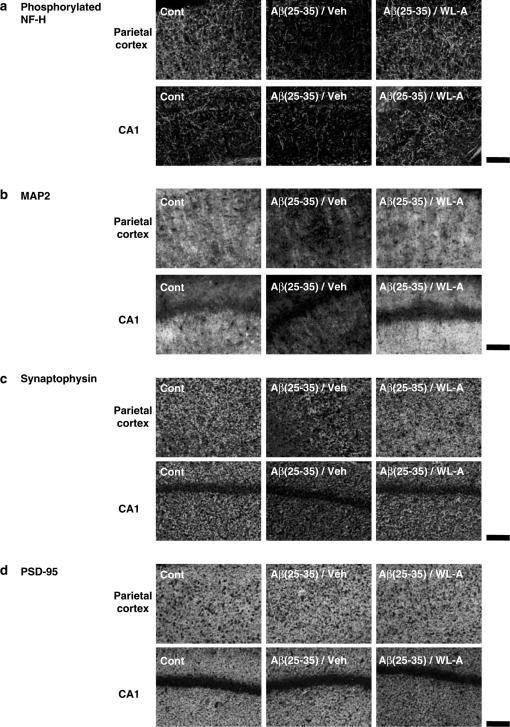
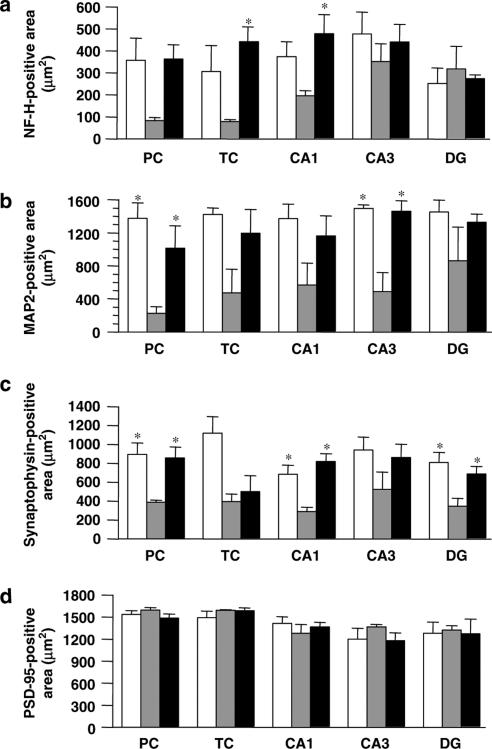
After the retention test, the expression levels of phosphorylated NF-H, MAP2, synaptophysin, and PSD-95 were measured in the mouse brain. We observed two cortical regions (parietal cortex and temporal cortex) and three hippocampal regions (CA1, CA3, and dentate gyrus) where neuronal degeneration occurred in Alzheimer's disease patients (De Kosky & Scheff, 1990; Heinonen et al., 1995) and Alzheimer's disease model mice (Games et al., 1995). In Aβ(25–35)-injected mice, phosphorylated NF-H-, MAP2-, and synaptophysin-positive areas were remarkably decreased in most regions compared with control mice (Figures 7a–c and 8a–c), while the administration of WL-A increased NF-H-, MAP2-, and synaptophysin-positive areas, compared with administration of the vehicle. These increases with WL-A reached the control level. It can be considered that these increases of neuritic and synaptic marker proteins contribute to the recovery of memory deficits of mice induced by Aβ(25–35). Unexpectedly, PSD-95-positive areas in Aβ(25–35)-injected mice did not differ from those in control mice in any regions (Figures 7d and 8d), and the administration of WL-A did not increase the expression of PSD-95 in any regions.
Figure 7.
Effects of withanolide A on neuritic atrophy and synaptic loss in vivo induced by Aβ(25–35). At 2 days after the retention test, the mice were decapitated, and their brains were coronally sliced. The slices were immunostained with phosphorylated NF-H (a), MAP2 (b), synaptophysin (c), and PSD-95 (d). Representative images of brain slices from each group are shown from the parietal cortex (upper) and the hippocampal CA1 (below). Scale=100 μm.
Figure 8.
Quantified effects of withanolide A (WL-A) on neuritic atrophy and synaptic loss in vivo induced by Aβ(25–35). NF-H- (a), MAP2- (b), synaptophysin- (c), and PSD-95- (d) positive areas were measured in the parietal cortex (PC) and temporal cortex (TC), and the hippocampal CA1, CA3, and dentate gyrus (DG). Control mice (open columns) were treated with an i.c.v. injection of saline and p.o. administration of the vehicle. Aβ(25–35) i.c.v.-injected mice were treated with p.o. administration of the vehicle (gray columns) or WL-A (black columns). The values represent the means and s.e.m. of three mice. *P<0.05 when compared with Aβ(25–35) plus the vehicle-treated group.
Discussion
We demonstrated for the first time in vitro and in vivo that WL-A was able to recover both neuritic atrophy and synaptic loss. Until now, several factors have been reported as candidates for neurite outgrowth enhancement. For example, physiological substances such as cholesterol (Fan et al., 2002) and estradiol (Cambiasso & Carrer, 2001; Audesirk et al., 2003), and isolated compounds from herbal drugs such as genipin (Yamazaki et al., 2001), ginsenoside Rb1 (Rudakewich et al., 2001), nardosinone (Li et al., 1999) were reported. Although the authors of these reports speculated that those substances could also enhance synaptic formation, no clear evidence has been demonstrated in damaged neuronal models. In this study, we demonstrated that the remarkable enhancement of axonal and dendritic regeneration and synaptic reconstruction was induced by WL-A in the damaged mouse brain as well as in the damaged cultured neurons. In addition, p.o. administration of WL-A improved the Aβ(25–35)-induced memory deficit of mice. This impairment of the memory disorder may result from the reconstruction of neuronal networks by WL-A.
Although the signal transduction mechanisms of WL-A remained unknown, one possibility was that WL-A stimulated signal cascades similar to β-estradiol. WL-A has a steroidal structure (Kuboyama et al., 2002). β-Estradiol is an endogenous factor that induces neurite arborization via extracellular signal-regulated kinase (ERK) (Dominguez et al., 2004), and enhances synaptophysin expression via membrane ER and p44 MAP kinase (Yokomaku et al., 2003). It is also reported that β-estradiol enhances PSD-95 transcription via the PI3-K following Akt pathway (Akama & McEwen, 2003). We are now analyzing the mechanism of WL-A compared with the signal cascade of β-estradiol.
In this study, NGF induced neuritic regeneration, but did not lead to the reconstruction of synapses. These findings may indicate that NGF can induce the regeneration of neurites, but not that of synapses. BDNF is another important neurotrophin that leads to neurite extension (Mc Allister et al., 1995) and increases the number of synaptophysin-immunoreactive puncta (Wang et al., 1995; Coffey et al., 1997), whereas BDNF (100 ng ml−1) did not induce neurite outgrowth in our study (data not shown). We are unable to account for the failure of BDNF to lead to neurite extension in cultured cortical neurons. However, we suggest that WL-A induced the growth of neurites and synapses via different mechanisms from those associated with BDNF or NGF.
WL-A did not rescue the neurons from cell death induced by Aβ(25–35) in vitro. Amyloid β has been reported to induce cell death via a variety of mechanisms (Yamada & Nabeshima, 2000). However, the mechanism of neuritic atrophy induced by amyloid β is thought to differ from that of cell death, according to the diversity of the observed time courses of cell death (Grace et al., 2002) and also as based on observed differences in amyloid β formation (Postuma et al., 2000). As previously described, neuritic atrophy induced by amyloid β is thought to be caused by impaired cell adhesion (Postuma et al., 2000; Grace & Busciglio, 2002). In addition, amyloid β induces neuritic deformation by the polymerization and aggregation of actins (Hiruma et al., 2003). Thus, WL-A might lead to the repair of, or compensate for, disorder of cell adhesion and cytoskeletal molecules.
Aβ(25–35) induced the decrease of PSD-95-positive areas in cultured neurons, but not in the mouse brain in this experiment. Since few studies have reported the effect of Aβ on the expression of PSD-95 in vitro or in vivo, it is unknown why the effect of Aβ(25–35) on the expression of PSD-95 was not identical in vitro and in vivo in this experiment. However, this suggests that the expression of PSD-95 may not contribute significantly to the formation of spatial memory.
Here, we demonstrated that WL-A could facilitate the regeneration of axons and dendrites, and this compound led to the dramatic reconstruction of pre- and postsynapses, when neuron damage had already progressed. Moreover, WL-A could ameliorate the memory deficit in mice, and could generate neurites and synapses in the cerebral cortex and the hippocampus. These effects of WL-A in vivo were maintained even after the discontinuance of drug administration. Therefore, WL-A has potential as an essentially useful drug to treat neurodegenerative diseases when used together with treatments preventing pathogenesis and neuronal death.
Acknowledgments
This study was supported by the Uehara Memorial Foundation and by a Grant-in-Aid for the 21st century CoE Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- MAP2
microtubule-associated protein 2
- NF-H
neurofilament-H
- NGF
nerve growth factor
- PSD-95
post-synaptic density-95
References
- AKAMA K.T., MCEWEN B.S. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/Protein kinase B pathway. J. Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUDESIRK T., CABELL L., KERN M., AUDESIRK G. Estradiol influences differentiation of hippocampal neurons in vitro through an estrogen receptor-mediated process. Neuroscience. 2003;121:927–934. doi: 10.1016/s0306-4522(03)00294-x. [DOI] [PubMed] [Google Scholar]
- BOBINSKI M., WEGIEL J., TARNAWSKI M., BOBINSKI M., REISBERG B., DE LEON M.J., MILLER D.C., WISNIEWSKI H.M. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J. Neuropath. Exp. Neurol. 1997;56:414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- CAMBIASSO M.J., CARRER H.F. Nongenomic mechanism mediates estradiol stimulation of axon growth in male rat hypothalamic neurons in vitro. J. Neurosci. 2001;66:475–481. doi: 10.1002/jnr.1238. [DOI] [PubMed] [Google Scholar]
- CANNING D.R., MC KEON R.J., DE WITT D.A., PERRY G., WUJEK J.R., FREDERICKSON R.C., SILVER J. Amyloid of Alzheimer's disease induces reactive gliosis that inhibits axonal outgrowth. Exp. Neurol. 1993;124:289–298. doi: 10.1006/exnr.1993.1199. [DOI] [PubMed] [Google Scholar]
- COFFEY E.T., AKERMAN K.E.O., COURTNEY M.J. Brain derived neurotrophic factor induces a rapid upregulation of synaptophysin and tau proteins via the neurotrophin receptor TrkB in rat cerebellar granule cells. Neurosci. Lett. 1997;277:177–180. doi: 10.1016/s0304-3940(97)00335-2. [DOI] [PubMed] [Google Scholar]
- DE KOSKY S., SCHEFF S.W. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- DICKSON T.C., VICKERS J.C. The morphological phenotype of β-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- DHULEY J.N. Nootropic-like effect of Ashwagandha (Withania somnifera L.) in mice. Phytother. Res. 2001;15:524–528. doi: 10.1002/ptr.874. [DOI] [PubMed] [Google Scholar]
- DOMINGUEZ R., JALAI C., DE LACALLE S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J. Neurosci. 2004;24:982–990. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN Q.-W., YU W., GONG J.-S., ZOU K., SAWAMURA N., SENDA T., YANAGISAWA K., MICHIKAWA M. Cholesterol-dependent modulation of dendritic outgrowth and microtubule stability in cultured neuron. J. Neurochem. 2002;80:178–190. doi: 10.1046/j.0022-3042.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN H.V., BRESLER T., GARNER C.C., ZIV N.E. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- GAMES D., ADAMS D., ALESSANDRINI R., BARBOUR R., BORTHELETTE P., BLACKWELL C., CARR T., CLEMENS J., DONALDSON T., GILLESPIE F., GUIDO T., HAGOPIAN S., JOHNSON-WOOD K., KHAN K., LEE M., LEIBOWITZ P., LIEBERBURG I., LITTLE S., MASLIAH E., MCCONLOGUE L., MONTOYA-ZAVALA M., MUCKE L., PAGANINI L., PENNIMAN E., POWER M., SCHENK D., SEUBERT P., SNYDER B., SORIANO F., TAN H., VITALE J., WADSWORTH S., WOLOZIN B., ZHAO J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- GRACE E.A., BUSCIGLIO J. Aberrant activation of focal adhesion proteins mediates fibrillar amyloid β-induced neuronal dystrophy. J. Neuorsci. 2002;23:493–502. doi: 10.1523/JNEUROSCI.23-02-00493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE E.A., RABINER C.A., BUSCIGLIO J. Characterization of neuronal dystrophy induced by fibrillar amyloid β: implications for Alzheimer' disease. Neuroscience. 2002;114:265–273. doi: 10.1016/s0306-4522(02)00241-5. [DOI] [PubMed] [Google Scholar]
- HEINONEN O., LEHTOVIRTA M., SOININEN H., HELISALMI S., MANNERMAA A., SORVARI H., KOSUNEN O., PALJARVI L., RYYNANEN M., RIEKKINEN P.J. Alzheimer pathology of patients carrying apolipoprotein E epsilon 4 allele. Neurobiol. Aging. 1995;16:505–513. doi: 10.1016/0197-4580(95)00076-q. [DOI] [PubMed] [Google Scholar]
- HIRUMA H., KATAKURA T., TAKAHASHI S., ICHIKAWA T., KAWAKAMI T. Glutamate and amyloid β-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J. Neurosci. 2003;23:8967–8977. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON M., GENTLEMAN S., LENNOX L., WARD L., GRAY T., RANDALL K., MORRELL K., LOWE J. The cortical neuritic pathology by Huntington's disease. Neuropathol. Appl. Neurobiol. 1995;21:18–26. doi: 10.1111/j.1365-2990.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- KNOWLES R.B., WYART C., BULDYREY S.V., CRUZ L., URBANC B., HASSELMO M.E., STANLEY H.E., HYMAN B.T. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBOYAMA T., TOHDA C., ZHAO J., NAKAMURA N., HATTORI M., KOMATSU K. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. NeuroReport. 2002;13:1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- LI P., MATSUNAGA K., YAMAMOTO K., YOSHIKAWA R., KAWASHIMA K., OHIZUMI Y. Nardosinone, a novel enhancer of nerve growth factor in neurite outgrowth from PC12D cells. Neurosci. Lett. 1999;273:53–56. doi: 10.1016/s0304-3940(99)00629-1. [DOI] [PubMed] [Google Scholar]
- LIBERSKI P.P., BUDKA H. Neuroaxonal pathology in Creutzfeldt–Jakob disease. Acta Neuropathol. 1999;97:329–334. doi: 10.1007/s004010050995. [DOI] [PubMed] [Google Scholar]
- MATTILA P.M., RINNE J.O., HELENIUS H., ROYTTA M. Neuritic degeneration in the hippocampus and amygdala in Parkinson's disease in relation to Alzheimer pathology. Acta Neuropathol. 1999;98:157–164. doi: 10.1007/s004010051064. [DOI] [PubMed] [Google Scholar]
- MAURICE T., LOCKHART B.P., PRIVAT A. Amnesia induced in mice by centrally administered β-amiloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- MC ALLISTER A.K., LO D.C., KATZ L.C. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- OKABE S., MIWA A., OKADO H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J. Neurosci. 2001;21:6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIKE C.J., WALENCEWICZ-WASSERMAN A.J., KOSMOSKI J., CRIBBS D.H., GLABE C.G., COTMAN C.W. Structure-activity analyses of β-amyloid peptides: contribution of the β25-35 region to aggregation and neurotoxicity. J. Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- POSTUMA R.B., HE W., NUNAN J., BEYREUTHER K., MASTERS C.L., BARROW C.J., SMALL D.H. Substrate-bound β-amyloid peptides inhibit cell adhesion and neurite outgrowth in primary neuronal cultures. J. Neurochem. 2000;74:1122–1130. doi: 10.1046/j.1471-4159.2000.741122.x. [DOI] [PubMed] [Google Scholar]
- RUDAKEWICH M., BA F., BENISHIN C.G. Neurotophic and neuroprotective actions of ginsenosides Rb1 and Rg1. Planta Med. 2001;67:533–537. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- SIMMONS L.K., MAY P.C., TOMASELLI K.J., RYDEL R.E., FUSON K.S., BRIGHAM E.F., WRIGHT S., LIBERBURG I., BECKER G.W., BREMS D.N., LI W.Y. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Mol. Pharmacol. 1994;45:373–379. [PubMed] [Google Scholar]
- STUDER L., SPENGER C., LUTHMAN J., SEILER R.W. NGF increases neuritic complexity of cholinergic interneurons in organotypic cultures of neonatal rat striatum. J. Comp. Neurol. 1994;340:281–296. doi: 10.1002/cne.903400212. [DOI] [PubMed] [Google Scholar]
- TERRY R.D., MASLIAH E., SALMON D.P., BUTTERS N., DE TERESA R., HILL R., HANSEN L.A., KATZMAN R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- TOHDA C., KUBOYAMA T., KOMATSU K. Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK-N-SH cells. NeuroReport. 2000;11:1981–1985. doi: 10.1097/00001756-200006260-00035. [DOI] [PubMed] [Google Scholar]
- TOHDA C., MATSUMOTO N., ZOU K., MESELHY M.R., KOMATSU K. Aβ(25-35)-induced memory impairment, axonal atrophy and synaptic loss are ameliorated by M1, a metabolite of protopanaxadiol-type saponins. Neuropsycopharmacology. 2004;29:860–868. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- TOHDA C., TAMURA T., KOMATSU K. Repair of amyloid β(25-35)-induced memory impairment and synaptic loss by a Kampo formula, Zokumei-to. Brain Res. 2003;990:141–147. doi: 10.1016/s0006-8993(03)03449-8. [DOI] [PubMed] [Google Scholar]
- WANG T., XIE K., LU B. Neurotrophins promote maturation of developing neuromascular synapses. J. Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA K., NABESHIMA T. Animal model of Alzheimer's disease and evaluation of anti-demenria drugs. Pharmacol. Ther. 2000;88:93–113. doi: 10.1016/s0163-7258(00)00081-4. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI M., CHIBA K., MOHRI T., HATANAKA H. Activation of the mitogen-activated protein kinase cascade through nitric oxide synthesis as a mechanism of neuritogenic effect of genipin in PC12h cells. J. Neurochem. 2001;79:45–54. doi: 10.1046/j.1471-4159.2001.00533.x. [DOI] [PubMed] [Google Scholar]
- YANKNER B.A., DUFFY L.K., KIRSCHNER D.A. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- YOKOMAKU D., NUMAKAWA T., NUMAKAWA Y., SUZUKI S., MATSUMOTO T., ADACHI N., NISHIO C., TAGUCHI T., HATANAKA H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol. Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- ZHANG W., BENSON D.L. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO J., NAKAMURA N., HATTORI M., KUBOYAMA T., TOHDA C., KOMATSU K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem. Pharm. Bull. 2002;50:760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]