Abstract
We previously showed that oestrogen confers cardioprotection by downregulating the cardiac β1-adrenoceptor (β1-AR). The present study examined the effect of oestrogen on the post β1-AR signalling cascade, with particular emphasis on the activity of protein kinase A (PKA) and its influence on the L-type Ca2+ channel.
Three groups of adult female Sprague–Dawley rats were used: sham-operated controls, bilaterally ovariectomized (Ovx) rats, and Ovx rats with oestrogen replacement (Ovx+E2), which restored the oestrogen concentration to normal.
The electrically induced intracellular Ca2+ transient (E[Ca2+]i), 45Ca2+-uptake through cardiac L-type Ca2+ channels (Ca2+ channels), heart rate and force of contraction in response to β-AR stimulation with 10 nM isoprenaline (Iso) in hearts from Ovx rats were significantly greater than those of control and Ovx+E2 rats. The basal and Iso-induced PKA activities were also higher in hearts from Ovx rats. KT5720, a selective PKA inhibitor, completely inhibited its potentiating effect on basal Ca2+ channel activity in the Ovx rat heart. On the other hand, expression of G proteins (Gαs and Gαi1−3), basal and forskolin-stimulated cAMP accumulation, and responsiveness of PKA to cAMP, were not altered by Ovx.
Interestingly, the PKA inhibitor at the same concentration significantly reduced the increases in PKA activity and Ca2+ channel activity upon β-AR stimulation in all three groups of rats and the inhibitions were significantly greater in the Ovx rat than in the other two groups of rats.
This study provides the first evidence that, in addition to downregulation of β1-AR shown previously, suppression of PKA activity, which is partly responsible for the suppressed Ca2+ channel activity, also determines the E[Ca2+]i and cardiac contractility following β-AR stimulation in the female rat.
Keywords: Oestrogen, β-adrenoceptor, protein kinase A, ovariectomy, L-type Ca2+ channel, electrically induced Ca2+ transient, 45Ca2+ uptake
Introduction
In 1980, Ciric and Susic observed that isoprenaline (Iso), a β-adrenoceptor (β-AR) agonist, affects heart rate to different extents in female rats in different phases of the estrous cycle, and in ovariectomized rats with and without oestrogen replacement. These observations suggest that oestrogen regulates the cardiovascular system through modulation of β-AR in the heart. It was also found that oestrogen deficiency produced by ovariectomy (Ovx) causes upregulation of β1-AR, an effect restored to the normal level by oestrogen replacement (Thawornkaiwong et al., 2003). This result suggests that oestrogen suppresses the expression of cardiac β1-AR. Recently, we extended this finding by demonstrating that incubation of ventricular myocytes from Ovx rats with 10−9 M 17β-estradiol for 24 h, which suppressed the enhanced expression of β1-AR, was accompanied by cardioprotection against Iso-induced ischaemic insult, whereas incubation of ventricular myocytes with 17β-estradiol for 12 h neither suppressed the expression of β1-AR nor conferred cardioprotection against ischaemic insult and β-AR stimulation. These observations were taken to indicate that the cardioprotective effect of oestrogen is due, at least partly, to downregulating β1-AR in the rat heart (Kam et al., 2004). Studies in other laboratories have shown increased activity and overexpression of the cardiac L-type Ca2+ channel (Ca2+ channel) in oestrogen receptor α (ERα)-knockout mice (Johnson et al., 1997). In addition, altered Ca2+ sensitivity of cardiac myofilaments in ovariectomized rats has been observed (Wattanapermpool, 1998). However, the influence of oestrogen on events downstream from the cardiac β1-AR signalling mechanism leading to changes in Ca2+ channel activity is not yet understood.
The sympathetic nervous system is one of the most important extrinsic mechanisms regulating cardiac function, mainly through activation of β-AR. There are three β-AR subtypes, namely β1-, β2- and β3-ARs. The roles of β1- and β2-ARs are reasonably well defined; both are involved in contractile functions and apoptosis via different signalling mechanisms (see Xiao et al., 2004). β3-AR is found in human and murine heart and its role is not well understood other than a negative inotropic action (see Conrath & Opthof, 2003). Stimulation of the β1-AR subtype increases developed contraction (inotropy) and accelerates relaxation (lusitropy) by activating the GTP-binding protein (Gαs)/adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) pathway (Bers, 2002). PKA phosphorylates several key regulatory proteins in excitation–contraction (E–C) coupling; its inotropic effect is mainly mediated through phosphorylation of the Ca2+ channels and thus increases the peak L-type Ca2+ current (Ica). This causes more Ca2+ release during excitation (Beuckelmann & Wier, 1988; Nabauer et al., 1989; Sperelakis & Wahler, 1998), which in turn increases the developed contraction. Recently, it has also been shown that stimulation of β2-AR also increases cAMP and PKA, leading to increased Ica via Ca2+ channels (Yatani et al., 1999). On the other hand, it has also been suggested that β-AR stimulation may increase the Ica via a PKA-independent activation of Ca2+ channels (Yatani & Brown, 1989; Yatani et al., 1999).
In a preliminary study, we determined the effect of oestrogen on the expression/activity of the intermediates in the Gαs/AC/cAMP/PKA pathway and found that the expression of Gαs and Gαi and cAMP accumulation were the same in the hearts from control, Ovx, and Ovx+E2 rats. On the other hand, the basal PKA activity was increased in hearts from Ovx rats. We therefore hypothesized that oestrogen might suppress PKA activity, thus decreasing Ca2+ channel activity and thereby reduce the cardiac response to β-AR stimulation.
To test this hypothesis, we first determined the effect of β-AR stimulation with Iso on PKA activity. Secondly, we determined the effect of PKA blockade on basal and Iso-stimulated Ca2+ channel activity in the hearts from sham-operated control, Ovx, and Ovx+E2 rats. The main finding was that Iso-induced PKA activity and Ca2+ channel activity were significantly higher in the hearts from Ovx rats. This was accompanied by significantly greater increases in the electrically induced intracellular Ca2+ transient (E[Ca2+]i), and cardiac contractility in hearts from Ovx rats over those from control and Ovx+E2 rats. So, besides suppressing β1-AR expression as observed in our previous study (Kam et al., 2004), oestrogen also suppressed PKA activity, thus decreasing Ca2+ channel activity in response to β-AR stimulation in the heart.
Methods
Experimental animals
Female Sprague–Dawley rats weighing 190–210 g were purchased from Charles River Breeding Laboratories (Wilmington, MA, U.S.A.) and randomly divided into two groups. One group underwent sham operation and served as normal control. The other group underwent bilateral Ovx (Kam et al., 2004). A dorsal midline skin incision was made caudal to the posterior border of the ribs. Using blunt dissection, the muscles of the posterior abdominal wall were separated and the abdominal cavity opened. The periovarian fat was gently grasped with forceps to lift and exteriorize the ovary, which was then removed. The uterine horn was returned into the abdomen, and the process was repeated on the other side. At 1 week after Ovx, a subgroup of the rats (Ovx+E2) was subcutaneously implanted with a 60-day release pellet containing 1.5 mg of 17β-estradiol (Innovative Research of America, Toledo, OH, U.S.A.). These pellets maintain oestrogen concentration within the physiological range. All surgical procedures were performed under anaesthesia with sodium pentobarbital (60 mg kg−1, i.p.; Abbott Laboratory, Chicago, U.S.A.).
The protocol was approved by the Committee on the Use of Experimental Animals for Teaching and Research, The University of Hong Kong.
Cardiac membrane preparation
Cardiac membrane was prepared from the left ventricle as previously described (Yu et al., 2001) with some modification. The rats were anaesthetized with sodium pentobarbitone (60 mg kg−1 i.p.) and given heparin (200 IU, i.v.) before decapitation by guillotine. The heart was excised rapidly and mounted on the Langendorff apparatus. The heart was perfused with saline (0.9% NaCl) for 5 min to wash out all the blood. The isolated left ventricle was minced into small pieces in homogenization buffer containing 20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EGTA, 1 mM DTT, and 0.4 mM PMSF. The minced left ventricle was homogenized with Polytron PT 35 homogenizer for 30 s. The rest of the isolating procedure was the same as described by Yu et al. (2001). The final pellet was dispersed with a Polytron in 400 μl of the membrane buffer. The resuspended pellets were stored at −80°C until use. The protein content was determined as described previously (Lowry et al., 1951).
Western blotting analysis
In all, 60 μg of membrane protein from the left ventricle was diluted in loading buffer (130 mM Tris-HCl, pH 8.0, 20% (v v−1) glycerol, 5% (w v−1) sodium dodecyl sulphate (SDS), 0.02% bromophenol blue, 2% DTT) and denatured for 5 min at 95°C. Samples were separated by 12% polyacrylamide gel electrophoresis in the presence of SDS and then electrophoretically transferred onto polyvinylidene difluoride membrane at 100 V for 1.5 h. The membrane was then incubated with anti-Gαs, anti-Gα1,2 and anti-Gαi3 (each at 1 : 1000) overnight at 4°C. After three 10-min washes in TBST (Tris-buffered saline, pH 7.4, 0.1% Tween-20) solution, HRP-linked anti-rabbit IgG at a dilution ratio of 1 : 1000 was used as secondary antibody and incubated for 1 h at room temperature, followed by three 10-min washes in TBST solution. Protein bands were detected using an enhanced chemiluminescence detection system and visualized by autoradiography. Densitometric analysis was conducted using the Syngene CCDBIO acquisition system and its analysis software (Hitachi Genetics System, Alameda, CA, U.S.A.).
Isolated perfused heart preparation
Rats were anaesthetized with sodium pentobarbitone (60 mg kg−1 i.p.) and given heparin (200 IU, i.v.) before decapitation by guillotine. The heart was removed immediately, mounted on a Langendorff apparatus and perfused retrogradely with Krebs–Henseleit solution (in mM: NaCl 118, KCl 4.7, CaCl2 1.25, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, and glucose 11) equilibrated with 95% O2+5% CO2 at a constant pressure of 80 cm H2O and temperature of 37°C.
A balloon inserted through the left atrium into the left ventricle was inflated by injecting 0.1 to 0.2 ml of saline to adjust the end-diastolic pressure to 6–10 mmHg. Cardiac parameters were monitored continuously by a PowerLab/4SD analog-to-digital converter (AD Instruments, Castle Hill, Australia).
Isolation of ventricular myocytes
Ventricular myocytes were isolated from the hearts of control, Ovx, and Ovx+E2 rats, using the collagenase perfusion method described previously (Wu et al., 1999). After isolation, they were allowed to stabilize for at least 30 min before experiments.
Measurement of [Ca2+]i
A spectrofluorometric method with Fura-2/AM as the Ca2+ indicator was used to load cells and measure [Ca2+]i as previously described (Wu et al., 1999). The myocytes selected for the study were rod shaped and quiescent with clear striations. They exhibited asynchronous contraction in response to 0.2 Hz electrical field stimulation with 15 ms pulse at 60 V through two platinum wires in the bathing chamber. The myocytes were superfused with a Krebs–bicarbonate buffer containing (in mM) 118 NaCl, 5 KCl, 1.2 MgSO4, 1.2 KH2PO4, 1.25 CaCl2, 25 NaHCO3, and 11 glucose and a gas phase of 95% O2–5% CO2, pH 7.4 throughout the study. After 15 min of stabilization in the bathing chamber, a single ventricular myocyte was selected. The changes in E[Ca2+]i was recorded for 10 min, which constituted the basal [Ca2+]i transient in response to electrical stimulation. Afterwards, the perfusate was changed to various Krebs–bicarbonate buffer containing different concentrations of Iso and the recording was continued for another 5–10 min to ensure that equilibrium with Iso had been reached. Fluorescence signals obtained at excitation wavelengths of 340 nm (F340) and 380 nm (F380) were recorded and stored in a computer for subsequent processing and analysis. The F340/F380 ratio represented cytosolic [Ca2+]i in the ventricular myocyte. The E[Ca2+]i, which represents the influx of Ca2+ via Ca2+ channels and the release of Ca2+ from the sarcoplasmic reticulum triggered by the Ca2+ influx, is directly correlated with cardiac contraction in the experimental conditions of the present study (Yu et al., 1999).
Measurement of 45Ca2+ uptake
Freshly isolated cells (2 × 105 ml−1) were equilibrated for 5–10 min at room temperature (22°C) in MEM solution, where the concentration of Ca2+ was gradually increased to 0.1 mM. In all, 100–200 μl aliquots were placed in plastic tubes containing 1–2 ml of solution A (in mM): NaCl 140, KCl 5, MgCl2 1, CaCl2 0.1 (45CaCl2 2 μCi ml−1) and HEPES-Tris 20 (pH 7.4; 22°C). The cell suspension (2 × 104 ml−1) was incubated for 2–20 min at room temperature. A specific inhibitor of L-type Ca2+ channels, nitrendipine (10 μM), a highly selective β1-AR antagonist, bisoprolol (1 μM) (McGavin & Keating, 2002), and a selective inhibitor of PKA, KT5720 (2 μM) (Kase et al., 1987; Haikala et al., 1997; Kiehn et al., 1998) were added to the cell suspension along with Iso. The incubation mixture was rapidly filtered through Whatman GF/C filters and washed three times with 4 ml of washing solution containing (in mM): NaCl 140, HEPES-Tris 10 (pH 7.4), and EGTA 0.05. After washing, the GF/C filters were added to 10 ml of scintillation cocktail and left for 40 min (Universal LSC cocktail for aqueous samples, Sigma, St Louis, U.S.A.). The radioactivity was then measured in a scintillation counter (LS 6500, Beckman).
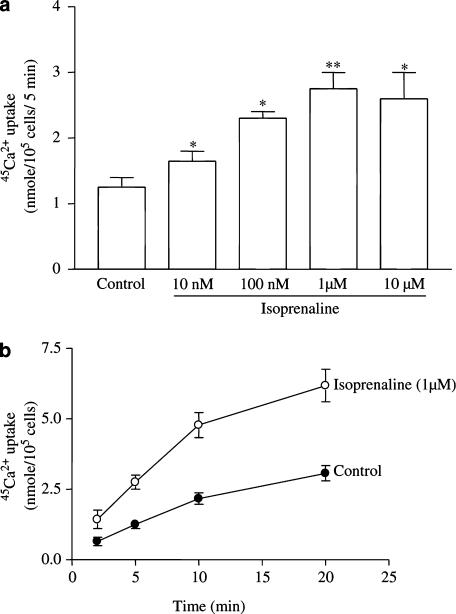
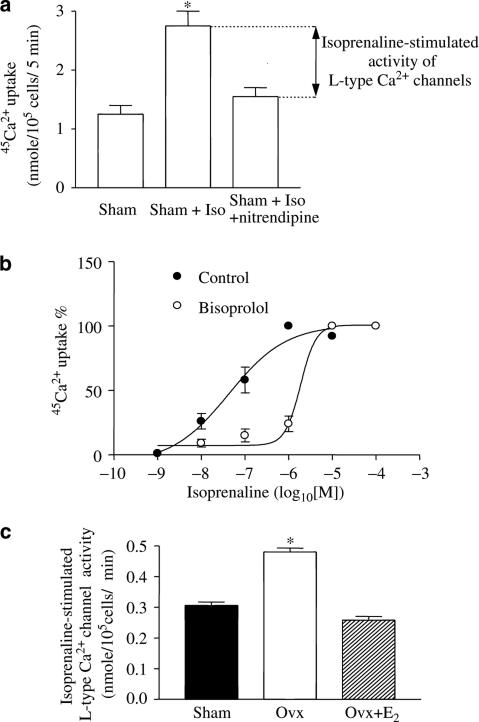
Iso increased the 45Ca2+ uptake in a concentration-dependent manner and 1 μM of the drug exerted a maximal effect (Figure 1a). Both basal and 1 μM Iso-stimulated 45Ca2+ uptake in solution A were linear from 2 to 10 min (Figure 1b). Since the linear part of the curve reflects the rate of 45Ca2+ uptake, we chose the 5 min point to evaluate the rate of 45Ca2+ uptake. In all subsequent experiments, the basal Ca2+ channel activity was defined as the difference between the basal rates of 45Ca2+ uptake in the presence and absence of 10 μM nitrendipine, a specific blocker of 45Ca2+ uptake through Ca2+ channels (Lubic et al., 1994). Iso-stimulated Ca2+ channel activity was defined as the difference between the rates of 45Ca2+ uptake in the presence of 1 μM Iso with/without 10 μM nitrendipine. A similar experimental protocol was used for determining Ca2+ channels activity and Iso-stimulated Ca2+ channel activity in myocytes from sham, Ovx, and Ovx+E2 rats.
Figure 1.
Effects of Iso on 45Ca2+ uptake in ventricular myocytes from control rats. (a) Concentration-related effects. (b) Time-dependent changes. Values are mean±s.e.m. of four independent experiments. *P<0.05 vs control. **P<0.01 vs control.
cAMP assay
cAMP content was determined as described previously (Bian et al., 2000). Briefly, 3 × 105 isolated myocytes were placed in wells to which different concentrations of forskolin were added and incubated for 10 min at 37°C. At the end of the treatment, cAMP was extracted and stored at −20°C for subsequent assay. Protein content was determined by Lowry's method (Lowry et al., 1951), and the intracellular cAMP content was measured with an assay kit (TRK 432; Amersham International, U.K.) according to the manufacturer's recommended protocol.
PKA activity
PKA activity was measured with a commercially available assay kit from Calbiochem®, using the manufacturer's recommended protocol. Briefly, left ventricular myocytes were sonicated in ice-cold extraction buffer and centrifuged at 14,000 × g for 5 min. PKA activity was measured by the incorporation of 32P from γ32P-ATP into Kemptide, the specific synthetic substrate for PKA. PKA sample (5 μl) was added to the reaction buffer and incubated at 30°C for 10 min and the reaction was stopped with 8 M guanidine hydrochloride. The mixture was transferred to a centrifugal ultrafiltration unit and washed three times with the buffer. The sample reservoir of the unit was then transferred to a scintillation vial with 10 ml of scintillation cocktail (Universal LSC cocktail for aqueous samples, Sigma). The radioactivity in the sample reservoir was measured in a scintillation counter (LS 6500, Beckman).
To study the effects of Iso on PKA activity, myocytes were incubated with 100 nM of Iso in the presence/absence of 1 μM bisoprolol for 10 min in serum-free DMEM with 0.5 mM 3-isobutyl-L-methylxanthine (IBMX) followed by sonication in ice-cold extraction buffer and centrifugation as described above. PKA activity was measured without exogenously added cAMP, using the manufacturer's recommended protocol.
To study the effect of cAMP on PKA activity, 5 μM of cAMP was added to the cellular extracts. The PKA activity was then measured according to the manufacturer's recommended protocol. The cAMP-induced increase in PKA activity was defined as the difference between the PKA activities in the presence and absence of 5 μM exogenously added cAMP.
Serum oestrogen level
Blood samples were collected from the rats after decapitation and serum E2 levels were measured using the solid-phase 125I radioimmunoassay technique (Diagnostic Research Laboratory, U.S.A.) according to the manufacturer's instructions. In addition to serum oestrogen level, the body weight/heart weight ratio was calculated as an idex of oestrogen depletion after Ovx.
Drugs and chemicals
Forskolin, type-1 collagenase, nitrendipine, anti-Gαs, anti-Gα1,2, and anti-Gα3 antibodies were from Sigma-Aldrich, St Louis U.S.A. Bisoprolol was from Tocris Cookson Ltd, Bristol, U.K. 45Ca2+ (74 MBq, 2.4 mCi ml−1) was from Amersham Pharmacia, Buckinghamshire, U.K. KT5720 was from Calbiochem, Darmstadt, Germany. HRP-conjugated anti-rabbit secondary antibody was from Santa Cruz Ltd, CA, U.S.A. The ECL detection kit was from Amersham Pharmacia. All drugs were dissolved in de-ionized H2O or Krebs solution except forskolin, which was dissolved in DMSO. The final concentration of DMSO was ⩽0.01%, which itself had no effect on the heart.
Statistical analysis
Data were expressed as mean±s.e.m. Two-way ANOVA with post hoc test was used to determine differences among multiple groups. The nonparametric Kruskal–Wallis test was used to analyse drug effects. Computer-assisted nonlinear regression analysis for sigmoidal dose–responses was used to determine EC50 (GraphPad Prism 3). A difference of P<0.05 was considered statistically significant.
Results
General features of experimental animals
At 6 weeks after Ovx, the rats had significantly reduced serum oestrogen levels accompanied by a significant increase in body weight. These changes were reversed in oestrogen-treated animals, with the exception of the heart/body weight ratio, which was significantly reduced in Ovx rats, but was not restored by oestrogen replacement (Table 1).
Table 1.
General feature of experimental animals
| Body weight (g) | Heart weight (g) | % Heart/body weight | Serum oestrogen (pg ml−1) | |
|---|---|---|---|---|
| Female (8) | 263±8.4 | 0.94±0.03 | 0.36±0.002 | 66±12.3 |
| Ovx (8) | 336±8.6* | 1.00±0.03 | 0.29±0.012* | 17±4.1* |
| Estrogen replacement (9) | 265±13.5 | 0.82±0.04 | 0.31±0.01* | 80±4.2* |
Each value represents the mean±s.e.m. The figures in parentheses indicate numbers of animals.
P<0.05 vs sham group.
Basal PKA and Ca2+ channel activity, cAMP accumulation, and expression of G-proteins in the heart from control, Ovx, and Ovx+E2 rats
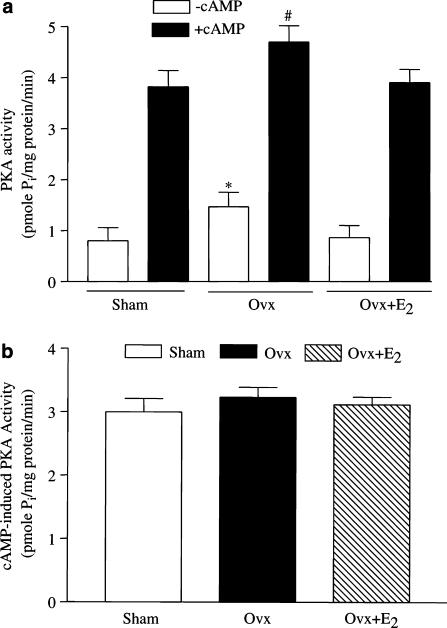
Basal PKA activity was significantly greater in myocytes from Ovx rats than in those from controls (1.47±0.28 vs 0.8±0.26 pmol Pi (mg protein)−1 min−1), and the increase was reversed by oestrogen replacement (Figure 2a). The PKA activity was markedly increased by challenge with 5 μM cAMP, in agreement with the previous observation (Pearson & Kemp, 1991). Interestingly, the cAMP-induced increase in PKA activity was the same among the three groups (Figure 2b), so the treatment did not change the responsiveness of PKA to cAMP.
Figure 2.
Enzymatic activity of PKA in ventricular myocytes from control, Ovx, and Ovx+E2 rats. (a) PKA activity. The activity of PKA was measured with/without added cAMP (5 μM) (b) cAMP-induced activity. The cAMP-induced PKA activity was defined as the difference in PKA activity with and without 5 μM cAMP. Values are expressed as mean±s.e.m. of triplicate measurements from three hearts in each group. *P<0.05 vs control group.
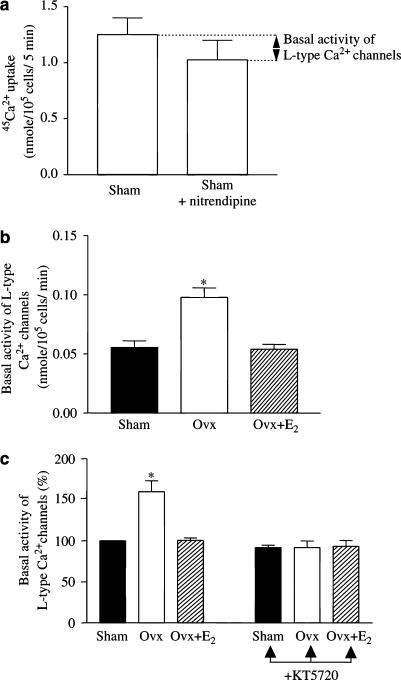
The basal Ca2+ channel activity was significantly higher in myocytes from Ovx rats than that of controls (0.11±0.0031 vs 0.069±0.0026 nmol (105 cells)−1 min−1) and the effect was reversed by oestrogen replacement (Figure 3b). Blockade of PKA with a selective inhibitor, KT5720 at 2 μM, a concentration shown in our lab to exert the maximal inhibitory effect, completely abolished the increase in basal Ca2+ channel activity in myocytes from Ovx rats without affecting the activity in myocytes from the other two groups. This indicates that a higher basal activity of PKA in myocytes from Ovx rats is responsible for the increased basal Ca2+ channel activity.
Figure 3.
Ca2+ channel activity in ventricular myocytes of control, Ovx, and Ovx+E2 rats – effect of PKA blockade. (a) 45Ca2+ uptake by ventricular myocytes from control rats. Basal Ca2+ channel activity was defined as the difference between basal 45Ca2+ uptake in the absence and presence of 10 μM nitrendipine, a selective L-type Ca2+ channel blocker. A similar experimental protocol was used for determining Ca2+ channel activity in myocytes from the other groups of rats. (b) Basal Ca2+ channel activity. (c) Effects of KT5720 (2 μM), a selective inhibitor of PKA, on basal Ca2+ channel activity (activity in myocytes from control rats expressed as 100%). Values are mean±s.e.m. of four independent experiments. *P<0.05 vs control and Ovx+E2 groups.
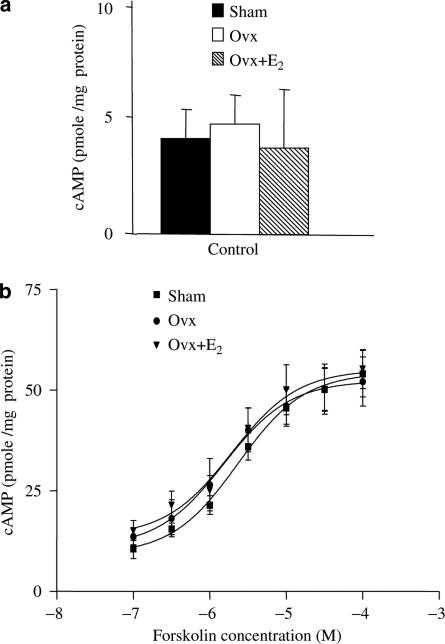
There was no difference in the basal cAMP accumulation among all three groups (Figure 4a). Nor was there any difference in their response to forskolin over the range from 10−7 to10−4 M (Figure 4b). So, the greater basal PKA activity in the hearts of Ovx rats was not due to a higher basal cellular cAMP concentration.
Figure 4.
Basal and forskolin-induced cAMP content in ventricular myocytes of control, Ovx, and Ovx+E2 rats. (a) Basal cAMP content and (b) concentration-related effect of forskolin. Cells were challenged with 100 nM to 0.1 mM forskolin for 10 min and cAMP was extracted and measured. All measurements were made in the absence of phosphodiesterase inhibitor. Values represent mean±s.e.m. of triplicate determinations from 3 hearts in each group. *P<0.05 vs corresponding value in the control group.
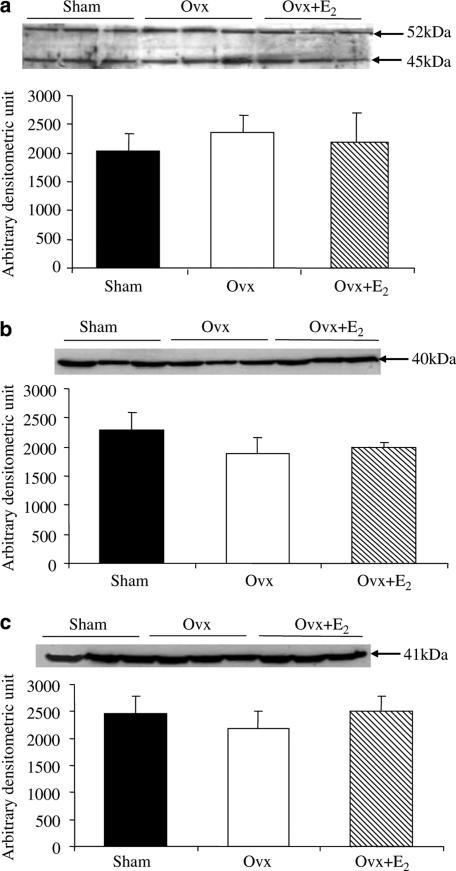
Figure 5a shows the sum of 45 and 52 kDa peptides detected by antibody to Gαs. Bands migrating at 40 and 41 kDa detected by antibodies to Gαi1−2 and Gαi3 are shown in Figure 5b and c, respectively. The amounts of Gαs, Gαi1−2, and Gαi3 proteins did not significantly differ among the three groups, so the higher basal PKA activity in the heart of Ovx rats was not due to changes in expression of G-proteins.
Figure 5.
Western blotting analysis of Gαs and Gαi1−3 in the membrane fraction of left ventricles. (a) Gαs protein, (b) Gαi1–2 protein, and (c) Gαi3 protein. Upper panels show representative Western blots for the respective proteins. Each lane represents the sample from one heart. Lower panels show quantified data. Values represent mean±s.e.m. of six independent experiments.
Effect of β-AR stimulation with Iso on PKA and Ca2+ channel activity, E[Ca2+], and contractile functions in the hearts from control, Ovx, and Ovx+E2 rats
To test the hypothesis that the female sex hormone suppresses cardiac responses to β-AR stimulation with Iso by suppressing PKA, we first determined the PKA activity following β-AR stimulation in all three groups of rats. Iso significantly increased the PKA activity in all groups and the increase was significantly greater in the hearts of Ovx than in those of sham and Ovx+E2 rats (Figure 6a). In the presence of 1 μM bisoprolol, a specific β1-AR antagonist, the PKA activity was reduced by 80%, confirming a receptor-mediated action (Figure 6b). This increased PKA activity may be responsible for the greater cardiac response to β-AR stimulation in Ovx rats. The Iso-stimulated PKA activity in Ovx rats was 1.17 pmol Pi (mg protein)−1 min−1 higher than in the control rats (Figure 6c), a much greater increase than that in basal PKA activity (0.67 pmol Pi (mg protein−1) min−1) shown in Figure 2a, so the significantly higher Iso-stimulated PKA activity in Ovx rats cannot be accounted for by the greater basal PKA activity.
Figure 6.
Effect of Iso on enzymatic activity of PKA in ventricular myocytes from control, Ovx, and Ovx+E rats. (a) Iso-stimulated PKA activity, (b) Iso-stimulated PKA activity in the presence of bisoprolol (1 μM), and (c) Iso-stimulated PKA activity. The Iso-stimulated PKA activity is defined as the difference in PKA activity with/without Iso preincubation in the absence/presence of bisoprolol, respectively. Values are expressed as mean±s.e.m. of triplicate measurements from three hearts in each group. *P<0.05 vs corresponding values without Iso. #P<0.05 vs corresponding values with Iso.
Iso-stimulated Ca2+ channel activity was concentration-dependent and antagonized by 1 μM bisoprolol (Figure 7b). This antagonist shifted the EC50 for Iso from 42.5 nM to 2.1 μM, indicating a β1-AR-mediated effect on 45Ca2+ uptake through Ca2+ channels. The Iso-stimulated Ca2+ channel activity in the heart of Ovx rats was much greater than those of the other two groups (Figure 7c). In addition, the increment was 0.22 nmol (105 cells)−1 min−1 over that of the control, a value much greater than the difference (0.041 nmol (105 cells)−1 min−1) in basal Ca2+ channel activity between Ovx and control rats (Figure 3b), showing that β-AR stimulation elicits an increase in Ca2+ channel activity that cannot be accounted for by greater basal activity.
Figure 7.
Effects of Iso on Ca2+ channel activity in ventricular myocytes from control, Ovx, and Ovx+E2 rats. (a) Effects of Iso on 45Ca2+ uptake in ventricular myocytes from control rats. The activity of the Iso-stimulated L-type Ca2+ channel was defined as the difference between Iso-stimulated 45Ca2+ uptake in the absence and presence of 10 μM nitrendipine. A similar experimental protocol was used for determining Ca2+ channel activity in myocytes from the other groups of rats. (b) Concentration-related effect of Iso on 45Ca2+ uptake in the presence and absence of 1 μM bisoprolol, a selective β1-AR antagonist. (c) Effects of Iso on Ca2+ channel activity in ventricular myocytes from control, Ovx, and Ovx+E2 rats. Values are mean±s.e.m. of four independent experiments. *P<0.05 vs control and Ovx+E2 groups.
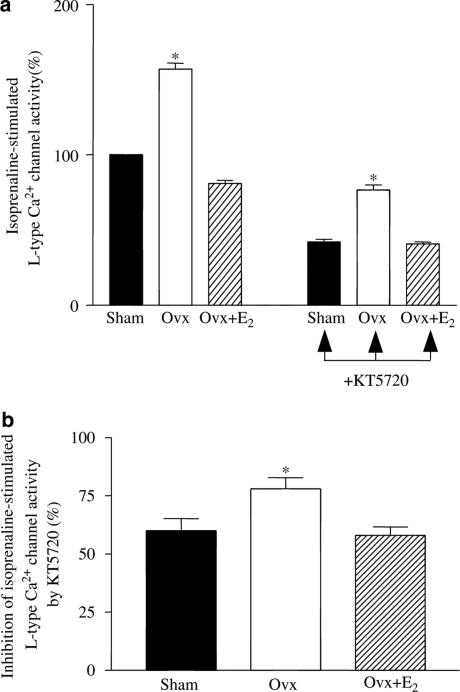
To determine whether the differences in the effects of β-AR activation on Ca2+ channel activity were PKA-dependent, we measured the effect of KT5720, which significantly reduced the Iso-stimulated 45Ca2+ influx via Ca2+ channels (Figure 8a). The degree of inhibition was significantly greater in myocytes from Ovx rats than in those from the other two groups, indicating a greater influence of PKA on Ca2+ channels (Figure 8b).
Figure 8.
Iso-stimulated L-type Ca2+ channel activity in ventricular myocytes from control, Ovx, and Ovx+E2 rats – effect of PKA blockade with KT5720. (a) Effects of Iso on Ca2+ channel activity in the absence (left) and presence (right) of KT5720 (2 μM) in ventricular myocytes from control, Ovx, and Ovx+E2 rats. The value in the myocytes from control rats was expressed as 100%. (b) Inhibition of Iso-stimulated Ca2+ channel activity by KT5720 (defined as the difference in the presence and absence of KT5720). Values are mean±s.e.m. of four independent experiments. *P<0.05 vs control and Ovx+E2 groups.
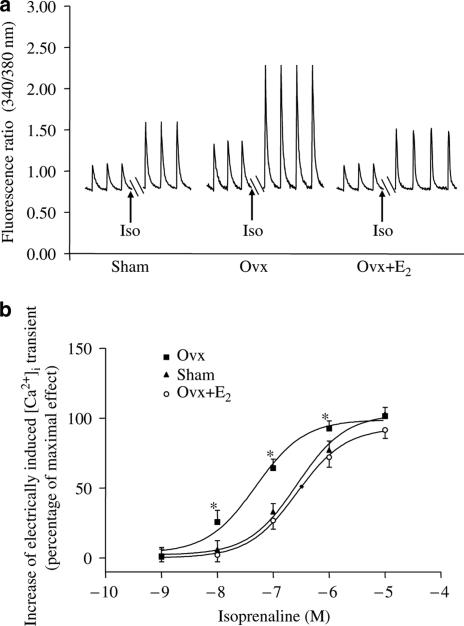
Iso (1 nM–10 μM) concentration dependently increased the E[Ca2+]i in cardiac myocytes from all groups of rats (Figure 9b). The maximum responses of myocytes from control, Ovx, and Ovx+E were 196±26, 225±31, and 187±36%, respectively. The corresponding EC50 values were 248±0.09, 51±0.16, and 267±0.05 nM. Over a concentration range from 10 nM to 1 μM Iso, the percentage increase in E[Ca2+]i was significantly higher in myocytes from Ovx rats than those from the other two groups and there was no significant difference between those from control and Ovx+E2 rats. So, β-AR stimulation in Ovx rats causes a significantly higher Ca2+ release during E–C coupling, which may in turn increase contraction.
Figure 9.
Concentration-related effects of Iso on E[Ca2+]i in ventricular myocytes from control, Ovx, and Ovx+E2 rats. (a) Representative traces showing the effect of 100 nM Iso and (b) concentration–response curve. Y-axis represents percentage of maximal effect. The effect of 10 μM Iso stimulation on E[Ca2+]i in sham rats was taken as 100. Values are mean±s.e.m. N=15–24 cells from three different rats in each group. *P<0.05 vs corresponding value in the control group.
As in the case of E[Ca2+]i, which is directly correlated to contraction (Yu et al., 1999), 10 nM of Iso significantly increased heart rate, LVDP, and ±dp/dtmax in all three groups and the increase over baseline was significantly greater in Ovx than those in sham and Ovx+E2 rats (Table 2), indicating significant higher excitation and contraction, and faster relaxation in the hearts from Ovx rats.
Table 2.
Cardiac variables in control, Ovx, and Ovx+E2 groups
| Variables | Baseline | Isoprenaline (10−8 M) | Fold-increase |
|---|---|---|---|
| LVDP (mmHg) | |||
| Con | 121±9 | 162±11 | 1.3±0.7 |
| Ovx | 181±12* | 452±23* | 2.5±0.6* |
| Ovx+E2 | 116±8 | 164±12 | 1.4±0.4 |
| LVEDP (mmHg) | |||
| Con | 10±1.4 | 11±1.6 | 1.1±0.2 |
| Ovx | 11±2.1 | 10.6±1.8 | 0.9±0.1 |
| Ovx+E2 | 10±1.8 | 12.3±1.9 | 1.2±0.3 |
| +dp/dtmax (mmHg/s) | |||
| Con | 2137±72 | 4274±83 | 2±0.2 |
| Ovx | 2991±65* | 8973±96* | 3±0.3* |
| Ovx+E2 | 2230±71 | 4727±75 | 2.1±0.3 |
| −dp/dtmax (mmHg/s) | |||
| Con | 1659±47 | 3318±76 | 2±0.2 |
| Ovx | 2654±36* | 8492±101* | 3.2±0.3* |
| Ovx+E2 | 1780±38 | 4094±65 | 2.3±0.4 |
| HR (beats/min) | |||
| Con | 221±17 | 354±12 | 1.2±0.5 |
| Ovx | 230±15 | 377±18* | 1.64±0.6* |
| Ovx+E2 | 232±12 | 362±14 | 1.2±0.5 |
Values are expressed as mean±s.e.m. (n=8 in each group).
+dp/dtmax, the velocity of contraction; −dp/dtmax, the velocity of relaxation, LVDP, left ventricular developed pressure; LVEDP, left ventricular end diastolic pressure; HR, heart rate.
P<0.05 vs Con.
Discussion
There is compelling evidence for oestrogen-mediated modulation of G-protein coupled receptors (GPCR) in the reproductive system (Roberts et al., 1977; Maggi et al., 1988; Pinto et al., 1997). A previous study from our laboratory showed that 17β-estradiol confers cardioprotection by inhibiting β1-AR expression and the accumulation of cAMP after β1-AR activation (Kam et al., 2004). In the present study, we further demonstrated that β-AR stimulation with Iso led to significantly greater increases in PKA activity, 45Ca2+ uptake through Ca2+ channels E[Ca2+]i, heart rate and contractility in the hearts of Ovx rats than in those from controls, and these responses were restored to normal by oestrogen replacement.
The most important finding of the present study was that the basal PKA activity was much higher in the hearts of Ovx rats than in controls, an effect reversed by oestrogen replacement. The increased basal PKA activity is responsible for a greater Ca2+ channel activity since blockade with its selective inhibitor, KT5720, completely abolished the effect. The higher PKA activity is not due to altered expression of G-proteins, or cAMP accumulation, or responsiveness of PKA to cAMP, as we found no change in the expression of G-proteins, or cAMP accumulation, or cAMP-induced PKA activity in the hearts from sham, Ovx, and Ovx+E2 rats. Following β-AR stimulation with Iso, PKA activity increased to a significantly greater extent in the hearts from Ovx rats than in the hearts of the other two groups of rats, and was accompanied by a significantly greater increase in Ca2+ channel activity, indicating a causal relationship. This greater increase in PKA activity cannot be accounted for by higher basal activity. Rather it is due to an increased influence from the β1-AR, which is upregulated in the heart of Ovx rats, as reflected by a greater cAMP accumulation in response to β-AR stimulation (Kam et al., 2004). So the oestrogenic environment downregulates the β1-AR and suppresses PKA activity. The suppressed PKA activity in turn reduces Ca2+ channel activity and associated cardiac functions.
In cardiomyocytes, Ca2+ flux through the Ca2+ channel triggers Ca2+ release from sarcoplasmic reticulum, leading to contraction, which is modified by sympathetic stimulation. The Ca2+ influx is potentiated by PKA-catalysed phosphorylation of the α1C and β2a subunits (Gao et al., 1997; Kapiloff, 2002). Since the basal PKA activity in the hearts of Ovx rats was significantly higher than in the other two groups, the basal conductance of Ca2+ channels would be expected to be higher. Indeed, our experiments demonstrated a 64% elevation. This increment was a function of PKA activity, since KT5720, at a concentration known to maximally inhibit it, restored the channel activity to normal in myocytes from Ovx rats. Previous studies reported that both the expression and activity of Ca2+ channels are significantly increased in ERα knockout mice (Johnson et al., 1997), and that oestrogen replacement suppresses both the expression and activity of cardiac Ca2+ channels in the Ovx rabbit (Patterson et al., 1998). We recently also observed that the number of L-type Ca2+ channels in left ventricular myocytes from Ovx rats was much higher than in those from control and Ovx+E2 rats (Kam & Wong, unpublished data). Based on these observations, we believe that both higher levels of PKA and increased density of L-type Ca2+ channels are responsible for the enhanced basal Ca2+ channel activity in the hearts of Ovx rats.
In contrast to basal Ca2+ transport, blockade of PKA with KT5720, at a concentration that completely abolished the effect of PKA on L-type Ca2+ channel activity, was unable to reduce the channel activity in Iso-treated myocytes from Ovx rats to that of myocytes from control and Ovx+E2 rats. This result would not be expected if β-AR and Ca2+ channel coupling occurred only through a PKA-dependent mechanism. There is evidence to support a mechanism independent of PKA. A recent study on CHW fibroblasts stably transfected with cardiac L-type Ca2+ channel α1 and β2 subunits along with either β1- or β2-AR revealed PKA-independent preferential coupling of the β1-AR to Ca2+ channels (Yatani et al., 1999). β1-AR may directly increase the Ca2+ channel activity in myocytes from Ovx rats without involving PKA. Furthermore, evidence supports a direct interaction of Gαs and Ca2+ channels with β-AR stimulation (Yatani & Brown, 1989).
We also found that both the basal and forskolin-stimulated cAMP accumulation was the same in all the experimental groups, so the oestrogen does not affect the accumulation of cAMP or its response to activation of AC. Since no phosphodiesterase inhibitor was used in the cAMP assay, the cellular cAMP content represents a balance between cAMP formation via AC and breakdown via PDE. The possibility that AC and PDE activities are altered by the oestrogenic environment cannot be excluded.
We found in the present study that 10−8 M Iso increased LVDP and +dp/dtmax by 2.5- and 3-fold, respectively in the hearts from Ovx rats, which were much greater than the corresponding increases in the normal female rat hearts (1.3- and 2-fold for LVDP and +dp/dtmax, respectively). The observation indicates a significantly greater increase in contractility following β1-AR stimulation in the hearts from Ovx rats. In addition to increased PKA activity shown in the present study, it also up-egulates the β1-AR in the hearts of Ovx rats (Kam et al., 2004) and increase in the Ca2+ sensitivity of myofilaments (Wattanapermpool, 1998) are also responsible for increased contractility.
β1-AR stimulation activates two signalling pathways. The first is the Gαs/AC/cAMP/PKA pathway, which leads to an increased influx of Ca2+ via the Ca2+ channel and to an increased contractility (Campbell & Strauss, 1995). The second is through a PKA-independent CaMKII-mediated apoptotic signalling pathway (Zhu et al., 2003). During myocardial ischaemia, an increased sympathetic discharge activates both pathways, leading to increased cardiac contractility and increased apoptosis. In addition to apoptosis, increased contraction at a time of insufficient oxygen may contribute to myocardial infarct. In a previous study, we showed that oestrogen confers cardioprotection against myocardial ischaemia and β-AR stimulation with Iso in vitro (Kam et al., 2004). In the present study, we found that oestrogen decreased the Iso-stimulated contraction. This suggested that it may confer cardioprotection by reducing cardiac contraction.
In conclusion, the present study provides the first evidence that oestrogen inhibits PKA activity, thus reducing Ca2+ influx via the Ca2+ channel, which is responsible for reduction in E[Ca2+]i and cardiac contraction. However, oestrogen does not alter the expression of Gαs and Gαi proteins, or cAMP accumulation, or the responsiveness of PKA to cAMP. Upon β-AR stimulation, the reduction in PKA activity in the presence of oestrogen is even greater than in the absence of the hormone, and is accompanied by greater reductions in Ca2+ channel activity and cardiac responses. So, in normal female rats, cardiac responses to β-AR stimulation are suppressed due to decreased basal PKA catalytic activity in addition to the downregulation of β1-AR shown previously (Kam et al., 2004).
Acknowledgments
We thank Dr I.C. Bruce for advice on the writing of the manuscript, Dr C.-M. Cao for advice on the measurement of cardiac variables, and Mr C.P. Mok for technical assistance. This study was supported by a grant to T.M.W. from the Research Grant Council of Hong Kong (HKU7312/01M). K.W.L.K. was supported by a Postgraduate Studentship from the University of Hong Kong and an Edward Youde Memorial Fund Scholarship.
Abbreviations
- β-AR
β-adrenoceptor
- Ca2+ channel
L-type Ca2+ channel
- cAMP
cyclic adenosine monophosphate
- E–C
excitation–contraction
- E[Ca2+]i
electrically induced intracellular Ca2+ transient
- Ica
L-type Ca2+ current
- Iso
isoprenaline
- Ovx
ovariectomy
- Ovx+E2
ovariectomy with oestrogen replacement
- PKA
protein kinase A
References
- BERS D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- BEUCKELMANN D.J., WIER W.G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J. Physiol. 1988;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIAN J.S, ZHANG W.M., PEI J.M., WONG T.M. The role of phosphodiesterase in mediating the effect of protein kinase C on cyclic AMP accumulation upon kappa-opioid receptor stimulation in the rat heart. J. Pharmacol. Exp. Ther. 2000;292:1065–1070. [PubMed] [Google Scholar]
- CAMPBELL D.L., STRAUSS H.C. Regulation of calcium channels in the heart. Adv. Second Messenger Phosphoprotein Res. 1995;30:25–88. doi: 10.1016/s1040-7952(05)80004-7. [DOI] [PubMed] [Google Scholar]
- CIRIC O., SUSIC D. Effect of isoprenaline on blood pressure and heart rate in different phases of the oestrous cycle. Endokrinologie. 1980;76:274–278. [PubMed] [Google Scholar]
- CONRATH C.E., OPTHOF T. Beta3-adrenoceptors in the heart. Cardiovasc. Res. 2003;56:353–356. doi: 10.1016/s0008-6363(02)00706-x. [DOI] [PubMed] [Google Scholar]
- GAO T., YATANI A., DELL'ACQUA M.L., SAKO H., GREEN S.A., DASCAL N., SCOTT J.D., HOSEY M.M. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- HAIKALA H., KAHEINEN P., LEVIJOKI J., LINDEN I.B. The role of cAMP- and cGMP-dependent protein kinases in the cardiac actions of the new calcium sensitizer, levosimendan. Cardiovasc. Res. 1997;34:536–546. doi: 10.1016/s0008-6363(97)00057-6. [DOI] [PubMed] [Google Scholar]
- JOHNSON B.D., ZHENG W., KORACH K.S., SCHEUER T., CATTERALL W.A., RUBANYI G.M. Increased expression of the cardiac L-type calcium channel in ooestrogen receptor-deficient mice. J. Gen. Physiol. 1997;110:135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAM K.W., QI J.S., CHEN M., WONG T.M. Oestrogen reduces cardiac injury and expression of beta1-adrenoceptor upon ischaemic insult in the rat heart. J. Pharmacol. Exp. Ther. 2004;309:8–15. doi: 10.1124/jpet.103.058339. [DOI] [PubMed] [Google Scholar]
- KAPILOFF M.S. Contributions of protein kinase A anchoring proteins to compartmentation of cAMP signaling in the heart. Mol. Pharmacol. 2002;62:193–199. doi: 10.1124/mol.62.2.193. [DOI] [PubMed] [Google Scholar]
- KASE H., IWAHASHI K., NAKANISHI S., MATSUDA Y., YAMADA K., TAKAHASHI M., MURAKATA C., SATO A., KANEKO M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- KIEHN J., KARLE C., THOMAS D., YAO X., BRACHMANN J., KUBLER W. HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J. Biol. Chem. 1998;273:25285–25291. doi: 10.1074/jbc.273.39.25285. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUBIC S.P., GIACOMINI K.M., GIACOMINI J.C. Increased 1,4-dihydropyridine binding sites in serum-stimulated cardiomyocyte hypertrophy. J. Pharmacol. Exp. Ther. 1994;270:697–701. [PubMed] [Google Scholar]
- MAGGI M., GENAZZANI A.D., GIANNINI S., TORRISI C., BALDI E., DI TOMASO M., MUNSON P.J., RODBARD D., SERIO M., DI TOMASSO M. Vasopressin and oxytocin receptors in vagina, myometrium, and oviduct of rabbits. Endocrinology. 1988;122:2970–2980. doi: 10.1210/endo-122-6-2970. [DOI] [PubMed] [Google Scholar]
- MCGAVIN J.K., KEATING G.M. Bisoprolol: a review of its use in chronic heart failure. Drugs. 2002;62:2677–2696. doi: 10.2165/00003495-200262180-00017. [DOI] [PubMed] [Google Scholar]
- NABAUER M., CALLEWAERT G., CLEEMANN L., MORAD M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- PATTERSON E., MA L., SZABO B., ROBINSON C.P., THADANI U. Ovariectomy and oestrogen-induced alterations in myocardial contractility in female rabbits: role of the L-type calcium channel. J. Pharmacol. Exp. Ther. 1998;284:586–591. [PubMed] [Google Scholar]
- PEARSON R.B., KEMP B.E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- PINTO F.M., MAGRANER J., AUSINA P., ANSELMI E., MARTIN J.D., CANDENAS M.L. Regulation by ooestrogens of tachykinin NK3 receptor expression in the rat uterus. Eur. J. Pharmacol. 1997;324:125–127. doi: 10.1016/s0014-2999(97)00150-7. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.M., INSEL P.A., GOLDFIEN R.D., GOLDFIEN A. Alpha adrenoreceptors but not beta adrenoreceptors increase in rabbit uterus with ooestrogen. Nature. 1977;270:624–625. doi: 10.1038/270624a0. [DOI] [PubMed] [Google Scholar]
- SPERELAKIS N., WAHLER G.M. Regulation of Ca2+ influx in myocardial cells by beta adrenergic receptors, cyclic nucleotides, and phosphorylation. Mol. Cell Biochem. 1998;182:19–28. doi: 10.1007/BF00242511. [DOI] [PubMed] [Google Scholar]
- THAWORNKAIWONG A., PREAWNIM S., WATTANAPERMPOOL J. Upregulation of beta 1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824. doi: 10.1016/s0024-3205(02)02473-6. [DOI] [PubMed] [Google Scholar]
- WATTANAPERMPOOL J. Increase in calcium responsiveness of cardiac myofilament activation in ovariectomized rats. Life Sci. 1998;63:955–964. doi: 10.1016/s0024-3205(98)00353-1. [DOI] [PubMed] [Google Scholar]
- WU S., LI H.Y., WONG T.M. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of kappa-opioid receptor. Circ. Res. 1999;84:1388–1395. doi: 10.1161/01.res.84.12.1388. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., ZHU W., ZHENG M., CHAKIR K., BOND R., LAKATTA E.G., CHENG H. Subtype-specific b-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol. Sci. 2004;25:358–365. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- YATANI A., BROWN A.M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989;245:71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- YATANI A., TAJIMA Y., GREEN S.A. Coupling of beta-adrenergic receptors to cardiac L-type Ca2+ channels: preferential coupling of the beta1 versus beta2 receptor subtype and evidence for PKA-independent activation of the channel. Cell Signal. 1999;11:337–342. doi: 10.1016/s0898-6568(98)00050-3. [DOI] [PubMed] [Google Scholar]
- YU X.C., DIAO T.M., PEI J.M., ZHANG W.M., WONG N.S., WONG T.M. Kappa-opioid receptor agonist inhibits the cholera toxin-sensitive G protein in the heart. J. Cardiovasc. Pharmacol. 2001;38:232–239. doi: 10.1097/00005344-200108000-00009. [DOI] [PubMed] [Google Scholar]
- YU X.C., WANG H.X., ZHANG W.M., WONG T.M. Cross-talk between cardiac kappa-opioid and beta-adrenergic receptors in developing hypertensive rats. J. Mol. Cell Cardiol. 1999;31:597–605. doi: 10.1006/jmcc.1998.0896. [DOI] [PubMed] [Google Scholar]
- ZHU W.Z., WANG S.Q., CHAKIR K., YANG D., ZHANG T., BROWN J.H., DEVIC E., KOBILKA B.K., CHENG H., XIAO R.P. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]