Abstract
Vasoactive intestinal peptide (VIP) has been demonstrated in intestinal mucosal neurones and elicits chloride secretion from enterocytes. These findings have led to the proposal that VIP is a secretomotor neurotransmitter. Confirmation of such a role may now be possible with the development of PG 97–269, a high-affinity, selective antagonist of VIP type 1 (VPAC1) receptor, which is expressed by gut epithelial cells. We have evaluated the VIP antagonism and antisecretory potential of this novel compound using in vitro and in vivo models of intestinal secretion.
Monolayers of the human colonic cell line (T84) and muscle-stripped preparations of rat jejunum and human ileum were set up in Ussing chambers for recording of transepithelial resistance and short-circuit current. Ussing chambers were modified to allow electrical stimulation of mucosal neurones. Effects of PG 97–269 on enterotoxin-induced secretion were investigated in perfused rat jejunum in vivo.
PG 97–269 competitively antagonised VIP in T84 monolayers. In rat jejunum and human ileum, responses to VIP were inhibited as were responses of rat jejunum to electrical stimulation of mucosal neurons.
In perfused rat jejunum, PG 97–269 abolished the effects of VIP on fluid and electrolyte transport and attenuated cholera toxin and Escherichia coli heat labile toxin-induced net fluid and electrolyte secretion.
PG 97–269 is a competitive antagonist of enterocyte VIP receptors and effectively inhibits responses of rat and human intestinal mucosa to VIP. Antagonism of secretory responses to electrical stimulation of mucosal neurons and lumenal application of enterotoxins imply a secretory role for VIP in these processes.
Keywords: Vasoactive intestinal peptide, VPAC1 receptor antagonist, intestinal secretion, cholera toxin, T84 cell line, human ileum, rat jejunum, enterocytes, enteric nerves
Introduction
Within the enteric nervous system (ENS), vasoactive intestinal polypeptide (VIP) is thought to act as a neurotransmitter, with a prosecretory function. Supporting evidence for such a role comes from immunohistochemical, functional and receptor binding studies. VIP was originally isolated from the porcine small intestine and later from the human and rat intestine (Said & Mutt, 1970; Dimaline & Dockray, 1978; Dimaline et al., 1984). VIP immunoreactivity has been demonstrated in neurons of both the myenteric and submucosal plexuses projecting to the epithelium and blood vessels (Bryant et al., 1976; Larsson et al., 1976; Costa et al., 1980). In addition, VIP receptors were found to be distributed throughout most layers of the gastrointestinal tract (Zimmerman et al., 1989). Initial functional evidence to support the role of VIP in intestinal secretion came from observations that intravenous infusions of VIP increased fluid accumulation in the small intestine of dogs, sheep and humans (Barbezat & Grossman, 1971; Krejs et al., 1980; Hyun et al., 1995). Also electrical field stimulation (EFS) of rabbit ileum mounted in Ussing chambers resulted in a tetrodotoxin-sensitive secretory response associated with release of VIP into the mucosal bathing solution (Gaginella et al., 1981).
Unequivocal evidence for a physiological role of VIP in intestinal secretion has not been forthcoming due to the lack of a potent and selective VIP antagonist. Several VIP antagonists have been developed and, in the main, fall into four categories: VIP analogues, VIP fragments, growth hormone-releasing factor analogues and hybrid peptides. The results of VIP antagonism studies have, however, been inconsistent and often not replicated between different models. Reddix et al. (1994) found that neural stimulation of guinea-pig colon evoked an increase in short-circuit current (Isc), which was attenuated by the VIP fragment, VIP (10–28). Yet in muscle-stripped rabbit colon, VIP (10–28) reduced secretion induced by VIP, but failed to affect electrical field-stimulated secretion in the presence of atropine (Percy et al., 1998). Furthermore, we and other workers have found VIP (10–28) to be an ineffective antagonist of VIP-induced secretion in rat jejunum and colon (Cox & Cuthbert, 1989; Burleigh & Kirkham, 1993). Discrepancies have also been found with the VIP analogue [4Cl-D-Phe6, Leu17]-VIP. Partial antagonism of VIP-induced secretion in a colonic tumour cell line (T84) has been reported (Pandol et al., 1986), although this analogue failed to antagonise VIP responses in rat jejunum and colon (Cox & Cuthbert, 1989; Schulzke et al., 1995). Neither of the growth hormone releasing factor (GRF) analogues, [AcTyr1, D-Phe2]GRF-(1–29)-NH2 and [AcTyr1]hGRF-(1–40)-OH, exhibited any competitive antagonism of VIP-induced secretion in rat jejunum; however, some noncompetitive inhibition was observed with [AcTyr1]hGRF-(1–40)-OH (Cox & Cuthbert, 1989). [AcTyr1]hGRF-(1–40)-OH exhibited no antagonism to VIP-induced secretion in rat colon (Burleigh & Kirkham, 1993).
Two VIP receptors (VPAC1 and VPAC2) have been identified in human and rat small intestine (Usdin et al., 1994; Adamou et al., 1995; Sreedharan et al., 1995). The affinity of VIP for the different VIP receptors does not differ greatly. The IC50s for rat VPAC1, VPAC2, and human VPAC1 and VPAC2 receptors were found to be 1, 8, 2 and 5 nM, respectively (Gourlet et al., 1997).
Following on from these investigations a novel compound, [Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17] VIP(3–7)/GRF(8–27) or PG 97–269, has been shown by radioligand binding studies and in vitro functional assays to be a high-affinity selective antagonist of VPAC1 receptors expressed in Chinese hamster ovary and LoVo cell membranes (Gourlet et al., 1997).
Our aims were to assess the antisecretory potential of this selective antagonist using in vitro and in vivo models of intestinal secretion. We have calculated the antagonist pKb value in a human colonic cell line and assessed the effectiveness of PG 97–269 against VIP-stimulated secretion in rat and human small intestine. As VIP has been implicated in the neural pathways involved in enterotoxin-induced secretion (Cassuto et al., 1981a; Mourad & Nassar, 2000), we investigated the effects of PG 97–269 on neurally stimulated secretion and, using a rat in vivo model, on cholera toxin (CT), Escherichia coli heat stable and heat labile toxin-induced secretion.
Methods
Cell culture
T84 cells, a human colonic epithelial cell line, were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, Wiltshire, U.K.) and used between passages 70 and 85. The methods have been described previously (Burleigh et al., 2000). In all, 1 million cells were seeded onto 12-mm internal diameter collagenised semipermeable membranes (Costar Snapwell inserts, 0.4 μM pore diameter), and after 9 or 11 days the inserts with attached confluent monolayers were used for experimentation. Maximum transepithelial resistance of T84 cell monolayers develops after 7 days (Barrett et al., 1991). In pilot experiments, confluence was assessed visually by photographing the developing monolayers at various times after ‘seeding' the Snapwell inserts. In all monolayers, confluence was monitored indirectly by transepithelial resistance measurements, and a resistance cutoff was not used.
In vitro preparations of rat and human intestine
Adult male Wistar rats (300–400 g, 12–14-weeks old, Charles River, U.K.) were killed by cervical dislocation and a section of mid-jejunum was removed and immediately bathed in warmed Kreb's physiological saline. Human terminal ileum (taken within 20 cm of the ileocaecal junction) was obtained from right hemicolectomy resections carried out for colon cancer and approval was obtained from the East London & City NHS Health Authority (T/1/11), Redbridge & Waltham Forest Health Authority (190) and Riverside Research Ethics Committee (RREC 2914). The muscle layers were peeled away by blunt dissection with the aid of a dissecting microscope. All experiments with muscle-stripped intestine were carried out in the presence of indomethacin (10−5 M). This reduced endogenous prostaglandin formation and provided a more stable level of basal Isc.
Isc measurements
T84 monolayers on 12 mm internal diameter Snapwell inserts or preparations of muscle-stripped intestinal tissue were placed into modified Ussing chambers for continuous recording of Isc as an indirect measurement of net chloride ion secretion. Monolayers were bathed on both sides with 5 ml of circulating, gassed Kreb's buffer kept at 37°C and gassed with a mixture of 5% CO2 in oxygen. Electrical measurements of Isc and transepithelial resistance were carried out as described previously (Burleigh et al., 2000). All drugs, in a maximum volume of 100 μl, were applied to the basolateral domain of monolayers unless indicated otherwise. No monolayer received more than one concentration of a given compound. Intestinal neurons were stimulated electrically by passing a current across muscle-stripped tissue sheets of rat and human small intestine set up in Ussing chambers. EFS of mucosal nerves was achieved by the addition of aluminium foil electrodes positioned diagonally on opposite sides of the tissue (Cooke, 1994). A rectangular unipolar direct current (120 pulses at 1 ms pulse width and 30 mA strength) was passed using a Grass S88 stimulator delivering pulses at 1 and 10 Hz frequencies. Peak increases in Isc evoked by EFS were measured and compared to baseline Isc measured immediately prior to EFS.
Model of in vivo intestinal secretion
We used a validated model of in vivo enterotoxin-induced secretion which has been previously described by our laboratory (Turvill et al., 1998). Experiments were carried out under a Home Office animal licence (PPL 70/5277). Male Wistar rats (180–220 g, 9–11weeks old, Tuck and Sons Ltd, Essex, U.K.) were fasted for 18 h with free access to water. Rats were anaesthetised with an intraperitoneal injection of sodium pentobarbitone (60 mg kg−1) while in the cage to reduce any stresses, and maintained throughout the experiments by interval intraperitoneal injections. All animals had a tracheostomy and were kept warm by heater pads at 37°C. The abdomen was opened by a midline incision and the small intestine mobilised. A perfusion cannula was placed in the jejunum 5 cm distal to the ligament of Treitz and secured by ligation. A further wide bore cannula was then secured in the distal jejunum creating a 20 cm intestinal loop. The intestine was flushed with 2 ml of 0.9% saline followed by air to expel remaining fluid. It was then returned to the abdomen, which was closed with a single suture. Bilateral femoral vein cannulae were placed for infusion of drugs. Net movement in fluid and electrolyte transport was then measured by in situ intestinal perfusion with a plasma electrolyte solution (PES) containing Na 140 mmol l−1, K 4 mmol l−1, Cl 104 mmol l−1, and HCO3 40 mmol l−1 to which 5 g/l of cold polyethylene glycol 4000 (PEG) and 4 μCi l−1 of [14C]-PEG were added as a nonabsorbable marker. The perfusate was infused at a rate of 0.5 ml min−1. After a 30 min period to establish steady state, perfusate was collected consecutively for three 20 min periods. Steady-state conditions in all experiments were demonstrated by less than 5% variation in fluid movement between consecutive collections. Furthermore, values were only accepted if recovery of radioactive PEG was 95–105%.
Enterotoxin-induced secretion was established by placing 50 μg of CT or E. coli heat labile toxin (LT), each in 2 ml of normal saline in the jejunal loop and incubating for 120 min, before PES perfusion was started. Intravenous infusion of VIP antagonists at 2 μg kg−1 min−1 or 0.9% NaCl was started directly after enterotoxins were instilled into the loops. [4Cl-D-Phe6, Leu17]-VIP only tested against CT.
In experiments, with E. coli heat stable toxin (STa), the prepared jejunum was perfused without the 2 h incubation used for CT and LT, with PES containing [14C]-PEG to which 200 μg l−1 of STa (equivalent to 50,000 mouse units) was added. After 30 min of perfusion to establish steady state, three 20 min consecutive collections of the effluent were obtained. Intravenous infusion of PG 97–269 or 0.9% NaCl was started 30 min before jejunal perfusion.
In a separate set of experiments, we studied the effects of the VIP antagonists on VIP-induced changes in fluid and electrolyte movement. PG 97–269 or [4Cl-D-Phe6, Leu17]-VIP (2 μg kg−1 min−1) were infused into one femoral vein and 30 min later VIP (0.02 μg kg−1 min−1) was infused into the second femoral vein. Jejunal perfusion was started 60 min after VIP infusion.
Analytical methods
[14C]-PEG concentrations in the effluents were measured by liquid scintillation spectroscopy in a Packard 2200CA TRI-CARB liquid scintillation analyser. Samples were mixed in a 1 : 10 ratio with Ultima Gold XR scintillation fluid (Packard, U.K.). The coefficient of variation of 12 samples from the same solution was 2.44%. All samples were measured in triplicate. Chloride concentrations were analysed using a Corning 945 chloride meter (Halstead, Essex, U.K.). The concentrations of sodium ions were measured in an IL 943 flame photometer (Instrumentation Laboratories, Warrington, U.K.). Positive values denote net absorption of fluid and electroytes and are expressed as microlitres per minute per gram and micromoles per minute per gram dry intestinal weight, respectively.
Statistics and Schild analysis
In vitro data are expressed as mean±s.e.m. or mean±95% confidence limits. In vivo data are expressed as median and interquartile ranges. Statistical significance was evaluated by the nonparametric two-tailed Mann–Whitney U-test. A Schild plot was constructed as follows: agonist concentration-ratios (CR) were obtained from individual EC50 values with and without antagonist present. The plot of log (CR-1) versus log antagonist molar concentration was analysed by linear regression. Antagonism was considered to be competitive in nature if the slope of the regression line was not significantly different from unity. In this case, the antagonist affinity was expressed as the pKb value (negative log of the antagonist dissociation constant) and, assuming a slope of unity, was calculated as the mean of the individual values obtained with the equation: pKb=log (CR-1)−log (molar antagonist concentration) (Jenkinson, 1991).
Materials
CT was obtained from the Swiss Serum and Vaccine Institute, Berne, Switzerland. 14C-PEG 4000 was obtained from Amersham Pharmacia Biotech, U.K. LT and STa toxins and VIP were all obtained from Sigma Chemical Co., Poole, U.K. The selective VIP1-receptor antagonist, [Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17] VIP(3–7)/GRF(8–27) or PG 97–269, was synthesised in the laboratories of Professor P. Robberecht of Universite Libre De Bruxelles, Bruxelles, Belgium. Snapwell cell monolayer supports were obtained from Costar U.K. Ltd, High Wycombe, Bucks, U.K.
Results
Basal Isc and transepithelial resistance values were: 1.6±0.4 μA cm−2 and 718±79 Ω cm2 for T84 monolayers (n=60), 17.6±3.2 μA cm−2 and 97.0±4.8 Ω cm2 for rat jejunum (n=18) and 26.2±7.1 μA cm−2 and 186±42 Ω cm2 for human ileum (n=9).
Evaluation of the potency of the VIP antagonist PG 97–269 using T84 cell monolayers
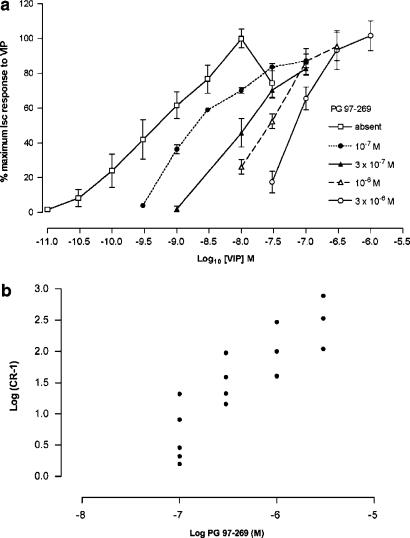
Owing to prolonged recovery times and marked desensitisation, individual monolayers were only exposed to a single concentration of VIP. Responses to VIP were expressed as a % of the maximum response, obtained in the absence of antagonist, and EC50 values were obtained graphically from concentration–response curves. VIP (3 × 10−11 to 3 × 10−7 M) increased Isc giving a maximum response of 54±3 μA cm−2 at 10−8 M and an EC50 value of 6.9±2.4 × 10−10 M (n=5). PG 97–269 (10−7 to 3 × 10−6 M) given 30 min before VIP, caused a parallel, rightward shift in concentration–response curves to VIP without any depression of the maximum response (Figure 1a). Schild analysis of the antagonism produced by PG 97–269 yielded a Schild plot with a slope of 1.22 (95% confidence limits 0.82–1.62, Figure 1b) and a calculated pKb value of 7.88±0.10.
Figure 1.
(a) Log concentration–response curves for VIP in T84 cells in the absence (n=5) or presence of PG 97–269 at a concentration of 10−7 M (n=5); 3 × 10−7 M (n=4); 10−6 M (n=4); and 3 × 10−6 M (n=3). Data are expressed as % of the maximum short circuit current (Isc) response to VIP and are given as mean±s.e.m. (b) Schild plot for antagonism of VIP-induced increases of Isc by PG 97–269. Slope was 1.22 (with 95% confidence limits of 0.82–1.62). pKb was calculated as 7.88±0.10.
Effects of VIP antagonism by PG 97–269 on muscle-stripped preparations of rat jejunum and human ileum
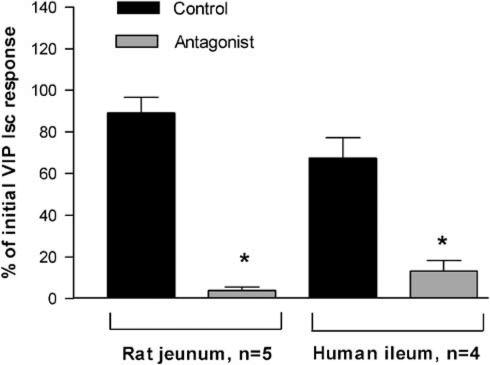
Cumulative concentration–response curves were obtained for VIP (3 × 10−9 to 1 × 10−6 M) on both preparations. Cumulative dosing is advantageous when recovery from agonist responses is prolonged as is the case with VIP on rat and human mucosal preparations. Cumulative dosing was only used to obtain an estimate of the concentration of VIP required to produce a submaximal response. There was a marked increase in response to each subsequent cumulative addition of VIP that produced a submaximal rsponse. For rat jejunum, a maximum response of 63.8±9.3 μA cm−2 and an EC50 value of 1.7±0.5 × 10−8 M (n=4) was obtained. For human ileum, the equivalent values were 110±12 μA cm−2 and 1.1±0.4 × 10−8 M (n=3). Submaximal responses to a single concentration of VIP (10−8 M) were obtained in individual preparations, one before and one 30 min after, administering PG 97–269 (1.25 × 10−5 M) or control vehicle (H2O). In both rat jejunum and human ileum, responses to VIP were considerably reduced when compared to control (P<0.05, Figure 2).
Figure 2.
Effects of the VIP antagonist, PG 97–269 on responses of rat jejunal and human ileal mucosa to VIP. Initial short-circuit current (Isc) response to VIP (10−8 M) obtained in the absence of antagonist. Second VIP response obtained in the absence (control, H2O) or presence of PG 97–269 (1.25 × 10−5 M) and expressed as % of initial response. Data given as mean±s.e.m., *P<0.05 compared to aqueous controls.
Rat jejunum responses to VIP (10−8 M) were not reduced in the presence of tetrodotoxin (TTX 10−6 M, n=4, P>0.05). In the presence of TTX (10−6 M), submaximal responses of rat jejunum to PGE2 (10−7 M) were not affected by PG 97–269 (1.25 × 10−5 M; 127±13 to 115±10 μA cm−2, n=4, P>0.05).
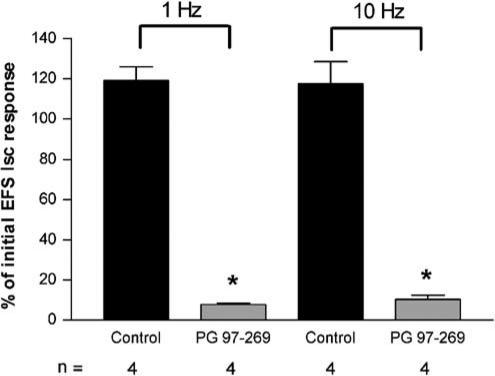
The effects of PG 97–269 on responses of rat jejunum to EFS of mucosal nerves were also assessed. Preliminary experiments were also performed with human ileum. For rat jejunum, responses to EFS at both 1 and 10 Hz were obtained. Either PG 97–269 (1.25 × 10−5 M) or H2O was then added 30 min before repeating EFS. In the presence of PG 97–269, responses to EFS were greatly reduced when compared to control (P<0.05, Figure 3). Responses to EFS (10 Hz) were abolished in the presence of TTX (10−6 M, n=5). Preliminary data obtained using human ileum suggested an inhibitory effect of PG 97–269 on EFS. In the presence of the antagonist (2.5 × 10−5 M), responses to 1 Hz stimulation were reduced by 64% compared to an 11% decrease in aqueous controls (n=2). PG 97–269 had no effect on basal Isc or transepithelial resistance of T84 cells and native tissue preparations.
Figure 3.
Effects of PG 97–269 on responses of rat jejunal mucosa to electrical field stimulation (EFS). Initial Isc responses to EFS (1 and 10 Hz, 120 pulses at 1 ms pulse width and 30 mA current strength) obtained in the absence of antagonist. Second response to EFS obtained in absence (control) or presence of PG 97–269 (1.25 × 10−5 M). Data expressed as mean±s.e.m. (n=4), *P<0.05 compared to aqueous controls.
Effect of VIP antagonism on rat jejunal fluid and electrolyte secretion induced by cholera and E. coli enterotoxins and VIP
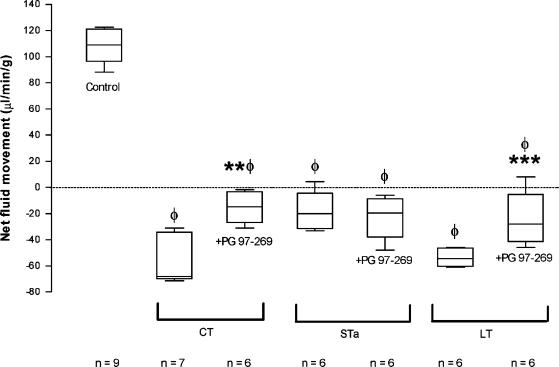
PG 97–269 (2 μg kg−1 min−1) attenuated CT- and LT-induced net fluid and electrolyte secretion but had no effect on STa-induced secretion (Figure 4, Table 1). PG 97–269 (2 μg kg−1 min−1) abolished the effects of VIP (0.02 μg kg−1 min−1) on fluid and electrolyte transport. By itself, PG 97–269 did not change basal levels of fluid and electrolyte transport (Table 1). [4Cl-D-Phe6, Leu17]-VIP infused at 2μg kg−1 min−1 did not influence the effects of VIP and CT on fluid and electrolyte transport (Table 1).
Figure 4.
Effects of PG 97–269 on CT-, LT- and STa-induced fluid secretion in rat jejunum. PG 97–269 was infused intravenously at 2 μg kg−1 min−1. Results are expressed as median (5th, 25th, 75th and 95th centiles). Positive values denote absorption and negative values denote secretion. ΦP<0.05 compared to controls; **P<0.05 compared to CT; ***P<0.05 compared to LT.
Table 1.
Effect of VIP antagonism with PG 97–269 and [4-Cl-D-Phe6, Leu17]-VIP (Phe6, Leu17) on CT, STa, LT and VIP-induced changes in net sodium and chloride flux
| n | Net fluid movement (μl min−1 g−1) | Net chloride movement (μmol min−1 g−1) | Net sodium movement (μmol min−1 g−1) | |
|---|---|---|---|---|
| Control | 9 | 109 (96 to 121) | 11 (9.9 to 12) | 12 (12 to 17) |
| PG 97–269 | 6 | 100.3 (89 to 105.7) | 10.2 (9 to 11.6) | 12 (10.3 to 13.7) |
| Phe6, Leu17 | 6 | 75.3 (41.5 to 94.1) | 7.5 (6 to 9) | 8 (3 to 11.5) |
| VIP | 11 | 82 (80 to 89.5)* | 8 (8 to 8.5)* | 11 (9 to 12)* |
| +PG 97–269 | 6 | 100.3 (89.4 to 109.7)** | 10.5 (8.9 to 11.5)** | 11.75 (10.2 to 13.8) |
| +Phe6, Leu17 | 6 | 63 (49 to 86) | 7 (4.5 to 8.8) | 6.5 (4 to 9) |
| CT | 7 | −68.1 (−34 to −70)* | −9 (−10.5 to −7)* | −13 (−14 to −9.5)* |
| +PG 97–269 | 6 | −14.7 (−3.3 to −27)*** | −1 (−2 to −1)*** | −4.35 (−5.7 to −2.6)*** |
| +Phe6, Leu17 | 6 | −35.5 (−13 to −66.8) | −7.5 (−10 to −5.5) | −10 (−13.5 to −6) |
| STa | 6 | −20 (−4.3 to −31.5)* | −2.3 (−5 to −7.5)* | −3.3 (−3.1 to −3.7)* |
| +PG 97–269 | 6 | −19.5 (−8.7 to −38) | −2.2 (−3.5 to −0.6) | −7 (−5.6 to −8.4) |
| LT | 6 | −54.4 (−46.5 to −50.5)* | −7 (−6.2 to −9)* | ND |
| +PG 97–269 | 6 | −28 (−5.5 to −41.4)**** | −3.55 (−2 to −4.9)**** | ND |
VIP was infused at 0.02 μg kg−1 min−1 and antagonists were infused at 2 μg kg−1 min−1. Data expressed as median (interquartile range). Positive values denote absorption and negative values denote secretion.
P<0.05 compared to controls;
P<0.05 compared to VIP 0.02;
P<0.05 compared to CT;
P<0.05 compared to LT.
ND, not done.
Discussion
The novel VIP receptor ligand, [Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17] VIP(3–7)/GRF(8–27) or PG 97–269, is a selective, high-affinity antagonist of VPAC1 receptors. Concentrations required for half-maximal inhibition of 125I-VIP binding on membranes from cells expressing rat VPAC1 and VPAC2 receptors and human VPAC1 and VPAC2 receptors were 10, 2000, 2 and 3000 nM, respectively, demonstrating selectivity for VPAC1 receptors. PG 97–269 also showed high-affinity competitive inhibition of VPAC1 receptor-mediated stimulation of adenylate cyclase. Inhibition constants (Ki) of PG 97–269 in Lo-Vo cells expressing human VPAC1 and CHO cells expressing rat VPAC1 receptors were 2 nM and 15 nM, respectively (Gourlet et al., 1997). In T84 cells, a pKb value of 7.88 was calculated for antagonism of secretory responses to VIP by PG 97–269. Assuming that the negative log of the Ki value theoretically corresponds to pKb (Jenkinson, 1991), a pKb value of 7.88 would be equivalent to a Ki value of 13 nM. Given variations in experimental conditions and a 1000-fold difference in affinity of the antagonist for VPAC1 compared to VPAC2 receptors, this is consistent with an action on VPAC1 receptors.
Clarification of the functional role played by VIP in intestinal secretion requires the availability of a high-affinity, selective antagonist of epithelial VIP receptors. PG 97–269 is more potent than other available VIP antagonists and selectivity of action is implied in the competitive nature of its antagonism in T84 cells.
In both rat and human native tissue preparations, VIP was less potent than in T84 cells. Such a discrepancy could not be explained by activation of different populations of VIP receptors as competition with 125I-VIP binding by VIP for rat VPAC1 or VPAC2 and human VPAC1 or VPAC2 receptors yielded IC50 values of 1, 8, 2 and 5 nM, respectively. Confirmation of an action on epithelial cells by VIP in native tissue preparations was demonstrated by the insensitivity of VIP responses to TTX. A possible explanation of reduced sensitivity to VIP is lower bioavailability at the receptor resulting from metabolism by endopeptidases (Ikezaki et al., 1998) and possibly diffusional barriers posed by the submucosa. Rapid degradation of VIP has been shown to occur when the peptide was added to the serosal domain of muscle-stripped rat colon set up in Ussing chambers (Schulzke et al., 1995). Alternatively, T84 cells and native epithelial tissue preparations may differ from each other in terms of number of VIP receptors per cell or the number of secretory, VIP receptor expressing cells per square centimetre.
PG 97–269 is an effective antagonist of secretory responses to VIP in both rat and human native tissue. However, the concentrations of antagonist required were greater than for T84 cells. This may be due to reduced bioavailability of the antagonist, as described above for VIP, as PG 97–269 is a hybrid VIP analogue. An alternative or additional explanation may be the existence of basic differences between T84 and native epithelial cells as discussed above. As PG 97–269 (1.25 × 10−5 M) had no effect on responses of rat jejunum to prostaglandin E2 (PGE2), it is unlikely that this higher concentration is exerting nonselective antisecretory effects. PGE2 like VIP induces secretion through activation of adenylate cyclase via G protein-coupled membrane receptors.
Availability of a potent and selective VIP antagonist, acting on epithelial VIP receptors, provided an opportunity to assess the role of VIP as a secretomotor neurotransmitter at the neuro-epithelial junction. The two main candidates for such a function are VIP and ACh (Goyal & Hirano, 1996). In rat jejunum at low and high frequencies of stimulation, VIP appears to play the dominant role in mediating responses, judging by the degree of antagonism to EFS by PG 97–269. However, caution should be exercised in excluding ACh. Small but significant reductions in responses to EFS were obtained with hyoscine (unpublished data). Moreover, synergistic interaction between secretagogues acting through different second messenger systems have been described (Cartwright et al., 1985). Thus, selective antagonism of VIP receptors in preventing elevation of cAMP would also diminish responses to released ACh, which acts through elevation of intracellular calcium. Preliminary data obtained using human ileum suggest that VIP also acts as a secretomotor neurotransmitter in this tissue. Originating with the work of Cassuto et al. (1979; 1982), there is increasing evidence that enterotoxins stimulate intramural secretomotor neurons (Cassuto et al., 1981b; Jodal et al., 1993; Turvill et al., 1998; 2000) in addition to their direct actions on epithelial cells. As PG 97–269 could inhibit responses to EFS of secretomotor neurons in vitro, we investigated the actions of the antagonist on responses to enterotoxins in vivo using an established model of enterotoxin-induced intestinal secretion (Turvill et al., 2000). PG 97–269 reduced the secretory actions of CT and LT, but not STa, in rat jejunum. Thus, neuronal release of VIP and its subsequent effects on fluid and electrolyte transport probably contribute to the secretory actions of CT and LT. In contrast, STa stimulation of secretomotor reflexes does not appear to involve VIP-ergic neurons. It is possible that STa-induced secretion is, in part, mediated through cholinergic secretomotor neurons. This is supported by Eklund et al. (1985), who have shown that atropine could reduce STa-induced secretion in an animal model. Furthermore, the lack of effect of PG 97–269 on STa-induced secretion suggests that nonspecific effects of the antagonist on secretion are unlikely.
We could not reproduce previous findings showing an in vivo antisecretory effect for [4Cl-D-Phe6, Leu17]-VIP. The discrepancy is difficult to explain as both investigations used similar protocols. Apart from reducing secretory action of CT, LT and STa there was also a dose-dependent inhibition of the actions of VIP (Mourad & Nassar, 2000). Assuming that no metabolism of the antagonist occurred over the 3 h period of the experiment and that the antagonist remained in the central plasma compartment (i.e. the smallest volume of distribution possible), then significant VIP antagonism was obtained at concentrations less than 1 μM of [4Cl-D-Phe6, Leu17]-VIP. This contrasts with in vitro studies where a concentration of 30 μM was required to produce partial inhibition of VIP effects in T84 cells (Pandol et al., 1986).
Although conceding that some in vitro studies failed to show VIP antagonists, including [4Cl-D-Phe6, Leu17]-VIP, as inhibitors of VIP, it was claimed that two in vivo investigations showed [4Cl-D-Phe6, Leu17]-VIP to antagonise or reverse secretory effects of VIP in the intestine (Mourad & Nassar, 2000). However, one of the studies was not carried out in vivo and more importantly, the authors could not demonstrate VIP antagonism using [4Cl-D-Phe6, Leu17]-VIP, from which they concluded that [4Cl-D-Phe6, Leu17]-VIP did not yield direct evidence for a role of VIP as a neurotransmitter in their system (Schulzke et al., 1995).
In conclusion, we have shown PG 97–269 to be a high-affinity, competitive antagonist of VPAC1 receptors in the T84 human epithelial cell line, with a pKb value of 7.88 against VIP-induced increases in Isc. PG 97–269 effectively inhibited secretory responses of rat jejunum and human ileum to VIP without affecting responses of rat jejunum to PGE2. Inhibitory actions of PG 97–269 on responses to mucosal nerve stimulation and enterotoxins support a role for VIP as a secretomotor neurotransmitter in rat and human intestine as well as its involvement in pathogenesis of CT- and LT-induced intestinal secretion.
Acknowledgments
We thank Professor Henry Binder for a helpful review of the manuscript. We are grateful to the Special Trustees of St Bartholomew's Hospital and The Stanley Thomas Johnson Foundation for financial support. We are also grateful to Ms Karin Fernandes for expert technical assistance with cell culture.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- CT
cholera toxin
- EFS
electrical field stimulation
- ENS
enteric nervous system
- Isc
short-circuit current
- LT
Escherichia heat labile toxin
- LoVo
human colon adenocarcinoma cell line
- PEG
polyethylene glycol
- PES
plasma electrolyte solution
- PG 97–269
a VPAC1 antagonist [Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17] VIP(3–7)/GRF(8–27)
- PGE2
prostaglandin E2
- STa
Escherichia coli heat stable toxin
- T84
human colon carcinoma cell line
- VIP
vasoactive intestinal peptide
References
- ADAMOU J.E., AIYAR N., VAN HORN S., ELSHOURBAGY N.A. Cloning and functional characterization of the human vasoactive intestinal peptide (VIP)-2 receptor. Biochem. Biophys. Res. Commun. 1995;209:385–392. doi: 10.1006/bbrc.1995.1515. [DOI] [PubMed] [Google Scholar]
- BARBEZAT G.O., GROSSMAN M.I. Intestinal secretion: stimulation by peptides. Science. 1971;174:422–424. doi: 10.1126/science.174.4007.422. [DOI] [PubMed] [Google Scholar]
- BARRETT K.E., BURLEIGH D.E., DHARMSATHAPHORN K. Lidamidine does not reverse T84 human colonic epithelial cell line short circuit current responses to vasoactive intestinal peptide. Br. J. Pharmacol. 1991;102:159. [Google Scholar]
- BRYANT M.G., POLAK M.M., MODLIN I., BLOOM S.R., ALBUQUERQUE R.H., PEARSE A.G. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet. 1976;1:991–993. doi: 10.1016/s0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- BURLEIGH D.E., FERNANDES K., PERRETT D. T(84) epithelial cells respond to 5-hydroxytryptamine when grown in serum-free media. Eur. J. Pharmacol. 2000;390:103–106. doi: 10.1016/s0014-2999(00)00013-3. [DOI] [PubMed] [Google Scholar]
- BURLEIGH D.E., KIRKHAM S.E. Lack of effect of three putative vasoactive intestinal peptide receptor antagonists on vasoactive intestinal peptide-induced secretory responses in rat colon. Eur. J. Pharmacol. 1993;249:239–242. doi: 10.1016/0014-2999(93)90439-o. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT C.A., MCROBERTS J.A., MANDEL K.G., DHARMSATHAPHORN K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J. Clin. Invest. 1985;76:1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSUTO J., FAHRENKRUG J., JODAL M., TUTTLE R., LUNDGREN O. Release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut. 1981a;22:958–963. doi: 10.1136/gut.22.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSUTO J., JODAL M., LUNDGREN O. The effect of nicotinic and muscarinic receptor blockade on cholera toxin induced intestinal secretion in rats and cats. Acta Physiol. Scand. 1982;114:573–577. doi: 10.1111/j.1748-1716.1982.tb07026.x. [DOI] [PubMed] [Google Scholar]
- CASSUTO J., JODAL M., SJOVALL H., LUNDGREN O. Nervous control of intestinal secretion. Clin. Res. Rev. 1981b;1:11–21. [Google Scholar]
- CASSUTO J.F., JODAL M.F., TUTTLE R.F., LUNDGREN O. The effect of lidocaine on the secretion induced by cholera toxin in the cat small intestine. Experientia. 1979;35:1467–1468. doi: 10.1007/BF01962788. [DOI] [PubMed] [Google Scholar]
- COOKE H.J.Neural and humoral regulation of small intestinal transport Physiology of the Gastrointestinal Tract 1994New York: Raven Press; 1307–1350.ed. Johnson L.R., pp [Google Scholar]
- COSTA M., FURNESS J.B., BUFFA R., SAID S.I. Distribution of enteric nerve cell bodies and axons showing immunoreactivity for vasoactive intestinal polypeptide in the guinea-pig intestine. Neuroscience. 1980;5:587–596. doi: 10.1016/0306-4522(80)90056-1. [DOI] [PubMed] [Google Scholar]
- COX H.M., CUTHBERT A.W. Secretory actions of vasoactive intestinal polypeptide, peptide histidine isoleucine and helodermin in rat small intestine: the effects of putative VIP antagonists upon VIP-induced ion secretion. Regul. Pept. 1989;26:127–135. doi: 10.1016/0167-0115(89)90004-9. [DOI] [PubMed] [Google Scholar]
- DIMALINE R., DOCKRAY G.J. Multiple immunoreactive forms of vasoactive intestinal peptide in human colonic mucosa. Gastroenterology. 1978;75:387–392. [PubMed] [Google Scholar]
- DIMALINE R., REEVE J.R., JR, SHIVELY J.E., HAWKE D. Isolation and characterization of rat vasoactive intestinal peptide. Peptides. 1984;5:183–187. doi: 10.1016/0196-9781(84)90204-3. [DOI] [PubMed] [Google Scholar]
- EKLUND S., JODAL M., LUNDGREN O. The enteric nervous system participates in the secretory response to the heat stable enterotoxins of Escherichia coli in rats and cats. Neuroscience. 1985;14:673–681. doi: 10.1016/0306-4522(85)90318-5. [DOI] [PubMed] [Google Scholar]
- GAGINELLA T.S., O'DORISIO T.M., HUBEL K.A. Release of vasoactive intestinal polypeptide by electrical field stimulation of rabbit ileum. Regul. Pept. 1981;2:165–174. doi: 10.1016/0167-0115(81)90010-0. [DOI] [PubMed] [Google Scholar]
- GOURLET P., DE NEEF P., CNUDDE J., WAELBROECK M., ROBBERECHT P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- GOYAL R.K., HIRANO I. The enteric nervous system. N. Engl. J. Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- HYUN H.S., ONAGA T., MINEO H., KATO S. Effect of vasoactive intestinal polypeptide (VIP) on the net movement of electrolytes and water and glucose absorption in the jejunal loop of sheep. J. Vet. Med. Sci. 1995;57:865–869. doi: 10.1292/jvms.57.865. [DOI] [PubMed] [Google Scholar]
- IKEZAKI H., PAUL S., ALKAN-ONYUKSEL H., PATEL M., GAO X.P., RUBINSTEIN I. Vasodilation elicited by liposomal VIP is unimpeded by anti-VIP antibody in hamster cheek pouch. Am. J. Physiol. 1998;275:R56–R62. doi: 10.1152/ajpregu.1998.275.1.R56. [DOI] [PubMed] [Google Scholar]
- JENKINSON J.H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol. Sci. 1991;12:53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- JODAL M., HOLMGREN S., LUNDGREN O., SJOQVIST A. Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology. 1993;105:1286–1293. doi: 10.1016/0016-5085(93)90130-5. [DOI] [PubMed] [Google Scholar]
- KREJS G.J., FORDTRAN J.S., FAHRENKRUG J., SCHAFFALITZKY DE MUCKADELL O.B., FISCHER J.E., HUMPHREY C.S., O'DORISIO T.M., SAID S.I., WALSH J.H., SHULKES A.A. Effect of VIP infusion in water and ion transport in the human jejunum. Gastroenterology. 1980;78:722–727. [PubMed] [Google Scholar]
- LARSSON L.I., FAHRENKRUG J., SCHAFFALITZKY D.M., SUNDLER F., HAKANSON R., REHFELD J.R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc. Natl. Acad. Sci. U.S.A. 1976;73:3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOURAD F.H., NASSAR C.F. Effect of vasoactive intestinal polypeptide (VIP) antagonism on rat jejunal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut. 2000;47:382–386. doi: 10.1136/gut.47.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDOL S.J., DHARMSATHAPHORN K., SCHOEFFIELD M.S., VALE W., RIVIER J. Vasoactive intestinal peptide receptor antagonist [4Cl-D-Phe6, Leu17] VIP. Am. J. Physiol. 1986;250:G553–G557. doi: 10.1152/ajpgi.1986.250.4.G553. [DOI] [PubMed] [Google Scholar]
- PERCY W.H., MERKWAN C.L., APPLEYARD C.B. Evidence that vasoactive intestinal polypeptide is not the principal secretomotor transmitter in rabbit distal colonic epithelium. Gastroenterology. 1998;114:A1172. [Google Scholar]
- REDDIX R., KUHAWARA A., WALLACE L., COOKE H.J. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. J. Pharmacol. Exp. Therap. 1994;269:1124–1129. [PubMed] [Google Scholar]
- SAID S.I., MUTT V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- SCHULZKE J.D., RIECKEN E.O., FROMM M. Distension-induced electrogenic Cl- secretion is mediated via VIP-ergic neurons in rat rectal colon. Am. J. Physiol. 1995;268:G725–G731. doi: 10.1152/ajpgi.1995.268.5.G725. [DOI] [PubMed] [Google Scholar]
- SREEDHARAN S.P., HUANG J.X., CHEUNG M.C., GOETZL E.J. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURVILL J.L., CONNOR P., FARTHING MJ.G. Neurokinin 1 and 2 receptors mediate cholera toxin secretion in rat jejunum. Gastroenterology. 2000;119:1037–1044. doi: 10.1053/gast.2000.18147. [DOI] [PubMed] [Google Scholar]
- TURVILL J.L., MOURAD F.H., FARTHING M.J.G. Crucial role for 5-HT in cholera toxin but not Escherichia coli heat- labile enterotoxin-intestinal secretion in rats. Gastroenterology. 1998;115:883–890. doi: 10.1016/s0016-5085(98)70260-4. [DOI] [PubMed] [Google Scholar]
- USDIN T.B., BONNER T.I., MEZEY E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN R.P., GATES T.S., MANTYH C.R., VIGNA S.R., WELTON M.L., PASSARO E.P., Jr., MANTYH P.W. Vasoactive intestinal polypeptide receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Neuroscience. 1989;31:771–783. doi: 10.1016/0306-4522(89)90440-5. [DOI] [PubMed] [Google Scholar]