Abstract
Maternal cocaine administration during pregnancy increased apoptosis in near-term fetal rat heart. The present study tested the hypothesis that prenatal cocaine exposure increases the heart susceptibility to ischemia/reperfusion injury in the offspring.
Pregnant Sprague–Dawley rats received cocaine (30 mg kg−1 day−1) or saline from days 15 to 21 of gestational age. Maternal body weights were not significantly different at the end of cocaine treatment, but body weights of offspring were decreased slightly at ages of 1, 3, and 7 days. Although heart-to-body weight ratio was not affected at all ages examined, prenatal cocaine significantly increased left ventricular myocyte size at an age of 30 days.
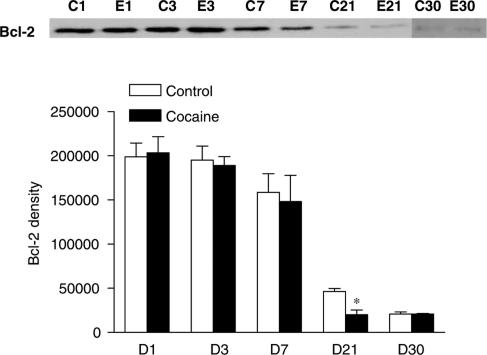
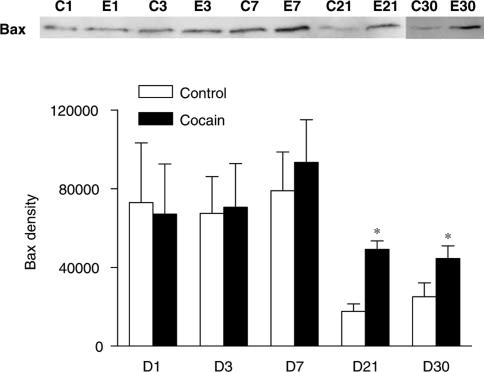
Additionally, prenatal cocaine increased DNA fragmentation measured in the hearts isolated from offspring of 1, 3, 7, and 21 days, but not of 30 days, with the peak at 3-day neonates. Antiapoptotic (Bcl-2 and Bcl-XL) and proapoptotic (Bax and Bad) proteins were expressed in neonatal rat hearts of both groups. Prenatal cocaine exposure decreased levels of Bcl-2 in 21-day and increased Bax in 21- and 30-day rat hearts.
In addition, hearts of 30-day-old male progeny were studied using the Langendorff preparation, and were subjected to 25 min of ischemia and 60 min of reperfusion. Preischemic baseline values of left ventricular (LV) function were the same between the two groups. However, prenatal cocaine exposure significantly attenuated postischemic recovery of LV function, and significantly increased elevated LV end diastolic pressure during reperfusion. This was associated with a significant increase in ischemia/reperfusion-induced LV myocardial infarct size.
The results suggest that prenatal cocaine exposure induces abnormal apoptosis and myocyte hypertrophy in postnatal heart, leading to an increased heart susceptibility to ischemic insults in postnatal life.
Keywords: Cocaine, pregnancy, rat, neonate, heart, apoptosis, ischemia, Bcl-2 proteins
Introduction
Cocaine abuse is a significant problem not only in the general population but also among pregnant women. It has been estimated that each year more than 100,000 infants who were exposed prenatally to cocaine are born in the U.S. Long-term cocaine abuse during pregnancy has been associated with numerous adverse perinatal outcomes, such as intrauterine growth retardation, preterm delivery, abruption placenta, and congenital anomalies (Holzman & Paneth, 1994). In addition to postnatal neurobehavioral alterations, maternal cocaine abuse clearly predisposes the fetus and neonate to various cardiovascular disorders. Developmental disorders observed in humans include congenital cardiac anomalies and altered cardiac function in newborns (Van de Bor et al., 1990; Lipshultz et al., 1991; Norris & Hill, 1992; Wiggins, 1992). It has been reported that cocaine abuse in pregnant mothers is associated with transient ST segment abnormalities in their infants, which are at risk for the development of transient myocardial ischemia in later adult life (Mehta et al., 1993).
The cardiotoxic effects of cocaine are likely to be multifactorial, and the mechanisms are not fully understood. Recently, we have demonstrated that maternal administration of cocaine during pregnancy causes activation of caspases and apoptotic cell death in near-term fetal rat heart in vivo in a dose-dependent manner (Xiao et al., 2001). It has been shown that cocaine can induce uterine artery vasoconstriction and cause fetal hypoxia (Woods et al., 1987). In addition, direct cytotoxic effects of cocaine on fetal cardiomyocytes have been demonstrated (Zhang et al., 1999; Xiao et al., 2000b). It has been demonstrated that cocaine induces time- and concentration-dependent increases in apoptosis in cultured fetal rat myocardial cells, which is associated with the release of cytochrome c from the mitochondria into the cytosol and subsequent activation of caspase 9 and caspase 3 (Xiao et al., 2000b). Apoptosis is a form of programmed cell death that plays a vital role in the maintenance of cell homeostasis in mature organisms and in morphogenesis during development (Thompson, 1995). During developmental period, either excessive and/or persistent cardiomyocyte apoptosis have been suggested to lead to a variety of cardiovascular disease (Haunstetter & Izumo, 1998; James, 1998; Fernandez et al., 2001; Gill et al., 2002). Recently, we have demonstrated that chronic hypoxia during pregnancy increases apoptosis in fetal rat heart, and hearts from adult offspring that were exposed to hypoxia before birth show greater myocardial damage after ischemia and reperfusion than do control hearts (Bae et al., 2003; Li et al., 2003).
Studies in rats have demonstrated that early postnatal apoptosis is essential for normal heart remodeling during postnatal maturation (Kajstura et al., 1995; Cook et al., 1999). Apoptosis in the heart was demonstrated in 1, 5, and 11 days after birth, and was dramatically decreased by 21 days (Kajstura et al., 1995). Both anti- and proapoptotic Bcl-2 family proteins are expressed in neonatal rat heart, and are differently regulated during postnatal development (Kajstura et al., 1995; Cook et al., 1999). We have demonstrated that cocaine-induced apoptosis in fetal rat heart in vivo is associated with a differential regulation of anti- and proapoptotic Bcl-2 family proteins (Xiao et al., 2001). To determine whether prenatal cocaine exposure affects postnatal heart development, the present study was designed to test the hypothesis that maternal cocaine administration during pregnancy increases apoptosis in the neonatal rat heart during early postnatal development, leading to an increased cardiac susceptibility to ischemic insults in postnatal life. We examined the effect of prenatal cocaine exposure on apoptosis and the expression of Bcl-2 family proteins in the heart of 1-, 3-, 7-, 21-, and 30-day-old rats. The effect of prenatal cocaine exposure on left ventricular (LV) function and susceptibility to ischemia and reperfusion injury was examined in 1-month-old male progeny.
Methods
Experimental animals
Time-dated pregnant Sprague–Dawley rats were purchased from Charles River Laboratories (Portage, MI, U.S.A.). The rats were randomly divided into two groups: (1) saline control, (2) cocaine 30 mg kg−1 day−1. Cocaine treatment rats received cocaine subcutaneously from days 15 to 21 of gestational age. Dilute concentration of cocaine (10 mg ml−1 saline) was used, and rats received cocaine (15 mg kg−1) twice daily at 10:00 hours and 16:00 hours at varying sites. No skin lesion was observed. Saline-injected pregnant rats served as controls. No fetal loss in the control and cocaine-treated groups were observed. After birth, neonatal pups were killed at days 1, 3, 7, 21, and 30 and hearts were isolated for measuring DNA fragmentation and Bcl-2 family proteins. Hearts were also isolated from 30-day-old male offspring for functional studies. All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University, and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of myocyte size
Myocyte size was measured as previously described (Li et al., 2004). Briefly, hearts were isolated from control and prenatally cocaine-treated rats at the age of 30 days and weighted. To measure myocyte size, tissue slices (4 μm thick) obtained from the middle position of the LV were stained with hematoxylin and eosin, viewed, and photographed by the microscope with the SPOT digital camera (Diagnostic Instruments; Sterling Heights, MI, U.S.A.). The cross-sectional area of myocytes was quantified by computerized planimetry (Image-Pro Plus) in a double-blind manner.
Measurement of DNA fragmentation by enzyme-linked immunosorbent assay (ELISA)
DNA fragmentation was quantified by specific determination of cytosolic mononucleosomes and oligonucleosomes using a commercial quantitative sandwich ELISA kit (Boehringer Mannheim) as described previously (Piot et al., 1997; Bae et al., 2003). Briefly, heart samples were put into 500 μl lysis buffer supplies in the kit, disintegrated by tissue grinder, and incubated for 30 min at room temperature. After centrifugation at 200 × g for 10 min, the supernatant (cytosolic fraction) was further diluted 40-fold in PBS buffer, and used as the antigen source in the sandwich ELISA. The values of absorbance were measured at 405/490 nm and the background value of the immunoassay was subtracted. The values obtained from cocaine treatment samples were normalized to the controls, allowing determination of the fold increase in DNA fragmentation.
Western blotting analysis
Protein levels of Bcl-2, Bcl-XL, Bad, and Bax were measured with Western blot analysis as previously described (Bae et al., 2003). Briefly, proteins were separated on 12% SDS–PAGE. The gel was then transferred to nitrocellulose membranes, and incubated with the primary antibodies for Bcl-2, Bcl-XL, Bad, and Bax (Santa Cruz Biotechnology; Santa Cruz, CA, U.S.A.). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham, Arlington Heights, IL, U.S.A.). Proteins were then visualized with an enhanced chemiluminescence detection system. Results were quantified with KODAK Electrophoresis Documentation and Analysis System and KODAK 1D Image Analysis Software.
Perfused rat harts subjected to ischemia and reperfusion
Hearts were excised rapidly from 30-day-old male progeny, and were retrogradely perfused via the aorta in a modified Langendorff apparatus under constant pressure (60 mmHg) with gassed (95% O2, 5% CO2) Krebs–Heinseleit buffer (KHB) at 37°C, as previously described (Li et al., 2003). A pressure transducer connected to a saline-filled balloon inserted into the LV was used to assess ventricular function by measuring the ventricular pressure (mmHg) and its first derivative (dP dt−1). LV end diastolic pressure (LVEDP) was set about 5 mmHg. After the baseline recording, hearts were subjected to 25 min of global ischemia by stopping the reperfusion, followed by 60 min of reperfusion. LV function parameters, LV developed pressure (LVDP), heart rate (HR), dP dtmax−1, dP dtmin−1, and LVEDP were continuously recorded with an on-line computer. Pulmonary artery effluent was collected as an index of coronary flow (CF).
Measurement of myocardial infarct size
At the end of reperfusion, LVs were collected, cut into four slices, incubated with 1% triphenyltetrazolium chloride (TTC) solution for 15 min at 37°C, and immersed in formalin for 30 min. Each slice was then photographed (Kodak digital camera) separately, and the areas of myocardial infarction (MI) in each slice were analyzed by computerized planimetry (Image-Pro Plus), corrected for the tissue weight, summed for each heart, and expressed as a percentage of the total LV weight.
Nitric oxide (NO) measurement
NO was measured by the chemiluminescence method as described previously (Xiao et al., 1999). The samples of the coronary effluent (60 μl) were injected into a gas purge vessel containing 5 ml vanadium (III)/HCl to react for 1 min and to reduce nitrite/nitrate in the sample back to NO. To achieve high reducing efficiency, the reduction was performed at 90°C. NO in the sample was then ‘stripped' into the head-space by helium bubbling (12 ml min−1) for 1 min. NO in the head-space was drawn into the NO analyzer (model 270B, Sievers Instruments; Boulder, CO, U.S.A.) and mixed with O3 in the front of a cooled Hamamatsu, red-sensitive photomutiplier tube. Signals from the detector were analyzed with the use of an on-line computer as area under the peak. The measurement reflected the combined concentrations of nitrite, nitrate, and NO (NOx), which were expressed as nmol min−1 CF.
Statistical analysis
Data were expressed as means±s.e.m. Statistical significance (P<0.05) was determined by analysis of variance (ANOVA) or unpaired t-test, where appropriate.
Results
Maternal and neonatal body weight
Maternal body weights were not significantly different between the saline control and cocaine-treated groups in the beginning of the treatment (275.4±11.6 g, n=5, for control vs 277.5±11.9 g, n=6, for cocaine, P>0.05) and at the end of the treatment (360.4±14.2 g vs 342.0±15.6 g, P>0.05). The gestational length was not significantly affected by cocaine, with 21.8±0.2 days in the control rats and 22.2±0.2 days in the cocaine-treated rats (P>0.05). As shown in Table 1, prenatal cocaine exposure caused a significant decrease in body weight of 1, 3, and 7 days neonates. However, at the age of 21 and 30 days, there were no differences in body weight between the two groups. The heart to body weight ratio was not different between the two groups at all ages examined (Table 1). Additionally, ratios of right ventricle (RV) weight to body weight (control: 0.067±0.001%; cocaine: 0.067±0.001%) and LV weight to body weight (control: 0.293±0.004%; cocaine: 0.292±0.005%) at the age of 30 days were not different between two groups (P>0.05, n=13). However, there was a significant increase in cross-sectional area of LV myocytes from 237.5±9.9 μm2 in control rats to 295.7±9.0 μm2 in the cocaine-treated animals (P<0.05) (Figure 1).
Table 1.
The effect of prenatal cocaine exposure on postnatal body and heart weight
| Postnatal age | Group | n | BW (g) | HW (g) | HW BW−1 (%) |
|---|---|---|---|---|---|
| Day 1 | Control | 5 | 6.63±0.20 | 0.03±0.00 | 0.44±0.01 |
| Cocaine | 5 | 5.93±0.16* | 0.03±0.00 | 0.45±0.01 | |
| Day 3 | Control | 5 | 8.92±0.30 | 0.05±0.00 | 0.54±0.01 |
| Cocaine | 5 | 7.84±0.21* | 0.04±0.00 | 0.53±0.01 | |
| Day 7 | Control | 5 | 17.2±0.60 | 0.09±0.00 | 0.51±0.01 |
| Cocaine | 5 | 15.5±0.40* | 0.08±0.00 | 0.55±0.02 | |
| Day 21 | Control | 5 | 54.4±1.97 | 0.27±0.01 | 0.51±0.01 |
| Cocaine | 5 | 56.1±2.37 | 0.28±0.01 | 0.49±0.03 | |
| Day 30 | Control | 5 | 173±17.3 | 1.07±0.05 | 0.58±0.02 |
| Cocaine | 5 | 180±18.4 | 1.12±0.08 | 0.58±0.02 |
Pregnant rats received daily dose of cocaine (30 mg kg−1) or saline as control from day 15 to day 21 of gestational age. BW, body weight; HW, heart weight;
P<0.05, cocaine vs control at the same age.
Figure 1.
Effect of prenatal cocaine exposure on LV myocyte size. Hearts were obtained from 30-day-old rats that were exposed to either saline control or cocaine (30 mg kg−1 day−1) before birth from days 15 to 21 of gestational age. Cross-sectional area (CSA) of myocytes in LV was measured as described in Methods. Data are mean±s.e.m. *P<0.05, cocaine vs control, n=7–8.
DNA fragmentation in neonatal hearts
As shown in Figure 2, prenatal cocaine exposure significantly increased DNA fragmentation in hearts of 1, 3, 7, and 21 days neonates, with the peak at 3-day-old neonates. However, at the age of 30 days, there was no significant difference in the level of DNA fragmentation between two groups.
Figure 2.
Effect of prenatal cocaine exposure on DNA fragmentation in neonatal rat hearts. Hearts were obtained from 1 (D1)-, 3 (D3)-, 7 (D7)-, 21 (D21)-, and 30 (D30)-day-old neonates that were exposed to either saline control or cocaine (30 mg kg−1 day−1) before birth from days 15 to 21 of gestational age. DNA fragmentation was determined using an ELISA kit as described in the Methods. Data are mean±s.e.m. *P<0.05, cocaine vs control, n=5–8.
Bcl-2 family proteins in neonatal hearts
The expression of antiapoptotic (Bcl-2 and Bcl-XL) and proapoptotic (Bad and Bax) members of the Bcl-2 family was examined in rat hearts by Western blot analysis. All four proteins were expressed in hearts of 1, 3, 7, 21, and 30 days neonates. There was a pattern of decrease in Bcl-2 and Bax expression during the first month of postnatal development (Figures 3 and 4). Compared with the control, there was a significant decrease in Bcl-2 protein levels in the heart of 21 days neonatal rats that were exposed to cocaine before birth (Figure 3). In contrast, prenatal cocaine treatment significantly increased Bax protein levels in the heart of 21 and 30 days neonatal rats (Figure 4). However, the expression levels (% of control) of neither of Bcl-XL (96±19 at day 1 and 101±19 at day 21) and Bad (101±6 at day 1 and 97±18 at day 21) changed by cocaine during the 21 days postnatal period.
Figure 3.
Effect of prenatal cocaine exposure on expression of Bcl-2 in neonatal rat hearts. Hearts were obtained from 1 (D1)-, 3 (D3)-, 7 (D7)-, 21 (D21)-, and 30 (D30)-day-old neonates that were exposed to either saline control (C) or cocaine (30 mg kg−1 day−1) (E) before birth from days 15 to 21 of gestational age. Protein levels of Bcl-2 were determined using Western blot analyses. Proteins were visualized with an enhanced chemiluminescence detection system, and were quantified with KODAK Electrophoresis Documentation and Analysis System and KODAK 1D Image Analysis Software. Data are mean±s.e.m. *P<0.05, cocaine vs control, n=4–7.
Figure 4.
Effect of prenatal cocaine exposure on the expression of Bax in neonatal rat hearts. Hearts were obtained from 1 (D1)-, 3 (D3)-, 7 (D7)-, 21 (D21)-, and 30 (D30)-day-old neonates that were exposed to either saline control (C) or cocaine (30 mg kg−1 day−1) (E) before birth from days 15 to 21 of gestational age. Protein levels of Bax were determined using Western blot analyses. Proteins were visualized with an enhanced chemiluminescence detection system, and were quantified with KODAK Electrophoresis Documentation and Analysis System and KODAK 1D Image Analysis Software. Data are mean±s.e.m. *P<0.05, cocaine vs control, n=4–8.
LV function and postischemic recovery
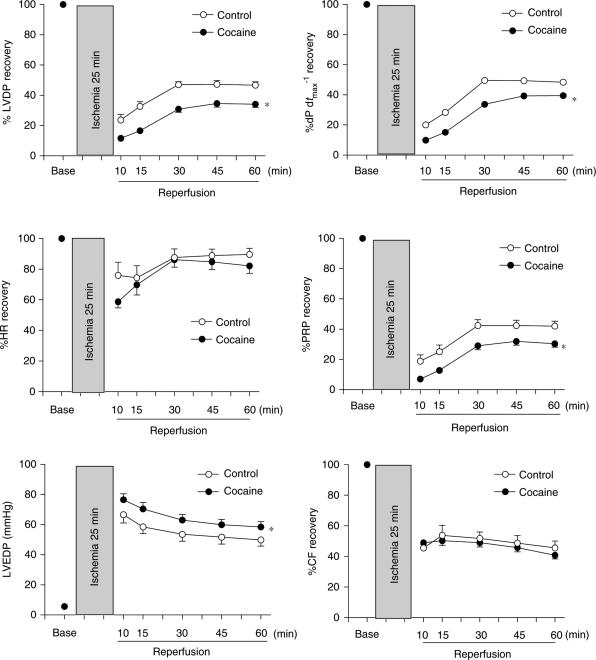
Using the Langendorff preparation, LV function was assessed in isolated hearts from 30-day-old neonatal rats that were exposed to either saline control or cocaine before birth. As shown in Table 2, there were no significant differences in LVDP, HR, dP dtmax−1, dP dtmin−1 and coronary flow at baseline levels between the two groups. Figure 5 shows the effect of 25-min ischemia followed by 60-min reperfusion on LV function in the control and cocaine-treated animals. Myocardial contraction was completely eliminated during 25-min ischemia, but gradually resumed when perfusion was restored. Compared with the control, there were significant decreases in postischemic recovery of LVDP, pressure-rate product (PRP), and dP dtmax−1 in cocaine-treated animals. However, postischemic recovery of HR and coronary flow was not significantly different between the two groups. Release of NO, measured as NOx levels in coronary effluent (nmol min−1), was not significantly different between the control (0.35±0.06) and cocaine-treated (0.42±0.11) hearts at baseline. Ischemia decreased NO release (nmol min−1) to the same extent at t0, t30 min, and t60 min of reperfusion in the control (0.51±0.12, 0.11±0.02, and 0.12±0.04, respectively) and cocaine-treated (0.61±0.13, 0.12±0.02, and 0.10±0.02, respectively) hearts during reperfusion. Ischemia and reperfusion caused an injury to the heart and resulted in elevated LVEDP during reperfusion in both groups. However, there was a significant increase in the ischemia/reperfusion-induced elevation of LVEDP in the cocaine-treated heart, as compared with the control heart (Figure 5). Consistent with the finding of LVEDP, prenatal cocaine treatment significantly increased myocardial infarct size that resulted from ischemia and reperfusion in LV (46.3±1.2% in cocaine vs 35.0±1.3% in control, P<0.05).
Table 2.
Preischemic LV function parameters
| Parameter | Control | Cocaine |
|---|---|---|
| LVDP (mmHg) | 108±5 | 108±5 |
| HR (beats min−1) | 309±10 | 296±13 |
| LVEDP (mmHg) | 5.62±0.85 | 5.22±0.72 |
| DP dtmin−1 (mmHg s−1) | 2280±10 | 2300±146 |
| DP dtmax−1 (mmHg s−1) | 3770±184 | 3380±354 |
| Coronary flow (ml min−1) | 8.07±0.97 | 7.87±0.66 |
Pregnant rats received daily dose of cocaine (30 mg kg−1) or saline as control from day 15 to day 21 of gestational age. Left ventricle (LV) function was measured in the hearts isolated from 30-day-old male progeny. LVDP, left ventricular developed pressure; HR, heart rate; LVEDP, left ventricular end-diastolic pressure. n=5 in each group.
Figure 5.
Effect of prenatal cocaine exposure on postischemic recovery of LV function in 1 month-old rats. Hearts were obtained from 30-day-old neonates that were exposed to either saline control or cocaine (30 mg kg−1 day−1) before birth from days 15 to 21 of gestational age, and were subjected to 25 min of ischemia and 60 min of reperfusion in the Langendorff preparation. LVDP, left ventricular developed pressure; HR, heart rate; PRP, pressure rate product (LVDP × HR); LVEDP, left ventricular end diastolic pressure; CF, coronary flow. Data were analyzed by two-way ANOVA with ischemia–reperfusion as one factor and cocaine treatment as the other. The asterisk (*) indicates a significant difference (P<0.05) from control for the entire curve. n=5.
Discussion
The present study has demonstrated for the first time in a rat model that prenatal cocaine exposure induces abnormal apoptosis and myocyte hypertrophy in early postnatal heart. This has been associated with a differential regulation of expression of antiapoptotic and proapoptotic Bcl-2 family proteins. The increased and persistent apoptosis in the heart during neonatal period is likely to affect the remodeling of myocardium shortly after birth, and result in an increase in cardiac susceptibility to ischemia and reperfusion injury in postnatal life.
In the present study, maternal cocaine administration resulted in a modest decrease in newborn body weight. We have previously demonstrated that cocaine administration during pregnancy decreases slightly body weight of near-term fetal rat (Xiao et al., 2001). Previous studies have shown that chronic daily doses of cocaine from 10 to 40 mg kg−1 during rat gestation produces plasma cocaine levels in the human use range (Spear et al., 1989). The present finding of about 11% reduction of newborn body weight in cocaine-treated animals closely resembles those reported for neonates of cocaine-using women (Zuckerman et al., 1989; Bandstra et al., 2001), suggesting a comparable model in the present study. The effect of cocaine on fetal growth may be due in part to potential fetal hypoxia. It has been reported that cocaine induces uterine artery vasoconstriction and causes fetal hypoxia (Woods et al., 1987). Whereas hypoxia caused an asymmetric growth restriction and increased fetal heart-to-body weight ratio (Murotsuki et al., 1997; Martin et al., 1998; Xiao et al., 2000a; Bae et al., 2003), cocaine did not affect heart-to-body weight ratio in fetal and neonatal rats (Xiao et al., 2001, present study), suggesting that cocaine may have other effects in addition to causing fetal hypoxia.
We have previously demonstrated that maternal cocaine administration produces a dose-dependent increase in caspase activities and apoptosis in fetal rat heart (Xiao et al., 2001). The present finding that prenatal cocaine exposure significantly increased DNA fragmentation of the heart during the neonatal period despite discontinuation of cocaine exposure after birth suggests that cocaine causes in utero programming of apoptotic pathways in the heart, which has lasting and persistent effect on heart development postnatally. Similar findings have been obtained with prenatal nicotine exposure, which causes a persistent brain cell loss by apoptosis during the first 2 weeks postpartum despite discontinuation of nicotine exposure at birth (Slotkin, 1998). Apoptosis plays a fundamental role in the morphogenesis and remodeling of mammalian heart during first 2 postnatal weeks (Kajstura et al., 1995; Fernandez et al., 2001). In the present study, we found that prenatal cocaine exposure-mediated increase in the level of DNA fragmentation in the heart was prominent in the first postnatal week, and was absent at the age of 30 days. As cardiomyocytes are highly differentiated cells and rarely replicate after birth, postnatal loss of cardiomyocytes through apoptosis is likely to result in a permanent reduction of the number of functioning units in the myocardium. Nevertheless, we found that heart weights were the same between the neonates from the control and cocaine-treated mothers, despite a possible apoptosis-dependent early loss in myocytes induced by prenatal cocaine treatment. Additionally, the ratios of RV weight to body weight and LV weight to body weight at the age of 30 days were not different between two groups. These results suggest that the heart may compensate for the loss of myocyte by remodeling through increasing myocyte size in the neonates. Indeed, the present study demonstrated that cross-sectional area of LV myocytes was significantly increased in the hearts of 30-day-old rats that were exposed to cocaine prenatally. This is in agreement with our previous findings that chronic hypoxia during pregnancy increases apoptosis in near-term fetal rat heart, which is associated with myocyte hypertrophy (Bae et al., 2003; Li et al., 2004). The myocyte hypertrophy was also evident in 2-month-old rat that were exposed to hypoxia before birth (Li & Zhang, unpublished observation). Although myocyte hypertrophy may compensate for the loss of myocytes and maintain cardiac function at the resting level, it may cause an increase in ischemic vulnerability of the heart at the same time. It has been demonstrated that hypertrophied heart decreases the tolerance of global ischemia and the recovering of postischemic cardiac function (Minami et al., 2000; Wambolt et al., 2001). Taken together, it is possible to speculate that increased apoptosis and subsequent myocyte hypertrophy play an important role in the increased cardiac vulnerability to ischemia and reperfusion injury in the rats that were exposed to cocaine before birth.
The present study has further revealed that prenatal cocaine exposure differentially regulates the postnatal developmental pattern of Bcl-2 family proteins in the heart. Prenatal cocaine produced a profound decrease in Bcl-2 protein and an increase in Bax protein in the heart of 21-day-old rats. The dissociation of the peak of DNA fragmentation changes at day 3 and the peak of changes in Bax and Bcl-2 proteins at day 21 rats suggest the involvement of factors other than Bcl-2 family proteins in cocaine-mediated apoptosis in the developing heart. At the age of 30 days, Bax levels were maintained significantly higher in the cocaine-treated heart as compared with the control, whereas Bcl-2 levels were the same between the two groups. This sustained increase in Bax/Bcl-2 ratio may suggest a significant decrease in protective mechanisms against apoptosis in the heart in response to stress such as ischemia and reperfusion (Adams & Cory, 1998; Haunstetter & Izumo, 1998; Gill et al., 2002; Hochhauser et al., 2003; Li et al., 2003; Gustafsson et al., 2004). Alternatively, it could be part of mechanisms in maintaining normal heart mass to compensate for the hypertrophy of the individual myocytes in cocaine-treated animals.
The present findings of decreased postischemic recovery of LV function and increased myocardial infarct size to ischemia and reperfusion in offspring of rats that were exposed to cocaine during gestation are intriguing. This is in agreement with our recent studies in which prenatal hypoxia significantly increased the sensitivity of adult offspring heart to ischemia and reperfusion injury (Li et al., 2003). Other studies have demonstrated that second-hand smoke exposure in utero for 3 weeks tends to increase infarct size in young pups that are subjected to 17 min of left coronary artery occlusion (Zhu et al., 1997). In addition, prenatal ethanol exposure promoted cardiac myocyte apoptosis, which may contribute to the depressed cardiac contractile function in adult offspring rats (Ren et al., 2002). These studies suggest that, in addition to undernutrition as originally proposed (Barker et al., 1989), many other factors could affect fetal development in utero, which has lasting and lifelong effects on the cardiovascular system. In the present study, hearts from prenatal cocaine-treated rats showed similar basal LV function as compared to those from control animals, suggesting that prenatal cocaine exposure did not influence contractility in the resting heart of offspring. Similar findings have been obtained in our recent studies in adult rats that were exposed to hypoxia before birth (Li et al., 2003). Since the hearts were perfused at a constant pressure, and the end-diastolic pressure was set at about 5 mmHg, the present study was not subject to differences in afterload, preload, or endogenous sympathetic tone. Given that NO plays an important role in modulating the severity of ischemia–reperfusion injury (Bolli, 2001), we measured NO release in coronary effluent. The present finding that prenatal cocaine treatment had no effect on preischemic or postischemic coronary flow rate is consistent with the results of no changes in coronary NO release between control and cocaine-treated rats. This suggests that the cocaine-induced increase in susceptibility of the heart to ischemia and reperfusion injury is not mediated by changes in coronary vasculature, but rather due to intrinsic changes in myocyte itself.
In conclusion, we have demonstrated for the first time, to our knowledge, in a rat model, that prenatal cocaine exposure significantly increases the susceptibility of the heart to ischemia and reperfusion injury by increasing myocardial infarct size and decreasing postischemic recovery of LV function. Although the mechanisms underlying this increased susceptibility are not clear at present, and are likely to be multiplex, the present study suggests that cocaine exposure during fetal development may cause in utero programming of apoptotic pathways, which may has lasting and lifelong importance on cardiac function, and may play an important role in the increased susceptibility of the heart to ischemia and reperfusion injury in postnatal life.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL-67745, HL-57787, HD-31226, and by Loma Linda University School of Medicine. We thank Guohu Li for his technical assistance.
Abbreviations
- CF
coronary flow
- HR
heart rate
- LV
left ventricle
- LVDP
left ventricle developed pressure
- LVEDP
left ventricle end diastolic pressure
- NO
nitric oxide
- PRP
pressure rate product
- RV
right ventricle
References
- ADAMS J.M., CORY S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- BAE S., XIAO Y.H., LI G., CASIANO C., ZHANG L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- BANDSTRA E.S., MORROW C.E., ANTHONY J.C., CHURCHILL S.S., CHITWOOD D.C., STEELE B.W., OFIR A.Y., XUE L. Intrauterine growth of full-term infants: impact of prenatal cocaine exposure. Pediatrics. 2001;108:1309–1319. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- BARKER D.J., OSMOND C., GOLDING J., KUH D., WADSWORTH M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLI R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J. Mol. Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- COOK S.A., SUGDEN P.H., CLERK A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ. Res. 1999;85:940–949. doi: 10.1161/01.res.85.10.940. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ E., SIDDIQUEE Z., SHOHET R. Apoptosis and proliferation in the neonatal murine heart. Dev. Dyn. 2001;221:302–319. doi: 10.1002/dvdy.1139. [DOI] [PubMed] [Google Scholar]
- GILL C., MESTRIL R., SAMALI A. Losing heart: the role of apoptosis in heart disease – a novel therapeutic target. FASEB J. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON A.B., TSAI J.G., LOGUE S.E., CROW M.T., GOTTLIEB R.A. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J. Biol. Chem. 2004;279:21233–21238. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]
- HAUNSTETTER A., IZUMO S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ. Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- HOCHHAUSER E., KIVITY S., OFFEN D., MAULIK N., OTANI H., BARHUM Y., PANNET H., SHNEYVAYS V., SHAINBERG A., GOLDSHTAUB V., TOBAR A., VIDNE B.A. Bax ablation protects against myocardial ischemia–reperfusion injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- HOLZMAN C., PANETH N. Maternal cocaine use during pregnancy and perinatal outcomes. Epidemiol. Rev. 1994;82:1111–1129. doi: 10.1093/oxfordjournals.epirev.a036156. [DOI] [PubMed] [Google Scholar]
- JAMES T.N. Normal and abnormal consequences of apoptosis in the human heart. Annu. Rev. Physiol. 1998;60:309–325. doi: 10.1146/annurev.physiol.60.1.309. [DOI] [PubMed] [Google Scholar]
- KAJSTURA J., MANSUKHANI M., CHENG W., REISS K., KRAJEWSKI S., REED J.C., QUAINI F., SONNENBLICK E.H., ANVERSA P. Programmed cell death and expression of the protooncogene bcl-2 in myocytes during postnatal maturation of the heart. Exp. Cell Res. 1995;219:110–121. doi: 10.1006/excr.1995.1211. [DOI] [PubMed] [Google Scholar]
- LI G., BAE S., ZHANG L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am. J. Physiol. 2004;286:H1712–H1719. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- LI G., XIAO Y.H., ESTRELLA J.L., DUCSAY C.A., GILBERT R.D., ZHANG L. Effect of prenatally chronic hypoxia on heart susceptibility to ischemia/reperfusion injury in adult rat. J. Soc. Gynecol. Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- LIPSHULTZ S.E., FRASSICA J.J., ORAV E.J. Cardiovascular abnormalities in infants prenatally exposed to cocaine. J. Pediatr. 1991;118:44–51. doi: 10.1016/s0022-3476(05)81842-6. [DOI] [PubMed] [Google Scholar]
- MARTIN C., YU A.Y., JIANG B.H., DAVIS L., KIMBERLY D., HOHIMER A.R., SEMENZA G.L. Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am. J. Obstet. Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- MEHTA S.K., FINKELHOR R.S., ANDERSON R.L., HARCAR-SEVCIK R.A., WASSER T.E., BAHLER R.C. Transient myocardial ischemia in infants prenatally exposed to cocaine. J. Pediatr. 1993;122:945–949. doi: 10.1016/s0022-3476(09)90025-7. [DOI] [PubMed] [Google Scholar]
- MINAMI Y., GOHRA H., SASAKI G., KATOH T., ZENPO N., ESATO K. Changes in left ventricular function after cardiac arrest and reperfusion in hypertropheral hearts. Ann. Thorac. Cardiovasc. Surg. 2000;6:309–314. [PubMed] [Google Scholar]
- MUROTSUKI J., CHALLIS J.R., HAN V.K., FRAHER L.J., GAGNON R. Chronic fetal placental embolization and hypoxemia cause hypertension and myocardial hypertrophy in fetal sheep. Am. J. Physiol. 1997;272:R201–R207. doi: 10.1152/ajpregu.1997.272.1.R201. [DOI] [PubMed] [Google Scholar]
- NORRIS M.K., HILL C.S. Assessing congenital heart defects in the cocaine-exposed neonate. Dimens. Crit. Care Nurs. 1992;11:6–12. doi: 10.1097/00003465-199201000-00003. [DOI] [PubMed] [Google Scholar]
- PIOT C.A., PADMANABAN D., URSELL P.C., SIEVERS R.E., WOLFE C.L. Ischemic preconditioning decreases apoptosis in rat heart in vivo. Circulation. 1997;96:1598–1604. doi: 10.1161/01.cir.96.5.1598. [DOI] [PubMed] [Google Scholar]
- REN J., WOLD L.E., NATAVIO M., REN B.H., HANNIGAN J.H., BROWN R.A. Influence of prenatal alcohol exposure on myocardial contractile function in adult rat heart: role of intracellular calcium and apoptosis. Alcohol Alcohol. 2002;37:30–37. doi: 10.1093/alcalc/37.1.30. [DOI] [PubMed] [Google Scholar]
- SLOTKIN T.A. Fetal nicotine or cocaine exposure: which one is worse. J. Pharmacol. Exp. Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- SPEAR L.P., FRAMBES N.A., KIRSTEIN C.L. Fetal and maternal brain and plasma levels of cocaine and benzoylecgonine following chronic subcutaneous administration of cocaine during gestation in rats. Psychopharmacology (Berl) 1989;97:427–431. doi: 10.1007/BF00439542. [DOI] [PubMed] [Google Scholar]
- THOMPSON C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- VAN de BOR M., WALTHER F.J., EBRAHIMI M. Decreased cardiac output in infants of mothers who abused cocaine. Pediatrics. 1990;85:30–32. [PubMed] [Google Scholar]
- WAMBOLT R.B., GRIST M., BONDY G.P., ALLARD M.F. Accelerated glycolysis and greater postischemic dysfunction in hypertrophied rat hearts are independent of coronary flow. Can. J. Cardiol. 2001;17:889–894. [PubMed] [Google Scholar]
- WIGGINS R.C. Pharmacokinetics of cocaine in pregnancy and effects on fetal maturation. Clin. Pharmacokinet. 1992;22:85–93. doi: 10.2165/00003088-199222020-00001. [DOI] [PubMed] [Google Scholar]
- WOODS J.R., PLESSINGER M.A., CLARK K.E. Effects of cocaine on uterine blood flow and fetal oxygenation. JAMA. 1987;257:957–961. [PubMed] [Google Scholar]
- XIAO D., DUCSAY C.A., ZHANG L. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J. Soc. Gynecol. Investig. 2000a;7:279–283. [PubMed] [Google Scholar]
- XIAO D.L., LIU Y., PEARCE W.J., ZHANG L. The release of endothelial nitric oxide in ovine uterine artery is up-regulated by pregnancy. Eur. J. Pharmacol. 1999;367:223–230. doi: 10.1016/s0014-2999(98)00951-0. [DOI] [PubMed] [Google Scholar]
- XIAO Y., HE J., GILBERT R.D., ZHANG L. Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. J. Pharmacol. Exp. Ther. 2000b;292:8–14. [PubMed] [Google Scholar]
- XIAO Y., XIAO D., HE J., ZHANG L. Maternal cocaine administration during pregnancy induces apoptosis in fetal rat heart. J. Cardiovasc. Pharmacol. 2001;37:639–648. doi: 10.1097/00005344-200106000-00001. [DOI] [PubMed] [Google Scholar]
- ZHANG L., XIAO Y., HE J. Cocaine and apoptosis in myocardial cells. Anat. Rec. 1999;257:1–9. doi: 10.1002/(SICI)1097-0185(19991215)257:6<208::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- ZHU B., SUN Y., SUDHIR K., SIEVERS R.E., BROWNE A.E., GAO L., HUTCHISON S.T., CHOU T.M., DEEDWANIA P.C., CHATTERJEE K., GLANTZ S.A., PARMLEY W.W. Effects of second-hand smoke and gender on infarct size of young rats exposed in utero and in the neonatal to adolescent period. JACC. 1997;30:1878–1885. doi: 10.1016/s0735-1097(97)00364-1. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN B., FRANK D.A., HINGSON R., AMARO H., LEVENSON S.M., KAYNE H., PARKER S., VINCI R., ABOAGYE K., FRIED L.E., CABRAL H., TIMPERI R., BAUCHNER H. Effects of maternal marijuana and cocaine use on fetal growth. N. Engl. J. Med. 1989;320:762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]