Abstract
Theophylline, a phosphodiesterase inhibitor and adenosine receptor antagonist, is used in asthma and chronic obstructive pulmonary disease (COPD) treatment. However, the relatively low effectiveness of theophylline have recently led to reduced usage. The goal of the present study was to identify a theophylline-like compound with improved effectiveness.
We discovered CGH2466, which not only antagonised the adenosine A1, A2b and A3 receptors with IC50 values of 19±4, 21±3 and 80±14 nM, respectively, but also inhibited the p38 mitogen-activated protein (MAP) kinases α and β and the phosphodiesterase 4D (PDE4D) isoenzyme with IC50 values of 187±18, 400±38 and 22±5 nM, respectively.
Despite similar potencies on individual targets, CGH2466 inhibited the production of cytokines and oxygen radicals by human peripheral blood leucocytes in vitro, more potently (IC50 values between 30 and 50 nM) than the standard p38 MAP kinase inhibitor SB203580 (30 nM to >1 μM), the PDE4 inhibitor cilomilast (120–400 nM) and the broad spectrum adenosine receptor antagonist CGS15943 (>10 μM).
When given either orally or locally into the lungs, CGH2466 (3 to 10 mg kg−1) inhibited the ovalbumin- or lipopolysaccharide-induced airway inflammation in mice more potently than the single receptor antagonists or enzyme inhibitors used alone.
In conclusion, CGH2466 through its combined activities at multiple targets exerted a powerful anti-inflammatory effect and therefore may have beneficial therapeutic value in diseases such as asthma and COPD.
Keywords: p38MAP kinase, phosphodiesterase, adenosine receptor, asthma, chronic obstructive pulmonary disease
Introduction
Asthma is a chronic inflammatory disorder of the airways characterised by infiltration of the lung with inflammatory cells such as eosinophils and lymphocytes, and by the presence of bronchial hyper-responsiveness to a variety of stimuli. Although effective therapies for the management of asthma, such as inhaled glucocorticosteroids and β2 adrenoceptor agonists, are available, they are limited by issues of side effects and compliance (Barnes, 1997). A clear medical need exists, therefore, for new therapies that match the effectiveness of existing treatments but are easier to use and have a more favourable side effect profile. New therapies are also required for a group of asthma patients whose symptoms are inadequately controlled or resistant to high dose of inhaled glucocorticosteroids (Barnes et al., 1998). In these patients, oral glucocorticosteroids are often employed despite the associated systemic side effects, but even then, patients may remain symptomatic. In contrast, currently available asthma therapies are largely ineffective in chronic obstructive pulmonary disease (COPD), characterised by slowly progressive and irreversible airway obstruction, mucus hypersecretion and infiltration of neutrophils and macrophages into the lungs (Jeffery, 2000). An urgent need for novel therapies is thus required to target all aspects of this disease.
Theophylline is being used in asthma and COPD treatment. Originally, it was mainly used as a bronchodilator, but this application has become less popular as β2 adrenoceptor agonists are more effective. Nowadays, theophylline tends to be added to these inhaled bronchodilators in patients with more severe asthma and COPD, but at the doses that are needed for bronchodilation, side effects are relatively common (Barnes, 2003). Although its mechanisms of action remains poorly understood, theophylline has been shown to inhibit different families of phosphodiesterases (PDE) as well as adenosine receptors (Rabe & Dent, 1998; Page, 1999; Yasui et al., 2000). At least some of the cardiovascular, gastrointestinal and central side effects seen with theophylline treatment can be attributed to general inhibition of PDEs in all tissues of the body. More recently, however, it has been demonstrated that theophylline used at lower concentrations to avoid many of the problems with side effects, still exert anti-inflammatory benefits and steroid sparing effects in asthma. This effect was attributed to adenosine receptor antagonism rather than PDE inhibition since these low doses of theophylline selectively blocked the adenosine monophosphate-induced bronchoconstriction in asthmatics (Evans et al., 1997; Rabe & Dent, 1998).
The goal of the present study was therefore to identify selective adenosine receptor antagonists, which may demonstrate the beneficial effects of theophylline but with an improved effectiveness. In a search for such an improved theophylline, we discovered CGH2466 a combined adenosine receptor antagonist, PDE4 and p38 mitogen-activated protein (MAP) kinase inhibitor, which demonstrated potent anti-inflammatory activities in various in vitro and in vivo models, suggesting that this compound might have therapeutic benefits in multiple inflammatory diseases including asthma and COPD.
Methods
Adenosine receptor assays
To determine the potency and selectivity of the different compounds at human adenosine receptor subtypes, the following assays were used. A1 receptor binding assay: Chinese hamster ovary cells expressing human A1 receptors (Novartis, Horsham, U.K.) were cultured in Nut.Mix.F-12 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine and 200 μg ml−1 geneticin. Confluent cells were scraped from the culture flasks and centrifuged at 1500 × g for 5 min. The pellet was homogenised in a glass homogeniser and centrifuged at 40,000 × g for 25 min. The final pellet was resuspended in the assay buffer (20 mM HEPES buffer containing 100 mM sodium chloride, 10 mM magnesium chloride and 2 U ml−1 adenosine deaminase, pH 7.4). The radioactive ligand, [propyl-3H], 8-cyclopentyl-1,3,dipropylxanthine (2 nM) and increasing concentrations of test compounds were added to the resulting membrane preparation (0.4 mg of protein ml−1) and incubated for 90 min at room temperature. Samples were harvested onto glass filters, scintillation fluid was added and counts per minute were measured using a Packard Topcount. A1 receptor functional assay: This assay measures the ability of A1 antagonists to inhibit I-AB-MECA-induced [35S]GTPγS binding to cellular membranes. The assay was performed in a white nonbinding surface 96-well Optiplate. Assay components were added as follows: 25 μl of assay buffer (20 mM HEPES, 10 mM MgCl2, 100 mM NaCl, 1 mM EDTA, 10 μg ml−1 saponin, 0.1% BSA, pH 7.4), 25 μl of 10 μM GDP, 25 μl of 1.25 nM [35S]GTPγS, 25 μl of 100 nM I-AB-MECA and 25 μl of increasing concentrations of the compound or vehicle. Membranes were diluted in assay buffer (100 μl, containing 10 μg ml−1 of adenosine deaminase) to 25 μg ml−1 and mixed with 50 μl of WGA SPA beads (5 mg ml−1) and added to each well. After incubation at room temperature for 90 min, the plate was centrifuged at 850 × g for 10 min, and immediately read on a Packard TopCount. A2a receptor binding assay: HEK-293 A2a membranes were suspended in assay buffer (50 mM Tris-HCl, 120 mM sodium chloride, 5 mM potassium chloride, 10 mM magnesium chloride, 2 mM calcium chloride, 2 U ml−1 adenosine deaminase, pH 7.4). The radioactive ligand, [3H]-ZM241385 (5 nM) and increasing concentrations of test compounds were added to the membrane preparation (0.4 mg of protein ml−1) and incubated for 60 min at room temperature. Samples were harvested onto glass filters, scintillation fluid was added and counts per minute were measured using a Packard Topcount. A2b receptor functional assay: A reporter gene assay using Chinese hamster ovary cells transfected both with a luciferase-expressing reporter plasmid and functional human adenosine A2b receptor (Novartis, Horsham, U.K.) was used. Cells were grown to confluency in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 0.4 mg ml−1 L-proline, 1 nM sodium selenite, 0.5 mg ml−1 hygromycin B and 1 mg ml−1 geneticin. For the assay, 50,000 cells well−1 were seeded onto 96-well plates and incubated for 24 h at 37°C, 5% CO2. Compounds were added to the cells and incubated for 30 min at 37°C prior to addition of increasing concentrations of 5′-N-ethylcarboxyamideoadenosine. After an incubation period of 3 h at 37°C, 5% CO2, 100 μl of Steady-Glo reagent was added and luminescence was read on a Topcount. A3 receptor binding assay: Chinese hamster ovary cells stably transfected with human A3 receptor (Novartis, Basel, Switzerland) were grown to confluency in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum and 2 mM L-glutamine. For the assay, 500,000 cells well−1 were seeded onto 96-well plates and incubated for 24 h at 37°C, 5% CO2. The radioactive ligand, [125I]N6-4-amino-3-iodobenzyladenosine-5′-N-methyluronamide (2 nM), and increasing concentrations of test compounds were added to the cells and incubated for 120 min at 4°C. Samples from the assay plate were harvested onto glass filters, scintillation fluid was added and counts per minutes were measured using a Packard Topcount. A3 receptor functional assay: This assay measures the ability of A3 antagonists to inhibit I-AB-MECA-induced [35S]GTPγS binding to cellular membranes. The assay was performed as described for the A1 functional assay using Chinese hamster ovary cells stably transfected with human A3 receptor (Novartis, Basel, Switzerland).
Inhibition of nucleotide PDE isoenzymes
PDE1 was purified from human lung obtained from patients undergoing surgery for lung cancer. PDE2 and 5 were purified from platelet concentrates obtained from the local blood transfusion centre (Trifilieff et al., 2002). Purification of human PDE3 and cloning and expression of human PDE4A, 4D and rat PDE4B were performed as previously described (Iwamura et al., 2001). PDE activity was determined using cAMP or cGMP as substrate (Engels et al., 1995).
Kinase assays
A phosphorylated form of human His-p38α MAP kinase (10 ng well−1) was used to phosphorylate the immobilised substrate GST-ATF-1 in the presence of 120 μM cold ATP. The phosphorylated GST-ATF-1 was detected by rabbit polyclonal antibodies, followed by biotin labelled goat anti-rabbit IgG, streptavidin-alkaline phosphatase and substrate. Phosphorylated form of His-p38bα, His-p38δ, and His-JNK1 MAP kinases (30, 3 and 30 ng well−1, respectively) of human origin were used to phosphorylate the immobilised substrate GST-ATF-2 in the presence of cold ATP (120 μM) (Revesz et al., 2000). The other kinase inhibition assays were performed under conditions optimised for each kinase and with ATP concentrations similar to the Km of the respective enzyme toward ATP: 8 μM (KDR-1, FGFR), 1 μM (c-Kit, c-Met), 13 μM (c-Abl), 2 μM (Her-1, Her-2), 20 μM (c-Src), 30 μM (IGF-1R) and 7.5 μM (CDK1). For tyrosine kinases, filter binding assays using recombinant GST-fused kinase domains of the receptors expressed in baculovirus and purified over glutathione sepharose were employed. [33P]ATP was used as the phosphate donor, and the polyGluTyr (4 : 1) peptide was used as the acceptor (Vangrevelinghe et al., 2003).
Human leucocyte-based assays
All the assays were performed as previously described with cells isolated from blood of normal individuals (Trifilieff et al., 2002). Neutrophils were stimulated with formyl-Met-Leu-Phe (1 μM) and the ability of the cells to generate superoxide anions during an oxidative burst was measured using a cytochrome c reduction assay. Mononuclear cells were stimulated either with anti-CD3 monoclonal antibodies (100 ng ml−1) or with LPS (10 μg ml−1) and interferon-gamma (IFN-γ) (50 ng ml−1) for IFN-γ and TNF-α measurement, respectively. After an incubation period of 20 h at 37°C, 5% CO2, supernatants were harvested and cytokine levels were measured by commercially available sandwich enzyme-linked immunosorbent assay.
Molecular modelling
Crystal structures of p38 MAP kinase (Wilson et al., 1996) and human phosphodiesterase PDE 4B2B (Xu et al., 2000) were downloaded from the Brookhaven Protein Data Bank (Berman et al., 2000). The PDB accession numbers are 1WFC (p38 MAP kinase) and 1F0J (PDE4B). The crystal structures were loaded into Sybyl (Tripos Inc., St Louis, MO, U.S.A.) and hydrogens added.
CGH2466 was docked manually into the ATP-binding pocket of p38 MAP. The ligand and surrounding residues were relaxed to a gradient of 0.05 kcal mol−1 Å−1 to resolve any bad contacts using the Tripos force field. The compound CGH2466 was manually docked into the PDE4 active site followed by a 50 ps molecular dynamics simulation and relaxation of the ligand and surrounding residues to 0.05 kcal mol−1 Å−1 using the Tripos force field.
In vivo models
Female BALB/C mice or C57BL/6 (8 weeks old) were purchased from Harlan (Oxon, U.K.). The animals were housed in plastic cages in an air-conditioned room at 24°C. Food and water were available ad libitum. The studies reported here conformed to the U.K. Animals (scientific procedures) Act 1986. All the in vivo procedures has been described previously in detail (Trifilieff et al., 2000; Corteling et al., 2002).
For the ovalbumin-induced eosinophilic airway inflammation, actively sensitised C57BL/6 mice were challenged with an aerosol of ovalbumin and killed after 48 h for bronchoalveolar lavage. At 15 min before and 24 h after the challenge, mice were intranasaly treated with compounds suspended in sterile phosphate-buffered saline (PBS) containing 2% dimethyl sulphoxide (50 μl). Control mice received 50 μl of vehicle. In some experiments, mice were orally treated 1 h before and 24 h after the challenge. The vehicle used for the oral treatment was Neoral® placebo containing 2% dimethyl sulphoxide (200 μl).
For the LPS-induced airway neutrophilic inflammation, BALB/c mice were intranasally challenged with 0.3 mg kg−1 of LPS and killed after 3 h for bronchoalveolar lavage. Mice were intranasaly treated, half an hour before the challenge, with compounds suspended in sterile PBS containing 2% dimethyl sulphoxide (50 μl). Control mice received 50 μl of vehicle. In some experiments, mice were orally treated 1 h before the challenge. The vehicle used for the oral treatment was Neoral® placebo containing 2% dimethyl sulphoxide (200 μl).
The doses for cilomilast (Griswold et al., 1998; Souza et al., 2001), SB203580 (Escott et al., 2000) and CGS15943 (Lappe et al., 1992; Hannon et al., 2002) were chosen from previous studies demonstrating activity in vivo.
Materials
CGH2466 and cilomilast were synthesised by The Department of Chemistry (Novartis, Horsham, U.K.). WGA SPA beads and the radiolabelled ligands were purchased from Amersham Biosciences (Chalfont, St Giles, U.K.). Cell culture reagents were from Invitrogen Ltd (Paisley, U.K.). HEK-293 A2a membranes were from Tocris Cookson (Bristol, U.K.). IFN-γ and TNF-α ELISAs were from R&D Systems (Abingdon, U.K.). All other reagents were obtained from Sigma-Aldrich (Gillingham, U.K.).
In vivo data analysis
Data are expressed as mean±s.e.mean (s.e.m.) Statistical comparisons were performed using Kruskal–Wallis test with Bonferroni correction for multiple comparison and a P-value of less than 0.05 was considered significant.
Results
In vitro effects of CGH2466
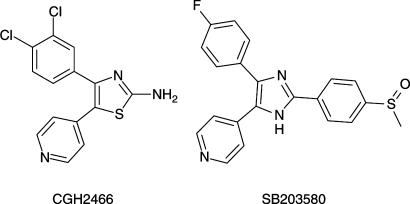
CGH2466 (2-amino-4-(3,4-dichlorophenyl)-5-pyridin-4-yl-thiazol, Figure 1) was evaluated as an adenosine receptor antagonist by binding and functional assays. The results showed that the compound was a potent binder of the adenosine A1 and A3 receptors, with no binding activity at the A2a receptor. Cell-based functional assays show that CGH2466 behaved as an antagonist at the A1, A2b and A3 receptors (Table 1). Since CGH2466 was structurally related to the well-known p38 MAP kinase inhibitor SB203580 (Figure 1) (Boehm et al., 1996), it was therefore tested on these enzymes and shown to be a potent inhibitor of the p38 MAP kinases α and β (Table 1). In order to further investigate a potential crossreactivity with other kinases, CGH2466 was screened in a number of other kinase assays (JNK1, CDK1, Her-1, Her-2, c-Abl, KDR1, c-Met, FGFR, c-Kit, IGF-1R, c-Src) and was found to be inactive (IC50>10,000 nM). In addition, screening against a panel of other selectivity assays revealed that the compound was also a powerful and rather selective PDE4D inhibitor (Table 1) with no or significantly lower potency on other members of the phosphodiesterase family, including PDE1, 2, 3, 5, 6 and 7.
Figure 1.
Structure of CGH2466 (2-amino-4-(3,4-dichlorophenyl)-5-pyridin-4-yl-thiazol) and SB203580 (4-(4-Fluorophenyl)-2-(4-methylsulfonylphenyl)-5(4-pyridyl) imidazole).
Table 1.
Antagonist and inhibitor profiles of CGH2466 and comparator compounds in in vitro assays
| IC50 (nM) | ||||
|---|---|---|---|---|
| CGH2466 | SB203580 | Cilomilast | CGS15943 | |
| (p38MAP kinase inhibitor) | (PDE4 inhibitor) | (adenosine receptors antagonist) | ||
| Adenosine A1 binding assay | 39±9 | >10,000 | NDa | 19±11 |
| Adenosine A1 functional assay (GTP-γ-S binding assay) | 19±4 | ND | ND | ND |
| Adenosine A2a binding assay | >10,000 | 251±45 | NDa | 9±1 |
| Adenosine A2b functional assay (reporter gene asssay) | 21±3 | >10,000 | >10,000 | 8±2 |
| Adenosine A3 binding assay | 38±11 | >10,000 | NDa | 41±4 |
| Adenosine A3 functional assay (GTP-γ-S binding assay) | 80±14 | NDa | NDa | NDa |
| p38α | 187±18 | 152±45 | >10,000 | 8124±1454 |
| p38β | 400±38 | NDa | NDa | NDa |
| PDE4A | 633±254 | >10,000 | 132±24 | >10,000 |
| PDE4B | 1287±356 | >10,000 | 98±11 | >10,000 |
| PDE4D | 22±5 | >10,000 | 14±2 | >10,000 |
| TNF-α production from monocytes | 26±10 | 34±25 | 392±230 | >10,000 |
| IFN-γ production from T cells | 49±27 | 622±313 | 337±147 | >10,000 |
| Neutrophils oxidative burst | 30±22 | >1,000 | 121±49 | >10,000 |
The human A1, A2b and A3 receptors were expressed in Chinese hamster ovary cells. The human A2a receptor was expressed in HEK-293 cells. For the enzymatic assays, recombinant human p38α, p38β, PDE4A, PDE4D and rat PDE4B were used. Leucocyte-based assays were performed on cells isolated from human blood. Data are expressed as mean±s.e.m. of at least three different determinations.
Not done.
Molecular modelling of CGH2466 at the P38 MAP kinase and PDE4 active site
Since we were quite surprised by the multiple effects of CGH2466, we used molecular modelling in order to dock this compound in both the P38 MAP kinase (Wilson et al., 1996) and the PDE4 (Xu et al., 2000) active sites.
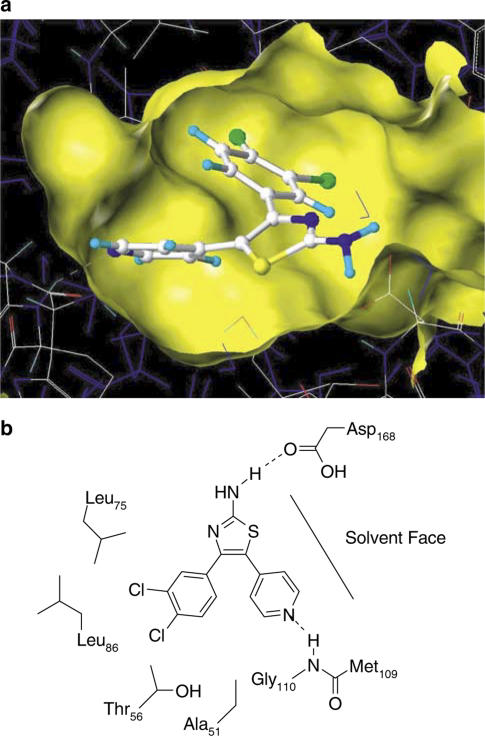
CGH2466 was docked into p38 MAP kinase using the coordinates of the ATP in the complex as a template. The ATP was then removed and the ligand relaxed to 0.05 kcal−1 mol−1 Å to resolve any bad contacts using the Tripos force field in SYBYL (Tripos Inc., St Louis, MO, U.S.A.). The overall orientation of CGH2466 is very similar to the published crystal structures of the pyridinylimidazoles (Wilson et al., 1997; Wang et al., 1998). As shown in Figure 2, the pyridine forms the expected hydrogen bond with the backbone carbonyl of Met109. The compound is, however, slightly twisted allowing for the formation of a hydrogen bond between the NH2 of the aminothiazole and the carbonyl of Asp168 (∼2 Å length). At the same time, this allows for space in the hydrophobic cleft to accommodate the rather bulky dichlorophenyl group.
Figure 2.
(a) Model of CGH2466 docked in the active site of p38 MAP kinase. The ligand is drawn in a ball and stick representation, and protein and water molecules in a wire frame representation. The surface of the binding site is shown in yellow. Parts of the surface have been removed for clarity. (b) Schematic representation of the binding mode of CGH2466 in p38 MAP kinase.
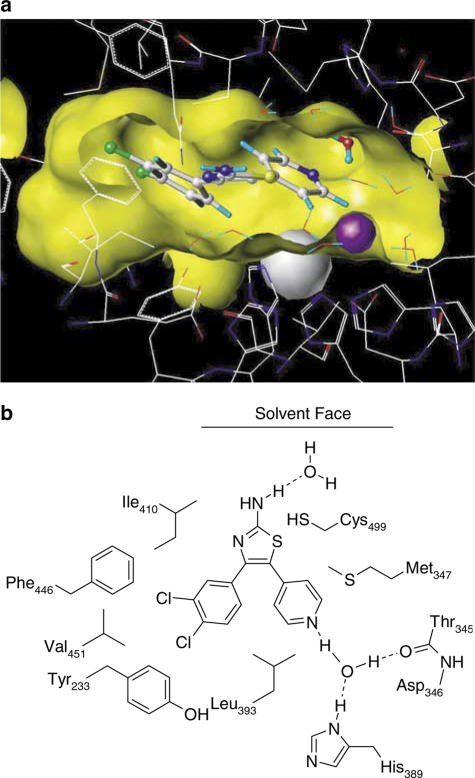
Our modelling work on the PDE4 active site is unable to account for the PDE4 subtype selectivity of our compound, since all the active site residues in PDE4A–D are identical. Nevertheless, docking of CGH2466 into the PDE4 active site followed by a 50 ps molecular dynamics simulation and relaxation of the ligand and surrounding residues to 0.05 kcal−1 mol−1 Å using the Tripos force field lead to a convincing fit of the compound into the narrow active site. The dichlorophenyl group is buried in a hydrophobic pocket formed by Ile410 and Tyr233. The pyridyl group forms a hydrogen bond to a water molecule bound to Thr345 and His389 (Figure 3).
Figure 3.
(a) Model of CGH2466 docked in the PDE4 active site looking from the solvent into the binding pocket. The ligand is drawn in a ball and stick representation, protein and water molecules in a wire frame representation and the metal ions as spheres. The surface of the binding site is shown in yellow. Parts of the surface have been removed for clarity. (b) Schematic representation of the binding mode of CGH2466 in PDE4.
CGH2466 is a more potent anti-inflammatory compound than individual p38 MAP kinase and PDE4 inhibitors or adenosine receptor antagonist in vitro
In order to get a better understanding of its potential as an anti-inflammatory drug candidate, the in vitro profile of CGH2466 was compared with a standard PDE4 inhibitor cilomilast (Christensen et al., 1998), the prototypical p38 MAP kinase inhibitor SB203580 (Boehm et al., 1996) and the broad spectrum adenosine antagonist CGS15943 (Kim et al., 1998). Table 1 summarises the activity of all four compounds at the key enzymes or receptors and in functional assays such as the LPS-induced TNF-α production by human peripheral blood mononuclear cells, the anti-CD3 antibody-induced IFN-γ production by human peripheral blood lymphocytes as well as the formyl-Met-Leu-Phe-induced oxidative burst from human peripheral blood neutrophils. As expected, cilomilast selectively inhibited PDE4 isoenzymes with no activity on p38 MAP kinase or adenosine receptors. The monocyte TNF-α and T-cell IFN-γ release as well as the oxidative burst in neutrophils were also inhibited by cilomilast. The standard p38 MAP kinase inhibitor SB203580 exhibited no PDE4 inhibitor activity but showed some binding activity at the adenosine A2a receptor. This compound also potently inhibited TNF-α secretion by monocytes. However, the inhibition of the IFN-γ production was much less pronounced and no activity in the oxidative burst assay was observed. The standard adenosine receptor antagonist CGS15943 demonstrated the expected profile on adenosine receptor subtypes and had no activity on p38 MAP kinase or PDE4 and showed no inhibitory effect in the cellular assays. Compared to these compounds, CGH2466 is similar in potency to SB203580 as a p38 MAP kinase inhibitor, is similarly active to cilomilast as a PDE4D inhibitor and showed a similar profile on A1, A2b and A3 adenosine receptors compared to CGS15943. In contrast to CGS15943, CGH2466 was inactive at the A2a receptor. However, despite the similarity between the different compounds with regard to their activity on selected enzymes or receptors, CGH2466 was the most potent inhibitor in all three leucocyte-based assays.
In vivo anti-inflammatory activities for CGH2466 in comparison with single individual p38 MAP kinase and PDE4 inhibitors or adenosine receptor antagonist
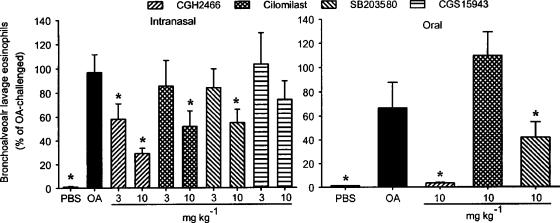
Based on the in vitro data, we were very much interested to analyse whether this broad anti-inflammatory potential of CGH2466 also translates into better efficacy in in vivo murine models of eosinophilic or neutrophilic lung infiltration. As shown in Figure 4, aerosol challenge of ovalbumin to sensitised mice increased eosinophil numbers recovered by bronchoalveolar lavage 48 h after the allergen challenge compared to vehicle-treated mice. Intranasal administration of CGH2466 in this animal model of asthmatic inflammation, 15 min before and 24 h after the challenge, inhibited the ovalbumin-induced airway eosinophilia in a dose-dependent manner, reaching significant levels at a dose of 3 and 10 mg kg−1 (Figure 4a). Similar results were found by analysing the numbers of lymphocytes and neutrophils (34±7 and 23±3% of control at 10 mg kg−1, respectively, data not shown). In contrast, none of the other compounds tested reached statistically significant inhibitions at a dose of 3 mg kg−1 on any cell type analysed. Significant but less pronounced inhibitions were obtained following treatment with 10 mg kg−1 cilomilast or SB203580 whereas CGS15943 was without significant effect. The three active compounds were also tested after oral administration. When given at a dose of 10 mg kg−1, 1 h before and 24 h after the challenge, CGH2466 completely inhibited the ovalbumin-induced bronchoalveolar lavage eosinophilia (Figure 4b). The p38 MAP kinase inhibitor also significantly suppressed the allergen-mediated eosinophilia but again to a lesser extent whereas cilomilast had no inhibitory effect at this dose.
Figure 4.
Effect of compounds on ovalbumin-induced bronchoalveolar lavage eosinophilia. Sensitised C57BL/6 mice were challenged with an aerosolised solution of either phosphate-buffered saline (PBS) or ovalbumin (OA). Compounds or vehicle were given (a) intransally 15 min before and 24 h after the ovalbumin challenge or (b) orally, at a dose of 10 mg kg−1, an hour before and 24 h after the challenge. Animals were killed 48 h after the challenge. Data are expressed as the mean±s.e.m. of two different experiments, each included six to eight mice per group. Significance versus vehicle-treated mice and ovalbumin-challenged animals is indicated by *(P<0.05).
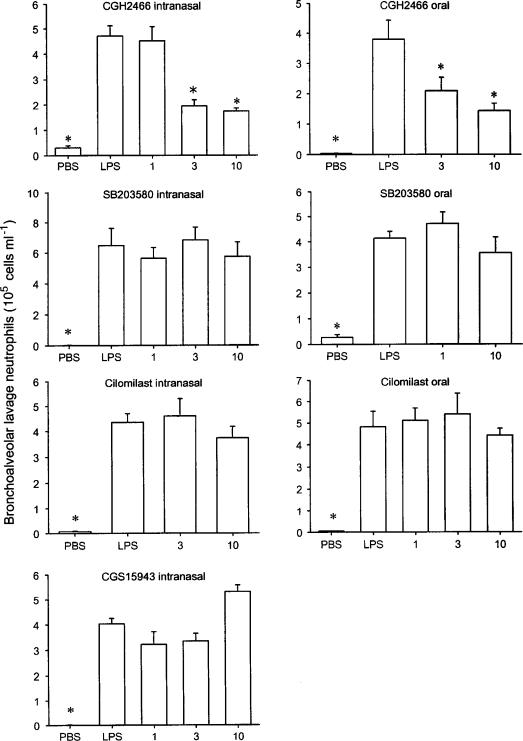
CGH2466 was also tested in a LPS-induced neutrophilic lung inflammation model (Figure 5). The intranasal administration of LPS to BALB/c mice induced a significant increase in neutrophil numbers present in bronchoalveolar lavage fluid within 3 h following the challenge. Half an hour pretreatment with CGH2466, via the intranasal route, significantly inhibited this LPS-induced neutrophil accumulation at doses of 3 and 10 mg kg−1. None of the other compounds tested showed any significant inhibition up to 10 mg kg−1. When given orally, half an hour before the LPS challenge, the compounds showed a similar efficacy profile. CGH2466 significantly inhibited the neutrophils influx at doses of 3 and 10 mg kg−1, whereas SB203580 and cilomilast were inactive up to 10 mg kg−1 (Figure 5).
Figure 5.
Effect of compounds on lipopolysaccharide-induced bronchoalveolar lavage neutrophilia. BALB/c mice were intranasally challenged with either phosphate-buffered saline (PBS) or lipopolysaccharide (LPS). Compounds or vehicle were given, intranasally or orally, 30 min or 1 h before the LPS challenge, respectively. Animals were killed 3 h after the challenge. Data are expressed as the mean±s.e.m. (n=8–10 mice per group). Significance versus vehicle-treated mice and LPS-challenged animals is indicated by *(P<0.05). Doses are in mg kg−1.
Discussion
The results of the present study clearly demonstrate the effectiveness of the novel and potent combined adenosine A1, A2b and A3 receptors antagonist, PDE4D and p38 MAP kinase inhibitor CGH2466 in reducing proinflammatory cytokine production in vitro and eosinophilic and neutrophilic pulmonary inflammation in vivo. The major findings of this study include: (1) the demonstration of potent antagonism of CGH2466 at the A1, A2b and A3 adenosine receptors, and inhibitory action at PDE4D and p38 MAP kinases; (2) potent and superior inhibition of proinflammatory cytokines production and release of oxygen radicals by human leucocytes compared to the nonselective adenosine receptor antagonist or enzyme inhibitors used alone and (3) more potent in vivo inhibition of ovalbumin-induced pulmonary eosinophilia and LPS-induced pulmonary neutrophilia compared to standard blockers of single proteins used alone.
Adenosine affects the functioning of a wide variety of cells by interacting with one or more of the G-protein coupled receptor subtypes, A1, A2a, A2b and A3 (Klotz, 2000). The two receptors that are believed to be central to the role of adenosine in the pathophysiology of asthma are the A2b and A3 receptors (Kohno et al., 1996; Feoktistov et al., 1998). However, the importance of these receptor types varies with the species studied (Fozard & Hannon, 2000). In human mast cells, it is the A2b receptor which facilitates allergen-induced preformed mediator and cytokine release when activated and A3 receptors appears to play a role in eosinophil activation and survival (Kohno et al., 1996). CGH2466 was found to have equal antagonist effects on A1, A2b and A3 but no antagonism on A2a. Indeed, the blockade of A2a receptors could be disadvantageous since the anti-inflammatory effects of endogenous adenosine are mediated by this site and this receptor is also associated with cardiovascular side effects (Thomas et al., 2000; Fozard & McCarthy, 2002). On this basis, an adenosine receptor antagonist such as CGH2466 without A2a activity would represent an improved theophylline since the beneficial effects should be maintained, or even enhanced, but the therapeutic index widened.
CGH2466 is not only a potent adenosine receptor antagonist but was also a PDE4 inhibitor. No inhibition of PDE1, 2, 3, 5, 6, 7 was found and within the PDE4 family, the compound was quite selective on PDE4D with about 30-fold less inhibition on PDE4A and 60-fold selectivity over PDE4B. PDE4 is the predominant family of PDEs in inflammatory cells including mast cell, eosinophils, T cells and monocytes, suggesting that PDE4 inhibitors would be useful as an anti-inflammatory treatment in allergic diseases such as asthma (Torphy, 1998). Indeed, multiple PDE4 inhibitors were tested in animal models of asthma and were found to potently reduce the eosinophil infiltration and the bronchial hyper-reactivity response to allergen (Teixeira et al., 1997). However, most of the PDE4 inhibitors tested in the clinic have had side effects, in particular nausea and vomiting, side effects that also limit the use of theophylline (Page, 1999). It is possible that the side effects observed with these types of PDE4 inhibitor is due to inhibition of particular subtypes of PDE4 and it is expected that subtype selective inhibitors may preserve the anti-inflammatory effect while having less propensity to side effects. It has indeed been suggested that PDE4D inhibition is likely to be responsible for emesis (Robichaud et al., 2002). The data supporting this hypothesis were generated in genetically modified mice using a surrogate marker for emesis (i.e. reversing of α-2-adrenoceptor-mediated anaesthesia). In man, however, the situation appears to be different, since cilomilast a relatively selective PDE4D inhibitor is only one of two PDE4 inhibitors that are currently in phase III studies for COPD. Although cilomilast showed some side effects in clinical studies, the adverse events have been generally mild to moderate, transient and self-limiting (Zussman et al., 2001; Gamble et al., 2003). Thus, CGH2466 based on its profile of antagonism at adenosine receptor and selective inhibition of PDE4D might prove to be a considerably improved theophylline.
Owing to the structural similarity of the compound class with the known p38 MAP kinase inhibitor SB203580, CGH2466 was also tested for inhibition of p38 MAP kinase and other kinases. CGH2466 showed inhibition of p38α and p38β but no inhibition for the other kinases tested. Two of the four known p38 MAP kinase isoforms (α, β, γ, δ) are considered important in processes critical to the inflammatory response and tissue remodelling. The prominent p38 MAP kinases in monocytes and macrophages are p38α and p38β and are best known for their effects in TNF-α production and signalling. Moreover, the production and action of many of the potential mediators of airway inflammation have been shown to be dependent upon the p38 MAP kinase cascade (Herlaar & Brown, 1999). In addition, p38 MAP kinase inhibitors have also been shown to reduce persistent airway eosinophilia and enhance eosinophil apoptosis, activities that provide additional mechanisms by which p38 MAP kinase inhibitors may provide a therapeutic benefit in chronic airway inflammation (Underwood et al., 2000). CGH2466 is indeed a potent inhibitor of TNF-α and IFN-γ production, however, most likely through a combination of inhibition of p38 MAP kinase and PDE4 inhibition. Moreover, this compound was also more active than the p38 MAP kinase inhibitor SB203580 in both in vivo models of eosinophilic and neutrophilic airway infiltration.
In conclusion, in a search for an improved theophylline with higher effectiveness, we discovered a compound which combines adenosine receptor antagonism, p38 MAP kinase and PDE4 inhibition and demonstrated potent anti-inflammatory effects in vitro and in vivo. This combination of activity appears to be far more efficacious as CGH2466 has a more pronounced anti-inflammatory response compared to the more selective enzyme inhibitors or nonselective adenosine receptor antagonist, used alone. Moreover, due to its selectivity for specific subtypes of adenosine receptors and PDE4 isoenzymes, CGH2466 might exhibit a better side effect profile than that of theophylline. Thus, CGH2466, by targeting key mechanisms known to be involved in chronic airway inflammatory responses, may have therapeutic utility in lung diseases such as asthma and COPD.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- IFN-γ
interferon-gamma
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- OA
ovalbumin
- PBS
phosphate-buffered saline
- PDE
phosphodiesterase
- TNF-α
tumour necrosis factor
References
- BARNES P.J. Current therapies for asthma. Promise and limitations. Chest. 1997;111:17S–26S. doi: 10.1378/chest.111.2_supplement.17s. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Theophylline: new perspectives for an old drug. Am. J. Respir. Crit. Care Med. 2003;167:813–818. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., PEDERSEN S., BUSSE W.W. Efficacy and safety of inhaled corticosteroids. New developments. Am. J. Respir. Crit. Care Med. 1998;157:S1–S53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- BERMAN H.M., WESTBROOK J., FENG Z., GILLILAND G., BHAT T.N., WEISSIG H., SHINDYALOV I.N., BOURNE P.E. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHM J.C., SMIETANA J.M., SORENSON M.E., GARIGIPATI R.S., GALLAGHER T.F., SHELDRAKE P.L., BRADBEER J., BADGER A.M., LAYDON J.T., LEE J.C., HILLEGASS L.M., GRISWOLD D.E., BRETON J.J., CHABOT-FLETCHER M.C., ADAMS J.L. 1-substituted 4-aryl-5-pyridinylimidazoles: a new class of cytokine suppressive drugs with low 5-lipoxygenase and cyclooxygenase inhibitory potency. J. Med. Chem. 1996;39:3929–3937. doi: 10.1021/jm960415o. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN S.B., GUIDER A., FORSTER C.J., GLEASON J.G., BENDER P.E., KARPINSKI J.M., DEWOLF W.E., JR, BARNETTE M.S., UNDERWOOD D.C., GRISWOLD D.E., CIESLINSKI L.B., BURMAN M., BOCHNOWICZ S., OSBORN R.R., MANNING C.D., GROUS M., HILLEGAS L.M., BARTUS J.O., RYAN M.D., EGGLESTON D.S., HALTIWANGER R.C., TORPHY T.J. 1,4-Cyclohexanecarboxylates: potent and selective inhibitors of phosophodiesterase 4 for the treatment of asthma. J. Med. Chem. 1998;41:821–835. doi: 10.1021/jm970090r. [DOI] [PubMed] [Google Scholar]
- CORTELING R., WYSS D., TRIFILIEFF A. In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacol. 2002;2:1. doi: 10.1186/1471-2210-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGELS P., SULLIVAN M., MULLER T., LUBBERT H. Molecular cloning and functional expression in yeast of a human cAMP-specific phosphodiesterase subtype (PDE IV-C) FEBS Lett. 1995;358:305–310. doi: 10.1016/0014-5793(94)01460-i. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J., BELVISI M.G., BIRRELL M.A., WEBBER S.E., FOSTER M.L., SARGENT C.A. Effect of the p38 kinase inhibitor, SB 203580, on allergic airway inflammation in the rat. Br. J. Pharmacol. 2000;131:173–176. doi: 10.1038/sj.bjp.0703605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D.J., TAYLOR D.A., ZETTERSTROM O., CHUNG K.F., O'CONNOR B.J., BARNES P.J. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N. Engl. J. Med. 1997;337:1412–1418. doi: 10.1056/NEJM199711133372002. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., POLOSA R., HOLGATE S.T., BIAGGIONI I. Adenosine A2B receptors: a novel therapeutic target in asthma. Trends Pharmacol. Sci. 1998;19:148–153. doi: 10.1016/s0165-6147(98)01179-1. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R., HANNON J.P. Species differences in adenosine receptor-mediated bronchoconstrictor responses. Clin. Exp. Allergy. 2000;30:1213–1220. doi: 10.1046/j.1365-2222.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R., MCCARTHY C. Adenosine receptor ligands as potential therapeutics in asthma. Curr. Opin. Investig. Drugs. 2002;3:69–77. [PubMed] [Google Scholar]
- GAMBLE E., GROOTENDORST D.C., BRIGHTLING C.E., TROY S., QIU Y., ZHU J., PARKER D., MATIN D., MAJUMDAR S., VIGNOLA A.M., KROEGEL C., MORELL F., HANSEL T.T., RENNARD S.I., COMPTON C., AMIT O., TAT T., EDELSON J., PAVORD I.D., RABE K.F., BARNES N.C., JEFFERY P.K. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;168:976–982. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- GRISWOLD D.E., WEBB E.F., BADGER A.M., GORYCKI P.D., LEVANDOSKI P.A., BARNETTE M.A., GROUS M., CHRISTENSEN S., TORPHY T.J. SB 207499 (Ariflo), a second generation phosphodiesterase 4 inhibitor, reduces tumor necrosis factor α and interleukin-4 production in vivo. J. Pharmacol. Exp. Ther. 1998;287:705–711. [PubMed] [Google Scholar]
- HANNON J.P., TIGANI B., WOLBER C., WILLIAMS I., MAZZONI L., HOWES C., FOZARD J.R. Evidence for an atypical receptor mediating the augmented bronchoconstrictor response to adenosine induced by allergen challenge in actively sensitized Brown Norway rats. Br. J. Pharmacol. 2002;135:685–696. doi: 10.1038/sj.bjp.0704516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERLAAR E., BROWN Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- IWAMURA H., SUZUKI H., UEDA Y., KAYA T., INABA T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB(2) receptor. J. Pharmacol. Exp. Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- JEFFERY P.K. Comparison of the structural and inflammatory features of COPD and asthma, Giles F. Filley Lecture. Chest. 2000;117:251–260. doi: 10.1378/chest.117.5_suppl_1.251s. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., DE ZWART M., CHANG L., MORO S., VON FRIJTAG DRABBE KUNZEL J.K., MELMAN N., IJZERMAN A.P., JACOBSON K.A. Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J. Med. Chem. 1998;41:2835–2845. doi: 10.1021/jm980094b. [DOI] [PubMed] [Google Scholar]
- KLOTZ K.N. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- KOHNO Y., JI X., MAWHORTER S.D., KOSHIBA M., JACOBSON K.A. Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood. 1996;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- LAPPE R.W., SHELDON J.H., COX B.F. Selective adenosine-2 agonist produces both direct and reflex tachycardia in normotensive rats. J. Cardiovasc. Pharmacol. 1992;19:460–463. doi: 10.1097/00005344-199203000-00025. [DOI] [PubMed] [Google Scholar]
- PAGE C.P. Recent advances in our understanding of the use of theophylline in the treatment of asthma. J. Clin. Pharmacol. 1999;39:237–240. [PubMed] [Google Scholar]
- RABE K.F., DENT G. Theophylline and airway inflammation. Clin. Exp. Allergy. 1998;28 Suppl 3:35–41. [PubMed] [Google Scholar]
- REVESZ L., DI PADOVA F.E., BUHL T., FEIFEL R., GRAM H., HIESTAND P., MANNING U., ZIMMERLIN A.G. SAR of 4-hydroxypiperidine and hydroxyalkyl substituted heterocycles as novel p38 map kinase inhibitors. Bioorganic Med. Chem. Lett. 2000;10:1261–1264. doi: 10.1016/s0960-894x(00)00200-6. [DOI] [PubMed] [Google Scholar]
- ROBICHAUD A., STAMATIOU P.B., JIN S.L.C., LACHANCE N., MACDONALD D., LALIBERTE F., LIU S., HUANG Z., CONTI M., CHAN C.C. Deletion of phosphodiesterase 4D in mice shortens {alpha}2-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J. Clin. Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-α on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., GRISTWOOD R.W., COOPER N., HELLEWELL P.G. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future. Trends Pharmacol. Sci. 1997;18:164–171. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- THOMAS T., ST LAMBERT J.H., DASHWOOD M.R., SPYER K.M. Localization and action of adenosine A2a receptors in regions of the brainstem important in cardiovascular control. Neuroscience. 2000;95:513–518. doi: 10.1016/s0306-4522(99)00473-x. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TRIFILIEFF A., EL-HASHIM A., BERTRAND C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1120–L1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- TRIFILIEFF A., WYSS D., WALKER C., MAZZONI L., HERSPERGER R. Pharmacological profile of a novel phosphodiesterase 4 inhibitor, 4-(8-benzo[1,2,5]oxadiazol-5-yl-[1,7]naphthyridin-6-yl)-benzoic acid (NVP-ABE171), a 1,7-naphthyridine derivative, with anti-inflammatory activities. J. Pharmacol. Exp. Ther. 2002;301:241–248. doi: 10.1124/jpet.301.1.241. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD D.C., OSBORN R.R., KOTZER C.J., ADAMS J.L., LEE J.C., WEBB E.F., CARPENTER D.C., BOCHNOWICZ S., THOMAS H.C., HAY D.W., GRISWOLD D.E. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J. Pharmacol. Exp. Ther. 2000;293:281–288. [PubMed] [Google Scholar]
- VANGREVELINGHE E., ZIMMERMANN K., SCHOEPFER J., PORTMANN R., FABBRO D., FURET P. Discovery of a potent and selective protein kinase CK2 inhibitor by high-throughput docking. J. Med. Chem. 2003;46:2656–2662. doi: 10.1021/jm030827e. [DOI] [PubMed] [Google Scholar]
- WANG Z., CANAGARAJAH B.J., BOEHM J.C., KASSISA S., COBB M.H., YOUNG P.R., ABDEL-MEGUID S., ADAMS J.L., GOLDSMITH E.J. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6:1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- WILSON K.P., FITZGIBBON M.J., CARON P.R., GRIFFITH J.P., CHEN W., MCCAFFREY P.G., CHAMBERS S.P., SU M.S. Crystal structure of p38 mitogen-activated protein kinase. J. Biol. Chem. 1996;271:27696–27700. doi: 10.1074/jbc.271.44.27696. [DOI] [PubMed] [Google Scholar]
- WILSON K.P., MCCAFFREY P.G., HSIAO K., PAZHANISAMY S., GALULLO V., BEMIS G.W., FITZGIBBON M.J., CARON P.R., MURCKO M.A., SU M.S. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem. Biol. 1997;4:423–431. doi: 10.1016/s1074-5521(97)90194-0. [DOI] [PubMed] [Google Scholar]
- XU R.X., HASSELL A.M., VANDERWALL D., LAMBERT M.H., HOLMES W.D., LUTHER M.A., ROCQUE W.J., MILBURN M.V., ZHAO Y., KE H., NOLTE R.T. Atomic structure of PDE4: insights into phosphodiesterase mechanism and specificity. Science. 2000;288:1822–1825. doi: 10.1126/science.288.5472.1822. [DOI] [PubMed] [Google Scholar]
- YASUI K., AGEMATSU K., SHINOZAKI K., HOKIBARA S., NAGUMO H., NAKAZAWA T., KOMIYAMA A. Theophylline induces neutrophil apoptosis through adenosine A2A receptor antagonism. J. Leukoc. Biol. 2000;67:529–535. doi: 10.1002/jlb.67.4.529. [DOI] [PubMed] [Google Scholar]
- ZUSSMAN B.D., BENINCOSA L.J., WEBBER D.M., CLARK D.J., COWLEY H., KELLY J., MURDOCH R.D., UPWARD J., WYLD P., PORT A., FUDER H. An overview of the pharmacokinetics of cilomilast (Ariflo), a new, orally active phosphodiesterase 4 inhibitor, in healthy young and elderly volunteers. J. Clin. Pharmacol. 2001;41:950–958. doi: 10.1177/00912700122010924. [DOI] [PubMed] [Google Scholar]