Abstract
Recently, we demonstrated that lipopolysaccharide (LPS)-induced fever could be suppressed by a selective mu-opioid receptor antagonist, indicating that the mu-opioid system is involved in the LPS fever. In the present study, to confirm the role of the mu-opioid system in the pathogenesis of LPS fever, we used mice lacking the mu-opioid receptor. In the wild type (WT), following intraperitoneal (i.p.) injection of 100 μg kg−1 of LPS, body temperature (Tb) increased approximately 1°C and remained elevated during the 360-min recording period. In the mu-opioid receptor knockout (MOR-KO) mice, the administration of 100 μg kg−1 i.p. of LPS did not induce fever during the recording period. Saline by itself, given i.p., did not alter the Tb, either in WT or MOR-KO. These results confirm that the mu-opioid system is involved in LPS-induced fever.
Keywords: Fever, lipopolysaccharide, mu-opioid receptor, body temperature
Introduction
It is generally believed that fever induced by lipopolysaccharide (LPS) is caused by the synthesis and release from monocytes and macrophages of a number of well-characterized pyrogenic factors, including interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and macrophage inflammatory protein-1 (MIP-1β) (Davidson et al., 1990; Long et al., 1990; Blatteis, 1992; Miñano et al., 1996). Prostaglandins, particularly PGE-2, have been proposed to be an essential mediator of the febrile response to most exogenous and endogenous pyrogens (Blatteis & Sehic, 1997). There is considerable evidence to support the involvement of mu-opioid receptors in fever production. IL-1β, which is generally thought to be the primary endogenous pyrogen, has been shown to induce β-endorphin release (Xin et al., 1997) and to modulate opioid receptor binding in the brain (Ahmed et al., 1985). It has been found that the febrile response of guinea pigs to both exogenous Escherichia coli and endogenous pyrogens (IL-6, TNF-α and IFN-α) was significantly attenuated by the prior subcutaneous injection of naloxone (Ahokas et al., 1985; Blatteis et al., 1991; Romanovsky et al., 1994; Zawada et al., 1997). The development of knockout (KO) mice with selective deletions of opioid receptor subtypes provides an opportunity to evaluate the effects of drugs in the absence of receptors rather than in the presence of blockade by pharmacological agents.
The purpose of the present study was to confirm the role of the mu-opioid receptor in LPS-induced fever by using mice lacking the mu-opioid receptor (MOR-KO).
Methods
Animals
KO mice were developed by disruption of exon-1 of the MOR-1 gene through homologous recombination as described previously (Schuller et al., 1999). The 129S6 × C57BL/6J chimeras were directly crossed with 129S6 mice to produce the inbred 129S6 MOR-1 mutant strain, while the 129S6 × C57BL/6J F1 mutants were produced by directly crossing F10 C57BL/6J MOR-1 KOs with the 129S6 MOR-1-deficient strain. Mice weighing 20–30 g were used in this study. They were housed five per cage for at least 1 week before surgery and were fed laboratory chow and water ad libitum. The ambient temperature was 22±2°C and a 12 h light/12 h dark cycle was used. All experiments were started between 09 : 00 and 10 : 00 h to minimize the effect of circadian variation in Tb. All animal use procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Surgery procedures
Mice were anesthetized with an intraperitoneal (i.p.) injection of a mixture of ketamine hydrochloride (100–150 mg kg−1) and acepromazine maleate (0.2 mg kg−1). An incision 0.5 cm in length was made along the linea alba, and the underlying tissue was dissected and retracted. A radio transmitter (model E-4000, Mini-Mitter Co. Inc., OR, U.S.A.) was then implanted in the i.p. space. After the radio transmitter was passed through the incision, the abdominal musculature and dermis were sutured independently. The animals were returned to individual cages in the environmental room.
Measurement of body temperature
At 1 week after surgery, the mice were tested in an environmental room (21±0.3°C ambient temperature and 52±2% relative humidity). After 1 h of adaptation, two readings were averaged to determine the baseline. During the recording period (pre- and postinjection), the Tb was measured at 15-min intervals. Either saline or drug was injected i.p., and Tb was measured by a biotelemetry system using calibrated radio transmitters. Signals from the transmitter were delivered through a computer-linked receiver. This method minimizes stress to animals during the Tb reading. Thus, the Tb could be monitored continuously and recorded without restraint or any disturbance to the animal.
Drugs
LPS from the phenol-extracted preparation of E. coli (0111:B4) was obtained from Sigma-Aldrich (St Louis, MO, U.S.A.) and dissolved in pyrogen-free saline.
Statistical analysis
All results were expressed as mean±s.e.m. Statistical analysis of differences between groups was determined by analysis of variance (ANOVA) followed by Tukey's test. A value of P less than 0.05 was considered statistically significant.
Results
Mean Tb before injection was 36.68±0.13°C for the WT group, 36.79±0.12 for MOR-KO, 36.74±0.14 for WT/saline group and 36.71±0.17 MOR-KO/saline group. There was no significant difference in baseline Tb among these groups.
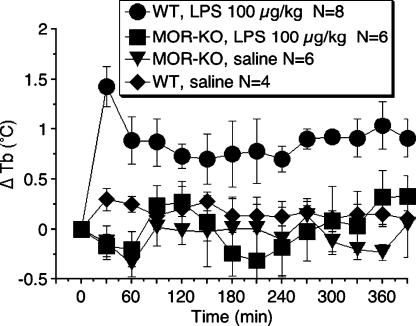
Animals were injected i.p. with either LPS (100 μg kg−1) or vehicle (sterile, pyrogen-free saline, 1 μg kg−1), and Tb monitored 360 min after injection (Figure 1). The administration of LPS (100 μg kg−1, i.p.) to WT caused an increase in Tb of approximately 1°C, which remained elevated throughout the 360-min recording period. However, LPS (100 μg kg−1, i.p.) administration to MOR-KO did not show any increase in Tb compared to WT (F3,40=2.84, P<0.001).
Figure 1.
Effects of LPS on body temperature response in WT or mu-opioid receptor knockout mice. LPS (100 μg kg−1) injected at time zero. Data are expressed as the mean±s.e.m. of body temperature. N, number of mice. ΔTb, change in body temperature from baseline (time 0).
Discussion
The present studies show that the i.p. injection of LPS (100 μg kg−1, i.p.) produced a significant elevation in Tb in WT during the 360-min recording period. However, the administration of LPS (100 μg kg−1, i.p.) to MOR-KO did not evoke any increase in Tb during the same recording period. These data further substantiate the finding that the presence of mu-opioid receptors is essential for LPS-induced fever in mice and confirm our previous pharmacological study showing that mu-opioid receptors mediate the fever induced by LPS (Benamar et al., 2000). The results are consistent with other previous pharmacological studies: naloxone, a general opioid receptor antagonist given i.p. antagonized the IL-6 response induced by i.c.v. or i.p. IL-1 (Bertolucci et al., 1996); the IL-6-induced fever can be blocked by pretreatment with a selective mu-opioid receptor antagonist (Benamar et al., 2002). In examining the effect of genetic ablation of mu-opioid receptors on Tb, we found that basal Tb was not significantly modified, suggesting that endogenous opioids do not exert a tonic control on Tb or that other systems may compensate for the absence of mu-opioid receptors.
Considerable evidence indicates that many circulating cytokines, such as IL-1β, IL-6, TNF-α, and others, act as endogenous pyrogens and are responsible for the induction and maintenance of fever (Kluger, 1991; Kozak et al., 1995). Given that cytokines are involved in LPS-induced fever and that evidence shows that mu-opioid receptor antagonists prevent the febrile effects of IL-6 and IL-1 (Xin & Blatteis, 1992; Benamar et al., 2002), an interaction between cytokines and the mu-opioid system may take place during the development of the fever induced by LPS.
In summary, these results further reinforce our earlier finding that the opioid system is involved in the pathogenesis of fever.
Acknowledgments
This work was supported by DA 06650 and DA 13429 from NIDA. We thank Drs Keith Latham and Ellen Unterwald for their help in breeding the knockout mice.
Abbreviations
- LPS
lipopolysaccharide
- MOR-KO
mu-opioid receptor knockout mice
- Tb
body temperature
- WT
wild type
References
- AHMED M.S., LLANOS-Q J., DINARELLO C.A., BLATTEIS C.M. Interleukin-1 reduces opioid binding in guinea pig brain. Peptides. 1985;6:1149–1154. doi: 10.1016/0196-9781(85)90442-5. [DOI] [PubMed] [Google Scholar]
- AHOKAS R.A., SEYDOUX J., LLANOS-Q J., MASHBURN T.A., JR, BLATTEIS C.M. Hypothalamic opioids and the acute-phase glycoprotein response in guinea pigs. Brain Res. Bull. 1985;15:603–608. doi: 10.1016/0361-9230(85)90210-2. [DOI] [PubMed] [Google Scholar]
- BENAMAR K., GELLER E.B., ADLER M.W. Effect of a μ-opioid receptor-selective antagonist on interleukin-6 fever. Life Sci. 2002;70:2139–2145. doi: 10.1016/s0024-3205(01)01535-1. [DOI] [PubMed] [Google Scholar]
- BENAMAR K., XIN L., GELLER E.B., ADLER M.W. Blockade of lipopolysaccharide-induced fever by a μ-opioid receptor-selective antagonist in rats. Eur. J. Pharmacol. 2000;401:161–165. doi: 10.1016/s0014-2999(00)00424-6. [DOI] [PubMed] [Google Scholar]
- BERTOLUCCI M., PEREGO C., DESIMONI M.G. Central opiate modulation of peripheral IL-6 in rats. Neuro. Rep. 1996;7:1181–1184. doi: 10.1097/00001756-199604260-00017. [DOI] [PubMed] [Google Scholar]
- BLATTEIS C.M.The pyrogenic action of cytokines Interleukin-1 in the Brain 1992Oxford: Pergamon Press; 93–114.eds. Rothwell, N.J. & Dantzer, R.D. pp [Google Scholar]
- BLATTEIS C.M., SEHIC E. Fever: how may circulating pyrogens signal the brain. News Physiol. Sci. 1997;12:1–9. [Google Scholar]
- BLATTEIS C.M., XIN L., QUAN N. Neuromodulation of fever: apparent involvement of opioids. Brain Res. Bull. 1991;26:219–223. doi: 10.1016/0361-9230(91)90230-h. [DOI] [PubMed] [Google Scholar]
- DAVIDSON A., MILTON A.S., ROTONDO D. A study of the pyrogenic actions of interleukin-1α and interleukin-1β: Interaction with a steroidal and non-steroidal antiinflammatory agent. Br. J. Pharmacol. 1990;100:542–546. doi: 10.1111/j.1476-5381.1990.tb15843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUGER M.J. Fever: role of pyrogens and cryogens. Physiol. Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZAK W., ZHENG H., CONN C.A., SOSZYNSKI D., VAN DER PLOEG L.H., KLUGER M.J. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- LONG N.C., OTTERNESS I., KUNKEL S., VANDER A.J., KLUGER M.J. Roles of interleukin 1β and tumor necrosis factor in lipopolysaccharide fever in rats. Am. J. Physiol. 1990;259:R724–R728. doi: 10.1152/ajpregu.1990.259.4.R724. [DOI] [PubMed] [Google Scholar]
- MIÑANO F.J., FERNÁNDEZ-ALONSO A., BENAMAR K., MYERS R.D., SANCIBRIÁN M., RUIZ R.M., ARMENGOL J.A. Macrophage inflammatory protein-1β (MIP-1β) produced endogenously in brain during E. coli fever in rats. Eur. J. Neurosci. 1996;8:424–428. doi: 10.1111/j.1460-9568.1996.tb01225.x. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SHIDO O., UNGAR A.L., BLATTEIS C.M. Peripheral naloxone attenuates lipopolysaccharide fever in guinea pigs by an action outside the blood–brain barrier. Am. J. Physiol. 1994;266:R1824–R1831. doi: 10.1152/ajpregu.1994.266.6.R1824. [DOI] [PubMed] [Google Scholar]
- SCHULLER A.G.P., KING M.A., ZHANG J., BOLAN E., PAN Y.-X., MORGAN D.J., CHANG A., CZICK M.E., UNTERWALD E.M., PATERNAK G.W., PINTAR J.E. Retention of heroin and morphine-6β-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat. Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- XIN L., BLATTEIS C.M. Hypothalamic neuronal responses to interleukin-6 in tissue slices: effects of indomethacin and naloxone. Brain Res. Bull. 1992;29:27–35. doi: 10.1016/0361-9230(92)90005-i. [DOI] [PubMed] [Google Scholar]
- XIN L., ZHAO S.F., GELLER E.B., MCCAFFERTY M.R., STERLING G.H., ADLER M.W. Involvement of β-endorphin in the preoptic anterior hypothalamus during interleukin-1β-induced fever in rats. Ann. N.Y. Acad. Sci. 1997;813:324–326. doi: 10.1111/j.1749-6632.1997.tb51714.x. [DOI] [PubMed] [Google Scholar]
- ZAWADA W.M., CLARKE J., RUWE W.D. Naloxone differentially alters fevers induced by cytokines. Neurochem. Int. 1997;30:441–448. doi: 10.1016/s0197-0186(96)00080-0. [DOI] [PubMed] [Google Scholar]