Abstract
We investigated muscarinic receptors in the detrusor and mucosa of the human bladder body. Radioligand-binding studies with [3H]QNB were conducted using specimens collected from patients (36–77 years) with normal bladder function, undergoing surgery. For RT–PCR, biopsies of normal bladder were obtained from patients (30–88 years) undergoing check cystoscopy.
Binding of [3H]QNB in detrusor (n=20) was of high affinity (KD 77.1 (55.2–99.0) pM) and capacity (Bmax 181±7 fmol mg protein−1). Similar values were obtained in mucosa (n=6) (KD 100.5 (41.2–159.9) pM; Bmax 145±9 fmol mg protein−1).
Competition-binding experiments in detrusor membranes with muscarinic receptor antagonists including trospium, darifenacin, 4-DAMP, methoctramine, AQ-RA 741, AF-DX 116 and pirenzepine indicated a receptor population of 71% M2, 22% M3 and 7% M1. In the mucosa, 75% of sites were M2 receptors, with 25% M3/M5.
Using RT–PCR, expression of M1, M2, M3 and M5 mRNA was demonstrated in both detrusor and mucosa.
The presence of a high density of mainly M2 muscarinic receptors in the mucosa appears to be a novel finding and raises the question of their physiological significance and the source of their endogenous ligand.
There was a negative correlation of receptor number (Bmax) with age in detrusor muscle from male patients (P=0.02). Quantitative competitive RT–PCR demonstrated a selective age-related decrease in mRNA for muscarinic M3 but not M2 receptors, in both male (P<0.0001) and female (P=0.019) detrusor. These findings correspond with reports of decreased detrusor contractility with ageing.
Keywords: Smooth muscle, detrusor, urothelium, urinary bladder, [3H]QNB, muscarinic receptors, human, M3, QCRT–PCR
Introduction
Five subtypes of G protein-coupled muscarinic receptors (M1–M5) have been cloned and pharmacologically characterised. In the urinary bladder, as in other smooth muscles, multiple muscarinic receptor subtypes have been identified (Eglen et al., 1996). Binding and immunoprecipitation studies in human detrusor (Nilvebrant et al., 1985; Wang et al., 1995; Goepel et al., 1998) have shown that the majority of muscarinic receptors present are of the M2 subtype. Although there is evidence that M2 receptors are of some functional importance (Matsui et al., 2002; Ehlert, 2003), particularly in pathological states (Braverman & Ruggieri, 2003; Pontari et al., 2004), functional experiments in M3 knockout mice (Matsui et al., 2000) and human detrusor strips (Chess-Williams et al., 2001; Fetscher et al., 2002) have demonstrated that muscarinic M3 receptors are the main mediators of the contractile response.
Muscarinic receptor antagonists (anticholinergics) are the mainstay of treatment for the overactive bladder (Andersson & Yoshida, 2003), for example, in patients with frequency and urgency of micturition, with or without urge incontinence. Unfortunately, their lack of organ selectivity has resulted in many patients experiencing dry mouth and/or constipation. Recently, muscarinic receptor antagonists with greater subtype selectivity (darifenacin and methoctramine) or higher potency (trospium) have become available, but have not been used to more precisely define receptor subtypes in the human detrusor.
Historically, the urothelium has been considered a simple inert barrier. However, the urothelium is metabolically active and some reviews of recent evidence suggest that the tissue acts as an important regulator of bladder contractility (Chess-Williams, 2002; Fry et al., 2004). Radioligand-binding studies with [3H]quinuclidinyl benzylate ([3H]QNB) in the pig urothelium have indicated that this tissue possesses a large number of muscarinic receptors (Hawthorn et al., 2000). Therefore, these urothelial receptors may represent a second site of action for the muscarinic receptor antagonists that are used to treat overactive bladder.
A recent large survey reported that 16.6% of European adults reported symptoms of overactive bladder, with the symptoms increasing consistently with advancing age (Milsom et al., 2001). Urodynamic tests have demonstrated an age-related reduction in bladder capacity, and an increased incidence of uninhibited contractions, decreased urinary flow rate and incomplete bladder emptying (Madersbacher et al., 1998). Despite these known age-related alterations in detrusor function, changes in muscarinic receptors with age have not been extensively studied in the human bladder.
The initial aims of this study were (1) to determine the affinity and density of muscarinic receptor proteins in both detrusor and mucosa (urothelium and lamina propria) of the human urinary bladder; (2) to use a range of muscarinic receptor antagonists to pharmacologically characterise the muscarinic receptor subtypes present in these regions; (3) to document the expression of muscarinic receptor subtype mRNA in detrusor and mucosa. During the course of this study, some age-related changes became apparent, and therefore we also examined (4) age-related changes in muscarinic receptor protein density (Bmax) in male detrusor, and (5) age-related changes in expression of M2 and M3 receptor mRNA in the detrusor from both males and females.
Methods
Patients and specimens
Collection of human bladder specimens was approved by the Human Ethics Committee of the University of New South Wales (HREC 03175). All patients displayed normal micturition frequency with no symptoms of urge incontinence or obstruction. Patients were characterised by their clinician as having no evidence of overactive bladder (frequency, nocturia with or without urge incontinence) nor features of outflow obstruction (poor stream, incomplete emptying). Previous pelvic radiotherapy or current bladder infection were exclusion criteria.
Bladder segment collection
Whole-wall segments of macroscopically normal bladder (body) were collected from 33 patients (25 males, 8 females; age range 36–77 years) undergoing open bladder surgery (15 cystectomy for malignancy, 15 radical prostatectomy for malignancy, two colposuspension and one ileal conduit). Bladder segments were placed immediately into ice-cold Krebs-Henseleit solution (composition in mM: NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5 and D-glucose 11.7), pre-gassed with carbogen (95% O2, 5% CO2). Specimens were immediately transported to the laboratory and transferred to fresh, cold, pre-gassed solution. They were either refrigerated overnight before dissection or dissected immediately (Zeng et al., 1995).
The bladder segments were first separated into detrusor muscle and mucosa (containing urothelium and lamina propria) and then cut into portions of approximately 500 mg, which were frozen in liquid nitrogen and then stored at −70°C until use in radioligand-binding experiments. All 33 detrusor specimens and eight (seven male and one female patients, age range 51–71 years) mucosal specimens were used for radioligand binding.
Bladder biopsy collection
Bladder biopsies (3 × 4 mm2, 5–20 mg) were obtained from 73 patients (41 males, 32 females, age range 30–88 years) undergoing check cystoscopy for previous bladder cancer or asymptomatic haematuria. Cold-cup biopsies were taken from a site 2 cm lateral and cephalad from the left ureteric orifice. They were washed in saline, followed by immediate collection into ice-cold RNA Later (Ambion) in the operating theatre. After overnight storage at 4°C, biopsies were dissected into detrusor muscle and mucosa (containing urothelium and lamina propria) and stored at −80°C until RNA was extracted (see below).
Radioligand binding studies
Membrane preparation
Radioligand binding studies were carried out on bladder segments as described previously (Mansfield et al., 2003). Approximately 500 mg detrusor muscle or mucosa was finely minced in ice-cold sodium phosphate buffer (10 ml, 50 mM Na2HPO4, pH 7.4) and homogenised with a Polytron (setting 5, for 3 × 10 s). The suspension was then centrifuged at 1000 × g for 15 min. The pellet was discarded and the supernatant re-centrifuged at 40,000 × g for 20 min. The final pellet was resuspended in 10 ml of 50 mM sodium phosphate buffer, pH 7.4.
Kinetic, saturation and competition studies
These were carried out using the nonselective radioligand [3H]QNB in a final volume of 0.5 ml of 50 mM phosphate buffer (pH 7.4) at 37°C. Nonspecific binding was defined in replicate tubes using 10 μM atropine. The incubation was initiated by addition of detrusor or mucosal membranes (2% wet weight final tissue concentration) to each tube. Binding of radioligand was <10% of total radioactivity in most experiments.
In preliminary studies, 200 pM [3H]QNB was incubated with human detrusor muscle membranes at six time points for up to 3 h. Equilibrium appeared to be reached at approximately 1 h, and an incubation time of 2 h was chosen for subsequent experiments. In saturation experiments, eight concentrations of [3H]QNB (15 pM to 2 nM) were incubated with detrusor or mucosal membranes for 2 h at 37°C. Protein content was determined by the Lowry method using BSA as a standard.
In competition studies, increasing concentrations of muscarinic receptor antagonists were incubated in 50 mM sodium phosphate buffer (pH 7.4) with detrusor or mucosal membranes and 200 pM [3H]QNB for 2 h, before filtration and washing as above. For most compounds, 13 concentrations of competitor were used.
Incubations were terminated by addition of 3 ml ice-cold 50 mM sodium phosphate buffer (pH 7.4). Membranes were filtered using a tissue harvester (Brandel Inc., Gaithersburg, U.S.A.) through GF/B filters (Whatman, Maidstone, U.K.) pre-soaked in sodium phosphate buffer containing 0.5% polyethyleneimine (PEI) and 10 μM atropine. The filters were washed three times with 3 ml of ice-cold buffer and then placed into scintillation vials containing 2 ml scintillant (Beckman Ready Safe, Fullerton, U.S.A.). Vials were left overnight before measurement of radioactivity using liquid scintillation spectrometry (TriCarb Model 1900TR, Packard, Meriden, U.S.A.).
Data analysis
For saturation studies, data from individual experiment were fitted with a one-site binding model using the nonlinear regression analysis program of GraphPad Prism (version 3.0, GraphPad Software Inc., San Diego, U.S.A.), to derive the binding parameters. The dissociation constant (KD, in pM) is expressed as geometric mean (95% confidence limits) and the maximum number of binding sites present (Bmax, in fmol mg protein−1) is expressed as mean±s.e.m.
For competition studies, all data were simultaneously analysed using the nonlinear regression analysis program of GraphPad Prism (version 3.0) and tested to determine if a one-site or two-site model was statistically preferred (F-test, P<0.05). Dissociation constants (Ki values with 95% confidence limits) of competitors for [3H]QNB-binding sites were calculated according to the formula Ki=IC50/(1+L/KD), where L is the concentration of radioligand, KD is the dissociation constant of the radioligand and IC50 denotes 50% inhibition of specific binding by the competitor. Global analyses of data were performed using GraphPad Prism (version 4.0).
Molecular studies
RNA extraction and RT–PCR
Total RNA was extracted from human bladder biopsy specimens of detrusor and mucosa, using the Epicentre RNA purification kit. Contaminating DNA was removed by two treatments with RNase-Free DNase at 37°C for 30 min. To monitor the quality of RNA and DNA contamination, RT–PCR amplification of the β-actin transcript using Access RT–PCR System was carried out in the presence and absence of Amv reverse transcriptase. Only good quality and DNA-free RNA samples were used for RT–PCR.
Expression of muscarinic receptor subtype transcripts in human bladder detrusor and mucosa was determined by RT–PCR with subtype gene-specific primers. For each muscarinic receptor subtype, a pair of primers were designed to amplify a region of the mRNA corresponding to fragments of receptor lacking any homology with other subtypes (Table 1). RT–PCR was performed using the Access RT–PCR System, following the manufacturer's instruction. Briefly, a 25 μl reaction mixture contained total RNA (100 ng for M2 and M3, and 500 ng for M1, M4 and M5), 0.4 μM sense primer, 0.6 μM antisense primer, 0.4 mM dNTPs, 2.5 mM MgSO4 (but 1.2 mM for M3), 2.5 U Amv reverse transcriptase and 2.5 U Tfl DNA polymerase. The RT–PCR reaction was conducted at 48°C for 45 min, 94°C for 2 min and 33 cycles (for M2 and M3) or 35 cycles (for M1, M4 and M5) of 94°C for 30 s, 56°C for 1 min and 70°C for 45 s, followed by a final extension at 70°C for 10 min. The concentration of MgSO4 was optimised and the PCR cycle number was chosen to be in the linear range of amplification, to allow for comparisons of the same receptor mRNA expressed in different regions and different age groups. Human brain total RNA (Becton Dickinson) was used for positive control.
Table 1.
Primer sequence for RT–PCR of muscarinic receptor subtypes and β-actin
| Code | Primer sequence (5′ → 3′) | GenBank accession no. | Sequence position | Fragment size (bp) |
|---|---|---|---|---|
| M1 | Sense: GCTCCCCAAATACAGTCAAGAG | NM_000738 | 1363–1384 | 338 |
| Antisense: CAGCAGCAGGCGAAAGGTGT | 1700–1681 | |||
| M2 | Sense: GATGGCCTGGAGCACAACA | NM_000739 | 757–775 | 358 |
| Antisense: GCTGCTTAGTCATCTTCACAATC | 1114–1092 | |||
| M3 | Sense: CGAGCAGATGGACCAAGAC | NM_000740 | 972–990 | 390 |
| Antisense: AGGTAGAGTGGCCGTGCTC | 1365–1347 | |||
| M4 | Sense: TCCAATGAGTCCAGCTCAGG | NM_000741 | 874–893 | 488 |
| Antisense: AGAGCATAGCAGGCAGGGTTG | 1361–1341 | |||
| Nested M4 | Sense: TCCAGATTGTGACGAAGCAG | NM_000741 | 1022–1041 | 202 |
| Antisense: AAGGCTAGCAGAATGGCAAA | 1204–1223 | |||
| M5 | Sense: GGACTATAAGTTCCGATTGGTG | NM_012125 | 1445–1466 | 295 |
| Antisense: GGTGACTGGGACACACTTG | 1739–1721 | |||
| β-Actin | Sense: ACGGGGTCACCCACACTGTGC | NM_001101 | 543–563 | 659 |
| Antisense: CTAGAAGCATTTGCGGTGGAC | 1201–1181 |
First-round PCR reactions demonstrated all five subtypes of muscarinic receptor cDNA fragments of expected size in the human brain, and all subtypes except M4 in the human bladder. The specificity of the primers and the authenticity of the corresponding PCR products were verified by digestion of PCR products with appropriate restriction enzymes, which were predicted from cDNA. M1 PCR products were digested with AvaI (generated fragments of 225 and 113 bp); M2 with HinfI (97, 111 and 150 bp); M3 with PstI (57, 139 and 198 bp); M4 with AvaI (302 and 186 bp); and M5 with DdeI (200 and 95 bp). As the M4 product was not detected in either detrusor or mucosa after first round PCR, nested-PCR reactions were performed with 1 : 10 and 1 : 100 dilutions of M4 first round PCR product as templates. Nested primer pairs for M4 receptor were used with Tfl polymerase: 94°C for 2 min, followed by 30 cycles of 94°C for 30 s, 56°C for 1 min and 70°C for 45 s, and a final elongation period at 70°C for 10 min.
In this study, we separated detrusor and mucosa and examined mRNA expression in each region independently. Expression of mRNA for calponin (smooth muscle marker) was used to check the dissections and, as expected, calponin mRNA expression was very high in detrusor muscle RNA, with very low or undetectable expression in the mucosa RNA (data not shown). The expression of calponin and β-actin gene transcripts was verified by RT–PCR. A PCR cycle of 25 amplifications was performed and extension time changed from 45 s to 2 min; otherwise the conditions were identical to RT–PCR for M2. PCR products were subjected to electrophoresis on 1.5% agarose gels containing ethidium bromide.
Quantitative competitive RT–PCR
The expression of M2 and M3 receptor mRNA from the male and female detrusor was quantified using quantitative competitive RT–PCR (QCRT–PCR), where a known amount of competitor RNA is added to the target RNA sample and both the competitor and target RNA are amplified in the same reaction (Dash et al., 2000, Holsinger et al., 2000).
Competitor RNA (internally deleted standard RNA) was produced for M2 and M3 muscarinic receptors. M2 and M3 cDNA fragments (358 and 390 bp, respectively) were generated by RT–PCR from human bladder total RNA and then internally deleted 150 and 57 bp, using restriction enzymes HinfI and PstI, respectively. The modified cDNAs were subcloned into a pTargetT vector and used as templates to synthesise competitor RNA by T7 polymerase. The competitor RNA concentration was quantified by spectrophotometric absorbance at 260 nm.
For QCRT–PCR, bladder total RNA (100 ng) was co-amplified with serial dilutions (30–0.03 pg) of competitor RNA, reverse transcribed with random hexamer (2 μM) and Amv reverse transcriptase, and the RT products were subsequently amplified by PCR with Tfl polymerase and a pair of M2 or M3 gene-specific primers (0.2 μM). The products were then separated by gel electrophoresis (2.5% agarose containing ethidium bromide) and quantified by densitometry (BioRad Gel Doc system).
Data analysis
The amount of sample mRNA (M2 or M3 mRNA per 100 ng total RNA) was calculated from the competition equivalence point, determined by plotting the log relative intensity of DNA bands (total bladder RNA per competitor RNA) versus the log of the known concentration of competitor RNA.
The QCRT–PCR data for M2 and M3 receptor expression in detrusor muscle were then normalised for the expression of β-actin (cytoskeletal protein) in the same sample. Expression of β-actin did not vary with age (see Results) and was considered to be a suitable housekeeping gene for internal standardisation of target gene expression. Densitometry results from QCRT–PCR gels and age-related changes in muscarinic receptor expression were analysed using the linear regression analysis program of GraphPad Prism (version 3.0).
Statistical tests
Paired comparisons were carried out using the Wilcoxon ranked pairs test, and unpaired comparisons were carried out using the Mann–Whitney test.
Materials
[3H]QNB (specific activity; 37 Ci mmol−1) was obtained from NEN (Boston, U.S.A.). Atropine, 4-DAMP, methoctramine and pirenzepine were obtained from Sigma (St Louis, U.S.A.). AQ-RA 741, and AF-DX 116 were gifts from Dr Karl Thomae GmbH (Biberach an der Riss, Germany). Darifenacin was a gift from Pfizer (Sandwich, U.K.). Trospium was supplied by Dr R. Pfleger GmbH (Bamberg, Germany). All other reagents were of analytical grade.
RNA extraction kits were obtained from Epicentre (Madison, U.S.A.). All other molecular reagents including Access RT–PCR kit, restriction enzymes, pTargetT vector, DNase treatment, RNasin and random hexamers were from Promega (Madison, U.S.A.).
Results
Radioligand binding studies in detrusor muscle membranes
Saturation studies
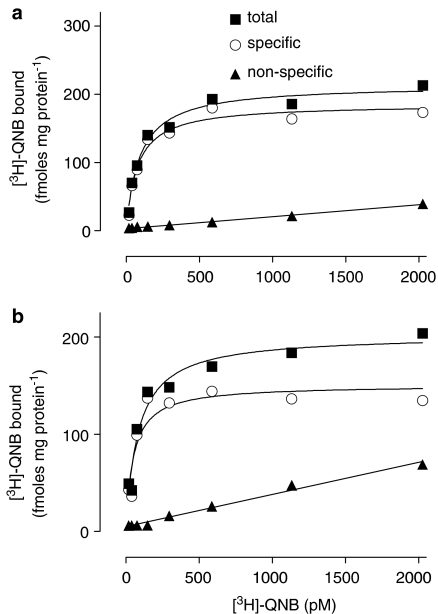
When detrusor muscle membranes were incubated for 2 h in the presence of increasing concentrations of [3H]QNB, specific binding was saturable at approximately 500 pM (Figure 1a). Nonlinear regression analysis indicated that specific binding of [3H]QNB was consistent with binding to a single-site rather than to a multiple-site model (nH 0.905), with KD 77.1 (55.2–99.0) pM and Bmax 181±7 fmol mg protein−1 (n=20). The data for the male group (KD 84.2 [60.4–108.0] pM; Bmax 172±11fmol mg protein−1; n=16) were not different from those for the small female group (KD 57.9 [26.7–89.1], n=4; Bmax 192±45 fmol mg protein−1; n=4).
Figure 1.
Results from one representative saturation-binding experiment (male, 63 years) with [3H]QNB. (a) Membranes prepared from the detrusor muscle. KD 75.9 pM; Bmax 186 fmol mg protein−1. (b) Membranes prepared from the mucosa. KD 50.7 pM; Bmax 151 fmol mg protein−1. Similar data were obtained from other specimens.
Competition studies
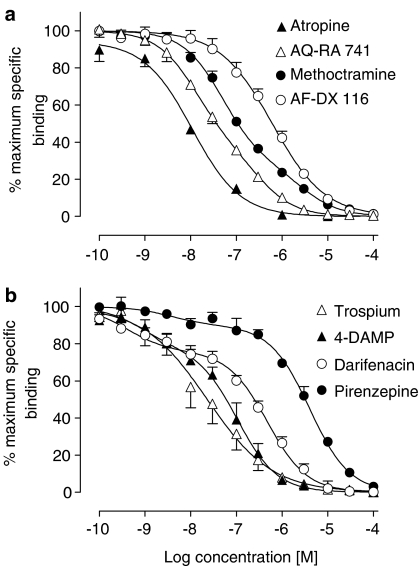
A number of muscarinic receptor antagonists were examined for their ability to compete with 200 pM [3H]QNB in detrusor muscle membranes (Table 2). The order of potency, based on IC50 values, was atropine>trospium>AQ-RA 741⩾4-DAMP>methoctramine⩾darifenacin>AF-DX 116>pirenzepine (Figure 2a, b). For some competitors, the competition curves displayed slope factors less than unity, and a two-site analysis resulted in significantly better resolution of some of these data sets (Table 2). The high-affinity component of binding of the muscarinic M2 receptor-preferring antagonists methoctramine and AQ-RA 741 represented 71 and 65% of total binding sites, respectively. In contrast, the high-affinity components of the muscarinic M3 receptor-preferring antagonists 4-DAMP and darifenacin represented 22 and 24% of total binding sites, respectively. Pirenzepine, which shows preference for M1 receptors, also bound to more than one site: it showed high affinity for a minority of sites (8%) and very low affinity for the remainder.
Table 2.
Dissociation constants (Ki) for muscarinic receptor antagonists in human detrusor muscle membranes
| Drug | N | Hill slopea | Ki (nM)b | Ki site 1 | Ki site 2 | % Ha, c |
|---|---|---|---|---|---|---|
| Atropine | 4 | 0.82±0.10 | 3.0 (2.1–4.3) | N/A | ||
| Trospium | 7 | 0.66±0.06 | 4.9 (3.6–6.7) | N/A | ||
| AQ-RA 741 | 6 | 0.73±0.03 | 12.3 (10.8–14.1) | 4.5 (2.8–7.2) | 97.5 (39.8–238) | 65±9 |
| 4-DAMP | 4 | 0.63±0.05 | 12.2 (9.2–16.4) | 0.23 (0.05–1.19) | 29.5 (18.5–47.1) | 22±6 |
| Methoctramine | 12 | 0.65±0.03 | 38.6 (33.4–44.7) | 13.7 (9.9–18.9) | 752 (336–1680) | 71±5 |
| Darifenacin | 11 | 0.52±0.05 | 42.0 (33.6–52.6) | 0.23 (0.11–0.53) | 146 (113–189) | 24±3 |
| AF-DX 116 | 6 | 0.75±0.05 | 170 (140–208) | N/A | ||
| Pirenzepine | 8 | 0.78±0.04 | 856 (734–998) | 0.42 (0.06–3.10) | 1134 (962–1340) | 8±2 |
Ki values were calculated according to the equation Ki=IC50/(1+200/77.1).
Data are expressed as mean±s.e.m.
Data are derived from simultaneous analysis of all competition curves and are expressed as geometric mean (95% confidence intervals).
% H, indicates % of high-affinity sites.
Figure 2.
Competition for specific binding of 200 pM [3H]QNB to human detrusor membranes by muscarinic receptor antagonists. Values are mean±s.e.m. (n=4–12).
The Ki values obtained from all competition experiments (Table 2) were correlated with the Ki values reported in the literature for the five cloned human muscarinic receptors (Table 3) by means of a log–log plot. A significant correlation (r2=0.978, P<0.0001) was found for the M2 receptor subtype, whereas extremely poor correlations were obtained for M1, M3, M4 and M5 receptor subtypes (r2=0.000, 0.004, 0.008, 0.000, respectively).
Table 3.
Reported Ki values (nM) for muscarinic ligands in cell lines expressing human muscarinic receptor subtypes
| Compound | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Trospiuma | 0.8 | 0.6 | 0.5 | 1.0 | 1.9 |
| Atropine | 0.3 | 0.8–1.3 | 0.2–0.3 | 0.1–1.2 | 0.2–0.8 |
| AQ-RA 741 | 29–62 | 3.7–4.4 | 55–86 | 1.5–15 | 732–831 |
| 4-DAMP | 0.6–1.2 | 4–9 | 0.4–1.0 | 0.7–1.7 | 0.6–1.3 |
| Methoctramine | 41–112 | 13–20 | 214–954 | 32–78 | 134–398 |
| Darifenacin | 7–35 | 44–77 | 0.8–1.3 | 18–46 | 5–9 |
| AF-DX 116 | 417–740 | 64–73 | 786–1290 | 211–545 | 5130 |
| Pirenzepine | 6–9 | 224–407 | 75–184 | 17–48 | 66–158 |
Values in bold indicate preferred receptor subtype.
Data from references cited in Mansfield et al. (2003).
Data from Madersbacher (2003).
As two-site analysis is inadequate to resolve the relative proportions when more than two sites are present, a global fit of the data was undertaken for darifenacin, methoctramine, AQ-RA 741 and pirenzepine, with shared proportions of each of the three sites. The analysis gave the following percentages for the three putative muscarinic receptor subtypes: M1 7.2±2.2%; M2 71.2±6.3%; and M3 21.5±5.9%. Correlation plots using log–log transformations of the appropriate Ki values obtained from this analysis of the data for each of the four ligands versus Ki values from the literature (Table 3) for M1, M2 and M3 receptors gave significant correlations with each receptor subtype (M1, r2=0.99, P<0.01; M2, r2=0.97, P<0.05; M3, r2=0.99, P<0.005).
Radioligand binding studies in mucosal membranes
Saturation studies
In mucosal membranes, specific binding of [3H]QNB was saturable at approximately 500 pM (Figure 1b). Binding occurred to a single site (nH 0.982) with KD 100.5 (41.2–159.9) pM and Bmax 145±9 fmol mg protein−1 (n=6). These studies were carried out in parallel with studies using detrusor membranes from the same patients (five males, one female); under these conditions, no significant differences between Bmax or KD values were seen between mucosa and detrusor (Bmax, P=0.33; KD, P=0.62; paired t-test, n=6).
Competition studies
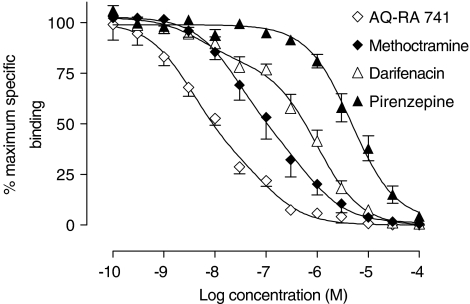
Limited competition studies (n=3–5) were also conducted in bladder mucosal membranes using the most selective antagonists available: methoctramine (M2>M4), darifenacin (M3>M5) and pirenzepine (M1>M4), as well as AQ-RA 741 (M2>M4, with very low affinity for M5 receptor subtype). The order of potency to inhibit specific binding of [3H]QNB was AQ-RA 741>methoctramine>darifenacin>pirenzepine (Figure 3, Table 4). Of these antagonists, only the data for darifenacin were best fitted to two sites, with 22% of sites showing high affinity. When the Ki values (Table 4) were correlated with those found in the literature for the five cloned human muscarinic receptors (Table 3), the only significant correlation was seen for M2 receptor subtype (P=0.004, r2=0.992). Based on the results obtained with pirenzepine (very low affinity, slope factor approaching unity), there was no evidence for the presence of M1 receptors in the mucosa using this technique.
Figure 3.
Competition for specific binding of 200 pM [3H]QNB binding to membranes from human bladder mucosa membranes by subtype-selective muscarinic receptor antagonists. Values are mean±s.e.m. (n=3–5).
Table 4.
Dissociation constants (Ki) for muscarinic receptor antagonists in human mucosal membranes
| Drug | N | Hill slopea | Ki (nM)b | Ki site 1 | Ki site 2 | % Ha, c |
|---|---|---|---|---|---|---|
| AQ-RA 741 | 3 | 0.69±0.05 | 3.5 (2.8–4.3) | N/A | ||
| Methoctramine | 5 | 0.71±0.07 | 38.8 (28.9–52.3) | N/A | ||
| Darifenacin | 5 | 0.67±0.05 | 144 (113–184) | 1.9 (0.4–10.0) | 325 (206–511) | 22±4 |
| Pirenzepine | 5 | 0.87±0.06 | 1600 (1320–1940) | N/A |
Ki values were calculated according to the equation Ki=IC50/(1+200/100.5).
Data are expressed as mean±s.e.m.
Data are derived from simultaneous analysis of all competition curves and are expressed as geometric mean Ki (95% confidence intervals).
% H, indicates % of high-affinity sites.
Slope factors were low for AQ-RA 741, methoctramine and darifenacin. A global fit of the data for these three competitors showed that two-site binding curves could be fitted: 24.8±5.4% of sites showed high affinity for darifenacin coupled with low affinity for both methoctramine and AQ-RA 741, while 75.2% of sites showed high affinity for methoctramine and AQ-RA 741 and low affinity for darifenacin, and represented M2 receptors. The Ki values obtained for darifenacin (5.5 nM (2.7–11.1)) and methoctramine (631 nM (256–1556)) at the minor site showed agreement with published values for M3 or M5 receptors, whereas that for AQ-RA 741 (63 nM (40–98)) was in better agreement with published values for M3 or M1 receptors (Table 3).
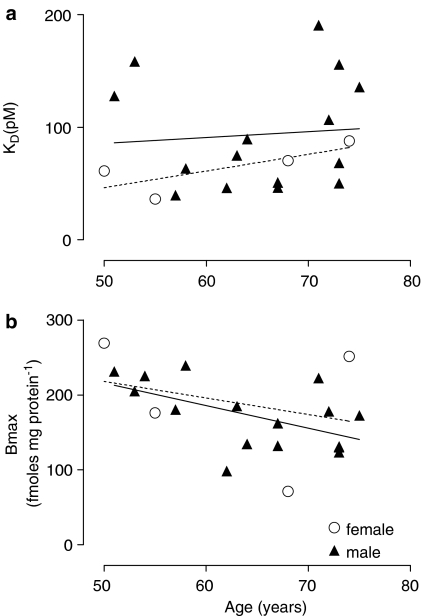
Radioligand binding studies – age-related trends in male bladder
There was no age-related change in KD of [3H]QNB in the male detrusor (Figure 4a). However, a significant decrease in Bmax was observed (Figure 4b) with increasing age, in male detrusor (51–75 years, r2=0.33, P=0.019, n=16) but not in female detrusor (r2=0.07, n=4). In the mucosa, no change in KD or Bmax with age was observed, but numbers were low (n=6).
Figure 4.
Age-related distribution of [3H]QNB-binding parameters in human detrusor membranes, from males (continuous regression line) and females (dashed regression line). (a) Absence of change in KD with age in either males (r2=0.006, n=15) or females (r2=0.59, P=0.23, n=4). (b) There was a significant inverse correlation (r2=0.33, P=0.019, n=16) between age and Bmax in males, but not (r2=0.07, n=4) in females.
When detrusor muscle data from males were grouped by age, the mean Bmax for the youngest patients (in the sixth decade) was 199±8 fmol mg protein−1 (age 55±1 years, n=5), with a significant decrease (P=0.013, one-way ANOVA) to 131±13 fmol mg protein−1 for patients in the seventh decade (age 65±1, n=5). For the patients in the eighth decade (age 73±1 years, n=6), the mean Bmax value was 147±11 fmol mg protein−1, no different from that of patients in the seventh decade. The number of muscarinic receptors decreased by 34% between 55 and 65 years of age and 26% between 55 and 73 years of age.
Molecular studies
RT–PCR
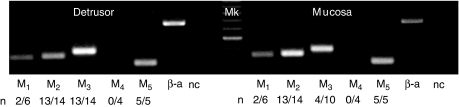
The expression of muscarinic receptor mRNA in human bladder detrusor and mucosa was examined by RT–PCR (Figure 5). In detrusor samples, expression of M2, M3 and M5 receptor mRNA was highly consistent, whereas that for M1 was variable. In mucosa, on the other hand, mRNA for M2 and M5 receptors was expressed consistently, whereas mRNA for M1 and M3 receptors was detected only in some samples. The densitometric analysis demonstrated that band intensities of M2 (n=14 matched pairs, P<0.001) and M3 mRNA (n=14 for detrusor and n=10 for mucosa, P<0.001) were greater in detrusor compared with mucosa. For both M1 mRNA (n=6) and M5 mRNA (n=5), no differences in expression were observed in detrusor compared with corresponding samples from the mucosa.
Figure 5.
Expression of mRNA for muscarinic receptor subtypes. The gel shows expression in one bladder biopsy (male, 86 years). Detrusor and mucosa both have bands of the expected size, indicating expression of M1, M2, M3 and M5 (338, 358, 390, 295 bp), but not M4 (488 bp). β-Actin (β-a) is used as a positive control (659 bp) and the negative control (nc) represents the RT–PCR for β-actin conducted in the absence of Amv (no reverse transcription); 100 bp marker (Mk). n indicates the number of specimens showing positive expression for each subtype, out of the total number of specimens tested.
Neither detrusor nor mucosa showed any visible expression of M4 receptor mRNA with RT–PCR, so nested-PCR reactions were performed to enhance the sensitivity. A weak band of M4 receptor cDNA (202 bp) was then detected in all four detrusor and mucosa RNA samples, indicating the presence of very low levels of M4 in human bladder.
Quantitative competitive RT–PCR studies in the male and female detrusor
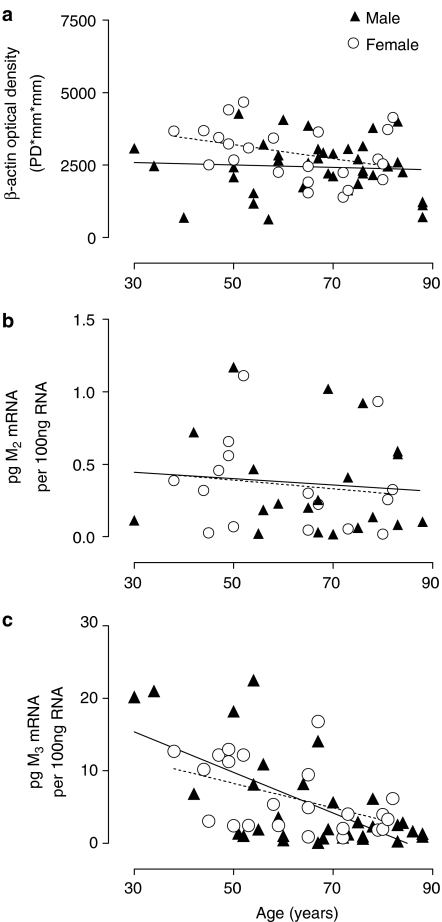
Following our observation of significant change in total muscarinic receptors in the male detrusor, we carried out QCRT–PCR studies in biopsy specimens of both male and female detrusor, to examine the expression of mRNA for the two major subtypes, M2 and M3 receptors. The amount of M2 or M3 mRNA in the total bladder RNA was calculated from the competition equivalence point, determined by plotting the log relative intensity of DNA bands (total bladder RNA relative to competitor RNA) versus the log of the known concentration of competitor RNA (Figure 6). The QCRT–PCR results were normalised to β-actin, as there was no change in β-actin expression with age (Figure 7a) in male (r2=0.004, P=0.69, n=38) or female (r2=0.128, P=0.09, n=23) detrusor.
Figure 6.
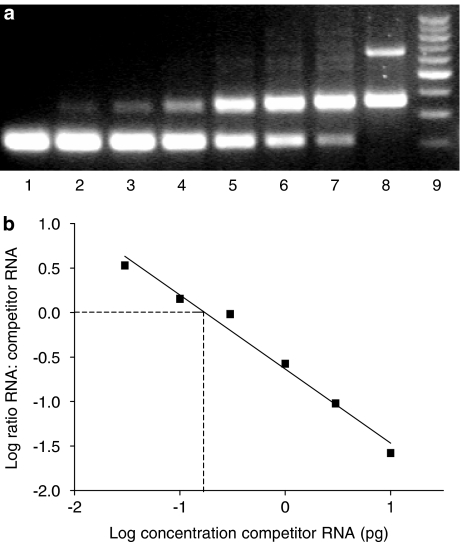
Calculation of M2 receptor mRNA expression in human bladder detrusor RNA with quantitative competitive RT–PCR. (a) Typical gel image (65 years old, male, 2.5% agarose) showing band intensities for M2 RT–PCR products obtained from bladder RNA (upper band, 350 bp) and competitor RNA (lower band 250 bp). Competitor RNA is present in tubes 1–7 as follows: lanes 1 and 2, 10 pg; lane 3, 3 pg; lane 4, 1 pg; lane 5, 0.3 pg; lane 6, 0.1 pg; lane 7, 0.03 pg; lane 8, 0 pg. Bladder RNA (100 ng) is present in tubes 2–8. Lane 8 also shows a band for the β-actin internal control at 650 bp. Lane 9 is a 100 bp marker. (b) Quantitative analysis of gel shown in panel (a). At the point where the log ratio is zero, PCR band densities from the bladder RNA and competitor RNA are equal. The amount of the competitor RNA at this point represents the amount of M2 mRNA in the bladder RNA (here, 0.174 pg per 100 ng total RNA).
Figure 7.
Age-related changes in mRNA expression in detrusor muscle, analysed using linear regression (males, solid line, females, dashed line). (a) β-Actin expression did not alter with age in male (r2=0.004, P=0.69, n=38) or female patients (r2=0.128, P=0.09, n=23). (b) M2 receptor mRNA expression remained constant with age in male (r2=0.009, P=0.69, n=20) and female patients (r2=0.019, P=0.60, n=16). (c) M3 receptor mRNA expression decreased with age in male (r2=0.41, P<0.0001, n=33) and female patients (r2=0.23, P=0.019, n=23). The level of muscarinic M2 and M3 receptor mRNA expression was determined using QCRT–PCR. Data were normalised to expression of β-actin.
There were no gender differences in the expression of M2 or M3 muscarinic receptor mRNA. The mean expression of M2 muscarinic receptor mRNA was 0.37±0.08 competitor RNA per 100 ng total RNA (male, n=20) and 0.35±0.08 competitor RNA per 100 ng total RNA (female, n=16), whereas that of M3 muscarinic receptor mRNA appeared higher, at 5.36±1.16 competitor RNA per 100 ng total RNA (male, n=33) and 6.23±1.00 competitor RNA per 100 ng total RNA (female, n=23).
The age-related expression of M2 and M3 muscarinic receptor mRNA is depicted in Figure 7b, c. The expression of M2 muscarinic receptor mRNA remained constant with age (male: r2=0.009, P=0.69, n=20 and female: r2=0.019, P=0.60, n=16), while an age-related decrease in expression of M3 receptor mRNA was demonstrated (male: r2=0.41, P<0.0001, n=33, female: r2=0.23, P=0.019, n=23) in detrusor.
Discussion
Detrusor muscle muscarinic receptors
Our binding study in detrusor muscle has used some new subtype-selective antagonists and more sophisticated radioligand-binding data analysis to confirm and extend previous findings that detrusor muscle contains not only M2 and M3 muscarinic receptor subtypes, but also M1 receptors. While the potent nonselective antagonists atropine and trospium showed only single-site binding, competition curves for the M1, M2 and M3 subtype-preferring antagonists could all be resolved into two-site binding (Table 2). However, global analysis is preferred when binding occurs to more than two sites. Global analysis of the data for the subtype preferring ligands, AQ-RA 741, darifenacin, methoctramine and pirenzepine showed the proportions of the three sites as 71 : 22 : 7 and the correlation plots of the appropriate Ki values showed good agreement with the presence of M2, M3 and M1 receptors, respectively.
The presence of these three muscarinic receptor subtypes is supported by results of the RT–PCR studies. However, the relative expression of the different muscarinic receptor subtypes varies, so that the ratio of M2 : M3 protein expression does not correlate with that of M2 : M3 mRNA expression. While it is possible to correlate changes in receptor expression within a receptor subtype (M3 mRNA and M3 protein), it is inappropriate to compare changes across different receptor subtypes (M2 compared to M3). As well as different PCR conditions, differences could be due to variation in mRNA translation rates, which are governed by a variety of hormonal and cellular events, and/or mRNA stability. Fraser & Lee (1995) reported that M3 receptor mRNA expression was extremely stable compared to M1, but to our knowledge no one has reported on the stability of M2 receptor mRNA.
A novel and potentially important finding was the expression of M5 receptor mRNA in the human detrusor (and mucosa). Most reports of M5 receptors have concerned their possible roles in the CNS and cerebral vasculature, although they have also been implicated in the eye, salivary gland and blood vessels (refer Eglen & Nahorski, 2000). The expression of M5 receptor mRNA has been shown previously in the bladder trigone (Sigala et al., 2002) and in other human peripheral tissues such as oesophageal smooth muscle (Preiksaitis et al., 2000). The roles of the M5 receptor in the bladder are at present unclear, but one speculative function might be in connection with blood vessels, which would be consistent with weak expression in both mucosa and detrusor. At present, there is no antagonist with high affinity that could have been used to define any M5 receptors. Instead, we used AQ-RA 741 (which possesses a 10- to 200-fold lower affinity for M5 receptors than for the other four subtypes, Table 3) and, although its competition curves could be resolved into two sites (Table 2), each had an affinity much higher than that reported for recombinant M5 receptors (Table 3).
M4 receptors were not detected in human detrusor (or mucosa) using either binding or RT–PCR. However, nested PCR revealed a very low level of expression of M4 receptor mRNA. There is evidence from functional studies for prejunctional inhibitory M4 receptors on cholinergic neurons in human bladder (Somogyi & de Groat, 1999; D'Agostino et al., 2000), which although of putative functional importance would not represent a significant proportion of total muscarinic receptors.
Mucosal muscarinic receptors
A novel result of our binding studies was the revelation of a substantial population of muscarinic receptors on the human urinary bladder mucosa, showing an affinity (KD) for [3H]-QNB and density (Bmax) similar to that in the detrusor. The results are similar to those from a recent binding study with the same radioligand in the pig urinary bladder mucosa, where the Bmax in the mucosa was 129 fmol mg protein−1 compared with 81 fmol mg protein−1 in the detrusor (Hawthorn et al., 2000).
The main muscarinic receptor subtype on the mucosa was the M2 receptor. Binding studies using four subtype-selective antagonists suggested the presence of a majority of M2 receptors and a minor population of darifenacin-preferring sites that might represent M5 and/or M3 receptors. Resolution of the inconclusive findings will rely on the future availability of a ligand with high subtype selectivity for the M5 receptor. In the mucosal biopsies, RT–PCR demonstrated consistent strong expression of M2 and M5 receptor mRNA, but inconsistent expression of M1 and M3 receptor mRNA. The exact localisation of these mucosal receptors is unclear and not elucidated in our study, but they could be expressed on urothelium, myofibroblasts, blood vessels and/or nerves.
The contribution of the urinary mucosa to bladder function is now the subject of intense research. Urothelial cells express vanilloid receptors (Birder et al., 2002) and muscarinic receptors (Hawthorn et al., 2000) and can respond to stretch and other stimuli by release of a number of agents including Ach (Yoshida et al., 2004), ATP (Ferguson et al., 1997; Fry et al., 2004) and other mediators (Birder et al., 2002), which may stimulate suburothelial nerves directly or via myofibroblasts (Fry et al., 2004). Recently, studies in pig and human bladder strips have suggested that activation of urothelial muscarinic receptors, by carbachol, leads to the release of an inhibitory factor that can regulate detrusor contractility (Templeman et al., 2002; Chaiyaprasithi et al., 2003). The role of muscarinic receptors in the human mucosa has not been extensively investigated but recent speculation has centred on possible afferent functions. It is now accepted that muscarinic antagonists act mainly during bladder filling to increase bladder capacity and decrease urge (Andersson & Yoshida, 2003), actions not necessarily related to inhibition of detrusor contraction. Therefore, the urothelial muscarinic receptors, present in unexpectedly high density, may represent another site of action for the muscarinic receptor antagonists. The source of the endogenous ligand for these receptors may not be neuronal Ach but may be Ach of urothelial origin (for discussion, refer Yoshida et al., 2004).
Age-related changes in muscarinic receptor density, expression and function
Several studies have examined changes in muscarinic receptors with age in rabbit (Latifpour et al., 1990) and rat (Ordway et al., 1986; Pagala et al., 2001) bladders, but results are variable, complex and region-dependent, and binding data do not necessarily correlate with functional data. In humans, previous urodynamic studies have shown that bladder capacity and urinary flow rate decline with age (Elbadawi et al., 1998, Madersbacher et al., 1998), while detrusor overactivity (involuntary detrusor contractions) becomes more common in the elderly. In the human detrusor, Yoshida et al. (2001) have demonstrated an age-related reduction in atropine-sensitive contractile responses elicited by electrical field stimulation and an age-related reduction in Ach release whose source appears to be predominantly urothelial (Yoshida et al., 2004). A large clinical study found a diminished efficacy of the muscarinic antagonist tolterodine in elderly patients (Michel et al., 2002). With the exception of an early study with [3H]NMS reporting no age-related differences in Bmax (Lepor et al., 1989), minimal information is available in the literature concerning the effect of age on the density and distribution of total or individual muscarinic receptor subtypes in human detrusor.
Our results using the technique of radioligand binding indicate a decrease in total muscarinic receptor numbers (Bmax) with age in male detrusor muscle (Figure 4b). These findings in male detrusor are unlikely to be due to obstruction due to prostatic hypertrophy, since such patients were excluded from our study. Furthermore, obstruction in the rat bladder leads to an increase in the number of total and M2 muscarinic receptors (Braverman & Ruggieri, 2003). This binding component of our study was unable to demonstrate which receptor subtype(s) was decreased with age. However, using quantitative competitive RT–PCR, we were able to show a decrease in the level of expression for M3 receptor mRNA with age in both male and female subjects, but no corresponding change for M2 receptor mRNA over a wide age range (30–88 years).
It would be simplistic and probably inaccurate to ascribe the decrease in total muscarinic receptor protein (Bmax) to the decrease in M3 receptor mRNA. The expressions of mRNA and protein do not always follow a simple correlation since many mechanisms may be involved in post-transcriptional control. While the expression of gene-specific mRNA can be accurately determined, reliable measurements of associated protein can be problematic. To date, there are no highly subtype-selective radioligands, antagonists, and antibodies commercially available for the muscarinic receptors. Until a reliable protein quantitative technique is developed for individual receptor subtypes, determination of a change in expression of mRNA is the only mainstream technique available to indicate corresponding protein changes in ageing or disease.
Conclusions
Our data from normal bladder show the existence of multiple muscarinic receptor subtypes in the human detrusor and mucosa. In the detrusor, evidence from both radioligand-binding and molecular studies is consistent with the presence of a majority of M2 and lesser populations of M3 and M1 receptors. Molecular studies also indicated a small population of M5 receptors. In the mucosa, both techniques revealed a significant population of M2 receptors. Minor populations of M5, M3 and M1 receptors were suggested by the molecular studies.
The age-related decline in total muscarinic receptor protein, and specifically in M3 receptor mRNA expression, may partly account for the previous urodynamic findings of reduced contractility/flow rate and incomplete emptying in the elderly, particularly in males. However, the relationship between the decrease in muscarinic receptor numbers with age and the development of conditions of bladder overactivity remains unclear.
Acknowledgments
We thank Dr K. Vaux and Dr S. Ehsman for kindly providing bladder specimens and Emma Schofield, Neville McDonnell and Arthur Denning for assistance with specimen collection. This study was supported by the National Health and Medical Research Council of Australia, the Australasian Urological Foundation and Dr R. Pfleger GmbH (Bamberg, Germany).
Abbreviations
- AF-DX 116
{[2-[(diethylamino)methyl]-1-piperidinyl]acetyl}5,11-dihydro-6H-pyrido[2,3b][1,4] benzodiazepine-6-one
- AQ-RA 741
(11-[[4-[4-(diethylamino)butyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3b][1,4]benzodiazepine-6-one
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine-methiodide
- QCRT–PCR
quantitative competitive reverse transcription PCR
- [3H]QNB
[3H]quinuclidinyl benzylate
References
- ANDERSSON K.E., YOSHIDA M. Antimuscarinics and the overactive detrusor – Which is the main mechanism of action. Eur. Urol. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., NAKAMURA Y., KISS S., NEALEN M.L.M., BARRICK S., KANAI A.J., WANG E., RUIZ G., DE GROAT W.C., APODACA G., WATKINS S., CATERINA M.J. Altered urinary bladder function in mice lacking the vanilloid receptor. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- BRAVERMAN A.S., RUGGIERI M.R. Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am. J. Physiol. 2003;285:R701–R708. doi: 10.1152/ajpregu.00009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIYAPRASITHI B., MANG C.F., KILBINGER H., HOHENFELLNER M. Inhibition of human detrusor contraction by a urothelium derived factor. J. Urol. 2003;170:1897–1900. doi: 10.1097/01.ju.0000091870.51841.ae. [DOI] [PubMed] [Google Scholar]
- CHESS-WILLIAMS R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton. Autocoid. Pharmacol. 2002;22:133–145. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- CHESS-WILLIAMS R., CHAPPLE C.R., YAMANISHI T., SELLERS D.J. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J. Auton. Pharmacol. 2001;21:243–248. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO G., BOLOGNESI M.L., LUCCHELLI A., VICINI D., BALESTRA B., SPELTA V., MELCHIORRE C., TONINI M. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: involvement of M4 receptor subtype. Br. J. Pharmacol. 2000;129:493–500. doi: 10.1038/sj.bjp.0703080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASH S., SAXENA R., MYUNG J., REGE T., TSUJI H., GAGLIO P., GARRY R.F., THUNG S.N., GERBER M.A. HCV RNA levels in hepatocellular carcinomas and adjacent non-tumorous livers. J. Virol. Meth. 2000;90:15–23. doi: 10.1016/s0166-0934(00)00199-3. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., NAHORSKI S.R. The muscarinic M(5) receptor: a silent or emerging subtype. Br. J. Pharmacol. 2000;130:13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHLERT F.J. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 2003;74:355–366. doi: 10.1016/j.lfs.2003.09.023. [DOI] [PubMed] [Google Scholar]
- ELBADAWI A., DIOKNO A.C., MILLARD R.J. The aging bladder – morphology and urodynamics. World J. Urol. 1998;16:S10–S34. doi: 10.1007/pl00014134. [DOI] [PubMed] [Google Scholar]
- FERGUSON D.R., KENNEDY I., BURTON T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism. J. Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FETSCHER C., FLEICHMAN M., SCHMIDT M., KREGE S., MICHEL M.C. M3 muscarinic receptors mediate contraction of human urinary bladder. Br. J. Pharmacol. 2002;136:641–644. doi: 10.1038/sj.bjp.0704781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER C.M, LEE N.H. Regulation of muscarinic receptor expression by changes in mRNA stability. Life Sci. 1995;56:899–906. doi: 10.1016/0024-3205(95)00026-3. [DOI] [PubMed] [Google Scholar]
- FRY C.H., IKEDA Y., HARVEY R., WU C., SUI G.P. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology. 2004;63 Suppl 1:24–31. doi: 10.1016/j.urology.2003.10.031. [DOI] [PubMed] [Google Scholar]
- GOEPEL M., GRONEWALD A., KREGE S., MICHEL M.C. Muscarinic receptor subtypes in porcine detrusor: comparison with humans and regulation by bladder augmentation. Urol. Res. 1998;26:149–154. doi: 10.1007/s002400050038. [DOI] [PubMed] [Google Scholar]
- HAWTHORN M.H., CHAPPLE C.R., COCK M., CHESS-WILLIAMS R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br. J. Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLSINGER R.M.D., SCHNARR J., HENRY P., CASTELO V.T., FAHNESTOCK M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction. Mol. Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- LATIFPOUR J., KONDO S., O'HOLLAREN B., MORITA T., WEISS R.M. Autonomic receptors in urinary tract: sex and age differences. J. Pharmacol. Exp. Ther. 1990;253:661–667. [PubMed] [Google Scholar]
- LEPOR H., GUP D., SHAPIRO E., BAUMANN M. Muscarinic cholinergic receptors in the normal and neurogenic human bladder. J. Urol. 1989;142:869–874. doi: 10.1016/s0022-5347(17)38933-4. [DOI] [PubMed] [Google Scholar]
- MADERSBACHER H.S.Trospium chloride Focusing on the Science of OAB Management 2003. ICS Symposium, Florence, Italy
- MADERSBACHER S., PYCHA A., SCHATZI G., MIAN C KLINGLER C.H., MARBERGER M. The ageing lower urinary tract: a comparative urodynamics study of men and women. Urology. 1998;51:206–212. doi: 10.1016/s0090-4295(97)00616-x. [DOI] [PubMed] [Google Scholar]
- MANSFIELD K.J., MITCHELSON F.J., MOORE K.H., BURCHER E. Muscarinic receptor subtypes in the human colon: lack of evidence for atypical subtypes. Eur. J. Pharmacol. 2003;182:101–109. doi: 10.1016/j.ejphar.2003.10.008. [DOI] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., FUJIKAWA T., JIANG J., TAKAHASHI S., MANABE T., TAKETO M. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J. Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., KARASAWA H., FUJIKAWA T., JIANG J., KOMIYA Y., TAKAHASHI S., TAKETO M. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL M.C., SCHNEIDER T., KREGE S., GOEPEL M. Does gender or age affect the efficacy and safety of Tolterodine. J. Urol. 2002;168:102–1032. doi: 10.1016/S0022-5347(05)64567-3. [DOI] [PubMed] [Google Scholar]
- MILSOM I., ABRAMS P., CARDOZO L., ROBERTS R.G., THUROFF J., WEIN A.J. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- NILVEBRANT L., ANDERSSON K.E., MATTIASSON A. Characterization of the muscarinic cholinoceptors in the human detrusor. J. Urol. 1985;134:418–423. doi: 10.1016/s0022-5347(17)47191-6. [DOI] [PubMed] [Google Scholar]
- ORDWAY G.A., ESBENSHADE T.A., KOLTA M.G., GERALD M.C., WALLACE L.J. Effect of age on cholinergic muscarinic responsiveness and receptors in the rat urinary bladder. J. Urol. 1986;136:492–496. doi: 10.1016/s0022-5347(17)44928-7. [DOI] [PubMed] [Google Scholar]
- PAGALA M.K., TETSOTI L., NAGPAL D., WISE G.J. Ageing effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J. Urol. 2001;166:721–725. [PubMed] [Google Scholar]
- PONTARI M.A., BRAVERMAN A.S., RUGGIERI M.R. The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am. J. Physiol. 2004;268:R874–R880. doi: 10.1152/ajpregu.00391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREIKSAITIS H.G., KRYSIAK P.S., CHRONES T., RAJGOPAL V., LAURIER L.G. Pharmacological and molecular characterisation of muscarinic receptor subtypes in human oesophageal smooth muscle. J. Pharmacol. Exp. Ther. 2000;295:879–888. [PubMed] [Google Scholar]
- SIGALA S., MIRABELLA G., PERONI A., PEZZOTTI G., SIMEONE C., SPANO P., CUNINO S.C. Differential gene expression of cholinergic muscarinic receptor subtypes in male and female normal human urinary bladder. J. Urol. 2002;60:719–725. doi: 10.1016/s0090-4295(02)01819-8. [DOI] [PubMed] [Google Scholar]
- SOMOGYI G.T., De GROAT W.C. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- TEMPLEMAN L., CHAPPLE C.R., CHESS-WILLIAMS R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J. Urol. 2002;167:742–745. doi: 10.1016/S0022-5347(01)69137-7. [DOI] [PubMed] [Google Scholar]
- WANG P., LUTHIN G.R., RUGGIERI M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA M., HOMMA Y., INADOME A., YONO M., SESHITA H., MIYAMOTO Y., MURAKAMI S., KAWABE K., UEDA S. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscle. Exp. Geront. 2001;36:99–109. doi: 10.1016/s0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., MIYAMAE K., IWASHITA H., OTANI M., INADOME A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- ZENG X.P., MOORE K.H., BURCHER E. Characterization of tachykinin NK2 receptors in human urinary bladder. J. Urol. 1995;153:1688–1692. [PubMed] [Google Scholar]