Abstract
Diadenosine polyphosphates (P1,P3-diadenosine triphosphate, Ap3A; P1,P4-diadenosine tetraphosphate, Ap4A; and P1,P5-diadenosine pentaphosphate, Ap5A) are vasoactive molecules. The experimental model of isolated rat renal glomeruli was used to investigate their effects on glomerular vasculature. We measured the changes of glomerular inulin space (GIS) as a marker of glomeruli contractility.
Ap4A and Ap5A induced concentration- and time-dependent reduction of GIS whereas Ap3A had no effect. The effects of Ap4A and Ap5A (both at 1 μM) were prevented by a nonselective P2 receptor antagonist, that is, suramin (10 μM) and P2Y receptor antagonist – reactive blue 2 (50 μM). However, the antagonist of P1 receptor, that is, theophylline (1 μM) and A1 receptor 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 10 μM) did not affect the responses of glomeruli to Ap4A or Ap5A.
Ap3A, in contrast to Ap4A and Ap5A, prevented angiotensin II-induced reduction of GIS in a concentration- and time-dependent manner. This effect was partially prevented by suramin and markedly reduced by reactive blue 2 and the specific antagonist of P2Y1 receptor – MRS 2179 (10 μM). However, theophylline and the specific antagonist of A2 receptor – 3,7-dimethyl-1-propargylxanthine (DMPX; 10 μM) – did not affect Ap3A action.
We indicate that diadenosine polyphosphates changed the glomerular volume via activation of P2 receptors. We suggest that extracellular Ap4A and Ap5A via P2X and P2Y receptors may decrease and Ap3A via, at least in part, P2Y1 receptors may increase filtration surface, which in turn may modify glomerular filtration rate.
Keywords: Diadenosine polyphosphates, renal glomeruli, GIS, purinoceptors
Introduction
The naturally occurring diadenosine polyphosphates (ApnAs, n=2–7) are recognised as vasoactive nucleotides. These molecules consist of two adenosine moieties connected via 5′-ribose linkage to both ends of the polyphosphate chain. ApnAs are present in all living cells and certain amounts of these are released into extracellular space during platelet aggregation, metabolic stress and neurotransmission (Luthje & Ogilvie, 1988; Schluter et al., 1994; Miras-Portugal et al., 1998). The effects of ApnAs are mediated via P1 and P2 receptors or dinucleotide receptors (Hoyle et al., 1996; Vahlensieck et al., 1996; Pintor et al., 1997; Ralevic & Burnstock, 1998; Verspohl et al., 1999). P1 receptors (G proteins coupled receptors) are classical receptors for adenosine and are further subdivided, according to convergent molecular, biochemical and pharmacological evidence into the following subtypes: A1, A2A, A2B and A3. These receptors have been cloned from several species (Ralevic & Burnstock, 1998). P2 receptors are divided into P2X (ligand-gated ion channels) and P2Y (G proteins coupled receptors). Up to this time, seven mammalian P2X receptors: P2X1–7 and eight mammalian P2Y receptors: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–13 and P2Y15 have been cloned (Illes & Ribeiro, 2004). There is also pharmacological evidence favouring the hypothesis that biological action of ApnAs is mediated by activation of the specific dinucleotide receptor (Pintor & Miras-Portugal, 1995; Diaz-Hernandez et al., 2001; Jimenez et al., 2002). However, this receptor has not been cloned yet. Vasomotor actions of ApnAs depend on the localisation of receptors and number of phosphate groups in the moiety (Ralevic et al., 1995; Van der Giet et al., 1997; Ralevic et al., 2001). Ap3A (P1,P3-diadenosine triphosphate) induces endothelium-dependent vasodilatation in the rat mesenteric arteries (Ralevic et al., 1995) and endothelium-independent vasodilatation in rabbit mesenteric arteries (Busse et al., 1988). Ap4A (P1,P4-diadenosine tetraphosphate) induces endothelium-independent vasoconstriction and endothelium-dependent vasodilatation in rabbit mesenteric arteries (Busse et al., 1988). Ap5A (P1,P5-diadenosine pentaphosphate) induces vasoconstriction in rat mesenteric arteries (Ralevic & Burnstock, 1991) and relaxation in the porcine coronary artery (Sumiyoshi et al., 1997).
There is also an increasing interest in the renal effects of ApnA (Hohage et al., 1996). Ap3A and Ap5A induce vasoconstriction of intralobular arteries (Gabriels et al., 2000). Bolus injection of Ap4A, Ap5A or Ap6A into the jugular vein decreases the renal blood flow (Khattab et al., 1998). It has been shown that Ap3A, Ap4A, Ap5A and Ap6A can influence the tone of resistance arteries in the human kidney (Steinmetz et al., 2003). We have shown that intravenous infusion of Ap4A reduces glomerular filtration rate (GFR) and evokes natriuresis and diuresis in rat (Szczepanska-Konkel et al., 2003). There are also reports describing action of ApnAs on renal cells. Ap3A, Ap4A, Ap5A and Ap6A induce depolarisation of mesangial cell membrane by activation of Cl− and nonselective conductance (Kleta et al., 1995). Diadenosine polyphosphates (Ap3A–Ap6A) modulate pH regulation of rat mesangial cells (Schulte et al., 1999). Moreover, these nucleotides increase intracellular calcium concentration (Tepel et al., 1996) and cause contraction of mesangial cells (Schlatter et al., 1995).
These reports have led us to focus our efforts on the investigation of the influence of ApnAs on glomerular microvasculature. The diameter of glomerular vessels is one of the factors that may alter GFR. In the present study, we examined the vasomotor properties of Ap3A, Ap4A and Ap5A in isolated rat renal glomeruli. The effects of nucleotides were evaluated based on changes of glomerular [3H]-inulin space (GIS).
Methods
Animals
Experiments were performed on male Wistar rats (weighing 220–250 g). The rats were housed in an animal care facility at the Medical University of Gdańsk and fed the standard pellet diet (Altromin GmbH, Lage, Germany). Experiments were approved by the local Ethics Committee for Animal Research.
Isolation of renal glomeruli
Rats were decapitated under diethyl ether anaesthesia (5 ml l air−1, inhalation for 1 min in a glass bell) and the kidneys were removed and placed in ice-cold phosphate-buffered saline (PBS; pH 7.4, composition in mM) containing NaCl 137, KCl 2.7, Na2HPO4 8.1, KH2PO4 1.5, CaCl2 0.9, MgCl2 0.49 and glucose 5.6. Glomeruli were isolated by gradual sieving technique (Misra, 1972). Briefly, the renal capsule was removed and the cortex was minced with a razor blade to a paste-like consistency and strained through a steel sieve (pore size 250 μm). The mash that passed through this sieve was suspended in ice-cold PBS. Then, the suspension passed through two consecutive steel sieves (120 and 70 μm). The glomeruli retained on the top of the 70 μm sieve were washed off with ice-cold PBS. Glomeruli were resuspended in ice-cold PBS buffer. The final suspension consisted of decapsulated glomeruli devoid of afferent and efferent arterioles. The tubular contamination was less than 5% as assessed under the light microscope. The entire procedure was carried out in an ice bath and took no more than 1 h.
Determination of glomerular inulin space
Glomerular inulin space (GIS) was measured according to the previously described method (Savin, 1986; Fujiwara et al., 1989) with our own modifications (Szczepanska-Konkel et al., 1991; Jankowski M et al., 2001a). Briefly, about 2000 glomeruli were suspended in 200 μl ice-cold PBS containing 1% bovine serum albumin. Samples were preincubated with 0.5 μCi [3H]inulin for 30 min at 37°C in a shaking water bath (1.7 Hz). Incubation was continued with varied concentrations of tested agents for the indicated time. The final volume of incubation mixture was 250 μl. Reactions were terminated by centrifugation in the following manner: suspension of glomeruli (200 μl) was transferred to a microtube containing 100 μl ice-cold silicone oil (Wacker Silicone) and centrifuged for 5 s at 5000 × g. Glomeruli were spun through the oil, forming a pellet on the bottom of the tube with incubation medium remaining behind. The tip of the microtube with the glomerular pellet was cutoff with a scalpel blade and content was resuspended in 500 μl 0.3% Triton X-100. Supernatant (20 μl) was taken from the medium above the oil and also transferred to the scintillation vial. After solubilisation, 2 ml of scintillation cocktail was added. Radioactivity of samples was measured in a liquid scintillation counter (LKB Wallace). GIS of a single glomerulus was calculated as follows:
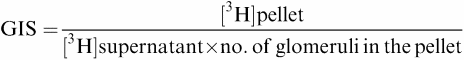 |
where [3H]pellet is measured in counts per minute (c.p.m.) and [3H]supernatant in c.p.m. divided by picolitres. The number of glomeruli in suspension medium was counted with a light microscope at low magnification. Each GIS determination was carried out in quadruplicate samples.
The results are expressed as picolitres per glomerulus or as a percentage of basal GIS value. Basal GIS value (mean±s.e.m.) of pooled calculations (n=36, control GIS before the start of incubation with tested agents) was 632±18 pl per glomerulus.
Experimental protocols
Measurements of GIS were performed according to the following protocols
Group 1. Effects of Ap3A, Ap4A and Ap5A on GIS of intact glomeruli: concentration- and time-dependency
For concentration–response curves, glomeruli were incubated for 5 min with Ap3A, Ap4A or Ap5A at concentration range 1 pM–100 μM each. For time–response curves, glomeruli were incubated with 1 μM tested dinucleotides for 0–10 min.
Group 2. Effect of P1 and P2 receptors antagonists on Ap4A and Ap5A action on GIS of intact glomeruli
Glomeruli were preincubated with theophylline (1 μM), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 10 μM), suramin (10 μM) or reactive blue 2 (50 μM) for 2 min and, thereafter, incubation was continued with 1 μM Ap4A or Ap5A for 5 min.
Group 3. Effects of Ap3A, Ap4A and Ap5A on GIS in the presence of angiotensin II: concentration- and time-dependency
For concentration–response curves, glomeruli were co-incubated with 1 μM angiotensin II and different concentrations of Ap3A, Ap4A or Ap5A (1 pM–1 μM) for 5 min. For time–response curves, glomeruli were co-incubated with 1 μM angiotensin II and 1 μM Ap3A, Ap4A or Ap5A for 0–10 min.
Group 4. Effect of P1 and P2 receptors antagonists on Ap3A action on GIS in the presence of angiotensin II
Glomeruli were preincubated with theophylline (1 μM), 3,7-dimethyl-1-propargylxanthine (DMPX; 10 μM), suramin (10 μM), reactive blue 2 (50 μM) or MRS 2179 (10 μM) for 2 min and, thereafter, incubation was continued with 1 μM angiotensin II and 1 μM Ap3A for 5 min.
Hydrolysis of Ap3A, Ap4A and Ap5A in suspension of glomeruli
The hydrolysis of dinucleotides by glomeruli was determined by measurement of ATP, adenosine, inosine and hypoxanthine concentration in suspension of glomeruli. About 2000 glomeruli were suspended in 200 μl ice-cold PBS and preincubated for 30 min at 37°C in shaking water bath (1.7 Hz). Then, incubation was continued with Ap3A, Ap4A or Ap5A (each at 1 μM) for the indicated time. Reactions were terminated by centrifugation (5 s at 5000 × g) of 200 μl of the suspension in the microtube containing 100 μl of ice-cold silicone oil (Wacker Silicone). Supernatant (150 μl) was taken from the medium above the oil and concentration of ATP, adenosine, inosine and hypoxanthine was measured immediately.
Analysis of metabolite formation from Ap3A, Ap4A and Ap5A
The concentration of ATP in suspension of glomeruli was measured using ATP Bioluminescence Assay Kit CLS II as per the manufacturer's instructions according to the published method (Kimmich et al., 1975). The concentration of adenosine, inosine and hypoxanthine in suspension of glomeruli was determined using the standard chemiluminescent method (Kather et al., 1987). Since adenosine was transformed to inosine, followed by conversion into hypoxanthine, the amount of inosine and hypoxanthine was pooled to that of adenosine to evaluate the total adenosine formation. The incubation medium (pH 8.2) contained 0.2 mM Na2HPO4/KH2PO4, 2.5 mM EDTA, 25 μM luminol and the following enzymes (all activities in U ml−1): 1.5 peroxidase, 40 xantine oxidase, 50 nucleoside phosphorylase, 2.4 adenosine deaminase. Luminescence was measured in room temperature with a photon-counting luminometer (TD-20/20) with autoinjector system.
Data analysis
Data were analysed statistically using Student's t-test or one-way analysis of variance, followed by a Dunnett's multiple comparison test using Sigma Stat 2.01 as appropriate. Differences were considered statistical significant where P was found to be <0.05. Data are shown as the mean±s.e.m. from n independent experiments.
Materials
Ammonium salts of Ap3A, Ap4A, Ap5A, MRS 2179, scintillation cocktail, suramin, reactive blue 2, theophylline, luminol, nucleoside phosphorylase were purchased from Sigma (St Louis, MO, U.S.A.), DPCPX and DMPX from RBI (Natick, MA, U.S.A.) and peroxidase, xantine oxidase, adenosine deaminase and ATP Bioluminescence Assay Kit CLS II from Roche (Mannheim, Germany). [3H]Inulin was obtained from DuPont NEN Products (Boston, MA, U.S.A.). All other agents were purchased from POCh (Gliwice, Poland).
Results
Hydrolysis of ApnA by isolated glomeruli
Ap3A, Ap4A and Ap5A were tested to evaluate their potential degradation by glomerular ecto-nucleotidases to vasoactive metabolites, that is, ATP and adenosine. The results are summarised in Table 1 (n=3). Luminescence analysis of the supernatant of glomeruli suspension incubated with 1 μM Ap3A did not reveal any detectable accumulation of ATP. With 1 μM Ap4A or Ap5A, however, there was significant accumulation of ATP after 1-min incubation. The maximal ATP concentration was detected during incubation with Ap4A 60.5±1.7 nM and it was three times more than with Ap5A. All tested dinucleotides were degraded to adenosine, inosine and hypoxanthine during 5-min incubation. Total concentrations of these metabolites increased about three-fold compared to basal value (control).
Table 1.
Hydrolysis of 1 μM ApnA by isolated rat glomeruli
| ApnA | ATP (nM) | Ado+Ino+Hyp (μM) | ||
|---|---|---|---|---|
| 1 min | 2 min | 5 min | 5 min | |
| Control | ND | ND | ND | 0.44±0.04 |
| Ap3A | ND | ND | ND | 1.39±0.16* |
| Ap4A | 60.5±1.7* | 54.1±0.6* | 45.4±1.1* | 1.31±0.15* |
| Ap5A | 17.5±0.6* | 15.7±0.8* | 21.4±0.2* | 1.13±0.06* |
n=3,
P<0.001,
ND=not detectable.
Effects of Ap3A, Ap4A and Ap5A on GIS of intact glomeruli
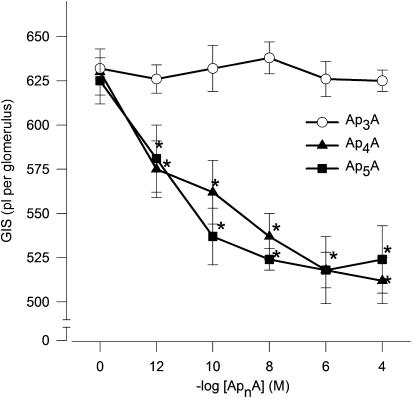
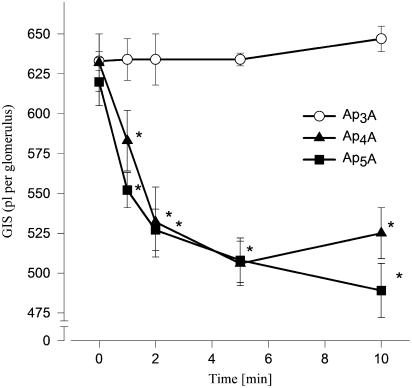
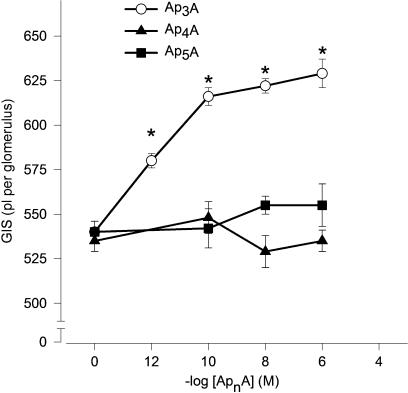
The actions of Ap4A and Ap5A were concentration dependent. Ap4A and Ap5A had an apparent EC50 of approximately 1 pM (Figure 1, n=5–6). By contrast, the actions of Ap3A were not concentration related in the range used in this study. The reduction of GIS induced by 1 μM Ap4A or Ap5A was significant in the 2nd min, reaching a maximum, that is, reduction of GIS about 15% in the 5th min (Figure 2, n=4–5).
Figure 1.
Concentration–response curves illustrating the constrictor effects of ApnAs on GIS. Glomeruli were incubated with Ap3A (open circles), Ap4A (filled triangles) or Ap5A (filled squares) at concentration range 1 pM–100 μM for 5 min. Results are expressed as the mean of absolute values±s.e.m. for n=5–6 experiments. *P<0.05 ApnAs vs basal value.
Figure 2.
Time–response curves illustrating the constrictor effects of ApnAs on GIS. Glomeruli were incubated with 1 μM Ap3A (open circles), Ap4A (filled triangles) or Ap5A (filled squares) for the indicated time. Results are expressed as the mean of absolute values±s.e.m. for n=4–5 experiments. *P<0.05 agent vs basal value.
Effect of P1 and P2 receptor antagonists on responses of glomeruli to Ap4A and Ap5A
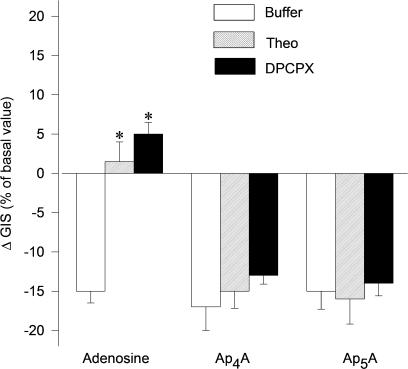
It is accepted that A1 receptors mediate vasoconstrictive response to adenosine. Therefore, in order to investigate the potential involvement of A1 receptors in ApnA-induced reduction of GIS, we used the nonselective antagonist of P1 receptor; theophylline (Theo) and selective antagonists of A1 receptor, that is, DPCPX. In the presence of Theo (1 μM) or DPCPX (10 μM) (Figure 3, n=4–5), responses of glomeruli to Ap4A and Ap5A were not significantly affected. The control response to 1 μM adenosine (Ado) was completely abolished. Theo and DPCPX alone did not exert any significant influence on GIS (data not shown).
Figure 3.
Effects of P1 receptor antagonists on contractile responses of glomeruli to Ap4A and Ap5A. Glomeruli were incubated with 1 μM theophylline (Theo, rising right columns), 10 μM DPCPX (selective A1 receptors antagonist, solid columns) or with buffer (open columns) for 2 min. Incubation was continued for 5 min with Ap4A, Ap5A and adenosine (each at 1 μM). Results are expressed as the percentage of changes of basal value±s.e.m. for n=4–5 experiments. *P<0.05 agent+antagonist vs agent+buffer.
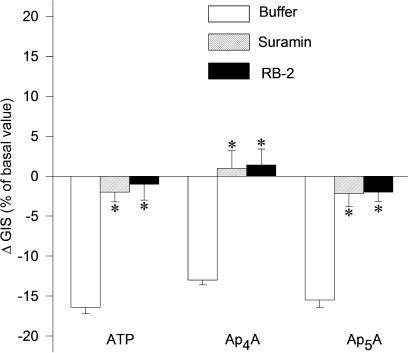
In another set of experiments, the glomeruli were preincubated with nonselective P2 receptor antagonist, that is, suramin (10 μM) or relatively selective P2 receptor antagonist, that is, reactive blue 2 (50 μM) to evaluate the involvement of P2 receptors in Ap4A- and Ap5A-induced reduction of GIS glomeruli. The responses to Ap4A, Ap5A and ATP as well, were almost completely abolished (Figure 4, n=3–4, P<0.05). Suramin and reactive blue 2 alone did not exert any significant influence on GIS (data not shown).
Figure 4.
Effects of P2 receptor antagonists on dilation responses of glomeruli to Ap4A and Ap5A. Glomeruli were incubated with buffer (open columns), 10 μM suramin (rising right columns) or 50 μM reactive blue 2 (RB-2, solid columns) for 2 min and then with ATP, Ap4A and Ap5A (each at 1 μM) for 5 min. Results are expressed as the percentage of changes of basal value±s.e.m. for n=3–4 experiments. *P<0.05 agent+antagonist vs agent+buffer.
Effects of Ap3A, Ap4A and Ap5A on GIS in the presence of angiotensin II
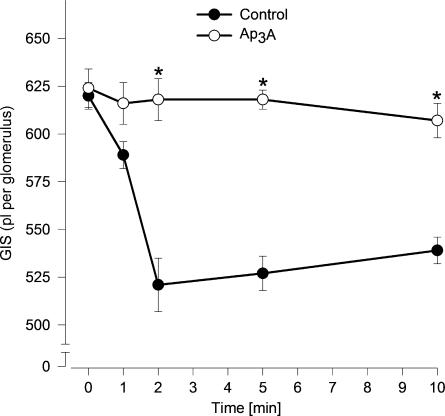
To evaluate the potential vasodilatory effects of ApnA, the responses of glomeruli to Ap3A, Ap4A and Ap5A were tested in the presence of a vasoconstrictive agent, that is, angiotensin II. It was published that angiotensin II-induced reduction of GIS and ATP prevented the action of angiotensin II in a concentration- and time-dependent manner (Jankowski M et al., 2001a). As shown in Figure 5 (n=5–6), Ap3A at concentration range 1 pM–1 μM prevented Ang II-induced reduction of GIS with apparent EC50 of approximately 1 pM. Ap4A and Ap5A (0.1 nM–1 μM) were not active in the concentration range used in this study. The effect of Ap3A was maximal in the 2nd min and stable during the 10 min of incubation (Figure 6, n=5–6).
Figure 5.
Concentration–response curves illustrating the dilator effects of ApnAs on GIS. Glomeruli were co-incubated with 1 μM angiotensin II and tested ApnA: Ap3A (open circles), Ap4A (filled triangles) and Ap5A (filled squares) at concentration range 1 pM–1 μM for 5 min. Results are expressed as the absolute values±s.e.m. for n=5–6 experiments. *P<0.05 ApnAs+Ang II vs Ang II+buffer.
Figure 6.
Time–response curves illustrating the dilator effects of ApnAs on GIS. Glomeruli were incubated with 1 μM Ap3A (open circles) or buffer (control; closed circles) in the presence of angiotensin II (1 μM) for the indicated time. Results are expressed as the absolute values±s.e.m. for n=5–6 experiments. *P<0.05 Ap3A vs control.
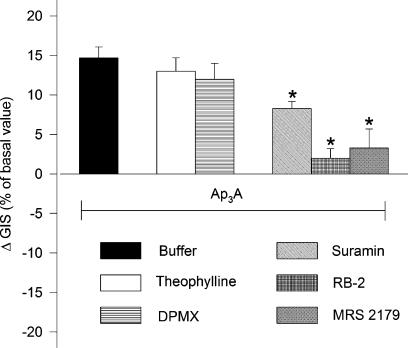
Effects of P1 and P2 receptor antagonists on responses of glomeruli to Ap3A in the presence of angiotensin II
To determine whether glomerular vasodilator response to Ap3A involves A2 receptors, Theo (1 μM) and selective A2 receptor antagonist – DMPX (10 μM) – were used. Following preincubation with Theo or DMPX, the maximal responses to Ap3A were not significantly affected (Figure 7, n=4–5). In order to determine whether P2 receptors are involved in Ap3A action, P2 receptor antagonists were used. The effect of Ap3A was almost completely abolished following preincubation of glomeruli with reactive blue 2 (50 μM) or selective P2Y1 receptor antagonist MRS 2179 (10 μM). The response to Ap3A was partially attenuated by suramin (P<0.05).
Figure 7.
Effects of P1 and P2 receptors antagonists on dilation responses of glomeruli to Ap3A. Glomeruli were preincubated with buffer (solid column) or P1 receptors antagonists: 1 μM theophylline (Theo, open columns), 10 μM DPMX (selective A2 receptors antagonist, horizontal lines column) or P2 receptors antagonists: 10 μM suramin (rising left column), 50 μM reactive blue 2 (horizontal cross hatch) and 10 μM MRS 2179 (selective P2Y1 receptors antagonist, diagonal cross hatch) for 2 min. Incubation was continued with angiotensin II (1 μM) and Ap3A (1 μM) for 5 min. Results are expressed as the percentage of changes of Ang II-treated glomeruli±s.e.m. for n=4–5 experiments. *P<0.05 Ap3A+antagonists vs Ap3A+buffer.
Discussion
The filtration of plasma, which occurs in renal glomeruli, is a fundamental physiological process. The glomerular tuft comprises capillary network supported by a central mesangium made up of smooth-muscle-derived cells, that is, mesangial cells and the dense fibrillar matrix (Lemley et al., 1992). It is known that mesangial cells contain well-developed contractile apparatus (Foidart et al., 1980; Anderson et al., 1981). Contraction of mesangial cells may lead to reduction of the effective filtration area, which, in turn, may reduce GFR. The studies on regulation of glomerular filtration have provided findings showing that ultrafiltration may be significantly changed as a result of mesangial cell contraction (Savin, 1986; Mene et al., 1989). The glomerular endothelial cells also influence the filtration process. These cells, under physiological conditions, are able to produce and release vasoactive substances, for example, nitric oxide, PGI2. These substances may influence mesangial cells tension and, in turn, GFR (Stockand & Sansom, 1998).
In this study, we investigated the action of diadenosine polyphosphates on contractility of glomeruli by measuring changes of extracellular volume (3H-labelled inulin space) of isolated decapsulated rat glomeruli. It has been shown that most of the extracellular space of decapsulated glomeruli is the intracapillary space that accounts for about 34% of total glomerular volume (Fujiwara et al., 1989). The intracapillary volume was calculated (GIS) after the separation of glomeruli from the medium. The basal GIS value we determined was 632±18 pl glomerulus−1. This value was comparable with other estimations (Fujiwara et al., 1984; Lewko et al., 1997). An increase or decrease of GIS reflects a relaxation or contraction of glomeruli, respectively. The validity of this method was endorsed by a previous study (Fujiwara et al., 1984; Kikkawa et al., 1986). It was confirmed that a decrease of glomerular diameter about 4% in response to angiotensin II was equivalent to about 10% decrease of GIS.
In the present study, we have investigated responses of isolated rat glomeruli to extracellular Ap3A, Ap4A and Ap5A. We have shown, for the first time, that incubation of intact glomeruli with Ap4A and Ap5A leads to concentration- and time-dependent reduction of glomerular volume. However, Ap3A did not affect the volume of native isolated glomeruli. Conversely, the potential vasodilatory activities of ApnA were investigated during co-incubation with angiotensin II (Ang II). This well-known vasoconstrictive agent induces stable in time reduction of GIS by about 20% (Fujiwara et al., 1984) or 16% (Figure 6). In our experiments, Ap3A prevented the action of Ang II in a concentration- and time-dependent manner. However, Ap4A and Ap5A did not affect the vasoconstrictive action of Ang II. These observations are in line with findings in various isolated vascular beds and arteries of several species (Busse et al., 1988; Van der Giet et al., 1997; Gabriels et al., 2000; Steinmetz et al., 2002). However, on the contrary to our observations, several investigators have shown that Ap4A and Ap5A possess the vasodilatory activity (Busse et al., 1988; van Ginneken et al., 2002). This bidirectional action of Ap4A and Ap5A observed by other investigators is probably due to, at least in part, characteristic for particular species and vessels distribution of purinoceptors, which may be simultaneously activated by ApnA (Steinmetz et al., 2000).
We have previously shown that extracellular ATP possesses unique vasomotor properties and acts bidirectionally on isolated glomeruli. ATP induces cGMP-dependent relaxation of angiotensin II-precontracted glomeruli (Jankowski M et al., 2001a) and induces contraction of intact glomeruli (Jankowski et al., 2000) with involvement of Rho-kinase in this process (Jankowski M et al., 2003). In contrast to ATP, the tested dinucleotides affect the glomerular volume only in one direction; Ap3A induces only relaxation whereas Ap4A and Ap5A induce only contraction of isolated glomeruli.
The effects of dinucleotides on glomeruli can be induced, at least in part, through their vasoactive metabolites, such as mononucleotides and adenosine. It has been reported for several cell types that dinucleotides are degraded by cell surface ecto-nucleotides that may be of importance for biological effects of extracellular dinucleotides (Verspohl et al., 1999). We analysed the ability of isolated glomeruli to hydrolyse ApnA and we observed significant release of adenosine (from Ap3A, Ap4A and Ap5A) and ATP (from Ap4A and Ap5A) during incubation (Table 1). From our previous work with ATP and adenosine in isolated glomeruli, it was suggested that dinucleotides might act through their vasoactive metabolites, that is, adenosine and ATP. We have previously shown that adenosine induces decrease of the glomerular volume and this effect is abolished in the presence of nonselective antagonist of P1 receptor, that is, theophylline (Jankowski M et al., 2001b). It is widely accepted that the vasoconstrictor response to adenosine is mediated via A1 receptors (Olivera et al., 1989). The expression of A1, A2A, A2B receptors has been observed on the cells of glomerulus (Inscho, 2001; Vitzthum et al., 2004). In order to investigate the potential involvement of adenosine receptors in ApnA-induced contraction and relaxation of glomeruli, we used the nonselective antagonist of P1 receptor; theophylline and selective antagonists of A1 receptor, that is, DPCPX and A2 receptor, that is, DMPX. Neither theophylline nor DPCPX prevented Ap4A- and Ap5A-induced contraction of glomeruli. Similarly, theophylline and DPMA did not prevent Ap3A-induced relaxation of glomeruli. These results suggest that P1 receptors, in particular A1 and A2 receptors, are probably not involved in vasoconstrictive and vasodilatory effects of ApnA. However, we cannot fully exclude that adenosine via P1 receptors, which appears in medium during incubation, modulates response of glomeruli to ApnA. Further studies are needed to investigate the cross-talk between P1 and P2 receptors during incubation of glomeruli with ApnA.
It has been previously reported that vascular effects of ApnA are mediated, at least in part, via P2 receptors. Ap3A and Ap5A evoke transient constrictions in vessels of the hydronephrotic rat kidney via P2 receptors (Gabriels et al., 2000). Ap5A mediates the decrease of mean arterial blood pressure by activation of P2Y1 receptors (Steinmetz et al., 2000). There is also pharmacological evidence showing that activity of Ap5A in the isolated perfused rat kidney is mediated by P2X receptors (Van der Giet et al., 1999). Ap4A and Ap5A are full agonists of rat P2X3 receptors and Ap4A is the partial agonist of rat P2X4 when expressed in Xenopus laevis oocytes (Wildman et al., 1999). It has been reported that Ap3A is a potent agonist of P2Y1 (Pintor et al., 1996). Our previous study has shown the functional expression of P2X receptors in isolated glomeruli (Jankowski et al., 2000). The expression of P2Y1, P2Y2, P2Y4, P2Y6 and P2X7 receptors has been observed in glomeruli (Inscho, 2001; Bailey et al., 2004).
The contraction and relaxation of glomeruli induced by ApnA can be compared to the glomerular effects of mononucleotides. ATP has been shown to be a potent agonist of P2X and P2Y receptors in the renal glomeruli (Jankowski M et al., 2001a; Bailey et al., 2004). The role of P2 receptors in response to ApnA was analysed using a common P2 receptor antagonists, that is, suramin (nonselective of P2 receptors) and reactive blue 2 (relatively selective of P2Y receptors). Both antagonists abolished the effects of Ap4A, Ap5A, and ATP as well, on isolated glomeruli. Conversely, the vasodilatory effect of Ap3A was partially prevented by suramin and markedly reduced by reactive blue 2. It has been previously shown that Ap3A is a potent agonist of P2Y1 receptors (Pintor et al., 1996). We have shown that the potent agonist of P2Y1 receptors, that is, 2-methythio-ATP induces the relaxation of glomeruli (Jankowski M et al., 2001a). Moreover, mRNA for P2Y1 receptors has been detected in isolated glomeruli (Bailey et al., 2004). Based on the above evidence we used MRS 2179: selective antagonist of P2Y1 receptor (Baurand et al., 2001). In our experiments, MRS 2179 abolished the vasodilatory effect of Ap3A. This finding strongly suggests that the Ap3A induces relaxation via P2Y1 receptors, which are probably located on endothelial cells. Similar effects were observed in rat mesenteric arterial bed where the effects of Ap3A were blocked by endothelium removal (Busse et al., 1988).
Our experiments show that the effects of Ap3A, Ap4A and Ap5A on glomerular volume are mediated via P2 receptors. However, it should be noted that ApnA are hydrolysed to mononucleotides, which may act on glomerular cells via P2 receptors. To our knowledge, it is not feasible to differentiate between effects evoked by ApnA per se and ATP and ADP in this experimental model. Hydrolysis of ApnA can be affected by agents that are antagonists of P2 receptors, for example, suramin, PPADS, Cibacron Blue. However, application of P2 inhibitors, for example, suramin will potentially obscure pharmacological experiments in which the involvement of P2 receptors in ApnA action on isolated glomeruli are investigated – because ApnA hydrolysis and P2 receptors are simultaneously blocked (Vollmayer et al., 2003).
Taken together, we have shown that Ap3A, Ap4A and Ap5A per se or via their degradation products, that is, ATP or ADP affect glomerular volume in a concentration of nmol range. Ap3A via P2Y1 receptors increase the glomerular volume but Ap4A and Ap5A via P2X and P2Y receptors decrease glomerular volume. These actions may subsequently lead to modification of GFR. Accordingly, we have previously shown that intravenous infusion of Ap4A reduces GFR (Szczepanska-Konkel et al., 2003). The concentrations of Ap3A–Ap6A in the human plasma are in μmol range under physiological conditions; however, a considerable portion of plasma ApnA is protein bound (Jankowski J et al., 2003). Thus, only a small portion of ApnA can affect their effectors, for example, microvasculature of glomeruli. It is believed that local concentrations of ApnA, that is, close to the place of its release, are important determinants of their effects. It can be assumed that local concentration of ApnA reaches 10 μM after ApnA release from platelets during their aggregation. The local concentration of ApnA may be higher in patients with essential hypertension because of elevated concentration of Ap5A and Ap6A in their platelets (Hollah et al., 2001). The elevated amounts of ApnA can be found also in platelets of haemodialysis patients and subsequently higher local concentration of ApnA can be reached after formation of a platelet thrombus. Therefore, ApnA are taken into consideration as an additional atherogenic factor in haemodialysis patients because of growth-stimulating effects on vascular smooth muscle cells (Jankowski J et al., 2001). Similarly, diadenosine polyphosphates are mesangial cell growth factors (Heidenreich et al., 1995) and may take part in pathogenesis of glomerulonephritis where platelet aggregation is one of the essential steps in the development of glomerular damage (Johnson et al., 1990; Harada et al., 2000; Rost et al., 2002).
The local concentration of Ap4A in plasma is increased also during ischaemia. Ap4A are released from myocardial granules into the blood stream of coronary vessels during ischaemia of the heart and play a role in cardioprotective function (Jovanovic et al., 1996; Luo et al., 2004). ApnAs may be released together with norepinephrine after sympathetic stimulation and hence may modulate the neural regulation of glomerular and tubular function (DiBona & Kopp 1997; Burnstock, 2004). This may be pathologically pronounced in patients with increased activity of sympathetic system, for example, essential hypertension (Hollenberg et al., 1975; Schlaich et al., 2004) or nephrotic syndrome (Herman et al., 1989).
In summary, the results of this study show that extracellular nucleotides, that is, Ap3A, Ap4A and Ap5A similar to vasoactive mononucleotides change glomerular volume. Ap4A and Ap5A via P2X and P2Y receptors may decrease and Ap3A via, at least in part, P2Y1 receptors may increase filtration surface, which in turn may modify GFR. It may be speculated that the physiological role of ApnAs may be the regulation of GFR per se or that they play the role of a long-term source of mononucleotides affecting glomerular function. Further studies are needed to determine the participation of particular cells of glomerulus in these actions. The present observations give an opportunity to develop new pharmacological tools that could be used to modulate renal function in different pathophysiological situations.
Acknowledgments
The study was supported by the State Committee for Scientific Research, Grant No. 4PO5A 019 19.
Abbreviations
- ApnAs
diadenosine polyphosphates
- Ap3A
P1,P3-diadenosine triphosphate
- Ap4A
P1,P4-diadenosine tetraphosphate
- Ap5A
P1,P5-diadenosine pentaphosphate
- DMPX
3,7-dimethyl-1-propargylxanthine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- GIS
glomerular inulin space
- MRS 2179
2′-deoxy-N6-methyl adenosine 3′5′-diphosphate diammonium salt
- PBS
phosphate-buffered saline
References
- ANDERSON J.M., GIMBRONE M.A., ALEXANDER R.W. Angiotensin II stimulates phosphorylation of the myosin light chain in cultured vascular smooth muscle cells. J. Biol. Chem. 1981;256:4693–4696. [PubMed] [Google Scholar]
- BAILEY M.A., TURNER C.M., HUS-CITHAREL A., MARCHETTI J., IMBERT-TEBOUL M., MILNER P., BURNSTOCK G., UNWIN R.J. P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol. 2004;96:79–90. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- BAURAND A., RABOISSON P., FREUND M., LEON C., CAZENAVE J.P., BOURGUIGNON J.J., GACHET C. Inhibition of platelet function by administration of MRS 2179, a P2Y1 receptor antagonist. Eur. J. Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Cotransmission. Curr. Opin. Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- BUSSE R., OGILVIE A., POHL U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am. J. Physiol. 1988;254:H828–H832. doi: 10.1152/ajpheart.1988.254.5.H828. [DOI] [PubMed] [Google Scholar]
- DIAZ-HERNANDEZ M., PINTOR J., CASTRO E., MIRAS-PORTUGAL M.T. Independent receptors for diadenosine pentaphosphate and ATP in rat midbrain single synaptic terminals. Eur. J. Neurosci. 2001;14:918–926. doi: 10.1046/j.0953-816x.2001.01703.x. [DOI] [PubMed] [Google Scholar]
- DIBONA G.F., KOPP U.C. Neural control of renal function. Physiol. Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- FOIDART J., SRAER J., DELARUE F., MAHIEU P., ARDAILLOU R. Evidence for mesangial glomerular receptors for angiotensin II linked to mesangial cell contractility. FEBS Lett. 1980;121:333–339. doi: 10.1016/0014-5793(80)80375-9. [DOI] [PubMed] [Google Scholar]
- FUJIWARA Y., KIKKAWA R., KITAMUTA E., TAKAMA T., SHIGETA Y. Angiotensin II effects upon glomerular intracapillary volume in the rat. Renal Physiol. 1984;7:344–348. doi: 10.1159/000172956. [DOI] [PubMed] [Google Scholar]
- FUJIWARA Y., KITAMUTA E., UEDA N., FUKUNAGA M., ORITA Y., KAMADA J. Mechanism of action of angiotensin II on isolated rat glomeruli. Kidney Int. 1989;36:985–991. doi: 10.1038/ki.1989.291. [DOI] [PubMed] [Google Scholar]
- GABRIELS G., ENDLICH K., RAHN K.H., SCHLATTER E., STEINHAUSEN M. In vivo effects of diadenosine polyphosphates on rat renal microcirculation. Kidney Int. 2000;57:2476–2484. doi: 10.1046/j.1523-1755.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- HARADA H., CHAN C.M., LOESCH A., UNWIN R., BURNSTOCK G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- HEIDENREICH S., TEPEL M., SCHLUTER H., HARRACH B., ZIDEK W. Regulation of rat mesangial cell growth diadenosine phosphates. J. Clin. Invest. 1995;95:2862–2867. doi: 10.1172/JCI117992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN P.J., SAWIN L.L., DIBONA G.F. Role of renal nerves in renal sodium retention of nephritic syndrome. Am. J. Physiol. 1989;256:F823–F829. doi: 10.1152/ajprenal.1989.256.5.F823. [DOI] [PubMed] [Google Scholar]
- HOHAGE H., REINHARDT C., BORUCKI U., ENCK G., SCHLUTER H., SCHLATTER E., ZIDEK W. Effects of diadenosine polyphosphates on renal function and blood pressure in anesthetised Wistar rats. J. Am. Soc. Nephrol. 1996;7:1216–1222. doi: 10.1681/ASN.V781216. [DOI] [PubMed] [Google Scholar]
- HOLLAH P., HAUSBERG M., KOSCH M., BARENBROCK M., LETZEL M., SCHLATTER E., RAHN K.H. A novel assay for determination of diadenosine polyphosphates in human platelets: studies in normotensive subjects and in patients with essential hypertension. J. Hypertens. 2001;19:237–245. doi: 10.1097/00004872-200102000-00010. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG N.K., ADAMS D.F., SOLOMON H., CHENITZ W.R., BURGER B.M., ABRAMS H.L., MERILL J.P. Renal vascular tone in essential and secondary hypertension. Medicine. 1975;54:29–44. doi: 10.1097/00005792-197501000-00002. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H., ZIGANSHIN A.U., PINTOR J., BURNSTOCK G. The activation of P1- and P2-purinoceptors in the guinea-pig left atrium by diadenosine polyphosphates. Br. J. Pharmacol. 1996;118:1294–1300. doi: 10.1111/j.1476-5381.1996.tb15536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILLES P., RIBEIRO J.A. Molecular physiology of P2 receptors in the central nervous system. Eur. J. Pharmacol. 2004;483:5–17. doi: 10.1016/j.ejphar.2003.10.030. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W. P2 receptors in regulation of renal microvascular function. Am. J. Physiol. 2001;280:F927–F944. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- JANKOWSKI J., HAGEMANN J., YOON M.S., VAN DER GIET M., STEPHAN N., ZIDEK W., SCHLUTER H., TEPEL M. Increased vascular growth in hemodialysis patients induced by platelet-derived diadenosine polyphosphates. Kidney Int. 2001;59:1134–1141. doi: 10.1046/j.1523-1755.2001.0590031134.x. [DOI] [PubMed] [Google Scholar]
- JANKOWSKI J., JANKOWSKI V., LAUFER U., VAN DER GIET M., HENNING L., TEPEL M., ZIDEK W., SCHLUTER H. Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler. Thromb. Vasc. Biol. 2003;23:1231–1238. doi: 10.1161/01.ATV.0000075913.00428.FD. [DOI] [PubMed] [Google Scholar]
- JANKOWSKI M., SZCZEPAŃSKA-KONKEL M., KALINOWSKI L., ANGIELSKI S. Bidirectional action of extracellular ATP on intracapillary volume of isolated rat renal glomeruli. J. Physiol. Pharmacol. 2000;51:491–496. [PubMed] [Google Scholar]
- JANKOWSKI M., SZCZEPAŃSKA-KONKEL M., KALINOWSKI L., ANGIELSKI S. Cyclic GMP-dependent relaxation of isolated rat renal glomeruli induced by extracellular ATP. J. Physiol. 2001a;530.1:123–130. doi: 10.1111/j.1469-7793.2001.0123m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANKOWSKI M., SZCZEPAŃSKA-KONKEL M., KALINOWSKI L., ANGIELSKI S. The role of P2Y-receptors in the regulation of glomerular volume. Med. Sci. Monit. 2001b;7:635–640. [PubMed] [Google Scholar]
- JANKOWSKI M., SZCZEPAŃSKA-KONKEL M., KALINOWSKI L., ANGIELSKI S. Involvement of Rho-kinase in P2Y-receptor-mediated contraction of renal glomeruli. Biochem. Biophys. Res. Commun. 2003;302:855–859. doi: 10.1016/s0006-291x(03)00272-9. [DOI] [PubMed] [Google Scholar]
- JIMENEZ A.I., CASTRO E., DELICADO E.G., MIRAS-PORTUGAL M.T. Specific diadenosine pentaphosphate receptor coupled to extracellular regulated kinases in cerebellar astrocytes. J. Neurochem. 2002;83:299–308. doi: 10.1046/j.1471-4159.2002.01111.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON R.J., GARCIA R.L., PRITZL P., ALPERS C.E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am. J. Physiol. 1990;136:369–374. [PMC free article] [PubMed] [Google Scholar]
- JOVANOVIC A., ZHANG S., ALEKSEEV A.E., TERZIC A. Diadenosine polyphosphate-induced inhibition of cardiac KATP channels: operative state-dependent regulation by a nucleoside diphosphate. Pflugers Arch. 1996;431:800–802. doi: 10.1007/BF02253848. [DOI] [PubMed] [Google Scholar]
- KATHER H., WIELAND E., WAAS W. Chemiluminescent determination of adenosine, inosine and hypoxantine/xantine. Anal. Biochem. 1987;163:45–51. doi: 10.1016/0003-2697(87)90091-1. [DOI] [PubMed] [Google Scholar]
- KHATTAB M., HOHAGE H., HOLLACH P.E., RAHN K.H., SCHLATTER E. Effects of diadenosine polyphosphates on systemic and regional hemodynamics in anesthtized rats. Kidney Blood Pressure Res. 1998;21:42–49. doi: 10.1159/000025842. [DOI] [PubMed] [Google Scholar]
- KIKKAWA R., KITAMURA E., FUJIWARA Y., ARIMURA T., HANEDA M., SHIGETA Y. Impaired contractile responsiveness of diabetic glomeruli to angiotensin II: A possible indication of mesangial dysfunction in diabetes mellitus. Biochem. Biophys. Res. Commun. 1986;136:1185–1190. doi: 10.1016/0006-291x(86)90459-6. [DOI] [PubMed] [Google Scholar]
- KIMMICH G.A., RANDLES J., BRAND J.S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme. Anal. Biochem. 1975;69:187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- KLETA R., HIRSH J., HEIDENREICH S., SCHLUTER H., ZIDEK W., SCHLATTER E. Effects of diadenosine polyphosphates, ATP and angiotensin II on membrane voltage and membrane conductances of rat mesangial cells. Pflugers Arch. 1995;430:713–720. doi: 10.1007/BF00386166. [DOI] [PubMed] [Google Scholar]
- LEMLEY K.V., ELGER M., KOEPPEN-HAGEMANN I., KRETZLER M., NAGATA N., SAKAI T., UIKER S., KRIZ W. The glomerular mesangium: capillary support function and its failure under experimental conditions. Clin. Invest. 1992;70:843–856. doi: 10.1007/BF00180755. [DOI] [PubMed] [Google Scholar]
- LEWKO B., WENDT U., SZCZEPAŃSKA-KONKEL M., STEPINSKI J., DREWNOWSKA K., ANGIELSKI S. Inhibition of endogenous nitric oxide synthesis activates particulate guanylyl cyclase in the rat renal glomeruli. Kidney Int. 1997;52:654–659. doi: 10.1038/ki.1997.379. [DOI] [PubMed] [Google Scholar]
- LUO J., JANKOWSKI V., GUNGAR N., NEUMANN J., SCHMITZ W., ZIDEK W., SCHLUTER H., JANKOWSKI J. Endogenous diadenosine tetraphosphate, diadenosine pentaphosphate, and diadenosine hexaphosphate in human myocardial tissue. Hypertension. 2004;43:1055–1059. doi: 10.1161/01.hyp.0000126110.46402.dd. [DOI] [PubMed] [Google Scholar]
- LUTHJE J., OGILVIE A. Catabolism of Ap4A and Ap3A in whole blood. The dinucleotides are long-lived signal molecules in the blood ending up as intracellular ATP in the erythrocytes. Eur. J. Biochem. 1988;173:241–245. doi: 10.1111/j.1432-1033.1988.tb13990.x. [DOI] [PubMed] [Google Scholar]
- MENE P., SIMONSON M.S., DUNN M.J. Physiology of the mesangial cell. Physiol. Rev. 1989;69:1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- MIRAS-PORTUGAL M.T., GUALIX J., PINTOR J. The neurotransmitter role of diadenosine polyphosphates. FEBS Lett. 1998;430:78–82. doi: 10.1016/s0014-5793(98)00560-2. [DOI] [PubMed] [Google Scholar]
- MISRA R.P. Isolation of glomeruli from mammalian kidneys by graded sieving. Am. J. Clin. Pathol. 1972;62:135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- OLIVERA A., LAMAS S., RODRIGUEZ-PUYOL D., LOPEZ-NOVOA J. Adenosine induces mesangial cell contraction by an A1-type receptor. Kidney Int. 1989;38:1300–1305. doi: 10.1038/ki.1989.126. [DOI] [PubMed] [Google Scholar]
- PINTOR J., KING B.F., MIRAS-PORTUGAL M.T., BURNSTOCK G. Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br. J. Pharmacol. 1996;119:1006–1012. doi: 10.1111/j.1476-5381.1996.tb15771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTOR J., MIRAS-PORTUGAL M.T. A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br. J. Pharmacol. 1995;115:895–902. doi: 10.1111/j.1476-5381.1995.tb15894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTOR J., PUCHE J.A., GUALIX J., HOYLE C.H., MIRAS-PORTUGAL T. Diadenosine polyphosphates evoke Ca2+ transients in guinea-pig brain via receptors distinct from those from ATP. J. Physiol. 1997;504.2:327–335. doi: 10.1111/j.1469-7793.1997.327be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Effects of purines and pyrimidines on rat mesenteric arterial bed. Circ. Res. 1991;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., HOYLE CH., BURNSTOCK G. Pivotal role of phosphate chain length in vasoconstrictor versus vasodilatator actions of adenine dinucleotides in rat mesenteric arteries. J. Physiol. 1995;483:703–713. doi: 10.1113/jphysiol.1995.sp020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., JANKOWSKI J., SCHLUTER H. Structure–activity relationship of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs) at P2 receptors in the rat mesenteric arterial bed. Br. J. Pharmacol. 2001;134:1073–1083. doi: 10.1038/sj.bjp.0704341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROST S., DANIEL C.H., SCHULZE-LOHOFF E., BAUMERT H.G., LAMBRECHT G., HUGO C.H. P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kidney Int. 2002;62:1659–1671. doi: 10.1046/j.1523-1755.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- SAVIN V.J. In vitro effects of angiotensin II on glomerular function. Am. J. Physiol. 1986;251:F627–F634. doi: 10.1152/ajprenal.1986.251.4.F627. [DOI] [PubMed] [Google Scholar]
- SCHLAICH M.P., LAMBERT E., KAYE D.M., KROZOWSKI Z., CAMPBELL D.J., LAMBERT G., HASTINGS J., AGGARWAL A., ESLER M.D. Sympathetic augmentation in hypertension. Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- SCHLATTER E., ANKORINA I., HAXELMANS S., KLETA R. Effects of diadenosine polyphosphates, ATP and angiotensin II on cytosolic Ca2+ activity and contraction of rat mesangial cells. Pflugers. Arch. 1995;430:721–728. doi: 10.1007/BF00386167. [DOI] [PubMed] [Google Scholar]
- SCHULTE E.A., HOHENDAHL A., STEGEMANN H., HIRSCH J.R., SALEH H., SCHLATTER E. Natriuretic peptides and diadenosine polyphosphates modulate pH regulation of rat mesangial cells. Cell Physiol. Biochem. 1999;9:310–322. doi: 10.1159/000016325. [DOI] [PubMed] [Google Scholar]
- SCHLUTER H., OFFERS E., BRUGGEMANN G., VAN DER GIET M., TEPEL M., NORDHOFF E., KARAS M., SPIEKER C., WITZEL H., ZIDEK W. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- STEINMETZ M., BIERER S., HOLLAH P., RAHN K.H., SCHLATTER E. Heterogeneous vascular effects of AP5A in different rat resistance arteries are due to heterogeneous distribution of P2X and P2Y1 purinoceptors. J. Pharmacol. Exp. Ther. 2000;294:1182–1187. [PubMed] [Google Scholar]
- STEINMETZ M., GABRIELS G., LE T.V., PIECHOTA H.J., RAHN K.H., SCHLATTER E. Vasoactivity of diadenosine polyphosphates in human small renal resistance arteries. Nephrol. Dial. Transplant. 2003;18:2496–2504. doi: 10.1093/ndt/gfg405. [DOI] [PubMed] [Google Scholar]
- STEINMETZ M., JANSSEN A.K., PELSTER F., RAHN K.H., SCHLATTER E. Vasoactivity of diadenosine polyphosphates in human small mesenteric resistance arteries. J. Pharmacol. Exp. Ther. 2002;302:787–794. doi: 10.1124/jpet.302.2.787. [DOI] [PubMed] [Google Scholar]
- STOCKAND J.D., SANSOM S.C. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol. Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- SUMIYOSHI R., NISHIMURA J., KAWASAKI J., KOBAYASHI S., TAKAHASHI S., KANAIDE H. Diadenosine polyphosphates directly relax porcaine coronary arterial smooth muscle. J. Pharmacol. Exp. Ther. 1997;283:548–556. [PubMed] [Google Scholar]
- SZCZEPAŃSKA-KONKEL M., LANGNER G., BEDNARCZUK G., STIEPANOW-TRZECIAK A., JANKOWSKI M., ANGIELSKI S. Renal haemodynamics and natriuretic responses to intravenous administration of diadenosine tetraphosphate (Ap4A) and nicotinamide adenine dinucleotides (NAD) in rat. J. Physiol. Pharm. 2003;54:163–173. [PubMed] [Google Scholar]
- SZCZEPAŃSKA-KONKEL M., REDLAK M., ANGIELSKI S. Glibenclamide-sensitive K+ channels are responsible for angiotensin II hypersensitive contraction and atrial natriuretic factor refractoriness of glomeruli in low-sodium rats. Biochem. Biophys. Res. Commun. 1991;181:871–876. doi: 10.1016/0006-291x(91)91271-d. [DOI] [PubMed] [Google Scholar]
- TEPEL M., HEIDENREICH S., SCHLUTER H., BEINLICH A., NOFER J.R., WALTER M., ASSMANN G., ZIDEK W. Diadenosine polyphosphates induce transplasma membrane calcium influx in cultured glomerular mesangial cells. Eur. J. Clin. Invest. 1996;26:1077–1084. doi: 10.1046/j.1365-2362.1996.400592.x. [DOI] [PubMed] [Google Scholar]
- VAHLENSIECK U., BOKNIK P., KNAPP J., MULLER F.U., NEUMANN J., HERZIG S., SCHLUTER H., ZIDEK W., DENG M.C., SCHELD H.H., SCHMITZ W. Negative chronotropic and inotropic effects of exerted by diadenosine hexaphosphate (AP6A) via A1-adenosine receptors. Br. J. Pharmacol. 1996;119:835–844. doi: 10.1111/j.1476-5381.1996.tb15748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER GIET M., CINKILIC O., JANKOWSKI J., TEPEL M., ZIDEK W., SCHLUTER H. Evidence for two different P2X-receptors mediating vasoconstriction of Ap5A and Ap6A in the isolated perfused rat kidney. Br. J. Pharmacol. 1999;127:1463–1469. doi: 10.1038/sj.bjp.0702667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER GIET M., KHATTAB M., BORGEL J., SCHLUTER H., ZIDEK W. Differential effects of diadenosine phosphate on purinoceptors in the rat isolated perfused kidney. Br. J. Pharmacol. 1997;120:1453–1460. doi: 10.1038/sj.bjp.0701074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN GINNEKEN E.E., RONGEN G.A., RUSSEL F.G., SMITS P. Diadenosine pentaphosphate vasodilates the forearm vascular bed: inhibition by theophylline and augmentation by dipyridamole. Clin. Pharmacol. Ther. 2002;71:448–456. doi: 10.1067/mcp.2002.124469. [DOI] [PubMed] [Google Scholar]
- VERSPOHL E.J., JOHANNWILLE B., KAISERLING-BUDDEMEIER I., SCHLUTER H., HAGEMANN J. Diadenosine polyphosphates in cultured vascular smooth-muscle cells and endothelium cells – their interaction with specific receptors and their degradation. J. Pharm. Pharmacol. 1999;51:1175–1781. doi: 10.1211/0022357991776714. [DOI] [PubMed] [Google Scholar]
- VITZTHUM H., WEISS B., BACHLEITNER W., KRÄMER B.K., KURTZ A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004;65:1180–1190. doi: 10.1111/j.1523-1755.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- VOLLMAYER P., CLAIR T., GODING J.W., SANO K., SERVOS J., ZIMMERMANN H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur. J. Biochem. 2003;270:2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., KING B.F., BURNSTOCK G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 1999;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]