Abstract
The limitations of traditional anticoagulants, heparin and warfarin, have prompted the development of new anticoagulant drugs for prevention and treatment of both venous and arterial thromboembolism. After a brief review of thrombogenesis and its regulation, this paper focuses on new anticoagulant agents in more advanced stages of clinical testing.
Keywords: Anticoagulant, antithrombotic, venous thromboembolism, arterial thromboembolism, direct thrombin inhibitor, pentasaccharide
Thrombogenesis and its regulation
Hemostasis, the physiologic response to vascular injury, results in the formation of a haemostatic plug that prevents blood loss. Under normal conditions, factors that promote blood coagulation are balanced by those that inhibit it. Pathologic thrombosis occurs when procoagulant stimuli overwhelm natural anticoagulant and fibrinolytic systems (Friedman, 1987).
Venous thrombi, which form under low flow conditions, are predominantly composed of fibrin and red cells. Thrombi may develop anywhere within the venous system, but most commonly arise in the deep veins of the leg (Lensing et al., 1999), through an interplay among venous stasis, hypercoagulability, and vessel wall damage (Lensing et al., 1999). Delayed emptying of the veins retard clearance of activated clotting factors. Hypoxemia caused by stasis results in activation of the endothelial cells lining the avascular valve cusps, a process exacerbated by inflammatory cytokines generated postoperatively or in medical illness. Leukocytes tethered to activated endothelial cells express tissue factor (TF), whereas platelets become activated and aggregate. Congenital or acquired disorders associated with hypercoagulability promote coagulation at these sites, thereby increasing the risk of thrombosis. Direct damage to the veins helps to explain the propensity to deep vein thrombosis after major orthopaedic surgery. Signs and symptoms develop when there is obstruction to venous outflow and inflammation of the vessel wall and perivascular tissue. Symptoms of pulmonary embolism can arise if segments of thrombus detach and embolize to the pulmonary circulation.
Arterial thrombosis usually is initiated by spontaneous or mechanical rupture of atherosclerotic plaque, a process that exposes thrombogenic material in the lipid-rich core of the plaque to the blood (Fuster, 1996). These thrombi form under high shear conditions and are composed primarily of platelet aggregates held together by fibrin strands. Obstruction of anterograde arterial flow leads to ischemia, which manifests as unstable angina or myocardial infarction in the case of coronary arteries, or stroke if cerebral vessels are involved (Fuster, 1996).
Blood constituents normally do not interact with intact endothelium. After damage to the endothelial lining of veins or arteries, platelets adhere to newly exposed subendothelial matrix components, particularly collagen and von Willebrand factor, via constitutively expressed receptors. Adherent platelets become activated, and recruit additional platelets by synthesizing thromboxane A2 and releasing adenosine diphosphate (ADP) (Davie, 1995). Platelet activation induces conformational changes in glycoprotein (GP) IIb/IIIa, one of the most abundant receptors on the platelet surface. By binding fibrinogen or, under high shear conditions, von Willebrand factor, conformationally activated GPIIb/IIIa crosslinks adjacent platelets (Davie, 1995), resulting in platelet aggregation.
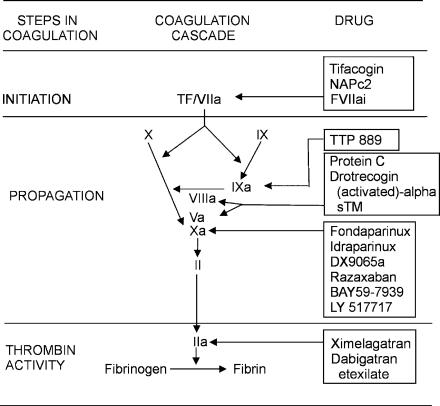
With damage to the vascular wall, TF-expressing cells are exposed to blood (Davie, 1995). This initiates coagulation in both the arteries and veins (Figure 1). TF binds activated factor VII (factor VIIa), which is found in small amounts in plasma, thereby forming factor VIIa/TF complex. This complex, also known as extrinsic tenase, activates factors IX and X, although factor X activation is more efficient (Davie, 1995). Factor Xa then converts small amounts of prothrombin to thrombin. This low concentration of thrombin is sufficient to activate factors V and VIII (key cofactors in coagulation), platelets, and platelet-bound factor XI.
Figure 1.
New anticoagulants and their targets in the coagulation pathway.
Propagation of coagulation is effected when factor IXa binds to factor VIIIa on the surface of activated platelets to form intrinsic tenase, a complex that efficiently activates factor X. Factor Xa binds to factor Va on the activated platelet surface to form prothrombinase, a complex that amplifies conversion of prothrombin to thrombin. Activation of platelet-bound factor XI by thrombin also promotes factor Xa generation (Davie, 1995). In the final step of coagulation, thrombin converts fibrinogen to fibrin and activates factor XIII, which stabilizes the platelet/fibrin thrombus by crosslinking the fibrin network.
Normally, the vessel wall inhibits thrombosis. Endothelial cells express thrombomodulin, a thrombin receptor, on their surface (Esmon & Owen, 1981). Once bound to thrombomodulin, the substrate specificity of thrombin is altered such that its procoagulant activities are abolished. Instead, thrombin's ability to activate protein C is enhanced about 1000-fold (Esmon et al., 1982; 1983). Activated protein C, along with its cofactor, protein S, acts as an inhibitor of coagulation by inactivating factors Va and VIIIa (Esmon, 1989), thereby attenuating thrombin generation. Although the density of thrombomodulin is greater on small vessels than it is on larger ones, large vessels also express more endothelial protein C receptor (EPCR) than smaller vessels. By binding protein C, EPCR localizes it in the vicinity of the thrombin/thrombomodulin complex (Laszik et al., 1997).
The endothelial surface also contains heparan sulfate, a heparin-like substance that catalyzes the antithrombin-mediated inhibition of thrombin, factor Xa, and other clotting enzymes (Lollar et al., 1984). Heparan sulfate and other glycosaminoglycans on the endothelial cell surface contribute to the binding of tissue factor pathway inhibitor (TFPI), a bivalent Kunitz-type inhibitor that blocks the initiation of coagulation by inhibiting TF-bound factor VIIa in a factor Xa-dependent fashion (Girard et al., 1990).
Endothelial cells also produce prostacyclin, nitric oxide, and ectoADPase, substances that inhibit platelet aggregation (Gayle et al., 1998). In addition, endothelial cells play a critical role in fibrinolysis. They synthesize and release tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA) and express annexin II on their surface (Huber et al., 2002). By serving as a coreceptor for t-PA and plasminogen, annexin II promotes plasmin generation on the endothelial cell surface (Brownstein et al., 2001).
New anticoagulants
When considering anticoagulants in current use, low-molecular-weight heparin is gradually replacing heparin for treatment of most patients with venous thromboembolism and acute coronary syndromes because low-molecular-weight heparin has similar efficacy but is more convenient and cost-effective than heparin in these patients (Weitz, 1997; Gould et al., 1999a, 1999b; Eikelboom et al., 2000; Quinlan et al., 2004). Despite advances with low-molecular-weight heparin, however, more potent anticoagulants are still required. Likewise, there is also a need for safer oral anticoagulants that do not require routine coagulation monitoring. Coumarin derivatives have a narrow therapeutic window and their metabolism is influenced by dietary factors and concomitant medications (Hirsh, 1991b). Consequently, time-consuming and expensive monitoring is required to ensure that a therapeutic anticoagulant effect is achieved.
New anticoagulant drugs target specific steps in coagulation. In general, anticoagulant strategies to inhibit thrombogenesis focus on blocking initiation of coagulation, preventing thrombin generation, or inhibiting thrombin. Initiation of coagulation can be inhibited by agents that target the factor VIIa/TF complex, whereas thrombin generation can be blocked by drugs that target factors IXa or Xa, or by inactivation of factors Va or VIIIa. Thrombin inhibitors prevent fibrin formation, block thrombin-mediated feedback activation of factors V, VIII, and XI, and attenuate thrombin-induced platelet aggregation.
Inhibitors of initiation of coagulation
Since the factor VIIa/TF complex initiates thrombosis (Davie, 1995), drugs that target this complex are potent inhibitors of coagulation. Agents in the most advanced stage of development are recombinant TFPI, nematode anticoagulant peptide (NAPc2), and active site-blocked factor VIIa (Factor VIIai).
TFPI
Based on studies in animals demonstrating that TFPI attenuates the coagulopathy and improves survival in sepsis models (Creasey et al., 1993; Elsayed et al., 1996; Bajaj & Bajaj, 1997), a recombinant form of TFPI (tifacogin) has been evaluated for this indication in humans. With promising phase II data (Abraham et al., 2001), TFPI was compared with placebo in a phase III clinical trial in 1754 patients with severe sepsis (Abraham et al., 2003). The primary end point, all-cause mortality at 28 days, was similar in both groups (34.2 and 33.9% with tifacogin and placebo, respectively) and the rate of bleeding was significantly higher with tifacogin than with placebo (6.5 and 4.8%, respectively). The utility of tifacogan is currently being evaluated in patients with severe community acquired pneumonia.
NAPc2
An 85-amino-acid anticoagulant protein isolated from the nematode, Ancylostoma caninum, NAPc2 binds to a noncatalytic site on both factor X and factor Xa and inhibits factor VIIa within the factor VIIa/TF complex (Stassens et al., 1996). Functionally, therefore, NAPc2 behaves much like TFPI. Since NAPc2 binds to factor X, as well as factor Xa, it has a half-life of almost 50 h after subcutaneous injection. Consequently, NAPc2 can be given on alternate days.
In a phase II study, NAPc2 showed promise in preventing venous thromboembolism after elective knee replacement surgery. Compared with historical controls, the efficacy and safety of NAPc2 were similar to those of low-molecular-weight heparin (Lee et al., 2001). However, prospective randomized trials are needed to confirm these findings. Currently, a series of phase II studies are underway to evaluate the utility of NAPc2 in patients with unstable angina or non-ST-myocardial infarction and in those undergoing percutaneous coronary interventions. In these trials, NAPc2 is added to routine therapy that includes aspirin, clopidogrel, heparin or low-molecular-weight heparin and, in some cases, a GPIIb/IIIa antagonist. The hemorrhagic consequences of adjunctive NAPc2 in these settings remain to be established and the long half-life of NAPc2 may be problematic if patients require urgent aortocoronary bypass surgery.
FVIIai
By competing with factor VIIa for TF binding, FVIIai, an inactivated form of factor VIIa, serves as a competitive inhibitor of TF-dependent factor IX or X activation. Based on promising preclinical studies in which FVIIai infusion prevented thrombus formation on artificial surfaces or injured vasculature (Jang et al., 1995; Banner et al., 1996; Arnljots et al., 1997; Harker et al., 1997; Golino et al., 1998; Giesen et al., 1999), factor VIIai with or without adjunctive heparin was compared with heparin alone in a phase II study of patients undergoing percutaneous coronary intervention (Lincoff, 2000). There was no significant difference between the two groups in the primary end point, a composite of death, myocardial infarction, need for urgent revascularization, abrupt vessel closure, or bailout use of GP IIb/IIIa antagonists, or heparin at day 7 or hospital discharge. Further, rates of major bleeding were similar in patients receiving factor VIIai or heparin. Owing to these disappointing results, factor VIIai has not been developed further for the treatment of arterial thrombosis.
Inhibitors of propagation of coagulation
Drugs that block factors IXa or Xa, or their respective cofactors, factor VIIIa or factor Va, inhibit the propagation of coagulation.
Factor IXa inhibitors
Factor IXa is essential for amplification of coagulation (Hoffman et al., 1995). Strategies to block factor IXa activity include active site-blocked factor IXa (Factor IXai), which competes with factor IXa for incorporation into the intrinsic tenase complex that assembles on the surface of activated platelets, and monoclonal antibodies against factor IX/IXa. Factor IXai inhibits clot formation in vitro and has been shown to attenuate injury-induced coronary artery thrombosis in a canine model (Benedict et al., 1991). One chimeric humanized derivative of an antibody against factor IX/IXa that inhibits factor XI-mediated activation of factor IX and blocks factor IXa activity has demonstrated antithrombotic activity in rat arterial thrombosis models (Bajaj et al., 1985; Feuerstein et al., 1999a, 1999b). TPP-889, an orally active factor IXa inhibitor, has completed phase I clinical testing, but neither it nor the parenteral factor IXa inhibitor has yet to reach phase II evaluation.
Factor Xa inhibitors
New factor Xa inhibitors include agents that block factor Xa indirectly or directly. The former act by catalyzing factor Xa inhibition by antithrombin. In contrast, direct factor Xa inhibitors bind directly to the active site of factor Xa, thereby blocking its interaction with its substrates. Unlike the heparin/antithrombin complex, direct factor Xa inhibitors not only inhibit free factor Xa but also inactivate factor Xa bound to platelets within the prothrombinase complex (Eisenberg et al., 1993; Brufatto & Nesheim, 2001; Rezaie, 2001). This property may endow these agents with an advantage over indirect factor Xa inhibitors. Synthetic pentasaccharides (fondaparinux and idraparinux), analogs of the pentasaccharide sequence of heparin that mediates its interaction with antithrombin (Herbert et al., 1998; Walenga et al., 2002), are new indirect factor Xa inhibitors. A parenteral (DX9065a) and several orally active direct factor Xa inhibitors (razaxaban, BAY 59-7939 and LY-51,7717) are currently undergoing phase II clinical testing.
Indirect factor Xa inhibitors
Fondaparinux and idraparinux have features that distinguish them from low-molecular-weight heparin (Table 1). Since they are too short to bridge antithrombin to thrombin, fondaparinux and idraparinux enhance the rate of factor Xa inactivation by antithrombin, thereby blocking thrombin generation, but have no effect on the rate of thrombin inhibition. Neither fondaparinux nor idraparinux interacts with plasma proteins other than antithrombin; as a result, these drugs produce a predictable dose response. Both agents have almost complete bioavailability after subcutaneous injection.
Table 1.
Comparison of the properties of LMWH, fondaparinux and idraparinux
| Feature | LMWH | Fondaprinux | Idraparinux |
|---|---|---|---|
| Mode of administration | Subcutaneous | Subcutaneous | Subcutaneous |
| Bioavailability | 80–90% | 100% | 100% |
| Half-life (h) | 4 | 17 | 80 |
| Target | Factor Xa and thrombin | Factor Xa | Factor Xa |
| Renal clearance | Yes | Yes | Yes |
| Neutralized by protamine sulfate | Partial | No | No |
Fondaparinux exhibits a dose-independent elimination half-life of approximately 17 h (Boneu et al., 1995) and can be administered subcutaneously once daily. Fondaparinux is not metabolized and clearance is almost exclusively by the kidney (Walenga et al., 1997). Consequently, dose adjustments are necessary in patients with renal insufficiency and fondaparinux should not be used in patients with renal failure. Although placental transfer of fondaparinux was not observed in a dually perfused human cotyledon model (Lagrange et al., 2002), limited clinical experience suggests that fondaparinux may pass the placental barrier in vivo, resulting in low but measurable antifactor Xa activity in umbilical cord blood (Dempfle, 2004). Fondaparinux does not bind to platelets or platelet factor 4 (PF4). As it does not induce formation of heparin/PF4 complexes that serve as the antigenic target for antibodies that cause heparin-induced thrombocytopenia, this condition is unlikely to occur with fondaparinux. Small studies have shown no crossreactivity of fondaparinux with sera from patients with heparin-induced thrombocytopenia (Elalamy et al., 1995; Walenga et al., 1996; Amiral et al., 1997).
Idraparinux, a hypermethylated derivative of fondaparinux, binds antithrombin with such high affinity that it assumes a plasma half-life of 80 h (Herbert et al., 1998), similar to that of antithrombin. Consequently, idraparinux can be given subcutaneously on a once-weekly basis.
Neither fondaparinux (Walenga et al., 2002) nor idraparinux interacts with protamine sulfate, the antidote for heparin. If uncontrolled bleeding occurs, a procoagulant, such as recombinant factor VIIa may be beneficial (Bijsterveld et al., 2002; 2004). However, recombinant factor VIIa is not available in all hospitals, and the drug is expensive and can induce thrombotic complications. Fondaparinux is degraded and inactivated by heparinases (Daud et al., 2001), so these agents offer promise as potential antidotes. In contrast, idraparinux is not susceptible to heparinase digestion. Owing to its long half-life, the absence of an antidote is a potential limitation of idraparinux.
Fondaparinux has been evaluated for prevention and treatment of venous thromboembolism and for treatment of arterial thrombosis. The antithrombotic efficacy of fondaparinux was demonstrated in four phase III trials comparing this agent with enoxaparin for thromboprophylaxis after surgery for hip fracture or for elective hip or knee arthroplasty (Eriksson B.I. et al., 2001; Bauer et al., 2001; Turpie et al., 2002a; Lassen et al., 2002). In these trials, which involved over 7300 patients, fondaparinux started 6 h after surgery reduced the risk of venous thromboembolism by approximately 55% compared with enoxaparin (Turpie et al., 2002b). Although major bleeding occurred more frequently in fondaparinux-treated patients (P=0.008), the incidence of bleeding in a critical organ or bleeding leading to death or reoperation was similar to that in patients receiving enoxaparin (Turpie et al., 2002b). These results may reflect the administration regimens rather than intrinsic differences in the efficacy of fondaparinux relative to enoxaparin. That is, the reduced risk of venous thromboembolism with fondaparinux may be due to the fact that it was started 6 h after surgery, whereas initiation of enoxaparin was delayed until 12–24 h after surgery. The earlier start with fondaparinux also could explain the increase in major bleeding observed with this agent. In support of this concept, post-hoc analysis reveals similar rates of major bleeding with fondaparinux and enoxaparin in those patients whose fondaparinux was started 6–8 h after surgery.
Extended prophylaxis with fondaparinux was studied in a phase III trial (PENTHIFRA-Plus) of 656 patients undergoing surgery for hip fracture. Prolonging the duration of prophylaxis with fondaparinux from 1 week to 4 weeks after hip fracture surgery reduced the primary end point, a composite of deep vein thrombosis detected on routine venography and symptomatic venous thromboembolism, from 35 to 1.4% (P<0.001) (Eriksson B.I. et al., 2003c). More importantly, the rate of symptomatic venous thromboembolism was reduced from 2.7 to 0.3% with extended fondaparinux prophylaxis (P=0.021).
Fondaparinux has also been evaluated for thromboprophylaxis in general medical and general surgical patients. In a double-blind randomized study of 849 medical patients 65 years or older, the primary end point (a composite of venographically diagnosed and symptomatic deep vein thrombosis, as well as nonfatal and fatal pulmonary embolism at day 15) occurred in 5.6% of those given fondaparinux and 10.5% of those who received placebo (P=0.03). Major bleeding while on therapy was infrequent (Cohen et al., 2003). In a double-blind phase III trial in which 2297 patients undergoing abdominal surgery were randomly assigned to receive prophylaxis with fondaparinux or dalteparin, the primary end point (a composite of venographically documented deep vein thrombosis symptomatic deep vein thrombosis, and nonfatal and fatal pulmonary embolism at postoperative day 30) occurred in 4.6 and 6.1% of patients, respectively (P=0.14). Major bleeding occurred in a similar proportion of patients in each treatment arm (Agnelli et al., 2003).
Fondaparinux also has been investigated for the initial treatment of venous thromboembolism. The results of the MATISSE DVT trial (Buller et al., 2004) and the MATISSE PE trial (Matisse Investigators, 2003) suggest that fondaparinux is as effective and safe as low-molecular-weight heparin or unfractionated heparin for initial treatment of patients with deep vein thrombosis and pulmonary embolism, respectively.
In a randomized, open-label, dose-finding trial (PENTALYSE), coadministration of fondaparinux and alteplase in ST-segment elevation myocardial infarction produced similar angiographic patency rates at 90 min as did treatment with heparin and alteplase (Coussement et al., 2001). Bleeding rates were also similar in those receiving fondaparinux and in those randomized to heparin. Fondaparinux also compared favorably with enoxaparin in a large phase II trial of patients with acute coronary syndrome without ST-segment elevation (PENTUA) (Fergusson, 2002); both the primary outcome (a composite of death, myocardial infarction, or recurrent angina at day 9) and bleeding occurred in similar proportions of patients randomized to fondaparinux or enoxaparin. Phase III trials with fondaparinux in patients with ST elevation and non-ST elevation myocardial infarction are well underway.
Once-weekly subcutaneous idraparinux was compared with warfarin in a phase II dose-finding trial of 659 patients with proximal deep vein thrombosis. Participants were randomized to warfarin or one of four doses of idraparinux after 5–7 days of initial therapy with enoxaparin (Persist Investigators, 2004). The rates of normalization and deterioration of ultrasonography and perfusion lung scanning were similar in all idraparinux dosing groups, and did not differ from that in the warfarin control group. However, there was a clear dose-response with respect to major bleeding in patients given idraparinux, with an unacceptably high frequency of bleeding in those given 10 mg of idraparinux. Two patients, both of whom received 5 mg of idraparinux, suffered a fatal bleed. Patients given the lowest dose of 2.5 mg had less bleeding than those randomized to warfarin (P=0.029). Phase III trials comparing 2.5 mg of idraparinux subcutaneously once-weekly with enoxaparin followed by warfarin for treatment of patients with deep vein thrombosis or pulmonary embolism are ongoing, as is another phase III study of once-weekly idraparinux versus warfarin in patients with atrial fibrillation.
Direct factor Xa inhibitors
Natural direct inhibitors of factor Xa include tick anticoagulant peptide (TAP) and antistasin. TAP (Vlasuk, 1993) and antistasin (Tuszyuski et al., 1987; Dunwiddie et al., 1989) were originally isolated from the soft tick and the Mexican leech, respectively. Both are available in recombinant forms. Although these agents have been shown to reduce arterial thrombosis (Orvim et al., 1995; Beimond et al., 1996) and restenosis (Ragosta et al., 1994) in animal models, because they are antigenic, neither has been tested in humans.
DX-9065a (Herbert et al., 1996; Murayama et al., 1996) is a synthetic nonpeptidic low-molecular-weight inhibitor that binds reversibly to the active site of factor Xa. It has been shown to be effective in thrombosis models in laboratory animals. DX-9065a exhibits oral bioavailability in animals but must be given in high doses to produce an antithrombotic effect (Herbert et al., 1996). In a phase I study in patients with stable coronary artery disease, intravenous DX-9065a appeared safe and did not cause excess bleeding (Dyke et al., 2002). In the XANADU-PCI pilot study (Alexander et al., 2004), which randomized 175 patients undergoing elective native-vessel percutaneous coronary interventions to different doses of DX9065a or to heparin, DX-9065a appeared to be a promising alternative to heparin. This possibility requires confirmation in phase III clinical trials.
An orally active agent, razaxaban is an aminobenzisoxazole that binds factor Xa with high affinity and is given twice daily. The antithrombotic potential of this agent was investigated in a phase II trial of thromboprophylaxis in knee arthroplasty patients in which participants were randomized to various doses of razaxaban or to enoxaparin for 10 days after surgery (Lassen et al., 2003). The primary end point, a composite of venographically detected deep vein thrombosis and symptomatic venous thromboembolism, occurred in 8.6% of patients given the lowest dose of razaxaban and in 15.9% of those treated with enoxaparin. Major bleeding occurred in 0.7% of patients given this dose of razaxaban and in none of those treated with enoxaparin. The three higher doses razaxaban arms of the study were stopped prematurely because of increased rates of major bleeding. Consequently, additional phase II studies are needed to identify the optimal doses of this agent.
BAY 59-7939 and LY-51,7717 are orally active, small molecule direct factor Xa inhibitors that are currently undergoing phase II testing for thromboprophylaxis in patients undergoing elective hip or knee arthroplasty and treatment of deep vein thrombosis.
Modulators of the protein C pathway
Factors VIIIa and Va, key cofactors for intrinsic tenase and prothrombinase, respectively, are critical for propagation of coagulation. Both cofactors are inactivated by activated protein C (along with its cofactor protein S). Strategies aimed at enhancing the protein C anticoagulant pathway include administration of protein C, activated protein C, or soluble thrombomodulin.
Protein C and activated protein C
Both plasma-derived and recombinant forms of protein C and activated protein C are available. Although promising results with protein C concentrates have been reported in patients with meningococcemia (Ettingshausen et al., 1999; White et al., 2000), activated protein C may be a better choice in patients with severe sepsis because inflammatory cytokines downregulate thrombomodulin expression on the endothelial surface. In a phase III trial in which intravenous recombinant activated protein C, drotrecogin alpha (activated), was compared with placebo in 1690 patients with severe sepsis, activated protein C produced a 19% reduction in 28-day mortality (from 30.8 to 24.7%, P=0.005) (Bernard et al., 2001). The rate of major bleeding was higher with activated protein C than with placebo (3.5 versus 2%, respectively; P=0.06). Based on these results and economic analyses (Manns et al., 2002) supporting the benefits of this agent, recombinant activated protein C is licensed in North America for adults with severe sepsis. An ongoing study is evaluating the utility of this agent in children with severe sepsis.
Soluble thrombomodulin
Soluble thrombomodulin is a recombinant analog of the extracellular domain of thrombomodulin (Parkinson et al., 1990). Like membrane-bound thrombomodulin, soluble thrombomodulin binds thrombin and converts it from a procoagulant enzyme into a potent activator of protein C. Recombinant soluble thrombomodulin has a half-life of 2–3 days after subcutaneous injection. This agent has been shown to have antithrombotic activity in a variety of animal models (Gomi et al., 1990; Aoki et al., 1994). In an open-label, dose-escalation study, soluble thrombomodulin attenuated coagulation abnormalities in patients with disseminated intravascular coagulation (Maruyama, 1999). In a phase II dose-ranging trial examining the utility of soluble thrombomodulin for thromboprophylaxis after elective hip arthroplasty, the primary end point (a composite of venographically detected deep vein thrombosis and symptomatic pulmonary embolism) occurred in 4.3% of the 94 patients given the lower dose of soluble thrombomodulin and in none of the 99 patients receiving the higher dose (Kearon et al., 2003). Major bleeding occurred in 1.6 and 5.7% of patients receiving the low or high dose of soluble thrombomodulin, respectively. Phase III clinical trials are necessary to compare soluble thrombomodulin with other forms of thromboprophylaxis, such as low-molecular-weight heparin or fondaparinux.
Thrombin inhibitors
Owing to its central role in coagulation and hemostasis, thrombin is an ideal target for new anticoagulants. The procoagulant effects of thrombin can be blocked either by inactivating the enzyme itself, or by preventing its generation. Indirect thrombin inhibitors, like unfractionated heparin, low-molecular-weight heparin, and dermatan sulfate act by catalyzing the naturally occurring thrombin inhibitors, antithrombin and/or heparin cofactor II (Hirsh, 1991a; Tollefsen, 1995; Weitz, 1997). Direct thrombin inhibitors bind directly to thrombin and block its interaction with substrates, thus preventing fibrin formation, thrombin-mediated activation of factors V, VIII, XI, or XIII, and thrombin-induced platelet aggregation. As a class, direct thrombin inhibitors have potential biologic and pharmacokinetic advantages over heparin. Unlike unfractionated heparin and low-molecular-weight heparin, direct thrombin inhibitors inactivate fibrin-bound thrombin (Hogg & Jackson, 1989; Weitz et al., 1990; 1998), in addition to fluid-phase thrombin. Consequently, direct thrombin inhibitors may attenuate thrombus accretion more effectively. In addition, direct thrombin inhibitors produce a more predictable anticoagulant effect than heparin because they do not bind to plasma proteins and are not neutralized by PF4 and high molecular-weight multimers of von Willebrand factor generated at sites of vascular injury (Stringer & Lindenfeld, 1992; Fox et al., 1993). Three parenteral direct thrombin inhibitors have been licensed in North America for limited indications. Hirudin (Potzch et al., 1994; Reiss et al., 1995; Greinacher et al., 1999a, 1999b; Farner et al., 2001; Lewis et al., 2001) and argatroban (Lewis et al., 2001; 2003) are approved for the treatment of heparin-induced thrombocytopenia, whereas bivalirudin is licensed as an alternative to heparin in patients undergoing percutaneous coronary indications (Bittl, 1995; Bittl et al., 1995; 2001; Kong et al., 1999; Lincoff et al., 2002; 2003; Weitz & Buller, 2002). Focusing on new agents, ximelagatran, a prodrug of melagtran, is the first orally available direct thrombin inhibitor and has undergone phase III clinical evaluation for prevention and treatment of venous thromboembolism and for prevention of cardioembolic events in patients with atrial fibrillation. Dabigatran etexilate (BIBR 1048), another oral direct thrombin inhibitor, is in a less advanced state of clinical development.
Ximelagatran
The first orally available direct thrombin inhibitor, ximelagatran is an uncharged lipophilic drug with little intrinsic activity against thrombin. This agent is a prodrug of melagatran, an active site-directed thrombin inhibitor. Ximelagatran is well absorbed from the gastrointestinal tract with a bioavailability of approximately 20% and undergoes rapid biotransformation to melagatran via two intermediate metabolites, H338/57 and H415/04 (Gustafsson et al., 2001; Eriksson U.G. et al., 2003a; Gustafsson, 2003). Melagatran levels peak in the blood within 2 h. Melagatran has a plasma half-life of 3–4 h in healthy volunteers and 4–5 h in patients. Owing to its short half-life, ximelagatran is administered twice daily. To-date, no foods or drugs have been documented to influence its absorption. Ximelagatran does not inhibit cytochrome P450 enzymes (Bredberg et al., 2003) and, therefore, has a low potential for drug–drug interactions. Ximelagatran produces a predictable anticoagulant response after oral administration and no coagulation monitoring appears to be necessary. As melagatran, the active agent, is eliminated via the kidneys, however, dose adjustments may be needed in patients with severe renal insufficiency, as evidenced by a creatinine clearance less than 30 ml min−1 (Eriksson U.G. et al, 2003b; Johansson et al., 2003).
Based on its pharmacological profile, ximelagatran has properties that endow it with potential advantages over vitamin K antagonists, such as warfarin (Table 2). One of the side effects of ximelagatran is elevation of liver transaminases. Elevations in alanine aminotrasferase of greater than three times the upper limit of normal occur in about 7.9% of patients receiving long-term therapy. Concomitant elevations in serum bilirubin occur in 0.5% of patients. Typically, changes in liver enzymes occur after 6 weeks to 4 months of therapy and are asymptomatic and reversible, even if the medication is continued. Although the increase in transaminases with ximelagatran appears to be benign, the long-term sequelae have yet to be determined. Consequently, it is likely that liver function tests will need to be monitored when initiating ximelagatran therapy. As with other direct thrombin inhibitors, there is no antidote for ximelagatran. Fortunately, this drug's short half-life makes it unlikely that this will be a substantial problem.
Table 2.
Potential advantages of ximelagatran over warfarin and their consequences
| Advantage | Consequence |
|---|---|
| Rapid onset of action | Obviates need for a parenteral anticoagulant when initiating therapy in patients with thrombosis or at high risk of thrombosis |
| No food or drug interactions | Predictable anticoagulant response |
| Wide therapeutic window | Can be given in fixed doses without routine coagulation monitoring |
| Short half-life | Reduced need for an antidote |
Ximelagatran has been evaluated for thromboprophylaxis in high-risk orthopedic patients, treatment of venous thromboembolism, prevention of cardioembolic events in patients with nonvalvular atrial fibrillation, and prevention of recurrent ischemia in patients with recent myocardial infarction. In phase II studies, ximelagatran, in combination with subcutaneous melagatran or as monotherapy, was shown to be safe and to have antithrombotic efficacy when used as prophylaxis against venous thromboembolism after elective hip or knee arthroplasty (Heit et al., 2001; Eriksson B.I. et al., 2002). Results of phase III studies suggest that the combination of a single subcutaneous injection of 3 mg melagatran preoperatively followed by oral ximelagatran (24 mg twice daily) postoperatively is more effective than enoxaparin for thromboprophylaxis after hip or knee arthroplasty, but may cause more bleeding (Eriksson B.I. et al., 2003a). While a single injection of subcutaneous melagatran postoperatively followed by ximelagatran (Eriksson B.I. et al., 2003b) or ximelagatran alone at a dosage of 24 mg twice daily (Colwell C.W. et al., 2003) appear less effective than enoxaparin in patients undergoing joint arthroplasty, postoperative ximelagatran in doses of 36 mg twice daily is more effective than warfarin for thromboprophylaxis after knee arthroplasty (Colwell C. et al., 2003; Francis C.S. et al., 2003).
Based on phase II data suggesting that ximelagatran also is effective for acute treatment of venous thromboembolism (Eriksson H. et al., 2003), a phase III trial comparing 6 months of ximelgatran monotherapy with low-molecular-weight heparin followed by warfarin in 2489 patients with venous thromboembolism was performed (Francis C.W. et al., 2003). The results suggest that oral ximelagatran is as effective and safe as conventional anticoagulation. Ximelagatran has been compared with placebo in 1233 patients who had completed a 6-month course of anticoagulant therapy for venous thromboembolism. Ximelegatran treatment significantly reduced the risk of recurrent thrombosis without increasing the risk of major bleeding (Schulman et al., 2003).
Promising data from phase II studies comparing ximelagatran with warfarin in patients with nonvalvular atrial fibrillation (Peterson, 2001; Petersen et al., 2003) prompted two phase III trials (Executive Steering Committee on behalf of the SPORTIF III Investigators, 2003; Cleland et al., 2004). In a randomized, open-label, parallel-group study of about 3400 patients at high risk of embolism (SPORTIF III), ximelgatran at a dose of 36 mg twice daily was at least as effective as warfarin targeted to an international normalized ratio (INR) of 2.0–3.0 in preventing stroke and systemic thromboembolism. Although the combined incidence of major and minor bleeding was significantly lower in those receiving ximelagatran than in those receiving warfarin, there was no statistically significant difference between the two groups with respect to major bleeding or intracranial hemorrhage. The results of a double-blinded, randomized trial comparing ximelagatran with warfarin (SPORTIF V) confirmed that unmonitored fixed-dose ximelagatran is as effective and safe as dose-adjusted warfarin. It is important to note that the SPORTIF trials compare ximelagatran with optimally controlled warfarin; using an expanded INR range of 1.8–3.2, over 80% of INR values were within range in these studies. In contrast, reports suggest that less than 50% of INR values are therapeutic when warfarin is managed in the community setting.
In the phase II ESTEEM trial (Wallentin et al., 2003), ximelagatran was compared with placebo in 1883 patients with ST elevation or non-ST elevation myocardial infarction within the past 14 days. All patients were given aspirin and optimal medical management that included statins and ACE inhibitors. Compared with placebo, all four ximelagatran doses significantly reduced the frequency of the primary end point, a composite of all-cause mortality, recurrent myocardial infarction, and severe recurrent ischemia. Major bleeding occurred in 1% of patients treated with placebo and 2% of those given ximelagran, a difference that is not statistically significant. Based on these data, a phase III trial is under consideration.
Dabigatran etexilate (BIBR 1048)
Dabigatran etexilate, another oral direct thrombin inhibitor (Gustafsson, 2003), is in a less advanced state of clinical development than ximelagatran. Like ximelagatran, dabigatran etexilate is a prodrug that is converted to its active metabolite dabigatran (BIBR 953), once it is absorbed from the gastrointestinal tract. Levels of dabigatran peak in the blood about 6 h after dabigatran etexilate administration and dabigatran is eliminated via the kidneys. The half-life of dabigatran is approximately 12 h, so the drug can be given once daily. Dabigatran and dabigatran etexilate have been shown to be effective in animal models of venous thrombosis (Wiene et al., 2001a, 2001b). While early clinical studies are promising (Stangier et al., 2003a, 2003b; Eriksson, B.I. et al., 2004), further work is needed to establish the safety and efficacy of this agent; dose finding studies for the prophylaxis and treatment of venous thromboembolism and for stroke prevention in atrial fibrillation are underway.
Conclusions
Although several promising new anticoagulants have been evaluated, the role of many of these agents remains to be delineated. Even if new anticoagulants prove superior to currently available agents, their advantages have to be substantial to offset additional costs.
Fondaparinux has been licensed for thromboprophylaxis in patients undergoing major orthopedic surgery and for the initial treatment of venous thromboembolism. Ongoing studies will determine the utility of this agent in patients with acute coronary syndromes.
Idraparinux and ximelagatran have the potential to streamline long-term anticoagulant therapy by eliminating the need for routine coagulation monitoring. With once-weekly subcutaneous administration, idraparinux is easy to administer and ongoing studies will determine its efficacy and safety relative to warfarin for treatment of venous thromboembolism and for prevention of cerebral and systemic embolism in patients with atrial fibrillation. The latter is an area where there is considerable room for improvement. Although warfarin is more effective than aspirin at reducing the risk of embolization in this setting, the need for frequent monitoring and difficulties in maintaining the INR within the therapeutic range limit its use (Albers et al., 2001).
As an orally active agent, ximelagatran shows promise for prevention and treatment of venous thrombosis. Based on the results of the SPORTIF III and V trials, unmonitored ximelagatran appears to be as effective and safe as dose-adjusted warfarin in this setting. With no need for coagulation monitoring, ximelagatran is more convenient than warfarin, a feature that may increase anticoagulant use in patients with atrial fibrillation. Still to be determined is the utility of ximelagatran in patients with mechanical valves and its safety in pregnancy. More information about the long-term hepatic effects of ximelagatran therapy is also needed.
Acknowledgments
Dr Bates is a recipient of a New Investigator Award from the Canadian Institutes of Health Research – University Industry (bioMérieux) program. Dr Weitz is the recipient of a Career Investigator Award from the Heart and Stroke Foundation of Canada and holds the Heart and Stroke Foundation of Ontario/J. Fraser Mustard Chair in Cardiovascular Research and the Canada Research Chair in Thrombosis.
Abbreviations
- ADP
adenosine diphosphate
- EPCR
endothelial protein C receptor
- Factor VIIa
activated factor VII
- Factor VIIai
active site-blocked factor VIIa
- GP
glycoprotein
- INR
international normalized ratio
- NAPc2
nematode anticoagulant peptide
- PF4
platelet factor 4
- TAP
tick anticoagulant peptide
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- t-PA
tissue plasminogen activator
- u-PA
urokinase plasminogen activator
References
- ABRAHAM E., REINHART K., OPAL S., DEMEYER I., DOIG C., RODRIGUEZ A.L., BEALE R., SVOBODA P., LATERRE P.F., SIMON S., LIGHT B., SPAPEN H., STONE J., SEIBERT A., PECKELSEN C., DE DEYNE C., POSTIER R., PETTILA V., ARTIGAS A., PERCELL S.R., SHU V., ZWINGELSTEIN C., TOBIAS J., POOLE L., STOLZENBACH J.C., CREASEY A.A., OPTIMIST TRIAL STUDY GROUP Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- ABRAHAM E., REINHART K., SVOBODA P., SEIBERT A., OLTHOFF D., DAL NOGARE A., POSTIER R., HEMPELMANN G., BUTLER T., MARTIN E., ZWINGELSTEIN C., PERCELL S., SHU V., LEIGHTON A., CREASEY A.A. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit. Care Med. 2001;29:8081–8089. doi: 10.1097/00003246-200111000-00007. [DOI] [PubMed] [Google Scholar]
- AGNELLI G., BERGQVIST D., COHEN A., GALLUS A., GENT M.A randomized double-blind study to compare the efficacy and safety of fondaparinux with dalteparin in the prevention of venous thromboembolism after high-risk abdominal surgery: the Pegasus Study J. Thromb. Haemost. 2003Suppl 1Abstr. #OC006
- ALBERS G.W., DALEN J., LAUPACIS A., MANNING W.J., PETERSEN P., SINGER D.E. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119:194S–206S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- ALEXANDER J.H., DYKE C.K., YANG H., BECKER R.C., HASSELBLAD V., ZILLMAN L.A., KLEIMAN N.S., HOCHMAN J.S., BERGER P.B., COHEN E.A., LINCOFF A.M., SAINT-JACQUES H., CHETCUTI S., BURTON J.R., BUERGLER J.M., SPENCE F.P., SHIMOTO Y., ROBERTSON T.L., KUNITADA S., BOVILL E.G., ARMSTRONG P.W., HARRINGTON R.A., The XaNADU-PCI PILOT Investigators Initial experience with factor Xa inhibition in percutaneous coronary intervention: the XaNADU-PIC Pilot. J. Thromb. Haemost. 2004;2:234–241. doi: 10.1111/j.1538-7933.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- AMIRAL J., LORMEAU J.C., MARFAING-KOKA A., VISSAC A.M., BOYER-NEUMANN C., TARDY B., HERBERT M., MEYER D. Absence of cross-reactivity of SR 90107A/ORG 31540 pentasaccharide with antibodies to heparin-PF4 complexes developed on heparin-induced thrombocytopenia. Blood Coagul. Fibrinolysis. 1997;8:114–117. doi: 10.1097/00001721-199703000-00005. [DOI] [PubMed] [Google Scholar]
- AOKI Y., OHISHI R., TAKEI R., MATSUZAKI O., MOHRI M., SAITOH K., GOMI K., SUGIHARA T., KIYOTO T., YAMAMOTO S. Effects of recombinant human soluble thrombomodulin (rhs-TM) on a rat model of disseminated intravascular coagulation with decreased levels of plasma antithrombin III. Thromb. Haemost. 1994;71:452–455. [PubMed] [Google Scholar]
- ARNLJOTS B., EZBAN M., HEDNER U. Prevention of experimental arterial thrombosis by topical administration of active site-inactivated factor VIIa. J. Vasc. Surg. 1997;25:341–346. doi: 10.1016/s0741-5214(97)70356-2. [DOI] [PubMed] [Google Scholar]
- BAJAJ M.S., BAJAJ S.P. Tissue factor pathway inhibitor: potential therapeutic applications. Thromb. Haemost. 1997;78:471–477. [PubMed] [Google Scholar]
- BAJAJ S.P., RAPAPORT S.I., MAKI S.L. A monoclonal antibody to factor IX that inhibits the factor VII: Ca potentiation of factor X activation. J. Biol. Chem. 1985;260:11574–11580. [PubMed] [Google Scholar]
- BANNER D.W., D'ARCY A., CHENE C., WINKLER F., GUHA A., KONIGSBERG W.H., NEMERSON Y., KIRCHHOFEV D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- BAUER K.A., ERIKSSON M.D., LASSEN M.R., TURPIE A.G.G., for the Steering Committee of the Pentasaccharide in Major Knee Surgery Study Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after major elective knee surgery. N. Engl. J. Med. 2001;345:1305–1310. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- BEIMOND B.J., FRIEDERICH P.W., LEVI M., VLASUK G.P., BULLER H.R., TEN CATE J.W. Comparison of sustained antithrombotic effects of inhibitors of thrombin and factor Xa in experimental thrombosis. Circulation. 1996;93:153–160. doi: 10.1161/01.cir.93.1.153. [DOI] [PubMed] [Google Scholar]
- BENEDICT C.R., RYAN J., WOLITZKY B., RAMOS R., GERLACH M., TIJBURG P., STERN D. Active site-blocked factor IXa prevents intravascular thrombus formation in the coronary vasculature without inhibiting extravascular coagulation in a canine thrombosis model. J. Clin. Invest. 1991;88:1760–1765. doi: 10.1172/JCI115495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNARD G.R., VINCENT J.L., LATERRE P.F., LA ROSA S.P., DHAINOUT J.R., LOPEZ-RODRIGUEZ A., STEINGRUB J.S., GARBER G.E., HELTERBRAND J.D., ELY E.W., FISHER C.W., JR Efficacy and safety of recombinant activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- BIJSTERVELD N.R., MOONS A.H., BOEKHOLDT S.M., VAN AKEN B.E., FENNEMA H., PETERS R.J., MEIJERS J.C., BULLER H.R., LEVI M. Ability of recombinant factor VIIa to reverse the anticoagulant effect of the pentasaccharide fondaparinux in healthy volunteers. Circulation. 2002;106:2550–2554. doi: 10.1161/01.cir.0000038501.87442.02. [DOI] [PubMed] [Google Scholar]
- BIJSTERVELD N.R., VINK R., VAN ARKEN B.E., FENNEMA H., PETERS R.J., MEIJERS J.C., BULLER H.R., LEVI M. Recombinant factor VIIa reverses the anticoagulant effect of the long-acting pentasaccharide idraparinux in healthy volunteers. Br. J. Haematol. 2004;124:653–658. doi: 10.1111/j.1365-2141.2003.04811.x. [DOI] [PubMed] [Google Scholar]
- BITTL J.A. Comparative safety profiles of hirulog and heparin in patients undergoing coronary angioplasty. The Hirulog Angioplasty Study Investigators. Am. Heart J. 1995;130:658–665. doi: 10.1016/0002-8703(95)90303-8. [DOI] [PubMed] [Google Scholar]
- BITTL J.A., CHAITMAN B.R., FEIT F., KIMBALL W., TOPOL E.J. Bivalirudin verus heparin during coronary angioplasty for unstable or post-infarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am. Heart. J. 2001;142:952–959. doi: 10.1067/mhj.2001.119374. [DOI] [PubMed] [Google Scholar]
- BITTL J.A., STRONY J., BRINKER J., AHMED WH MECKEL C.R., CHAITMAN B.R., MARAGANORE J., DEUTCH E., ADELMAN B., for the Hirulog Angioplasty Study Investigators Treatment with bivalirudin (hirulog) as compared with heparin during coronary angioplasty for unstable or post-infarction angina. N. Engl. J. Med. 1995;333:764–769. doi: 10.1056/NEJM199509213331204. [DOI] [PubMed] [Google Scholar]
- BONEU B., NECCIARI J., CARIOU R., SIE P., GABAIG A.M., KIEFFER G., DICKINSON J., LAMOND G., MOELKER H., MANT T. Pharmacokinetics and tolerance of the natural pentasaccharide (SR90107A/ ORG31540) with high affinity to antithrombin III in man. Thromb. Haemost. 1995;74:1468–1473. [PubMed] [Google Scholar]
- BREDBERG E., ANDERSSON T.B., FRISON L., THURESSON A., JOHANSSON S., ERIKSSON-LEPKOWSKA M., LARSSON M., ERIKSSON U.G. Ximelagatran, an oral direct thrombin inhibitor, has a low potential for cytochrome P450-mediatged drug–drug interactions. Clin. Pharmacokinet. 2003;42:765–777. doi: 10.2165/00003088-200342080-00005. [DOI] [PubMed] [Google Scholar]
- BROWNSTEIN C., FALCONE D.J., JACOVINA A& HAJJAR K.A., HAJJAR K.A. A mediator of cell surface-specific plasmin generation. Ann. NY Acad. Sci. U.S.A. 2001;947:143–155. [PubMed] [Google Scholar]
- BRUFATTO N., NESHEIM M.E. The use of prothrombin (S525C) labelled with fluorescein to directly study the inhibition of prothrombinase by antithrombin during prothrombin activation. J. Biol. Chem. 2001;276:17663–17671. doi: 10.1074/jbc.M011586200. [DOI] [PubMed] [Google Scholar]
- BULLER H.R., DAVIDSON B.L., DECOUSUS H., GALLUS A., GENT M., PIOVELLA F., PRINS M.H., RASKOB G., SEGERS A.E., CARIOU R., LEEUWENKAMP O., LENSING A.W., MATISSE Investigators Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann. Intern. Med. 2004;140:867–873. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- CLELAND G.F., FREEMANTLE N., KAYE G., NASIR M., VELAVAN P., LALUKOTA K., MUDAWI T., SHELTON R., CLARK A.L., COLETTA A.P. Clinical trial update from the American Heart Association meeting: Omega-3 fatty acids and arrhythmia risk in patients with an implantable defibrillator, ACTIV in CHR, VALIANT, the Hanover autologous bone marrow transplantation study, SPORTIF V, ORGIT and PAD and DEFINITE. Eur. J. Heart Failure. 2004;6:109–115. doi: 10.1016/j.ejheart.2003.12.002. [DOI] [PubMed] [Google Scholar]
- COHEN A.T., GALLUS A.S., LASSEN M.R., TOMKOWSKI W., TURPIE A.G.G., DAVIDSON B.L., CARIOU R.G., LENSING A.W.A., EGBERTS J.F.M.Fondaparinux vs placebo for the prevention of venous thromboembolism in acutely ill medical patients (ARTEMIS) J. Thromb. Haemost. 2003Suppl. 1Abstr. #P2046
- COLWELL C., BERKOWTIZ S.D., COMP P.C., LIEBERMAN J.R., GINSBERG J.S., for the EXULT B Investigators Randomized double-blind comparison of ximelagatran, an oral direct thrombin inhibitor and warfarin to prevent venous thromboembolism (VTE) after total knee replacement Blood 200310214Abstr. 39 [Google Scholar]
- COLWELL C.W., BERKOWITZ S.D., DAVIDSON B.L., LOTKE P.A., GINSBERG J.S., LIEBERMAN J.R., NEUBAUER J., MCELHATTAN J.L., PETERS G.R., FRANCIS C.W. Comparison of ximelagatran, an oral direct thrombin inhibitor with enoxaparin for the prevention of venous thromboembolism following total hip replacement. A randomized, double-blind study. J. Thromb. Haemost. 2003;1:2119–2130. doi: 10.1046/j.1538-7836.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- COUSSEMENT P.K., BASSAND J.P., CONVENS C., VROLIX M., BOLAND J., GROLLIER G., MICHELS R., VAHANIAN A., VANDERHEYDEN M., RUPPRECHT H.J., VAN DE WERF F. A synthetic factor Xa inhibitor ( ORG31540/SR9017A) as an adjunct to fibrinolysis in acute myocardial infarction. Eur. Heart J. 2001;22:1716–1724. doi: 10.1053/euhj.2001.2777. [DOI] [PubMed] [Google Scholar]
- CREASEY A.A., CHANG A.C., FEIGEN L., WUN T.C., TAYLOR FB JR., HINSHAW L.B. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J. Clin. Invest. 1993;91:2850–2856. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUD A.N., AHSAN A., IQBAL O., WALENGA J.M., SILVER P.J., AHMAD S., FAREED J. Synthetic heparin pentasacchardie depolymerization by heparinase: I: Molecular and biological implications. Clin. Appl. Thromb. Haemost. 2001;7:58–64. doi: 10.1177/107602960100700112. [DOI] [PubMed] [Google Scholar]
- DAVIE E.W. Biochemical and molecular aspects of the coagulation cascade. Thromb. Haemost. 1995;75:1–6. [PubMed] [Google Scholar]
- DEMPFLE C.H. Minor transplacental passage of fondaparinux in vivo (Letter to the Editor) N. Engl. J. Med. 2004;350:1914–1915. doi: 10.1056/NEJM200404293501825. [DOI] [PubMed] [Google Scholar]
- DUNWIDDIE C., THORNBERRY N.A., BULL H.G., SARDANA M., FRIEDMAN P.A., JACOBS J.W., SIMPSON E. Antistasin: a leech-derived inhibitor of factor Xa: kinetic analysis of enzyme inhibition and identification of the reactive site. J. Biol. Chem. 1989;264:16694–16699. [PubMed] [Google Scholar]
- DYKE C.K., BECKER R.C., KLEIMAN N.S., HOCHMAN J.S., BOVILL E.G., LINCOFF A.M., GERSTENBLITH G., DZAVIK V., GARDNER L.H., HASSELBLAD V., ZILLMAN L.A., SHIMOTO Y., ROBERTSON T.L., KUNITADA S., ARMSTRONG P.W., HARRINGTON R.A. First experience with direct factor Xa inhibition in patients with stable coronary disease: a pharmacokinetic and pharmacodynamic evaluation. Circulation. 2002;105:2385–2391. doi: 10.1161/01.cir.0000016351.12759.52. [DOI] [PubMed] [Google Scholar]
- EIKELBOOM J.W., ANAND S.S., MALMBERG K., WEITZ J.I., GINSBERG J.S., YUSUF S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndromes without ST-elevation: a meta-analysis. Lancet. 2000;355:1936–1942. doi: 10.1016/S0140-6736(00)02324-2. [DOI] [PubMed] [Google Scholar]
- EISENBERG P.R., SIEGEL J.E., ABENDSCHEIN D.R., MILETICH J.B. Importance of factor Xa in determining the procoagulant activity of whole-blood clots. J. Clin. Invest. 1993;191:1877–1883. doi: 10.1172/JCI116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELALAMY I., LECRUBIER C., POTEVIN F., ABDELOUAHED M., BARA L., MARIE J.P., SAMAMA M. Absence of in vitro cross-reaction of pentasaccharide with the plasma heparin dependent factor of twenty-five patients with heparin associated thrombocytopenia. Thromb. Haemost. 1995;74:1384–1385. [PubMed] [Google Scholar]
- ELSAYED Y.A., NAKAGAWA K., KAMIKUBO Y.I., ENJYOJI K.I., KATO H., SUEISHI K. Effects of recombinant human tissue factor pathway inhibitor on thrombus formation and its in vivo distribution in a rat DIC model. Am. J. Clin. Pathol. 1996;106:574–583. doi: 10.1093/ajcp/106.5.574. [DOI] [PubMed] [Google Scholar]
- ERIKSSON B.I., AGNELLI G., COHEN A.T., DAHL O.E., LASSEN M.R., MOURET P., ROSENCHER N., KALEBO P., PANFILOV S., ESKILSON C., ANDERSSON M., FREIJ A., EXPRESS Study Group The direct thrombin inhibitor melagatran followed by ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. J. Thromb. Haemost. 2003a;1:2490–2496. doi: 10.1111/j.1538-7836.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- ERIKSSON B.I., AGNELLI G., COHEN A.T., DAHL O.E., MOURET P., ROSENCHER N., ESKILSON C., NYLANDER I., FRISON L., OGREN M. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee joint replacement. Thromb. Haemost. 2003b;89:288–296. [PubMed] [Google Scholar]
- ERIKSSON B.I., BAUER K.A., LASSEN M.R., TURPIE A.G.G., for the Steering Committee of the Pentasaccharide in Hip Fracture Surgery Study Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N. Engl. J. Med. 2001;345:1340–1342. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- ERIKSSON B.I., BERGQVIST D., KALEBO P., DAHL O.E., LINDBRATT S., BYLOCK A., FRISON L., ERIKSSON U.G., WELIN L., GUSTAFSSON D. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet. 2002;360:1441–1447. doi: 10.1016/s0140-6736(02)11469-3. [DOI] [PubMed] [Google Scholar]
- ERIKSSON B.I., DAHL O.E., AHNFELT L., KALEBO P., STANGIER J., NEHMIZ G., HERMANSSON K., KOHLBRENNER V. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO 1. J. Thromb. Haemost. 2004;2:1573–1580. doi: 10.1111/j.1538-7836.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- ERIKSSON B.I., LASSEN M.R., PENTasaccharide in Hip-FRActure Surgery Plus Investigators Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicentre, randomized, placebo-controlled, double-blind study. Arch. Intern. Med. 2003c;163:1733–1742. doi: 10.1001/archinte.163.11.1337. [DOI] [PubMed] [Google Scholar]
- ERIKSSON H., WAHLANDER K., GUSTAFSSON D., for the THRIVE I Investigators A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J. Thromb. Haemost. 2003;1:41–47. doi: 10.1046/j.1538-7836.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- ERIKSSON U.G., BREDBERG U., GISLEN K., JOHANSSON L.C., FRISON L., AHNOFF M., GUSTAFSSON D. Pharmacokinetics and pharmacodynamic of ximelagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur. J. Clin. Pharmcol. 2003a;59:35–43. doi: 10.1007/s00228-003-0565-7. [DOI] [PubMed] [Google Scholar]
- ERIKSSON U.G., JOHANSSON S., ATTMAN P.O., MULEC H., FRISON L., FAGER G., SAMUELSSON O. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin. Pharmcokinet. 2003b;42:743–753. doi: 10.2165/00003088-200342080-00003. [DOI] [PubMed] [Google Scholar]
- ESMON C.T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J. Biol. Chem. 1989;264:4743–4746. [PubMed] [Google Scholar]
- ESMON C.T., ESMON N.L., HARRIS K.W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J. Biol. Chem. 1982;257:7944–7948. [PubMed] [Google Scholar]
- ESMON C.T., OWEN W.B. Identification of an endothelial cell cofactor for the thrombin-catalyzed activation of protein C. Proc. Natl. Acad. Sci. U.S.A. 1981;78:224–229. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESMON N.L., CARROLL R.C., ESMON C.T. Thrombomodulin blocks the ability of thrombin to activate platelets. J. Biol. Chem. 1983;258:12268–12273. [PubMed] [Google Scholar]
- ETTINGSHAUSEN C.E., VELDMANN A., BEEG T., SCHNEIDER W., JAGER G., KREUZ W. Replacement therapy with protein C concentrate in infants and adolescents with meningococcal sepsis and purpura fulminans. Semin. Thomb. Hemost. 1999;25:537–541. doi: 10.1055/s-2007-994962. [DOI] [PubMed] [Google Scholar]
- EXECUTIVE STEERING COMMITTEE ON BEHALF OF THE SPORTIF III INVESTIGATORS Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- FARNER B., EICHER P., KROLL H., GREINACHER A. A comparison of danaparoid and lepirudin in heparin-induced thrombocytopenia. Thromb. Haemost. 2001;85:950–957. [PubMed] [Google Scholar]
- FERGUSSON J.J. Meeting highlights – American Heart Association scientific sessions 2001. Circulation. 2002;105:e37–e41. [Google Scholar]
- FEUERSTEIN G.Z., PATEL A., TOOMEY J.R., BUGELSKI P., NICHOLS A.J., CHURCH W.R., VALOCIK R., KOSTER P., BAKER A., BLACKBURN M.N. Antithrombotic efficacy of a novel murine antihuman factor IX antibody in rats. Arterioscler Thromb. Vasc. Biol. 1999a;19:2554–2562. doi: 10.1161/01.atv.19.10.2554. [DOI] [PubMed] [Google Scholar]
- FEURERSTEIN G.Z., TOOMEY J.R., VALOCIK R., KOSTER P., PATEL A., BLACKBURN M.N. An inhibitory anti-factor IX antibody effectively reduces thrombus formation in a rat model of venous thrombosis. Thromb. Haemost. 1999b;92:1443–1450. [PubMed] [Google Scholar]
- FOX I., DAWSON A., LOYNDS P., EISNER J., FINDLEN K., LEVIN E., HANSON D., MANT T., WAGNER J., MARAGANORE J. Anticoagulant activity of Hirulog, a direct thrombin inhibitor, in humans. Thromb. Haemost. 1993;69:157–163. [PubMed] [Google Scholar]
- FRANCIS C.S., BERKOWITZ S.D., COMP P.C., LIEBERMAN J.R., GINSBERG J.S., PAIMENT G., PETERS G.R., ROTH A.W., MCELHATTAN J., COLWELL C.W. for the EXULT A Study Group Comparison of ximelagatran with warfarin for prevention of venous thromboembolism after total knee replacement. N. Engl. J. Med. 2003;349:1703–1712. doi: 10.1056/NEJMoa035162. [DOI] [PubMed] [Google Scholar]
- FRANCIS C.W., GINSBERG J.S., BERKOWITZ S.D., BOUNAMOUX H., DAVIDSON B.L., ERIKSSON H., FIESSINGER J.N., HUISMANN M., LUNDSTROM T., NYSTROM P., for the THRIVE Treatment Study Investigators Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute symptomatic deep vein thrombosis, with or without pulmonary embolism: the THRIVE Treatment Study Blood 2003102Suppl.6aAbstract 7 [Google Scholar]
- FRIEDMAN D.G.The structure of thrombi Hemostasis and Thrombosis: Basic Principles and Clinical Practice 1987Philadelphia: JB Lippincott; 2nd edn. ed. Colman RW, Hirsh J, Marder V, Salzman EW.Chapter 71 [Google Scholar]
- FUSTER V. Elucidation of the role of plaque instability and rupture in acute coronary events. Curr. Opin. Cardiol. 1996;11:351–360. doi: 10.1016/s0002-9149(99)80467-6. [DOI] [PubMed] [Google Scholar]
- GAYLE R.B., III, MALISZEWSKI C.R., GIMPEL S.C., SCHOENBORN M.A., CASPARY R.G., RICHARDS C., BRASEL K., PRICE V., DROSOPOULOS J.H., ISLAM N., ALYONYCHEVEA T.N., BOREKMAN M.J., MARCUS A.J. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J. Clin. Invest. 1998;101:1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIESEN P.L., RAUCH U., BOHRMANN B., KLING D., ROQUE M., FALLON J.T., BADIMON J.J., HIMBER J., RIEDERER M.A., NEMERSON Y. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD T.J., MACPHAIL L.A., LIKERT K.M., NOVOTNY W.F., MILETICH J.P., BROZE GJ JR. Inhibition of factor VIIa-tissue factor coagulation activity by a hybrid protein. Science. 1990;248:1421–1424. doi: 10.1126/science.1972598. [DOI] [PubMed] [Google Scholar]
- GOLINO P., RAGNI M., CIRILLO P., D'ANDREA D., SCOGNAMIGLIO A., RAVERA A., BUONO C., EZBAN M., CORCIONE N., VIGORITO F., CONDORELLI M., CHIARELLO M. Antithrombotic effects of recombinant human, active site-blocked factor VIIa in a rabbit model of recurrent and arterial thrombosis. Circul. Res. 1998;82:39–46. doi: 10.1161/01.res.82.1.39. [DOI] [PubMed] [Google Scholar]
- GOMI K., ZUSHI M., HONDA G., KAWAHARA S., MATSUZAKI O., KARABAYASHI T., YAMAMOTO S., MARUYAMA I., SUZUKI K. Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood. 1990;75:1396–1399. [PubMed] [Google Scholar]
- GOULD M.K., DEMBITZER A.D., DOYLE R.L., HASTIE T.J., GARBER A.M. Low-molecular-weight heparins compared with unfractionated heparin for treatment of deep venous thrombosis. A meta-analysis of randomized controlled trials. Ann. Intern. Med. 1999a;130:800–809. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- GOULD M.K., DEMBITZER A.D., SANDERS G.D., GARBER A.M. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysis. Ann. Intern. Med. 1999b;130:789–799. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- GREINACHER A., JANSSENS U., BERG G., BOCK M., KWASNY H., KEMKES-MATTHES B., EICHLER P., VOLPEL H., POTZSCH B., LUZ M., for the Heparin-Associated Thrombocytopenia Study (HAT) Investigators Lepirudin (recombinant hirudin) for parenteral anticoagulation in patients with heparin-induced thrombocytopenia. Circulation. 1999a;100:587–593. doi: 10.1161/01.cir.100.6.587. [DOI] [PubMed] [Google Scholar]
- GREINACHER A., VOLPEL H., JANSSENS U., HACH-WUNDERLE V., KEMKES-MATTHES B., EICHLER P., MUELLER-VELTEN H.G., POTZSCH B., for the HIT Investigators Group Recombinant hirudin (lepirudin) provides safe and effective anticoagulation in patients with heparin-induced thrombocytopenia: a prospective study. Circulation. 1999b;99:73–80. doi: 10.1161/01.cir.99.1.73. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON D. Oral direct thrombin inhibitors in clinical development. J. Int. Med. 2003;254:322–334. doi: 10.1046/j.1365-2796.2003.01225.x. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON D., NYSTROM J., CARLSSON S., BREDBERG U., ERIKSSON U., GYZANDER E., ELG M., ANTONSSON T., HOFFMAN K., UNGELL A., SORESNSEN H., NAGARD S., ABRAHAMSSON A., BYLUND R. The direct thrombin inhibitor melagatran and its oral prodrug H376/95: intestinal absorption properties, biochemical and pharmacodynamic effects. Thromb. Res. 2001;101:171–181. doi: 10.1016/s0049-3848(00)00399-6. [DOI] [PubMed] [Google Scholar]
- HARKER L.A., HANSON S.R., KELLY A.B. Antithrombotic strategies targeting thrombin activities, thrombin receptors and thrombin generation. Thromb. Haemost. 1997;78:736–741. [PubMed] [Google Scholar]
- HEIT J.A., COLWELL D.W., FRANCIS C.W., GINSBERG J.S., BERKOWITZ S.D., WHILLPLE J., PETERS G., for the AstraZeneca Arthroplasty Study Group Comparison of the oral direct thrombin inhibitor ximelagatran with enoxaparin as prophylaxis against venous thromboembolism after total knee replacement. Arch. Intern. Med. 2001;161:2215–2221. doi: 10.1001/archinte.161.18.2215. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M., BERNAT A., DOL F., HERAULT J.P., CREPON B., LORMEAU J.C. DX-9065a, a novel synthetic, selective and orally active inhibitor of factor Xa: in vitro and in vivo studies. J. Pharmacol. Exper. Ther. 1996;276:1030–1038. [PubMed] [Google Scholar]
- HERBERT J.M., HERAULT J.P., BERNAT A., VAN ARMSTERDAM R.G., LORMEAU J.C., PETITOU M., VANBOECKEL C., HOFFMAN P., MEULEMAN D.G. Biochemical and pharmacological properties of SANORG 340006, a potent and long-acting synthetic pentasaccharide. Blood. 1998;91:4197–4205. [PubMed] [Google Scholar]
- HIRSH J. Heparin. N. Engl. J. Med. 1991a;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- HIRSH J. Oral anticoagulant drugs. N. Engl. J. Med. 1991b;324:1865–1875. doi: 10.1056/NEJM199106273242606. [DOI] [PubMed] [Google Scholar]
- HOFFMAN M., MONROE D.M., OLIVER J.A., ROBERTS H.R. Factors IXa and Xa play distinct roles in tissue-dependent initiation of coagulation. Blood. 1995;86:1794–1801. [PubMed] [Google Scholar]
- HOGG P.I., JACKSON C.M. Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: implications for heparin efficacy. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3619–3623. doi: 10.1073/pnas.86.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBER D., CRAMER E.M., KAUFMANN J.E., MEDA P., MASSE J.M., KRUITHOF E.K., VISCHER U.M. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002;99:3637–3645. doi: 10.1182/blood.v99.10.3637. [DOI] [PubMed] [Google Scholar]
- JANG Y., GUZMAN L.A., LINCOFF A.M., GOTTWAUNER-WOLF M., FORUDI F., HART C.E., COURTMAN D.W., EZBAN M., ELLIS S.G., TOPOL E.J. Influence of blockade at specific levels of the coagulation cascade on restenosis in a rabbit atherosclerotic femoral artery injury model. Circulation. 1995;92:3041–3050. doi: 10.1161/01.cir.92.10.3041. [DOI] [PubMed] [Google Scholar]
- JOHANSSON L.C., FRISON L., LOGREN U., FAGER G., GUSTAFSSON D., ERIKSSON U.G. Influence of age on the pharmacokinetics and pharmacodynamics of ximelagatran, an oral direct thrombin inhibitor. Clin. Pharmacokinet. 2003;42:381–392. doi: 10.2165/00003088-200342040-00006. [DOI] [PubMed] [Google Scholar]
- KEARON C., COMP P., DOUKETIS J.D., ROYDS R., YAMADA K., Gent M.A dose–response study of a recombinant human soluble thrombomodulin (ART-123) for prevention of venous thromboembolism after unilateral total hip replacement J. Thromb. Haemost. 2003Suppl. IAbstr. #OC330 [DOI] [PubMed]
- KONG D.F., TOPOL E.J., BITTL J.A., WHITE H.D., THEROUX P., HASSELBLAD V., CALIFF R.M. Clinical outcomes of bivalirudin for ischemic heart disease. Circulation. 1999;100:2049–2053. doi: 10.1161/01.cir.100.20.2049. [DOI] [PubMed] [Google Scholar]
- LAGRANGE F., VERGNES C., BRUN J.L., PAOLUCCI F., NADAL T., LENG J.J., SAUX M.C., BANWARTH B. Absence of placental transfer of pentasaccharide (Fondaparinux, Arixtra) in the dually perfused human cotyledon in vitro. Thromb. Haemost. 2002;87:831–835. [PubMed] [Google Scholar]
- LASSEN M.R., BAUER K.A., ERIKSSON B.I., TURPIE A.G.G., for the European Pentasaccharide Hip Elective Surgery Study (EPHESUS) Steering Committee Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002;359:1715–1720. doi: 10.1016/S0140-6736(02)08652-X. [DOI] [PubMed] [Google Scholar]
- LASSEN M.R., DAVIDSON B.L., GALLUS A., PINEO G., ANSELL J., DEITCHMAN D.A phase II randomized, double-blind, five-arm, parallel-group, dose–response study of a new oral directly-acting factor Xa inhibitor, Razaxaban, for the prevention of deep vein thrombosis in knee replacement surgery Blood 200310215aAbstract #41 [Google Scholar]
- LASZIK Z., MITRO A., TAYLOR F.B., JR, FERRELL G., ESMON C.T. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- LEE A., AGNELLI G., BULLER H., GINSBERG J., HEIT J., ROTE W., VLASUK G., COSTANTINI L., JULIAN J., COMP P., VAN DER MEER J., PIOVETTA F., RASKOB G., GENT M. Dose–response study of recombinant factor VIIa/tissue factor inhibitor recombinant nematode anticoagulant protein c2 in prevention of postoperative venous thromboembolism in patients undergoing total knee replacement. Circulation. 2001;104:74–78. doi: 10.1161/hc2601.091386. [DOI] [PubMed] [Google Scholar]
- LENSING A.W.A., PRANDONI P., PRINS M.H., BULLER H.R. Deep vein thrombosis. Lancet. 1999;353:479–485. doi: 10.1016/s0140-6736(98)04298-6. [DOI] [PubMed] [Google Scholar]
- LEWIS B.E., WALLIS D.E., BERKOWITZ SD MATTHAI W.H., FAREED J., WALENGA J.M., BARTHOLOMEW J., SHAM R., LERNER R.G., ZEIGLER Z.R., RUSTAGI P.K., JANG J.K., RIFKIN S.D., MORAN J., HURSTING M.J., KELTON J.G., ARG-911 Study Investigators Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- LEWIS B.E., WALLIS D.E., LEYA F., HURSTIN M.J., KELTON J.G., for the Argatroban-915 Investigators Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch. Intern. Med. 2003;163:1849–1856. doi: 10.1001/archinte.163.15.1849. [DOI] [PubMed] [Google Scholar]
- LINCOFF A.M.First clinical investigation of a tissue-factor inhibitor administered during percutaneous coronary revascularization: a randomized, double-blinded, dose-escalation trial assessing safety and efficacy of FFR-FVIIa in percutaneous transluminal coronary angioplasty (ASIS) trial JACC 200036312Abstract [Google Scholar]
- LINCOFF A.M., BITTL J.A., HARRINGTON R.A., FEIT F., KLEIMAN N.S., JACKMAN J.D., SAREMBOCK I.J., COHEN D.J., SPRIGGS D., EBRAHIMI R., KEREN G., CARR J., COHEN E.A., BETRIU A., DESMET W., KEREIAKES D.J., RUTSCH W., WILCOX R.G., DE FEYTER P.J., VAHANIAN A., TOPOL E., REPLACE-2 Investigators Bivalirudin and provisional GPIIb/IIIa blockade compared with heparin and planned GPIIb/IIIa blockade during percutaneous coronary intervention. REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- LINCOFF A.M., KLEIMAN N.S., KOTTKE-MARCHANT K., MAIERSON E.S., MARESH K., WOLSKI K.E., TOPOL E.J. Bivalirudin with planned or provisional abciximab versus low-dose heparin and abciximab during percutaneous coronary revascularization: results of the Comparison of Abciximab Complications with Hirulog for Ischemic Events Trial (CACHET) Am. Heart J. 2002;143:847–853. doi: 10.1067/mhj.2002.122173. [DOI] [PubMed] [Google Scholar]
- LOLLAR P., MACINTOSH S.C., OWEN W.B. Reaction of antithrombin III with thrombin bound to the vascular endothelium: analysis in a recirculating perfused rabbit heart preparation. J. Biol. Chem. 1984;259:4335–4338. [PubMed] [Google Scholar]
- MANNS B.J., LEE H., DOIG C.J., JOHNSON D., DONALDSON C. An economic evaluation of activated protein C treatment for severe sepsis. N. Engl. J. Med. 2002;347:993–1000. doi: 10.1056/NEJMsa020969. [DOI] [PubMed] [Google Scholar]
- MARUYAMA I. Recombinant thrombomodulin and activated protein C in the treatment of disseminated intravascular coagulation. Thromb. Haemost. 1999;82:718–721. [PubMed] [Google Scholar]
- MATISSE INVESTIGATORS Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N. Engl. J. Med. 2003;349:1695–1702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- MURAYAMA N., TANAKA M., KUNITADA S., YUMADA H., INOUE T., TERADA Y., FUJITA M., IKEDA Y. Tolerability, pharmacokinetics and pharmacodynamics of DX-9065a, a new synthetic potent anticoagulant and specific factor Xa inhibitor, in healthy male volunteers. Clin. Pharmacol. Ther. 1996;66:258–264. doi: 10.1016/S0009-9236(99)70033-0. [DOI] [PubMed] [Google Scholar]
- ORVIM U., BARSTAD R.M., VLASUK G.P., SAKARIASSEN K.S. Effect of selective factor Xa inhibition on arterial thrombus formation triggered by tissue factor/factor VIIa or collagen in an ex vivo model of shear-dependent human thrombogenesis. Arterioscl. Throm. Vasc. Biol. 1995;15:2188–2194. doi: 10.1161/01.atv.15.12.2188. [DOI] [PubMed] [Google Scholar]
- PARKINSON J.F., GRINNELL B.W., MOORE R.E., HOSKINS J., VLAHOS C.J., BANG N.U. Stable expression of a secretable deletion mutation of recombinant human thrombomodulin in mammalian cells. J. Biol. Chem. 1990;265:12602–12610. [PubMed] [Google Scholar]
- PERSIST INVESTIGATORS. A novel long-acting synthetic factor Xa inhibitor (SanOrg34006) to replace warfarin for secondary prevention in deep vein thrombosis. A phase II evaluation. J. Thromb. Haemost. 2004;2:47–53. doi: 10.1111/j.1538-7836.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- PETERSON P.A long-term follow-up of ximelagatran as an oral anticoagulant for the prevention of stroke and systemic embolism in patients with atrial fibrillation Blood 200198Suppl.2953Abstract [Google Scholar]
- PETERSEN P., GRIND M., ADLER J., SPORTIF II Investigators Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a dose-guiding tolerability, and safety study. J. Am. Coll. Cardiol. 2003;41:1445–1451. doi: 10.1016/s0735-1097(03)00255-9. [DOI] [PubMed] [Google Scholar]
- POTZCH B., IVERSEN S., REISS F.C. Recombinant hirudin as an anticoagulant in open-heart surgery: a case-report. Ann. Hematol. 1994;68:A53. [Google Scholar]
- QUINLAN D.J., MCQUILLAN A., EIKELBOOM J.W. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2004;140:175–183. doi: 10.7326/0003-4819-140-3-200402030-00008. [DOI] [PubMed] [Google Scholar]
- RAGOSTA M., GIMPLE L.W., GERTZ S.D., DUNWIDDIE C.T., VLASUK G.P., HABER H.L., POWERS E.R., ROBERTS W.C., SAREMBOCK I.J. Specific factor Xa inhibition reduces restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation. 1994;89:1262–1271. doi: 10.1161/01.cir.89.3.1262. [DOI] [PubMed] [Google Scholar]
- REISS F.C., LOWER C., SELLIG C. Recombinant hirudin as a new anticoagulant during cardiac operations instead of heparin: successful for aortic valve replacement in man. J. Thorac. Cardiovasc. Surg. 1995;11:265–267. doi: 10.1016/S0022-5223(05)80033-9. [DOI] [PubMed] [Google Scholar]
- REZAIE A.R. Prothrombin protects factor Xa in the prothrombinase complex from inhibition by heparin-antithrombin complex. Blood. 2001;97:2308–2313. doi: 10.1182/blood.v97.8.2308. [DOI] [PubMed] [Google Scholar]
- SCHULMAN S., WAHLANDER K., LUNDSTROM T., CLASON S.B., ERIKSSON H., for the THRIVE III Investigators Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N. Engl. J. Med. 2003;349:1713–1721. doi: 10.1056/NEJMoa030104. [DOI] [PubMed] [Google Scholar]
- STANGIER J., LIESENFELD K.H., TROCONIZ C.H., TILLMANN I.F., SCHAEFER H.G., HERMANSSON K., ERIKSSON B.I.The effect of BIBR953ZW, the active form of the oral direct thrombin inhibitor BIBR1048, on the prolongation of the aPTT and ECT in orthopedic patients: a population pharmacodynamic study J. Thromb. Haemost. 2003a1Suppl. 1Abstract P1916) [Google Scholar]
- STANGIER J., NEHMIZ G., LIESENFELD K.N., HOLZSCHUH I., HERMANSSON K., SVAERD R., DAHL O.E., AHNFELT L., ERIKSSON B.I.Pharmacokinetics of BIBR953ZW, the active form of the oral direct thrombin inhibitor dabigatran etexilate, in patients undergoing hip replacement J. Thromb. Haemost. 2003b1Suppl. 1abstract P1916 [DOI] [PubMed] [Google Scholar]
- STASSENS P., BERGUM P.W., GANSEMANS Y., JESPERS L., LAROCHE Y., HUANG S., MAKI S., MESSENS J., LAUWEREYS M., CAPPELLO M., HOTEZ P.J., LASTERS I., VLASUK G.D. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRINGER K.A., LINDENFELD J. Hirudins: antithrombin anticoagulants. Ann. Pharmacother. 1992;26:1535–1540. doi: 10.1177/106002809202601211. [DOI] [PubMed] [Google Scholar]
- TOLLEFSEN D.M. Insight into the mechanism of action of heparin cofactor II. Thromb. Haemost. 1995;74:1209–1274. [PubMed] [Google Scholar]
- TURPIE A.G.G., BAUER K., ERIKSSON B.I., LASSEN M.R., for the Pentathlon 2000 Study Steering Committee Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002a;359:1721–1726. doi: 10.1016/S0140-6736(02)08648-8. [DOI] [PubMed] [Google Scholar]
- TURPIE A.G.G., BAUER K.A., ERIKSSON B.I., LASSEN M.R., for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. A meta-analysis of 4 randomized double-blind studies. Arch. Intern. Med. 2002b;162:1833–1840. doi: 10.1001/archinte.162.16.1833. [DOI] [PubMed] [Google Scholar]
- TUSZYUSKI G., GASIC T.B., GASIC G.J. Isolation and characterization of antistasin. J. Biol. Chem. 1987;262:9718–9723. [PubMed] [Google Scholar]
- VLASUK G.P. Structural and functional characterization of tick anticoagulant peptide (TAP): a potent and selective inhibitor of blood coagulation factor Xa. Thromb. Haemost. 1993;70:212–216. [PubMed] [Google Scholar]
- WALENGA J., JESKE W., BARA L., SAMAMA M.M., FAREED J. Biochemical and pharmacological rationale for the development of a synthetic heparin pentasaccharide. Thromb. Res. 1997;86:1–36. doi: 10.1016/s0049-3848(97)00042-x. [DOI] [PubMed] [Google Scholar]
- WALENGA J.M., JESKE W.P., SAMAMA M.M., FRAPAISE F.X., BICK R.L., FAREED J. Fondaparinux: a synthetic heparin pentasaccharide as a new antithrombotic agent. Expert. Opin. Invest. Drugs. 2002;11:397–404. doi: 10.1517/13543784.11.3.397. [DOI] [PubMed] [Google Scholar]
- WALENGA J.M., KOZA M.J., LEWIS B.E., PIFARRE R. Relative heparin-induced thrombocytopenic potential of low molecular weight heparins and new antithrombotic agents. Clin. Appl. Thromb. Hemost. 1996;2 Suppl. 1:S21. [Google Scholar]
- WALLENTIN L., WILCOX R.G., WEAVER W.D., EMANUELSSON H., GOODVIN A., NYSTROM P., BYLOCK A., for the ESTEEM Investigators Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet. 2003;362:789–792. doi: 10.1016/S0140-6736(03)14287-0. [DOI] [PubMed] [Google Scholar]
- WEITZ J., LESLIE B., HUDOBA M. Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation. 1998;97:544–552. doi: 10.1161/01.cir.97.6.544. [DOI] [PubMed] [Google Scholar]
- WEITZ J.I. Low-molecular-weight heparins. N. Engl. J. Med. 1997;337:688–698. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- WEITZ J.I., BULLER H.R. Direct thrombin inhibitors in acute coronary syndromes: present and future. Circulation. 2002;105:1004–1011. doi: 10.1161/hc0802.104331. [DOI] [PubMed] [Google Scholar]
- WEITZ J.I., HUDOBA M., MASSEL D., MARAGANORE J., HIRSH J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J. Clin. Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE B., LIVINGSTONE W., MURPHY C., HODGSON A., RAFFERTY M., SMITH O.D. An open-label study of the adjuvant hemostatic support with protein C replacement therapy in purpura fulminans-associated meningococcemia. Blood. 2000;96:3719–3724. [PubMed] [Google Scholar]
- WIENE W., NAR H., RIES U.J.Antithrombotic effects of the direct thrombin inhibitor BIBR953ZW and its orally active prodrug BIBR1048MS in a model of venous thrombosis in rabbits Thromb. Haemost. 2001aSuppl.Abstract OC583
- WIENE W., NAR H., RIES U.J.Effects of the direct thrombin inhibitor BIBR953ZW and its orally active prodrug BIBR1048MS on experimentally-induced clot formation and template bleeding time in rats Thromb. Haemost. 2001b12Suppl.Abstract P761 [PubMed] [Google Scholar]