Abstract
Efforts to determine the role of stretch-dependent K+ (SDK) channels in enteric inhibitory neural responses in gastrointestinal muscles are difficult due to a lack of blocking drugs for SDK channels.
SDK channels are blocked by sulfur-containing amino acids. These compounds reduced the open probability of SDK channels in on and off-cell patches of murine colonic myocytes. L-Methionine was the most selective and had little or no effect on other known K+ conductances in colonic myocytes.
Application of L-cysteine, L-methionine or DL-homocysteine depolarized intact muscles and enhanced spontaneous contractions. D-Stereoisomers of these amino acids were less effective than L-stereoisomers.
Pretreatment of muscles with tetrodotoxin, NW-nitro-L-arginine or 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one reduced the depolarization responses to these compounds, suggesting that spontaneous neural activity and release of NO tonically activates SDK channels.
Nitrergic responses to nerve stimulation were reduced by sulfur-containing amino acids.
These data suggest that nitrergic inhibitory junction potentials are mediated, in part, by activation of SDK channels in murine colonic muscles.
Keywords: Gastrointestinal motility, inhibitory junction potential, membrane potential, potassium channel
Introduction
NO is released by non-adrenergic, non-cholinergic (NANC) inhibitory neurons in a variety of tissues, including gastrointestinal (GI) smooth muscles. NO serves as the primary enteric inhibitory neurotransmitter in GI muscles, and nitrergic neurons regulate gut tone, phasic contractile amplitude and frequency, and inhibitory reflexes (Sanders & Ward, 1992). At least a portion of the mechanical effect of NO are a consequence of hyperpolarization of membrane potential that results in reduced smooth muscle excitability. Recent studies have suggested that part of the hyperpolarizing effects of NO may be mediated by stretch-dependent K+ (SDK) channels that are expressed in GI smooth muscles (Koh & Sanders, 2001). An obstacle to testing this hypothesis in intact GI muscles, however, is the lack of drugs with specificity for blocking two-pore K+ channels. For example, we reported previously that known K+ channel blockers (4-aminopyridine (4-AP), tetraethyl ammonium (TEA), quinine, etc.) have only weak actions on SDK channels (Koh & Sanders, 2001).
Our previous studies have suggested that the two-pore K+ channel, TREK-1, encodes SDK channels in murine GI smooth muscle cells (Koh et al., 2001). In the present study, we noted that sulfur-containing amino acids block SDK channels in colonic myocytes, and we hypothesized that compounds of this class might be effective in blocking NO-dependent responses in intact muscles. One report in the literature supports this idea in that cysteine inhibited relaxations of sheep urethra in response to nitrergic stimulation (Garcia-Pascual et al., 2000). Here we have investigated the effects of the sulfur-containing amino acids in muscle strips of the mouse colon using intracellular electrophysiological techniques and contractile experiments. Our results indicate that homocysteine, cysteine and methionine blocked SDK channels in colonic myocytes and depolarized membrane potentials and increased the frequency of contractions in intact muscles. These compounds also inhibited responses to the NO donor sodium nitroprusside (SNP) and neurally released NO. The effects of sulfur-containing amino acids cannot be explained by block of other known K+ channels, and we suggest that these compounds act through block of SDK channels.
Methods
Animals and tissue preparation
BALB/c mice (20–30 days old, Harlan–Sprague–Dawley; Indianapolis, IN, U.S.A.) of either sex were anesthetized with chloroform inhalation and killed by cervical dislocation. The use and treatment of animals were reviewed and approved by the Institutional Animal Use and Care Committee at the University of Nevada.
Segments of the proximal colon from 1 cm distal to the ileocecal sphincter were removed through a midline abdominal incision and opened along the mesenteric border. Luminal contents were removed by washing with Krebs–Ringer bicarbonate solution (KRB), and the cleaned tissue sheets were pinned down onto a Sylgard base with the mucosa facing up. The mucosa was removed leaving the tunica muscularis and remnants of the submucosa.
Mechanical responses
Mechanical responses were performed using standard organ-bath techniques. Strips of muscle (10 × 5 mm) were cut from the tunica muscularis by sharp dissection. The muscles were attached with sutures to a fixed mount within the organ bath and to an isometric strain gauge (World Precision Instruments, Sarasota, FL, U.S.A.). The muscles were immersed in oxygenated KRB and maintained at 37.5±0.5°C. The muscles were set at Lo by applying 0.1–0.3 g of basal tension and then allowed to equilibrate for 1–2 h with constant perfusion with fresh KRB. Contractions of the muscles were monitored, digitized and stored using AcqKnowledge software (Biopac Systems Inc., Santa Barbara, CA, U.S.A.) running on a PC-style computer. Contractions were quantified by calculations of area above the baseline using the AcqKnowledge software. Drugs were diluted to the desired concentrations and applied to the muscles by switching the perfusion to the drug-containing solution.
Intracellular microelectrode recordings and electrical field stimulation
Muscle strips of similar dimensions were also used for electrophysiology experiments. The muscle strips were pinned to the base of a recording chamber with the submucosal surface of the circular muscle layer facing upwards. The muscles were maintained in oxygenated KRB at 37.5±0.5°C for an equilibration period of 1 h before recordings were initiated. During electrical recordings, nifedipine or nicardipine (1 μM) was added to the KRB solution to reduce movement and facilitate intracellular impalements.
Circular muscle cells were impaled with glass microelectrodes filled with 3 M KCl and having tip resistances of 50–80 MΩ. Transmembrane potential was measured with a high-input impedance amplifier (model Duo 773; World Precision Instruments, Sarasota, FL, U.S.A.) and data were stored and analyzed by computer using AcqKnowledge software (version 3.5.7, Biopac Systems Inc.). Neural responses were evoked by transmural electrical field stimulation (EFS) applied through two platinum wires placed along both sides and parallel to the long axis of the preparation. The electrodes were connected via a stimulus isolation unit (Grass SIU 5A) to an electrical stimulator (Grass 588; Grass, Quincy, MA, U.S.A.). Square wave pulses of 0.5 ms duration at supramaximal voltage were applied as trains 1–30 Hz to activate intrinsic neurons. Responses to EFS were blocked by tetrodotoxin (TTX; EMD Biosciences Inc., La Jolla, CA, U.S.A.), demonstrating the neural origin of responses.
Solutions and drugs for intracellular microelectrode recordings
The KRB used in this study contained (in mM) 120.4 NaCl, 5.9 KCl, 15.5 NaHCO3, 11.5 glucose, 1.2 MgCl2, 1.2 NaH2PO4 and 2.5 CaCl2. This solution had a pH of 7.3–7.4 at 37.5°C when bubbled to equilibrium with 97% O2–3% CO2. Nicardipine, nifedipine, L-methionine, D-methionine, L-cysteine, D-cysteine, DL-cystine, DL-homocysteine, SNP, TTX, NW-nitro-L-arginine (L-NA), N-ethylmaleimide (NEM), sodium hydrosulfide (NaHS), 4-AP, TEA, glibenclamide (GBC) and 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Preparation of isolated myocytes
The proximal colon tissues were opened along the mesenteric border and mucosa and submucosa were removed in Ca2+-free, phosphate-buffered saline (PBS) containing (in mM) 125 NaCl, 5.36 KCl, 15.5 NaOH, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose and 11 N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES). Small pieces of muscle were cut and incubated for 8–12 min at 37°C in a Ca2+-free solution containing 4 mg ml−1 fatty acid-free bovine serum albumin, 2 mg ml−1 trypsin inhibitor and 1 mg ml−1 collagenase. After enzymatic treatment, the muscles were washed with Ca2+-free solution and agitated gently to create a cell suspension.
Dispersed smooth muscle cells were stored at 4°C in Ca2+-free solution. Cells were transferred from the refrigerator to the recording chamber. Drops of the cell suspensions were placed on a glass coverslip forming the bottom of a 300 μl chamber mounted on an inverted microscope with a thermostatically regulated stage and allowed to adhere to the bottom of the chamber for 5 min prior to recording.
Voltage-clamp methods
The single-channel and whole-cell voltage-clamp techniques were used to record membrane currents from dissociated smooth muscle cells. Membrane currents were amplified by a List EPC-7 (List Electronics, Darmstadt, Germany) or Axopatch 1D (Axon Instruments, Foster City, CA, U.S.A.) and digitized with an A/D converter (Labmaster or Digidata 1322A, Axon Instruments). For the single recording of K+ channels, the bath solution contained (mM) 140 KCl, 1 EGTA, 0.61 CaCl2 and 10 HEPES adjusted to pH 7.4 with Tris. The pipette solution for asymmetrical K+ gradients was 5 mM KCl and 135 mM NaCl instead of the 140 mM KCl used in the bath solution. Charybdotoxin (200 nM) was added to the pipette solution to inhibit large-conductance Ca2+-activated K+ channels. Data were collected at 4 kHz and filtered at 1 kHz via Bessel filter and digitized on line using pClamp software (version 5.1.1 or version 9.0, Axon Instruments). The data were analyzed with use of pClamp software (version 9.0, Axon Instruments) Origin software (MicroCal Software) to obtain amplitude histogram and channel activity (NPo, where N is the number of channels in the patch and Po is the probability of a channel being open). NPo was determined from 30 s or 1 min of channel recording. Membrane stretch was elicited by applying suction (negative pressure) to a side port of the patch pipette holder. The degree of negative pressure was calibrated with a pressure transducer (Koh & Sanders, 2001). The whole-cell patch-clamp technique was used to record membrane currents from dissociated murine colonic smooth muscle cells. Pipette resistances were 1–4 MΩ, and the uncompensated series resistance was between 2 and 4 MΩ. The linear leak current was subtracted digitally. The external solution used in conventional whole-cell recordings contained (in mM) 135 NaCl, 5 KCl, 2 CaCl2, 1.2 MgCl2, 10 glucose and 10 HEPES, adjusted to pH 7.4 with Tris (CaPSS). For measuring KATP currents, the external solution for asymmetrical K+ gradients was 140 mM KCl instead of the 5 mM KCl and 135 mM NaCl used in the bath solution. The pipette solution contained (mM) 130 KCl, 5 MgCl2, 2.7 K2ATP, 0.1 Na2GTP, 2.5 creatine phosphate disodium, 5 HEPES and 1 EGTA, adjusted to pH 7.2 with Tris. To measure the spontaneous transient outward current (STOC) activity, we performed perforated (amphotericin B, final concentration 230–270 μg ml−1) whole-cell configuration.
Statistical analysis
All data are expressed as mean±standard error of the means. Student's paired t-tests were used to determine if data sets differed, and P-values of less than 0.05 were taken to indicate significant differences between sets of observations. The n values reported in the text refer to the number of recordings from the muscle strips, which is equivalent to the number of animals used unless otherwise stated.
Results
Sulfur-containing amino acids block SDK channels
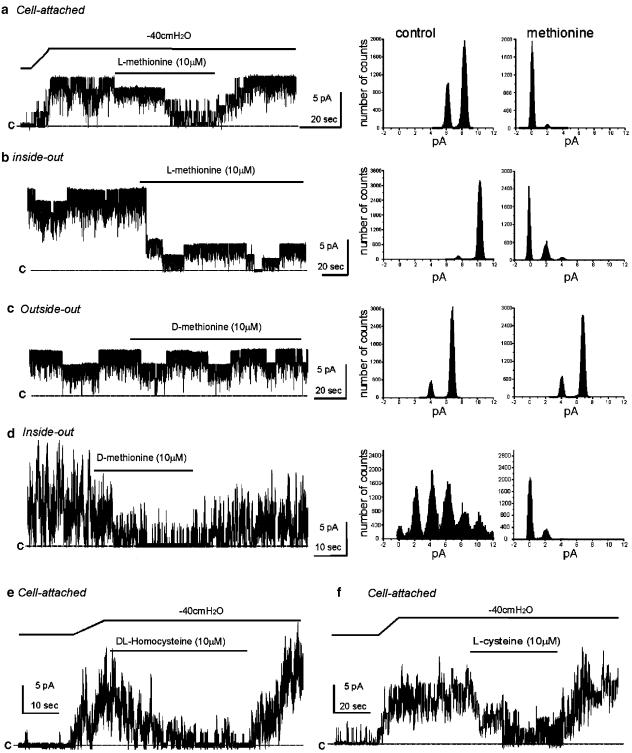
In studies to evaluate the channel blocking capabilities of several compounds, we noted that sulfur-containing amino acids were capable of blocking SDK channels. Negative pressure (−40 cmH2O) was applied to on-cell patches to increase the NPo of SDK channels from 0 to 3.8±0.6 (n=4). After steady-state activation of SDK channel, L-methionine (10 μM) inhibited channel activity (NPo=0.4±0.8, P<0.01 compared to negative pressure control). Normal sensitivity to stretch was restored by washout of methionine (Figure 1a). In the cell-attached configuration, DL-homocysteine and L-cysteine (10 μM) also blocked SDK channel openings (NPo=4.2±0.9 for control under negative pressure (−40 cmH2O) vs NPo=0.9±0.6 for after DL-homocysteine, n=4; NPo=4.8±1.5 under similar control negative pressure vs NPo=1.5±0.8 after L-cysteine, n=4; see Figure 1e and f). Patch excision also caused an increase in the open probability of channels (NPo=4.5±0.5, n=4). L-Methionine reduced NPo of SDK channels to 0.8±0.4 (P<0.01) in the inside-out configuration (Figure 1b). D-Methionine (10 μM) had no effect in the outside-out configuration (NPo=3.9±0.4 compared to control NPo=3.7±0.5, n=4; Figure 1c). However, the cytoplasmic application of D-methionine in the inside-out configuration decreased NPo of SDK channels from 4.2±1.0 to 0.6±0.5 (n=4). These data suggest that methionine acts at the cytoplasmic side of channels.
Figure 1.
Inhibition of SDK channels by methionine in murine colonic myocytes. (a) Negative pressure (−40 cmH2O) applied to patch pipettes (black bar) increased channel activity in cell-attached patches of murine colonic myocytes. L-Methionine decreased NPo (see amplitude histograms before and in the presence of L-methionine to right of trace in (a); cell was held at 0 mV in asymmetrical K+ (5/140 mM). (b) Excising patches to inside-out configuration increased open probability of SDK channels. NPo was decreased by L-methionine in the bathing solution. Right panels demonstrate amplitude histograms before and in the presence of methionine. (c) D-Methionine had no effect on open probability of SDK channels in the outside-out configuration. Right panels demonstrate amplitude histograms before and in the presence of methionine. (d) D-Methionine decreased NPo of SDK channels in the outside-out configuration. Right panels demonstrate amplitude histograms before and in the presence of D-methionine. (e and f) Negative pressure (−40 cmH2O) applied to patch pipettes (black bar) increased channel activity in cell-attached patches. DL-Homocysteine and L-cysteine decreased NPo. The ‘c' in each trace denotes the 0 current line when all SDK channels were closed.
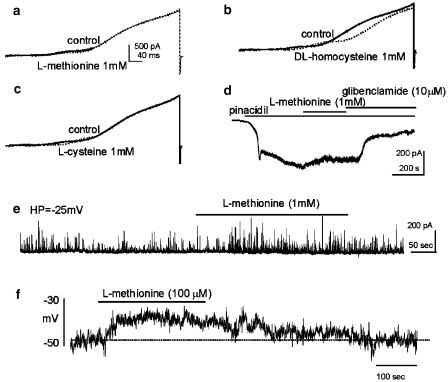
We also tested the nonspecific effects of sulfur-containing amino acids on other known K+ conductances in GI muscles using whole-cell voltage-clamp protocols. Cells were depolarized repetitively to +80 mV with voltage-ramp protocols from a holding potential of −80 mV in asymmetrical K+ gradients (5/140 mM), and current responses were averaged before and after application of drugs. L-Methionine had no discernable effect on net outward currents (n=4; Figure 2a), but DL-homocysteine (n=4; Figure 2b) and L-cysteine (n=4; Figure 2c) shifted the voltage dependence of activation of the delayed rectifier component of the outward current, which has been previously characterized (see Koh et al., 1999), toward more positive potentials. DL-Homocysteine decreased the maximum outward current amplitude (i.e. from 901±52 to 508±53 pA at +20 mV, P<0.001) at the most positive potential range tested (i.e. >20 mV). We also examined the effects of methionine on KATP currents. Cells were held at −60 mV using symmetrical K+ solution (140/140 mM), and pinacidil (1 μM) was used to activate KATP currents (which were inward currents under these conditions). Pinacidil activated inward currents (−356±43 pA, n=4) under these conditions. L-Methionine (1 mM) had no effect on pinacidil-induced currents (−351±47 pA). However, GBC (10 μM) suppressed 89±4% of pinacidil-activated currents (Figure 2d). In other experiments, we tested the effects of methionine on STOCs. Cells were held at −25 mV using perforated patch techniques to elicit STOCs, which are composed of currents through BK and SK channels in colonic myocytes (Kong et al., 2000). STOC activity was not significantly changed by methionine, that is, from 372±98 to 397±105 events per 100 s (n=3; Figure 2e). These studies showed that homocysteine and cysteine may have minor nonspecific effects on outward currents at physiological potentials, but methionine does not appear to affect any of the major K+ conductances in these cells. Methionine was therefore considered to be a useful reagent to test the role of SDK channels in the electrical activity of intact muscles.
Figure 2.
Effects of sulfur-containing amino acids on outward currents in murine colonic myocytes. Current responses were evoked from a holding potential of −80 mV with 400-ms ramp voltage pulses to +80 mV in CaPSS (see Methods). (a–c) Current responses before (solid lines) and in the presence of methionine, homocysteine or cysteine (dotted lines in each pane), respectively. (d) Pinacidil (1 μM) activated a large, steady-state, GBC-sensitive inward (10 μM) current in symmetrical K+ (140/140 mM) at a holding potential of −60 mV. This current was not blocked by methionine (1 mM). (e) Holding cells at −25 mV with amphotericin-permeabilized patch techniques often activated STOCs. Methionine did not change STOC activity significantly. (f) Methionine depolarized isolated colonic myocytes under current-clamp (I=0) condition. The dashed line denotes resting membrane potential under control condition.
We also performed current-clamp (I=0) experiments on isolated colonic myocytes to examine the effects of methionine on membrane potential. L-Methionine (100 μM) depolarized isolated smooth muscle cells from −46±1 to −36±3 mV (n=4, P<0.05; Figure 2f).
Mechanical responses
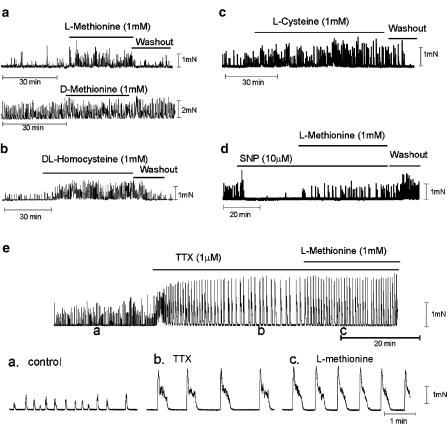
The effects of sulfur-containing amino acids were tested on the mechanical activity of colonic muscle strips. L-Methionine (1 mM) reversibly increased the area of contractions (9.4±1.9 vs 3.5±0.6 mN min−1 in control, P<0.05, n=9; Figure 3a). However, the application of D-methionine (1 mM) did not affect the spontaneous mechanical activity (e.g. 22.0±3.5 vs 22.7±4.2 mN min−1 for D-methionine, n=5). DL-Homocysteine (1 mM; Figure 3b) and L-cysteine (1 mM; Figure 3c) also increased the area of contractions (e.g. 16.7±4.3 vs 7.3±2.7 mN min−1 for DL-homocysteine, P<0.05, n=5; 18.1±4.7 vs 9.0±1.9 mN min−1 for L-cysteine, P<0.05, n=7). The effects of all the amino acids tested were reversible upon washout (see Figure 3a–d). TTX (1 μM) dramatically increased the amplitude of spontaneous contractions from 0.9±0.2 to 1.6±0.1 mN; even in the presence of TTX, sulfur-containing amino acids increased the area of contractions (22.5±5.9 vs 32.7±7.1 mN min−1 for methionine, n=3; Figure 3e) due to increased frequency (0.6±0.2 vs 1.6±0.2 cycle min−1 for methionine). There was no significant effect of the sulfur-containing amino acids on tone.
Figure 3.
Effects of sulfur-containing amino acids on the mechanical activities of murine colonic stripes. Colonic muscles displayed spontaneous contractile activity. (a–c) L-Methionine, DL-homocysteine and L-cysteine increased the amplitude and frequency of spontaneous contractions (indicated by black bars). The lower trace in panel a shows the lack of effect of D-methionine on spontaneous mechanical activity. (d) SNP (10 μM) decreased spontaneous contractile activity. Methionine partially reversed the inhibitory effect of SNP. (e) TTX (1 μM) increased the amplitude and the regularity of contractions. Methionine increased the frequency of contractions. Insets in (e) show contractions at points denoted by (a–c) at faster sweep speeds.
The nitric oxide donor SNP (10 μM) reduced the area of spontaneous contractions (from 20.7±8.1 to 3.7±1.4 mN min−1 in control, n=4, P<0.05), and these effects were partially reversed by adding methionine (1 mM) in the continued presence of SNP (i.e. 6.7±2.3 mN min−1, n=4, P<0.05, compared to the maximal SNP effect; Figure 3d).
Effects of sulfur-containing amino acids on membrane potential
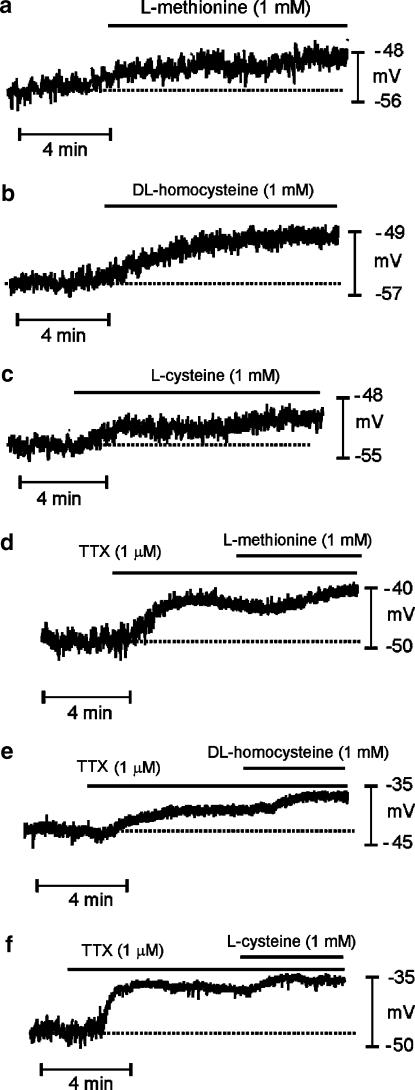
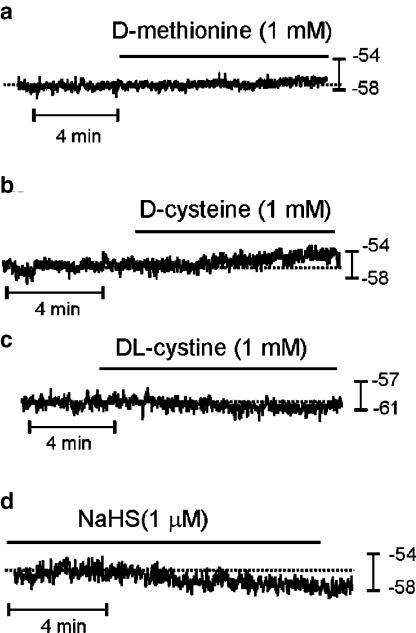
L-Methionine, DL-homocysteine and L-cysteine (all 1 mM) caused depolarization (Figure 4a–c and summarized in Table 1), and these effects were rapidly reversible upon washout. We observed no effect on membrane potential from these compounds when they were applied at concentrations of <100 μM. TTX (1 μM) depolarized membrane potential from −48±1 to −43±1 mV (P<0.05, n=26). In the presence of TTX, addition of L-methionine, DL-homocysteine and L-cysteine caused additional depolarization of membrane potential (Figure 4d–f and Table 1). We also examined whether D-isomers of methionine and cysteine had depolarizing actions on colonic smooth muscles (Figure 5a–c). D-Cysteine (1 mM) caused a small, but significant, depolarization (i.e. −48±1 mV with D-cysteine vs −52±1 mV for control, P<0.05, n=8; Figure 5b), but D-methionine had no effect (−56±2 mV with 1 mM D-methionine vs −56±2 mV for control, P>0.05, n=6; Figure 5a). The depolarization caused by D-cysteine (5±1 mV) was significantly less than that caused by L-cysteine (7±1 mV, P<0.01).
Figure 4.
Effects of sulfur-containing amino acids on the membrane potential of murine proximal colon. Intracellular microelectrode recordings were made from circular muscle cells of intact colonic muscle strips. (a–c) Methionine, homocysteine and cysteine, respectively, caused depolarization. (d–f) TTX (1 μM) also caused depolarization. Methionine, homocysteine and cysteine, added in the continued presence of TTX, caused further depolarization; however, the magnitude of depolarization was significantly reduced later. Dashed lines in each panel denote membrane potentials under control conditions.
Table 1.
Effects of sulfur-containing amino acids on membrane potentials in the absence and presence of TTX
| Control | L-mtn | Control | DL-hct | Control | L-cyst | |
|---|---|---|---|---|---|---|
| Absence of TTX | −51±2 | −44±2 (11)a | −50±1 | −43±2 (11)a | −48±2 | −40±2 (11)a |
| Presence of TTX | −43±2 | −36±2 (8)a | −44±1 | −36±1 (9)a | −43±1 | −35±1 (9)a |
Values are mean±s.e. (mV). L-mtn, L-methionine; DL-hct, DL-homocysteine; L-cyst, L-cysteine.
P<0.05 and values in parentheses denote number of muscle strips.
Figure 5.
Effects of isomers of sulfur-containing amino acids on the resting membrane potential of murine proximal colon. (a) D-Methionine did not affect the membrane potentials. (b) D-Cysteine caused a small degree of depolarization that was significantly less than that caused by L-cysteine. (c) DL-Cystine did not affect membrane potentials. (d) NaHS hyperpolarized membrane potential. The dashed line indicates the original resting potentials.
To determine whether it was mainly the presence of sulfur in these amino acids that affected membrane potential, we tested the effect of DL-cystine (1 mM), which has two sulfur atoms in its structure. DL-Cystine had no effect on the resting membrane potential (−57±1 mV in DL-cystine vs −57±2 mV in control, P>0.05, n=6; Figure 5c). We also tested NaHS, which dissociates to Na+ and HS−, to determine whether the action of the sulfur-containing amino acids could be ascribed to HS− (Figure 5d). At concentrations of 1, 10 and 100 μM, however, NaHS caused significant hyperpolarization (i.e. −58±2 vs −54±2 mV in control for 1 μM, P<0.01, n=7; −57±2 vs −51±2 mV in control for 10 μM, P<0.01, n=6; −58±3 vs −51±2 mV in control for 100 μM, P<0.001, n=6).
Effects of sulfur-containing amino acids in the presence and absence of NO-dependent mechanisms
The NO donor SNP (1 and 10 μM) induced hyperpolarization from −50±1 to −59±1 mV (1 μM SNP, P<0.05, n=15), and from −45±1 to −62±1 mV in SNP (10 μM, P<0.05, n=18). In the presence of SNP (1 and 10 μM), application of L-methionine, DL-homocysteine and L-cysteine significantly induced depolarization (see Table 2 and Figure 6a–c). Most GI muscles have tonic inhibitory drive due to the spontaneous production and release of NO. L-NA (100 μM), an inhibitor of nitric oxide synthase, caused depolarization from −55±1 to −42±1 mV (P<0.05, n=21), suggesting a substantial basal production of NO in murine colonic muscles (see Shuttleworth et al., 1995; 1997). After treatment with L-NA, L-methionine, DL-homocysteine and L-cysteine (all at 1 mM) caused small, but significant, depolarization (see Table 2 and Figure 6d–f). The degree of depolarization after L-NA treatment was significantly less (P<0.01) than that in muscles not treated with L-NA.
Table 2.
Effects of sulfur-containing amino acids on membrane potentials in the presence of SNP, L-NA and ODQ
| Control | L-mtn | Control | DL-hct | Control | L-cyst | |
|---|---|---|---|---|---|---|
| Presence of SNP (1 μM) | −57±1 | −52±1 (5)a | −59±2 | −51±2 (5)a | −60±2 | −50±2 (5)a |
| Presence of SNP (10 μM) | −64±2 | −57±2 (6)a | −62±1 | −53±2 (6)a | −61±1 | −51±2 (6)a |
| Presence of L-NA (100 μM) | −42±2 | −40±2 (7)a | −41±1 | −39±1 (7)a | −41±1 | −39±1 (7)a |
| Presence of ODQ (10 μM) | −45±3 | −41±2 (4)a | −45±3 | −41±2 (4)a | −43±1 | −40±2 (4)a |
Values are mean±s.e. (mV). Control values refer to the membrane potential after treatment with SNP, L-NA and ODQ. L-mtn, L-methionine; DL-hct, DL-homocysteine; L-cyst, L-cysteine.
P<0.05 and values in parentheses denote number of muscle strips.
Figure 6.
Effects of sulfur-containing amino acids in the presence of SNP and L-NA on the membrane potential of murine proximal colon. (a–c) SNP induced large hyperpolarization in colonic smooth muscles. Methionine, homocysteine and cysteine partially restored membrane potentials hyperpolarized by SNP (10 μM). (d–f) L-NA (100 μM) caused large depolarization. Methionine, homocysteine and cysteine further depolarized membrane potentials in the presence of L-NA, but these responses were significantly less than that observed in the absence of L-NA. Dashed lines in each panel indicate membrane potentials before the addition of drugs.
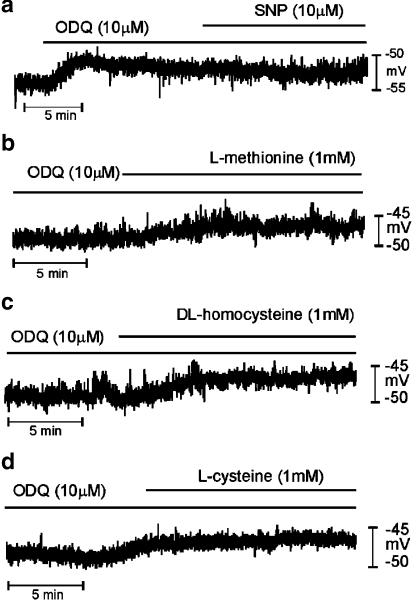
We also tested the effects of the soluble guanylate cyclase inhibitor ODQ. ODQ (10 μM) depolarized colonic cells from −50±1 to −44±1 mV (P<0.05, n=12). In the presence of ODQ, L-methionine, DL-homocysteine and L-cysteine (1 mM) caused further depolarization (see Table 2 and Figure 7). The degree of depolarization after ODQ treatment was significantly less (P<0.05) than that in muscles not treated with this compound. These results indicate that the depolarization caused by sulfur-containing amino acids depends substantially on the level of NO in colonic muscles, but a portion of depolarization may occur through a mechanism independent of NO and cGMP.
Figure 7.
Effects of sulfur-containing amino acids in the presence of ODQ on the membrane potential of murine proximal colon. (a) ODQ caused depolarization of membrane potential. SNP had no effect on membrane potential in the presence of ODQ (10 μM). (b–d) Methionine, homocysteine and cysteine caused depolarization under preincubated ODQ, but these responses were significantly less than that observed in the absence of ODQ.
Influence of K+ channel blocking drugs and thiol-specific agents on depolarization caused by sulfur-containing amino acids
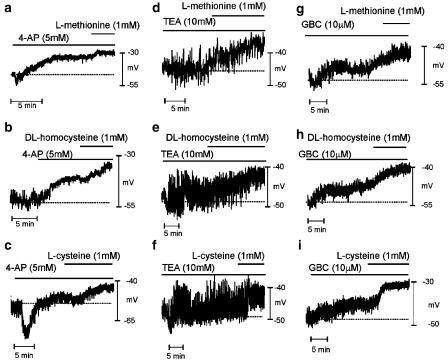
To further investigate the possible mode of action of the sulfur-containing amino acids, we have tested the effects of these sulfur-containing amino acids on a background of known K+ channel blocking drugs or thiol-specific agents. Addition of 4-AP (5 mM, a blocker of delayed rectifier K+ channels) induced transient hyperpolarization, followed by depolarization from −47±2 to −35±1 mV (P<0.05, n=15). The initial hyperpolarization was likely to be due to release of NO from intrinsic neurons (Du et al., 1994). In the presence of 4-AP, L-methionine (P<0.01, n=6), DL-homocysteine (P<0.05, n=4) and L-cysteine (P<0.01, n=5) caused further depolarization (see Table 3 and Figure 7a–c). TEA (10 mM), a blocker of BK and IK channels and several other voltage-dependent K+ channels, had no significant effect on the resting membrane potential (−47±2 vs −48±2 mV under control conditions, P>0.05, n=13) but increased the noise level in membrane potential recordings. TEA had no effect on the depolarization caused by L-methionine, DL-homocysteine and L-cysteine (1 mM) (see Table 3 and Figure 8d–f).
Table 3.
Effects of sulfur-containing amino acids on membrane potentials in the presence of K channel blockers and NEM
| Control | L-mtn | Control | DL-hct | Control | L-cyst | |
|---|---|---|---|---|---|---|
| Presence of 4-AP (5 mM) | −37±3 | −33±2 (6)a | −42±2 | −36±2 (4)a | −40±3 | −33±3 (5)a |
| Presence of TEA (10 mM) | −46±3 | −39±3 (5)a | −48±2 | −38±2 (4)a | −50±2 | −44±2 (4)a |
| Presence of GBC (10 μM) | −51±2 | −45±2 (4)a | −51±2 | −43±1 (4)a | −45±2 | −37±3 (4)a |
| Presence of NEM (10 μM) | −59±3 | −54±3 (4)a | −56±4 | −50±3 (4)a | −62±4 | −51±3 (4)a |
Values are mean±s.e. (mV). Control values refer to the membrane potential after treatment with 4-AP, TEA, GBC and NEM. L-mtn, L-methionine; DL-hct, DL-homocysteine; L-cyst, L-cysteine; 4-AP, 4-aminopyridine; TEA, tetraethyl ammonium; GBC, glibenclamide; NEM, N-ethylmaleimide.
P<0.05 and values in parentheses denote number of muscle strips.
Figure 8.
Effects of sulfur-containing amino acids in the presence of K+ channel blockers on the membrane potential of murine proximal colon. (a–c) 4-AP (5 mM) caused depolarization of colonic muscles. Methionine, homocysteine and cysteine caused significant depolarization in the presence of 4-AP. In a few cases, 4-AP induced transient hyperpolarization followed by depolarization (panel c). (d–f) TEA (10 mM) induced considerable noise in membrane potential recordings. Methionine, homocysteine and cysteine depolarized membrane potentials in the presence of TEA. (g–i) GBC (10 μM) caused depolarization and methionine, homocysteine and cysteine further depolarized the membrane potentials in the presence of GBC. Dashed lines in each panel indicate the membrane potential before addition of drugs.
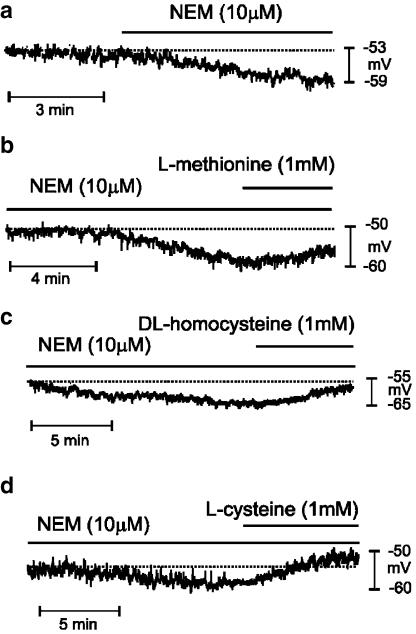
We have previously shown that there is resting activity of KATP channels in murine colonic muscles (Koh et al., 1998). In the present experiments, the KATP channel blocker GBC (10 μM) depolarized colonic cells from −54±2 to −49±2 mV (P<0.05, n=12). In the presence of GBC, further depolarization was caused by L-methionine, DL-homocysteine and L-cysteine (see Table 3 and Figure 8g–i). We also tested the thiol-specific reagent NEM. NEM (10 μM) caused hyperpolarization (i.e. from −54±2 to 59±2 mV, P<0.05, n=12). L-methionine, DL-homocysteine and L-cysteine caused depolarization in the presence of NEM (see Table 3 and Figure 9).
Figure 9.
Effects of sulfur-containing amino acids in the presence of NEM on the membrane potential of murine proximal colon. (a) NEM (10 μM) induced hyperpolarization. (b–d) Methionine, homocysteine and cysteine depolarized the membrane potentials in the presence of NEM. Dashed lines in each panel indicate the membrane potential before addition of drugs.
Sulfur-containing amino acids reduce the NO-dependent effects of enteric inhibitory nerve stimulation
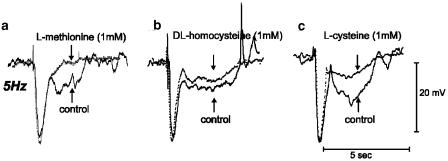
In the above studies, we found that the depolarizing effects of sulfur-containing amino acids are, in part, related to the amount of NO produced by colonic muscles. Thus, we tested whether responses to nitrergic nerve stimulation are inhibited by sulfur-containing amino acids. Transmural EFS, with parameters set to activate intrinsic nerves (0.5 ms pulses, 150 V, 5 Hz), evoked inhibitory junction potentials (IJPs) with fast and slow components in the murine colon. We have described the characteristics of these responses previously (Shuttleworth et al., 1999). IJPs in the murine colon consist of a fast component of hyperpolarization followed by a slower component. The latter is dependent upon synthesis and release of NO from enteric inhibitory neurons (Shuttleworth et al., 1999). L-Methionine, DL-homocysteine and L-cysteine (all at 1 mM) had not effect on the amplitude and duration of the fast component of IJPs (i.e. 26±1 vs 27±18 mV for methionine, n=16, 5 Hz for 1 s), but these compounds significantly reduced the second (nitrergic) component (Figure 10). For example, in the presence of 1 mM L-methionine, the peak amplitude of slow IJP was reduced from 10±1 to 7±1 mV (5 Hz for 1 s, n=16, P<0.001; Figure 10a). DL-Homocysteine and L-cysteine (1 mM) also reduced the amplitude (i.e. 12±1 vs 9±1 mV, n=12, P<0.01 for DL-homocysteine; 11±1 vs 7±1 mV, n=12, P<0.01 for L-cysteine) and duration (i.e. 3.8±0.1 vs 3.1±0.1 s, n=12, P<0.01 for DL-homocysteine; 4.0±0.2 vs 3.3±0.1 s, n=12, P<0.01 for L-cysteine) of the nitrergic component of the IJPs (Figure 10b and c).
Figure 10.
Effects of sulfur-containing amino acids on junction potentials elicited with single electrical field stimuli of murine proximal colon. IJPs, consisting of fast (apamin-sensitive) and slow (nitrergic) components, were elicited by EFS (five pulses delivered in 1 s; solid lines). (a–c) Methionine, homocysteine and cysteine inhibited the slow component without significant effect on the fast component (dashed lines).
Discussion
SDK channels have been associated with cGMP-dependent responses to NO in GI smooth muscles and may mediate a portion of the NO-dependent hyperpolarization that is generated by activation of enteric inhibitory neurons in GI muscles (Koh & Sanders, 2001). This hypothesis has been difficult to test since specific blockers of SDK channels have not been available. In the present study, we found that several sulfur-containing amino acids block SDK channels in murine colonic myocytes, and we have used the blocking action of these compounds to test the role of SDK channels in maintenance of resting potential, regulation of contractile frequency and responses to NO. L-Methionine, L-cysteine and DL-homocysteine depolarized membrane potential, enhanced contractile frequency and partially reversed the hyperpolarization induced by the NO donor SNP in intact muscles. Depolarization effects of L-methionine, L-cysteine and DL-homocysteine were greatly reduced by treatment of muscles with L-NA or ODQ, compounds known to interfere with nitrergic responses in GI muscles (cf. Franck et al., 1997). Responses to L-cysteine may have been contaminated with some nonselective actions on K+ channels other than SDK, but L-methionine had no significant effect on whole-cell currents or KATP currents known to contribute to resting potentials of murine colonic muscles. These data suggest that SDK channels have important roles in regulating the electrical activity of colonic muscles. Much of the contribution of SDK channels in regulating membrane potential and setting basal excitability may be the result of stimulation from tonic production of NO and basal activation of cGMP-dependent mechanisms in colonic muscles. SDK channels also participate in the hyperpolarization responses elicited by release of NO from nitrergic nerves.
Ion channels activated by plasma membrane stretch have been observed in numerous cell types and under diverse experimental conditions. In GI smooth muscle cells, three types of mechanosensitive ion channels have been identified that can generate inward current: (i) swelling-activated chloride channels (Dick et al., 1998), (ii) stretch activated nonselective cation channels (Waniishi et al., 1997) and (iii) Ca2+ channels (Farrugia et al., 1999). Activation of these conductances can lead to depolarization and contraction. Contraction, however, is not the major response to stretch in many regions of the GI tract and this is an important feature of GI muscles since it allows expansion of organs without expulsion of contents (i.e. the reservoir function, which is a primary pattern of GI motility). For example, the colon displays a reservoir function that allows volume expansion without increasing intraluminal pressure. The existence of a reservoir function in GI organs suggests that, in addition to neural control, smooth muscles may express a mechanism to stabilize the membrane potential, control excitability and mediate responses to stretch. We attribute these functions to SDK channels in colonic myocytes (Koh & Sanders, 2001), and the present study shows that blockers of these channels depolarize membrane potential and increase contractile frequency (Koh et al., 2001). We have not yet tested the effects of sulfur-containing amino acids on stretch responses of intact muscles.
Our data suggest that SDK channels, either in smooth muscle cells or interstitial cells of Cajal (ICC), which are electrically coupled to smooth muscle cells, are tonically activated in colonic muscles via NO-dependent and NO-independent mechanisms. The latter could be due to the stretch applied to muscles to facilitate intracellular impalements in small strips of colonic muscle. Effects of L-methionine, L-cysteine and DL-homocysteine on membrane potential were significantly reduced by L-NA and ODQ, suggesting that ongoing production of NO within colonic muscles, and background activation of guanylyl cyclase are important factors in maintaining activation of SDK channels in murine colonic cells. Depolarization caused by sulfur-containing amino acids was also reduced by TTX, suggesting that a significant portion of the basal NO in colonic muscles is due to release from spontaneous motor neuron firing. These data are consistent with the concept of tonic inhibitory drive in GI organs (Wood, 1972). Data in the present study expand this concept and suggest that the portion of the inhibitory drive that is mediated by spontaneous nitrergic nerve activity is due to postjunctional activation of SDK channels in smooth muscle and/or ICC.
We have not yet conducted a detailed study of the block of SDK channels by sulfur-containing amino acids, but a few observations suggest preliminary insights into the blocking actions of these compounds. Application of L-methionine and D-methionine to the cytoplasmic surface of patches effectively blocked SDK channels; however, application of D-methionine to the extracellular surface was not effective. This suggests that these compounds block primarily from the cytoplasmic aspect of the channel. Block of SDK in whole tissues must, therefore, rely upon amino-acid transporters to deliver the amino acid to the cytoplasmic surface. D-Isomers of methionine and cysteine were less effective than L-isomers in producing membrane potential effects in whole tissues. This could either be due to reduced uptake of D-isomers or stereo-specificity in the block of SDK channels. Most of the known K+ channels were not particularly sensitive to the sulfur-containing amino acids, but L-cysteine had some effect on the delayed rectifier portion of the outward current in colonic myocytes. This crossreactivity with delayed rectifier channels may explain why D-cysteine produced some depolarization (albeit reduced from the effects of L-cysteine), while D-methionine was without effects.
L-NA (which presumably greatly reduced basal production of NO in our experiments, as shown previously; see Shuttleworth et al., 1995) produced much greater depolarization than any of the sulfur-containing amino acids. Similarly, the sulfur-containing amino acids only partially reversed the hyperpolarization due to SNP. It is possible that these differences could be due to incomplete block of SDK channels in the intact muscles. It is also possible that nitrergic hyperpolarization result from activation of more than a single class of K+ channels. We have reported previously that three K+ conductances are activated by NO-dependent mechanisms in colonic myocytes: (i) 80 pS channels (KNO1), very small channels (4 pS, KNO2) and 270 pS Ca2+-activated K+ channels (BK channels) (Koh et al., 1995). Subsequent studies showed that the 80 pS conductance was due to SDK channels. Thus, incomplete block of nitrergic responses by sulfur-containing amino acids may be due to an inability of these compounds to block all of the conductances responsible for hyperpolarization responses to nitrergic stimulation.
GI muscles from mutant mice that lack specific types of ICC have greatly attenuated responses to enteric nerve stimulation (Burns et al., 1996; Ward et al., 2000). These studies, and others showing a close synaptic relationship between ICC and enteric motor nerve terminals (cf. Wang et al., 2000), have suggested that the predominant innervations of GI muscles, including nitrergic innervations, occur through contacts between enteric motor nerve terminals and ICC (see review by Ward & Sanders, 2001). In the present study, we found that sulfur-containing amino acids also decreased nitrergic postjunctional responses to transmural nerve stimulation. Thus, it is likely that a portion of the SDK channels in GI tissues may be expressed by ICC. In fact, ICC contain an abundance of SDK channels (S.D. Koh and K.M. Sanders, unpublished observations), and since these channels are activated by NO, it is possible that SDK channels in ICC mediate at least a portion of the nitrergic IJP.
In summary, SDK channels are abundantly expressed in colonic smooth muscle cells and are responsive to stretch and nitrergic stimulation (Koh & Sanders, 2001). Lack of specific blockers of SDK channels has made it difficult to clearly assign functions to this conductance. In the present study, we have found that sulfur-containing amino acids are capable of blocking SDK channels, and there are relatively minor nonspecific effects of these agents on other known K+ conductances. L-Methionine, L-cysteine and DL-homocysteine caused membrane depolarization, stimulation of basal contractile activity and partially reversed the hyperpolarization to the NO donor SNP and reduced neurally induced nitrergic stimulation. It is possible that other compounds, mimicking key molecular elements in sulfur-containing amino acids, might produce even more selective block of SDK channels at lower concentrations. These compounds might provide prokinetic functions in GI organs.
Acknowledgments
National Institutes of Health Grant P01 DK41315 supported this work.
Abbreviations
- 4-AP
4-aminopyridine
- EFS
electrical field stimulation
- GBC
glibenclamide
- GI
gastrointestinal
- IJP
inhibitory junction potential
- KATP
ATP-sensitive K+ channel
- KRB
Krebs–Ringer bicarbonate solution
- L-NA
NW-nitro-L-arginine
- NANC
non-adrenergic, non-cholinergic
- NEM
N-ethylmaleimide
- NPo
N the number of channels in the patch and Po the probability of a channel being open
- ODQ
1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one
- SDK
stretch-dependent K+
- SNP
sodium nitroprusside
- STOCs
spontaneous transient outward currents
- TEA
tetraethyl ammonium
- TTX
tetrodotoxin
References
- BURNS A.J., LOMAX A.E., TORIHASHI S., SANDERS K.M., WARD S.M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK G.M., BRADLEY K.K., HOROWITZ B., HUME J.R., SANDERS K.M. Functional and molecular identification of a novel chloride conductance in canine colonic smooth muscle. Am. J. Physiol. 1998;275:C940–C950. doi: 10.1152/ajpcell.1998.275.4.C940. [DOI] [PubMed] [Google Scholar]
- DU C., CARL A., SMITH T.K., SANDERS K.M., KEEF K.D. Mechanism of cyclic AMP-induced hyperpolarization in canine colon. J. Pharmacol. Exp. Ther. 1994;268:208–215. [PubMed] [Google Scholar]
- FARRUGIA G., HOLM A.N., RICH A., SARR M.G., SZURSZEWSKI J.H., RAE J.L. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology. 1999;117:900–905. doi: 10.1016/s0016-5085(99)70349-5. [DOI] [PubMed] [Google Scholar]
- FRANCK H., SWEENEY K.M., SANDERS K.M., SHUTTLEWORTH C.W. Effects of a novel guanylate cyclase inhibitor on nitric oxide-dependent inhibitory neurotransmission in canine proximal colon. Br. J. Pharmacol. 1997;122:1223–1229. doi: 10.1038/sj.bjp.0701487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-PASCUAL A., LABADIA A., COSTA G., TRIGUERO D. Effects of superoxide anion generators and thiol modulators on nitrergic transmission and relaxation to exogenous nitric oxide in the sheep urethra. Br. J. Pharmacol. 2000;129:53–62. doi: 10.1038/sj.bjp.0703000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., BRADLEY K.K., RAE M.G., KEEF K.D., HOROWITZ B., SANDERS K.M. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys. J. 1998;75:1793–1800. doi: 10.1016/S0006-3495(98)77621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., CAMPBELL J.D., CARL A., SANDERS K.M. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J. Physiol. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., MONAGHAN K., SERGEANT G.P., RO S., WALKER R.L., SANDERS K.M., HOROWITZ B. TREK-1 regulation by nitric oxide and cGMP dependent protein kinase: an essential role in smooth muscle inhibitory neurotransmission. J. Biol. Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- KOH S.D., SANDERS K.M. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J. Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., WARD S.M., DICK G.M., EPPERSON A., BONNER H.P., SANDERS K.M., HOROWITZ B., KENYON J.L. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J. Physiol. 1999;515 Part 2:475–487. doi: 10.1111/j.1469-7793.1999.475ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG I.D., KOH S.D., SANDERS K.M. Purinergic activation of spontaneous transient outward currents in guinea-pig taenia colonic myocytes. Am. J. Physiol. Cell Physiol. 2000;278:C352–C362. doi: 10.1152/ajpcell.2000.278.2.C352. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M., WARD S.M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am. J. Physiol. 1992;262:G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- SHUTTLEWORTH C.W., CONLON S.B., SANDERS K.M. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br. J. Pharmacol. 1997;120:707–713. doi: 10.1038/sj.bjp.0700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUTTLEWORTH C.W., SWEENEY K.M., SANDERS K.M. Evidence that nitric oxide acts as an inhibitory neurotransmitter supplying taenia from the guinea-pig caecum. Br. J. Pharmacol. 1999;127:1495–1501. doi: 10.1038/sj.bjp.0702674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUTTLEWORTH C.W., WEINERT J.S., SANDERS K.M., BUXTON I.L. Detection of nitric oxide release from canine enteric neurons. J. Auton. Nerv. Syst. 1995;56:61–68. doi: 10.1016/0165-1838(95)00050-6. [DOI] [PubMed] [Google Scholar]
- WANG X.Y., SANDERS K.M., WARD S.M. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000;302:331–342. doi: 10.1007/s004410000272. [DOI] [PubMed] [Google Scholar]
- WANIISHI Y., INOUE R., ITO Y. Preferential potentiation by hypotonic cell swelling of muscarinic cation current in guinea pig ileum. Am. J. Physiol. 1997;272:C240–C253. doi: 10.1152/ajpcell.1997.272.1.C240. [DOI] [PubMed] [Google Scholar]
- WARD S.M., BECKETT E.A., WANG X., BAKER F., KHOYI M., SANDERS K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., SANDERS K.M. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat. Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- WOOD J.D. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am. J. Physiol. 1972;222:118–125. doi: 10.1152/ajplegacy.1972.222.1.118. [DOI] [PubMed] [Google Scholar]