Abstract
We investigated the role of mast cells in granuloma-associated angiogenesis in rat by using: (i) a mast cell membrane stabilizer, ketotifen; (ii) a mast cell depleting agent, compound 48/80. Moreover, we focused on the role of chymases, which exhibit proinflammatory and proangiogenic properties by using: (i) chymostatin, an inhibitor of chymase activity; (ii) a specific antisense oligonucleotide (AS-ODN) designed against rat mast cell protease-5 (rMCP-5), the most abundantly expressed chymase in the skin.
The formation of granuloma was evaluated, as wet weight, 96 h after the subcutaneous implant of two λ-carrageenin (1%)-soaked sponges on the back of male Wistar rats. Angiogenesis was evaluated as haemoglobin content in the granulomatous tissue and as level of tumour necrosis factor-α (TNF-α) in the exudates.
A single injection of ketotifen (1–5–25 mg kg−1 i.p.) significantly reduced granuloma formation by 31.6, 44.6 and 71.9%, and haemoglobin content by 17.0, 35.0 and 66.2%, suggesting that the release of mediator(s) from mast cells modulates the process. Chymostatin (5–10 nmol−1 site−1 day−1) reduced granuloma-associated angiogenesis by 57.3 and 70.0%.
RT–PCR analysis showed that rMCP-5 mRNA amounts were significantly reduced by rMCP-5 AS-ODN (1.25–2.5–5.0 nmol site−1) by 69.5, 72.5 and 81.8%. In parallel experiments, rMCP-5 AS-ODN (1.25, 2.5, 5.0 nmol site−1) strongly reduced granuloma weight (26.1, 45.0 and 56.3%) and haemoglobin content (22.2, 50.4, 62.03%), suggesting that the observed effect is mediated through an antisense mechanism.
In conclusion, these data suggest that: (i) inhibition of mast cell mediators release may represent a novel strategy to modulate angiogenesis; (ii) among the chymase family, rMCP-5 is a key promoter of angiogenesis in the rat.
Keywords: Angiogenesis, chronic inflammation, chymases, granuloma formation, ketotifen, mast cells, rMCP-5
Introduction
Angiogenesis, the multistep process leading to new capillary formation from the pre-existing ones (Jackson et al., 1997; Risau, 1997), plays a central role in many physiological and pathological events (Breier et al., 1992; Sueishi et al., 1997), including tumour growth (Folkman, 1995). The progress of chronic and proliferative diseases, such as granuloma formation, as well as rheumatoid arthritis, atherosclerosis and solid tumour growth also depends on angiogenesis (Jackson et al., 1997), required not only for the maintenance of tissue perfusion but also to allow cellular traffic supporting chronicity (Colville-Nash et al., 1995).
Angiostatic steroids, cortisone and tetrahydrocortisol (Crum et al., 1985), frequently co-administered with heparin, are currently used to modulate the vascular density during chronic inflammation (Colville-Nash & Scott, 1992; Colville-Nash et al., 1995; Hori et al., 1996). Furthermore, antiangiogenic therapy combined with conventional anticancer therapies seems to be promising in the treatment of patients with cancer. Therefore, new antiangiogenic agents may represent useful therapeutic tools for the control of both chronic inflammatory diseases and cancer.
Mast cells are highly specialized immune effector cells that, according to the granule content, can be divided into two subpopulations: mucosal mast cells residing mainly in the mucosa of the respiratory and intestinal tracts, and connective tissue mast cells mainly occurring in skin, peritoneum, airways and joints (Irani et al., 1986; Karlson et al., 2002). Mast cells are topographically associated with microvessels (Rhodin & Fujita, 1989; Rakusan et al., 1990) and their number rises in several angiogenesis-dependent events such as ovulation, atherosclerosis, rheumatoid arthritis (Mican & Metcalfe, 1990) and tumour growth (Ribatti et al., 2001) supporting their involvement particularly connective tissue mast cells, in angiogenesis (Meininger & Zetter, 1995).
The role of mast cells in angiogenesis is mediated by the release of their stored and newly synthesized mediators following either classical immunological IgE-dependent activation or in response to a variety of stimuli (Leal-Berumen et al., 1994; Iuvone et al., 1999). Mast cell mediators include cytokines, tumour necrosis factor-α (TNF-α) (Gordon & Galli, 1990), vascular endothelial growth factor (VEGF) (Grutzkau et al., 1998), histamine (Sorbo et al., 1994) and a family of serine proteases (Solivan et al., 2002), all of them exhibiting proangiogenic properties. Therefore, modulation of mast cell granule content release may be a promising and until now uninvestigated pharmacological approach to control angiogenesis-dependent events. This strategy can be afforded by the use of mast cell membrane stabilizer, as ketotifen.
In the last years, among all mast cell mediators, an increased interest is raised around chymases as proangiogenic effectors. In fact, crude chymase extract from human skin are able to increase vascular permeability in guinea pig (He & Walls, 1998) and to mediate angiogenesis in hamster (Muramatsu et al., 2000a, 2000b). Chymases belong to a family of serine proteases classified either as chymases showing chymotrypsin-like substrate specificity, or tryptases showing trypsin-like specificity. Based on phylogenetic relationships, the chymase family can be further subdivided into two groups: α- and β- chymases (Chandrasekharan et al., 1996). In nonrodent mammals, only one α-chymase has been found, whereas in rodents several β-chymases are expressed in addition to α-chymases. Five of the 10 rat mast cell proteases (rMCP-1 to rMPC-5) are considered to display chymase activity (Lutzelschwab et al., 1997). The rMCP-5 (initially designated as rMCP-3) is the α-chymase most abundantly expressed in the rat skin by connective tissue mast cells (Ide et al., 1995). Until now the role of rMCP-5 in angiogenesis-dependent events has not been yet showed.
The aim of our study was to evaluate the role of mast cell degranulation and the involvement of mast cell released mediators in the development of granuloma-dependent angiogenesis, in a model of carrageenin-soaked sponge implants in rat. For this purpose we used ketotifen, as a mast cell stabilizer, and compound 48/80, as a mast cell depleting agent. Furthermore, we focused our interest on the role of rMCP-5, the most abundant chymase expressed in the skin, in this model, by using a selective antisense oligonucleotide (AS-ODN) against rMCP-5 mRNA sequence.
Methods
Sponge implantation
Male Wistar rats were housed in a temperature- and humidity-controlled environment, on a 12 h light/dark cycle with free access to food and water. All procedures we used were as humane as possible and complied with international guidelines for animal care; all efforts were made to minimize the animal's suffering and the number of animals used.
Sponges were implanted as described by Iuvone et al. (1994). Briefly, two polyether sponges (0.5 × 1.5 × 2.0 cm) weighing 0.035±0.002 g were implanted subcutaneously on the back of male Wistar rats (200–220 g) under general anaesthesia (pentobarbital 60 mg kg−1 i.p.). Sponges and surgery tools were sterilized by autoclaving for 20 min at 120°C. 1% λ-carrageenin (0.5 ml sponge−1) in pyrogen-free saline, or saline as control, were injected into each sponge. The rats were killed 96 h after sponge implantation. In preliminary time course experiments, the effect of carrageenin on granuloma formation was studied at 24–48–96 h as well as 8 and 16 days. Since the granulomatous tissue peaked at 96 h, this time point was used in all the further experiments.
Treatments
Ketotifen fumarate (1–5–25 mg kg−1 i.p.) was given into the sponge at time 0, that is only once after sponge implantation. Depletion of mediators from mast cells was achieved according to Di Rosa et al. (1971). Briefly, rats were depleted of their stored mediators by repeated injection of compound 48/80. A solution (0.1% w/v) of compound 48/80 in saline was given i.p. morning and evening for eight doses, starting from an evening dose. The employed doses were 0.6 mg kg−1 for the first six injections and 1.2 mg kg−1 for the last two doses. Sponges were implanted 5–6 h after the last 48/80 injection. In some experiments, clorpheniramine maleate (1–5–25 mg kg−1 i.p.), an anti-H1 antagonist, was given at time 0, in parallel to ketotifen, to compare the anti-H1 effects.
Chymostatin (5–10 nmol site−1) was injected into each sponge daily in a volume of 100 μl sponge−1. The rMCP-5 AS-ODN (1.25–2.5–5.0 nmol site−1) was given into each sponge once, at time 0, in a volume of 100 μl sponge−1. Phosphorothioate oligonucleotides (antisense and sense) used in this study were designed and synthesized by Tib-Biomol (Roche, Brescia, Italy) and their sequences are the following: AS-ODN 5′-gXTXgX CXCXCX CXCXgX AXTXgX AXTXCX TXCXCX CXCXA (X=tioate); S-ODN: 5′-TXgXgX gXgXAX gXAXTX CXAXTX CXgXgX gXgXgX CXAXC.
Collection of tissue and exudate
After 96 h from implantation, the sponges were removed, centrifuged at 800 × g for 10 min; the exudate fluids into the sponge were collected, measured and stored at −20°C for the assays. The exudate volume, measuring 1.0±0.2 ml, did not change over the time course period and treatments.
The granulomatous tissue around the sponge was dissected by surgical blade, the fresh weight determined and after the tissue was quickly frozen in liquid nitrogen and stored in sterile vials at −80°C until used.
Histology of granulomatous tissue
After 96 h from sponge implants, the granulomatous tissue around the sponge was removed and fixed in 10% formalin. Thin (0.5 μm) paraffined section were prepared and stained with toluidine blue according to Iuvone et al. (1999) and then processed for light microscopy examination. Mast cell counting was performed on five randomly selected sections using a × 100 objective lens making a difference from deep blue (not degranulated) and light blue (degranulated) mast cells.
Evaluation of haemoglobin content
Neovascularization was assessed by measuring the concentration of haemoglobin in the granulomatous tissues. Granuloma was weighted and homogenized on ice with a Polytron homogenizer (Kinematica AG) in 1 × PBS (4 ml each gram of wet weight) according to the method described by Muramatsu et al. (2000b). Briefly, after centrifugation at 2500 × g for 20 min at 4°C, the supernatant was further centrifuged at 5000 × g for 30 min and haemoglobin concentration in the supernatant was determined spectrophotometrically at 450 nm by using the haemoglobin assay kit (Sigma Diagnostic). The haemoglobin content was expressed as mg haemoglobin g−1 of wet weight.
TNF-α determination
The levels of TNF-α in the exudates were determined by ELISA. Briefly, ELISA plates (NUNC) were coated overnight at 4°C with a monoclonal anti-mouse TNF-α antibody (R&D System, Europe Ltd, Abingdon, U.K.) in PBS pH 7.4 (2.5 μg ml−1). The wells were washed twice with PBS 0.05% Tween 20 (PBS/Tween), blocked with 1% BSA in PBS for 2 h at 37°C, and incubated with a rat TNF-α protein standard or the sample derived from sponges, for 1 h at room temperature. Plates were washed with PBS/Tween and incubated with a polyclonal anti-mouse/rat TNF-α antibody biotin conjugate (Amersham Biosciences, Milano, Italy) for 1 h at room temperature. The antigen bound antibody complexes were detected with extravidin peroxidase conjugate and visualized with tetramethylbenzidine, reading plates at 405 nm by an ELISA spectrophotometer (Titertek Multiscan MCC/340).
Western blot analysis
Immunoblot analysis of chymase protein was performed on granulomatous tissue from both λ-carrageenin alone and λ-carrageenin plus AS-ODN (1.25–2. 5–5 nmol site−1)-treated animals. Cytosolic fraction proteins, prepared as previously described (Esposito et al., 2002), were mixed with Laemli loading buffer in a ratio of 1:1, boiled for 3 min and centrifuged at 10,000 × g for 10 min. Protein concentration was determined and equal amounts (50 μg lane−1) of each sample were electrophoresed in a 12% discontinuous polyacrilamide minigel; thereafter, proteins were transferred onto nitrocellulose membrane, according to the manufacturer's instructions (Bio-Rad). The membranes were saturated by incubation at 4°C overnight with 10% non-fat dry milk in PBS and then incubated with anti-human skin chymase antibody (1 μg ml−1) (Neomarkers) for 2 h at room temperature. The membranes were washed three times with 1% Triton X-100 in PBS and then incubated with anti-mouse immunoglobulins coupled to peroxidase (1 : 2000) (Amersham). The immune complexes were visualized by the chemiluminescence method (ECL-Amersham).
mRNA analysis
The mRNA level of rMCP-5 in granulomatous tissue was determined using the semiquantitative RT–PCR method. Total RNA was extracted from tissue samples by use of an ultrapure TRIzol reagent (GibcoBRL) as directed by the manufacturer. RNA (5 μg) was then reverse-transcribed in 20 μl with 200 U of Superscript II RNase H-Reverse Transcriptase (Invitrogen s.r.l., Milano, Italy) in the presence of random hexamers (5 μM), 20 U of RNasin (Promega Italia s.r.l., Milano, Italy), dNTPs (1 mM), for 1 h at 42°C. PCR was performed on 1 μl of the reverse transcription reaction mixture in a final volume of 50 μl with 2.5 U of Taq polymerase (Roche Diagnostics S.p.A., Monza, Italy) and 5 pmol of the appropriate primers. The PCR-primers were selected according to the rat rMCP-5 cDNA sequence (accession number U67908) (forward primer 5′-TCCTGCAAACACTTCACCAG-3′ nucleotide position 554–574 in the cDNA, reverse primer 5′-CGAGATCCAGAGTTAATTCT-3′ positions 771–791 in the cDNA) to yield a product of 236 bp; and rat β-actin cDNA (accession number X03672) (forward primer 5′-GGCACCACACCTTCTACA-3′ nucleotide positions 330–348, and reverse primer 5′-CAGGAGGAGCAATGATCT-3′ nucleotide position 1056–1074) to yield a product of 700 bp. To obtain linear amplification curves, the cDNA mixtures were subjected to 30, 35 and 40 cycles for rMCP-5 and 10, 15, 20 cycles for the β-actin control, under the following conditions: denaturating at 95°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1 min. The final extension step was performed at 72°C for 10 min.
A 25 μl aliquot of PCR products were electrophoretically fractionated through 1% agarose gel; transferred onto a Hybond-N+membrane (Millipore) and hybridized with rMCP-5 or β-actin cDNA probes made using a random-priming kit (Boerhingher-Mannheim) according to the manufacturer's instructions. The rMCP-5 and β-actin mRNA levels were determined by PhosphorImager analysis (Storm840-Amersham).
Statistical analysis
Results were expressed as the mean±s.e.mean of n animals where each value is the average of responses in duplicate sites. Statistical comparisons were made by one-way-ANOVA followed by Bonferroni's test for multiple comparisons. A P-value⩽than 0.05 was considered to be significant.
Drugs
All the used drugs, unless otherwise stated, were supplied from Sigma-Aldrich (Milano, Italy).
Results
Granuloma formation and mast cell degranulation
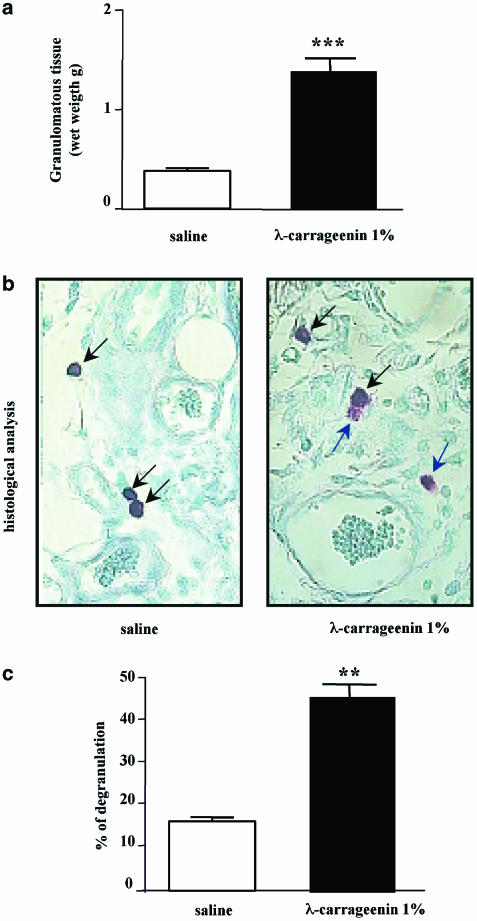
Implants of λ-carrageenin (1%)-soaked sponge on the back of rats caused a significant increase, at 96 h, of granuloma formation in comparison to implants of saline-soaked sponges (1.39±0.14 vs 0.38±0.03 g) (Figure 1a). Histological analysis (Figure 1b) showed that mast cell degranulation occurs at 96 h in the tissue around carrageenin-soaked sponge in comparison to the tissue around saline-soaked sponges with a percentage of degranulated mast cells of 43±2.5% vs 12±0.9% (Figure 1c).
Figure 1.
Granuloma formation and mast cell degranulation in λ-carrageenin-soaked sponge implants in rat. (a) Granuloma was induced by implantation of two polyether sponges soaked with 1% (w/v) λ-carrageenin (0.5 ml sponge−1) or saline (0.5 ml sponge−1) as control. Granuloma formation around the sponge was evaluated 96 h after implantation and expressed as wet weight. (b) A representative histological analysis of rat granulomatous tissue around (1%) λ-carrageenin-soaked sponge implants and saline-soaked sponge implants in rats at 96 h. Mast cell degranulation was evaluated on microscopically visible connective mast cells stained with 0.05% (w/v) toluidine blue and counterstained with 0.1% (w/v) nuclear fast red (magnification × 100). (c) A differentiation between not degranulated (deep blue-black arrow) and degranulated (light blue-blue arrow) mast cells, in both saline and 1% λ-carrageenin, was performed. Results are expressed as percentage of degranulation (%). **P<0.01 and ***P<0.001 vs saline.
Effect of ketotifen on granuloma formation
Single injection of ketotifen (1–5–25 mg kg−1) at time 0, that is, the same day of sponge implantation, reduced significantly and in a dose-related manner granuloma formation, evaluated as wet weight (0.95±0.09, 0.77±0.02, 0.39±0.04 g), in comparison to carrageenin-soaked sponges (1.39±0.14 g). Since ketotifen has a stabilizing effect on mast cell membrane dissociated from its anti-H1 effect, in some experiments chlorpheniramine maleate (1–5–25 mg kg−1), an anti-H1 receptor antagonist, was given at time 0 in parallel to ketotifen to compare the effects of both compounds on granuloma formation. Chlorpheniramine maleate (1–5–25 mg kg−1) was not able to inhibit granuloma formation (1.3±0.13, 1.25±0.08, 1.15±0.10 g) in comparison to carrageenin-soaked sponges (1.39±0.14 g) (Figure 2). Therefore, our data suggest that ketotifen, by inhibiting the initial release of mediators from mast cells, is able to modulate granuloma formation.
Figure 2.
Effect of ketotifen and chlorpheniramine on granuloma formation. Granuloma was induced by (1%) λ-carrageenin- or saline-soaked sponge implants in rats. Ketotifen fumarate and chlorpheniramine maleate (1–5–25 mg kg−1) were given intraperitoneally at time 0, that is, the same day of sponge implantation. Granuloma formation was evaluated 96 h after implantation and expressed as wet weight. Bars represent the mean±s.e. of n=6 experiments in duplicate. ***P<0.001 vs saline; °°°P<0.001 and °°P<0.01 vs carrageenin alone.
Effect of mast cell depletion on granuloma formation
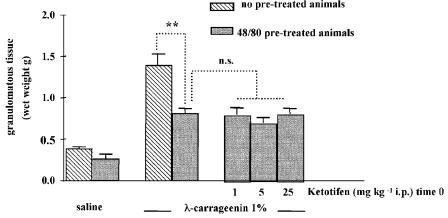
In order to confirm the importance of mast cell released mediators in the initial stage of the granuloma formation process, some experiments were performed in rats previously depleted of mast cell granule content according to the protocol described by Di Rosa et al. (1971). Rats receiving compound 48/80 for 4 days before carrageenin-soaked sponge implants, developed a granulomatous tissue at 96 h that was significantly increased in comparison to control rats (0.81±0.06 vs 0.26±0.005 g), but smaller in comparison to rats that did not receive compound 48/80 (1.39±0.14 vs 0.38±0.03 g) (Figure 3), confirming that some mast cell mediator(s) released at an initial stage might be important for the subsequent development of granulomatous tissue around the sponge. When ketotifen (1–5–25 mg kg−1) was given at time 0 to compound 48/80-treated rats it failed to inhibit granuloma formation (0.80±0.08, 0.70±0.06, and 0.81±0.08 g) (Figure 3). These results indicate that when mast cells have already been depleted ketotifen is without effect.
Figure 3.
Effect of ketotifen on granuloma formation in compound 48/80 pretreated rats. Granuloma was induced by (1%) λ-carrageenin- or saline-soaked sponge implants in rats (no pretreated animals). In order to confirm the importance of mast cell-released mediators in granuloma formation, in some experiments rats were previously depleted of mast cell granule content by using compound 48/80 (48/80 pretreated animals) according to the protocol described in the methods. Ketotifen fumarate (1–5–25 mg kg−1) was given intraperitoneally at time 0, that is, the same day of sponge implantation to 48/80 pretreated animals. Granuloma formation was evaluated 96 h after implantation and expressed as wet weight. Bars represent the mean±s.e. of n=4 experiments in duplicate. **P<0.01 vs no pretreated animals.
Effect of ketotifen on angiogenesis
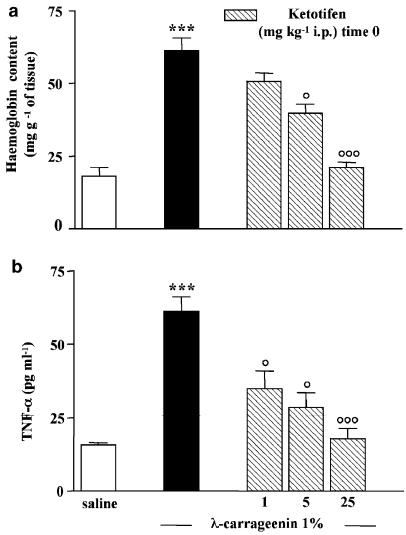
As angiogenesis markers we considered both haemoglobin content in the granulomatous tissue and levels of TNF-α, a proangiogenic cytokine, in the exudates at 96 h. Haemoglobin content in the granulomatous tissue around carrageenin-soaked sponges was significantly increased in comparison to saline-soaked sponges (61.4±4.2 mg haemoglobin g−1 of tissue vs 18.12±3.05 mg haemoglobin g−1 of tissue) and it was drastically reduced, in a dose-related manner, by ketotifen (1–5–25 mg kg−1) given at time 0, by 17±0.9, 35±1.7, 66±3.2% (Figure 4a). TNF-α levels in the exudates into the sponges at 96 h were increased in carrageenin-soaked sponges in comparison to saline-soaked sponges (61.3±5.0 vs 15.8±0.75 pg ml−1). Ketotifen treatment significantly reduced TNF-α levels into the exudates by 43±2.0, 53±2.7, 71±3.5% (Figure 4b).
Figure 4.
Effect of ketotifen on angiogenesis. Granuloma was induced by (1%) λ-carrageenin- or saline-soaked sponge implants in rats. Ketotifen fumarate (1–5–25 mg kg−1) was given intraperitoneally at time 0, that is, the same day of sponge implantation. Angiogenesis was evaluated at 96 h after implantation as (a) haemoglobin content in the tissue and (b) TNF-α levels in the exudates. Bars represent the mean±s.e. of n=4 experiments in duplicate. ***P<0.001 vs saline; °P<0.05 and °°°P<0.001 vs carrageenin alone.
Effect of chymostatin on granuloma formation and angiogenesis
In order to investigate the possible involvement of chymases in granuloma-associated angiogenesis, we used chymostatin, an inhibitor of chymase activity.
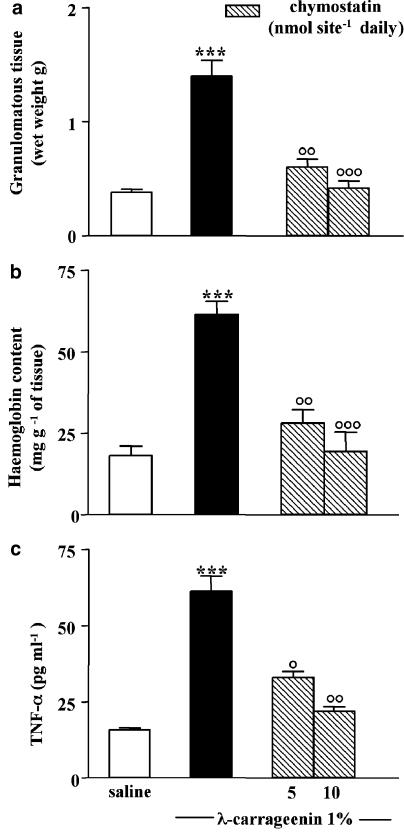
Treatment with chymostatin (5–10 nmol site−1 daily), injected into the sponge, significantly inhibited granuloma formation, expressed as wet weight, by 57±3.5 and 70±2.8% (Figure 5a).
Figure 5.
Effect of chymostatin on granuloma formation and angiogenesis. Granuloma was induced by (1%) λ-carrageenin- or saline-soaked sponge implants in rats. Chymostatin (5–10 nmol site−1) was given daily into the sponge. (a) Granuloma formation was evaluated 96 h after implantation and expressed as wet weight. Angiogenesis was evaluated at 96 h as (b) haemoglobin content in the tissue and (c) TNF-α levels in the exudates. Bars represent the mean±s.e. of n=6 experiments in duplicate. ***P<0.001 vs saline; °P<0.05 °°P<0.01 and °°°P<0.001 vs carrageenin alone.
Moreover, chymostatin (5–10 nmol site−1 daily) inhibited angiogenesis, evaluated as haemoglobin content into the tissue (54±2.8 and 68±5.2%) (Figure 5b) and TNF-α levels into the exudates (56±3.4 and 64±3.0%) (Figure 5c).
Effect of λ-carrageenin on rMCP-5 mRNA
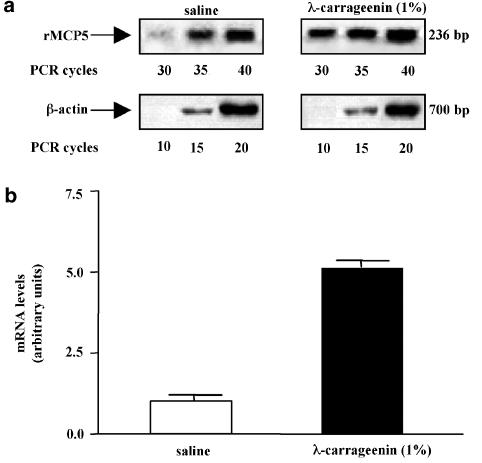
Additional experiments were performed in order to better understand the role of chymases, especially of rMCP-5, the most abundantly expressed chymase in the skin (Ide et al., 1995) during granuloma-dependent angiogenesis. For this purpose, we determined the level of rMCP-5 mRNA in the granulomatous tissue around sponge implants after 96 h. We subjected total RNA to reverse transcription (RT) reaction and the resulting cDNA to semiquantitative PCR utilizing primers specific for rMCP-5. The rMCP-5 cDNA was subjected to Southern-blot analysis and quantified by PhosphorImager. We also amplified the same cDNA preparation with primers for β-actin mRNA, as an internal control. As shown in Figure 6, levels of rMPC-5 mRNA were significantly increased (four-fold) in the granulomatous tissue of λ-carrageenin-soaked sponges in comparison to tissue from saline-soaked sponges. These findings demonstrate that λ-carrageenin increases rMCP-5 mRNA levels, which in turn may be responsible for the proinflammatory and proangiogenic effect observed in our experiments.
Figure 6.
Expression of rMCP-5 in granulomatous tissue. (a) RT–PCR assay of rMCP-5 mRNA levels in animals injected with saline solution or with (1%) λ-carrageenin. The expression of β-actin, a housekeeping gene, was used as a control of cDNA quantity. PCR was allowed to proceed for 30, 35, and 40 cycles using rMCP5 primers and for 10, 15, and 20 cycles using β-actin primers. The results demonstrate a linear relationship along the cycles. (b) Quantitative analysis of the results of three independent experiments expressed in arbitrary units after normalization to β-actin mRNA levels.
Effect of AS-ODN on rMCP-5 mRNA
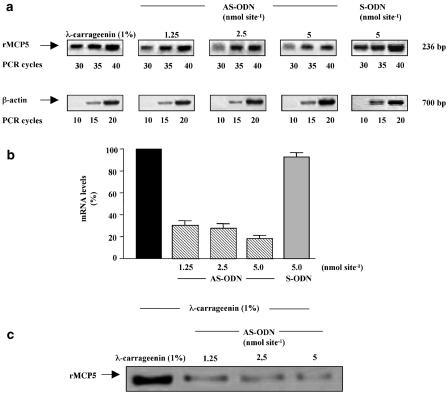
Since we demonstrate that rMCP-5 RNA levels are increased in our experimental model, we used an antisense technology to negatively modulate the expression of rMPC-5. For this purpose, a novel 20 base phosphorothioate ODN, in antisense (AS-ODN) and sense (S-ODN) orientation, targeted to a specific coding region of rMCP-5 mRNA was synthesized by Roche and administered into the sponge (1.25, 2.5 and 5 nmol site−1) in the presence of 1% λ-carrageenin at time 0. Since phosphorothioate AS-ODNs have been shown commonly to act through an RNase H-dependent mRNA cleavage mechanism in cells (Crooke, 1993), antisense activity was determined by means of a semiquantitative RT–PCR. Total RNA was extracted from granulomatous tissue 96 h after sponge implants. As control total RNA extracted from granulomatous tissue around λ-carrageenin-soaked sponges was used. A representative analysis of the electrophoretically separated products, derived from AS-ODN treated and nontreated tissues, is shown in Figure 7. Local injections of rMCP-5 AS-ODN (1.25, 2.5, 5 nmol site−1) caused a dose-dependent reduction in rMCP-5 mRNA levels by 69.5±3.9, 72.5±4.3, 81.8±2.7% relative to control ratio. The specificity of AS-ODN effect was verified by using rMCP-5 S-ODN. In fact, the local injections of rMCP-5 S-ODN (5 nmol site−1) did not cause changes in rMCP-5 mRNA levels (Figure 7a, b). The inhibition of rMCP-5 expression was also verified at the protein level by Western blot experiments (Figure 7c). These results demonstrate that AS-ODN is able to induce a decrease of rMCP-5 mRNA levels through an antisense mechanism.
Figure 7.
Reduction in rMCP-5 mRNA expression following treatment with phosphorothioate AS-ODN. (a) RT–PCR assay of rMCP-5 mRNA levels in animals injected with (1%) λ-carrageenin in the absence or in presence of rMCP-5 AS-ODN (1.25–2.5–5 nmol site−1) or S-ODN (5 nmol site−1). PCR was allowed to proceed as described in the legend to Figure 6a. (b) Quantitative analysis of the results of three independent experiments after normalization to β-actin mRNA levels calibrated to the signal of animals injected with (1%) λ-carrageenin (100%). (c) Western blot analysis of chymase protein levels in animals injected with (1%) λ-carrageenin in the absence or in presence of increasing amounts of AS-ODN.
Effect of AS-ODN on granuloma-associated angiogenesis
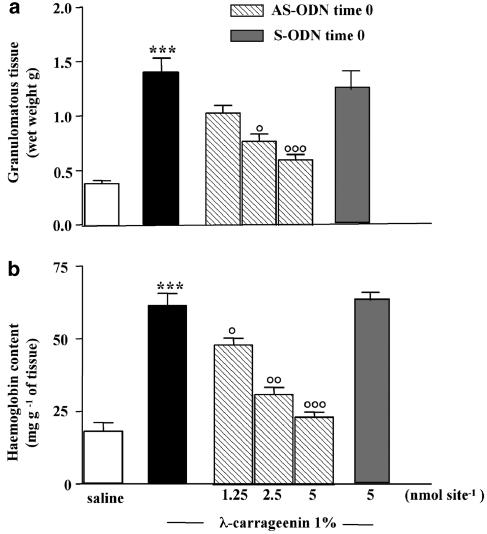
When the rMCP-5 AS-ODN (1.25,. 2.5, 5.0 nmol site−1) was injected into the sponge at time 0, it reduced, significantly and in a dose-related manner, granuloma formation (Figure 8a) evaluated as wet weight (1.03±0.08, 0.77±0.07, 0.60±0.05 g vs control 1.39±0.14 g) (Figure 8a) and haemoglobin content (47.9±2.3, 30.7±2.5, 23.0±1.8 mg haemoglobin g−1 of tissue vs control 61.4±4.2 mg haemoglobin g−1 of tissue) (Figure 8b), thus paralleling the effects obtained with mRNA analysis. The sense rMPC-5 ODN (5 nmol site−1 given at time 0), designed in the same region, was without effect on granuloma formation and haemoglobin content (Figure 8).
Figure 8.
Effect of AS-ODN against rMCP-5 on granuloma formation and related angiogenesis. Granuloma was induced by (1%) λ-carrageenin- or saline-soaked sponge implants in rats. rMCP-5 AS-ODN (1.25–2.5–5 nmol site−1) and S-ODN (5 nmol site−1) were given into the sponge at time 0, that is, the same day of sponge implantation. (a) Granuloma formation was evaluated after 96 h from implantation and expressed as wet weight. (b) Granuloma-associated angiogenesis was evaluated as haemoglobin content in the tissue. Bars represent the mean±s.e. of n=6 experiments in duplicate. ***P<0.001 vs saline; °P<0.05, °°P<0.01 and °°°P<0.001 vs carrageenin alone.
Discussion
Mast cells are involved in several pathological events, since they can release, following activation, a plethora of both preformed and newly synthesized mediators (Galli, 2000; Galli et al., 2002). In this study, we investigated the role of mast cells and their mediators in angiogenesis associated to granuloma formation 96 h after carrageenin-soaked sponge implants in rat. Subcutaneous implantation of carrageenin-soaked sponges induced granuloma-associated angiogenesis that was paralleled by mast cell degranulation in the tissue surrounding the sponge. At the best of our knowledge, this is the first direct evidence that mast cells degranulate in granulomatous tissue around carrageenin-soaked sponge implant in the rat. In our experiments we used ketotifen to stabilize mast cell membrane. In fact, dissociated from its antihistaminic action (Martin & Romer, 1978), ketotifen exhibits a stabilizing effect on mast cell membranes (Martin & Baggiolini, 1981; Grant et al., 1990) and it has been extensively used, by several investigators, to prevent mast cell degranulation (Brown et al., 1998; Gilles et al., 2003). Ketotifen strongly inhibited both granuloma formation and angiogenesis. Notably, ketotifen was given only once at the time of sponge implantation suggesting that inhibition of the prestored mediator release from mast cells may represent an important step for the subsequent modulation of the process. These data are in agreement with our previous results showing the protective effect of ketotifen in a model of acute inflammation in rat skin induced by LPS (Iuvone et al., 1999) and extend our results to a model of chronic inflammation, such as granuloma formation. Our results are also in agreement with data showing that mast cell stabilization with cromolyn, shortly after adjuvant immunization, reduced later vasculitis in the rats, suggesting that mast cells play an early role in the initiation of vasculitis (Johnston et al., 1998). On the other hand, a classic anti-H1 antagonist, chlorpheniramine maleate, did not inhibit neoangiogenesis, a result apparently in contrast with other results in the literature showing that anti-H1 actually inhibit angiogenesis (Sorbo et al., 1994). The failure of chlorpheniramine maleate in inhibiting granuloma-associated angiogenesis maybe due to our experimental protocol in which chlorpheniramine maleate was only given once, at the time of sponge implantation. Therefore, chlorpheniramine maleate was not able to control a long-lasting inflammatory process brought about by the sponge implantation. Thus, in fact, we observed that when anti-H1 was given daily, it was able to inhibit granuloma formation (unpublished results), according to the data reported in the literature. In animals pretreated with compound 48/80, to deplete mast cells from the prestored mediators, granuloma formation and angiogenesis were significantly reduced suggesting that some prestored mediator(s), released by mast cells at an initial stage of the experiment, may have a key role in modulating the later angiogenic process. Among the several preformed mediators from mast cells we focused our interest on chymases, a family of serine proteases, stored in mast cell granules exhibiting proinflammatory and proangiogenic properties (He & Walls, 1998; Muramatsu et al., 2000b). Chymostatin, an inhibitor of chymase enzymatic activity, strongly reduced both granuloma formation and associated angiogenesis, indicating that mast cell chymases play a key role in carrageenin-induced granuloma formation. These data are in agreement with the inhibitory effect exhibited by chymostatin and other chymase inhibitor on granuloma formation and angiogenesis induced by basic fibroblast growth factor (bFGF) in hamster (Muramatsu et al., 2000a; 2003). The pro-angiogenic effect of mast cell chymases may depend on their ability to cleave angiotensin I into its active fragment, angiotensin II (Reilly et al., 1982). This cleavage occurs in several species, including hamster, dog and human (Muramatsu et al., 2000b), although it has not been yet clearly demonstrated in rats. In a recent study in hamster sponge granulomas (Katada et al., 2002), it has been suggested that chymases, by increasing the local production of angiotensin II, upregulates VEGF-mRNA and thereby induces angiogenesis. Nevertheless, another interesting mechanism of the possible proangiogenic effect of chymases may be related to their ability to cleave a variety of other substrates, for example, metalloproteases (Lees et al., 1994), progelatinase B and pro-collagen (Fukami et al., 1998), all of them involved in the extracellular matrix degradation that occurs during angiogenesis. The above-mentioned or other mechanisms of action may be responsible for the proinflammatory and proangiogenetic effects of chymase in carrageenin-induced-granuloma formation in rats.
Moreover, RNA analysis demonstrates for the first time that the expression of rMCP-5, the most representative chymase in the rat skin (Ide et al., 1995), was remarkably increased by λ-carrageenin in the granulomatous tissue, suggesting that the proinflammatory and proangiogenic effects of λ-carrageenin may be mediated, at least in part, through the upregulation of rMCP-5 mRNA levels. Furthermore, we used an antisense technology to modulate the increased expression of rMPC-5 mRNA by using a novel rMCP-5 AS-ODN. The results of semiquantitative RT–PCR and western blotting demonstrate the ability of the rMCP-5 AS-ODN to reduce rMCP-5 mRNA and consequently protein levels; moreover, rMCP-5 AS-ODN is also able to reduce both granuloma formation and associated angiogenesis. All these findings demonstrate a key role of rMCP-5 mRNA transcript in this model.
In conclusion, the results reported here show that controlling mast cell degranulation can modulate granuloma formation and related angiogenesis. Since ketotifen inhibits the release of preformed mediators from mast cells, its possible use in the pharmacological control of angiogenetic processes may be worth considered.
Moreover, here for the first time we use a specific AS-ODN, designed against rMCP-5, which has represented a useful tool to investigate the effective contribute of this chymase in modulate granuloma-dependent angiogenesis in rats. Since this AS-ODN acts by reducing, through an antisense mechanism, rMCP-5 mRNA levels, we propose that this AS-ODN may be of considerable value for controlling angiogenesis-related pathological states. In any case, despite the fact that a number of potential limitations for antisense technology have been reported (Stein, 1995; Wagner, 1995), this approach to novel drug discovery remains very attractive for the high specificity of AS-ODN in their inhibitory effects against the molecular target.
Acknowledgments
We are grateful to Dr Pasquale Maffia for his help in performing histology. This work was supported by ‘Cofinanziamento MURST'.
Abbreviations
- AS-ODN
antisense oligonucleotide
- bFGF
basic fibroblast growth factor
- PBS
phosphate-buffered saline
- rMCP-5
rat mast cell protease-5
- RT–PCR
reverse transcriptase polymerase chain reaction
- S-ODN
sense oligonucleotide
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
References
- BREIER G., ALBRECHT U., STERRER S., RISAU W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:524–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- BROWN J.F., CHAFEE K.A., TEPPERMAN B.L. Role of mast cells, neutrophils and nitric oxide in endotoxin-induced damage to the neonatal rat colon. Br. J. Pharmacol. 1998;123:31–38. doi: 10.1038/sj.bjp.0701576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANDRASEKHARAN U.M., SANKER S., GLYNIAS M.Y., KARNIK S.S., HUSAIN A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271:502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., ALAM C.A.S., APPLETON I., BROWN J.R., SEED M., WILLOUGHBY D.A. The pharmacological modulation of angiogenesis in chronic granulomatous inflammation. J. Pharmacol. Exp. Ther. 1995;274:1463–1471. [PubMed] [Google Scholar]
- COLVILLE-NASH P.R, SCOTT D.L. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann. Reheum. Dis. 1992;51:919–925. doi: 10.1136/ard.51.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOKE S.T. Progress toward oligonucleotide therapeutics: pharmacodynamic properties. FASEB J. 1993;7:533–539. doi: 10.1096/fasebj.7.6.7682523. [DOI] [PubMed] [Google Scholar]
- CRUM R., SZABO S., FOLKMAN J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230:1375–1378. doi: 10.1126/science.2416056. [DOI] [PubMed] [Google Scholar]
- DI ROSA M., GIROUD J.P., WILLOUGHBY D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- ESPOSITO G., LIGRESTI A., IZZO A.A., BISOGNO T., RUVO M., DI ROSA M., DI MARZO V., IUVONE T. The endocannabinoid system protects rat glioma cells against HIV-1 Tat protein-induced cytotoxicity. Mechanism and regulation. J. Biol. Chem. 2002;277:50348–50354. doi: 10.1074/jbc.M207170200. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- FUKAMI H., OHUNISHI H., MIYAZAKI M. Chymase: its pathophysiological roles and inhibitors. Curr. Pharm Des. 1998;4:439–453. [PubMed] [Google Scholar]
- GALLI S.J. Mast cells and basophils. Curr. Opin. Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- GALLI S.J., WEDEMEYER J., TSAI M. Analyzing the roles of mast cells and basophils in host defense and other biological responses. Int. J. Hematol. 2002;75:363–369. doi: 10.1007/BF02982125. [DOI] [PubMed] [Google Scholar]
- GILLES S., ZAHLER S., WELSCH U., SOMMERHOFF C.P., BECKER B.F. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc. Res. 2003;60:608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- GORDON J.R., GALLI S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- GRANT S.M., GOA K.L., FITTON A., SORKIN E.M. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drug. 1990;40:412–448. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- GRUTZKAU A., KRUGER-KRASAGAKES S., BAUMEISTER H., SCHWARTZ C., KOGEL H., WELKER P., LIPPERT U., HENZ B.M., MOLLER A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol. Biol. Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE S., WALLS A.F. The induction of a prolonged increase in microvascular permeability by human mast cell chymase Eur. J. Pharmacol. 1998;352:91–98. doi: 10.1016/s0014-2999(98)00343-4. [DOI] [PubMed] [Google Scholar]
- HORI Y., HU D.E., YASUI K., SMITHER R.L., GRESHAM G.A., FAN T.P. Differential effects of angiostatic steroids and dexamethasone on angiogenesis and cytokine levels in rat sponge implants. Br. J. Pharmacol. 1996;118:1584–1591. doi: 10.1111/j.1476-5381.1996.tb15578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDE H., ITOH H., TOMITA M., MURAKUMO Y., KOBAYASHI H., MARUYAMA H., OSADA Y., NAWA Y. Cloning of the cDNA encoding a novel rat mast-cell proteinase, rMCP-3, and its expression in comparison with other rat mast-cell proteinases. Biochem. J. 1995;311:675–680. doi: 10.1042/bj3110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRANI A.A., SCHECHTER N.M., CRAIG S.S., DE BLOIS G., SCHWARTZ L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUVONE T., CARNUCCIO R., DI ROSA M. Modulation of granuloma formation by endogenous nitric oxide. Eur. J. Pharmacol. 1994;265:89–92. doi: 10.1016/0014-2999(94)90227-5. [DOI] [PubMed] [Google Scholar]
- IUVONE T., VAN DEN BOSSCHE R., D'ACQUISTO F., CARNUCCIO R., HERMAN A.G. Evidence that mast cell degranulation, histamine and tumour necrosis factor alpha release occur in LPS-induced plasma leakage in rat skin. Br. J. Pharmacol. 1999;128:700–704. doi: 10.1038/sj.bjp.0702828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON J.R., SEED M.P., KIRCHER C.H., WILLOUGHBY D.A., WINKLER J.D. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- JOHNSTON B., BURNS A.R., KUBES P. A role for mast cells in the development of adjuvant-induced vasculitis and arthritis. Am. J. Pathol. 1998;152:555–613. [PMC free article] [PubMed] [Google Scholar]
- KARLSON U., PEJLER G., FRÖMAN G., HELLMAN L. Rat mast cell protease 4 is a beta-chymase with unusually stringent substrate recognition profile. J. Biol. Chem. 2002;277:18579–18585. doi: 10.1074/jbc.M110356200. [DOI] [PubMed] [Google Scholar]
- KATADA J., MURAMATSU M., HAYASHI I., TSUTSUMI M., KONISHI Y., MAJIMA M. Significance of vascular endothelial cell growth factor upregulation mediated via a chymase-angiotensin-dependent pathway during angiogenesis in hamster sponge granulomas. J. Pharmacol. Exp. Ther. 2002;302:949–956. doi: 10.1124/jpet.102.034231. [DOI] [PubMed] [Google Scholar]
- LEAL-BERUMEN I., CONLON P., MARSHALL J.S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J. Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- LEES M., TAYLOR D.J., WOOLLEY D.E. Mast cells proteinases activate precursor forms of collagenase and stromelysin, but not gelatinase A and B. Eur. J. Biochem. 1994;223:171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- LUTZELSCHWAB C., PEJLER G., AVESKOGH M., HELLMAN L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J. Exp. Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN U., BAGGIOLINI M. Dissociation between the anti-anaphylactic and the anti-histaminic actions of ketotifen Naunyn-Schmied. Arch. Pharmacol. 1981;316:186–189. doi: 10.1007/BF00505315. [DOI] [PubMed] [Google Scholar]
- MARTIN U., ROMER D. The pharmacological properties of a new, orally active antianaphylactic compound: ketotifen, a benzocycloheptathiophene. Arzneim. Forsch./Drug Res. 1978;28:770–782. [PubMed] [Google Scholar]
- MEININGER C.J., ZETTER B.R. Mast cells and angiogenesis. Sem. Cancer Biol. 1995;3:73–79. [PubMed] [Google Scholar]
- MICAN J.M., METCALFE D.D. Arthritis and mast cell activation. J. Allergy Clin. Immunol. 1990;86:677–683. doi: 10.1016/s0091-6749(05)80240-4. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., KATADA J., HATTORI M., HAYASHI I., MAJIMA M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur. J. Pharmacol. 2000a;402:181–191. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., KATADA J., HAYASHI I., MAJIMA M. Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J. Biol. Chem. 2000b;275:5545–5552. doi: 10.1074/jbc.275.8.5545. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., YAMADA M., TAKAI S., MIYAZAKI M. Suppression of basic fibroblast growth factor-induced angiogenesis by a specific chymase inhibitor, BCEAB, through the chymase-angiotensin-dependent pathway in hamster sponge granulomas. Br. J. Pharmacol. 2003;137:554–560. doi: 10.1038/sj.bjp.0704893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKUSAN K., SARKAR K., TUREK Z., WICKER P. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ. Res. 1990;66:511–516. doi: 10.1161/01.res.66.2.511. [DOI] [PubMed] [Google Scholar]
- REILLY C.F., TEWKSBURY D.A., SCHECHTER N.M., TRAVIS J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J. Biol. Chem. 1982;257:8619–8622. [PubMed] [Google Scholar]
- RHODIN J.A.G., FUJITA H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J. Submicrosc. Cytol. Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- RIBATTI D., VACCA A., NICO B., CRIVELLATO E., RONCALI L., DAMMACCO F.The role of mast cells in tumour angiogenesis Br. J. Haematol. 20011151–9.(Review) [DOI] [PubMed] [Google Scholar]
- RISAU W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- SOLIVAN S., SELWOOD T., WANG Z.M., SCHECHTER N.M. Evidence for diversity of substrate specificity among members of the chymase family of serine proteases. FEBS Lett. 2002;512:133–138. doi: 10.1016/s0014-5793(02)02242-1. [DOI] [PubMed] [Google Scholar]
- SORBO J., JAKOBSSON A., NORRBY K. Mast-cell histamine is angiogenic through receptors for histamine1 and histamine2. Int. J. Exp. Pathol. 1994;75:43–50. [PMC free article] [PubMed] [Google Scholar]
- STEIN C.A. Does antisense exist. Nature Med. 1995;1:1119–1121. doi: 10.1038/nm1195-1119. [DOI] [PubMed] [Google Scholar]
- SUEISHI K., YONEMITSU Y., NAKAGAWA K., KANEDA Y., KUMAMOTO M., NAKASHIMA Y. Atherosclerosis and angiogenesis. Its pathophysiological significance in humans as well as in an animal model induced by the gene transfer of vascular endothelial growth factor. Ann. N.Y. Acad. Sci. 1997;811:311–322. doi: 10.1111/j.1749-6632.1997.tb52011.x. [DOI] [PubMed] [Google Scholar]
- WAGNER R.W. The state of the art in antisense research. Nat. Med. 1995;1:1116–1118. doi: 10.1038/nm1195-1116. [DOI] [PubMed] [Google Scholar]