Abstract
The disposition kinetics of [3H]taurocholate ([3H]TC) in perfused normal and cholestatic rat livers were studied using the multiple indicator dilution technique and several physiologically based pharmacokinetic models.
The serum biochemistry levels, the outflow profiles and biliary recovery of [3H]TC were measured in three experimental groups: (i) control; (ii) 17α-ethynylestradiol (EE)-treated (low dose); and (iii) EE-treated (high dose) rats. EE treatment caused cholestasis in a dose-dependent manner.
A hepatobiliary TC transport model, which recognizes capillary mixing, active cellular uptake, and active efflux into bile and plasma described the disposition of [3H]TC in the normal and cholestatic livers better than the other pharmacokinetic models.
An estimated five- and 18-fold decrease in biliary elimination rate constant, 1.7- and 2.7-fold increase in hepatocyte to plasma efflux rate constant, and 1.8- and 2.8-fold decrease in [3H]TC biliary recovery ratio was found in moderate and severe cholestasis, respectively, relative to normal.
There were good correlations between the predicted and observed pharmacokinetic parameters of [3H]TC based on liver pathophysiology (e.g. serum bilirubin level and biliary excretion of [3H]TC). In conclusion, these results show that altered hepatic TC pharmacokinetics in cholestatic rat livers can be correlated with the relevant changes in liver pathophysiology in cholestasis.
Keywords: Taurocholate, 17α-ethynylestradiol, cholestasis, Bsep, Ntcp, Oatp
Introduction
Hepatic cholestasis is the result of defective bile salt transport or physical obstruction of the bile duct and manifests itself in clinical, biochemical, and histological forms. Estrogen is known to be an important inducer of intrahepatic cholestasis in pregnancy, which is characterized by biochemical cholestasis and dermatological symptoms (Kullak-Ublick & Meier, 2000b). Ethynylestradiol administration is known to cause hepatic cholestasis in experimental animals (Gumucio & Valdivieso, 1971; Bouchard et al., 1993; Jacquemin et al., 1993; Crocenzi et al., 2001; 2003). The underlying mechanism is still not fully elucidated, but D-ring glucuronide conjugates such as estradiol 17β-glucuronide have been implicated as causative agents (Meyers et al., 1980; Vore et al., 1983). It has been suggested that estrogen glucuronides impair bile salt excretion (Adinolfi et al., 1984), increase the permeability of tight junctions (Kan et al., 1989), decrease bile flow (Meyers et al., 1980; Vore et al., 1983) and sinusoidal membrane fluidity (Alvaro et al., 1991). In cholestasis transport of bile salts such as taurocholate (TC) is affected. The ATP-dependent transport of TC in the plasma membrane vesicles of EE-treated rats is decreased due to a reduction of the bile salt export pump (Bsep) on the protein level by approximately 50% (Bossard et al., 1993; Lee et al., 2000).
The active transport of TC across the apical (canalicular) hepatocyte membrane has been reported as the rate-limiting step in the overall blood-to-bile transfer process (Crocenzi et al., 2004). The active extraction of TC across the basolateral (sinusoidal) hepatocyte membrane has also been proposed to initiate the process of canalicular membrane insertion of Bsep (Misra et al., 1999; 2003; Kullak-Ublick et al., 2004). TC-mediated bile acid secretion and recruitment of ABC (ATP binding cassette) transporters are phosphatidylinositol 3-kinase (PI3K) dependent and require an intact microtubular apparatus (Misra et al., 2003).
In this study, we examined the hepatic pharmacokinetics of [3H]taurocholate ([3H]TC) in normal and cholestatic perfused rat livers using the multiple indicator dilution technique. Our goal was to define the effect of cholestasis on TC hepatic pharmacokinetics and to examine which pharmacokinetic parameters related to the severity of the cholestasis. Animal groups used were EE low-dose-treated group, EE high-dose-treated group, and control (vehicle treatment only) group. As in a previous study, a physiologically based hepatic disposition model consisting of a mixture of two inverse Gaussian density functions and a barrier-limited and space-distributed liver model were used to fit the reference marker data and provide estimates of the sinusoidal space and cellular volumes (Hung et al., 2002b). The [3H]TC data were then evaluated by fitting the outflow time profiles and the markers with various physiologically based pharmacokinetic models. A redefined well-mixed model, hepatobiliary TC transport model was found to optimally describe the data and to provide estimates of the pharmacokinetic parameters for hepatocellular influx (kin), efflux (kout), and biliary elimination (kbe) rate constants for [3H]TC. The parameters were then related to changes in liver pathophysiology (serum bilirubin level and TC biliary recovery).
Methods
Chemicals
All chemicals were purchased from Sigma Chemical Company (Castle Hill, NSW, Australia). [3H]TC, [3H]water, and [14C]sucrose were purchased from New England Nuclear (Boston, MA, U.S.A.).
Cholestatic rat model
All animal studies were approved by the Animal Ethics Committee of the University of Queensland, Queensland, Australia. The cholestatic rat model was established using male Wistar rats of approximately 300 g body weight. The procedure is a modification of the protocol given by Crocenzi et al. (2001). Rats were housed in groups of six each (three groups) and maintained on a standard diet and water ad libitum. EE was dissolved in a propylene glycol vehicle. The animals were injected with EE solution (low-dose-treated group: 2.5 mg kg−1, daily; high-dose-treated group: 5 mg kg−1, daily) or injected with propylene glycol only (control group) subcutaneously on 10 consecutive days.
Serum biochemistry determination
The impairment of liver function was assessed by taking blood samples from the tail vein before (day 0) and after (day 10) EE treatment. Serum bilirubin, alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured on a Hitachi 747 analyzer (Tokyo, Japan).
In situ rat liver perfusions
The in situ perfused rat liver preparation used in this study has been described previously (Cheung et al., 1996). Briefly, untreated and EE-treated male Wistar rats (300–350 g body weight) were anesthetized by an interperitoneal injection of xylazine 10 mg kg−1 (Bayer, Australia) and ketamin-hydrochloride 80 mg kg−1 (Parnell Laboratories, Australia). Following laparotomy animals were heparinized (heparin sodium, David Bull Laboratories, Australia, 200 U) via the inferior vena cava. The bile duct was cannulated with PE10 (Clay Adams, NJ, U.S.A.). The portal vein was then cannulated using an intravenous catheter and the liver was perfused via this cannula with MOPS (4-morpholinepropanesulfonic acid)-buffered Ringer solution (pH 7.4), which contains 2% bovine serum albumin and 15% (v v−1) prewashed canine red blood cells (School of Veterinary Sciences, The University of Queensland, Brisbane, Australia) and was oxygenated using a silastic tubing lung ventilated with oxygen. The perfusion system used was nonrecirculating and employed a peristaltic pump (Cole-Parmer, IL, U.S.A.). Following the initiation of liver perfusion, animals were killed by thoracotomy and the thoracic inferior vena cava was cannulated using PE 240 (Clay Adams). Oxygen consumption, bile flow, perfusion pressure and macroscopic appearance were used to assess liver viability. All parameters (see Results) were within normal values for the isolated perfused rat liver preparation (Cheung et al., 1996).
Bolus studies
Livers were perfused at a rate of 15 ml min−1. After a 10-min perfusion stabilization period, aliquots (50 μl) of buffer solution containing radio-labelled solutes or Evans Blue were administered through the portal vein cannula. Each liver received a bolus injection containing [3H]TC (3 × 106 dpm), Evans Blue dye (3 mg ml−1) and [14C]sucrose (1.5 × 106 dpm). The total perfusion time for each liver was less than 1 h. Outflow samples were collected using a fraction collector over 3½ min. These samples were centrifuged and aliquots (100 μl) of the supernatant that did not include red blood cells were taken for scintillation counting using a MINAXI beta TRI-CARB 4000 series liquid scintillation counter (Packard Instruments Co., U.S.A.). Aliquots of 100 μl were also removed from the outflow samples for absorption spectrophotometric analysis of Evans Blue dye at 620 nm using a Spectracount plate counter (Packard).
[3H]TC recovery in bile
Bile was collected continuously for 20 min after bolus injection using PE-10 tubing and quantified by weight. Aliquots (100 μl) of bile were taken for scintillation counting. The ratio of [3H]TC recovery in bile was determined from the dose injected, the amount of bile produced and the radioactivity of [3H]TC in the bile sample.
Fraction unbound of [3H]TC
Perfusate binding experiments were carried out in 2% bovine serum albumin MOPS-buffered Ringer solution (pH 7.4), containing 15% (v v−1) prewashed canine red blood cells, and incubated at 37°C in a water bath for 30 min. The unbound fraction of [3H]TC in perfusate plasma was determined using an ultrafiltration method. A 1.0 ml aliquot sample (in triplicate) was placed in a Millipore Microcon YM-30 ultrafiltration device (30,000 molecular wt CO; Millipore, Bedford, MA, U.S.A.) and centrifuged at 3000 × g for 10 min. The ultrafiltrate was then assayed by scintillation counting. The fraction unbound in perfusate (fup) was determined as the ratio of the free dpm to total dpm of [3H]TC. A similar technique was employed to measure the fraction of [3H]TC unbound in the cell (fuc) as described previously (Hung et al., 2002a).
Data analysis
A mixture of two inverse Gaussian density functions with correction for catheter effects was used to describe the outflow concentration–time profiles of Evans Blue dye-labelled albumin and [14C]sucrose. A detailed description of the mathematical theory and modelling methods has been reported previously (Varin & Huet, 1985; Hung et al., 2001; 2002b).
The outflow concentrations for [3H]TC are presented as outflow fraction/ml. The resulting outflow concentration–time profiles were analyzed using one of the following physiologically based two-phase organ pharmacokinetic models:
Hepatobiliary TC transport model
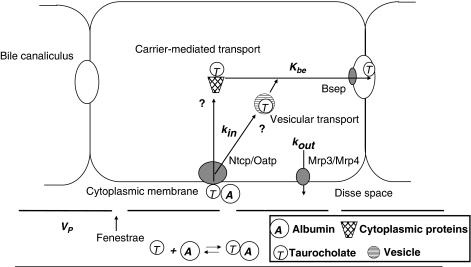
This model is a reparameterized form of the well-mixed model, which assumes quasi-instantaneous intracellular distribution equilibrium, that is, a cellular space that is well-mixed perpendicular to flow direction (Weiss et al., 2000). With respect to the assumptions of cellular distribution kinetics this model is identical with the two-compartment dispersion model (Roberts et al., 1988; Evans et al., 1993). Here the model (illustrated in Figure 1) highlights the key factors, which influence TC hepatocellular transmembrane exchange and biliary clearance.
Figure 1.
Schematic overview of taurocholate (TC) hepatocellular transmembrane exchange and biliary intrinsic clearance in the hepatobiliary TC transport model. TC uptake via the Na+-TC cotransporting polypeptide, Ntcp, or organic anion-transporting polypeptide, Oatp, and efflux via the multidrug resistance protein 3 or 4, Mrp3 or Mrp4 with influx and efflux rate constants kin and kout, respectively. TC biliary export is mediated by the bile salt export pump, Bsep with biliary elimination rate constant kbe. The intracellular trafficking of bile acids is presently not well understood but two possible mechanisms have been proposed: (1) vesicular transport; (2) carrier-mediated transport by cytoplasmic proteins. Nevertheless, these trafficking effects (e.g. binding and diffusion) do not take into account for modelling due to the consideration that uptake and excretion are the rate determinants in this model.
The model recognizes TC hepatic transport by (1) influx from the plasma across the cytoplasmic membrane via the Na+-TC cotransporting polypeptide (Ntcp) or organic anion-transporting polypeptide (Oatp) into the hepatocytes with influx rate constant kin; (2) efflux from the hepatocytes into the plasma via the multidrug resistance protein 3 or 4 (Mrp3 or Mrp4); also, a small fraction of bile salts can efflux from the hepatocytes into the plasma by cholehepatic shunting (Meier & Stieger, 2002) with efflux rate constant kout; (3) excretion from the hepatocytes into the bile via the canalicular membrane mediated by the Bsep with biliary elimination rate constant kbe. As the majority of [3H]TC is excreted into the bile canaliculus in unchanged form (Akita et al., 2001), the irreversible disappearance of TC into the bile from bolus dosing is therefore regarded as the elimination clearance in this model.
The Laplace domain function for the pharmacokinetics of TC f̂y(s) in the cell can be described as
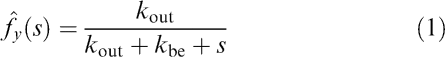 |
where s is the Laplace variable.
The Laplace domain function (transit time density function) f̂(s) for TC extraction across the liver can then be derived in terms of the transit time density of nonpermeating reference molecules f̂p(s), in this study Evans Blue dye-labelled albumin f̂alb(s), and the density function of successive sojourn times f̂(s) of the drug molecules into the cellular space
Hepatobiliary TC transport model with slow hepatocellular binding
This model assumes rapid intrahepatocellular diffusion but a slow binding of TC to immobile intrahepatocellular sites, with the consequence that the cytoplasmic equilibration process is determined by the binding and unbinding rate constants kon and koff following an instantaneous distribution of unbound TC in the hepatocellular volume (Hung et al., 2001).
Hepatobiliary TC transport model with hepatocellular transport being defined by slow diffusion of bound TC
This model assumes that diffusion in hepatocytes is a rate-limiting function and only the intrahepatocellular bound TC is contributing significantly to diffusion (Luxon & Weisiger, 1992).
A nonparametric estimate of the hepatic extraction ratio, retention of [3H]TC in the liver, and normalized variance was determined from the outflow concentration versus time profile as described previously (Hung et al., 2001).
Statistical analysis
All data are presented as mean±standard deviation unless stated otherwise. Stepwise regression analysis was performed using the program SPSS 10.1 for Windows. Statistical analysis was performed using Tukey's post hoc test or regression analysis where appropriate. A P<0.05 was taken as significant. The SCIENTIST model selection criterion (MSC), a modified Akaike Information Criterion (normalized to the number of data points), was used to compare the models with regard to ‘goodness of fit'. The most appropriate model – from a purely statistical point of view – is that with the largest MSC.
Results
Table 1 lists the experimental parameters of the perfusion studies in the three experimental animal groups. These parameters were comparable to those reported previously (Cheung et al., 1996; Hung et al., 2002b; 2003). The control group showed a significantly smaller liver weight compared to EE-treated low- and high-dose-treated rats. There were no significant differences of body weight between the EE-treated and control rats. The EE high-dose-treated group had a significantly lower bile flow than the low-dose group and the control group. There were no significant differences between the three animal groups with regard to perfusion rate, perfusion pressure, and liver oxygen consumption during the perfusion studies.
Table 1.
Comparison of liver perfusion parameters in the various animal groups (mean±s.d., n=6)
| Parameter | Animal group | Pa | ||
|---|---|---|---|---|
| Control | EE-treated (low dose)b | EE-treated (high dose) | ||
| Body weight (g) | 340±9.79 | 340±11.2 | 337±14.8 | — |
| Liver weight (g) | 10.3±1.01 | 12.4±1.23 | 12.8±1.55 | α*, γ* |
| Perfusion rate (ml min−1 g−1liver) | 1.31±0.05 | 1.26±0.07 | 1.29±0.09 | — |
| Perfusion pressure (cm H2O) | 12.4±1.12 | 12.8±1.69 | 13.1±1.99 | — |
| Bile flow (μl min−1 g−1 liver) | 1.47±0.25 | 0.91±0.23 | 0.51±0.27 | α‡, β*, γ† |
| O2 consumption (μmol min−1 g−1 liver) | 1.44±0.21 | 1.37±0.25 | 1.29±0.34 | — |
Tukey post hoc: only significant differences shown (α: control cf EE-treated (high dose); β: EE-treated (low dose) cf EE-treated (high dose); γ: control cf EE-treated (low dose);
P<0.05;
P<0.01;
P<0.001).
17α-ethynylestradiol.
Table 2 lists the serum biochemical parameters in the three animal groups. The EE high-dose-treated group had significantly higher of serum biochemical parameter level compared to the control group. However, the EE high-dose-treated group showed significant higher bilirubin and ALP levels than the EE low-dose-treated group but no significant differences for AST, and ALT levels.
Table 2.
Comparison of serum biochemical parameters in the various animal groups (mean±s.d., n=6)
| Parameter | Animal group | Pa | ||
|---|---|---|---|---|
| Control | EE-treated (low dose)b | EE-treated (high dose) | ||
| Bilirubin (μmol l−1) | 0.67±0.52 | 1.33±0.52 | 3.33±1.03 | α‡, β†, γ* |
| ALP (U l−1)c | 250±16.7 | 308±26.2 | 352±30.5 | α‡, β*, γ‡ |
| AST (U l−1)d | 55.8±6.77 | 59.7±5.54 | 66.5±5.41 | α* |
| ALT (U l−1)e | 54.2±6.34 | 57.2±8.82 | 65.2±5.61 | α† |
Tukey post hoc: only significant differences shown (α: control cf EE-treated (high dose); β: EE-treated (low dose) cf EE-treated (high dose); γ: control cf EE-treated (low dose);
P<0.05;
P<0.01;
P<0.001).
17α-ethynylestradiol.
Alkaline phosphatase (ALP).
Aspartate aminotransferase (AST).
Alanine aminotransferase (ALT).
The results of the study determining fraction unbound in the perfusate showed a high perfusate binding of [3H]TC (fup=0.15) and the respective results of a study to determine the cellular fraction unbound of [3H]TC showed that the EE high-dose-treated rats had higher fuc values (lower tissue binding) compared to the low-dose-treated and control rats (0.34, 0.29, and 0.26, respectively).
Table 3 shows the results of nonparametric moments analysis for [3H]TC in the three animal groups. The EE high-dose-treated group had significantly smaller hepatic extraction ratio values and larger retention of [3H]TC in the liver than the low-dose group and the control group. However, the normalized variance for [3H]TC did not appear to be related to EE treatment. Also included in Table 3 are the results of [3H]TC biliary recovery for a period of 20 min following bolus-1 injection in the experimental animal groups. The EE high-dose-treated group had a significantly lower [3H]TC biliary recovery than the low-dose group and the control group.
Table 3.
Comparison of nonparametric moments and [3H]taurocholate ([3H]TC) biliary recovery ratio in the various animal groups (mean±s.d., n=6)
| Moment results | Animal group | Pa | ||
|---|---|---|---|---|
| Control | EE-treated (low dose) | EE-treated (high dose) | ||
| Hepatic extraction ratio | 0.96±0.02 | 0.91±0.03 | 0.87±0.02 | α‡, β*, γ† |
| Retention of [3H]TC in the liver (s) | 9.35±1.30 | 12.7±1.97 | 16.8±2.10 | α‡, β†, γ† |
| Normalized variance | 1.54±0.32 | 1.51±0.38 | 1.44±0.38 | — |
| [3H]TC biliary recovery ratiob | 0.45±0.09 | 0.25±0.06 | 0.16±0.06 | α‡, β†, γ* |
Tukey post hoc test: only significant differences shown (α: control cf EE-treated (high dose); β: EE-treated (low dose) cf EE-treated (high dose); γ: control cf EE-treated (low dose);
P<0.05;
P<0.01;
P<0.001).
Determined from the dose injected, the amount of bile produced and a collection period of 20 min following bolus injection.
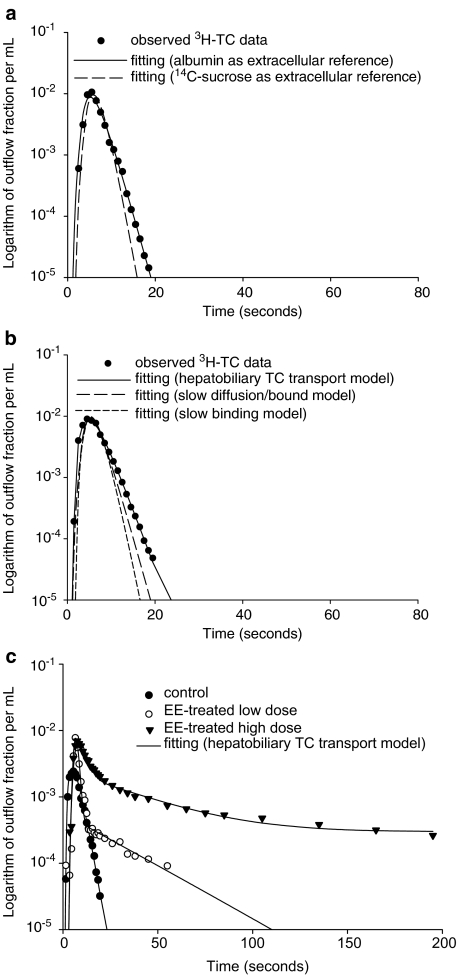
Figure 2a shows the fits for [3H]TC using either albumin or [14C]sucrose as the sinusoidal space reference. It is evident that the fitting of [3H]TC is best described using albumin as a reference, consistent with the high perfusion medium (2% bovine serum albumin) binding. It is also evident from Figure 2b that the outflow–time profiles for [3H]TC in control rats, using albumin as the sinusoidal space reference, is better fitted by the hepatobiliary TC transport model than the slow binding and slow diffusion/bound models. Application of the hepatobiliary TC transport model (equations 1 and 2) to describe typical outflow concentration–time profiles for [3H]TC in the three animal groups used is shown in Figure 2c (data weighted, 1/yobs). The EE high-dose-treated group had the largest area under the curve (lowest hepatic extraction) of the [3H]TC outflow curve of all animal groups.
Figure 2.
(a) Comparison of the data fitting results using Evans Blue (EB) labelled albumin or [14C]sucrose as the extracellular reference for [3H]TC. The lines represent the fits of the profiles to the hepatobiliary TC transport model. (b) Comparison of the data fitting results for [3H]TC using three physiologically based pharmacokinetic models: a hepatobiliary TC transport model, a slow binding model, and a slow diffusion/bound model. Albumin was used as the extracellular reference for all models. (c) Typical data fitting (data weighted, 1/yobs) results for [3H]TC in the various animal groups in the regressions using the hepatobiliary TC transport model.
Table 4 shows the kinetic parameters derived from [3H]TC hepatic outflow data using the hepatobiliary TC transport model. The kbe value of the EE high-dose-treated group is significantly smaller than that of the low-dose-treated and the control group, but there is no significant difference in kin values between all three animal groups. In contrast, the EE high-dose-treated rats showed significantly larger kout values compared to the low-dose-treated and control rats.
Table 4.
Comparison of kinetic parameters derived from the hepatobiliary taurocholate transport model fitting for [3H]TC in the various animal groups (mean±s.d., n=6)
| Kinetic parameters | Animal group | Pa | ||
|---|---|---|---|---|
| Control | EE-treated (low dose) | EE-treated (high dose) | ||
| kin (s−1)b | 1.41±0.35 | 1.21±0.39 | 1.15±0.29 | — |
| kout (s−1)c | 0.03±0.01 | 0.05±0.01 | 0.08±0.01 | α‡, β‡, γ† |
| kbe (s−1)d | 0.36±0.15 | 0.07±0.02 | 0.02±0.01 | α‡, β*, γ‡ |
Tukey post hoc test: only significant differences shown (α: control cf EE-treated (high dose); β: EE-treated (low dose) cf EE-treated (high dose); γ: control cf EE-treated (low dose);
P<0.05;
P<0.01;
P<0.001).
Plasma to hepatocyte influx rate constant.
Hepatocyte to plasma efflux rate constant.
Biliary elimination rate constant.
Discussion
Estrogens are a well-known cause of intrahepatic cholestasis, induced by pregnancy, administration of oral contraceptives, or postmenopausal replacement therapy (Schreiber & Simon, 1983). EE has been extensively used to induce experimental cholestasis in animals (Crocenzi et al., 2001; 2003). In this study EE treatment caused cholestasis in male rats in a dose-dependent manner. The EE high-dose-treated group had the highest serum bilirubin, ALP, AST and ALT levels (Table 2), lowest bile flow during perfusion (Table 1), and [3H]TC biliary recovery (Table 3) of all experimental groups.
Simon and co-workers have previously reported that endogenous estrogens alter the number of sinusoidal bile acid transporters and/or decrease membrane lipid fluidity. The initial sodium-dependent uptake of [3H]TC was 75% greater in hepatocytes from males than from either intact or oophorectomized females rats (Simon et al., 1999). Therefore, the majority of estrogen-induced cholestasis studies cited in the literature made use of male rats as the standard animal model (Crocenzi et al., 2001; Sanchez Pozzi et al., 2003).
In general, the cytoplasmic distribution of a solute after crossing the cell membrane can be described using at least three different modelling approaches. The well-mixed model (barrier-limited tissue distribution model) is traditionally used in most organ models of solute disposition such as the two-compartment dispersion model (Roberts et al., 1988; Evans et al., 1993), being based on an assumed quasi-instantaneous tissue equilibration due to rapid diffusion and binding. The slow diffusion/bound model (Luxon & Weisiger, 1992) assumes a diffusion limited transport of solute in the cell due to an instantaneous binding to mobile carrier proteins so that intrahepatic transport is defined by diffusion of the binding proteins. The third model, the slow binding model (Weiss et al., 2000), assumes a slow binding to cellular sites after instantaneous distribution throughout the cytosol. In the present study, we found that a hepatobiliary TC transport model (a reparameterized form of well-mixed model) best described the hepatic pharmacokinetics of TC in normal and cholestatic livers (Figure 2). The model used Evans Blue-labelled bovine serum albumin as the sinusoidal space reference solute, recognizing that TC is highly bound to bovine serum albumin. The fraction of TC unbound in the perfusate found in this work (2% albumin, 15% v v−1 prewashed canine RBCs) of 0.15 is a value consistent with the reported binding of TC to perfusate albumin (fu: 0.15–0.16) (Smallwood et al., 1988) and the lack of bile acid binding to red blood cell ‘ghosts'(Accatino & Simon, 1976). The model also assumed that the uptake and excretion of TC are the main rate determinants for TC transport and that intracellular trafficking by intracellular diffusion of free and bound TC is sufficiently rapid so that intracellular binding and diffusion are not rate limiting. It is possible that the known TC protein binding facilitated transport (Blitzer & Lyons, 1985) may result in apparent ‘well-stirred' model behavior as noted by Morgan et al. (1990). The poorer fitting of [3H]TC data with both the slow diffusion/bound and the slow binding models compared to the ‘well-stirred' hepatobiliary TC transport model was seen as supportive of the assumption that the intrahepatocellular transport processes were not rate limiting for TC.
Further, the existence of at least two possible bile salt transport mechanisms was recognized, namely: (1) vesicular transport and (2) carrier-mediated transport by cytoplasmic proteins (glutathione S-transferases, liver fatty acid binding protein, and dehydrogenases) (Stolz et al., 1989; Dietrich et al., 1995; Takikawa et al., 1996; Agellon & Torchia, 2000). Given that the majority of TC is excreted into the bile canaliculus in unchanged form (Akita et al., 2001), the effect of cholestasis on TC metabolism was not quantified in this study.
The EE high-dose-treated rats showed significantly larger kout values compared to the low-dose-treated and control rats. It is possible that the increase in kout observed with EE treatment is at least in part due to the significant EE-induced increase in fuc and to a potential upregulation of the efflux transporter. An upregulation of Mrp3 has been reported in obstructive cholestasis (Donner & Keppler, 2001).
As shown in Figure 2c, EE treatment affected [3H]TC outflow concentration time profiles in rat livers in a dose-dependent manner. The high-dose-treated group has the largest area under the curve (Figure 2c). The outflow profiles can be characterized as having three different phases: a rapid increase, a fast decrease and a slow decrease (tail phase). The patterns of slower decline of fast down phase and larger slow down phase in the two cholestatic groups (EE high- and low-dose-treated groups) are consistent with smaller hepatic extraction ratio values and larger hepatic retention values of [3H]TC (Table 3). These results are in contrast to the multiple factors we have reported as responsible for the decreased hepatic extraction ratio of [3H]TC in carbon tetrachloride-induced fibrotic/cirrhotic animals (Hung et al., 2002b). The architectural changes (collagenization, capillarization, defenestration, and nodularity) in fibrotic/cirrhotic livers and a reduction in cellular active transport capacity all contribute to the reduced uptake of TC under these conditions (Tsujii et al., 1991; de Caestecker et al., 1995). The increased retention of [3H]TC in the liver after EE treatment (Table 3) is mainly determined by a reduction in biliary clearance. However, a small contribution may be due to the increased liver mass following EE treatment. A larger liver weight (Table 1), sinusoidal space (Table 4), and hepatocellular volume (Table 4) were found in both cholestatic groups, which represent a larger sinusoidal and cellular distribution volume for [3H]TC.
In general, hepatic extraction of solute is sensitive to changes in liver perfusate flow, permeability-surface area product (sinusoidal space), hepatocyte to plasma efflux, and intrinsic clearance (Siebert et al., 2004). In this work we found that the decreased hepatic extraction of TC results from both a decreased kbe and an increased kout with an unchanged kin (Table 4) in cholestasis. Simon et al. (1996) and Geier et al. (2003) have previously reported that estrogen-induced cholestasis results in a downregulation of all basolateral organic anion transporters in rats. Accordingly, the EE-treated rats should have smaller kin values than the control rats. Conversely, our results showed similar kin values for the EE-treated and the control rats. We were uncertain whether Ntcp or Oatps had been affected by EE as we have previously shown (using an identical methodology) that cirrhosis does downregulate these transporters (Hung et al., 2002b).
Under normal physiologic conditions, there is no appreciable intrahepatocellular bile acid build up in the liver due to efficient biliary exporting mechanisms. At the canalicular hepatocyte membrane, excretion of monovalent bile salts (e.g. TC) is mediated by the Bsep (Kullak-Ublick et al., 2004). The specific amount and function of Bsep in the canalicular membrane increases with higher concentrations of TC and cAMP in the hepatocyte (Kipp & Arias, 2000). Bsep is a susceptible target for inhibition by estrogen metabolites, which can cause retention of bile salts and consequently intrahepatic cholestasis (Kullak-Ublick et al., 2000c). Canalicular efflux of monovalent bile salts and divalent sulfated or glucuronidated bile salts is mediated by Bsep and Mrp2, respectively (Kullak-Ublick et al., 2004). The expression of both canalicular bile salt transporters is decreased in cholestasis (Simon et al., 1996; Green et al., 1997; Trauner, 1997; Beuers et al., 1999; Paulusma et al., 2000). Decreased Mrp2 expression may lead to compensatory increase in the expression of basolateral Mrp3 and Mrp1, which mediate the sinusoidal efflux of anionic conjugates, monovalent bile salts and divalent bile salt conjugates during cholestasis to avoid hepatotoxic injury (Kullak-Ublick et al., 2000a; 2004). Keppler and co-workers have recently reported that human MRP4 mediates bile salt transport across the basolateral membrane by cotransport with glutathione (GSH) and may function as an overflow pathway during impaired bile salt secretion across the canalicular membrane into bile (Rius et al., 2003). Zelcer et al. (2003) have also shown that cholestatic estrogens inhibited the MRP4-mediated transport of estradiol 17-β-D-glucuronide. In addition, some amounts of bile salts re-enter the plasma by another route, the cholehepatic shunting under cholestatic conditions (Meier & Stieger, 2002).
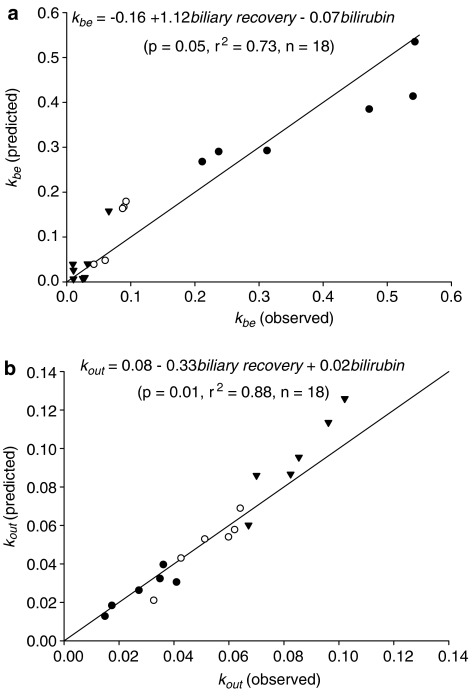
In this study, we estimated the severity of cholestasis by comparing (1) hepatocyte to plasma efflux rate constant; (2) biliary elimination constant; (3) TC biliary recovery; and (4) biochemical levels between control and EE-treated animals. An estimated five- and 18-fold decrease in kbe, 1.7- and 2.7-fold increase in kout, 1.8- and 2.8-fold decrease in TC biliary recovery, two and five times increase in serum bilirubin level, and 1.2 and 1.4 times increase in serum ALP level were found in moderate and severe cholestasis, respectively. Stepwise regression analysis showed that TC biliary recovery and serum bilirubin concentrations were the best predictors of kbe and kout. The regressions obtained are shown in Figure 3a, b. A high correlation between bilirubin and ALP (ALP=220+35.4 bilirubin; r2=0.93, n=18) precluded an examination of ALP as a determinant of kbe and kout. Given that kin was nearly unaffected by cholestasis (Table 4), these strong relationships are consistent with the notion that cholestasis reduced TC extraction by reducing biliary elimination and increasing hepatocellular to plasma efflux.
Figure 3.
Comparison of predicted and observed pharmacokinetic parameters of [3H]TC in the three animal groups. (a) biliary elimination rate constant (kbe); (b) hepatocyte to plasma efflux rate constant (kout).
The bile flow and TC biliary recovery ratio are reported in Table 1 and Table 3 represent a short experimental time frame (observation time 20 min) of TC hepatic disposition, and therefore must be viewed differently from the elimination prameters given in Table 4. These, the TC biliary elimination (kbe, model-derived kinetic parameter) represent a longer time frame (a theoretical prediction) of TC hepatic pharmacokinetics. The results show that EE-induced cholestasis has a higher impact on long-term kinetics (18-fold decrease in biliary elimination) than on relatively short term ones (three-fold decrease in both bile flow and TC biliary recovery ratio).
In conclusion, our results have shown that the hepatic disposition of TC can be directly related to the degree of cholestasis. The hepatobiliary TC transport model was found to describe the [3H]TC data in the isolated perfused normal and cholestatic rat livers best. The changes in hepatic extraction ratio in the cholestatic animal groups are consistent with a reduction in biliary elimination and an increased hepatocyte to plasma efflux of TC, this latter effect being possibly due to an increase in the hepatocellular fraction unbound of TC or a potential upregulation of the efflux transporter in cholestasis. Interestingly, there were no significant differences of plasma to hepatocyte influx constants between the three animal groups (regardless of EE treatment). There were good correlations between the predicted and observed pharmacokinetic parameters (biliary elimination and hepatocyte to plasma efflux constants) of TC based on TC biliary recovery and serum bilirubin levels, whereas no significant correlations between plasma to hepatocyte influx constant and liver pathophysiological results were found. This indicates that biliary elimination and hepatocyte to plasma efflux may be more important than plasma to hepatocyte influx in determining the hepatic disposition of TC in the cholestatic rat liver.
Acknowledgments
We acknowledge the support of the National Health and Medical Research Council of Australia and the Queensland and New South Wales Lions Kidney and Medical Research Foundation.
Abbreviations
- Bsep
bile salt export pump
- EE
17α-ethynylestradiol
- fuc
unbound fraction in the cell
- fup
unbound fraction in the perfusate
- kbe
biliary elimination rate constant
- kin
plasma to hepatocyte influx rate constant
- kout
hepatocyte to plasma efflux rate constant
- Mrp
multidrug resistance protein
- Ntcp
Na+-TC cotransporting polypeptide
- Oatp
organic anion-transporting polypeptide
- TC
taurocholate
References
- ACCATINO L., SIMON F.R. Identification and characterization of a bile acid receptor in isolated liver surface membranes. J. Clin. Invest. 1976;57:496–508. doi: 10.1172/JCI108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADINOLFI L.E., UTILI R., GAETA G.B., ABERNATHY C.O., ZIMMERMAN H.J. Cholestasis induced by estradiol-17 beta-D-glucuronide: mechanisms and prevention by sodium taurocholate. Hepatology. 1984;4:30–37. doi: 10.1002/hep.1840040106. [DOI] [PubMed] [Google Scholar]
- AGELLON L.B., TORCHIA E.C. Intracellular transport of bile acids. Biochim. Biophys. Acta. 2000;1486:198–209. doi: 10.1016/s1388-1981(00)00057-3. [DOI] [PubMed] [Google Scholar]
- AKITA H., SUZUKI H., ITO K., KINOSHITA S., SATO N., TAKIKAWA H., SUGIYAMA Y. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim. Biophys. Acta. 2001;1511:7–16. doi: 10.1016/s0005-2736(00)00355-2. [DOI] [PubMed] [Google Scholar]
- ALVARO D., ANGELICO M., CANTAFORA A., GAUDIO E., GANDIN C., SANTINI M.T., MASELLA R., CAPOCACCIA L. Improvement of estradiol 17 beta-D-glucuronide cholestasis by intravenous administration of dimethylethanolamine in the rat. Hepatology. 1991;13:1158–1172. [PubMed] [Google Scholar]
- BEUERS U., PROBST I., SOROKA C., BOYER J.L., KULLAK-UBLICK G.A., PAUMGARTNER G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology. 1999;29:477–482. doi: 10.1002/hep.510290227. [DOI] [PubMed] [Google Scholar]
- BLITZER B.L., LYONS L. Enhancement of Na+-dependent bile acid uptake by albumin: direct demonstration in rat basolateral liver plasma membrane vesicles. Am. J. Physiol. 1985;249:G34–G38. doi: 10.1152/ajpgi.1985.249.1.G34. [DOI] [PubMed] [Google Scholar]
- BOSSARD R., STIEGER B., O'NEILL B., FRICKER G., MEIER P.J. Ethinylestradiol treatment induces multiple canalicular membrane transport alterations in rat liver. J. Clin. Invest. 1993;91:2714–2720. doi: 10.1172/JCI116511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHARD G., YOUSEF I.M., TUCHWEBER B. Influence of oral treatment with ursodeoxycholic and tauroursodeoxycholic acids on estrogen-induced cholestasis in rats: effects on bile formation and liver plasma membranes. Liver. 1993;13:193–202. doi: 10.1111/j.1600-0676.1993.tb00630.x. [DOI] [PubMed] [Google Scholar]
- CHEUNG K., HICKMAN P.E., POTTER J.M., WALKER N.I., JERICHO M., HASLAM R., ROBERTS M.S. An optimized model for rat liver perfusion studies. J. Surg. Res. 1996;66:81–89. doi: 10.1006/jsre.1996.0376. [DOI] [PubMed] [Google Scholar]
- CROCENZI F.A., MOTTINO A.D., ROMA M.G. Regulation of synthesis and trafficking of canalicular transporters and its alteration in acquired hepatocellular cholestasis. Experimental therapeutic strategies for its prevention. Curr. Med. Chem. 2004;11:501–524. doi: 10.2174/0929867043455918. [DOI] [PubMed] [Google Scholar]
- CROCENZI F.A., MOTTINO A.D., CAO J., VEGGI L.M., POZZI E.J., VORE M., COLEMAN R., ROMA M.G. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G449–G459. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- CROCENZI F.A., SANCHEZ POZZI E.J., PELLEGRINO J.M., FAVRE C.O., RODRIGUEZ GARAY E.A., MOTTINO A.D., COLEMAN R., ROMA M.G. Beneficial effects of silymarin on estrogen-induced cholestasis in the rat: a study in vivo and in isolated hepatocyte couplets. Hepatology. 2001;34:329–339. doi: 10.1053/jhep.2001.26520. [DOI] [PubMed] [Google Scholar]
- DE CAESTECKER J.S., JAZRAWI R.P., NISBETT J.A., JOSEPH A.E., MAXWELL J.D., NORTHFIELD T.C. Direct assessment of the mechanism for a raised serum bile acid level in chronic liver disease. Eur. J. Gastroenterol. Hepatol. 1995;7:955–961. doi: 10.1097/00042737-199510000-00009. [DOI] [PubMed] [Google Scholar]
- DIETRICH A., DIEMINGER W., FUCHTE K., STOLL G.H., SCHLITZ E., GEROK W., KURZ G. Functional significance of interaction of H-FABP with sulfated and nonsulfated taurine-conjugated bile salts in rat liver. J. Lipid Res. 1995;36:1745–1755. [PubMed] [Google Scholar]
- DONNER M.G., KEPPLER D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- EVANS A.M., HUSSEIN Z., ROWLAND M. Influence of albumin on the distribution and elimination kinetics of diclofenac in the isolated perfused rat liver: analysis by the impulse-response technique and the dispersion model. J. Pharmaceut. Sci. 1993;82:421–428. doi: 10.1002/jps.2600820417. [DOI] [PubMed] [Google Scholar]
- GEIER A., DIETRICH C.G., GERLOFF T., HAENDLY J., KULLAK-UBLICK G.A., STIEGER B., MEIER P.J., MATERN S., GARTUNG C. Regulation of basolateral organic anion transporters in ethinylestradiol-induced cholestasis in the rat. Biochim. Biophys. Acta. 2003;1609:87–94. doi: 10.1016/s0005-2736(02)00657-0. [DOI] [PubMed] [Google Scholar]
- GREEN R.M., GOLLAN J.L., HAGENBUCH B., MEIER P.J., BEIER D.R. Regulation of hepatocyte bile salt transporters during hepatic regeneration. Am. J. Physiol. 1997;273:G621–G627. doi: 10.1152/ajpgi.1997.273.3.G621. [DOI] [PubMed] [Google Scholar]
- GUMUCIO J.J., VALDIVIESO V.D. Studies on the mechanism of the ethynylestradiol impairment of bile flow and bile salt excretion in the rat. Gastroenterology. 1971;61:339–344. [PubMed] [Google Scholar]
- HUNG D.Y., BURCZYNSKI F.J., CHANG P., LEWIS A., MASCI P.P., SIEBERT G.A., ANISSIMOV Y.G., ROBERTS M.S. Fatty acid binding protein is a major determinant of hepatic pharmacokinetics of palmitate and its metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G423–G433. doi: 10.1152/ajpgi.00328.2002. [DOI] [PubMed] [Google Scholar]
- HUNG D.Y., CHANG P., CHEUNG K., MCWHINNEY B., MASCI P.P., WEISS M., ROBERTS M.S. Cationic drug pharmacokinetics in diseased livers determined by fibrosis index, hepatic protein content, microsomal activity, and nature of drug. J. Pharmacol. Exp. Ther. 2002a;301:1079–1087. doi: 10.1124/jpet.301.3.1079. [DOI] [PubMed] [Google Scholar]
- HUNG D.Y., CHANG P., CHEUNG K., WINTERFORD C., ROBERTS M.S. Quantitative evaluation of altered hepatic spaces and membrane transport in fibrotic rat liver. Hepatology. 2002b;36:1180–1189. doi: 10.1053/jhep.2002.36820. [DOI] [PubMed] [Google Scholar]
- HUNG D.Y., CHANG P., WEISS M., ROBERTS M.S. Structure-hepatic disposition relationships for cationic drugs in isolated perfused rat livers: transmembrane exchange and cytoplasmic binding process. J. Pharmacol. Exp. Ther. 2001;297:780–789. [PubMed] [Google Scholar]
- JACQUEMIN E., DUMONT M., MALLET A., ERLINGER S. Ursodeoxycholic acid improves ethinyl estradiol-induced cholestasis in the rat. Eur. J. Clin. Invest. 1993;23:794–802. doi: 10.1111/j.1365-2362.1993.tb00733.x. [DOI] [PubMed] [Google Scholar]
- KAN K.S., MONTE M.J., PARSLOW R.A., COLEMAN R. Oestradiol 17 beta-glucuronide increases tight-junctional permeability in rat liver. Biochem. J. 1989;261:297–300. doi: 10.1042/bj2610297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPP H., ARIAS I.M. Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters. Semin. Liver Dis. 2000;20:339–351. doi: 10.1055/s-2000-9388. [DOI] [PubMed] [Google Scholar]
- KULLAK-UBLICK G.A., BEUERS U., PAUMGARTNER G. Hepatobiliary transport. J. Hepatol. 2000a;32:3–18. doi: 10.1016/s0168-8278(00)80411-0. [DOI] [PubMed] [Google Scholar]
- KULLAK-UBLICK G.A., MEIER P.J. Mechanisms of cholestasis. Clin. Liver Dis. 2000b;4:357–385. doi: 10.1016/s1089-3261(05)70114-8. [DOI] [PubMed] [Google Scholar]
- KULLAK-UBLICK G.A., STIEGER B., MEIER P.J. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- KULLAK-UBLICK G.A., STIEGER B., HAGENBUCH B., MEIER P.J. Hepatic transport of bile salts. Semin. Liver Dis. 2000c;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- LEE J.M., TRAUNER M., SOROKA C.J., STIEGER B., MEIER P.J., BOYER J.L. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–172. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- LUXON B.A., WEISIGER R.A. A new method for quantitating intracellular transport: application to the thyroid hormone 3,5,3′-triiodothyronine. Am. J. Physiol. 1992;263:G733–G741. doi: 10.1152/ajpgi.1992.263.5.G733. [DOI] [PubMed] [Google Scholar]
- MEIER P.J., STIEGER B. Bile salt transporters. Annu. Rev. Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- MEYERS M., SLIKKER W., PASCOE G., VORE M. Characterization of cholestasis induced by estradiol-17 beta-D-glucuronide in the rat. J. Pharmacol. Exp. Ther. 1980;214:87–93. [PubMed] [Google Scholar]
- MISRA S., UJHAZY P., VARTICOVSKI L., ARIAS I.M. Phosphoinositide 3-kinase lipid products regulate ATP-dependent transport by sister of P-glycoprotein and multidrug resistance associated protein 2 in bile canalicular membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5814–5819. doi: 10.1073/pnas.96.10.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISRA S., VARTICOVSKI L., ARIAS I.M. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G316–G324. doi: 10.1152/ajpgi.00048.2003. [DOI] [PubMed] [Google Scholar]
- MORGAN D.J., STEAD C.K., SMALLWOOD R.A. Kinetic assessment of apparent facilitation by albumin of cellular uptake of unbound ligands. J. Pharmacokinet. Biopharm. 1990;18:121–135. doi: 10.1007/BF01063555. [DOI] [PubMed] [Google Scholar]
- PAULUSMA C.C., KOTHE M.J., BAKKER C.T., BOSMA P.J., VAN BOKHOVEN I., VAN MARLE J., BOLDER U., TYTGAT G.N., OUDE ELFERINK R.P. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver. Hepatology. 2000;31:684–693. doi: 10.1002/hep.510310319. [DOI] [PubMed] [Google Scholar]
- RIUS M., NIES A.T., HUMMEL-EISENBEISS J., JEDLITSCHKY G., KEPPLER D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38:374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- ROBERTS M.S., DONALDSON J.D., ROWLAND M. Models of hepatic elimination: comparison of stochastic models to describe residence time distributions and to predict the influence of drug distribution, enzyme heterogeneity, and systemic recycling on hepatic elimination. J. Pharmacokinet. Biopharm. 1988;16:41–83. doi: 10.1007/BF01061862. [DOI] [PubMed] [Google Scholar]
- SANCHEZ POZZI E.J., CROCENZI F.A., PELLEGRINO J.M., CATANIA V.A., LUQUITA M.G., ROMA M.G., RODRIGUEZ GARAY E.A., MOTTINO A.D. Ursodeoxycholate reduces ethinylestradiol glucuronidation in the rat: role in prevention of estrogen-induced cholestasis. J. Pharmacol. Exp. Ther. 2003;306:279–286. doi: 10.1124/jpet.103.049940. [DOI] [PubMed] [Google Scholar]
- SCHREIBER A.J., SIMON F.R. Estrogen-induced cholestasis: clues to pathogenesis and treatment. Hepatology. 1983;3:607–613. doi: 10.1002/hep.1840030422. [DOI] [PubMed] [Google Scholar]
- SIEBERT G.A., HUNG D.Y., CHANG P., ROBERTS M.S. Ion-trapping, microsomal binding, and unbound drug distribution in the hepatic retention of basic drugs. J. Pharmacol. Exp. Ther. 2004;308:228–235. doi: 10.1124/jpet.103.056770. [DOI] [PubMed] [Google Scholar]
- SIMON F.R., FORTUNE J., IWAHASHI M., BOWMAN S., WOLKOFF A., SUTHERLAND E. Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am. J. Physiol. 1999;276:G556–G565. doi: 10.1152/ajpgi.1999.276.2.G556. [DOI] [PubMed] [Google Scholar]
- SIMON F.R., FORTUNE J., IWAHASHI M., GARTUNG C., WOLKOFF A., SUTHERLAND E. Ethinyl estradiol cholestasis involves alterations in expression of liver sinusoidal transporters. Am. J. Physiol. 1996;271:G1043–G1052. doi: 10.1152/ajpgi.1996.271.6.G1043. [DOI] [PubMed] [Google Scholar]
- SMALLWOOD R.H., MORGAN D.J., MIHALY G.W., JONES D.B., SMALLWOOD R.A. Effect of plasma protein binding on elimination of taurocholate by isolated perfused rat liver: comparison of venous equilibrium, undistributed and distributed sinusoidal, and dispersion models. J. Pharmacokinet. Biopharm. 1988;16:377–396. doi: 10.1007/BF01062552. [DOI] [PubMed] [Google Scholar]
- STOLZ A., TAKIKAWA H., OOKHTENS M., KAPLOWITZ N. The role of cytoplasmic proteins in hepatic bile acid transport. Annu. Rev. Physiol. 1989;51:161–176. doi: 10.1146/annurev.ph.51.030189.001113. [DOI] [PubMed] [Google Scholar]
- TAKIKAWA H., SUGIYAMA Y., FERNANDEZ-CHECA J.C., KUHLENKAMP J., OOKHTENS M., KAPLOWITZ N. Evidence that interference with binding to hepatic cytosol binders can inhibit bile acid excretion in rats. Hepatology. 1996;23:1642–1649. doi: 10.1002/hep.510230647. [DOI] [PubMed] [Google Scholar]
- TRAUNER M. Molecular alterations of canalicular transport systems in experimental models of cholestasis: possible functional correlations. Yale J. Biol. Med. 1997;70:365–378. [PMC free article] [PubMed] [Google Scholar]
- TSUJII T., MORITA T., KUBO R., YAMADA M., YAMAO J., MATSUMURA Y., FUJIMOTO T., FUKUI H., OKAMOTO Y. Glucagon-induced alteration of serum bile acid level in patients with liver cirrhosis. Gastroenterology. 1991;100:1671–1677. doi: 10.1016/0016-5085(91)90668-b. [DOI] [PubMed] [Google Scholar]
- VARIN F., HUET P.M. Hepatic microcirculation in the perfused cirrhotic rat liver. J. Clin. Invest. 1985;76:1904–1912. doi: 10.1172/JCI112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORE M., HADD H., SLIKKER W., JR Ethynylestradiol-17 beta D-ring glucuronide conjugates are potent cholestatic agents in the rat. Life Sci. 1983;32:2989–2993. doi: 10.1016/0024-3205(83)90650-1. [DOI] [PubMed] [Google Scholar]
- WEISS M., KUHLMANN O., HUNG D.Y., ROBERTS M.S. Cytoplasmic binding and disposition kinetics of diclofenac in the isolated perfused rat liver. Br. J. Pharmacol. 2000;130:1331–1338. doi: 10.1038/sj.bjp.0703448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELCER N., REID G., WIELINGA P., KUIL A., VAN DER HEIJDEN I., SCHUETZ J.D., BORST P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem. J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]