Abstract
Nucleotides like ATP and UTP act as potent extracellular signalling molecules. Released from sympathetic nerve endings as cotransmitters of noradrenaline or paracrine from nonexcitatory cells, they activate specific receptors (ion-gated P2X1–7 and G-protein-coupled P2Y1,2,4,6,11–15). Which of these subtypes, however, are able to modulate vasoconstriction in the kidney is unclear.
Wild-type- and P2Y4-receptor-deficient mice kidneys were isolated and perfused with Krebs–Henseleit solution. Pressor responses to renal nerve stimulations (RNS) and added drugs were recorded. Release of endogenous noradrenaline was measured by HPLC.
RNS (1–15 Hz) induced a frequency-dependent increase in the perfusion pressor (14.2±5.1–67.3±6.9 mmHg) and noradrenaline release (1.4±0.3–24.2±3.4 ng g−1 kidney).
Pressor responses to RNS were not (1–2 Hz) or only partially (5–15 Hz) blocked by the α-adrenoceptor antagonist phentolamine (1 μM). Combination of phentolamine and the P2-receptor blocker PPADS (5 μM) prevented RNS-induced pressor responses. The P2X1,3-receptor selective antagonist NF279 (10 μM) reduced RNS-induced pressor responses in a frequency-dependent manner.
Perfusion of ATP, ADP, UTP, UDP and α,β-meATP concentration dependently increased perfusion pressor with the following rank order of potency α,β-meATP>ADP≈ATP≈UDP⩾UTP. NF279 (10 μM) reduced α,β-meATP- (0.1 μM) (21.7±3.9% of control) but not UTP- (0.3 μM) (102.6±15.3% of control) induced pressor responses. No differences in nucleotide-induced effects were detected among wild-type and P2Y4-receptor knockout mice.
Continuous perfusion of α,β-meATP (0.01 μM) potentiated UTP-, UDP- and ATP-γS-induced pressor responses.
Neuronally and paracrine-released nucleotides evoked renal vasoconstriction by activation of P2X1,3- and P2Y6-like receptors in mice. Pretreatment with the P2X1,3-receptor agonist α,β-meATP potentiated P2Y6-like receptor-mediated vasoconstrictions.
Keywords: P2X receptors, P2Y receptors, ATP, ADP, UTP, UDP, sympathetic nervous system, kidney, renal vasoconstriction, renovascular, cotransmission
Introduction
It is now widely accepted that extracellular purine and pyrimidine nucleotides (mainly ATP, ADP, UTP- and UDP) have widespread and specific signalling actions in many tissues including the kidney (Abbracchio and Burnstock, 1998; Chan et al., 1998a, 1998b; Oberhauser et al., 1999; Vonend et al., 2002). Nucleotides mediate numerous long- and short-term effects, depending on which nucleotide receptor subtypes and which cell types were activated. This not only includes mitogenic effects like cell proliferation, cell differentiation, but also vascular effects, like vasoconstriction and vasodilation (von Kugelgen et al., 1995; Ralevic & Burnstock, 1998; Rump et al., 1998; Vonend et al., 2003).
The sympathetic nervous system plays a major role in regulating renovascular resistance by releasing noradrenaline and ATP (Oberhauser et al., 1999). In addition, sympathetic overactivity is closely related to essential hypertension. The main focus in the past, however, was on studying the role of the classical sympathetic neurotransmitter noradrenaline in hypertensive disease (Esler et al., 2001). Accordingly, renal noradrenaline spillover is more pronounced in patients with essential (Esler et al., 2001) and renovascular hypertension (Johansson et al., 1999) and is used as an independent predictor of cardiovascular risk (Zoccali et al., 2002). Since complete blockade of noradrenaline receptors (α-adrenoceptors) does not prevent nerve stimulation-induced vasoconstrictions, the importance of focusing on nonadrenergic sympathetic neurotransmission by ATP and UTP was obvious, in particular, since large amounts of extracellular nucleotides can also be released from nonexcitatory cells, in a paracrine and autocrine manner (Ralevic & Burnstock, 1998; Lazarowski & Boucher, 2001; Vonend et al., 2002; Bell et al., 2003). Extracellular nucleotides mediate their function via P2X and P2Y receptors. Seven ionotropic P2X-receptor subtypes (P2X1–7) and nine G-protein-coupled P2Y-receptor subtypes (P2Y1,2,4,6,11–15) have been identified in human and other vertebrate genomes (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000). Owing to the variety of subtypes and the lack of selective agonists and antagonists available, a distinct attribution of certain functions to a single receptor has been always very difficult (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000), in particular, when the observed effects were possibly mediated by more than one P2-receptor subtype. With specific gene targeting, it now became possible to knockout single P2-receptor subtypes in order to allocate or exclude their role in ATP- and UTP-mediated effects.
Although many P2 subtypes are identified in detail at the molecular level (Ralevic & Burnstock, 1998; North, 2002; von Kugelgen & Wetter, 2000), their physiological function and their potential interaction in the kidney has yet to be explored. Experiments were performed to demonstrate the release of extracellular nucleotides from sympathetic nerve endings and identify the receptor subtypes that mediate vasoconstriction in kidneys of wild-type and P2Y4-receptor knockout mice. In addition, ATP, UTP and analogues were perfused exogenously in order to reach potential P2 receptors not activated by neuronally released ATP or UTP. Finally, it has been analysed whether there is a crosstalk of different P2-receptor subtypes detectable.
Methods
C57/Bl6 mice were used and obtained from Janvier (Belgium), except in experiments using P2Y4-receptor knockout mice (P2Y4 0/−). The knockout mice were generated and characterized by Bernard Robaye and Jean-Marie Boeynaems (Robaye et al., 2003). In experiments with P2Y4 0/− mice, their wild-type (P2Y4 0/+) littermates were used as control animals. All mice were 70±15 days old, weighting between 21 and 26 g. The investigations were performed in accordance with institutional guidelines. Mice were anaesthetized intraperitoneally with Ketanest® and Xylazin® (0.168 mg and 8 μg g−1 body weight). Kidneys were isolated microscopically (Olympus CO11) and perfused with Krebs–Henseleit solution according to an amended method described previously for isolated perfused rat kidneys (Oberhauser et al., 1999). The right renal artery was cannulated via the upper aorta, and the left renal artery via the lower aorta with polyethylene tubing (Portex, Germany; internal diameter 0.28 mm) tubing and perfused in situ at a constant rate of 7.2 ml min−1 g−1 kidney. All connected tissue including the capsule of both kidneys was carefully removed before the renal veins and ureters were cut. Bipolar platinum electrodes were placed around the renal arteries to stimulate the renal sympathetic nerves. The kidneys were transferred into a jacketed glass chamber maintained at a temperature of 37°C. The perfusion medium was gassed continuously with a mixture of 95% O2 and 5% CO2 and passed through a 0.45 μm filter before it reached the kidney. The perfusate was allowed to drip out of the cut end of the renal vein and ureter and was then collected. Perfusion pressure was monitored continuously with a Statham P23 Db pressure transducer (Gould, Oxnard, CA, U.S.A.) coupled to a Watanabe pen recorder (Graphtec Corp., Tokyo, Japan).
Renal nerve stimulations (RNS) 1–15 Hz
At 10 min after preparation, 5 Hz for 30 s (1 ms pulse width, 40 mA) and a bolus injection of 60 mM KCl were delivered to test the viability of the preparation, followed by a stabilization period of 30 min. Renal nerve stimulations (RNS) at 1, 2, 5, 7.5, 10 and 15 Hz (1 ms pulse width, 40 mA) were applied in time intervals of 5 min (S1). After a recovery period of 20 min, a second RNS sequel was performed with or without drugs (S2). In some experiments, a third RNS sequel was performed (S3). Where applicable, drugs were added to the perfusion solution 10 min before S2 and S3. Results were illustrated as raw mean Δ perfusion pressor in mmHg (Δ perfusion pressor=perfusion pressor at stimulation−baseline perfusion pressor) or in % of Δ perfusion pressor at 15 Hz S1.
To estimate stimulation-induced release of endogenous noradrenaline in some experiments, cocaine (10 μM) and corticosterone (20 μM) were added after preparation to the perfusion solution in order to prevent neuronal and extraneuronal uptake of released noradrenaline, respectively. The kidneys were stimulated as described above. The 3-min fractions of the effluent were collected throughout the stimulations (1–15 Hz) into vials containing 167 μl of 1 M HCl, 13.3 μl of 0.067 M EDTA and 3.3 μl of 1 M Na2SO3. Noradrenaline was extracted (absorbed with aluminium oxide, eluted with HClO4) and determined by reversed-phase HPLC (Waters ECD-detection, M32). The amount of noradrenaline present in each sample was corrected for recoveries (average percent recovery of noradrenaline-HCl was 59.2±8.6%; n=6). RNS-induced outflow of noradrenaline was determined as the difference between the content of noradrenaline present in the two 3-min samples collected immediately after the onset of stimulation and the spontaneous noradrenaline outflow (noradrenaline content in the 3-min sample collected immediately before RNS).
Drug response curves
At 10 min after preparation, a bolus injection of 60 mM KCl was delivered to test the viability of the preparation followed by a stabilization period of 30 min. Drugs were added to the perfusion line by a perfusion apparatus (Braun, Germany) with an additional flow rate of 0.158 ml min−1. Drug concentrations in the perfusion apparatus were adjusted to the perfusion flow rate, in order to achieve the chosen end-concentrations in the perfusion line. The resulting increase in the perfusion pressor was negligible. The time interval between the agonist concentrations was 10 min. The perfusion of drugs was stopped when the pressor responses had reached a maximum, or when no effects were observed for 3 min. Results were illustrated in % of Δ perfusion pressor of maximal response.
Consecutive single dose drug stimulations
After testing the viability (60 mM KCl) and stabilization (30 min), a single drug concentration was infused two times (S1, S2) into the perfusion line using a perfusion apparatus (Braun, Germany). The time interval between S1and S2 was 10 min to demonstrate receptor desensitization and 20 min to investigate the effect of NF279, α,β-meATP and noradrenaline. The drugs were given 10 min before S2. Results were illustrated in % of Δ perfusion pressor in S1.
Modified protocol for experiments with P2Y4-receptor wild-type and knockout mice
Owing to the limited availability of P2Y4-receptor knockout mice and their wild-type littermates, the following protocols were used.
At 10 min after preparation, 5 Hz for 30 s (1 ms pulse width, 40 mA) and a bolus injection of 60 mM KCl were delivered to test the viability of the preparation followed by a stabilization period of 30 min. RNS at 1, 2 and 5 Hz (1 ms pulse width, 40 mA) were applied in time intervals of 5 min. After a recovery period of 20 min, a noradrenaline drug response curve was carried out as described above.
In the other experimental set-up, drug response curves for ATP or UTP were performed as described. The drug response curves were followed by consecutive single dose drug stimulations where applicable as described above.
Statistical analysis
All data were expressed as mean±s.e.m. Differences were analysed by two-factorial ANOVA for repeated measurements followed by unpaired Student's t-test. Probability levels of P<0.05 were statistically significant. The number of experiments indicates the number of individual kidneys.
RT–PCR to demonstrate P2X- and P2Y-receptor expression
Intrarenal arteries were isolated in ice-cold phosphate-buffered saline under microscopical (Olympus CO11) assistance. The tissue was homogenized and total RNA was isolated with Trizol-Reagent (Invitrogen, Germany) according to the manufacturer's protocol. Following DNA digestion (RNase-free DNase/Invitrogen), cDNA was synthesized according to the supplier's protocol using oligo-dt primer and reverse transcriptase (Superscript, Invitrogen). The amplification was performed with specific primers for mouse P2X1,3, and P2Y1,2,6 receptors and the positive control GAPDH (housekeeping gene) (Table 1) with 10% of the first-strand cDNAz and 1.5 U of Platinum Taq Polymerase (Invitrogen) in a volume of 50 μl. After 5 min, 95°C followed by 35 cycles consisting of 1 min 95°C, 1 min 58°C, 1 min 72°C and for termination 8 min 72°C, 10 μl of the reaction products were analysed on a 2% agarose gel stained with ethidium bromide. RT–PCR performed without reverse transcriptase was used to test for contamination with genomic DNA.
Table 1.
Primer-sequences and expected amplification product sizes for GAPDH and P2X1,3-, P2Y1,2,6-receptor RT–PCR analysis
| Gene | Sense | Antisense | Product size (bp=base pairs) |
|---|---|---|---|
| P2X1 | 5′-AAAGCCCAAGGTATTCGCACAG-3′ | 5′-GATACCAACCACCCCGCCCTTCTC-3′ | 348 |
| P2X3 | 5′-TCTGTCCGTCGGACCTGTGAGAT-3′ | 5′-AAGCGGATGCCGAAAGCCTTCA-3′ | 491 |
| P2Y1 | 5′-TGGCGTGGTGTACCCTCTCAAGTC-3′ | 5′-ACCGTGCTCGCAAATTCATCGTT-3′ | 410 |
| P2Y2 | 5′-AGACCTGGAACCCTGGAATAGCA-3′ | 5′-ACCCCGGGCGTAGTAATAAACCAA-3′ | 280 |
| P2Y6 | 5′-ATAACTACGCCAGAGGGGACCACT-3′ | 5′-GGCGGGCCATGCGACAATA-3′ | 402 |
| GAPDH | 5′-GGGCAAGGTCATCCCTGAGCTGAA-3′ | 5′-GAGGTCCACCACCCTGTTGCTGTA-3′ | 323 |
Drugs and vehicles
The Krebs–Henseleit solution had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 0.45, NAHCO3 25, KH2PO4 1.03, D-(+)-glucose 11.1, Na2EDTA 0.067 and ascorbic acid 0.07. The following drugs were purchased: (±)-noradrenaline HCl, corticosterone, phentolamine-HCL, PPADS tetrasodium salt, ATP, UTP, UDP, α,β-meATP, ATP-γS (Sigma, Germany); cocaine HCl (Merk, Germany); NF 279 (Tocris, U.S.A.) Drugs were dissolved in distilled water before being diluted with Krebs–Henseleit solution, except corticosterone (absolute ethanol).
Results
Role of extracellular nucleotides on sympathetic neurotransmission
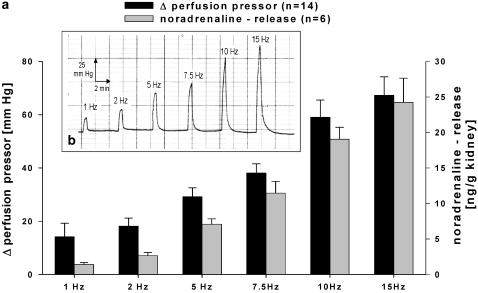
Mice kidneys were isolated and perfused with a constant flow of perfusion solution. Using platinum electrodes placed around the renal artery, RNS were performed to induce sympathetic transmitter release. RNS cause a frequency-dependent increase in perfusion pressor (Figure 1a and b) accompanied by a frequency-dependent increase in noradrenaline release (Figure 1a).
Figure 1.
(a and b) Effect of RNs (1–15 HZ) on pressor responses (black columns) and noradrenaline release (grey columns) in isolated perfused mouse kidney (a). Panel b shows a representative trace of pressor responses to RNS and panel a gives mean data±s.e.m.
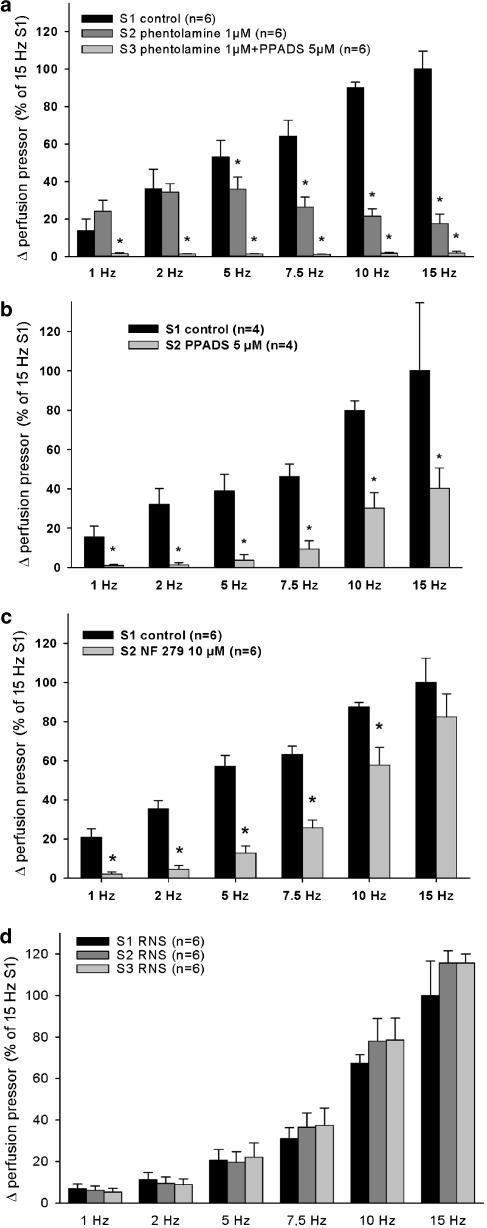
Pressor responses to RNS were not (1–2 Hz) or only partially (5–15 Hz) blocked by the nonselective α-adrenoceptor antagonist phentolamine (1 μM) (Figure 2a). The increase in perfusion pressor induced by exogenously applied noradrenaline (10 μM) was totally prevented by 1 μM phentolamine (data not shown), confirming a complete blockade of postsynaptic α-adrenoceptors. A combination of phentolamine (1 μM) and the nonselective P2-receptor blocker PPADS (5 μM) totally prevented RNS-induced pressor responses (Figure 2a).
Figure 2.
(a–d) Effect of α-adrenoceptor blockade by phentolamine (a), P2-receptor blockade by PPADS (a and b) and P2X1,3-receptor blockade by NF279 (c) on RNs- (1–15 HZ) induced pressor responses. Up to three consecutive RNS sequels (S1, S2, S3) were performed. Experiments with no drugs in S1, S2 and S3 demonstrate stable RNS-induced pressor responses (d). S1 without drugs serves as the control stimulation. All data is expressed in mean±s.e.m. % of S1/15 Hz response. *P<0.05 indicates a significant effect of each drug compared to S1 control.
PPADS (5 μM) alone, in the absence of phentolamine, blocked RNS-induced pressor responses at lower frequencies <5 Hz almost completely and decreased RNS-induced pressor responses significantly at ⩾5 Hz (Figure 2b). The P2X1,3-receptor subtype selective antagonist NF279 (10 μM) alone, reduced the RNS-induced pressor responses in a frequency-dependent manner (Figure 2c). The reduction of NF279 was more potent at lower frequencies (1, 2.5 and 5 Hz). Control experiment with no drugs present in S1, S2 and S3 showed comparable pressor responses throughout the RNS sequels (Figure 2d).
Vascular effects of non-neuronally released nucleotides
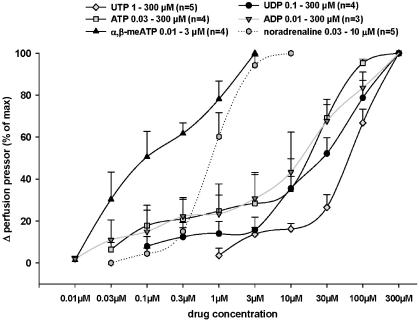
Concentration response curves were performed for noradrenaline and various P2-receptor agonists (Figure 3). Noradrenaline and the P2-receptor agonists ATP, ADP, UTP, UDP and α,β-meATP concentration dependently induced an increase in the perfusion pressor. The nucleotides had the following rank order of potency: α,β-meATP>ADP≈ATP≈UDP⩾UTP.
Figure 3.
Effect of noradrenaline and various P2-receptor agonists on the perfusion pressor of isolated perfused mouse kidneys. Drugs were added to the perfusion solution at increasing concentrations. The drug-mediated increase in the perfusion pressor is illustrated in % of Δ perfusion pressor of maximal response induced by each drug (data given in mean±s.e.m.).
In another set-up, two consecutive pressor responses (S1, S2) in a time interval of 10 min were induced by α,β-meATP (0.1 μM) or UTP (300 μM). The first drug-induced pressor response was set to 100%. S2 was significantly reduced by α,β-meATP- (65.7±8.6% of S1, n=7) but not by UTP- (100.4±3.4% of S1, n=7) induced pressor responses.
In experiments with two consecutive pressor responses (S1, S2) in a time interval of 20 min, the P2X1,3-receptor inhibitor NF279 (10 μM), given 10 min before S2, significantly reduced α,β-meATP- (0.1 μM) (21.7±3.9% of control, n=6) but not UTP- (0.3 μM) (102.6±15.3% of control, n=4) induced pressor responses.
Receptor classification using P2Y4-receptor knockout mice
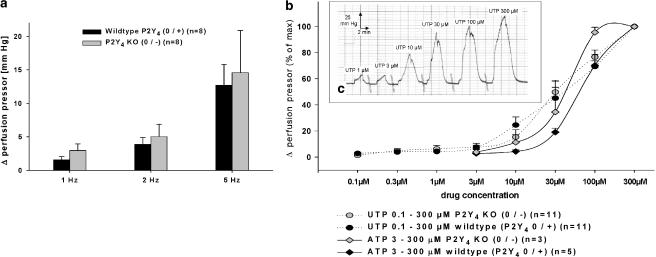
Experiments with P2Y4-receptor knockout mice (P2Y4 0/−) and their wild-type littermates (P2Y4 0/+) demonstrated no significant differences in changes in the perfusion pressor to RNS 1, 2, 5 Hz (Figure 4a) and exogenously perfused noradrenaline 0.01–0. 3 μM (data not shown). Drug response curves using ATP and UTP revealed no significant differences in the perfusion pressor between P2Y4-receptor knockout mice (P2Y4 0/−) and their wild-type littermates (P2Y4 0/+) (Figure 4b and c).
Figure 4.
(a–c) RNS- 1–5 Hz) (a) and drug (ATP, UTP) (b and c) induced pressor responses on isolated perfused kidneys of P2Y4-receptor knockout (P2Y4 0/−) and wild-type (P2Y4 0/+) mice. RNS-induced pressor responses are illustrated in mean Δ perfusion pressor (mmHg)±s.e.m. Drug-mediated pressor responses are given in % of Δ perfusion pressor of maximal response induced by each drug (mean±s.e.m.). No significant differences were detected between knockout and wild-type mice. Panel c shows a representative UTP dose–response curve of a P2Y4-receptor knockout mouse (P2Y4 0/−).
Interaction of P2X and P2Y receptors
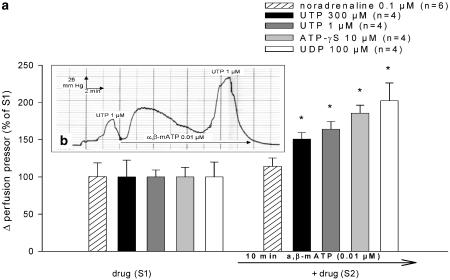
In experiments with two consecutive drug-induced pressor responses (S1, S2) in a time interval of 20 min, pretreatment with the P2X1,3-receptor agonist α,β-meATP (0.01 μM), given 10 min before S2, significantly potentiated UTP- (1 μM, 300 μM), UDP- (100 μM) and ATP-γS - (10 μM) induced pressor responses (Figure 5a and b). α,β-meATP (0.01 μM) failed to potentiate pressor responses induced by noradrenaline (0.1 μM) significantly (Figure 5a). The baseline perfusion pressor right before S2 was increased by α,β-meATP (0.01 μM) to 22.7±3.2 mmHg (n=22).
Figure 5.
(a and b) Effect of α,β-meATP on noradrenaline-, UTP-, UDP- and ATP-γS-induced pressor responses. α,β-meATP was added to the perfusion solution 10 min before the second drug-induced pressor responses (S2). The drug concentration of each drug was identical in S1 and S2. The first drug-induced pressor response (S1) was set to 100%. The effect of α,β-meATP on S2 is illustrated in % of S1 (mean±s.e.m. panel a). *P<0.05 indicates a significant effect of α,β-meATP. Panel b shows a representative trace of two consecutive UTP- (1 μM) induced pressor responses (S1, S2) and the effect of α,β-meATP on S2.
When kidneys were pretreated with continuous perfusion of noradrenaline (3 nM) instead of α,β-meATP (0.01 μM) 10 min before S2, pressor responses induced by UTP (30 μM/100 μM) were not potentiated significantly (115.2±10.5% of S1, n=6/116.0±11.2% of S1, n=4). The increase in baseline perfusion pressor right before S2 was 19.1±2.9 mmHg (n=10) when using 3 nM noradrenaline.
A significantly potentiated UTP- (30 μM) induced pressor response by perfusion of α,β-meATP (0.01 μM) was also observed in kidneys of P2Y4 (0/−)-receptor knockout mice (236.5±22.3% of S1, n=7) and their wild-type (P2Y4 0/+) littermates (235.1±43.9% of S1, n=4).
P2X- and P2Y-receptor expression in mouse kidney
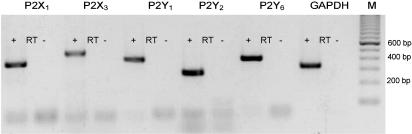
RT–PCR analyses of RNA extracted from intrarenal arteries of wild-type mice using specific primer pairs demonstrated P2X1,3- and P2Y1,2,6-receptor RNA expression within the tissue (Figure 6).
Figure 6.
Expression of P2X1,3-, P2Y1,2,6-receptor and GAPDH mRNA in mouse intrarenal arteries. RT–PCRs with specific primer pairs were performed. Amplification products for P2X1,3-, P2Y1,2,6-receptor and GAPDH mRNA with the expected size are shown in a representative ethidium bromide-stained gel. (+/−) indicates with and without reverse transcriptase, M=100 bp marker.
Discussion
Neuronally released nucleotides activate postsynaptic P2 receptors
Stimulation of sympathetic nerve endings results in an increase in the perfusion pressor as an indicator of renal vasoconstriction. This effect has been mainly attributed to the release of the classical sympathetic neurotransmitter noradrenaline (Bohmann et al., 1993). The release of noradrenaline from renal sympathetic nerve endings has been well documented in various species including humans (Vonend et al., 2002). In accordance with these observations, the present study demonstrated a stimulation-induced and frequency-dependent noradrenaline release in isolated perfused mouse kidneys. In contrast to the noradrenaline release, the observed pressor responses were resistant to α-adrenoceptor blockade, in particular, at physiologically relevant frequencies like 1 and 2 Hz. Since the α-adrenoceptor blockade-resistant pressor responses were blocked by the nonselective P2-receptor blocker PPADS, the observed effects were most likely to be mediated by neuronally released extracellular nucleotides. The significant reduction of sympathetic nerve stimulation-induced vasoconstriction, even in the absence of α-adrenoceptor blockade, by incubation with the selective P2X1,3-receptor antagonist NF279 implies a predominant role of the P2X1,3-receptor subtype in regulating renovascular resistance by neuronally released ATP. Our observations are in accordance with immunohistochemical studies showing P2X1-receptor expression within the smooth muscle layer of intrarenal vessels, in particular, in the glomerular vas afferens (Chan et al., 1998a, 1998b; Turner et al., 2003). Even more, Inscho and co-workers found a significantly disturbed vascular autoregulation within the kidney of P2X1 knockout mice (Inscho et al., 2003). However, in the actual set of experiments, the nonselective P2X- and P2Y-receptor antagonist PPADS reduced RNS-induced pressor responses more potently than the P2X1,3-receptor antagonist NF279. This suggests additional involvement of P2Y receptors. A contribution of P2Y4 receptors, however, can be excluded, since no significant differences in RNS-induced pressor responses in wild-type and P2Y4-receptor knockout mice were apparent.
P2Y6-like receptor-mediated renal vasoconstriction
With concentration response curves using the P2X1,3-receptor agonist α,β-meATP, the existence of functional P2X1,3 receptors could be confirmed. However, also UTP concentration dependently increased the renal perfusion pressor. This nucleotide is known to activate P2Y2,4 and P2Y6 receptors, but is unable to stimulate P2X receptors. In addition, preincubation with the P2X1,3-receptor antagonist NF279 reduced α,β-meATP but not UTP-induced pressor responses. This indicates that there are at least two different P2-receptor families involved in regulating renovascular resistance: the P2X1,3 receptor and in addition a P2Y receptor. Similar findings could be observed in rat mesenteric (Vial & Evans, 2002). To narrow down which P2Y-receptor subtype plays a role in nucleotide-induced renal vasoconstriction, experiments with the P2Y4 and P2Y6-receptor agonists UDP (von Kugelgen & Wetter, 2000) and the P2Y1,12 and P2Y6 receptor agonist ADP (Chang et al., 1995; von Kugelgen & Wetter, 2000) were added. Since ATP, UTP and also UDP and ADP showed a similar potency, the involvement of P2Y4 or P2Y6 receptors is feasible. UDP does not seem to activate P2Y2 receptors, making their involvement less likely (von Kugelgen & Wetter, 2000). To further discriminate between vasoconstrictive P2Y4 and P2Y6 receptors, experiments were performed using P2Y4-receptor knockout mice. However, when comparing P2Y4-receptor knockout mice with their wild-type littermates, no significant differences in RNS-induced and exogenously applied ATP- and UTP-mediated pressor responses were obvious. This excludes a significant role of vasoconstrictor P2Y4 receptors and suggests involvement of P2Y6-like receptors in mediating pressor responses in the kidney by non-neuronally released ATP and UTP.
However, characterization of P2 receptors by agonists and antagonists varies between tissues and species. We therefore cannot exclude involvement of other P2 receptors. To finally prove that P2Y6-receptors play an important role in regulating renovascular resistance, experiments using P2Y6-receptor knockout mice should be performed. Unfortunately, these mice are not available at present.
The involvement of P2Y6-like receptors in mediating vasoconstrictions on resistance vessels has been also suggested by others (Lewis et al., 2000; Vial & Evans, 2002; Malmsjo et al., 2003). Vial and Evans observed UTP- and UDP-evoked contractions on isolated perfused mesenteric arteries of P2X1-receptor-deficient mice, and thus favour the presence of vasoconstrictor P2Y6-like receptors in these vessels (Vial & Evans, 2002).
The existence of potentially vasoconstrictive and ATP-sensitive P2X1,3 and P2Y1,2,6 receptors could be confirmed by RT–PCR analysis of renovascular tissue. However, since intrarenal arteries of mice are very small, a possible contamination with other renal tissue cannot be excluded.
Interaction of P2X and P2Y receptors
Prolonged application of α,β-meATP leads, after activation of the P2X1,3 receptor, to its partial desensitization, so that only a small vasoconstriction could be observed after several minutes. Ennion and co-workers could show that this effect is possibly due to desensitization and internalization of the P2X1 receptor (Ennion & Evans, 2001). Stimulation with UTP or UDP on top of α,β-meATP, however, still produced renal vasoconstriction. This observation supports the existence of a vasoconstrictor P2Y receptor, which regulates renovascular resistance in addition to the above-characterized P2X1,3 receptor. Even more, the UTP- and UDP-evoked contractions were significantly augmented by α,β-meATP pretreatment, a phenomenon that has also been found in other vascular systems (von Kugelgen et al., 1987; Miyagi and Zhang, 2004). Owing to the various sources of extracellular nucleotides, the vascular P2 receptors have developed strategies to protect themselves from being constantly stimulated, such as desensitization, receptor internalization and ectonucleotidase-mediated nucleotide breakdown (Ralevic & Burnstock, 1998; North, 2002). In contrast to other studies, where P2-receptor agonists desensitized the contractile action of each other (Ralevic & Burnstock, 1998; Shaver, 2001), in this experimental set-up pretreatment with the P2X1,3 receptor agonist α,β-meATP augmented the vascular response of UTP. One possible explanation is that UTP reactivates P2X1,3 receptors. However, pretreatment with α,β-meATP may increase the sensitivity of P2Y receptors. The P2Y4 receptor can be excluded as a candidate, since α,β-meATP also potentiated UTP- and UDP-evoked contractions in P2Y4-receptor knockout mice. Another reason for the potentiating effect of α,β-meATP on UTP- and UDP-evoked contractions could be due to inhibition of ectonucleotidase activity. These enzymes break down extracellular nucleotides by dephosphorylation and might be inhibited by α,β-meATP (Ralevic & Burnstock, 1998). However, α,β-meATP also potentiated contractions evoked by ATP-γS, a P2Y-receptor agonist, which is resistant to these ectonucleotidase enzymes (Burnstock, 1996; Shaver, 2001). This indicates that α,β-meATP-induced potentiation is not predominantly mediated by inhibition of ectonucleotidase activity. This potentiating effect does not seem to affect adrenergic neurotransmission, since pressor responses induced by noradrenaline were not altered significantly by α,β-meATP application. Furthermore, to exclude possible nonspecific potentiating effects due to an elevated vascular tone, as observed before the second stimulation, mouse kidneys were preconstricted with noradrenaline. However, when noradrenaline was used instead of α,β-meATP, no significant potentiation of UTP-induced pressor responses was apparent.
In the present study, we have shown that extracellular nucleotides play a major role in regulating renovascular resistance. On the one hand, extracellular nucleotides are released from sympathetic nerve endings as cotransmitters of noradrenaline and activate P2X1,3 and possibly P2Y receptors. Even more, extracellular nucleotides and not noradrenaline are the predominant neurotransmitters at certain frequencies. On the other hand, millimolar amounts of extracellular nucleotides can be released from nonexcitatory cells, in particular, in pathophysiological conditions (North, 2002; Novak, 2003) and potentially evoke renal vasoconstrictions by activating P2Y6-like receptors. Using biosensor techniques, Bell and co-workers recently discovered a tubular glomerular feedback mechanism mediated by ATP released from macula densa cells providing evidence for a novel cell-to-cell signalling by ATP and a potential source for P2Y6-receptor-mediated vascular regulation (Bell et al., 2003). Finally, it could be demonstrated that there is a crosstalk of both P2-receptor subtypes. Pretreatment with the P2X1,3 and receptor agonist α,β-meATP leads to a potentiation of P2Y6-like receptor-mediated vasoconstriction. Sympathetic overactivity or inflammation releases large amounts of extracellular nucleotides and might so influence renovascular resistance. To find out the underlying cellular signalling mechanism of this receptor crosstalk, further experiments are necessary.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (RU 401/5–7). The expert technical assistance of Bettina Priesch is greatly acknowledged.
Abbreviations
- ANOVA
analysis of variance
- RNS
renal nerve stimulation
- RT–PCR
reverse transcriptase polymerase chain reaction
- s.e.m.
standard error of mean
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- BELL P.D., LAPOINTE J.Y., SABIROV R., HAYASHI S., PETI-PETERDI J., MANABE K., KOVACS G., OKADA Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHMANN C., SCHOLLMEYER P., RUMP L.C. Alpha 2-autoreceptor subclassification in rat isolated kidney by use of short trains of electrical stimulation. Br. J. Pharmacol. 1993;108:262–268. doi: 10.1111/j.1476-5381.1993.tb13472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G.P2 purinoceptors: historical perspective and classification Ciba. Found. Symp. 19961981–28.discussion 29–34 [DOI] [PubMed] [Google Scholar]
- CHAN C.M., UNWIN R.J., BARDINI M., OGLESBY I.B., FORD A.P., TOWNSEND-NICHOLSON A., BURNSTOCK G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am. J. Physiol. 1998a;274:F799–F804. doi: 10.1152/ajprenal.1998.274.4.F799. [DOI] [PubMed] [Google Scholar]
- CHAN C.M., UNWIN R.J., BURNSTOCK G. Potential functional roles of extracellular ATP in kidney and urinary tract. Exp. Nephrol. 1998b;6:200–207. doi: 10.1159/000020524. [DOI] [PubMed] [Google Scholar]
- CHANG K., HANAOKA K., KUMADA M., TAKUWA Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J. Biol. Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- ENNION S.J., EVANS R.J. Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/s0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- ESLER M., RUMANTIR M., KAYE D., JENNINGS G., HASTINGS J., SOCRATOUS F., LAMBERT G. Sympathetic nerve biology in essential hypertension. Clin. Exp. Pharmacol. Physiol. 2001;28:986–989. doi: 10.1046/j.1440-1681.2001.03566.x. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., COOK A.K., IMIG J.D., VIAL C., EVANS R.J. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J. Clin. Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON M., ELAM M., RUNDQVIST B., EISENHOFER G., HERLITZ H., LAMBERT G., FRIBERG P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C. UTP as an extracellular signaling molecule. News Physiol. Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- LEWIS C.J., ENNION S.J., EVANS R.J. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J. Physiol. 2000;527 Part 2:315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSJO M., HOU M., PENDERGAST W., ERLINGE D., EDVINSSON L. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between contractile cerebrovascular P2 receptors. Eur. J. Pharmacol. 2003;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- MIYAGI Y., ZHANG J.H. Aalpha,beta-methylene ATP enhances P2Y4 contraction of rabbit basilar artery. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1546–H1551. doi: 10.1152/ajpheart.00926.2003. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- NOVAK I. ATP as a signaling molecule: the exocrine focus. News Physiol. Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- OBERHAUSER V., VONEND O., RUMP L.C. Neuropeptide Y and ATP interact to control renovascular resistance in the rat. J. Am. Soc. Nephrol. 1999;10:1179–1185. doi: 10.1681/ASN.V1061179. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROBAYE B., GHANEM E., WILKIN F., FOKAN D., VAN DRIESSCHE W., SCHURMANS S., BOEYNAEMS J.M., BEAUWENS R. Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol. Pharmacol. 2003;63:777–783. doi: 10.1124/mol.63.4.777. [DOI] [PubMed] [Google Scholar]
- RUMP L.C., OBERHAUSER V., VON KUGELGEN I. Purinoceptors mediate renal vasodilation by nitric oxide dependent and independent mechanisms. Kidney Int. 1998;54:473–481. doi: 10.1046/j.1523-1755.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- SHAVER S.R. P2Y receptors: biological advances and therapeutic opportunities. Curr. Opin. Drug. Discov. Dev. 2001;4:665–670. [PubMed] [Google Scholar]
- TURNER C.M., VONEND O., CHAN C., BURNSTOCK G., UNWIN R.J. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol. Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., HAUSSINGER D., STARKE K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch. Pharmacol. 1987;336:556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., KRUMME B., SCHAIBLE U., SCHOLLMEYER P.J., RUMP L.C. Vasoconstrictor responses to the P2x-purinoceptor agonist beta, gamma-methylene-L-ATP in human cutaneous and renal blood vessels. Br. J. Pharmacol. 1995;116:1932–1936. doi: 10.1111/j.1476-5381.1995.tb16685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KUGELGEN I., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- VONEND O., GROTE T., OBERHAUSER V., VON KUGELGEN I., RUMP L.C. P2Y-receptors stimulating the proliferation of human mesangial cells through the MAPK42/44 pathway. Br. J. Pharmacol. 2003;139:1119–1126. doi: 10.1038/sj.bjp.0705358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VONEND O., OBERHAUSER V., VON KUGELGEN I., APEL T.W., AMANN K., RITZ E., RUMP L.C. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int. 2002;61:1617–1626. doi: 10.1046/j.1523-1755.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- ZOCCALI C., MALLAMACI F., PARLONGO S., CUTRUPI S., BENEDETTO F.A., TRIPEPI G., BONANNO G., RAPISARDA F., FATUZZO P., SEMINARA G., CATALIOTTI A., STANCANELLI B., MALATINO L.S., CATELIOTTI A. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]