Abstract
The effect of nitric oxide (NO) on aqueous humour formation (AHF) and intraocular pressure (IOP) was studied using NO donors, sodium azide (AZ) and sodium nitroprusside (SNP).
Using the porcine arterially perfused eye preparation, drug effects on AHF and IOP were measured by fluorescein dilution and manometry, respectively. Perfusion pressure of the ocular vasculature was also monitored using digital pressure transducer and pen recorder.
L-Arginine (1.0 mM), a precursor of NO, but not D-arginine (1.0 mM), the inactive analogue, produced a significant reduction in AHF (28.5%) and IOP (21.1%). L-NAME (L-nitro-L-arginine) (10–100 μM), an NO synthase inhibitor, had no effect on AHF and IOP. However, L-NAME (100 μM) completely reversed L-arginine's effect.
AZ and SNP reduced the AHF and IOP dose-dependently. AZ at 100 nM, 1 and 10 μM reduced AHF by 26.0, 39.7 and 51.7% and IOP by 10.8, 17.3 and 24.0%, respectively. SNP at 1, 10 and 100 μM reduced the AHF by 6.0, 24.2 and 35.4% and IOP by 3.5, 9.5 and 15.5%, respectively. 8-pCPT-cGMP (8-para-chlorophenyl-thioguanosine-3′,5′-cyclic guanosine monophosphate, 10 μM), a cGMP analogue, also reduced the AHF (34.9%) and IOP (15.9%).
The effects of AZ and SNP on the AHF and IOP were blocked by a soluble guanylate cyclase inhibitor ODQ (10 μM), whereas ODQ alone or combined with 8-pCPT-cGMP had no effect on the AHF and IOP.
None of the drugs had any significant effect on ocular vasculature.
The reduction of the AHF and IOP in the arterially perfused pig eye by nitrovasodilators is likely to involve the NO-cGMP pathway.
Keywords: Aqueous humour formation, cGMP, intraocular pressure, NO donors, pig eye
Introduction
Nitric oxide (NO) is an important biomolecule responsible for many biological activities. It is widely believed that the biological effects of NO donors, such as nitrovasodilators, are due to the release of NO which activates soluble guanylate cyclase (sGC) (Feelisch & Noack, 1987). The biological functions of NO are so diverse that it has been described as a second messenger, autocrine, paracrine, neurotransmitter, hormonal (Murad, 1998), cytoprotective (Wang et al., 2002) and a cytotoxic (Kroncke et al., 1997) agent. The role of NO has been studied extensively in many tissues including the eye. (Chiou, 2001) Although many have reported that NO donors reduce intraocular pressure (IOP) in normal and glaucomatous animals (Nathanson, 1992; Schuman et al., 1994; Wang & Podos, 1995) and human (Chuman et al., 2000), the exact effect of NO on the aqueous humour (AH) flow remains unclear. Owing to the dominant role of NO in vasodilatation and relaxation of smooth muscle, attention has been given mostly to ocular blood flow (Schmetterer & Polak, 2001) or trabecular meshwork and ciliary muscle relaxation (Wiederholt et al., 1994) and AH outflow (Schuman et al., 1994; Behar-Cohen et al., 1996). In those studies that have reported on the role of NO on AH outflow and IOP, data are conflicting. Some studies showed that both topical and intraocular administration of NO donors reduced the IOP (Nathanson, 1992; Behar-Cohen et al., 1996; Kotikoski et al., 2003b), but others reported that topical NO donors increased IOP without affecting aqueous humour formation (AHF) (Larsson et al., 1995) or outflow facility (Krupin et al., 1977). Yet, other studies showed that NO donors has no effect on the IOP in rabbit (Taniguchi et al., 1998) or human (Kiss et al., 1999).
Recently sodium azide (AZ), a nitrovasodilator, was shown to be able to lower the IOP in an isolated bovine eye by acting on the ciliary epithelial cells (CE) but not by relaxing the vascular smooth muscles (Millar et al., 1997; 2001). The significance of NO on AHF was further highlighted by the fact that L-NAME (L-nitro-L-arginine), a nitric oxide synthase (NOS) inhibitor, reduced the aqueous humour formation (AHF) in anaesthetised rabbit (Kiel et al., 2001). However, it was postulated that this reduction in the AHF was due to ciliary vasoconstriction, that is, blood-flow dependent. To complicate matter further, it was later reported that isosorbate-5-mononitrate, a NO donor, did not reduce AHF in healthy human volunteers (Kotikoski et al., 2003a). Since the AHF is an important determinant of IOP, we study the role of NO donors on the AH dynamics. Our hypothesis is that NO may directly regulate the AHF in addition to its effects on ocular vasculature and AH outflow. Indirect support to our hypothesis is now emerging. For instance, constitutive NOS (cNOS) activity has been detected in the bovine (Geyer et al., 1997) and porcine (Meyer et al., 1999) ciliary processes; basal nitric oxide production in the human and porcine ciliary processes has been shown to be inhibited by L-NAME (Haufschild et al., 2000); carbachol and NO can inhibit Na-K-ATPase activity of the bovine ciliary processes (Ellis et al., 2001) and stimulation of NO/cGMP pathway was shown to depolarise the epithelial transmembrane potential in porcine ciliary processes (Fleischhauer et al., 2000). In addition, NO has also been shown to be involved in fluid transport in other epithelia including kidney (Ortiz & Garvin, 2002) and salivary gland (Lomniczi et al., 1998).
The aim of the present study was to investigate the effect of NO donors on the AHF and IOP in isolated arterially perfused pig eyes.
Methods
Preparation of isolated eye
Fresh pig eyes were obtained from an abattoir and transported to the laboratory on ice-cold Krebs' solution. Perfusion of the eyes was started immediately after arrival. The postmortem time ranged from 9 to 35 min with an average of 23.0±0.5 min (n=226). The detailed methods of cannulation and perfusion of the eyes, as well as the estimation of the AHF rate have been described earlier (Wilson et al., 1993; Shahidullah et al., 2003). Briefly, excess adnexal tissues were trimmed away and care was taken not to damage the blood vessels running over the posterior surface of the globe and along the optic nerve. The ophthalmic artery was cleared of fat and cannulated distal to the point where it divides into the two long posterior ciliary arteries. The eye was placed in a circulating warming jacket at 37°C and covered with an insulated plastic cup. The ophthalmic artery was perfused with Krebs' solution at 37°C, which contained (mM): NaCl, 118; KCl, 4.0; MgSO4, 1.2; CaCl2, 2.0; NaHCO3, 25; KH2PO4, 1.2; glucose, 10.0; ascorbate 0.05; glutathione, 1.0 and allopurinol, 1.8. The pH of the Krebs' solution was adjusted to 7.4 by bubbling with O2 containing 5% CO2. Allopurinol, a xanthine oxidase inhibitor, has been added to the perfusate to combat oxidative damage and reperfusion injury.
The eyes were perfused with a digital peristaltic pump (Watson Marlow, 505S) to induce flow through the vasculature. The arterial pressure was recorded with a pressure transducer (Harvard Apparatus, 60–3003) and pen recorder (Kipp & Zonen, BD 112). Flow was commenced at 0.2 ml min−1 and increased to 1.5 ml min−1 in the first hour. Flow rate was increased in 10–20 steps, one increment in every 2.5–5 min. The number of steps and size of the increment to reach the optimum flow rate (1.5 ml min−1) depended on the arterial pressure of the eye, which was monitored continuously. The preparations were said to be valid if the pressure was below 140 mm Hg.
Estimation of AHF rate
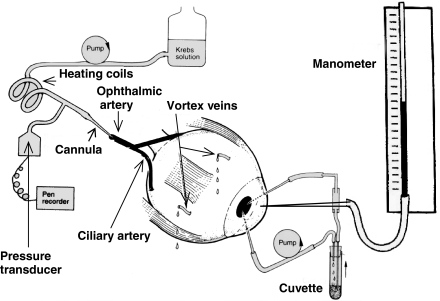
After the perfusion was set up and the eye appeared firm, the anterior chamber was cannulated with three 23G needles. The first 23G needle was connected by silicon rubber tubing (i.d., 0.5 mm; wall thickness 1.8 mm) through a Watson–Marlow peristaltic pump to a cuvette in a spectrophotometer (Pharmacia Biotech, Spectronic 2000). It returned to the anterior chamber via a second 23G needle to constitute the anterior chamber circulating system. The third needle connected the anterior chamber to a water manometer for measuring the IOP. The two circulating needles in the anterior chamber were kept wide apart to optimise mixing (Figure 1).
Figure 1.
Schematic diagram of the in vitro whole eye preparation for simultaneous measurement of AHF, IOP, arterial flow and arterial pressure.
The cuvette and the tubing system were prefilled with 1.04 ml of an AH substitute (AHS) comprising (mM): NaCl, 110; KCl, 3; CaCl2, 1.4; MgCl2, 0.5; KH2PO4, 0.9; NaHCO3, 30; glucose, 6; ascorbate, 3 and fluorescein sodium, 0.0186. The pH of this solution was adjusted to 7.6 by bubbling with 95% O2 and 5% CO2. This fluid was circulated through the anterior chamber at a rate of 0.2 ml min−1. The absorbance was recorded at 490 nm every 5 min using a computer-controlled spectrophotometer. A steady state, that is, optimal mixing of fluorescein in the anterior chamber, was achieved within 40–60 min.
The AHF rate was estimated from the rate of fluorescein dilution (decrease in absorbance) of the anterior chamber circulating system. As the AH was continuously secreted into and drained from the anterior chamber, the initial concentration of fluorescein in the anterior chamber circulating system was diluted with time. At constant AHF rate, a plot of loge (absorbance of fluorescein) against time (min) gave a straight line whose slope was taken as the rate constant for AHF (Kout min−1). Regression lines representing the dilution of fluorescein in the AH were constructed using the ANOVA statistical package by Minitab. The Kout value for the initial 30 min, that is, before the addition of drugs, was used as the control value to compare against that for the subsequent 40 min to an hour of drug-induced conditions.
The calculation of the AHF rate (in μl min−1) of the in vitro eye has been described previously (Wilson et al., 1993). In order to calculate the AHF rate, the volume of the anterior chamber is needed. The AHF rate can then be calculated as the product of (Vac+Vs) and Kout min−1 (μl min−1).
The relationship which allows this calculation is: F0 × Vs=Fm (Vs+Vac), where F0 represents the initial fluorescein concentration in the cuvette and tubing system prior to cannulation of the anterior chamber; Fm is the fluorescein concentration immediately after anterior chamber perfusion starts if instantaneous mixing was achieved; Vs represents the volume of the cuvette and the tubing system and Vac is the volume of the anterior chamber. Fm was obtained by the extrapolation of the control decay curve to time zero, that is, when the fluorescein solution was first circulated into the anterior chamber. Vac was obtained from the above relationship as
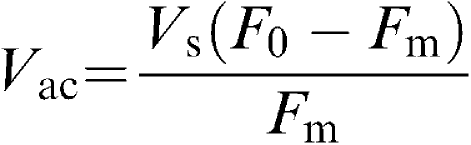 |
Measurement of IOP
IOP was measured with a water manometer which was connected to the anterior chamber via a 23G needle. The silicon rubber tubing (diameter, 0.5 mm) connecting the needle and the manometer was filled with AHS. Eyes showing IOP above 20 mmHg (270 mmH2O) were excluded from this study.
Measurement of vascular pressure
The arterial pressure was continuously monitored via a digital pressure transducer (Harvard Apparatus, 60–3003) and a dual channel chart recorder (Kipp and Zonen, BD 112). Eyes showing vascular perfusion pressure above 140 mmHg were excluded from the study.
A schematic diagram of the in vitro whole eye preparation for simultaneous measurement of AHF, IOP and vascular pressure is shown in Figure 1.
Drugs and chemicals
AZ, sodium nitroprusside (SNP), L-NAME, L-arginine, D-arginine, glutathione, 8-para-chlorophenyl-thioguanosine-3′,5′-cyclic guanosine monophosphate (8-pCPT-cGMP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), allopurinol and dimethyl sulphoxide (DMSO) were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). All other chemicals used in the Krebs' solution and AHS were of analytical grade and were obtained either from Sigma Chemical Co. or from Fisher Scientific Products (Pittsburgh, PA, U.S.A.). Stock solutions of drugs were prepared by dissolving either in distilled water or in DMSO according to their solubility. Stock solutions of drugs were added to the perfusate to obtain intended final concentration.
Statistical analysis of data
Results were expressed as the mean±s.e.m. of separate experiments. Statistical comparisons were made by paired two-tailed Student's t-test and analysis of variance followed by the Bonferroni post hoc multiple comparison tests. A probability (P) value of <0.05 was considered significant.
Results
Baseline AHF rate, IOP and arterial pressure
The mean baseline AHF rate, IOP and arterial perfusion pressure for all the eyes used in this investigation were 2.7±0.5 (n=100) μl min−1; 11.8±0.2, (n=100) mmHg and 53.52±2.26 (n=100) mmHg, respectively. The mean baseline as well as drug-treated values for AHF rate, IOP and arterial pressure for the various treatment groups are shown in Tables 1, 2 and 3 respectively.
Table 1.
Effects of drugs on AHF in isolated arterially perfused pig eye
| Drug/vehicle | Concentration (μM) | AHF (Kout.min−1 × 10−4)a (μl min−1)b (mean±s.e.m.) | n | P (ns=not significant) | % Change | |
|---|---|---|---|---|---|---|
| Baseline/control | Drug treated | |||||
| DMSO | 0.05% | 26.1±1.8 (2.7±0.2) | 28.2±2.0 (2.9±0.2) | 6 | 0.38 (ns) | −8.0 |
| L-NAME | 100 | 24.0±2.3 (2.7±0.2) | 22.6±2.4 (2.4±0.2) | 10 | 0.33 (ns) | 5.8 |
| L-NAME | 10 | 36.8±4.7 (3.7±0.5) | 33.7±0.5 (3.5±0.5) | 4 | 0.53(ns) | 8.4 |
| L-Arginine | 1000 | 28.1±3.6 (2.9±0.4) | 20.1±4.4 (2.1±0.4) | 5 | <0.01 | 28.5 |
| D-Arginine | 1000 | 24.2±1.8 (2.5±0.2) | 22.4±1.6 (2.3±0.2) | 6 | 0.52 (ns) | 8.3 |
| L-Arginine+L-NAME | 1000+100 | 26.5±1.8 (2.7±0.2) | 26.5±2.0 (2.7±0.2) | 6 | 0.98 (ns) | 0.0 |
| Sodium azide (AZ) | 10 | 25.5±1.3 (2.6±0.1) | 12.3±1.3 (1.2±0.1) | 5 | <0.001 | 51.7 |
| AZ | 1.0 | 23.4±0.9 (2.4±0.1) | 14.3±1.7 (1.4±0.2) | 6 | <0.01 | 39.7 |
| AZ | 0.1 | 29.2±2.7 (3.0±0.3) | 21.6±1.6 (2.2±0.2) | 5 | <0.01 | 26.0 |
| AZ+ODQ | 10+10 | 21.6±0.6 (2.2±0.1) | 20.5±0.7 (2.1±0.1) | 6 | 0.38 (ns) | 5.1 |
| SNP | 100 | 27.4±1.5 (2.8±0.2) | 17.6±2.0 (1.8±0.2) | 7 | <0.01 | 35.4 |
| SNP | 10 | 23.1±1.2 (2.4±0.1) | 17.5±1.0 (1.8±0.1) | 7 | <0.05 | 24.2 |
| SNP | 1.0 | 28.3±2.2 (2.9±0.2) | 26.6±3.4 (2.7±0.3) | 6 | 0.61 (ns) | 6.0 |
| SNP+ODQ | 100+10 | 29.7±2.8 (3.0±0.3) | 26.0±3.0 (2.7±0.3) | 5 | 0.07 (ns) | 12.45 |
| 8-pCPT-cGMP | 10 | 25.2±1.2 (2.6±0.1) | 16.4±1.1 (1.7±0.1) | 6 | <0.01 | 34.9 |
| 8-pCPT+ODQ | 10+10 | 27.1±1.9 (2.8±0.2) | 17.0±1.0 (1.7±0.2) | 6 | <0.01 | 37.3 |
| ODQ | 10 | 27.6±1.9 (2.8±0.2) | 24.3±1.0 (2.5±0.1) | 4 | 0.25 (ns) | 11.9 |
AHF was expressed as the rate constant (Kout min−1 × 10−4), defined as the slope of regression line drawn on LN (Loge) of the changes of absorbance with time (min) and was shown as mean±s.e.m.
Values enclosed between parentheses indicates mean AHF rate in terms of μl min−1. AHF rate measured during the first 30 min prior to drug addition was taken as the control values. AHF rate for the subsequent 40–60 min after establishment of drug effects was taken as treated values. Note that after the addition of drug, 20 min stabilisation period was allowed to establish drug effect. A probability (P) of 0.05 or less was considered significantly different from control.
Table 2.
Effects of drugs on IOP in isolated arterially perfused pig eye
| Drug/vehicle | Concentration (μM) | IOP (slope × 104)a (mmHg)b (mean±s.e.m.) | n | P (ns=not significant) | % Change | |
|---|---|---|---|---|---|---|
| Baseline/control | Drug treated | |||||
| DMSO | 0.05% | −0.4±2.0 (11.1±0.7) | 1.3±3.0 (11.2±1.0) | 8 | 0.62 (ns) | 0.4 |
| L-NAME | 100 | −9.3±6.0 (12.5±1.1) | −4.0±3.0 (12.1±1.0) | 10 | 0.27 (ns) | −0.5 |
| L-NAME | 10 | −0.1±4.0 (11.5±1.4) | 0.3±2.0 (11.6±1.3) | 4 | 0.94 (ns) | 3.2 |
| L-Arginine | 1000 | 6.2±11.0 (13±0.4) | −22.1±3.0 (11.5±0.4) | 5 | <0.05 | 12.1 |
| D-Arginine | 1000 | −7.7±2.0 (12.0±0.1) | 5.6±11.0 (11.6±0.3) | 6 | 0.33 (ns) | 3.2 |
| L-Arg+L-NAME | 1000 +100 | 0.1±1.0 (10.4±0.8) | −3.1±1.0 (10.2±0.7) | 6 | 0.26 (ns) | 2.3 |
| Sodium azide (AZ) | 10 | 1.4±3.0 (12.4±0.6) | −45.4±2.0 (9.4±0.5) | 5 | <0.001 | 24.0 |
| AZ | 1.0 | −1.0±2.0 (11.8±1.2) | −33.6±4.0 (9.7±1.0) | 6 | <0.01 | 17.3 |
| AZ | 0.1 | 1.5±3.0 (10.9±0.8) | −19.2±4.0 (9.7±1.0) | 5 | <0.01 | 10.8 |
| AZ+ODQ | 10 +10 | 1.0±2.0 (12.8±0.7) | −3.9±4.0 (12.3±0.8) | 6 | 0.27 (ns) | 3.7 |
| SNP | 100 | −3.6±5.0 (13.0±1.0) | −29.8±3.0 (11.0±0.8) | 7 | <0.001 | 15.6 |
| SNP | 10 | 6.9±5.0 (12.2±0.8) | −2.2±6.0 (11.1±1.3) | 7 | <0.01 | 9.5 |
| SNP | 1.0 | −1.2±1.0 (10.5±0.8) | −5.4±3.0 (10.2±0.8) | 6 | 0.16 (ns) | 3.5 |
| SNP+ODQ | 100 +10 | −1.4±1.0 (13.1±1.5) | −0.7±1.0 (13.0±1.4) | 5 | 0.64 (ns) | 1.2 |
| 8-pCPT-cGMP | 10 | 1.0±2.0 (10.9±1.0) | −27.8±3.0 (9.2±0.9) | 6 | <0.001 | 15.9 |
| 8-pCPT+ODQ | 10 +10 | 1.4±2.0 (11.0±0.9) | −20.6±3.0 (9.2±1.0) | 6 | <0.001 | 16.6 |
| ODQ | 10 | −5.7±2.6 (11.6±0.7) | −1.2±0.5 (11.3±0.7) | 4 | 0.18 (ns) | 2.8 |
IOP was expressed as the slope of regression line drawn on LN (Loge) of the changes of IOP with time (min) (slope × 104) and was shown as mean±s.e.m.
Values enclosed between parentheses indicates mean IOP in terms of mmHg. IOP recorded during the first 30 min prior to drug addition was taken as the control values. IOP recorded for the subsequent 40–60 min after establishment of drug effects was taken as treated values. Note that after the addition of drug, 20 min stabilisation period was allowed to establish drug effect. A probability (P) of 0.05 or less was considered significantly different from control.
Table 3.
Effects of drugs on ocular vasculature in isolated arterially perfused pig eye
| Drug/vehicle | Concentration (μM) | Arterial pressure (mmHg) (mean±s.e.m.) | n | P | % Change | |
|---|---|---|---|---|---|---|
| Control | Treated | |||||
| DMSO | 0.05% | 54.25±6.29 | 51.17±9.60 | 6 | >0.05 | 2.07 |
| L-NAME | 100 | 51.00±15.64 | 53.25±15.43 | 10 | >0.05 | 4.41 |
| L-NAME | 10 | 58.33±6.03 | 61.44±5.70 | 4 | >0.05 | 5.33 |
| L-Arginine | 1000 | 64.60±13.98 | 67.20±15.77 | 5 | >0.05 | 4.02 |
| D-Arginine | 1000 | 46.33±0.95 | 45.00±1.58 | 6 | >0.05 | 2.88 |
| L-Arg+L-NAME | 1000 +100 | 56.50±9.33 | 58.83±9.20 | 6 | >0.05 | 4.13 |
| Sodium azide (AZ) | 10 | 62.60±11.88 | 62.00±9.94 | 5 | >0.05 | 0.96 |
| AZ | 1 | 48.67±10.14 | 50.33±8.85 | 6 | >0.05 | 3.43 |
| AZ | 0.1 | 47.80±14.22 | 47.40±11.97 | 5 | >0.05 | 0.84 |
| AZ+ODQ | 10+10 | 52.17±12.88 | 53.00±12.76 | 6 | >0.05 | 1.60 |
| SNP | 100 | 53.53±7.14 | 52.00±6.16 | 7 | >0.05 | 2.93 |
| SNP | 10 | 46.43±11.96 | 45.57±11.82 | 7 | >0.05 | 1.85 |
| SNP | 1 | 48.50±7.24 | 51.00±7.38 | 6 | >0.05 | 5.15 |
| SNP+ODQ | 100 +10 | 49.00±7.97 | 49.20±8.78 | 5 | >0.05 | 0.41 |
| 8-pCPT-cGMP | 10 | 48.67±7.15 | 49.00±8.19 | 6 | >0.05 | 0.68 |
| 8-pCPT+ODQ | 10+10 | 60.75±12.88 | 59.25±13.11 | 6 | >0.05 | 2.47 |
| ODQ | 10 | 60.75±13.1 | 59.00±12.39 | 4 | >0.05 | 2.90 |
The arterial pressure was recorded in mmHg and was shown as mean±s.e.m. The control arterial pressures represent the average arterial pressure of the eyes of each treatment group before addition of drug. The treated arterial pressures represent the average arterial pressure of the respective eyes at the end of 60 min of drug treatment. Note that for each group of eyes, arterial pressure recorded during the first 30 min prior to drug addition was taken as the control values. Pressure recorded for the subsequent 40–60 min after establishment of drug effects was taken as treated values.
Effect of DMSO on AHF and IOP
In order to see if DMSO used in dissolving the drugs had any effect on the AHF or IOP, we perfused the eye with Kreb's solution containing DMSO alone. The data showed that DMSO has no effect on the AHF and IOP (Tables 1 and 2) and the AHF rate of the whole experimental period did not change significantly.
Effect of L-NAME and L-arginine on AHF and IOP
We tested two concentrations of L-NAME (10 and 100 μM), an NOS inhibitor, and found no effect on either the AHF (Table 1) or IOP (Table 2). We then used L-arginine, a physiological precursor of NO, to investigate its effect on the AHF and IOP. L-Arginine (1.0 mM) produced significant reduction in AHF and IOP (Table 1) by 28.5, 12.1%, respectively. However, addition of D-arginine (1.0 mM), an inactive analogue of L-arginine, produced no significant effect on either the AHF or IOP (Tables 1 and 2). When L-NAME (100 μM) and L-arginine (1 mM) were used together, the L-arginine's effects on AHF and IOP was effectively abolished.
Effect of AZ and SNP on AHF and IOP
The vasodilator drug AZ, which acts through the generation of NO, produced significant and concentration-dependent reduction of the AHF at the three concentrations used (10, 1 and 0.1 μM). The reductions with the three concentrations used were 51.7, 39.7 and 26.0%, respectively (Table 1). All three concentrations reduced the IOP significantly in a dose-dependent manner. The reductions were 24.0, 17.3 and 10.8%, respectively (Table 2).
The NO donor SNP also reduced AHF and IOP at concentrations of 10 and 100 μM. The AHF was reduced by 35.4 and 24.2% (Table 1), and the IOP was also depressed by 15.6 and 9.5%, respectively (Table 2). Lower concentration of SNP (1 μM) failed to affect both the AHF and IOP significantly.
The effect of AZ and SNP on AHF and IOP is inhibited by ODQ
We used ODQ, a specific inhibitor of sGC, to study its influence on the AZ and SNP-induced reduction of AHF and IOP. ODQ (10 μM) reversed both the AHF and IOP-reducing effect of AZ and SNP (Tables 1 and 2).
Effect of 8-pCPT-cGMP and/or ODQ on AHF and IOP
To further explore whether AZ and SNP acted through cGMP in the pig eye preparation, we studied the effect of 8-pCPT-cGMP, a cell-permeable and a stable analogue of cGMP, on the AHF and IOP. 8-pCPT-cGMP (10 μM) reduced both AHF and IOP by 34.9% (Table 1) and 15.9% (Table 2), respectively. However, the addition of ODQ (10 μM) failed to reverse the AHF and IOP-reducing effect of 8-pCPT-cGMP (Tables 1 and 2). ODQ (10 μM) alone had no effect on the AHF or IOP.
Thus, our data indicate that the reduction in AHF rate in the in vitro pig eye model may involve the NO/sGC/cGMO pathway. Figure 2 is a simplified diagram which shows the interaction between NO and cyclic GMP (cGMP).
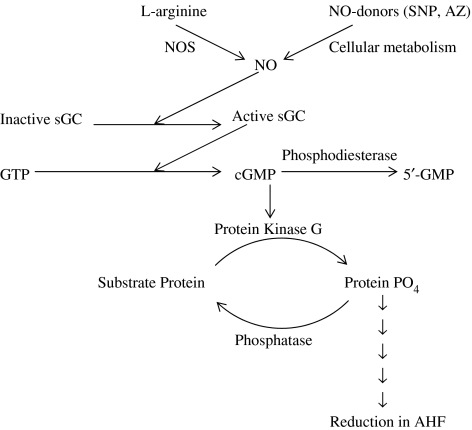
Figure 2.
Physiological actions of NO in reducing AHF. Endogenous NO is produced by NOS isoforms from L-arginine. Exogenous NO is produced from a class of compounds called NO donors, when administered to biological system. Once produced, NO diffuses into the cytoplasm, interacts and activates the enzyme sGC. Activated sGC hydrolyses GTP to produce cGMP. cGMP activates cGMP-dependent protein kinase (PKG). PKG in turn phosphorylates specific substrate proteins. The substrate proteins may itself constitute the transporter or ion channel, phosphorylation of which causes to affect the physiological function. Phosphorylated substrate proteins may also interact in subsequent steps with the final functional protein to produce the ultimate physiological action. The actions of phosphoproteins are usually terminated by a class of enzymes called phosphatases, which remove the phosphate group from the serine/threonine or tyrosine residues of the substrate protein to bring it back to the resting state.
Effects of drugs on the arterial pressure of the in vitro pig eye
The mean arterial pressures of the in vitro pig eyes ranged from 47±2 and 67±6 mmHg in the present study. The average arterial pressure for all the treatment groups was 53.52±2.26 mmHg (n=100). None of the drugs used in the present investigation had any significant effect on the arterial pressure of the in vitro pig eyes (Table 3).
Discussion
We have used an in vitro pig eye preparation to investigate two well-known NO donors – AZ and SNP – for their effects on the AHF, IOP and ocular vasculature. Pig eye are similar to the human eye both in terms of the size and anatomy and it has been considered as a good animal model for human eye (Beauchemin, 1974; Simoens et al., 1992). Therefore, we chose to use pig eyes to study the physiology of the AHF.
In addition to the NO donors, we have also used L-NAME, an inhibitor of NOS, to block the physiological production of NO and L-arginine, a nontoxic physiological precursor of NO to mimic the action of NO donors. Physiologically, NO is produced from L-arginine via various isoforms of NOS. Since it is widely believed that NO acts through the production of cGMP by activation of sGC, we used 8-pCPT-cGMP, a stable and cell-permeable analogue of cGMP and ODQ, a specific inhibitor of sGC, to simulate and to block the action of NO donors, respectively.
To the best of our knowledge, the present work represents the first attempt to study the effect of NO donors on the AHF of pig eye. The AHF and IOP in the isolated pig eye preparation were reduced dose-dependently by both AZe and SNP. In earlier investigations, it had been reported that both sodium azide and atriopeptin reduced the AHF in isolated arterially perfused bovine eye (Millar et al., 1997; 2001). NO had also been shown to modulate the secretion in a number of other epithelial tissues. Thus, the present observation of reduction of the AHF and IOP by AZ and SNP is in agreement with previous studies in ocular and other tissues. However, a recent study has shown that a single 10 mg oral dose of isosorbide-5-nomonitrate did not produce any significant effect on the AHF or IOP in healthy volunteers (Kotikoski et al., 2003a). For oral uptake, this drug will be digested and metabolised in the blood circulation so that the amount of NO released is likely to decrease with time. In addition, in the present study, it was clear that the AHF and IOP reducing effects of the NO donors were concentration-dependent. Therefore, sufficient amount of the drug will be required at the ciliary body to elicit a similar reduction in the AHF and IOP in vivo. It would have been useful to see if the final drug concentration in the blood circulation or at the ciliary epithelium was adequate to elicit any effect in the above human study. However, the authors were unable to use higher doses because of the potential side effects of the drugs. In contrast, the advantage of the present in vitro setup was that it allowed desired concentration of drugs (up to 100 μM) be quickly and directly perfused to the ciliary body without being metabolised in the blood stream.
The isolated perfused pig eye setup has several other advantages: (1) this system is free from systemic effects in terms of absorption and metabolism of drugs; (2) the isolated whole eye is free from the influence of any nervous or hormonal effects; (3) it allows simultaneous measurements of the arterial flow rate, arterial pressure, AHF and IOP; (4) it allows the use of high drug concentration that would otherwise be impossible in live animals; (5) it also allows both baseline and control data to be obtained from the same eye; (6) the whole organ preparation involves no traumatic isolation of the ciliary body and therefore preserves the integrity of the CE preparation; (7) lastly, instead of killing live animals for experiments, pig eyes are readily available from local abattoirs. However, it also has a few drawbacks technically: (1) reperfusion of the eye may be hindered by blood clot in the ocular vasculature if the eyes are not fresh enough; (2) occasionally, fine air bubbles in the perfusion system can block small arteries and thereby increase the perfusion pressure beyond acceptable limit of 140 mmHg; (3) despite adequate precautions, some blood vessels are severed in the dissection process and perfusate may leak out from the eye. In our experience, these drawbacks were easily identified and did not cause significant problem in the study.
The mean baseline AHF rate in the isolated perfused pig eye, as determined by fluorescein dilution technique, was 2.7±0.5 (n=100) μl min−1 and the mean anterior chamber volume was 237.4±15.5 (n=100) μl. There are few published data on the AHF and IOP of in vivo pig eye for comparison with our in vitro results. In big eye such as that in the bovine, the anterior chamber volume was found to be 1.69 ml and the AHF rate was also found to be high at 12 μl min−1 (Wilson et al., 1993). In in vivo human eye, the anterior chamber volume was reported to be 247 μl (Toris et al., 1999) with an AHF rate of 2.56 μl min−1 (Kaplan et al., 1996). Given the comparable size between the pig and human eyes, the present data on both the AHF and anterior chamber volume of pig eye are in a similar order of magnitude as the human eye.
The mean baseline IOP of the in vitro pig eye was 11.8±0.2, (n=100) mmHg. In this model, we removed most of the extraocular muscles and cut short all the vortex veins to encourage venous drainage and facilitate perfusion of the eye. Thus, there would be little vortex, orbital or ophthalmic venous pressures. Although there may be a small pressure in the remaining episcleral veins, this is likely to be small as well. Therefore, without these opposing pressures, the IOP recorded in the isolated pig eye is expected to be lower than the in vivo data. In fact, in a similar setup, the isolated bovine eye gave a lower IOP value of 9.4 mmHg (Wilson et al., 1993) than that recorded in the calves, 16.5 mmHg (Woellfel et al., 1964).
There are growing evidences that NO is involved in the modulation of AHF in human and animals. For example, cNOS activity has been detected in the bovine ciliary processes (Geyer et al., 1997), and neuronal and inducible NOS have also been localised in the porcine CE (Meyer et al., 1999). L-NAME inhibitable NO production has been demonstrated in the isolated human and porcine ciliary processes (Haufschild et al., 2000). Cyclic AMP, forskolin and isoproterenol have been shown to stimulate NO production in the pig ciliary processes (Liu et al., 1999). The nitrite/NO production in pig ciliary processes induced by norepinephrine, an adrenergic agonist, can be blocked by L-NAME, an NOS inhibitor (Liu et al., 1998).
The present data in the pig eye indicates that NO reduces the AHF and IOP via the NO-sGC-cGMP pathway. Exactly how cGMP modulates the ultimate effector (ion transporter, channel or other transport molecules) in the AHF of pig ciliary body remains to be elucidated. Na-K-ATPase is known to be important in AHF (Riley & Kishida, 1986) and it has been identified in the CE of rabbit (Flugel & Lutjen-Drecoll, 1988), ox (Ghosh et al., 1990), rat and mouse (Wetzel & Sweadner, 2001). NO and carbachol have been shown to inhibit the Na-K-ATPase activity in the bovine ciliary processes. This inhibition was correlated with an increase in the production of cGMP and was blocked by L-NAME and ODQ (Ellis et al., 2001). NO, NO donors or cGMP have also been reported to inhibit the bicarbonate and chloride transport in thick ascending limb of kidney by inhibiting Na-K-2Cl cotransporter (Ortiz et al., 2001). Na-K-2Cl cotransporter is a secondary active transport system which is thought to play an important role in the AHF in many species, including human (Hochgesand et al., 2001), rabbit (Crook et al., 2000) and ox (Do & To, 2000). Whether NO modulates the AHF via the Na-K-ATPase or Na-K-Cl or other transport proteins awaits further experimentation.
Recently, it has been reported that stimulation of NO/cGMP pathway induced membrane potential depolarisation in the porcine ciliary processes, which was predicted to lead to an increase in AHF (Fleischhauer et al., 2000). However, in the rabbit CE, cGMP produced an increase in the short circuit current when it was applied to the stromal side of the ciliary body, but decreased it when it was applied to the aqueous side (Carre & Civan, 1995). Therefore, it is difficult to predict the effect of cGMP on the AHF because of this ‘sidedness' effect of cGMP. We have attempted to directly study the effect of cGMP on the fluid formation in an in vitro eye. Although the exact molecular events in terms of ion transport are still unknown, the present data has shown that cGMP decreased the AHF as well as IOP via sGC pathway. It would be very interesting to correlate the electrophysiological data with the fluid formation rate in future studies.
In the present investigation, we found no effect on ocular vasculature with any of the drugs used. The lack of vasodilating effect of AZ, SNP, L-arginine, or 8-pCPT-cGMP on ocular vasculature is not entirely unexpected. Apparently, these data are in contrast with some published reports, particularly reports involving anaesthetised pigs (Jacot et al., 1998) and in isolated bovine posterior ciliary artery sections (Delaey & Van de Voorde, 1998). However, we observed similar results previously in arterially perfused bovine whole eye preparation where atriopeptin, which also acts through cGMP, reduced AHF without any effect on the ocular vasculature (Millar et al., 1997). Furthermore, vascular relaxation by AZ can readily be detected in arterially perfused eye when precontracted by norepinephrine (Millar et al., 2001).
A probable explanation for the lack of vascular effects of NO donors might be that the uveal vessels of the perfused eye, particularly the small diameter vessels, were already maximally dilated due to the mechanical shear stress of the fluid flow. In fact, in the normal pig the in vivo mean arterial pressure has been reported to be 95±18 mmHg (Gelzer et al., 2004). The present study has recorded a lower mean arterial pressure (53.52±2.26 mmHg, n=100). The fact that L-NAME failed to produce any effect on these dilated vessels argues against the notion that vasodilatation was due to NO. In addition, the perfusate (Krebs' solution) did not contain L-arginine, and there would not be sufficient L-arginine for the NOS enzyme to produce adequate NO for vasodilatation in the system. This suggestion is further supported by the fact that L-NAME was effective in reversing the L-arginine induced AHF reduction only in the presence of L-arginine. However, neither L-arginine nor L-NAME nor their combination has any significant effect on the vasculature of the present system. Thus, dilatation of ocular vessels in our system was unlikely to be due to NO. It has been shown that in the isolated perfused eye, the vasodilatation was mediated by endothelium-derived hyperpolarising factors (EDHF) (McNeish et al., 2001). Release of EDHF by vascular endothelium due to shear stress has been documented (Macedo & Lautt, 1998). The influence of endothelial mediators increases with decreasing diameter of porcine ocular vessels (Haefliger et al., 1993). It was also shown that most of the resistance in the perfused porcine eye is offered by the small arterioles with diameters of 100 μm or less (Meyer et al., 1993). Small arteries may be more prone to shear stress due to their thinner vessel wall. Thus, it may not be surprising that the maximally dilated vessels demonstrated no further dilatation with NO donors.
Although the exact reason for the lack of vascular response of the isolated pig eye to SNP or AZ is yet to be understood, the fact that there was no vascular response by the drugs has facilitated the interpretation that the action of NO was exerted primarily on the CE. In other words, the effects of NO donors on the AHF and IOP reductions can be attributed to the CE but not the ciliary blood flow. Furthermore, we have shown earlier that the effect of nitrovasodilator on the IOP of the isolated perfused bovine eye was not via vasodilatation, but by direct action at the CE (Millar et al., 2001). Recently, using the same in vitro pig eye system, we have observed that significant variation of the arterial perfusion flow or pressure did not affect the AHF rate even when the arterial flow is increased by 100% or decreased by more than 75% (unpublished data). Similar results have been found by Kiel and Reitsamer in anesthetised rabbits (Reitsamer & Kiel, 2003).
In the present investigation, reduction of AHF was found to be about double the reduction of IOP in terms of percentage. For example, while10 μM AZ produced about 52% reduction in AHF, it produced only 24% reduction in IOP (Table 1 vs Table 2). Similar trends were found with all effective drugs at all concentrations used in this study (Table 1 vs Table 2). There are three plausible interpretations for this fall in IOP: it may due to (i) a reduction in AHF with unchanged outflow facility at the trabecular meshwork; (ii) a combined effect of reduction in AHF and increase in trabecular outflow facility or (iii) a combined effect of increases in both AHF and outflow facility. Both the (i) and (ii) are possible explanations to the observation. However, condition (iii) where both the AHF and outflow facility increase is unlikely. Assuming the AHF remains unchanged, increase in the outflow facility will improve the ease of aqueous outflow and decrease both the IOP and volume of the anterior chamber. Although the AHF rate is unchanged, such decrease in anterior chamber volume will lead to an increase in the fluorescein dilution rate, which is always translated into as an increase, but not a decrease, in AHF. However in the above experimental observation, the rate of fall in absorbance was not accelerated but slowed down. It showed that the AHF was reduced and the IOP was decreased by a significant amount. In fact, the setup is more likely to underestimate the decrease of AHF if the outflow facility is increased. Similarly, this logic also argues against the possibility of any increase in the AHF with NO donors in the present experiment. However, the exact contribution of AHF rate and outflow facility to the reduction in IOP is unclear and awaits further experimentation. Although a number of reports have shown that NO donors reduced IOP by increasing trabecular outflow facility, we cannot confirm if NO has a dual effect of decreasing AHF and increasing outflow facility in the present study. We may reasonably conclude that NO donors reduce IOP at least in part by reducing AHF.
Nipradilol, a novel antiglaucoma drug, has been shown to improve retinal blood flow (Kida et al., 2001) and increased the optic nerve head circulation in human (Mizuno et al., 2002). Nipradilol has also been shown to offer neuroprotection in rats mainly through its NO releasing property (Mizuno et al., 2001; Nakazawa et al., 2002). The present results have shown that NO donors reduce the AHF and demonstrated ocular hypotensive action. Considering its effects of reducing AHF, increasing AH outflow and improving retinal circulation (Haefliger et al., 1994), NO and its donors have the potential to become the next generation of pharmacological treatment for glaucoma patients.
Acknowledgments
This work was supported by a grant from the Hong Kong Polytechnic University, and Grants A-PE59, YY32, GT595 and GT807. We are thankful to Dr W.S. Wilson, IBLS, University of Glasgow, for his advice in preparing the manuscript. We are grateful to the Sheung Shui Abattoir for the supply of pig eyes. We acknowledge the technical help of Forrest, Sing, Rachel, Bena and Coni.
Abbreviations
- AH
aqueous humour
- AHF
aqueous humour formation
- AHS
aqueous humour substitute
- AZ
sodium azide
- cAMP
cyclic AMP
- CE
ciliary epithelium
- cGMP
cyclic GMP
- DMSO
dimethyl sulphoxide
- EDHF
endothelium-derived hyperpolarizing factor
- IOP
intraocular pressure
- Kout
rate constant for aqueous humour formation
- L-NAME
L-nitro-L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- 8-pCPT-cGMP
8-para-chlorophenyl-thioguanosine-3′,5′-cyclic guanosine monophosphate
- sGC
soluble guanylate cyclase
- SNP
sodium nitroprusside
References
- BEAUCHEMIN M.L. The fine structure of the pig's retina. Graefes. Arch. Clin. Exp. Ophthalmol. 1974;190:27–45. doi: 10.1007/BF00414333. [DOI] [PubMed] [Google Scholar]
- BEHAR-COHEN F.F., GOUREAU O., D'HERMIES F., COURTOIS Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest. Ophthalmol. Vis. Sci. 1996;37:1711–1715. [PubMed] [Google Scholar]
- CARRE D.A., CIVAN M.M. cGMP modulates transport across the ciliary epithelium. J. Membr. Biol. 1995;146:293–305. doi: 10.1007/BF00233949. [DOI] [PubMed] [Google Scholar]
- CHIOU G.C. Review: effects of nitric oxide on eye diseases and their treatment. J. Ocul. Pharmacol. Therapeut. 2001;17:189–198. doi: 10.1089/10807680151125555. [DOI] [PubMed] [Google Scholar]
- CHUMAN H., CHUMAN T., NAO-I N., SAWADA A. The effect of L-arginine on intraocular pressure in the human eye. Curr. Eye Res. 2000;20:511–516. [PubMed] [Google Scholar]
- CROOK R.B., TAKAHASHI K., MEAD A., DUNN J.J., SEARS M.L. The role of NaKCl cotransport in blood-to-aqueous chloride fluxes across rabbit ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2000;41:2574–2583. [PubMed] [Google Scholar]
- DELAEY C., VAN DE VOORDE J. The effect of NO donors on bovine retinal small arteries and posterior ciliary arteries. Invest. Ophthalmol. Vis. Sci. 1998;39:1642–1646. [PubMed] [Google Scholar]
- DO C.W., TO C.H. Chloride secretion by bovine ciliary epithelium: a model of aqueous humor formation. Invest. Ophthalmol. Vis. Sci. 2000;41:1853–1860. [PubMed] [Google Scholar]
- ELLIS D.Z., NATHANSON J.A., RABE J., SWEADNER K.J. Carbachol and nitric oxide inhibition of Na,K-ATPase activity in bovine ciliary processes. Invest. Ophthalmol. Vis. Sci. 2001;42:2625–2631. [PubMed] [Google Scholar]
- FEELISCH M., NOACK E.A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur. J. Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- FLEISCHHAUER J.C., BENY J.L., FLAMMER J., HAEFLIGER I.O. NO/cGMP pathway activation and membrane potential depolarization in pig ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2000;41:1759–1763. [PubMed] [Google Scholar]
- FLUGEL C., LUTJEN-DRECOLL E. Presence and distribution of Na+/K+-ATPase in the ciliary epithelium of the rabbit. Histochemistry. 1988;88:613–621. doi: 10.1007/BF00570332. [DOI] [PubMed] [Google Scholar]
- GELZER A.R., ATTMANN T., RADICKE D., NYDAM D., CANDINAS R., LUTTER G. Effects of acute systemic endothelin receptor blockade on cardiac electrophysiology in vivo. J. Cardiovasc. Pharmacol. 2004;44:564–570. doi: 10.1097/00005344-200411000-00008. [DOI] [PubMed] [Google Scholar]
- GEYER O., PODOS S.M., MITTAG T. Nitric oxide synthase activity in tissues of the bovine eye. Graefes. Arch. Clin. Exp. Ophthalmol. 1997;235:786–793. doi: 10.1007/BF02332864. [DOI] [PubMed] [Google Scholar]
- GHOSH S., FREITAG A.C., MARTIN-VASALLO P., COCA-PRADOS M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J. Biol. Chem. 1990;265:2935–29340. [PubMed] [Google Scholar]
- HAEFLIGER I.O., FLAMMER J., LUSCHER T.F. Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest. Ophthalmol. Vis. Sci. 1993;34:1722–1730. [PubMed] [Google Scholar]
- HAEFLIGER I.O., MEYER P., FLAMMER J., LUSCHER T.F. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology. Surv. Ophthalmol. 1994;39:123–132. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- HAUFSCHILD T., TSCHUDI M.R., FLAMMER J., LUSCHER T.F., HAEFLIGER I.O. Nitric oxide production by isolated human and porcine ciliary processes. Graefes. Arch. Clin. Exp. Ophthalmol. 2000;238:448–453. doi: 10.1007/s004170050377. [DOI] [PubMed] [Google Scholar]
- HOCHGESAND D.H., DUNN J.J., CROOK R.B. Catecholaminergic regulation of Na-K-Cl cotransport in pigmented ciliary epithelium: differences between PE and NPE. Exp. Eye Res. 2001;72:1–12. doi: 10.1006/exer.2000.0927. [DOI] [PubMed] [Google Scholar]
- JACOT J.L., O'NEILL J.T., SCANDLING D.M., WEST S.D., MCKENZIE J.E. Nitric oxide modulation of retinal, choroidal, and anterior uveal blood flow in newborn piglets. J. Ocul. Pharmacol. Therapeut. 1998;14:473–489. doi: 10.1089/jop.1998.14.473. [DOI] [PubMed] [Google Scholar]
- KAPLAN B.H., KALINA P.H., LARSSON L.I., PACH J.M., BRUBAKER R.F. Aqueous humor flow in unilateral carotid stenosis. J. Glaucoma. 1996;5:237–240. [PubMed] [Google Scholar]
- KIDA T., SUGIYAMA T., HARINO S., KITANISHI K., IKEDA T. The effect of nipradilol, an alpha-beta blocker, on retinal blood flow in healthy volunteers. Curr. Eye Res. 2001;23:128–132. doi: 10.1076/ceyr.23.2.128.5475. [DOI] [PubMed] [Google Scholar]
- KIEL J.W., REITSAMER H.A., WALKER J.S., KIEL F.W. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp. Eye Res. 2001;73:355–364. doi: 10.1006/exer.2001.1050. [DOI] [PubMed] [Google Scholar]
- KISS B., DALLINGER S., FINDL O., RAINER G., EICHLER H.G., SCHMETTERER L. Acetazolamide-induced cerebral and ocular vasodilation in humans is independent of nitric oxide. Am. J. Physiol. 1999;276:R1661–R1667. doi: 10.1152/ajpregu.1999.276.6.R1661. [DOI] [PubMed] [Google Scholar]
- KOTIKOSKI H., OKSALA O., VAPAATALO H., AINE E. Aqueous humour flow after a single oral dose of isosorbide-5-mononitrate in healthy volunteers. Acta. Ophthalmol. Scand. 2003a;81:355–360. doi: 10.1034/j.1600-0420.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- KOTIKOSKI H., VAPAATALO H., OKSALA O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr. Eye Res. 2003b;26:119–123. doi: 10.1076/ceyr.26.2.119.14511. [DOI] [PubMed] [Google Scholar]
- KRONCKE K.D., FEHSEL K., KOLB-BACHOFEN V. Nitric oxide: cytotoxicity versus cytoprotection – how, why, when, and where. Nitric Oxide. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- KRUPIN T., WEISS A., BECKER B., HOLMBERG N., FRITZ C. Increased intraocular pressure following topical azide or nitroprusside. Invest. Ophthalmol. Vis. Sci. 1977;16:1002–1007. [PubMed] [Google Scholar]
- LARSSON L.I., MAUS T.L., BRUBAKER R.F., NATHANSON J.A. Topically applied hydralazine: effects on systemic cardiovascular parameters, blood-aqueous barrier, and aqueous humor dynamics in normotensive humans. J. Ocul. Pharmacol. Therapeut. 1995;11:145–156. doi: 10.1089/jop.1995.11.145. [DOI] [PubMed] [Google Scholar]
- LIU R., FLAMMER J., HAEFLIGER I.O. Isoproterenol, forskolin, and cAMP-induced nitric oxide production in pig ciliary processes. Invest. Ophthalmol. Vis. Sci. 1999;40:1833–1837. [PubMed] [Google Scholar]
- LIU R., FLAMMER J., LUSCHER T.F., HAEFLIGER I.O. Adrenergic agonist-induced nitrite production in isolated pig ciliary processes. Graefes. Arch. Clin. Exp. Ophthalmol. 1998;236:613–616. doi: 10.1007/s004170050130. [DOI] [PubMed] [Google Scholar]
- LOMNICZI A., SUBURO A.M., ELVERDIN J.C., MASTRONARDI C.A., DIAZ S., RETTORI V., MCCANN S.M. Role of nitric oxide in salivary secretion. Neuroimmunomodulation. 1998;5:226–233. doi: 10.1159/000026342. [DOI] [PubMed] [Google Scholar]
- MACEDO M.P., LAUTT W.W. Shear-induced modulation of vasoconstriction in the hepatic artery and portal vein by nitric oxide. Am. J. Physiol. 1998;274:G253–G260. doi: 10.1152/ajpgi.1998.274.2.G253. [DOI] [PubMed] [Google Scholar]
- MCNEISH A.J., WILSON W.S., MARTIN W. Dominant role of an endothelium-derived hyperpolarizing factor (EDHF)-like vasodilator in the ciliary vascular bed of the bovine isolated perfused eye. Br. J. Pharmacol. 2001;134:912–920. doi: 10.1038/sj.bjp.0704332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER P., CHAMPION C., SCHLOTZER-SCHREHARDT U., FLAMMER J., HAEFLIGER I.O. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr. Eye Res. 1999;18:375–380. doi: 10.1076/ceyr.18.5.375.5355. [DOI] [PubMed] [Google Scholar]
- MEYER P., FLAMMER J., LUSCHER T.F. Endothelium-dependent regulation of the ophthalmic microcirculation in the perfused porcine eye: role of nitric oxide and endothelins. Invest. Ophthalmol. Vis. Sci. 1993;34:3614–3621. [PubMed] [Google Scholar]
- MILLAR J.C., SHAHIDULLAH M., WILSON W.S. Atriopeptin lowers aqueous humor formation and intraocular pressure and elevates ciliary cyclic GMP but lacks uveal vascular effects in the bovine perfused eye. J. Ocul. Pharmacol. Therapeut. 1997;13:1–11. doi: 10.1089/jop.1997.13.1. [DOI] [PubMed] [Google Scholar]
- MILLAR J.C., SHAHIDULLAH M., WILSON W.S. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J. Ocul. Pharmacol. Therapeut. 2001;17:225–234. doi: 10.1089/108076801750295263. [DOI] [PubMed] [Google Scholar]
- MIZUNO K., KOIDE T., SAITO N., FUJII M., NAGAHARA M., TOMIDOKORO A., TAMAKI Y., ARAIE M. Topical nipradilol: effects on optic nerve head circulation in humans and periocular distribution in monkeys. Invest. Ophthalmol. Vis. Sci. 2002;43:3243–3250. [PubMed] [Google Scholar]
- MIZUNO K., KOIDE T., YOSHIMURA M., ARAIE M. Neuroprotective effect and intraocular penetration of nipradilol, a beta-blocker with nitric oxide donative action. Invest. Ophthalmol. Vis. Sci. 2001;42:688–694. [PubMed] [Google Scholar]
- MURAD F.Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone Recent Prog. Horm. Res. 19985343–59.; discussion 59–60 [PubMed] [Google Scholar]
- NAKAZAWA T., TOMITA H., YAMAGUCHI K., SATO Y., SHIMURA M., KUWAHARA S., TAMAI M. Neuroprotective effect of nipradilol on axotomized rat retinal ganglion cells. Curr. Eye Res. 2002;24:114–122. doi: 10.1076/ceyr.24.2.114.8162. [DOI] [PubMed] [Google Scholar]
- NATHANSON J.A. Nitrovasodilators as a new class of ocular hypotensive agents. J. Pharmacol. Exp. Therapeut. 1992;260:956–965. [PubMed] [Google Scholar]
- ORTIZ P.A., GARVIN J.L. Role of nitric oxide in the regulation of nephron transport. Am. J. Physiol. –Renal Fluid Electrol. Physiol. 2002;282:F777–F784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- ORTIZ P.A., HONG N.J., GARVIN J.L. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(−) cotransporter activity. Am. J. Physiol. – Renal Fluid Electrol. Physiol. 2001;281:F819–F825. doi: 10.1152/ajprenal.2001.281.5.F819. [DOI] [PubMed] [Google Scholar]
- REITSAMER H.A., KIEL J.W. Relationship between ciliary blood flow and aqueous production in rabbits. Invest. Ophthalmol. Vis. Sci. 2003;44:3967–3971. doi: 10.1167/iovs.03-0088. [DOI] [PubMed] [Google Scholar]
- RILEY M.V., KISHIDA K. ATPases of ciliary epithelium: cellular and subcellular distribution and probable role in secretion of aqueous humor. Exp. Eye Res. 1986;42:559–568. doi: 10.1016/0014-4835(86)90046-1. [DOI] [PubMed] [Google Scholar]
- SCHMETTERER L., POLAK K. Role of nitric oxide in the control of ocular blood flow. Prog. Retinal Eye Res. 2001;20:823–847. doi: 10.1016/s1350-9462(01)00014-3. [DOI] [PubMed] [Google Scholar]
- SCHUMAN J.S., ERICKSON K., NATHANSON J.A. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp. Eye Res. 1994;58:99–105. doi: 10.1006/exer.1994.1199. [DOI] [PubMed] [Google Scholar]
- SHAHIDULLAH M., WILSON W.S., YAP M., TO C.H. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest. Ophthalmol. Vis. Sci. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- SIMOENS P., DE SCHAEPDRIJVER L., LAUWERS H. Morphologic and clinical study of the retinal circulation in the miniature pig. A: Morphology of the retinal microvasculature. Exp. Eye. Res. 1992;54:965–973. doi: 10.1016/0014-4835(92)90161-k. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T., KAWAKAMI H., SAWADA A., IWAKI M., TSUJI A., SUGIYAMA K., KITAZAWA Y. Effects of nitric oxide synthase inhibitor on intraocular pressure and ocular inflammation following laser irradiation in rabbits. Curr. Eye Res. 1998;17:308–315. doi: 10.1076/ceyr.17.3.308.5225. [DOI] [PubMed] [Google Scholar]
- TORIS C.B., YABLONSKI M.E., WANG Y.L., CAMRAS C.B. Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol. 1999;127:407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- WANG R.F., PODOS S.M. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp. Eye Res. 1995;60:337–339. doi: 10.1016/s0014-4835(05)80116-2. [DOI] [PubMed] [Google Scholar]
- WANG Y., VODOVOTZ Y., KIM P.K., ZAMORA R., BILLIAR T.R. Mechanisms of hepatoprotection by nitric oxide. Ann. NY Acad. Sci. 2002;962:415–422. doi: 10.1111/j.1749-6632.2002.tb04085.x. [DOI] [PubMed] [Google Scholar]
- WETZEL R.K., SWEADNER K.J. Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2001;42:763–769. [PubMed] [Google Scholar]
- WIEDERHOLT M., STURM A., LEPPLE-WIENHUES A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest. Ophthalmol. Vis. Sci. 1994;35:2515–2520. [PubMed] [Google Scholar]
- WILSON W.S., SHAHIDULLAH M., MILLAR C. The bovine arterially-perfused eye: an in vitro method for the study of drug mechanisms on IOP, aqueous humour formation and uveal vasculature. Curr. Eye Res. 1993;12:609–620. doi: 10.3109/02713689309001840. [DOI] [PubMed] [Google Scholar]
- WOELLFEL C.G., ROUSSEAU J.E., KERSTING E.J., NEILSEN S.W., LUCAS J.J. Intraocular pressurein vitamin A deficient Hilstein male calves. J. Dairy Sci. 1964;47:655–657. [Google Scholar]