Abstract
The present study evaluated the effect of interferon-γ (IFN-γ) on intestinal Na+/H+ exchange (NHE) activity and the intracellular signaling pathways set into motion after IFN-γ receptor activation.
Caco-2 cells express endogenous NHE1, NHE2 and NHE3 proteins, as detected by immunoblotting. Short- (0.5 h) and long- (24 h) term exposure of Caco-2 cells to IFN-γ resulted in a concentration-dependent decrease in NHE activity. Inhibition of NHE activity by IFN-γ was absent in cariporide-treated cells, but not in cells treated with S-3226. The long-term exposure to IFN-γ was accompanied by a 20% increase in surface NHE1 abundance and no changes in total NHE1 abundance.
Inhibition of Raf1, mitogen-activated protein kinase kinase (MAPKK/MEK) and p38 MAPK with, respectively, GW 5074, PD 98059 and SB 203580 and downregulation of protein kinase C (PKC) with phorbol-12,13-dibutyrate (100 nM for 24 h) prevented inhibition of NHE activity by IFN-γ (0.5 and 24 h exposure). The signal transducer and activator transcription factor 1 (STAT1) inhibitor epigallocatechin-3-gallate (EGCG) prevented inhibition of NHE activity by long- but not the short-term treatment with IFN-γ.
Treatment with IFN-γ activated phospho-p38 MAPK, this effect being detected as early as 1 h, persisting over 3 h and decreasing after 24 h. IFN-γ produced a sustained action of phospho-STAT1 that was prevented by EGCG and partially attenuated by SB 203580 and insensitive to downregulation of PKC.
In conclusion, short- and long-term inhibition of NHE1 activity by IFN-γ involves a complex signaling pathway that includes PKC activation and STAT1 phosphorylation, respectively, but is not accompanied by downregulation of NHE1.
Keywords: Interferon-γ, Na+/H+ exchanger, second messengers, Caco-2 cells
Introduction
The dominant symptom in both Crohn's disease (CD) and ulcerative colitis (UC) is diarrhea associated with autonomic nerve dysfunction (Magro et al., 2002), and recent evidence suggests that this may relate to a reduction in electrolyte absorption rather than increases in electrolyte secretion (Sundaram et al., 1997). In agreement with this view is the finding that the colonic mucosa of inflammatory bowel disease (IBD) patients responds poorly to secretagogues and that Na+ absorption is diminished (Sandle et al., 1990). Consistent with these findings is the observation that interferon-γ (IFN-γ), a cytokine suggested to be involved in the pathophysiology of IBD, reduces agonist-induced and cAMP-induced Cl− intestinal secretion (Colgan et al., 1994). IFN-γ has also been shown to downregulate the cystic fibrosis conductance regulator (Colgan et al., 1994), the Na+-K+-2Cl− cotransporter (Zund et al., 1996; Fish et al., 1999), the Na+/H+ exchanger (NHE) (Rocha et al., 2001) and the Na+-K+-ATPase (Sugi et al., 2001). IFN-γ has direct effects on intestinal epithelial cells, such as changes in cell morphology and decreased levels of proteins involved in transport and barrier function (Madara & Stafford, 1989; Adams et al., 1993; Besançon et al., 1994; Colgan et al., 1994; Fish et al., 1999; Sugi et al., 2001), but the overall cytoskeletal structure appears to be maintained (Madara & Stafford, 1989). CD is a Th1-mediated disease, and an increase in IFN-γ-secreting cells and IFN-γ mRNA levels has been described in intestinal lesions of patients with CD (Podolsky, 1991; Breese et al., 1993; Niessner & Volk, 1995; Adorini & Francesco, 1997).
In general, the signal transduction pathway initiated by binding of IFN-γ to its receptor leads first to Janus kinase (JAK) 1 and 2 activation and their association with the IFN-γ receptor (Haque & Williams, 1998). JAK then phosphorylates IFN-γ receptor on specific tyrosines, which serve as docking sites for the signal transducer and activator transcription factor 1 (STAT1). Recently, IFN-γ was also shown to stimulate mitogen-activated protein kinase (MAPK) activity, namely extracellular signal-regulated kinases (ERK1 and ERK2) (Liu et al., 1994) and p38 MAPK (Verma et al., 2002). Activation of MAPKs is controlled via membrane-associated signaling complexes and involves a network that includes Ras protein(s), the Raf family (Raf-1, B-Raf, A-Raf and MEKK) of serine kinases and MAPK kinases, MEK1 and MEK2 as upstream regulators of ERK1 and ERK2 and MEK3 and MEK6 as upstream regulators of p38 MAPK (Pearson et al., 2001). Recently, we have reported on the effects of IFN-γ on intestinal Na+-K+-ATPase activity and the intracellular signaling pathways involved in cultured human intestinal epithelial Caco-2 cells, using probes that interact with Raf-1, MAPK/ERK kinase (MEK), ERK2, p38 MAPK, protein kinase C (PKC) and STAT1, and measuring the degree of activation of p38 MAPK and STAT1. It was shown that the decrease in Na+-K+-ATPase activity by IFN-γ may involve the activation of PKC downstream STAT1 phosphorylation and Raf-1, MEK, ERK2 and p38 MAPK pathways, in a complex sequence of events (Magro et al., 2004).
In the present study, the short- and long-term regulation of the intestinal NHE by IFN-γ was evaluated and the intracellular signaling pathways set into motion after IFN-γ receptor activation identified. For this purpose, human intestinal epithelial Caco-2 cells, which endogenously express NHE1, NHE2 and NHE3 isoforms, were used. A decrease in NHE1 activity after acute and chronic exposure to IFN-γ involving different signaling pathways is reported. The short-term regulation of NHE activity by IFN-γ involves mainly the activation of Raf-1, MEK, p38 MAPK and PKC with NHE1 as an effector protein for PKC. The transduction mechanisms set into motion by long-term exposure to IFN-γ involve STAT1 activation with p38 MAPK playing a minor role in SER701 phosphorylation of STAT1.
Methods
Cell culture
Caco-2 cells (ATCC 37-HTB; passages 39–49) were obtained from the American Type Culture Collection (Rockville, MD, U.S.A.) and maintained in a humidified atmosphere of 5% CO2–95% air at 37°C. Cells were grown in minimal essential medium (Sigma Chemical Company, St Louis, MO, U.S.A.) supplemented with 106 U l−1 penicillin G, 250 μg l−1 amphotericin B, 100 ng l−1 streptomycin (Sigma), 20% fetal bovine serum (Sigma) and 25 mmol l−1 N-2-hydroxyethylpiperazine-N′-2-ethanosulfonic acid (HEPES; Sigma). For subculturing, the cells were dissociated with 0.05% trypsin-EDTA, split 1 : 3 and subcultured in Costar Petri dishes with 21 cm2 growth area (Costar, Badhoevedorp, The Netherlands). For studies on NHE activity, the cells were seeded in 96-well plates. The cell medium was changed every 2 days, and the cells reached confluence after 5 days of initial seeding. For 24 h prior to each experiment, the cell medium was free of fetal bovine serum. Experiments were generally performed 2 days after cells reached confluence, usually 7 days after the initial seeding; each cm2 contained about 100 μg of cell protein.
NHE activity
NHE activity was assayed as the initial rate of intracellular pH (pHi) recovery after an acid load imposed by 20 mM NH4Cl followed by removal of Na+ from the Krebs' modified buffer solution (in mM: NaCl 140, KCl 5.4, CaCl2 2.8, MgSO4 1.2, NaH2PO4 0.3, HEPES 10, glucose 5 (pH 7.4)) in the absence of CO2/HCO3. In these experiments, NaCl was replaced by an equimolar concentration of tetramethylammonium chloride (TMA). pHi measurements were performed in cells cultured in 96-well plates, as previously described (Gomes et al., 2001; Gomes & Soares-da-silva, 2002a; Pedrosa et al., 2004). Briefly, cells were loaded in serum-free medium with 5 μM BCECF/AM, the membrane-permeant acetoxymethyl ester derivative of 2′,7′-bis (carboxyethyl)-5,6-carboxyfluorescein (BCECF), for 40 min at 37°C in 5% CO2–95% air atmosphere. The cells were washed free of dye and, unless stated otherwise, the test compounds were added to the extracellular fluid 0.5 h before starting the sodium-dependent pHi recovery. Cells were placed in the sample compartment of a dual-scanning microplate spectrofluorometer (Spectramax Gemini, Molecular Devices, Sunnyvale, U.S.A.) and fluorescence was monitored every 17 s alternating between 440 and 490 nm excitation at 535 nm of emission, with a cutoff filter of 530 nm. The ratio of intracellular BCECF fluorescence at 490 and 440 nm was converted to pHi by comparison with values from an intracellular calibration curve using the 10 μM nigericin and high-K+ method with pHi ranging from pH 6.6 to 7.8 (Gomes et al., 2001; Gomes & Soares-da-silva, 2002a).
In experiments aimed to evaluate the sensitivity of the sodium-dependent pHi recovery to selective inhibitors of NHE isoforms, cells were treated with increasing concentrations of amiloride, EIPA, cariporide, S-3226 or vehicle for 0.5 h before starting the sodium-dependent pHi recovery. Amiloride and EIPA are both more effective in inhibiting NHE1 than NHE3, although EIPA is relatively more potent in inhibiting NHE1 (Noel & Pouyssegur, 1995). On the other hand, cariporide and S-3226 are relatively selective for inhibition of NHE1 and NHE3 isoforms, respectively (Schwark et al., 1998). Differentiation between NHE1 and NHE2 can be achieved while employing selective concentrations of cariporide; NHE1 is fully inhibited by low concentrations (1 μM), whereas inhibition of NHE2 requires much higher concentrations of cariporide (25 μM) (Bachmann et al., 1998).
In experiments in which the effect of IFN-γ on NHE activity was tested, cells were treated with a given concentration of IFN-γ (from 10 to 1000 U ml−1) or vehicle for 24, 3 or 0.5 h before starting the sodium-dependent pHi recovery. In some experiments, cariporide (300 nM) or S-3226 (300 nM) were coincubated with IFN-γ (1000 U ml−1) for 0.5 h before starting the sodium-dependent pHi recovery. Assessment of signaling pathways used by IFN-γ was conducted by using a single concentration (1000 U ml−1) and a 0.5 and 24 h exposure of Caco-2 monolayers to human IFN-γ. Cells were pretreated with inhibitors of interest 0.5 h prior to the addition of IFN-γ, which was subsequently added to the culture well (inhibitor not washed out).
In experiments aimed to evaluate the effect of PKC and p38 MAPK activation on NHE activity, cells were treated with increasing concentrations of phorbol-12,13-dibutyrate (PDBu) and anisomycin, respectively, for 30 min before starting the sodium-dependent pHi recovery. In some experiments, cells were pretreated with inhibitors of interest 1 h prior to the addition of PDBu or anisomycin.
Measurement of cell viability
Cell viability was measured as previously described (Pedrosa & Soares-da-silva, 2002) using calcein-AM (Molecular Probes, Eugene, OR, U.S.A.). The membrane-permeant calcein-AM, a nonfluorescent dye, is taken up and converted by intracellular esterases to membrane-impermeant calcein, which emits green fluorescence. After treatment with IFN-γ (1000 U ml−1) or vehicle for 24 h, cells were washed twice with Hanks' medium (medium composition (in mM): NaCl 137, KCl 5, MgSO4 0.8, Na2HPO4 0.33, KH2PO4 0.44, CaCl2 0.25, MgCl2 1.0, Tris-HCl 0.15, sodium butyrate 1.0 (pH 7.4)) and loaded with 2 μM calcein-AM in Hanks' medium at room temperature for 30 min. Fluorescence was measured at 485 nm excitation and 530 nm emission wavelengths in a multiplate reader (Spectromax Gemini, Molecular Devices). To determine minimum staining for calcein-AM (calceinmin), six wells were treated with ethanol 30 min before calcein-AM addition. The percent viability was then calculated as [(calceinsample−calceinmin)/(calceincontrol−calceinmin)] × 100.
NHE immunoblotting
Caco-2 cells cultured to 90% of confluence were washed twice with PBS and total cell protein was extracted for NHE1, NHE2 and NHE3 detection. Briefly, to obtain total cell extract, cells were lysed by brief sonication (15 s) in lysis buffer with protease inhibitors (150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg ml−1 PMSF, and aprotinin and leupeptin 2 μg ml−1 each) and incubated on ice for 1 h. After centrifugation (16,000 × g, 30 min, 4°C), the supernatant was mixed in 6 × sample buffer (0.35 M Tris-HCl, 4% SDS, 30% glycerol, 9.3% DTT (pH 6.8), 0.01% bromophenol blue) and boiled for 5 min. The proteins (50 μg) were subjected to SDS–PAGE (10% SDS–polyacrylamide gel) and electrotransfered onto nitrocellulose membranes. The transblot sheets were blocked with 5% of nonfat dry milk in 25 mM Tris-HCl (pH 7.5), 150 mM NaCl and 0.1% Tween 20 overnight at 4°C. Then, the membranes were incubated with appropriately diluted antibodies: rabbit anti-NHE1, anti-NHE2 or anti-NHE3 polyclonal isoform specific antibodies (Alpha Diagnostics, Autogenbioclear, Wiltshire, U.K.) and the anti-β-actin primary antibody (Upstate Biotechnologies). The reaction was detected by peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and ECL system (Amersham Life, Arlington Heights, IL, U.S.A.). The densities of the appropriate bands were determined using Quantity One imaging software (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Protein concentration was measured using the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and bovine serum albumin as standard.
Cell surface biotinylation
Cell surface biotinylation was used to determine apical membrane NHE1 expression in Caco-2 cells treated with IFN-γ (1000 U ml−1) or vehicle for 24 h. Briefly, confluent cells were rinsed twice with ice-cold PBS with 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS-Ca-Mg). The apical surface was then exposed to 500 μg ml−1 of Sulfo-NHS-Biotin (Pierce, Rockford, IL, U.S.A.) in biotinylation buffer (10 mM triethanolamine, 2 mM CaCl2, 150 mM NaCl (pH 7.4)) for 20 min with horizontal motion at 4°C. After labeling, the cells were rinsed with quenching solution (PBS-Ca-Mg with 100 mM glycine) and cells lysed with RIPA buffer with protease inhibitors (150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg ml−1 PMSF, and aprotinin and leupeptin 2 μg ml−1 each), briefly sonicated and incubated on ice for about 1 h. After centrifugation (16,000 × g, 30 min, 4°C), the supernatant was adjusted to 3.4 mg ml−1 and the biotinylated protein precipitated overnight at 4°C with 100 μl of streptavidin-agarose beads (Pierce, Rockford, IL, U.S.A.) in a total volume of 500 μl. The streptavidin-agarose beads were washed twice with PBS and the bound proteins solubilized with SDS sample buffer (0.125 M Tris-HCl, 4% SDS, 20% glycerol, 2% 2-mercaptoethanol (pH 6.8)) and subjected to SDS–PAGE and blotting for NHE1 as described in NHE immunoblotting.
p38 MAPK immunoprecipitation and immunoblotting
p38 MAPK activity in Caco-2 cells exposed to IFN-γ (1000 U ml−1) and anisomycin (100 nM) for 1, 3 and 24 h was analyzed by immunoblotting using the p38 MAPK assay kit (Cell Signaling Technology™) according to the manufacturer's protocol. Briefly, cells were washed twice with PBS, lysed with cell lysis buffer and briefly sonicated (15 s). A monoclonal phospho-specific antibody to p38 MAPK (Thr180/Tyr182) was used to immunoprecipitate active p38 MAPK from cell lysates overnight at 4°C. Beads were sedimented by centrifugation (1300 r.p.m., 1 min, 4°C) and the pellet was washed with lysis buffer and kinase buffer. The pellet was then suspended in kinase buffer supplemented with 200 μM ATP and 2 μg ATF-2 fusion protein and incubated for 30 min at 30°C. The p38 active MAPK ATF-2-induced phosphorylation reaction was terminated by addition of SDS sample buffer (187.5 mM Tris–HCl (pH 6.8), 6% w v−1 SDS, 30% glycerol, 150 mM DTT, 0.03% w v−1 bromophenol blue). Samples were denatured at 95°C for 5 min and loaded onto 10% SDS–PAGE and electrophoretically transferred onto nitrocellulose membranes. The transblot sheets were blocked for 1 h with 5% of nonfat dry milk in 25 mM Tris-HCl (pH 7.5), 150 mM NaCl and 0.1% Tween 20 at room temperature. Then, the membranes were incubated with phospho-ATF-2 (Thr71) antibody (1 : 1000) and the immunocomplexes detected by the Phototope®-HRP Western detection kit.
STAT1 immunoblotting
Total and phosphorylated STAT1 protein levels in Caco-2 cells exposed to IFN-γ (1000 U ml−1) and anisomycin (100 nM) for 1, 3 and 24 h were analyzed using the PhosphoPlus® Stat1 (Tyr701) antibody kit (Cell Signaling Technology™) according to the manufacturer's protocol. In some experiments, cells were pretreated with inhibitors of interest 0.5 h prior to the addition of IFN-γ, which was subsequently added to the culture well (inhibitor not washed out). Briefly, cells (9.5 cm2) were washed twice with PBS, lysed with 200 μl SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 2% w v−1 SDS, 10% glycerol, 50 mM DTT, 0.1% w v−1 bromophenol blue) and briefly sonicated (15 s). Samples were then denatured for 5 min at 95°C, loaded (5 μl) onto 10% SDS–PAGE and electrophoretically transferred onto nitrocellulose membranes. The transblot sheets were blocked for 3 h with 5% of nonfat dry milk in 25 mM Tris-HCl (pH 7.5), 150 mM NaCl and 0.1% Tween 20 at room temperature. Then, the membranes were incubated with phospho-Stat1 or Stat1 antibody (1:1000) and the immunocomplexes detected with the Phototope®-HRP Western detection kit.
Data analysis
Geometric means are given with 95% confidence limits and arithmetic means are given with s.e.m. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Student's t-test or the Newman–Keuls test for multiple comparisons. A P-value less than 0.05 was assumed to denote a significant difference.
Drugs
Anisomycin, (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC) and human IFN-γ were obtained from Sigma (Chemical Company, St Louis, MO, U.S.A.). GW 5074 (3-(3,5-dibromo-4- hydroxybenzylidene-5-iodo-1,3-dihydro-indol-2-one) was purchased from Tocris (Tocris Cookson Inc., Avonmouth, U.K.). PD 98059 (2′-amino-3′-methoxyflavone) and SB 203580 (4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole) were obtained from Calbiochem (Calbiochem-Novabiochem Corporation, San Diego, CA, U.S.A.). Cariporide (4-isopropyl-3-methylsulfonylbenzoyl-guanidine methanesulfonate) and S-3226 (3-(54)-N-isopropylidene-2-methyl-acrylamide dihydrochloride) were kindly provided Dr H.J. Lang from Aventis Pharma Deutschland (Frankfurt, Germany). Stock solutions of anisomycin, EGCG, EGC, GW 5074, PD 98059, SB 203580, cariporide and S-3226 were prepared in 100% DMSO and working solutions contained 0.1% DMSO. Stock solutions of IFN-γ were prepared in water.
Results
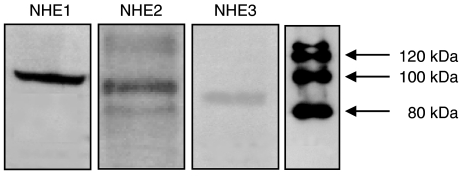
In the present study, NHE activity was assayed in Caco-2 cells loaded with a pH-sensitive dye (BCECF) as the Na+-dependent recovery of pHi measured after an acid load imposed by 20 mM NH4Cl followed by removal of Na+ from the Krebs modified buffer solution in the absence of CO2/HCO3. After acidification, Caco-2 cells showed a rapid alkalinization on addition of 140 mM Na+. This alkalinization process was inhibited by amiloride (1 mM), EIPA (10 μM) and cariporide (1 μM), but was insensitive to S-3226 (1 μM). Cariporide (IC50=5 [2, 18] nM) was considerably more potent than EIPA (IC50=24 [12, 49] nM) and amiloride (IC50=11 [3, 34] μM) inhibiting NHE activity in Caco-2 cells. Differences in sensitivity to these inhibitors are in agreement with the observation that Caco-2 cells express different NHE subtypes, namely NHE1, NHE2 and NHE3 isoforms (Biemesderfer et al., 1997; Karim et al., 1999). The presence of NHE1, NHE2 and NHE3 isoforms was confirmed by immunoblotting, as shown in Figure 1. The anti-NHE1, anti-NHE2 and anti-NHE3 protein antibodies recognized single bands of about 100, 90 and 85 kDa in Caco-2 cells. The size of the NHE isoforms identified in this study is comparable to the size previously reported (Noel & Pouyssegur, 1995). In polarized cells in culture, the NHE3 isoform is exclusively restricted to the apical membranes; however, the NHE1 isoform is expressed in the apical and basal lateral membranes (Noel et al., 1996).
Figure 1.
Expression of NHE1, NHE2 and NHE3 isoforms in Caco-2 cells. Immunoblots were repeated three times. NHE1 ∼100 kDa, NHE2 ∼90 kDa and NHE3 ∼85 kDa.
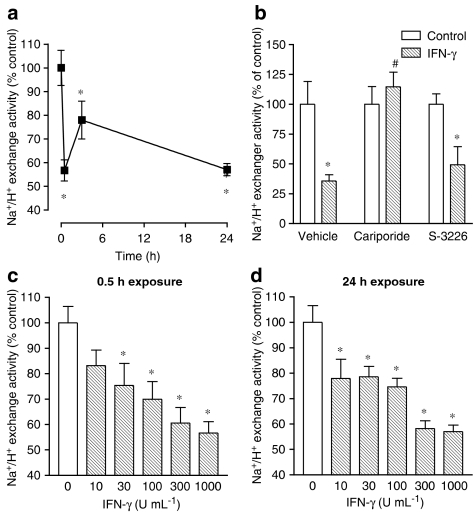
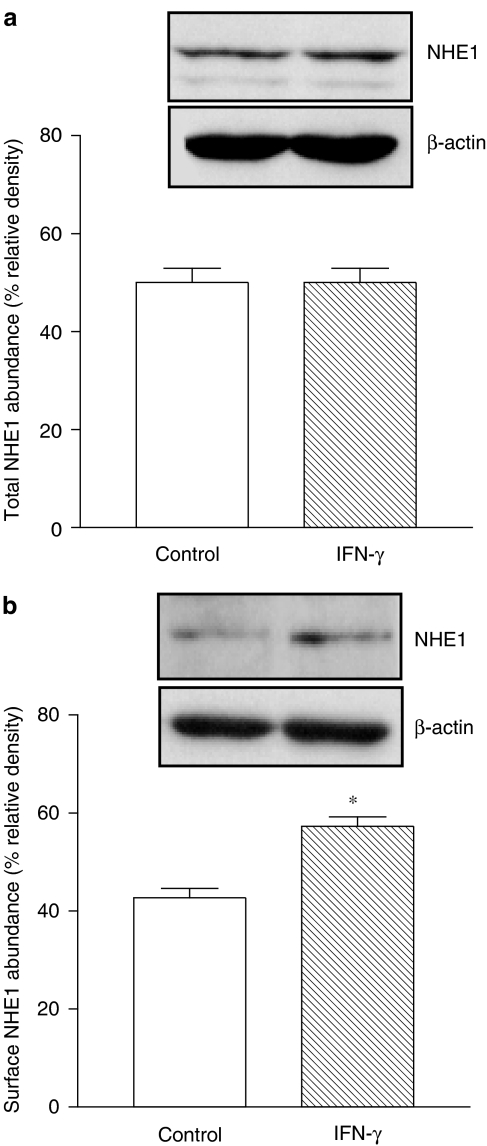
As indicated in Figure 2a, the maximal inhibitory effect of IFN-γ (1000 U ml−1) on NHE activity was obtained at 0.5 h with sustained inhibition up to 24 h exposure. Exposure of Caco-2 cells to 1000 U ml−1 IFN-γ during 24 h did not reduce cell viability when compared to vehicle-treated cells (102.7±4.1 vs 100±3.9% of live cells). To evaluate which NHE isoform was involved in the inhibitory effect of IFN-γ, cells were coincubated with IFN-γ (1000 U ml−1) plus cariporide (300 nM) or S-3226 (300 nM) for 30 min before starting the sodium-dependent pHi recovery. As shown in Figure 2b, inhibition of NHE activity by IFN-γ (1000 U ml−1, 0.5 h exposure) was absent in cells treated with cariporide (300 nM), but not in cells treated with S-3226 (300 nM), suggesting that NHE1 is the predominant isoform inhibited by the cytokine (Figure 2b). The short- (0.5 h) and long-term (24 h) treatment with increasing concentrations of human IFN-γ (10–1000 U ml−1) resulted in a concentration-dependent decrease in NHE activity that was identical with either exposure time (Figure 2c and d). To evaluate whether inhibition of NHE1 by IFN-γ was related to downregulation of NHE1, Caco-2 cells were treated with IFN-γ (1000 U ml−1) for 24 h and the abundance of total NHE1 and the abundance of biotinylated NHE1 quantified. As shown in Figure 3a, the abundance of total NHE1 was not altered after the long-term exposure (24 h) to IFN-γ, but the abundance of biotinylated NHE1 was significantly increased (Figure 3b). The abundance of total or surface NHE1 was corrected for the amount of β-actin.
Figure 2.
(a) Time-dependent effect of IFN-γ (1000 U ml−1) on NHE activity in cultured Caco-2 cells. (b) Effect of cariporide (300 nM) and S-3226 (300 nM) on the IFN-γ- (1000 U ml−1) induced inhibition of NHE activity in cultured Caco-2 cells. Concentration-dependent effect of IFN-γ (1000 U ml−1) after (c) 0.5 h and (d) 24 h of exposure. Columns or symbols represent means of seven experiments per group; vertical lines indicate s.e.m. values. *P<0.05, values significantly different from control values using the Newman–Keuls test; #P<0.05, values significantly different from values with IFN-γ alone using the Newman–Keuls test.
Figure 3.
Abundance of (a) total NHE1 and (b) surface NHE1 in Caco-2 cells treated for 24 h with IFN-γ (1000 U ml−1). Western blots were repeated 4–6 times. Columns indicate relative density and represent the mean of five separate experiments; vertical lines indicate s.e.m. values. *P<0.05, values significantly different from corresponding control values using the Student's t-test.
The next series of experiments were intended to explore the signaling pathways from IFN-γ receptor stimulation downstream to NHE, namely the involvement of STAT, MAPK and PKC. STAT1, Raf-1, MEK1, ERK1/2 and p38 MAPK can be probed with specific inhibitors. EGCG specifically inhibits the tyrosine phosphorylation of STAT1, but not of STAT3 (Menegazzi et al., 2001); EGC does not interfere with the tyrosine phosphorylation of STAT1 (Menegazzi et al., 2001). GW 5074 is a selective Raf-1 kinase inhibitor (Lackey et al., 2000). PD 98059 inhibits activation of MEK and therefore blocks the activation of ERK1/2 (Davies et al., 2000). PD 98059 binds to MEK1 preventing its activation by upstream protein kinases, such as Raf (Alessi et al., 1995). PD 98059 is therefore not a protein kinase inhibitor, but a compound that stops one protein kinase (Raf) from activating another (MEK1) (Davies et al., 2000). p38 MAPK α and β can be inhibited selectively with SB 203580 (Saklatvala et al., 1996; Davies et al., 2000). Assessment of signaling pathways used by IFN-γ was conducted by using a single concentration (1000 U ml−1) and a 0.5 and 24 h exposure of Caco-2 monolayers to human IFN-γ. Cells were pretreated with inhibitors of interest 0.5 h prior to the addition of IFN-γ, which was subsequently added to the culture well (inhibitor not washed out). As shown in Table 1, the Raf-1 inhibitor GW 5074, the MEK1 inhibitor PD 98059 and the p38 MAPK inhibitor SB 203580 effectively prevented the short-term inhibitory effect of IFN-γ on NHE activity. By contrast, the STAT1 inhibitor EGCG (Table 1) failed to alter the short-term inhibitory effect of IFN-γ on NHE activity. The long-term inhibitory effects of IFN-γ (24 h exposure) were also evaluated, and the Raf-1 inhibitor GW 5074 (Table 1) markedly attenuated the long-term inhibitory effect of IFN-γ (24 h exposure) on NHE activity. From the results shown above, one would expect the involvement of MAPK downstream MEK. To test this assumption, cells were treated with PD 98059 (10 μM) and SB 203580 (10 μM). As shown during the short-term treatment with IFN-γ, both PD 98059 and SB 203580 completely attenuated the inhibition of NHE activity after long-term exposure to IFN-γ. The STAT1 inhibitor EGCG (Table 1), but not the inactive analog EGC, prevented the long-term inhibitory effect of IFN-γ (24 h exposure) on NHE activity. In the short-term series of experiments, NHE activity (in pH units s−1) was not affected by GW 5074 (0.0033±0.0002 vs 0.0026±0.0002), EGC (0.0020±0.0003 vs 0.0030±0.0003) and EGCG (0.0020±0.0003 vs 0.0024±0.0003), whereas a significant (P<0.05) increase in NHE was observed after treatment with PD 98059 (0.0028±0.0003 vs 0.0053±0.0002) or SB 203580 (0.0028±0.0003 vs 0.0040±0.0003). In the long-term series of experiments, NHE activity (in pH units s−1) was not affected by GW 5074 (0.0041±0.0003 vs 0.0050±0.0003), EGC (0.0033±0.0003 vs 0.0033±0.0002), EGCG (0.0033±0.0003 vs 0.0030±0.0003), PD 98059 (0.0050±0.0003 vs 0.0053±0.0002) or SB 203580 (0.0050±0.0003 vs 0.0058±0.0005).
Table 1.
Effect of GW 5074 (20 nM), PD 98059 (10 μM), SB 203580 (10 μM), EGCG (20 μM) and EGC (20 μM) on NHE activity after short- (0.5 h) and long-term (24 h) exposure to IFN-γ (1000 U ml−1) in Caco-2 cells
| Short-term | Long-term | |
|---|---|---|
| Vehicle | 100±7 | 100±9 |
| IFN-γ | 69±7* | 57±3* |
| GW 5074 | 100±9 | 100±8 |
| GW 5074+IFN-γ | 112±7 | 99±7 |
| Vehicle | 100±7 | 100±10 |
| IFN-γ | 57±4* | 64±9* |
| PD 98059 | 100±14 | 100±6 |
| PD 98059+IFN-γ | 95±9 | 104±6 |
| Vehicle | 100±10 | 100±13 |
| IFN-γ | 64±9* | 58±2* |
| SB 203580 | 100±8 | 100±5 |
| SB 203580+IFN-γ | 116±4 | 114±10 |
| Vehicle | 100±9 | 100±13 |
| IFN-γ | 52±6* | 58±2* |
| EGCG | 100±12 | 100±10 |
| EGCG+IFN-γ | 62±8* | 103±12 |
| EGC | 100±8 | 100±11 |
| EGC+IFN-γ | 68±4* | 52±5* |
Values indicate means of 12 experiments per group.
P<0.05, values significantly different from control values using the Student's t-test. The NHE activity (in pH units s−1) in vehicle-treated cells in the short- and long-term stimulation series was 0.0033±0.0002 and 0.0050±0.0003, respectively.
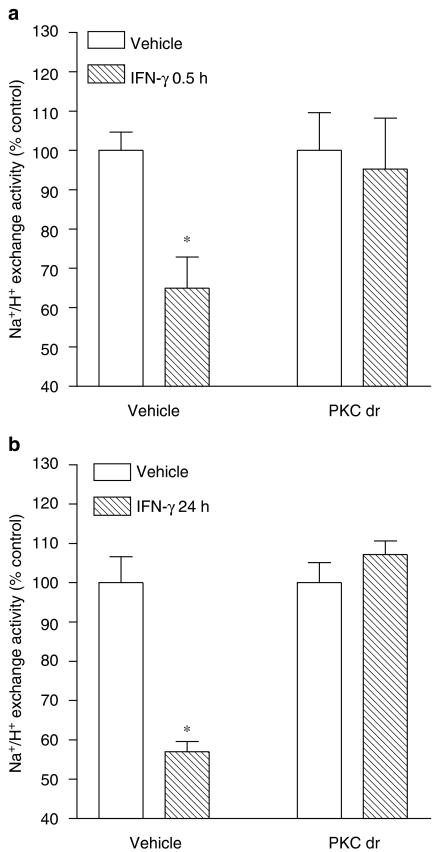
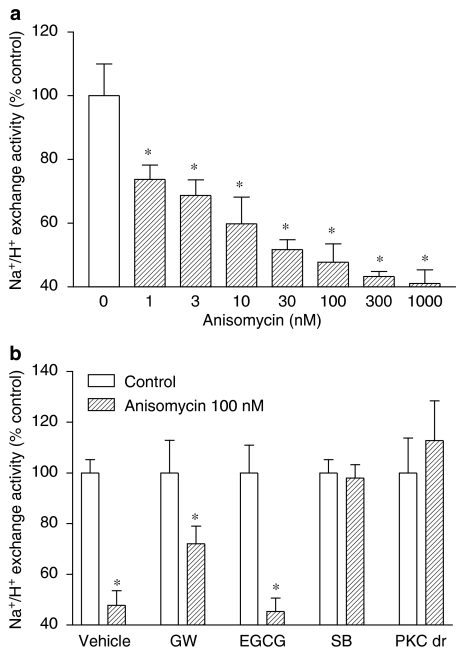
Because there is evidence that PKC may function as a serine kinase for STAT1 and as an upstream regulator of the p38 MAPK that plays an important role in biological responses to IFN-γ (Watanabe et al., 1995), it was decided to test whether PKC is involved in inhibition of NHE activity by IFN-γ. Treatment of Caco-2 cells with PDBu (1–1000 nM) for 0.5 h resulted in marked inhibition of NHE activity (IC50=2.3 [0.1, 52.0] nM). Downregulation of phorbol ester-sensitive PKC isoforms, obtained with overnight (∼18 h) incubation of cells in the presence of PDBu (100 nM) (Gomes & Soares-da-silva, 2002b; Pedrosa et al., 2004), prevented the short-term (0.5 h exposure) inhibitory effects of PDBu on NHE activity (Table 2). NHE activity (in pH units s−1) was significantly (P<0.05) reduced after PKC downregulation (0.0030±0.0002 vs 0.0020±0.0001). On the other hand, the short-term inhibitory effects of PDBu on NHE activity were not altered by the Raf-1 inhibitor GW 5074 and the STAT1 inhibitor EGCG (Table 2) or the MEK1 inhibitor PD 98059 and the p38 MAPK inhibitor SB 203580 (Table 2). To inhibit the signaling pathway initiated with PKC activation that appears to be involved after short- and long-term exposure to IFN-γ, it was decided to downregulate PKC activity, as described above. As shown in Figure 4, downregulation of phorbol ester-sensitive PKC isoforms also markedly attenuated the short-term (Figure 4a) and the long-term (Figure 4b) inhibitory effects of IFN-γ on NHE activity.
Table 2.
Inhibitory effect of PDBu (100 nM) on NHE activity, effect of downregulation of phorbol ester-sensitive PKC isoforms (PKC dr, overnight treatment with 300 nM PDBu) on NHE activity after 0.5 h exposure to PDBu and effect of PD 98059 (10 μM), SB 203580 (10 μM), GW 5074 (150 nM) and EGCG (20 μM) on NHE activity after 0.5 h exposure to PDBu
| Vehicle | PDBu | |
|---|---|---|
| Vehicle | 100±6 | 45±3* |
| PKC dr | 100±6 | 100±8 |
| PD 98059 | 100±11 | 65±4* |
| SB 203580 | 100±5 | 67± 6* |
| GW 5074 | 100±11 | 63±4* |
| EGCG | 100±9 | 59±6* |
Values indicate means of 13 experiments per group.
P<0.05, values significantly different from control values using the Newman–Keuls test. The NHE activity in pH units s−1 was as follows: vehicle, 0.0030±0.0002; PKC dr, 0.0020±0.0001; GW 5074, 0.0030±0.0003; PD 98059, 0.0025±0.0002; SB 203580, 0.0025±0.0003; EGCG, 0.0035±0.0003.
Figure 4.
Effect of downregulation of phorbol ester-sensitive PKC isoforms (PKC dr) on (a) short-term and (b) long-term inhibitory effects of IFN-γ (1000 U ml−1) on NHE activity. Columns represent means of seven experiments per group; vertical lines indicate s.e.m. values. *P<0.05, values significantly different from control values using the Student's t-test. The NHE activity in pH units s−1 was as follows: vehicle, 0.0030±0.0002; PKC dr, 0.0020±0.0002.
The role of p38 MAPK in inhibiting NHE activity was also examined in Caco-2 cells treated for 0.5 h with the p38 MAPK agonist anisomycin. As shown in Figure 5a, anisomycin inhibited in a concentration-dependent manner NHE activity in Caco-2 cells. The inhibitory effects of anisomycin (100 nM) on NHE activity were not altered by the Raf-1 inhibitor GW 5074 and the STAT1 inhibitor EGCG, but were completely prevented by the p38 MAPK inhibitor SB 203580 (Figure 5b) and by the downregulation of phorbol ester-sensitive PKC isoforms (Figure 5b).
Figure 5.
(a) Concentration-dependent effect of anisomycin (1–1000 nM) on NHE activity. (b) Effect of GW 5074 (150 nM), PD 98059 (10 μM), SB 203580 (10 μM), EGCG (20 μM) and downregulation of phorbol ester-sensitive PKC isoforms (PKC dr) on NHE activity after 0.5 h exposure to anisomycin (100 nM). Columns represent means of seven experiments per group; vertical lines indicate s.e.m. values. *P<0.05, values significantly different from control values using the Newman–Keuls test. The NHE activity (in pH units s−1) was as follows: vehicle, 0.0030±0.0005; GW 5074, 0.0030±0.0005; EGCG, 0.0040±0.0004; SB 203580, 0.0033±0.0003; PKC dr, 0.0020±0.0003.
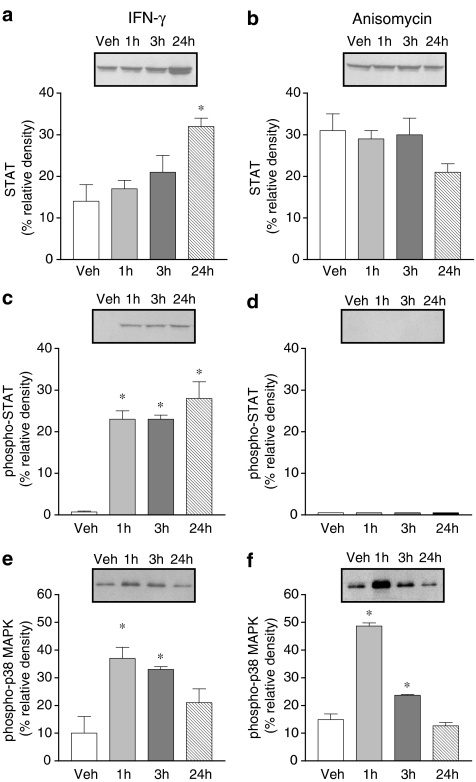
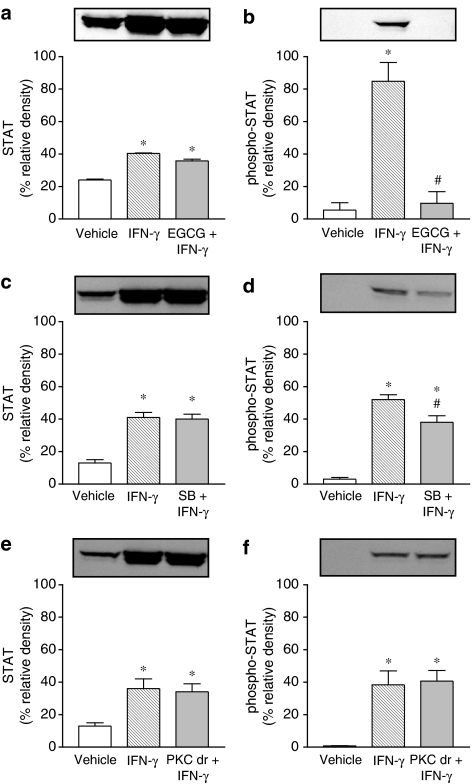
STAT, phospho-STAT and phospho-p38 MAPK activation was evaluated in Caco-2 cells exposed to IFN-γ (1000 U ml−1) and anisomycin (100 nM) for 1, 3 and 24 h. Treatment with IFN-γ for 24 h markedly increased total STAT and phospho-STAT (Figure 6a and c). However, 1 and 3 h treatment with IFN-γ was found to increase phospho-STAT only (Figure 6a and c). Treatment with IFN-γ resulted in marked activation of pospho-p38 MAPK, this effect being detected as early as 1 h, persisting over 3 h and decreasing after 24 h (Figure 6e). By contrast, the p38 MAPK activator anisomycin did not alter the expression of total STAT (Figure 6b) and phospho-STAT (Figure 6d) was not detected, although it markedly increased phospho-p38 MAPK (Figure 6f). The effects of anisomycin on phospho-p38 MAPK were evident as early as 1 h and faded after 3 h (Figure 6f). Activation of phospho-STAT after 24 h exposure to IFN-γ (1000 U ml−1) was completely prevented by EGCG (20 μM) (Figure 7b) and partially attenuated by SB 203580 (10 μM) (Figure 7d). Downregulation of PKC following overnight exposure of cells to 100 nM PDBu failed to alter the activation of phospho-STAT after 24 h exposure to IFN-γ (Figure 7f).
Figure 6.
Abundance of total STAT1, phospho-STAT1 and phospho-p38 MAPK in Caco-2 cells treated with vehicle and IFN-γ (1000 U ml−1) or anisomycin (100 nM) for 1, 3 and 24 h.
Figure 7.
Effect of EGCG (20 μM), SB 203580 (10 μM) and PKC downregulation (PKC dr; overnight treatment with 100 nM PDBu) on the abundance of STAT1 and phospho-STAT1 in Caco-2 cells treated for 24 h in the absence (vehicle) and presence of IFN-γ (1000 U ml−1).
Discussion
The present study was aimed at evaluate the effect of IFN-γ on intestinal NHE activity and identify the intracellular signaling pathways set into motion after IFN-γ receptor activation. A decrease in NHE1 activity after acute and chronic exposure to IFN-γ that is not accompanied by downregulation of NHE1 and involves different signaling pathways is reported. The short-term regulation of NHE activity by IFN-γ involves mainly the activation of Raf-1, MEK, p38 MAPK and PKC with NHE1 as an effector protein for PKC. The transduction mechanisms set into motion by long-term exposure to IFN-γ involve STAT1 activation with p38 MAPK playing a minor role in SER701 phosphorylation of STAT1.
Although inhibition of NHE activity by IFN-γ has been reported before, the signaling pathways that initiate the IFN-γ-induced inhibition and downregulation of NHE have not been examined. Some studies suggested that inhibition of NHE2 and NHE3 activities by IFN-γ could be mediated by downregulation of NHE2 and NHE3 (Rocha et al., 2001), but provided no insights into downstream events. On the other hand, these authors showed that downregulation of NHE2 and NHE3 occurred just after prolonged exposure to IFN-γ (24 and 48 h), being devoid of effects as early as after 2 h exposure to the cytokine (Rocha et al., 2001). The results in the present study provide evidence that inhibition of NHE activity by IFN-γ occurs as early as 0.5 h exposure with a magnitude similar to that observed at 24 h. However, it is likely that the NHE isoform involved in IFN-γ-mediated events is NHE1, although Caco-2 cells used in the present study expressed NHE1, NHE2 and NHE3, as previously descried (Janecki et al., 1999). There is no evidence in the literature for the presence of isoforms NHE4, NHE5 and NHE6 in Caco-2 cells. Differences between data provided by Rocha et al. (2001) and the present study may relate to differences in the origin of the cells; the present study was performed with the Caco-2 parental cell line, whereas the studies by Rocha et al. (2001) were performed in Caco-2BBe clonal cells (Peterson & Mooseker, 1992). NHE2 and NHE3 mainly serve the important purpose of acting as transport pathways whenever luminal Na+ is present (Wormmeester et al., 1998), the inhibition of which may be regarded as a potential cause of inflammation-associated diarrhea (Rocha et al., 2001). The ubiquitously expressed NHE1 primarily serves the purpose of pHi and cell volume regulation, cellular growth and differentiation (Yun et al., 1995; Wakabayashi et al., 1997), the inhibition of which may also reduce the uptake of sodium chloride and water from the inflamed colonic lumen and thus contribute to diarrhea. On the other hand, it is interesting to underline the observations that levels of NHE1 protein and mRNA were significantly decreased in the colonic mucosa of patients afflicted with both CD and UC (Khan et al., 2003); a decrease in pHi in colonocytes from patients with CD was also described (Rowe & Bobar, 1995). This goes well with the suggestion that increases in NHE1 expression may contribute to pHi and to cell growth and repair in colitis, and therefore, may be beneficial (Khan et al., 1998). Recent studies have also implicated NHEs in the development of inflammatory response. It has been shown that amiloride reduced IL-8 production in response to IL-1β (Nemeth et al., 2002a) and prevented the LPS-mediated IkB degradation (Nemeth et al., 2002b). Furthermore, infection with Escherichia coli produced stimulation of NHE1 and NHE2, which was accompanied by significant reduction in NHE3 exchange activity (Hecht et al., 2004).
Transduction mechanisms set into motion during short-term activation of IFN-γ receptors in Caco-2 cells appear to involve the activation of Raf-1, MEK, p38 MAPK and PKC pathways. This is evidenced by the sensitivity to GW 5074, PD 98059, SB 203580 and PKC downregulation of the IFN-γ-induced inhibition of NHE1 activity. Although the sequence of events may be not entirely apparent, the findings reported here suggest different types of interactions between the intervening molecular entities with NHE1 as an effector protein for PKC. Firstly, the sensitivity to SB 203580, but not to GW 5074, of the anisomycin-induced inhibition of NHE activity supports the view that activation of p38 MAPK is downstream Raf-1 activation. Secondly, activation of p38 MAPK by IFN-γ most likely occurs upstream PKC, as evidenced by the finding that downregulation of PKC prevents the anisomycin-induced inhibition of NHE activity. Thirdly, downregulation of PKC isoforms sensitive to PDBu prevents inhibition of NHE by IFN-γ, anisomycin and PDBu. Finally, inhibition of NHE activity by short-term exposure to PDBu was insensitive to the Raf-1 inhibitor GW 5074, the MEK1 inhibitor PD 98059 and the p38 MAPK inhibitor SB 203580. Several other observations support the suggested sequence of events. In fact, MEKK1 was found to scaffold the ERK2 cascade by binding to each of the three protein kinases of the module, Raf-1, MEK1 and ERK2 (Karandikar et al., 2000). The positions of the binding sites on MEKK1 suggest that the three kinases may be closely packed on the MEKK1 surface. Because MEK1 binds to both Raf-1 and ERK2 (Fukuda et al., 1997), the proximity of their binding sites on MEKK1 might allow these kinases to interact with each other, thereby stabilizing the heteromeric MEKK1 scaffolded complex. Although the relationship between IFN-γ, Raf/MEK/MAPK cascade and PKC is not apparent, several studies have suggested that IFN-γ mediates its cellular effects via activation of PKC (Benveniste et al., 1991; Lee et al., 1995; Lin et al., 1996; Flaishon et al., 2001; Chang et al., 2002). There is also evidence that IFN-γ causes a rapid but transient increase in diacylglycerol, the endogenous activator of PKC (Benveniste et al., 1991). Another finding that should be underlined concerns differences between NHE inhibition at 0.5 and 3 h exposure to IFN-γ, which could be related to the degree of temporal activation of p38 MAPK.
The long-term regulation of NHE1 activity by IFN-γ, in contrast to that observed during the short-term exposure to the cytokine, is suggested to primarily involve the activation of STAT1. In fact, the STAT1 inhibitor EGCG prevented both the inhibition of NHE activity and activation of phospho-STAT1 associated with the long-term exposure to IFN-γ. Although preventable by the Raf-1 inhibitor GW 5074, the MEK1 inhibitor PD 98059 and the p38 MAPK inhibitor SB 203580, it is likely that activation of Raf-1, MEK and p38 MAPK during long-term exposure to IFN-γ may correspond to events associated with the acute IFN-γ-induced NHE1 inhibition. Alternatively, the binding of MEK1 to both Raf-1 and ERK2 and their vicinity with STAT1 might allow these kinases to interact with each other, which modulates both the Raf/MEK/MAPK and JAK/STAT signaling cascades (Stancato et al., 1998). However, the results presented here may envision a relationship between p38 MAPK and STAT1 during long-term exposure to IFN-γ. In fact, the prolonged exposure to SB 203580 partially prevented the increase in phospho-STAT1 expression after long-term exposure to IFN-γ. This is compatible with the observation that when the IFN-γ-induced increase in phospho-STAT1 expression attained its maximum (24 h), the increase in phospho-p38 MAPK was no longer observed. On the other hand, the PKC-mediated events following the long-term exposure to IFN-γ most likely correspond to events associated with the acute IFN-γ-induced NHE1 inhibition and are not related to STAT1 phosphorylation. This possibility is supported by the observation that downregulation of phorbol ester-sensitive PKC isoforms, following overnight exposure of cells to 100 nM PDBu, failed to reduce the activation of phospho-STAT1 after 24 h exposure to IFN-γ. Taken together, the results presented here strongly suggest that downstream IFN-γ receptor activation to long-term regulation of NHE1 in Caco-2 cells, there is a chain of events comprising STAT1 activation with p38 MAPK playing a minor role in SER701 phosphorylation of STAT1. It is likely that STAT1 may have a role in the increased abundance of cell surface NHE1, which is presently under investigation. However, it is possible that increases in cell surface NHE1 may just relate to an adaptation to the prolonged IFN-γ-induced inhibition of NHE1 activity.
In conclusion, the short- and long-term exposure to IFN-γ results in decreases in NHE1 activity that do not result from downregulation of NHE1 and involves different signaling pathways. The short-term regulation of NHE activity by IFN-γ involves mainly the activation of Raf-1, MEK, p38 MAPK and PKC with NHE1 as an effector protein for PKC. The transduction mechanisms set into motion by long-term exposure to IFN-γ involve STAT1 activation with p38 MAPK playing a role in SER701 phosphorylation of STAT1. Keeping with the view that increases in IFN-γ-secreting cells and IFN-γ mRNA levels have been found in intestinal lesions of patients afflicted with IBD (Breese et al., 1993; Niessner & Volk, 1995) and increases in NHE1 expression may contribute to cell growth and repair in colitis (Khan et al., 1998), it might be then therapeutically adequate to prevent the IFN-γ-mediated inhibition of NHE1 activity.
Acknowledgments
This work was supported by Grant POCTI/SAU/14010/98 from Fundação para a Ciência e a Tecnologia.
Abbreviations
- CD
Crohn's disease
- EGC
(–)-epigallocatechin
- EGCG
(−)-epigallocatechin-3-gallate
- ERK
extracellular signal-regulated kinase
- IFN-γ
interferon-γ
- IBD
inflammatory bowel disease
- JAK
Janus kinase
- MAPK
mitogen-activated protein kinase
- MAPKK
mitogen-activated protein kinase kinase
- MEK
MAPK/ERK kinase
- PDBu
phorbol-12,13-dibutyrate
- PKC
protein kinase C
- STAT1
signal transducer and activator transcription factor 1
- UC
ulcerative colitis
References
- ADAMS R.B., PLANCHON S.M., ROCHE J.K. IFN-gamma modulation of epithelial barrier function: time course, reversibility, and site of cytokine binding. J. Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- ADORINI L., FRANCESCO S. Pathogenesis and immunotherapy of autoimmune diseases. Immunol. Today. 1997;18:209–211. doi: 10.1016/s0167-5699(97)01031-1. [DOI] [PubMed] [Google Scholar]
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BACHMANN O., SONNENTAG T., SIEGEL W.K., LAMPRECHT G., WEICHERT A., GREGOR M., SEIDLER U. Different acid secretagogues activate different Na+/H+ exchanger isoforms in rabbit parietal cells. Am. J. Physiol. 1998;275:G1085–G1093. doi: 10.1152/ajpgi.1998.275.5.G1085. [DOI] [PubMed] [Google Scholar]
- BENVENISTE E.N., VIDOVIC M., PANEK R.B., NORRIS J.G., REDDY A.T., BENOS D.J. Interferon-gamma-induced astrocyte class II major histocompatibility complex gene expression is associated with both protein kinase C activation and Na+ entry. J. Biol. Chem. 1991;266:18119–18126. [PubMed] [Google Scholar]
- BESANÇON F., PRZEWLOCKI G., BARÓ I., HONGRE A.S., ESCANDE D., EDELMAN A. Interferon-gamma downregulates CFTR gene expression in epithelial cells. Am. J. Physiol. Cell Physiol. 1994;267:C1398–C1404. doi: 10.1152/ajpcell.1994.267.5.C1398. [DOI] [PubMed] [Google Scholar]
- BIEMESDERFER D., RUTHERFORD P.A., NAGY T., PIZZONIA J.H., ABU-ALFA A.K., ARONSON P.S. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am. J. Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- BREESE E., BRAEGGER C.P., CORRIGAN C.J. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunol. Today. 1993;78:127–131. [PMC free article] [PubMed] [Google Scholar]
- CHANG Y.-J., HOLTZMAN M.J., CHEN C.-C. Interferon-gamma-induced epithelial ICAM-1 expression and monocyte adhesion. Involvement of protein kinase C-dependent c-SRC tyrosine kinase activation pathway. J. Biol. Chem. 2002;277:7118–7126. doi: 10.1074/jbc.M109924200. [DOI] [PubMed] [Google Scholar]
- COLGAN S.P., PARKOS C.A., MATTHEWS J.B., D'ANDREA L., AWTREY C.S., LICHTMAN A.H., DELP-ARCHER C., MADARA J.L. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 1994;267:C402–C410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISH S.M., PROUJANSKY R., REENSTRA W.W. Synergistic effects of interferon-gamma and tumor necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAISHON L., LANTNER F., HERSHKOVIZ R., LEVO Y., SHACHAR I. Low levels of IFN-gamma down-regulate the integrin-dependent adhesion of B cells by activating a pathway that interferes with cytoskeleton rearrangement. J. Biol. Chem. 2001;276:46701–46706. doi: 10.1074/jbc.M103484200. [DOI] [PubMed] [Google Scholar]
- FUKUDA M., GOTOH Y., NISHIDA E. Interaction of MAPK with MAPK kinase: its possible role in the control of nucleocytoplasmic transport of MAPK. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMES P., SOARES-DA-SILVA P. Na+/H+ exchanger activity and dopamine D1-like receptor function in two opossum kidney cell clonal sublines. Cell. Physiol. Biochem. 2002a;12:259–268. doi: 10.1159/000067896. [DOI] [PubMed] [Google Scholar]
- GOMES P., SOARES-DA-SILVA P. Role of cAMP–PKA–PLC signaling cascade on dopamine-induced PKC-mediated inhibition of renal Na+-K+-ATPase activity. Am. J. Physiol. Renal Physiol. 2002b;282:F1084–F1096. doi: 10.1152/ajprenal.00318.2001. [DOI] [PubMed] [Google Scholar]
- GOMES P., VIEIRA-COELHO M.A., SOARES-DA-SILVA P. Ouabain-insensitive acidification by dopamine in renal OK cells: primary control of the Na+/H+ exchanger. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R10–R18. doi: 10.1152/ajpregu.2001.281.1.R10. [DOI] [PubMed] [Google Scholar]
- HAQUE S.J., WILLIAMS B.R. Signal transduction in the interferon system. Semin. Oncol. 1998;25:14–22. [PubMed] [Google Scholar]
- HECHT G., HODGES K., GILL R.K., KEAR F., TYAGI S., MALAKOOTI J., RAMASWAMY K., DUDEJA P.K. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- JANECKI A.J., MONTROSE M.H., TSE C.M., DE MEDINA F.S., ZWEIBAUM A., DONOWITZ M. Development of an endogenous epithelial Na+/H+ exchanger (NHE3) in three clones of caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277:G292–G305. doi: 10.1152/ajpgi.1999.277.2.G292. [DOI] [PubMed] [Google Scholar]
- KARANDIKAR M., XU S., COBB M.H. MEKK1 binds Raf-1 and the ERK2 cascade components. J. Biol. Chem. 2000;275:40120–40127. doi: 10.1074/jbc.M005926200. [DOI] [PubMed] [Google Scholar]
- KARIM Z.G., CHAMBREY R., CHALUMEAU C., DEFONTAINE N., WARNOCK D.G., PAILLARD M., POGGIOLI J. Regulation by PKC isoforms of Na+/H+ exchanger in luminal membrane vesicles isolated from cortical tubules. Am. J. Physiol. 1999;277:F773–F778. doi: 10.1152/ajprenal.1999.277.5.F773. [DOI] [PubMed] [Google Scholar]
- KHAN I., AL-AWADI F.M., ABUL H. Colitis-induced changes in the expression of the Na+/H+ exchanger isoform NHE-1. J. Pharmacol. Exp. Ther. 1998;285:869–875. [PubMed] [Google Scholar]
- KHAN I., SIDDIQUE I., AL-AWADI F.M., MOHAN K. Role of Na+/H+ exchanger isoform-1 in human inflammatory bowel disease. Can. J. Gastroenterol. 2003;17:31–36. doi: 10.1155/2003/673819. [DOI] [PubMed] [Google Scholar]
- LACKEY K., CORY M., DAVIS R., FRYE S.V., HARRIS P.A., HUNTER R.N., JUNG D.K., MCDONALD O.B., MCNUTT R.W., PEEL M.R., RUTKOWSKE R.D., VEAL J.M., WOOD E.R. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- LEE Y.J., PANEK R.B., HUSTON M., BENVENISTE E.N. Role of protein kinase C and tyrosine kinase activity in IFN-gamma-induced expression of the class II MHC gene. Am. J. Physiol. Cell Physiol. 1995;268:C127–C137. doi: 10.1152/ajpcell.1995.268.1.C127. [DOI] [PubMed] [Google Scholar]
- LIN H.Y., THACORF H.R., DAVIS F.B., DAVIS P.J. Potentiation by thyroxine of interferon-gamma-induced antiviral state requires PKA and PKC activities. Am. J. Physiol. Cell Physiol. 1996;271:C1256–C1261. doi: 10.1152/ajpcell.1996.271.4.C1256. [DOI] [PubMed] [Google Scholar]
- LIU M.K., BROUWNSEY R.W., REINER N.E. Gamma interferon induces rapid and coordinate activation of mitogen-activated protein kinase (extracellular signal-regulated kinase) and calcium-independent protein kinase C in human monocytes. Infect. Immun. 1994;62:2722–2731. doi: 10.1128/iai.62.7.2722-2731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADARA J.L., STAFFORD J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGRO F., FRAGA S., RIBEIRO T., SOARES-DA-SILVA P. Intestinal Na+-K+-ATPase activity and molecular events downstream of interferon-gamma receptor stimulation. Br. J. Pharmacol. 2004;142:1281–1292. doi: 10.1038/sj.bjp.0705895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGRO F., VIEIRA-COELHO M.A., FRAGA S., SERRAO M.P., VELOSO F.T., RIBEIRO T., SOARES-DA-SILVA P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig. Dis. Sci. 2002;47:216–224. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- MENEGAZZI M., TEDESCHI E., DUSSIN D., PRATI A.C., CAVALIERI E., MARIOTTO S., SUZUKI H. Anti-interferon gamma action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. FASEB J. 2001;15:1309–1311. doi: 10.1096/fj.00-0519fje. [DOI] [PubMed] [Google Scholar]
- NEMETH Z.H., DEITCH E.A., LU Q., SZABO C., HASKO G. NHE blockade inhibits chemokine production and NF-kappaB activation in immunostimulated endothelial cells. Am. J. Physiol. Cell Physiol. 2002a;283:C396–C403. doi: 10.1152/ajpcell.00491.2001. [DOI] [PubMed] [Google Scholar]
- NEMETH Z.H., DEITCH E.A., SZABO C., MABLEY J.G., PACHER P., FEKETE Z., HAUSER C.J., HASKO G. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002b;283:G122–G132. doi: 10.1152/ajpgi.00015.2002. [DOI] [PubMed] [Google Scholar]
- NIESSNER M., VOLK B.A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reverse transcribed polymerase chain reaction (RT–PCR) Clin. Exp. Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOEL J., POUYSSEGUR J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. Am. J. Physiol. 1995;268:C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- NOEL J., ROUX D., POUYSSEGUR J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. J. Cell Sci. 1996;109:929–939. doi: 10.1242/jcs.109.5.929. [DOI] [PubMed] [Google Scholar]
- PEARSON G., ROBINSON F., GIBSON T.B., XU B.E., KARANDIKAR M., BERMAN K., COBB M.H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- PEDROSA R., GOMES P., SOARES-DA-SILVA P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell. Physiol. Biochem. 2004;14:91–100. doi: 10.1159/000076930. [DOI] [PubMed] [Google Scholar]
- PEDROSA R., SOARES-DA-SILVA P. Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine. Br. J. Pharmacol. 2002;137:1305–1313. doi: 10.1038/sj.bjp.0704982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON M.D., MOOSEKER M.S. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J. Cell Sci. 1992;102:581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- PODOLSKY D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991;325:928–935. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- ROCHA F., MUSCH M.W., LISHANSKIY L., BOOKSTEIN C., SUGI K., XIE Y., CHANG E.B. IFN-gamma downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am. J. Physiol. Cell Physiol. 2001;280:C1224–C1232. doi: 10.1152/ajpcell.2001.280.5.C1224. [DOI] [PubMed] [Google Scholar]
- ROWE W.A., BOBAR S.M. Intracelular pH of colonocytes is altered in patients with Crohn's disease. Gastroenterology. 1995;108:A319. [Google Scholar]
- SAKLATVALA J., RAWLINSON L., WALLER R.J., SARSFIELD S., LEE J.C., MORTON L.F., BARNES M.J., FARNDALE R.W. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J. Biol. Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- SANDLE G.I., HIGGS N., CROWE P., MARSH N.N., VENKATESAN S., PETERS T.J. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- SCHWARK J.R., JANSEN H.W., LANG H.J., KRICK W., BURCKHARDT G., HROPOT M. S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch. 1998;436:797–800. doi: 10.1007/s004240050704. [DOI] [PubMed] [Google Scholar]
- STANCATO L.F., YU C.-R., PETRICOIN E.F., III, LARNER A.C. Activation of Raf-1 by interferon and oncostatin M requires expression of the Stat1 transcription factor. J. Biol. Chem. 1998;273:18701–18704. doi: 10.1074/jbc.273.30.18701. [DOI] [PubMed] [Google Scholar]
- SUGI K., MUSCH M.W., FIELD M., CHANG E.B. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393–1403. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- SUNDARAM U., WISEL S., RAJENDRAN V., WEST A.B. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 1997;273:G913–G919. doi: 10.1152/ajpgi.1997.273.4.G913. [DOI] [PubMed] [Google Scholar]
- VERMA A., DEB D.K., SASSANO A., KAMBHAMPATI S., WICKREMA A., UDDIN S., MOHINDRU M., VAN BESIEN K., PLATANIAS L.C. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J. Immunol. 2002;168:5984–5988. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI S., SHIGEKAWA M., POUYSSEGUR J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol. Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- WATANABE I., HORIUCHI T., FUJITA S. Role of protein kinase C activation in synthesis of complement components C2 and factor B in interferon-gamma-stimulated human fibroblasts, glioblastoma cell line A172 and monocytes. Biochem. J. 1995;305:425–431. doi: 10.1042/bj3050425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORMMEESTER L., SANCHEZ DE MEDINA F., KOKKE F., TSE C.M., KHURANA S., BOWSER J., COHEN M.E., DONOWITZ M.M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am. J. Physiol. Cell Physiol. 1998;274:C1261–C1272. doi: 10.1152/ajpcell.1998.274.5.C1261. [DOI] [PubMed] [Google Scholar]
- YUN C.H., TSE C.M., NATH S.K., LEVINE S.A., BRANT S.R., DONOWITZ M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am. J. Physiol. Gastrointest. Liver Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- ZUND G., MADARA J.L., DZUS A.L., AWTREY C.S., COLGAN S.P. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J. Biol. Chem. 1996;271:7460–7464. doi: 10.1074/jbc.271.13.7460. [DOI] [PubMed] [Google Scholar]