Abstract
The present study examined the peripheral effects of vasoactive intestinal peptide (VIP) on rat knee joint blood flow during acute and chronic inflammation. The involvement of joint nerves and synovial mast cells on these effects was also investigated.
Prior to blood flow assessment, animals were deeply anaesthetised with ethyl carbamate (urethane; 2 mg kg−1 i.p.). Local application of VIP (10−13–10−9 mol) onto the capsular surface of normal rat knee joints caused a dose-dependent increase in synovial perfusion with an ED50 of 1.2 × 10−11 mol. The dilator effect of the peptide was transient with the maximal response occurring approximately 1 min after drug administration.
VIP-induced vasodilatation was blocked by co-administration of the VIP receptor antagonist VIP6–28 (10−9 mol). The inhibitory effect of the antagonist was consistent across the entire VIP dose range (P=0.01).
The vasoresponsiveness to VIP was significantly attenuated in acutely inflamed joints; however, surgical denervation of acutely inflamed knees re-established the vasodilator effect of the neuropeptide.
Topical application of VIP to 1- and 3-week adjuvant monoarthritic knees produced a hyperaemic response, which was not significantly different from normal (P=0.06 and 0.73 for 1- and 3-week adjuvant treated joints, respectively).
Stabilisation of synovial mast cells by disodium cromoglycate (cromolyn) pretreatment did not alter the vasoresponsiveness to VIP in acute or chronically inflamed joints.
The vasodilatatory effect of VIP is lost during acute knee joint inflammation and this abrogated effect is neurally dependent. In the chronic phase of knee joint inflammation, VIP-mediated hyperaemia recovers to normal levels. Synovial mast cells do not influence the vasomotor effects of exogenously applied VIP in inflamed knee joints.
Keywords: Arthritis, blood flow, knee joint nerves, mast cells, neurogenic inflammation, neuropeptides, vasoactive intestinal peptide
Introduction
Vasoactive intestinal peptide (VIP) is a 28 amino-acid neuropeptide, which was first identified in porcine intestine (Said & Mutt, 1970) and which is structurally similar to pituitary adenylate cyclase-activating polypeptide, secretin and glucagon (Klimaschewski, 1997). VIP has been localised in the terminal branches of peripheral nerves supplying a large number of mammalian organ systems (Larsson et al., 1976; Uddman et al., 1981), where it acts as an autonomic nonadrenergic, noncholinergic neuropeptide transmitter. In the knee joint, VIP is present in postganglionic sympathetic and sensory nerve fibres innervating the joint capsule (Abramovici et al., 1991; Catre & Salo, 1999; Buma et al., 2000) and is predominantly found in nerve fibres that are in close proximity to synovial blood vessels (Ackermann et al., 2001). This observation suggests that VIP-containing nerves have the potential to modulate knee joint vasomotor activity, although this association has never been tested.
VIP is known to act on two G-protein-coupled receptors, viz VPAC1 and VPAC2, although a weak affinity to the PAC1 receptor has also been described (Harmar et al., 1998). In the rat, VPAC receptors have been identified on vascular smooth muscle (Huang et al., 1993) and activation of these receptors by VIP causes a dose-dependent stimulation of adenylyl cyclase leading to cAMP generation and enhanced protein kinase A activity. These events result in the sequestration of intracellular Ca2+ leading to vascular smooth muscle relaxation. This vasodilator effect of VIP has been demonstrated in several organ systems such as the brain (Heistad et al., 1980), uterus (Clark et al., 1981), pancreas (Jin et al., 2001) and skin (Williams, 1982). The recent identification of VPAC receptors in human synovium (Takeba et al., 1999) is a further indication of VIP involvement in articular vasoregulation; however, confirmation of this physiological role has yet to be established.
A considerable amount of evidence suggests that the peripheral nervous system is involved in the development and progression of joint inflammation (Levine et al., 1985a; 1986). Inflammatory neuropeptides such as substance P and calcitonin gene-related peptide cause vasodilatation in joints (Levine et al., 1984; Lam & Ferrell, 1989; Cambridge & Brain, 1992; McDougall et al., 1995; McMurdo et al., 1997), while opioid peptides such as endomorphin-1 and nociceptin induce marked synovial hypoaemia (Barin & McDougall, 2003; McDougall, 2003; McDougall et al., 2004). Although it is known that the autonomic nervous system contributes to the development and severity of arthritis (Levine et al., 1986), the identification and mode of action of specific mediators involved in this process are not well understood. A number of reports suggest that VIP may be involved in the process of joint inflammation since a significant amount of the peptide has been found in the synovial fluid of patients with rheumatoid arthritis (Lygren et al., 1986; Larsson et al., 1991).
Mast cells are immune cells widely dispersed in mucosal and connective tissues and are inherently involved in modulating tissue inflammation. Activation of mast cells results in cellular degranulation and the release of a plethora of inflammatory mediators into the extracellular environment. Interestingly, among the agents known to induce mast cell degranulation are certain neuropeptides including VIP (Skofitsch et al., 1983; Piotrowski & Foreman, 1985). The identification of mast cells in close proximity to nerve endings and blood vessels in joints (Levine et al., 1985b; Johnston et al., 1998) suggests that the vasomotor effects of VIP may be influenced by the products of synovial mast cell degranulation. One such agent is mast cell tryptase, which enzymatically degrades VIP, thereby controlling its biological activity (Caughey et al., 1988; Naukkarinen et al., 1994). Thus, mast cell stimulation has the potential to regulate VIP responses by deactivating any unbound peptide before it reaches its site of action.
The aim of the present study was to assess the vasoactive effects of VIP on joint blood flow, and to determine whether these responses are altered by acute and chronic joint inflammation. A further consideration was to assess the contribution of synovial mast cells and joint nerves to the vasomotor profile of VIP in these joints.
Methods
Animals
This study used a total of 64 outbred male Wistar rats (240–445 g; Charles River Laboratories, MA, U.S.A.) that were housed two per cage at room temperature in a 12-h light/dark cycle with rodent food and water available ad libitum. All surgical and experimental procedures used in this study had prior approval from the University of Calgary Animal Care Committee, which follows the guidelines established by the Canadian Council for Animal Care.
The rats were deeply anaesthetised with urethane (25% stock solution, 2 g kg−1, i.p.), and the lack of a hindpaw pinch reflex was used to confirm depth of anaesthesia. To permit unrestricted breathing, the trachea was isolated and cannulated. Mean arterial blood pressure was continuously recorded via a cannula (Portex Fine Bore Tubing, 0.5 mm ID, 1.00 mm OD; SIMS Portex Ltd, Kent, England) containing heparinised saline (100 U ml−1) introduced into the left carotid artery. The cannula was attached to a pressure transducer (1050 Pressure Transducer, Stoelting Co., IL, U.S.A.), which was in series with a blood pressure monitor (Pressure Monitor BP-1, World Precision Instruments, FL, U.S.A.).
Induction of acute and chronic knee joint inflammation
Two distinct models of arthritis were used in this experiment based on their ability to produce a maximal inflammatory reaction at different time points. Thus, the kaolin/carrageenan model results in joint inflammation within hours after induction, while the adjuvant monoarthritis model is more suitable for the assessment of chronic inflammatory changes. Prior to inflammation induction, knee joint diameters were measured using electronic digital callipers (Mitutoyo Instruments, Tokyo, Japan) oriented medio-laterally across the midline of the articulating surfaces. Diameters were compared before and upon completion of inflammation development.
Acute inflammation was induced in the right knee joint by intra-articular injection of kaolin and carrageenan. Under isoflurane anaesthesia (2–5% isoflurane; 100% O2 at 1 l min−1), 0.2 ml of 2% kaolin, was injected through a 26-gauge needle into the posterior and anterior synovial cavity, followed by repeated limb extensions and flexions for 10 min to ensure adequate dispersion of the suspension within the joint and to cause articular abrasion. Next, 0.2 ml of 2% carrageenan was injected into the joint by the same procedure. These animals were allowed to recover for 3 h prior to the blood flow experiments.
For chronic inflammation, 23 rats were anaesthetised with 2–5% isoflurane (100% O2 at 1 l min−1), and a localised monoarthritis was induced as previously described (Donaldson et al., 1993; McDougall et al., 1995). The right knee joint was shaved and 0.2 ml of Freund's complete adjuvant (heat-killed Mycobacterium tuberculosis, 1 mg ml−1) was injected through a 26-guage needle into the knee joint with 0.1 ml being introduced into the posterior compartment of the joint and 0.1 ml being injected anteriorly. Animals were allowed to recover for 1 or 3 weeks prior to the blood flow experiments.
Since VIP-induced vasomotor control was found to be altered in acutely inflamed joints only, experiments were designed to determine whether joint nerves were contributing to this vascular dysfunction. As such, eight acutely inflamed rats underwent articular denervation. Following deep anaesthesia with 2–5% isoflurane (100% O2 at 1 l min−1), a longitudinal incision was made in the inguineal region of the right hindlimb and under sterile conditions a 5 mm section of the saphenous nerve was isolated and resected. The skin wound was closed with Vetbond skin glue (3M, St Paul, MN, U.S.A.), and these animals were allowed to recover for 1 week. This period of time has previously been found to ensure complete degeneration of almost all articular nerves in the rat knee (Ferrell et al., 1997). On the day of experimentation, acute kaolin/carrageenan inflammation was induced in the denervated joint. Following the terminal blood flow experiments, the saphenous nerve was examined to ensure that the surgical section was extant.
Assessment of knee joint perfusion
To assess the vasoactive effects of topically applied VIP on knee joint perfusion, the skin and overlying fascia of the antero-medial aspect of the knee was removed while viewing through a binocular dissection microscope. Removal of fascia served to enhance the accession of the peptide to synovial blood vessels, and permitted unobstructed knee joint perfusion measurements to be attained. The animals were placed in dorsal recumbency on an electric-heated blanket (TR-100, Fine Science Tools Inc., Vancouver, Canada) and their internal body temperature was maintained at 37°C as measured by a rectally inserted electronic thermometer. To prevent dehydration of the exposed joint capsule, the knee was frequently washed with warm (37°C) physiological saline (0.9% NaCl).
Laser Doppler perfusion imaging (LDI; Moor Instruments Ltd, Devon, U.K.), a technique described previously in more detail (Karimian et al., 1995), was used to assess knee joint perfusion. Briefly, the animals were placed 30 cm below the LDI scanner head with their knees in a resting position. The LDI scanned the exposed anteromedial aspect of the knee joint capsule in a raster pattern with a low power (1 mW) red laser (633 nm) and the backscattered Doppler-shifted photons were collected by a photodetector located in the LDI head. At each point in the scan, the LDI processor analysed the backscattered light dynamically to generate a flux value, which is the product of circulating erythrocyte speed and concentration. Flux values were colour-coded and formed the basis of the two-dimensional LDI perfusion image of the joint capsule. Proprietary perfusion image processing software (Moor Instruments Ltd, Devon, U.K.) was used to calculate an overall average perfusion measure for the antero-medial knee joint capsule.
Experimental procedure
To determine the time course of topical VIP-induced vasoactivity in the joint, a continuous single point perfusion measurement of a representative area of the synovium was recorded before (control) and after topical application of VIP to the exposed knee. In this mode, the laser beam of the LDI remained stationary and was directed at a discrete locus on the joint capsule, which was randomly chosen and usually in the vicinity of an observable microvascular network. When a stable baseline recording was achieved, 100 μl of 10−9 mol VIP was superfused over the surface of the knee as a single bolus and changes in tissue perfusion were continuously acquired to generate a real-time vasomotor profile of neuropeptide activity.
For dose–response determination of VIP, full LDI scans of the joint were performed. Prior to drug administration, a control (saline wash – 0.9% NaCl, 37°C) perfusion measurement of the joint was recorded. VIP (dose range 10−13–10−9 mol) was administered topically as a warm (37°C) 100 μl bolus to the exposed joint and synovial perfusion was measured at 0, 1, 2 and 5 min following administration of each dose. Drug doses were randomised to prevent any potential tachyphylactic effects and saline washes of the exposed capsule were performed between doses to remove residual peptide and prevent tissue dehydration. In other experiments, VIP was co-administered with the specific VPAC receptor antagonist VIP6–28 (10−9 mol; 100 μl bolus) and the vasoactivity profile of VIP in the presence of the VPAC receptor antagonist was repeated.
Involvement of synovial mast cells
Although it has been established that connective tissue type mast cells are present in rat knee joint synovium (Levine et al., 1990) and that VIP has a secretagogue action on mast cells (Skofitsch et al., 1983; Piotrowski & Foreman, 1985), experiments were undertaken to study a potential modulatory effect of these cells on VIP-mediated vasomotor control. Preliminary experiments were undertaken to assess the vasoactive effects of synovial mast cell activation with the mast cell degranulating compound 48/80 (50 μg topical, 37°C, 50 μl bolus). Upon establishment of the effects of synovial mast cell degranulation by compound 48/80 on joint capsular perfusion, experiments were carried out in a separate group of rats in which synovial mast cells were stabilised by preadministration of disodium cromoglycate (cromolyn, 20 mg kg−1, 37°C), a well-known mast cell stabiliser. This dose has previously been found to completely block mast cell activation following compound 48/80 challenge in other tissues (Hannon et al., 1995). VIP dose–response curves were then repeated in the presence of cromolyn to prevent mast cell degranulation.
Drugs and reagents
Disodium cromoglycate (cromolyn), compound 48/80, Freund's complete adjuvant, λ-carrageenan, kaolin, urethane, VIP and VIP6–28 were all purchased from Sigma-Aldrich Ltd, Ontario, Canada. All drugs and reagents were dissolved in 0.9% sodium chloride. Both VIP and VIP6–28 were diluted to their final concentrations, alliquotted at 250 μl, and stored at −20°C until required.
Statistical analysis
Statistical examination of the results was assessed with GraphPad Prism software (GraphPad Software, Inc., CA, U.S.A.). All data sets were normally distributed and, therefore, tested with parametric statistical analyses. Analysis of variance (ANOVA) was used to assess significant differences in perfusions within (one-way ANOVA) and between (two-way ANOVA) different animal groups. Student's t-tests were used to compare paired data sets. A significance level of P<0.05 was used for each test and all reported values represent means±s.e.m. for ‘n' observations.
Results
Changes in knee joint diameter
At 3 h after intra-articular kaolin/carrageenan injection, ipsilateral joint diameters increased by 13.2±4.2% in the acutely inflamed knee (Figure 1). Chronic inflammation induced by intra-articular injection of Freund's complete adjuvant showed a more pronounced swelling of the knee with 1-week animals exhibiting a 55.2±2.0% increase in joint diameter compared to the preinjection time point. By 3 weeks of chronic inflammation, joint swelling started to show signs of recovery with knee diameters only increasing by 28.7±4.2%.
Figure 1.
Changes in knee joint diameter following arthritis induction. Compared to pretreated control, knee joint diameter increased significantly in acutely inflamed, 1-, and 3-week chronically inflamed joints (*P=0.02; **P<0.005; ***P<0.0005; paired Student's t-test). Data are means±s.e.m.
VIP effects on normal knee joint perfusion
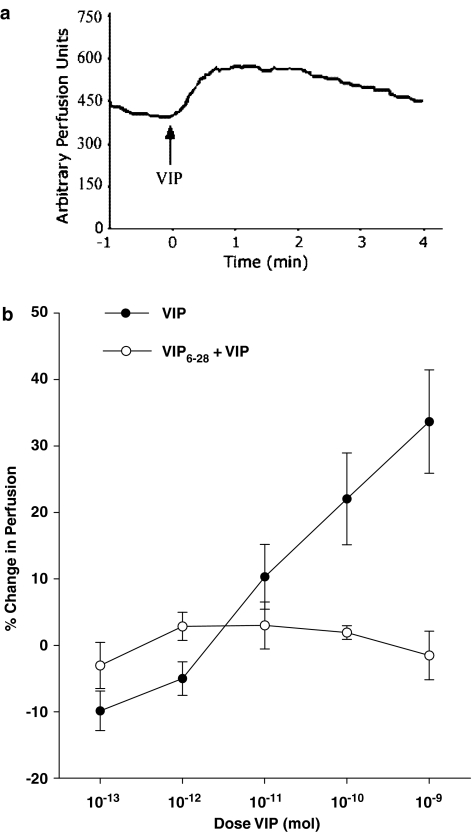
Topical application of VIP to normal knee joint capsules induced a transient rise in articular perfusion. Maximal vasodilatation was observed 1 min after peptide administration, followed by a gradual decline towards baseline perfusion levels over the succeeding 4 min (Figure 2a). Local delivery of VIP across the dose range 10−13–10−9 mol induced a dose-dependent increase in perfusion (Figure 2b). The maximal increase in perfusion (33.7±7.8%) occurred with the highest dose of the peptide and the calculated ED50 of this effect in normal knees was found to be 1.2 × 10−11 mol. The VPAC receptor antagonist VIP6–28 completely blocked VIP vasodilatation across the full dose range (Figure 2b). It should be noted that the local administration of VIP and VIP6–28 had no effect on systemic blood pressure (Table 1), confirming that all vasomotor changes were localised to the joint and not influenced by centrally mediated baroreflex events. Previously, we have shown that the vehicle (0.9% NaCl, 37°C) alone has no vasoactive effects when administered to the exposed knee joint (Barin & McDougall, 2003; McDougall, 2003).
Figure 2.
Representative single point perfusion measurement of VIP dilator response (a). Topical application of 10−9 mol VIP to the rat knee at 0 min resulted in a rapid increase in synovial blood flow with a maximal effect occurring 1 min after administration. Joint perfusion gradually returned to control levels approximately 4 min after VIP application. VIP caused a dose-dependent (P<0.0001, one-way ANOVA, n=15) vasodilatation of articular blood vessels (b), which was significantly attenuated (P=0.01; two-way ANOVA, n=6–15) in the presence of the VIP antagonist VIP6–28. Data are means±s.e.m.
Table 1.
Mean arterial pressure (MAP) values during administration of VIP and VIP6–28
| VIP | Control | 10−13 mol | 10−12 mol | 10−11 mol | 10−10 mol | 10−9 mol | P-value |
|---|---|---|---|---|---|---|---|
| MAP (mmHg) | 74.1±2.7 | 74.2±2.7 | 76.5±3.2 | 75.6±4.0 | 74.3±3.2 | 69.7±3.1 | 0.75 (n=15–16) |
| VIP6–28 | Control | 10−9 mol | P-value | ||||
| MAP (mmHg) | 75.0±6.0 | 78.7±5.9 | 0.24 (n=7) |
Data are means±s.e.m.
Effect of acute inflammation on VIP-mediated vasodilator responses
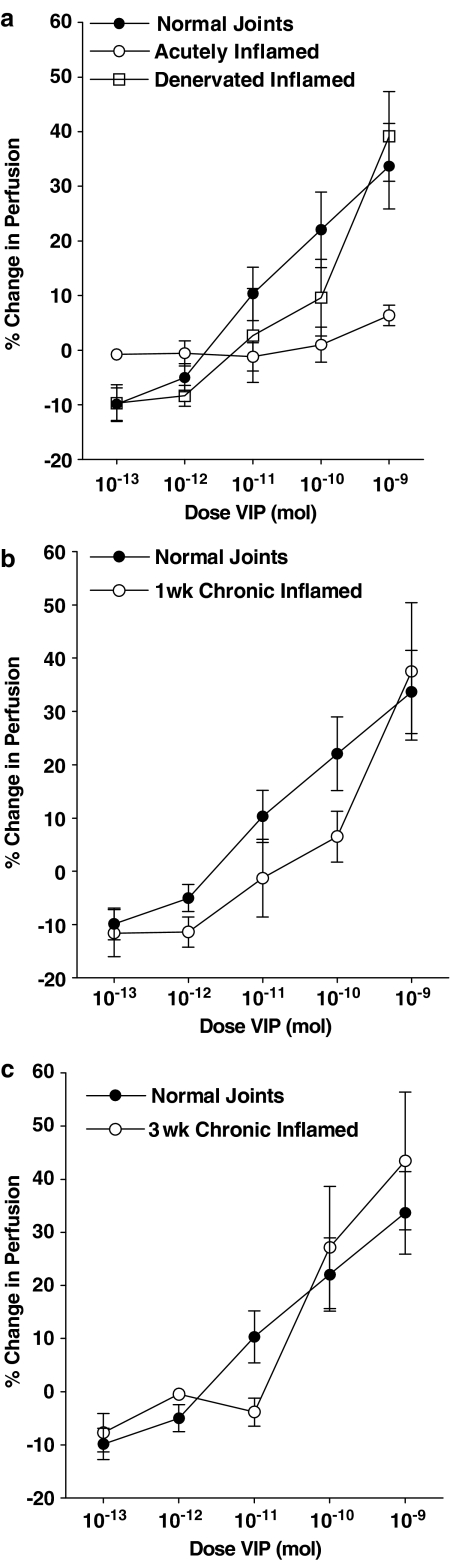
In acutely inflamed knee joints, topically applied VIP had no effect on synovial perfusion at any of the doses tested. Comparison of the VIP-induced vasodilator response between normal and kaolin/carrageenan inflamed joints by a two-way ANOVA confirmed the loss of VIP-mediated vasodilatation in the acutely inflamed joints (Figure 3a). To test neuronal involvement in this lack of vasoresponsiveness to exogenous VIP, the dilator effect of the neuropeptide was re-assessed in denervated acutely inflamed knee joints (Figure 3a). In this denervated group, VIP caused a vasodilatation, which was not significantly different from normal joints.
Figure 3.
Vasomotor effects of VIP in normal joints compared to, acute, acute/denervated (a), 1 week (b) and 3 week (c) adjuvant chronically inflamed knees. The vasodilator effect of VIP in normal joints was significantly reduced (P=0.02, two-way ANOVA, n=6–10) by acute inflammation but re-established in surgically denervated acutely inflamed knee joints such that the vasoresponsiveness to the neuropeptide was not significantly different from normal. VIP-mediated vasodilatation in 1- and 3-week chronically inflamed knees was not statistically different from normal control animals. Mean basal perfusion values for the different animal groups were 441PU (normal), 710PU (acutely inflamed), 347PU (denervated acutely inflamed), 424PU (1-week chronically inflamed), and 776PU (3-week chronically inflamed). Data are means±s.e.m.
Effect of chronic inflammation on VIP-mediated vasodilator response
At 1-week following adjuvant monoarthritis induction, VIP administration resulted in a dose-dependent vasodilatation, wherein the highest dose of the peptide induced a 37.5±12.9% increase in perfusion (Figure 3b). The vasodilatatory response to VIP in 1-week adjuvant inflamed joints was not statistically different from normal.
In the 3-week adjuvant inflamed group, it was observed that VIP again produced a dose-dependent vasodilatation in these joints (Figure 3c), which was also not significantly different from the effects of VIP in normal joints.
Effect of VIP6–28 on normal and acutely inflamed joint basal perfusion
Topical application of 10−9 mol VIP6–28 caused normal knee joint basal blood flow to fall by about 10% (Figure 4). In acutely inflamed knees, VIP6–28 had no significant effect on synovial perfusion.
Figure 4.
Topical application of 10−9 mol VIP6–28 to normal rat knees caused basal perfusion to fall from 467PU to 414PU, whereas administration of the antagonist to acutely inflamed knees had no significant effect on joint blood flow. (**P<0.005 paired Student's t-test; n=7. NS – not significantly different) Means±s.e.m. are shown.
Role of mast cells in the articular vasoresponsiveness to VIP
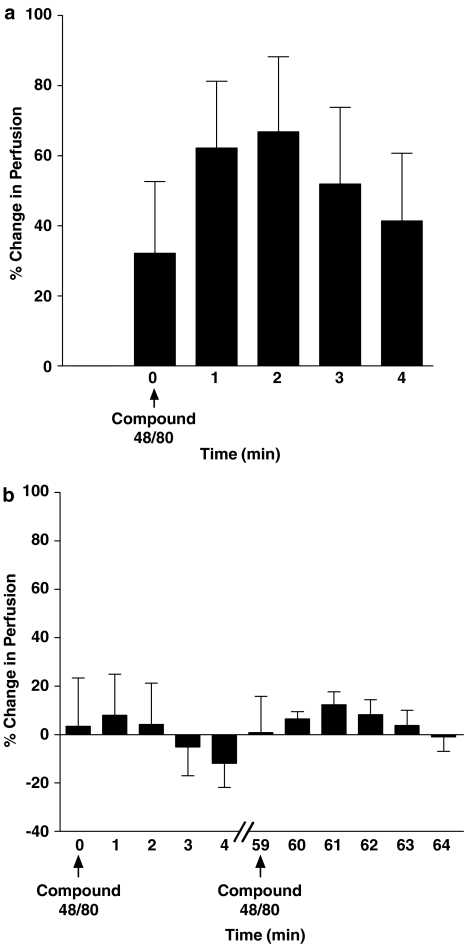
To test the stabilising effects of cromolyn on synovial mast cells, preliminary experiments were carried out using the mast cell degranulator compound 48/80. Topical administration of compound 48/80 induced a marked (66.8±21.4%) increase in joint capsular perfusion, which was maximal at 2 min followed by a gradual recovery of perfusion towards control levels (Figure 5a). In a separate group of animals, a single application of cromolyn (20 mg kg−1, topical) to the joint capsule 5 min prior to compound 48/80 challenge completely blocked the response to the mast cell degranulator (Figure 5b). Cromolyn stabilisation of mast cells was sustained in these joints for over an hour as compound 48/80 application after 60 min still failed to cause articular hyperaemia.
Figure 5.
Effect of the mast cell degranulator compound 48/80 on control articular perfusion (a) and following synovial mast cell stabilisation with cromolyn pretreatment (b). In control joints, compound 48/80 caused a noticeable increase in knee joint perfusion within 2 min after its topical application to the knee. Blood flow gradually recovered thereafter. Mast cell stabilisation with cromolyn prevented this hyperaemic response, which was maintained for at least 60 min. Data are presented as means±s.e.m.
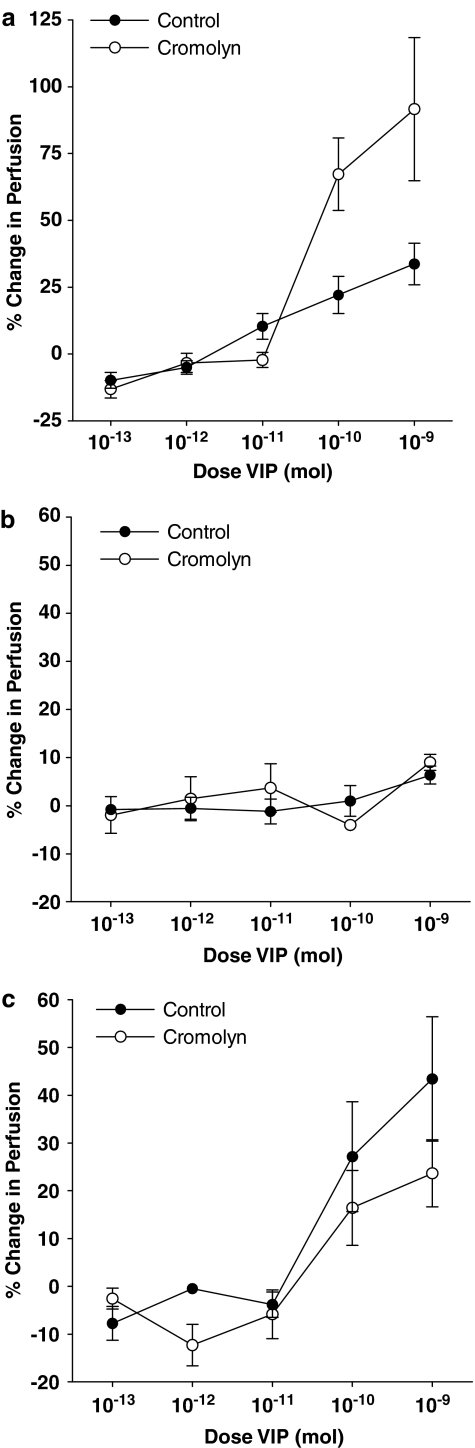
Stabilisation of synovial mast cells in normal rat knee joints led to an augmentation of VIP dilator effects, which was most conspicuous with the top two doses of the neuropeptide (Figure 6a). In acutely inflamed knee joints, mast cell stabilisation with cromolyn pretreatment had no significant effect on the vasoactive profile of VIP as compared to the vasoresponsiveness of noncromolyn treated acutely inflamed joints (Figure 6b). Similarly, VIP-mediated synovial vasodilatation in 3-week chronically inflamed joints was also unaffected by cromolyn application (Figure 6c).
Figure 6.
Effect of mast cell stabilisation on VIP-mediated vasodilatation in normal (a), acute (b), and 3-week chronically inflamed (c) knee joints. The vasodilator effect of VIP in normal knee joints was significantly augmented in cromolyn treated animals (P=0.001, two-way ANOVA, n=7–15). Cromolyn pretreatment of acute and chronically inflamed joints had no significant effect on the vasoactivity profile of VIP observed in untreated inflamed joints. Data are shown as means±s.e.m.
Discussion
The intriguing discovery of gastrointestinal peptides in the synovial fluid of arthritis patients led to the speculation of their involvement in the pathogenesis and progression of inflammatory joint disease (Lygren et al., 1986). In particular, the gut peptide VIP has been detected in arthritic joints and the concentration of the peptide is significantly reduced by intra-articular corticosteroid therapy (Lygren et al., 1986; Ahmed et al., 1995b). Although the occurrence of VIP in joints has been known for nearly two decades, the role of the neuropeptide in articular vasomotor function has never been examined. The present study shows for the first time that local administration of VIP to the rat knee joint caused a marked increase in capsular perfusion. The vasodilatatory action of the neuropeptide was rapid in onset (within 1 min after application) and was conspicuously transient. The inhibition of this response by the selective antagonist VIP6–28 indicates that VIP exerts its hyperaemic effect via VPAC receptors located within the knee joint. Interestingly, topical application of VIP6–28 alone to normal knees caused a mild but significant decrease in synovial blood flow indicative of tonic VIP release under basal conditions. The function of this basal VIP release is probably to partially offset sympathetic vasoconstrictor tone in synovial blood vessels as has been found with other dilator neuropeptides (Ferrell et al., 1997; McMurdo et al., 1997; McDougall et al., 1999).
At low doses, VIP appeared to cause a slight vasoconstriction that was inhibited by co-administration of VIP6–28. It is not clear whether this phenomenon is artefactual or if indeed low dose VIP elicits a vasoconstrictor response either directly on the blood vessels or via a secondary pathway (e.g. activation of postganglionic sympathetics). Further research is required to test the validity of this unexpected result.
Loss of VIP-mediated vasodilatation during acute inflammation
Exogenous administration of VIP to acutely inflamed knee joints had no effect on joint blood flow indicating that the dilator effect of the peptide is lost in these joints. One explanation for this lack of a hyperaemic response to VIP could be due to the synovial microvasculature already being maximally vasodilated such that further vascular smooth muscle relaxation is unattainable in this model. However, basal joint perfusion in the 3-week adjuvant inflamed group of animals was comparable to acutely inflamed knees yet VIP was still able to increase synovial blood flow in the chronically inflamed joints. Furthermore, the vasodilator effect of other neuropeptides has been shown to be augmented in this inflammatory model (Lam & Ferrell, 1993), indicating that supplementary vasorelaxation is still possible in these knees. An alternative reason for the loss of VIP-mediated vasodilatation is that acute joint inflammation causes a downregulation and/or desensitisation of synovial VPAC receptors. This scenario is supported by the fact that administration of the VIP antagonist VIP6–28 also had no effect on synovial perfusion in acutely inflamed joints compared to the distinct hypoaemic response observed in normal knees. If VPAC receptors are indeed downmodulated in acutely inflamed knees, then this would explain why VIP6–28 is unable to impart competitive antagonism in kaolin/carrageenan treated knees. Indeed, VPAC receptor downmodulation has been suggested as a possible cause for the failure of VIP to inhibit inflammatory cytokine production by synoviocytes procured from inflamed joints (Foey et al., 2003). Future studies examining VPAC receptor expression and function through, for example, changes in VIP-mediated cAMP production are required to test whether acute inflammation does indeed cause VPAC receptor downregulation/desensitisation in this model.
A further explanation for the lack of VIP-mediated vasodilatation during acute inflammation is that there may be enhanced phosphodiesterase IV (PDE IV) activity in these joints leading to the rapid destruction of cAMP levels. In vitro studies have shown that exposure of human monocytes to an acute inflammatory stimulus prevents VIP-induced cAMP production, which can be reversed by treatment with the PDE IV inhibitor rolipram (Foey et al., 2003). Whether a similar phenomenon is occurring in the present study is possible; however, it would not explain why VIP-mediated vasodilatation is restored following surgical denervation of the kaolin/carrageenan treated joint. This particular observation suggests that neurotransmitters released into the joint during acute inflammation inhibit VIP vasoresponsiveness either directly or by altering VPAC receptor availability. The most palpable neuromediator that could cause receptor loss is VIP itself, which has been shown to cause rapid desensitisation followed by internalisation of VPAC receptors (Robberecht et al., 1989; Langlet et al., 2004). By denervating the joint, the principal source of endogenous VIP was removed and VPAC receptor number would be preserved. Henceforth, exogenously administered VIP would continue to dilate synovial blood vessels as shown here. We postulate, therefore, that induction of acute joint inflammation leads to the peripheral release of VIP into the joint, thereby contributing to an inflammatory hyperaemia. Subsequent accumulation of VIP in the joint would likely have a negative effect on VPAC receptor availability, such that exogenous application of the neuropeptide could no longer exert its vasodilatatory effect. Future studies examining synovial VIP levels in an acute inflammatory model are however required to corroborate this hypothesis.
VIP responses in chronically inflamed joints
In adjuvant monoarthritic joints, the vasomotor effects of VIP returned such that up to the third week of adjuvant-induced inflammation, VIP-induced hyperaemia was not significantly different from normal joints. The recovery of VIP-mediated vasomotor effects in chronically inflamed knee joints is a novel finding since several studies have consistently shown that other vasodilatatory mediators lose their effectiveness in the chronic stages of joint inflammation (McDougall et al., 1995; 1998; 1999). This discrepancy in vasomotor responsiveness is again probably linked to endogenous peptide levels in the synovial fluid of chronically arthritic joints. Several reports indicate that intraarticular concentrations of the inflammatory neuropeptides substance P and calcitonin gene-related peptide are elevated in chronically inflamed knee joints (Lygren et al., 1986; Larsson et al., 1991; Ahmed et al., 1995a). The genes that express these neuropeptides are also upregulated in chronic arthritis (Donaldson et al., 1992; 1995) so that there is continuous production and peripheral release of these peptides culminating in the suppression of receptor function on the blood vessels of arthritic joints. Conversely, VIP expression is unaltered in adjuvant monoarthritic animals (Donaldson et al., 1992) and concomitantly the amount of VIP in chronically inflamed knees is normal (Ahmed et al., 1995b; Buma et al., 2000). This maintenance of synovial VIP levels suggests that VPAC receptor availability would be unaffected by chronic inflammation, and this would account for the preservation of VIP vasomotor activity described here.
Despite its classical pro-inflammatory characteristics, it has been speculated that VIP may act as an anti-inflammatory agent in certain tissues including joints (Firestein, 2001). For example, chronic systemic administration of VIP has been shown to ameliorate the incidence and severity of joint swelling and articular degeneration in the mouse collagen-induced arthritis model (Delgado et al., 2001). Furthermore, VIP treatment was shown to inhibit arthritis progression by downregulating inflammatory cytokine production and macrophage chemotaxis. Whether VIP-induced hyperaemia of monoarthritic joints described here could be a contributing factor in this proposed antiarthritic effect of the neuropeptide by promoting joint vascular nourishment and soft tissue healing requires further clarification.
Role of synovial mast cells on VIP-induced vasodilatation
The recent discovery of VPAC receptors on the surface of connective tissue mast cells (Groneberg et al., 2003) and the degranulatory effect of VIP (Skofitsch et al., 1983; Piotrowski & Foreman, 1985) has led to the suggestion that mast cells may participate in the local control of VIP activity. For example, synovial mast cells contain the serine protease tryptase (de Paulis et al., 1996), which degrades VIP thereby limiting the biological action of the peptide (Caughey et al., 1988; Naukkarinen et al., 1994). In the current investigation, stabilisation of normal knee joint mast cells with cromolyn caused a conspicuous augmentation of VIP-mediated hyperaemia with the top two doses of the peptide. This increased response is probably due to cromolyn inhibition of tryptase release from synovial mast cells allowing the dilator capacity of VIP to remain maximal. The fact that lower doses of VIP were unaffected by cromolyn treatment indicates that, at least in the rat knee joint, the secretagogue action of VIP (with concomitant tryptase release) only occurs with higher doses of the neuropeptide. Whether this is due to VIP being unable to access mast cell VPAC receptors because of the route of administration, or whether VIP must first reach a threshold concentration before triggering mast cell degranulation cannot be determined from the present data.
In acutely inflamed joints, preadministration of cromolyn did not influence VIP vasoresponsiveness, which may be reflective of mast cell VPAC receptor downregulation. Indeed, mast cells procured from acute lesions of atopic dermatitis patients show reduced VPAC receptor expression and localisation indicative of inflammation-induced receptor downregulation (Groneberg et al., 2003). In the chronic arthritic models, the recovery of VIP-mediated vasodilatation was not influenced by the presence of cromolyn. Thus, while vascular VPAC receptors regain functionality in the adjuvant monoarthritic joint, mast cell VPAC receptors appear to remain ineffective. We are confident that synovial mast cells were stabilised in this study since the dilator action of the degranulating agent compound 48/80 was blocked by cromolyn treatment and the duration of effect was comparable to the time required to carry out a full VIP dose–response comparison.
In addition to its degradative effect on VIP, mast cell tryptase can also activate the proteinase activated receptor-2 (PAR-2) by exposing a tethered ligand domain that binds to the cleaved receptor (Molino et al., 1997). In joints, PAR-2 activation has been found to be proinflammatory and has been implicated in the development of chronic inflammatory joint disease (Ferrell et al., 2003). Indeed, local delivery of PAR-2 agonists to mouse knee joints causes profound synovial vasodilatation. In the present investigation, VIP is not causing mast cell tryptase release in acute and chronically inflamed joints since the hyperaemic effect of VIP was not altered by cromolyn treatment. This observation provides further evidence that synovial mast cells are not influenced by VIP in inflamed knee joints.
In conclusion, this study shows for the first time that local administration of VIP to normal rat knee joints induces a dose-dependent vasodilatation of synovial blood vessels that is VPAC receptor mediated. This dilator effect of the neuropeptide seems to be self-limited by its capacity to degranulate connective tissue mast cells, leading to the release of mast cell tryptase, which rapidly degrades unbound VIP, thereby curtailing the biological activity of the peptide. The hyperaemic action of exogenous VIP is lost in acutely inflamed joints and this inhibitory effect is neurally dependent. During the chronic phase of joint inflammation there is a gradual recovery of vascular VPAC receptor function and the re-occurrence of a vasodilator profile. Synovial mast cells do not influence VIP effects in inflamed joints although future studies are required to determine the precise interaction between VIP and the host of mast cell mediators released into an arthritic joint.
Acknowledgments
The financial support of the Arthritis Society of Canada, the Alberta Heritage Foundation for Medical Research (AHFMR), and the Canadian Institutes of Health Research (CIHR) are gratefully acknowledged. JJMcD is an AHFMR Scholar and an Arthritis Society/CIHR New Investigator.
Abbreviations
- LDI
laser Doppler imager
- MAP
mean arterial pressure
- VIP
vasoactive intestinal peptide
References
- ABRAMOVICI A., DAIZADE I., YOSIPOVITCH Z., GIBSON S.J., POLAK J.M. The distribution of peptide-containing nerves in the synovia of the cat knee joint. Histol. Histopathol. 1991;6:469–476. [PubMed] [Google Scholar]
- ACKERMANN P.W., LI J., FINN A., AHMED M., KREICBERGS A. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J. Orthop. Res. 2001;19:372–378. doi: 10.1016/S0736-0266(00)90029-9. [DOI] [PubMed] [Google Scholar]
- AHMED M., BJURHOLM A., SCHULTZBERG M., THEODORSSON E., KREICBERGS A. Increased levels of substance P and calcitonin gene-related peptide in rat adjuvant arthritis. A combined immunohistochemical and radioimmunoassay analysis. Arthritis Rheum. 1995a;38:699–709. doi: 10.1002/art.1780380519. [DOI] [PubMed] [Google Scholar]
- AHMED M., BJURHOLM A., SRINIVASAN G.R., LUNDBERG T., THEODORSSON E., SCHULTZBERG M., KREICBERGS A. Capsaicin effects on substance P and CGRP in rat adjuvant arthritis. Regul. Peptides. 1995b;55:85–102. doi: 10.1016/0167-0115(94)00095-f. [DOI] [PubMed] [Google Scholar]
- BARIN A.K., MCDOUGALL J.J. Endomorphin-1 causes synovial hypoaemia in rat knee joints via a capsaicin-sensitive neural pathway. Neurosci. Lett. 2003;344:21–24. doi: 10.1016/s0304-3940(03)00405-1. [DOI] [PubMed] [Google Scholar]
- BUMA P., ELMANS L., VAN DEN BERG W.B., SCHRAMA L.H. Neurovascular plasticity in the knee joint of an arthritic mouse model. Anat. Rec. 2000;260:51–61. doi: 10.1002/1097-0185(20000901)260:1<51::AID-AR60>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- CAMBRIDGE H., BRAIN S.D. Calcitonin gene-related peptide increases blood flow and potentiates plasma protein extravasation in the rat knee joint. Br. J. Pharmacol. 1992;106:746–750. doi: 10.1111/j.1476-5381.1992.tb14404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATRE M.G., SALO P.T. Quantitative analysis of the sympathetic innervation of the rat knee joint. J. Anat. 1999;194:233–239. doi: 10.1046/j.1469-7580.1999.19420233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUGHEY G.H., LEIDIG F., VIRO N.F., NADEL J.A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J. Pharmacol. Exp. Ther. 1988;244:133–137. [PubMed] [Google Scholar]
- CLARK K.E., MILLS E.G., STYS S.J., SEEDS A.E. Effects of vasoactive polypeptides on the uterine vasculature. Am. J. Obstet. Gynecol. 1981;139:182–188. doi: 10.1016/0002-9378(81)90443-9. [DOI] [PubMed] [Google Scholar]
- DELGADO M., ABAD C., MARTINEZ C., LECETA J., GOMARIZ R.P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- DONALDSON L.F., HARMAR A.J., MCQUEEN D.S., SECKL J.R. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Mol. Brain Res. 1992;16:143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- DONALDSON L.F., MCQUEEN D.S., SECKL J.R. Neuropeptide gene expression and capsaicin-sensitive primary afferents: maintenance and spread of adjuvant arthritis in the rat. J. Physiol. 1995;486.2:473–482. doi: 10.1113/jphysiol.1995.sp020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON L.F., SECKL J.R., MCQUEEN D.S. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J. Neurosci. Methods. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- FERRELL W.R., LOCKHART J.C., KARIMIAN S.M. Tachykinin regulation of basal synovial blood flow. Br. J. Pharmacol. 1997;121:29–34. doi: 10.1038/sj.bjp.0701095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRELL W.R., LOCKHART J.C., KELSO E.B., DUNNING L., PLEVIN R., MEEK S.E., SMITH A.J., HUNTER G.D., MCLEAN J.S., MCGARRY F., RAMAGE R., JIANG L., KANKE T., KAWAGOE J. Essential role for proteinase-activated receptor-2 in arthritis. J. Clin. Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRESTEIN G.S. VIP: a very important protein in arthritis. Nat. Med. 2001;7:537–538. doi: 10.1038/87859. [DOI] [PubMed] [Google Scholar]
- FOEY A.D., FIELD S., AHMED S., JAIN A., FELDMANN M., BRENNAN F.M., WILLIAMS R. Impact of VIP and cAMP on the regulation of TNF-alpha and IL-10 production: implications for rheumatoid arthritis. Arthritis Res. Ther. 2003;5:R317–R328. doi: 10.1186/ar999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRONEBERG D.A., WELKER P., FISCHER T.C., DINH Q.T., GRUTZKAU A., PEISER C., WAHN U., HENZ B.M., FISCHER A. Down-regulation of vasoactive intestinal polypeptide receptor expression in atopic dermatitis. J. Allergy Clin. Immunol. 2003;111:1099–1105. doi: 10.1067/mai.2003.1477. [DOI] [PubMed] [Google Scholar]
- HANNON J.P., PFANNKUCHE H.J., FOZARD J.R. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br. J. Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMAR A.J., ARIMURA A., GOZES I., JOURNOT L., LABURTHE M., PISEGNA J.R., RAWLINGS S.R., ROBBERECHT P., SAID S.I., SREEDHARAN S.P., WANK S.A., WASCHEK J.A. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- HEISTAD D.D., MARCUS M.L., SAID S.I., GROSS P.M. Effect of acetylcholine and vasoactive intestinal peptide on cerebral blood flow. Am. J. Physiol.: Heart Circ. Physiol. 1980;239:H73–H80. doi: 10.1152/ajpheart.1980.239.1.H73. [DOI] [PubMed] [Google Scholar]
- HUANG M., SHIRAHASE H., RORSTAD O.P. Comparative study of vascular relaxation and receptor binding by PACAP and VIP. Peptides. 1993;14:755–762. doi: 10.1016/0196-9781(93)90109-t. [DOI] [PubMed] [Google Scholar]
- JIN C., NARUSE S., KITAGAWA M., ISHIGURO H., NAKAJIMA M., MIZUNO N., KO S.B., HAYAKAWA T. The effect of calcitonin gene-related peptide on pancreatic blood flow and secretion in conscious dogs. Regul. Peptides. 2001;99:9–15. doi: 10.1016/s0167-0115(01)00214-2. [DOI] [PubMed] [Google Scholar]
- JOHNSTON B., BURNS A.R., KUBES P. A role for mast cells in the development of adjuvant-induced vasculitis and arthritis. Am. J. Pathol. 1998;152:555–563. [PMC free article] [PubMed] [Google Scholar]
- KARIMIAN S.M., MCDOUGALL J.J., FERRELL W.R. Neuropeptidergic and autonomic control of the vasculature of the rat knee joint revealed by laser Doppler perfusion imaging. Exp. Physiol. 1995;80:341–348. doi: 10.1113/expphysiol.1995.sp003851. [DOI] [PubMed] [Google Scholar]
- KLIMASCHEWSKI L. VIP – a ‘very important peptide' in the sympathetic nervous system. Anat. Embryol. 1997;196:269–277. doi: 10.1007/s004290050096. [DOI] [PubMed] [Google Scholar]
- LAM F.Y., FERRELL W.R. Inhibition of carrageenan-induced joint inflammation by substance P antagonist. Ann. Rheum. Dis. 1989;48:928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAM F.Y., FERRELL W.R. Acute inflammation in the rat knee joint attenuates sympathetic vasoconstriction but enhances neuropeptide-mediated vasodilatation assessed by laser Doppler perfusion imaging. Neuroscience. 1993;52:443–449. doi: 10.1016/0306-4522(93)90170-k. [DOI] [PubMed] [Google Scholar]
- LANGLET C., GASPARD N., NACHTERGAEL I., ROBBERECHT P., LANGER I. Comparative efficacy of VIP and analogs on activation and internalization of the recombinant VPAC2 receptor expressed in CHO cells. Peptides. 2004;25:2079–2086. doi: 10.1016/j.peptides.2004.08.017. [DOI] [PubMed] [Google Scholar]
- LARSSON J., EKBLOM A., HENRIKSSON K., LUNDBERG T., THEODORSSON E. Concentration of substance P, neurokinin-A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand. J. Rheumatol. 1991;20:326–335. doi: 10.3109/03009749109096808. [DOI] [PubMed] [Google Scholar]
- LARSSON L.I., FAHRENKRUG J., SCHAFFALITZKY DE MUCKADELL O., SUNDLER F., HAKANSON R., REHFELD J.R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc. Natl. Acad. Sci. U.S.A. 1976;73:3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE J.D., CLARK R., DEVOR M., HELMS C., MOSKOWITZ M.A., BASBAUM A.I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984;226:547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- LEVINE J.D., CODERRE T.J., COVINSKY K., BASBAUM A.I. Neural influences on synovial mast cell density in rat. J. Neurosci. Res. 1990;26:301–307. doi: 10.1002/jnr.490260306. [DOI] [PubMed] [Google Scholar]
- LEVINE J.D., COLLIER D.H., BASBAUM A.I., MOSKOWITZ M.A., HELMS C.A. Hypothesis: the nervous system may contribute to the pathophysiology of rheumatoid arthritis. J. Rheumatol. 1985a;12:406–411. [PubMed] [Google Scholar]
- LEVINE J.D., DARDICK S.J., ROIZEN M.F., HELMS C., BASBAUM A.I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J. Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE J.D., MOSKOWITZ M.A., BASBAUM A.I. The contribution of neurogenic inflammation in experimental arthritis. J. Immunol. 1985b;135:843s–847s. [PubMed] [Google Scholar]
- LYGREN I., ØSTENSEN M., BURHOL P.G., HUSBY G. Gastrointestinal peptides in serum and synovial fluid from patients with inflammatory joint disease. Ann. Rheum. Dis. 1986;45:637–640. doi: 10.1136/ard.45.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDOUGALL J.J. Involvement of sympathetic efferents but not capsaicin-sensitive afferents in nociceptin-mediated dual control of rat synovial blood flow. Am. J. Physiol.: Regul. Integr. Comp. Physiol. 2003;284:R1477–R1485. doi: 10.1152/ajpregu.00733.2002. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., BARIN A.K., MCDOUGALL C.M. Loss of vasomotor responsiveness to the μ-opioid receptor ligand endomorphin-1 in adjuvant monoarthritic rat knee joints. Am. J. Physiol.: Regul. Integr. Comp. Physiol. 2004;286:R634–R641. doi: 10.1152/ajpregu.00464.2003. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., ELENKO R.D.V., BRAY R.C. Cholinergic vasoregulation in normal and adjuvant monoarthritic rat knee joints. J. Auton. Nerv. System. 1998;72:55–60. doi: 10.1016/s0165-1838(98)00087-3. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., FERRELL W.R., BRAY R.C. Neurogenic origin of articular hyperemia in early degenerative joint disease. Am. J. Physiol. 1999;276:R345–R352. doi: 10.1152/ajpregu.1999.276.3.R745. [DOI] [PubMed] [Google Scholar]
- MCDOUGALL J.J., KARIMIAN S.M., FERRELL W.R. Prolonged alteration of sympathetic vasoconstrictor and peptidergic vasodilator responses in rat knee joints by adjuvant-induced arthritis. Exp. Physiol. 1995;80:349–357. doi: 10.1113/expphysiol.1995.sp003852. [DOI] [PubMed] [Google Scholar]
- MCMURDO L., LOCKHART J.C., FERRELL W.R. Modulation of synovial blood flow by the calcitonin gene-related peptide (CGRP) receptor antagonist, CGRP(8–37) Br. J. Pharmacol. 1997;121:1075–1080. doi: 10.1038/sj.bjp.0701237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECHTER N., WOOLKALIS M., BRASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- NAUKKARINEN A., HARVIMA I.T., AALTO M.L., HORSMANHEIMO M. Mast cell tryptase and chymase are potential regulators of neurogenic inflammation in psoriatic skin. Int. J. Dermatol. 1994;33:361–366. doi: 10.1111/j.1365-4362.1994.tb01069.x. [DOI] [PubMed] [Google Scholar]
- DE PAULIS A., MARINO I., CICCARELLI A., DE CRESCENZO G., CONCARDI M., VERGA L., ARBUSTINI E., MARONE G. Human synovial mast cells. I. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum. 1996;39:1222–1233. doi: 10.1002/art.1780390723. [DOI] [PubMed] [Google Scholar]
- PIOTROWSKI W., FOREMAN J.C. On the actions of substance P, somatostatin, and vasoactive intestinal polypeptide on rat peritoneal mast cells and in human skin. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;331:364–368. doi: 10.1007/BF00500821. [DOI] [PubMed] [Google Scholar]
- ROBBERECHT P., DE NEEF P., WAELBROECK M., TASTENOY M., CHRISTOPHE J. VIP and related peptides induce rapid homologous desensitization in the human lymphoma SUP T1 cell line. Peptides. 1989;10:441–446. doi: 10.1016/0196-9781(89)90056-9. [DOI] [PubMed] [Google Scholar]
- SAID S.I., MUTT V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- SKOFITSCH G., DONNERER J., PETRONIJEVIC S., SARIA A., LEMBECK F. Release of histamine by neuropeptides from the perfused rat hindquarter. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983;322:153–157. doi: 10.1007/BF00512389. [DOI] [PubMed] [Google Scholar]
- TAKEBA Y., SUZUKI N., KANEKO A., ASAI T., SAKANE T. Evidence for neural regulation of inflammatory synovial cell functions by secreting calcitonin gene-related peptide and vasoactive intestinal peptide in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2418–2429. doi: 10.1002/1529-0131(199911)42:11<2418::AID-ANR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., ALUMETS J., EDVINSSON L., HAKANSON R., SUNDLER F. VIP nerve fibres around peripheral blood vessels. Acta Physiol. Scand. 1981;112:65–70. doi: 10.1111/j.1748-1716.1981.tb06783.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J. Vasoactive intestinal polypeptide is more potent than prostaglandin E2 as a vasodilator and oedema potentiator in rabbit skin. Br. J. Pharmacol. 1982;77:505–509. doi: 10.1111/j.1476-5381.1982.tb09324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]