Abstract
Mahanine, a naturally occurring carbazole alkaloid in some Asian vegetables, has been shown to exhibit antimutagenicity, antimicrobial activity, cytotoxicity, and other biological properties.
In the present study, we investigated the effect of mahanine on the activation of the apoptotic pathway in human leukemia U937 cells. Various end points were used to screen for apoptosis: Morphological changes in cells, the relative numbers of viable and apoptotic cells; translocation of membrane bound phosphatidylserine and DNA analysis.
We found that mahanine-induced apoptosis in U937 cells involved activation of caspases, including caspase-3, release of cytochrome c into cytosol, loss of mitochondrial membrane permeability, and decreased levels of cellular ATP.
Pretreatment of cells with cyclosporine A, prior to/concomitant with exposure to mahanine, effectively prevented the deleterious effects of the alkaloid on cellular integrity and viability.
As mitochondrial permeability is known to be important in the regulation of cytochrome c release, our observations indicate that mitochondria are the principal target of mahanine. More specifically, we propose that mahanine causes the mitochondrial membranes to lose their permeability, resulting in caspase-3 activation and apoptosis.
Keywords: Mahanine, apoptosis, cyclosporine A, mitochondrial-permeability transition
Introduction
Natural products account for more than 40% of all pharmaceuticals on the market today. From 1941 to 2002, over 50% of all the drugs, or new drug entities, available for cancer treatment were derived from natural resources (Newman et al., 2003). Most anticancer drugs, whether synthetic chemicals or natural products, interact with DNA or its precursors, and cause irreversible damage to DNA and inhibit the synthesis of new genetic materials. They also enhance apoptosis in a variety of premalignant or malignant cell types, although their discovery in the initial era of cancer chemotherapy largely depended on animal experiment, through screening against animal tumor system. However, the increased understanding of apoptotic pathway has directed attention in searching potential chemotherapeutic agent by evaluating its action in cellular or molecular level in vitro before applying on animal models in vivo or clinical trials.
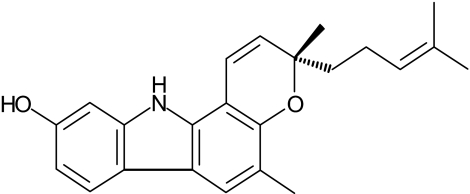
Mahanine (3,11-dihydro-3,5-dimethyl-3-(4-methyl-3-pentenyl)-pyrano[3,2-a]carbazol-9-ol) (Figure 1) is a carbazole alkaloid and is a major constituent of the edible parts of the Thai vegetable Micromelum minutum (Nakahara et al., 2002). The compound has also been reported to be present in the curry leaf plant Murraya koenigii and in some related species. These plants are cultivated in South and Southeast Asia (Ramsewak et al., 1999) and are therefore readily available sources of the compound. Mahanine has been shown to exhibit a wide range of pharmacological effects: antimutagenicity against heterocyclic amines; antimicrobial activity against Gram positive bacteria; and an anti-inflammatory effect (Ramsewak et al., 1999; Tachibana et al., 2001; Nakahara et al., 2002). In our previous study, mahanine was identified as a potent apoptosis-inducing agent in HL-60 cells as it caused multiple morphological and biochemical features typical of apoptotic cell death (Roy et al., 2004). The present study was undertaken to obtain an in-depth understanding of the molecular and cellular mechanisms underlying mahanine-induced apoptosis.
Figure 1.
Structure of mahanine, a carbazole alkaloid extracted from M. minutum.
Apoptosis, or programmed cell death, is involved in many normal biological processes, such as cell turnover, the immune system, embryonic development, metamorphosis, and hormone-dependent atrophy, as well as in pathological processes such as in some forms of chemically induced cell death. Deregulation of apoptosis underlies the pathogenesis of many human diseases from neurodegenerative and autoimmune disorders to neoplasia, and also in acute organ failure. Apoptotic cell death consists of multiple molecular cascades from an initial triggering event to the eventual disruption of cellular structure. The signals that initiate the cell death pathway fall into two broad categories, intrinsic and extrinsic. In both categories, proteolytic caspase cascades result in the cleavage of various intracellular proteins and in the activation of caspase activated DNase (CAD). CAD is responsible for internucleosomal DNA fragmentation (Enari et al., 1998) and is believed to be activated by the effector caspase, caspase-3. However, activation of effector caspase/s requires involvement of initiator caspase/s (Thornberry & Lazebnik, 1998). In the intrinsic apoptotic pathway, various intracellular stimuli, including DNA damage, glucocorticoids, redox balance alterations, enhanced ceramide generation, and loss of growth factor signals can initiate loss of mitochondrial transmembrane potential, activation of mitochondrial permeability transition (MPT), enhancement of MPT pore opening, and mitochondrial volume enlargement that results in the release of cytochrome c (Liu et al., 1996), endonuclease G (Li et al., 2001), apoptosis inducing factor (Susin et al., 1999) to the cytosol. Cytochrome c is released from the inner mitochondrial space to the cytosol where it forms a complex in the cytosol, called the apoptosome, with apoptotic protease activating factor-1 (apaf-1) and caspase-9. In turn, caspase-9 activation triggers that of caspase-3. In contrast, the alternative extrinsic pathway involves the activation of death receptors, such as Fas/CD95 or TNF receptor 1 and then recruitment of caspase-8 (Huang et al., 1999), which ultimately activates the effector caspase, caspase-3 (Boldin et al., 1996; Muzio et al., 1996; Li et al., 1998; Gross et al., 1999). Of these two categories of apoptotic pathway, the intrinsic mitochondria-dependent pathway is seen in most settings in vertebrate cells and possesses tremendous medical and pharmaceutical implications. A number of diseases states, for example HIV-1 infection, liver disease, cardiac infraction, cerebral damage, neurodegenerative diseases, cancer, and acute toxic induced cell death, show mitochondrial dysfunction. This may involve deregulation of MPT or MPT pore opening, mutation in the mitochondrial DNA, and dysfunction of mitochondrial respiratory chain enzymes. There is compelling evidence that ameliorating mitochondrial dysfunction can provide clinical benefit to many diseases including obesity, cancer, cardiovascular diseases, diabetes, and neurodegenerative diseases (Howell, 2002). Investigation of mitochondrial dysfunction, with its potential benefit of identifying drug candidates, has become an exciting area of research. Cancer cells resist the induction of MPT activation or MPT pore opening; these aspects of mitochondrial function are therefore of interest as potential targets for cancer therapy. Recently, it has been shown that many chemotherapeutic agents, such as saturosporine, act directly on mitochondria in tumor cells and induce apoptosis through mitochondrial dependent pathways (Costantini et al., 2000; Preston et al., 2001). Many experimental drugs for cancer treatment have also been shown to act on mitochondria, although it is not clear whether alterations in mitochondrial function are responsible for the anticancer effect. This makes it very important to conduct a systematic and comprehensive study to identify drug targets in the mitochondria.
The role of the mitochondria in the mechanism of cell death has been studied extensively. Cyclosporine A is an immunosuppressant that is widely used in organ transplantation to reduce allograft rejection and in the treatment of autoimmune disorders. At the cellular level, this compound forms complexes with a 17 kDa cyclophilin-family protein Cyp-M, a reaction that is believed to be involved in inhibiting MPT (Connern & Halestrap, 1992; Nicolli et al., 1996). Cyclosporine A can therefore be used to investigate the role of MPT in the mechanism of cell death. The present study was initiated to explore the in-depth mechanism in mahanine induced cell death. Here, we demonstrate that mahanine disrupts mitochondrial function in U937 cells. A number of apoptotic features are seen in these cells after mahanine treatment: loss of cell viability, fragmentation of nucleosomal DNA, loss of MPT, translocation of mitochondrial cytochrome c to the cytosol, and activation of caspase-3. These features are ameliorated by the treatment with cyclosporine A. Our results provide the first clear demonstration of the role of mitochondrial transition pores in mahanine-induced cell death.
Methods
Materials
Mahanine was purified (>95%, HPLC) from leaves of M. minutum by the method described previously (Nakahara et al., 2002). A stock solution (10 mM) was prepared by dissolving the crystallized compound in absolute ethanol. Fluorogenic caspase substrates were obtained from Peptide Institute Inc., Osaka, Japan. Benzyloxycarbonyl-Asp-CH2OC(0)-2,6-dichlorobenzene(Z-Asp-CH2-DCB), cyclosporine A and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbo-cyanine iodide (JC-1) were obtained from Wako Pure Chemicals, Osaka, Japan. For Western blotting, anticaspase-3 IgG was obtained from Oncogene Research Products (San Diego, CA, U.S.A.); anticytochrome c IgG was obtained from Pharmingen (San Diego, CA, U.S.A.). Unless otherwise indicated, the remaining chemicals were purchased from Sigma (St Louis, MO, U.S.A.).
Cell culture
Human myeloid leukemic cells (U937) were obtained from Riken Gene Bank, Tsukuba, Japan. Cells were maintained in RPMI-1640 (Gibco BRL Life Technologies, Grand Island, NY, U.S.A.) supplemented with 10% fetal bovine serum (FBS) and antibacterial antimycotic solution. Cells were grown at 37°C in a humidified incubator with a 5% CO2 atmosphere and were used for assays during the exponential phase of growth.
Cell treatment
U937 cells were grown in the culture media for 24 h. Mahanine, dissolved in absolute ethanol, was added to the culture media at a final concentration of 0–15 μM for the indicated periods in each experiment. An equal amount of ethanol was added to all treatments and the concentration never exceeded 0.1% of the culture media. No additives were given to the control cultures. Cyclosporine A was dissolved in dimethyl sulfoxide. In some experiments, cells were preincubated with 1–4 μM cyclosporine A for 1 h prior to mahanine exposure and then co-treated during the mahanine exposure period.
Quantitative analysis of viable and apoptotic cells
Cell proliferation activity was measured by counting viable cells using the trypan blue exclusion method. A fluorescent DNA-binding dye, Hoechst 33258, was used to define nuclear chromatin condensation as a quantitative index of apoptotic cell as described previously (Harada-Shiba et al., 1998; He et al., 2000). Briefly, cells were harvested and washed with phosphate-buffered saline (PBS) (pH 7.0). The washed cells were fixed with methanol/acetic acid (v v−1 3 : 1) at 4°C for 5 min, and stained with Hoechst 33258 dye for 10 min at room temperature; nuclear morphology was examined by fluorescence microscopy. The percentage of apoptotic cells was calculated as: (the number of apoptotic cells/number of total cells) × 100. Each experiment was conducted in triplicate and repeated three times.
ATP determination
Intracellular ATP was measured luminometricaly using an ATP assay kit based on the bioluminescent measurement of ATP. Briefly, U937 cells (1.5 × 106 cells 5 ml−1), with or without preincubation with cyclosporine A, were exposed to mahanine in a range of concentrations from 0 to 10 μM for 10 h and collected in 1 ml eppendorf tubes. After two washes with ice-cold PBS, cells were lysed with ATP-releasing reagent provided in the kit. Luciferin and luciferase were then added to the reaction chamber. Bioluminescence, which is linear to ATP content, was recorded on a luminometer. Total ATP content in the sample was determined by running an internal standard, and then expressed as a percentage of that of untreated (control) cells.
Analysis of DNA fragmentation
DNA fragmentation was assessed in U937 cells treated either with mahanine alone or after preincubation with cyclosporine A (3.5 μM) and Z-Asp-CH2-DCB (a broad caspase inhibitor) (100 μg ml−1). The amount of DNA fragmentation was measured using the terminal deoxynucleotidyl transferase mediated fluorescent isothiocyanate deoxyuridine triphosphate (FITC-dUTP) nick end labeling (TUNEL) assay (APO-DIRECT, Phoneix Flow Systems Inc., San Diego, CA, U.S.A.) and flow cytometry on a FACsort (Becton Dickinson). Internucleosomal DNA fragmentation was analyzed by agarose gel electrophoresis. U937 cells (1.25 × 106 cells 5 ml−1) were treated with 0, 6, 8, and 10 μM mahanine for 10 h, or with 7 μM mahanine preincubated with 0–4 μM cyclosporine A. Some U937 cells were cultured for 5 h with 5 μM actinomycin D (ActD) as a positive control. After treatment, cells (1 × 106) were washed once with PBS (4°C, pH 7.4) and then lysed with 15 μl buffer containing 50 mM Tris-HCl (pH 7.8), 10 mM ethylenediaminetetraacetic acid (EDTA), 0.5% sodium dodecyl sulfate (SDS) for 15 min on ice. The cell lysate was then treated with RNase (2 μl, 10 mg ml−1) for 30 min at 50°C, and finally digested with proteinase K (2 μl, 10 mg ml−1) for 1 h at 50°C. The whole lysate was then analyzed by gel electrophoresis on 2% agarose gel. Control cells were incubated with culture media alone.
Flow cytometry analysis for DNA distribution
The numbers of cells in each phase of the cell cycle were determined using a FACSort flow cytometer after staining the DNA with propidium iodide (PI). Briefly, U937 cells (1.25 × 106 cells 5 ml−1), with or without preincubation with cyclosporine A, were exposed to various concentrations of mahanine (as indicated in the figures). Cells were collected by centrifugation, washed with PBS, and fixed in 70% ethanol at −20°C for more than 12 h. Fixed cells were washed with PBS, incubated with 100 μg ml−1 RNase for 30 min at 37°C, and stained with 50 μg ml−1 PI for 20 min on ice. The stained cells were analyzed on a FACSort flow cytometer (Becton Dickinson) for relative DNA content. Cells that had not been exposed to any treatment were used as a control.
Flow cytometry analysis for discriminating apoptotic and necrotic cells
Dual staining of cells with annexin V and PI has become a useful tool for distinguishing apoptotic from necrotic and living cells (Koopman et al., 1994). The method was used for quantitative analysis of apoptotic and necrotic cells according to the procedures recommended by the manufacturer of the Annexin V-FITC apoptosis detection kit (Immunotech, Marseille, France). Briefly, cells (1 × 105 cells ml−1) were exposed to mahanine for 6 h and stained with annexin V-FITC and PI according to the manufacturer's protocol. Untreated cells were used as controls. The stained cells were then analyzed on a FACSort (Becton Dickinson). Annexin V is a protein that binds to a phosphatidylserine residue that is exposed on the surface of apoptotic but not normal cells. The PI is used to stain the cellular DNA of cells whose cell membrane has been totally compromised.
Assay for caspase-like activity
Activities of caspase-3, -8, and -9 like proteases were determined using corresponding fluorogenic substrates. Briefly, both untreated control cells or cells after mahanine treatment were washed with ice cold PBS and lysed in lysis buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 1% NP 40, 2 mM EDTA, 1 mM dithiothreitol (DTT), 1% phenylmethylsulfonyl fluoride (PMSF), 10 μg ml−1 of each of leupeptin and aprotinin) for 30 min on ice followed by centrifugation at 15,000 × g for 10 min. In total, 20 μl of protein solution containing 30 μg protein was mixed with 10 μl of 10 mM fluorogenic report substrate specific for each caspase: Ac-DEVD-MCA for caspase-3, Ac-IETD-MCA for caspase-8, Ac-LEHD-MCA for caspase-9. The reaction mixture was added to 2970 μl of assay buffer (20 mM HEPES (pH, 7.4), 0.1 M NaCl, 5 mM DTT, 0.1% NP 40) and incubated at 37°C for 1 h. Liberation of MCA was monitored using a spectrofluorometer at excitation and emission wavelengths of 360 and 450 nm, respectively. Untreated cells were used as a control.
Western blot analysis
To detect caspase-3 and its cleaved products, 40 μg of cell homogenates (prepared as described above for the caspase activity assay) were separated on 15% SDS–PAGE gels. For cytochrome c analysis, cells were suspended in buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES (pH 7.5), 0.2 mM EGTA, 1 mM MgCl2, 5 mM glutamic acid, 5 mM malate, 0.1 mM PMSF, 5 μg ml−1 pepstatin A, 10 μg ml−1 leupeptin and 2 μg ml−1 aprotonin. Digitonin (4 μg ml−1) was then added to the suspension and cell lysis was confirmed using trypan blue. The cell suspension was centrifuged at 5000 × g for 10 s at 4°C. The supernatant was collected and further centrifuged at 12,000 × g for 10 min at 4°C. The final supernatant was presumed to be the cytosolic fraction and the pellet was considered to be the mitochondrial fraction. Proteins of both cytosolic (30 μg) and mitochondrial (15 μg) fractions were separated on 15% SDS–PAGE gels. After separation, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). The membranes were incubated overnight with blocking solution (5% non-fat dry milk in TBS containing 0.1% Tween 20). They were then incubated in Tris-buffered saline-Tween buffer with primary antibodies against caspase-3 (1 : 1000) and cytochrome c (1 : 2000). After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse IgG (1 : 1250), and visualized by incubation of the membranes with a chemiluminescence Western blot kit (ECL, Amersham Bioscience, Bukinghamshire, England).
Mitochondrial permeability transition (MPT) assay
For MPT assay, control and cyclosporine A treated U937 cells were exposed to various concentrations of mahanine. After two washes with ice-cold PBS in a 5-ml polystyrene tube, cells were resuspended in PBS containing 2 μg ml−1 JC-1 and kept in the dark. After 20 min, the cells were washed twice with PBS and analyzed using a FACSort (Becton Dickinson). The photo multiplier values of the detectors were set according to Cossarizza et al. (1993).
Analysis of data
The data are expressed as means±s.e.m. The Student–Newman–Keuls post hoc comparison and ANOVA were used to detect statistically significant differences (P⩽0.05) between treatment groups. All the figures shown in this article were obtained from at least three independent experiments.
Results
Mahanine suppresses cell proliferation and induces apoptosis in U937 cells
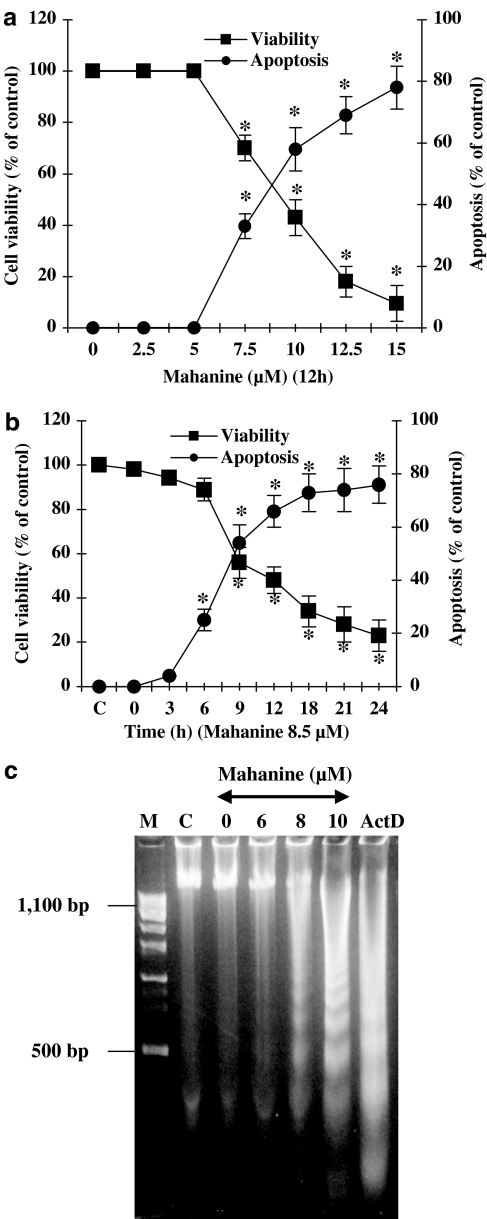
We first examined the influence of mahanine on cell viability in two separate experiments. In the first, cells were exposed to a range of concentrations of the alkaloid for a fixed period of 12 h. In the second, cells were treated with 8.5 μM of mahanine for a variable length of time. The results of these experiments are summarized in Figure 2a and b, respectively. A dose of up to 5 μM of mahanine had no discernible effect on cell viability or apoptosis after 12 h of treatment (Figure 2a). At higher doses cell viability declined and apoptosis increased in a reciprocal manner. Similarly, cell viability fell and apoptosis rose with time when cells were cultured in the presence of 8.5 μM of mahanine (Figure 2b). From our observations, we estimate IC50 values of 8.25 μM for cell viability and 8.7 μM for apoptosis at 12 h.
Figure 2.
Mahanine inhibits cell proliferation and induces apoptosis in U937 cells. (a and b) Dose and time dependent response of U937 cells to mahanine. Cells were incubated with the indicated concentration of mahanine for 0–24 h. The data show that mahanine increased number of apoptotic cells and decreased cell viability in both experiments. Cell viability was determined using the trypan-blue exclusion assay, and the number of apoptotic cells was counted after staining with Hoechst 33258. Data are mean values from three separate experiments and bars indicate standard deviations. *P<0.05 as compared with controls. (c) Mahanine-induced DNA fragmentation in U937 cells. Cells were incubated with the indicated amount of mahanine for 10 h. DNA was extracted as described in ‘Methods' and visualized on agarose gels. M, marker DNA; C, cells incubated with media alone; ActD, cells incubated with actinomycin D (positive control).
To assess the degree of apoptosis, we analyzed the formation of fragmented DNA in mahanine-treated cells by agarose gel electrophoresis. Figure 2c shows that at 10 h, 6.0, 8.0 or 10.0 μM mahanine treatment resulted in the formation of nucleosomal DNA fragmentation with 100–1000 bp oligonucleosomal fragments in U937 cells. Under these conditions, U937 cells treated with 5 μM actinomycin D for 5 h was used as a reference of positive control. We used agarose gel electrophoresis to analyze the formation of fragmented DNA in cells cultured in the presence of mahanine. After 10 h of culture, nucleosomal DNA fragmentation in the range 100–1000 bp were observed after treatment with mahanine doses greater than 6 μM, with an apparently increased fragmentation at higher doses (Figure 2c). The actinomycin D treated positive control showed the expected increase in DNA fragmentation.
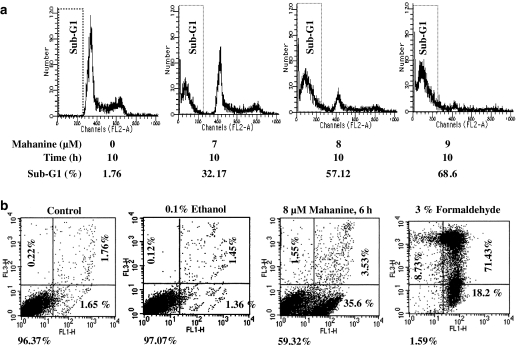
Our flow cytometric analysis of cell cycle revealed an increase in the subdiploid population of cells after treatment with mahanine (Figure 3a); this increase was consistent with that expected from the apoptosis results described above. After 10 h of culture with 8.0 μM of mahanine, more than 50% of the U937 cells were subdiploid. Double staining with annexin V and PI showed that more than 95% of the cells in control cultures were viable, as indicated by the low-intensity staining of both dyes. In contrast, 35% of cells cultured for 6 h in the presence of 8 μM of mahanine were classified as apoptotic due to their high annexin V and low PI staining (Figure 3b).
Figure 3.
(a) Flow cytometric analysis of apoptotic U937 cells. U937 cells were treated with mahanine as indicated, and then evaluated for DNA content after PI staining. (b) Determination of necrotic versus apoptotic cells in mahanine-treated U937 cell cultures. Cells were incubated with 8 μM mahanine for 6 h or 3% formaldehyde for 30 min on ice (positive control). Control cells were incubated with media alone. After incubation cells were double stained with annexin V and propidium iodide for FACS analysis. Lower left (LL), living cells, lower right (LR) apoptotic cells, upper right (UR) necrotic cells. The data are derived from at least three independent experiments.
Mahanine induces caspase-like activation
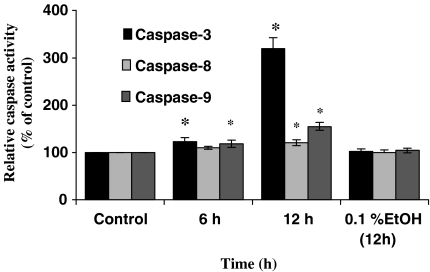
Recent studies showed that activation of caspases is an important factor in the induction of apoptosis (for a review see Cohen, 1997). We estimated the levels of one effector caspase, caspase-3, and two initiator caspases, caspase-8 and -9, in the cytosolic extract of U937 cells using fluorogenic specific caspase substrates. The cells were cultured in the presence of 7.5 μM mahanine for 6 or 12 h. The level of caspase-3 and -9 rose significantly after mahanine treatment compared with control cultures (Figure 4). The level of caspase-8 was not significantly altered by mahanine treatment at 6 h, while that showed a significant rise at the 12 h interval (Figure 4).
Figure 4.
Mahanine activates caspase-like activities in U937 cells. Time courses of mahanine-mediated activation of caspase-3, -8, and -9 in U937 cells. U937 cells were incubated with 7.5 μM mahanine for the indicated time and analyzed for caspase-like activities as described in ‘Methods'. The data are means of three separate experiments. *P<0.05 as compared with controls. Control cells were incubated with media alone.
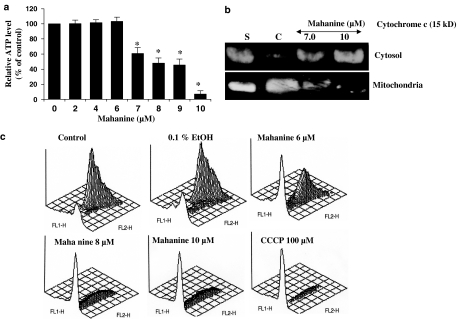
Impairment of mitochondrial function in U937 cells by mahanine
To determine whether mitochondria play any role in mahanine-induced apoptosis in U937 cells, we first investigated alteration of mitochondrial function by examining cellular ATP levels. Figure 5a shows the dose-dependent response of mahanine-inhibited cellular ATP level in U937 cells. The cells were incubated with various doses of mahanine for 10 h and their levels of ATP measured. We found no significant change in cellular ATP level when cells were treated with mahanine at concentrations of 6 μM and lower. At 7.0 μM a decrease in cellular ATP level (60% of the control) was detected; the level decreased further to 10% when cells were treated with 10 μM mahanine. We next examined the effect of mahanine on cytochrome c release by evaluating cytosolic and mitochondrial cytochrome c levels in U937 cells treated for 10 h with different concentrations of mahanine. As shown in Figure 5b, a remarkable release of cytochrome c from the mitochondrial to cytosolic compartments occurred. To further explore the consequences of mahanine on mitochondria, we investigated whether mahanine produced any alteration in mitochondrial membrane potential. U937 cells were treated with mahanine, stained with JC-1 and analyzed by flow cytometry with a FACSort cytometer (Becton Dickinson). We found that mahanine treatment at doses higher than 6 μM resulted in an increase in the intensity of the green fluorescence (530 nm) peak by the FL1 detector and a concomitant decrease in the red fluorescence (575 nm) peak by the FL2 detector, indicative of reduced mitochondrial membrane potential (Figure 5b). The FL2 (red) fluorescence of U937 cells treated with 100 μM carbonylcyanide m-chlorophenylhydrazone (CCCP) completely disappeared and used as reference for depolarized mitochondria (positive control). Overall, our observations show that loss of mitochondrial function is the critical step in mahanine-induced apoptosis.
Figure 5.
Mahanine inhibits cellular ATP level, induced cytochrome c release and MPT loss in U937 cells. (a) U937 cells were incubated with mahanine for 10 h, and collected for total cellular ATP measurement as described in ‘Methods'. Values are means of three separate experiments and bars indicate standard deviations. *P<0.05 as compared with controls. (b) Effects of mahanine on cytochrome c translocation in U937 cells. U937 cells were incubated with mahanine as indicated for 10 h. Cytosolic and mitochondrial fractions were separated as described in ‘Methods' and analyzed by Western blot for cytochrome c. S, standard cytochrome c; C, cells incubated with media alone (control). (c) Mahanine-induced mitochondrial membrane potential loss in U937 cells. U937 cells were incubated with the indicated amount of mahanine for 10 h. Cells were stained with JC-1 and analyzed by FACS. FL1 indicates green fluorescence (log scale), FL2 red fluorescence (log scale). One representative experiment of the three performed is shown. Control cells were incubated with media alone.
Cyclosporine A prevents mahanine-induced apoptosis
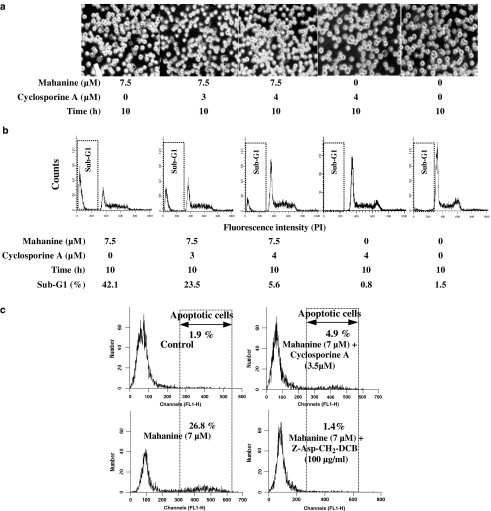
We used cyclosporine A, a potent and specific inhibitor of the MPT pore to investigate further the role of the mitochondria in mahanine-induced apoptosis. In this experiment, U937 cells were incubated with cyclosporine A for 1 h before mahanine treatment. We found that the typical mahanine-induced morphological changes in the cells, visible by light microscopy (Figure 6a), were not present in cells pretreated with cyclosporine A. This observation is the first indication that cyclosporine A can protect U937 cells from the apoptogenic effect of mahanine. Next, we estimated the number of apoptotic cells by FACS. After cyclosporine A pretreatment, there was a significant decrease in the population of subdiploid cells after mahanine treatment, compared with the cultures treated only with mahanine (Figure 6b).
Figure 6.
Effects of cyclosporine A on mahanine-induced morphological changes and apoptosis in U937 cells. U937 cells, with or without preincubation with cyclosporine A, were incubated with mahanine for 10 h. After incubation, cell morphology was examined by light microscopy (a); DNA content was measured by FACS after staining DNA with propidium iodide (b). The results are from a representative example of the three independent experiments performed. (c) Cells were preincubated with cyclosporine A and a general caspase inhibitor for 1 h. Following incubation with 10 μM mahanine for 10 h, DNA breaks in U937 cells were determined using the TUNEL assay and FACS. Control cells were incubated with media alone. One representative experiment of the three performed is shown.
Next, we used another methodology to assess the effects of cyclosporine A in the prevention of mahanine-induced apoptosis. For this purpose, the TUNEL assay was used to detect DNA breaks in U937 cells. In cells treated with mahanine alone, 26.8±4.5% cells showed DNA breaks detectable by TUNEL; cyclosporine A pretreatment reduced the rate of cells with fragmented DNA to 4.9±2.6% (Figure 6c).
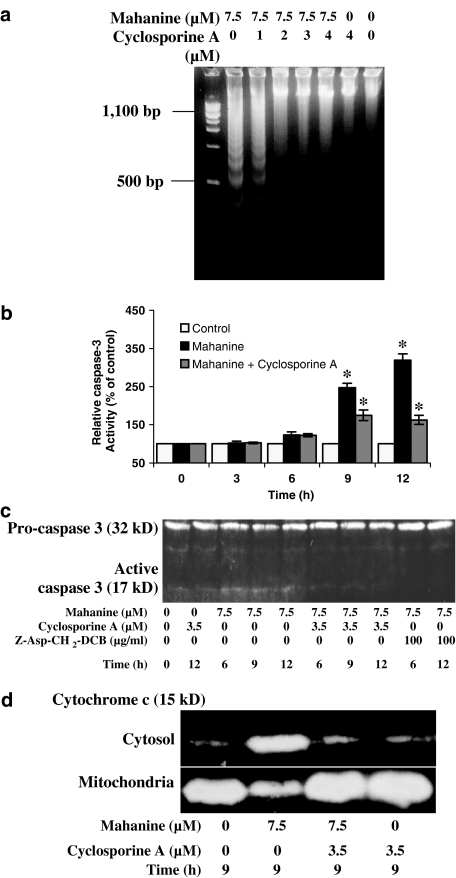
To further characterize the protective effect of cyclosporine A against nuclear fragmentation in U937 cells, cells were cultured for 1 h with cyclosporine A and then cultured with mahanine for 10 h (Figure 7a). DNA was extracted from the cell cultures at the end of this period. In mahanine treated cells, DNA appears as a ladder-like pattern. However, the degree of ladder formation was substantially decreased in the cells treated with cyclosporine A before mahanine treatment (Figure 7a).
Figure 7.
Cyclosporine A prevents mahanine-induced DNA fragmentation, expression of caspase-3 and cytochrome c release in to cytosol in U937 cells. Cells were pretreated with cyclosporine A for 1 h. Mahanine was then added to the culture as indicated in the figures. (a) After 10 h, DNA fragmentation was evaluated by agarose gel electrophoresis. (b) Cells were incubated with 3.5 μM cyclosporine A for 1 h, and then with 7 μM mahanine for the indicated time. Cytosolic extract was analyzed for caspase-3 activity assay as described in ‘Methods'. Data are means of three independent experiment, bars represents the standard deviation. *P<0.05 as compared with controls. (c) In a separate experiment, cells were pre-incubated with cyclosporine A and Z-Asp-CH2-DCB, a general caspase inhibitor, for 1 h, followed by mahanine treatment as indicated in the figure. Cytosolic extracts were prepared, and equal amounts of proteins (40 μg) were separated on SDS–PAGE gels and analyzed using Western blots for caspase-3 activation. (d) Inhibition of cytochrome c release into the cytosol by cyclosporine A was determined by evaluating cytosolic and mitochondrial fractions of U937 cells, with and without pre-incubation with cyclosporine A. The results are representative of three independent experiments with a similar pattern (a, c and d).
Cyclosporine A prevents caspase-3 activation
We next examined the effect of cyclosporine A pretreatment on mahanine-induced caspase activation. U937 cells were pretreated with cyclosporine A and then cultured with mahanine for 3, 6, 9 and 12 h. Cytosolic extracts were prepared and analyzed to determine procaspase-3 expression and caspase-3 activity. Procaspase-3 levels fall in cells treated with mahanine, and there is a corresponding rise in caspase-3 activity. Pretreatment with cyclosporine A caused a decrease in caspase-3 activity in mahanine treated cells (Figure 7b and c).
Cyclosporine A prevents mahanine induced cytochrome c release in U937 cells
There is increasing evidence to suggest that mitochondria play a pivotal role in the induction of apoptosis by releasing cytochrome c, which in turn drives formation of a high molecular weight caspase activating complex termed AIF (apoptosis inducing factor) (Li et al., 1997; Cain et al., 1999; Zou et al., 1999). To examine the release of cytochrome c, we conducted Western blot analysis of both cytosolic and mitochondrial fractions. We found that mahanine induced cytochrome c release from mitochondria and, as a result, the level of cytochrome c in the mitochondria decreased while that in the cytosol increased. As part of this study we also examined whether cyclosporine A played a role in preventing cytochrome c effluxion from mitochondria. We found that cyclosporine A pretreatment (1 h) clearly blocked cytochrome c release into the cytosol (Figure 7d).
Cyclosporine A pretreatment blocks mahanine-induced mitochondrial alterations
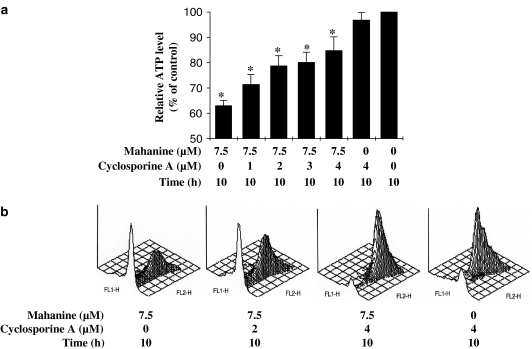
To further explore the actions of cyclosporine A on mitochondrial function and integrity, we examined cellular ATP levels and MPT in U937 cells after pretreatment with various concentrations of cyclosporine A before mahanine treatment. We found that cyclosporine A pretreatment significantly increased cellular ATP levels compared to those in cells treated with mahanine alone (Figure 8a). Cells cultured with 7.5 μM mahanine had 63% of the ATP levels present in untreated controls; however, pretreatment with 4 μM cyclosporine A increased ATP levels up to 83% of control levels (Figure 8a). JC-1 staining was used to measure MPT (Cossarizza et al., 1993). FACS analysis demonstrated that fluorescence of the aggregated JC-1 (FL2) was lower after mahanine treatment compared to controls; after pretreatment with cyclosporine A fluorescence was restored to control levels (Figure 8b). The results indicate that mahanine-induced changes of mitochondrial function or mitochondrial integrity, as reflected in ATP level and MPT analysis, were prevented or blocked by cyclosporine A.
Figure 8.
Cyclosporine A prevented mitochondrial functional loss, and formation of DNA breaks in mahanine-induced apoptotic cells. A 1 h pretreatment and 10 h co-treatment with cyclosporine A was given to U937 cells exposed to mahanine (7.5 μM). (a) Cells were then collected for total cellular ATP measurement as described in ‘Methods'. Values are means±s.e., n=3. *P<0.05 as compared with controls. (b) Cells were stained with JC-1 and mitochondrial permeability loss was determined by evaluating the fluorescence of aggregated JC-1, as described in ‘Methods' (results are representative of three independent experiments).
Discussion
Mitochondrial dysfunction, or deregulation of mitochondrial activity, is often associated with the development of cancer. Many anticancer agents, both natural and synthetic, mediate their chemotherapeutic effect by enhancing apoptotic cell death through mitochondrial membrane permeabilization (Costantini et al., 2000). Development of cytotoxic drugs that target mitochondria has become an increasingly important strategy for killing tumor cells (Howell et al., 2003; Morisaki & Katano, 2003).
Mahanine, and some of its analogs, are known for their cytotoxic activity against some cancer cell lines (Bowen & Perera, 1982; Wu, 1991). Here, we observed that mahanine induced cell death in U937 cells by targeting mitochondria and causing release of cytochrome c into the cytosol. U937 cells incubated with mahanine, at doses ranging from 7 to 10 μM, manifest a range of apoptotic characteristics such as reduction in cell viability, a shrunken cellular appearance, a ladder-like pattern of DNA fragmentation, reduced DNA content and translocation of phosphatidylserine to the outer leaflet of the plasmamembrane. All these data indicated that mahanine induces cell death in U937 cells via the apoptotic pathway.
In the study we also observed that mahanine causes caspase-3 activation and translocation of cytochrome c from the mitochondrial compartment to the cytosol. To study the effect of mahanine on mitochondrial function loss, we measured cellular ATP levels. Mahanine, at doses 7–9 μM, decreased the cellular ATP level to 60–40% of that of control cells. Besides the lower cellular ATP level, we also confirmed that mahanine depolarizes mitochondrial membrane potential. For this, we used the mitochondrial selective dye JC-1 that diffuses into the cell, accumulates in mitochondria and forms J-aggregates in the presence of high membrane potential. Our results demonstrated that mahanine-treated U937 cells exhibited lower mitochondrial membrane potential and, therefore, less mitochondrial stability than the control cells. We suggest that there are many reasons to believe that this is the most fundamental event in mahanine induced-apoptosis in U937 cells. Cells undergoing apoptosis have been shown to exhibit an increase in cytosolic cytochrome c with a correlated decrease in mitochondria. These cells also show a decrease in cellular ATP levels and reduced mitochondrial membrane potential.
It is widely considered that cytochrome c release into the cytosol is required to activate the caspase cascades that are essential for an apoptotic response. In the present study, we used cyclosporine A to investigate the ability of mahanine to induce mitochondrial functional loss and to activate caspases, particularly caspase-3. Cyclosporine A functions at the mitochondrial level, and is believed to bind at the MPT pores, thereby increasing the probability of pore closer and preventing the breakdown of inner mitochondrial membrane potential (Samali et al., 1998). Our results demonstrate that cyclosporine A pretreatment opposes mahanine action to increase cell viability and maintain normal cell morphology. Moreover, our results indicate that cyclosporine A acts at regulatory points controlling cell viability and cell morphology. Cyclosporine A pretreatment also decreases the number of apoptotic cells, inhibits DNA fragmentation, and suppresses caspase-3 activation. Our observations clearly demonstrate that cyclosporine A has a protective effect on those aspects of mitochondrial function that are targeted by mahanine.
The activation of caspases is a hallmark of apoptosis. Activated caspases cleave a variety of target proteins including poly(ADP-ribose) polymerase (Tewari et al., 1995; Nicholson & Thornberry, 1997), DNA-dependent protein kinase (Song et al., 1996), sterol regulatory dependent binding protein (Wang et al., 1996), and thereby disable cellular process and break down cellular structure. In addition, activated caspase leads to the cleavage of DFF-45, a 45 kDa DNA fragmentation factor and CAD inhibitor (Sakahira et al., 1998). Cleavage of DFF-45 ultimately activates a pathway leading to genomic DNA fragmentation, a hallmark of apoptosis (Liu et al., 1997; Wolf et al., 1999). In several model studies, it has been shown that caspase activation, particularly caspase-3, requires cytochrome c. Cytochrome c forms a 700 kDa Apaf-1 complex called apoptosome by binding with caspase-9, an event that leads to caspase-3 activation. Our measurements, by fluorometric enzyme assay and Western blotting, have shown that cyclosporine A pretreatment prevented caspase-3 activation. We therefore evaluated cytochrome c release into cytosol in cells pretreated with cyclosporine A and incubated with mahanine. Cyclosporine A pretreatment clearly prevented translocation of cytochrome c from mitochondria into the cytosol; this effect provides a direct link between mitochondria and mahanine-induced apoptosis in U937 cells. It is widely believed that cytochrome c release into the cytosol occurs in response to the collapse of mitochondrial transmembrane potential (Green & Reed, 1998). We assessed mitochondrial membrane potential, using the JC-1 dye, in cells treated with cyclosporine A and mahanine or with mahanine alone. Our results confirmed that mitochondrial membrane potential collapsed in mahanine-treated cells and further showed that it was protected when cells were pretreated with cyclosporine A. The inner membrane of the mitochondrion is the site for the electron transport chain and the generation of ATP. Measurement of intracellular ATP level in cells pretreated with cyclosporine A and then incubated with mahanine showed that the pretreatment prevented the drop in ATP levels normally associated with exposure to the alkaloid, that is, the pretreatment maintained mitochondrial function.
Thus, the cytotoxic effect of mahanine can be compared with that of many anticancer drugs that induce apoptosis in tumor cells by permeabilizing mitochondrial membranes. These drugs include 2-chloro-2′-deoxyadenosine, ciprofloxacin, etoposide, paclitaxel, and resveratrol (for a review see Debatin et al., 2002). The effect of these drugs on the permeability of mitochondrial membranes is prevented by cyclosporine A, a compound that blocks the opening of the permeability transition pore complex. This is analogous to the effect observed here of cyclosporine A ameliorating mahanine-induced mitochondrial dysfunction in the U937 cancer cell line. Recently, various other mitochondria-permeabilizing drugs have been developed (for a review see Debatin et al., 2002; Howell et al., 2003). In the future, this study may be a useful aid to finding new mitochondria-targeting drugs for destroying cancer cells.
In summary, we have shown that mahanine induces a dose-dependent increase in apoptotic cells and a concomitant decrease of cell viability in cultured U937 cells. Mahanine-induced apoptosis in U937 cells was associated with cytochrome c release into the cytosol, and with subsequent activation of caspase-9 and -3. Moreover, cyclosporine A protected U937 cells from mahanine-induced apoptosis by inhibiting cytochrome c release into cytosol, by preventing caspase-3 activation, and by opposing mitochondrial membrane permeabilization. Since impairment of mitochondrial function is often associated with tumor development, substances such as mahanine that target mitochondria and execute apoptosis through the intrinsic pathway could represent promising new agents for the prevention of tumor development or for the treatment of cancer.
Acknowledgments
We are indebted to Dr Hiroshi Shinmoto and his colleagues (National Food Research Institute, Tsukuba, Japan) for their facilities in FACS analysis. This work was supported by JIRCAS under the Visiting Research Fellowship Program.
Abbreviations
- Ac-DEVD-MCA
acetyl-L-aspartyl-L-glutamiyl-L-valyl-L-aspartic acid-(4-methyl-coumaryl-7-amide)
- Ac-IETD-MCA
acetyl-L-isoleucyl-L-glutamyl-L-threonyl-aspartic acid a-(4-methyl-coumaryl-7-amide)
- Ac-LEHD-MCA
acetyl-L-leucyl-L-glutamyl-L-histidyl-L-aspartic acid a-(4-methyl-coumaryl-7-amide)
- CAD
caspase activated DNase
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolylcarbo-cyanine iodide
- MPT
mitochondrial permeability transition
- PBS
phosphate-buffered saline
- PI
propidium iodide
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- Z-Asp-CH2-DCB
benzyloxycarbonyl-Asp-CH2OC (0)-2,6-dichlorobenzene
References
- BOLDIN M.P., GONCHAROV T.M., GOLTSEV Y.V., WALLACH D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- andTNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- BOWEN I.H., PERERA K.P.W.C. Alkaloids, coumarins and flavonoids of Micromelum zeylanicum. Phytochemistry. 1982;21:433–437. [Google Scholar]
- CAIN K., BROWN D.G., LANGLAIS C., COHEN G.M. Caspase activation involves the formation of the aposome, a large (∼700 kDa) caspase-activating complex. J. Biol. Chem. 1999;274:22686–22692. doi: 10.1074/jbc.274.32.22686. [DOI] [PubMed] [Google Scholar]
- COHEN G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNERN C.P., HALESTRAP A.P. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem. J. 1992;284:381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSSARIZZA A., BACCARANI-CONTRI M., KALASHNIKOVA G., FRANCESCHI C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem. Biophys. Res. Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- COSTANTINI P., JACOTOT E., DECAUDIN D., KROEMER G. Mitochondrion as a novel target of anticancer chemotherapy. J. Natl. Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- DEBATIN K.M., PONCET D., KROEMER G. Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- ENARI M., SAKAHIRA H., YOKOYAMA H., OKAWA K., IWAMATSU A., NAGATA S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- GREEN D.R., REED J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- GROSS A., MCDONNELL J.M., KORSMEYER S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- HARADA-SHIBA M., KINOSHITA M., KAMIDO H., SHIMOKADO K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J. Biol. Chem. 1998;273:9681–9687. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- HE J., XIAO Y.H., ZHANG L. Cocaine induces apoptosis in human coronary artery endothelial cells. J. Cardiovasc. Pharmacol. 2000;35:572–580. doi: 10.1097/00005344-200004000-00010. [DOI] [PubMed] [Google Scholar]
- HOWELL N. Navigating between Scylla and Charybdis: mitochondria are both precedented and novel targets for drug development. Drug Dev. Res. 2002;57:75–82. [Google Scholar]
- HOWELL N., TAYLOR S.W., FAHY E., MURPHY A., GHOSH S.S. Restoring energy in a power crisis: mitochondrial targets for drug development. Targets. 2003;2:208–216. [Google Scholar]
- HUANG D.C., HAHNE M., SCHROETER M., FREI K., FONTANA A., VILLUNGER A., NEWTON K., TSCHOPP J., STRASSER A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proc. Natl. Acad. Sci. U.S.A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOPMAN G., REUTELINGSPERGER C.P., KUIJTEN G.A., KEEHNEN R.M., PALS S.T., VAN OERS M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- LI H., ZHU H., XU C.J., YUAN J. Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- LI L.Y., LUO X., WANG X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- LI P., NIJHAWAN D., BUDIHARDJO I., SRINIVASULA S.M., AHMAD M., ALNEMRI E.S., WANG X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- LIU X., KIM C.N., YANG J., JEMMERSON R., WANG X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- LIU X., ZOU H., SLAUGHTER C., WANG X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- MORISAKI T., KATANO M. Mitochondria-targeting therapeutic strategies for overcoming chemoresistance and progression of cancer. Curr. Med. Chem. 2003;10:2517–2521. doi: 10.2174/0929867033456431. [DOI] [PubMed] [Google Scholar]
- MUZIO M., CHINNAIYAN A.M., KISCHKEL F.C., O'ROURKE K., SHEVCHENKO A., NI J., SCAFFIDI C., BRETZ J.D., ZHANG M., GENTZ R., MANN M., KRAMMER P.H., PETER M.E., DIXIT V.M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- NAKAHARA K., TRAKOONTIVAKORN G., ALZOREKY N.S., ONO H., ONISHI-KAMEYAMA M., YOSHIDA M. Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutum. J. Agric. Food. Chem. 2002;50:4796–4802. doi: 10.1021/jf025564w. [DOI] [PubMed] [Google Scholar]
- NEWMAN D.J., CRAGG G.M., SNADER K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D.W., THORNBERRY N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- NICOLLI A., BASSO E., PETRONILLI V., WENGER V.R., BERNARDI P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- PRESTON T.J., ABADI A., WILSON L., SINGH G. Mitochondrial contributions to cancer cell physiology: potential for drug development. Adv. Drug Deliv. Rev. 2001;49:45–61. doi: 10.1016/s0169-409x(01)00127-2. [DOI] [PubMed] [Google Scholar]
- RAMSEWAK R.S., NAIR M.G., STRASBURG G.M., DEWITT D.L., NITISS J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999;47:444–447. doi: 10.1021/jf9805808. [DOI] [PubMed] [Google Scholar]
- ROY M.K., VIPAPORN N.T., TRAKOONTIVAKORN G., NAKAHARA K. Mechanism of mahanine induced cell death in HL-60 cells. Biochem. Pharma. 2004;67:41–51. doi: 10.1016/j.bcp.2003.07.021. [DOI] [PubMed] [Google Scholar]
- SAKAHIRA H., ENARI M., NAGATA S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- SAMALI A., ZHIVOTOVSKY B., JONES D.P., ORRENIUS S. Detection of pro-caspase-3 in cytosol and mitochondria of various tissues. FEBS Lett. 1998;431:167–169. doi: 10.1016/s0014-5793(98)00740-6. [DOI] [PubMed] [Google Scholar]
- SONG Q., LEES-MILLER S.P., KUMAR S., ZHANG Z., CHAN D.W., SMITH G.C., JACKSON S.P., ALNEMRI E.S., LITWACK G., KHANNA K.K., LAVIN M.F. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 1996;15:3238–3246. [PMC free article] [PubMed] [Google Scholar]
- SUSIN S.A., LORENZO H.K., ZAMZAMI N., MARZO I., SNOW B.E., BROTHERS G.M., MANGION J., JACOTOT E., COSTANTINI P., LOEFFLER M., LAROCHETTE N., GOODLETT D.R., AEBERSOLD R., SIDEROVSKI D.P., PENNINGER J.M., KROEMER G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- TACHIBANA Y., KIKUZAKI H., LAJIS N.H., NAKATANI N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001;49:5589–5594. doi: 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- TEWARI M., QUAN L.T., O'ROURKE K., DESNOYERS S., ZENG Z., BEIDLER D.R., POIRIER G.G., SALVESEN G.S., DIXIT V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., LAZEBNIK Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- WANG X., ZELENSKI N.G., YANG J., SAKAI J., BROWN M.S., GOLDSTEIN J.L. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 1996;15:1012–1020. [PMC free article] [PubMed] [Google Scholar]
- WOLF B.B., SCHULER M., ECHEVERRI F., GREEN D.R. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 1999;274:30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- WU T.S. Murrayamine-A, -B, -C (+) mahanine, carbazole alkaloids from Murraya euchrestifolia. Phytochemistry. 1991;30:1048–1051. [Google Scholar]
- ZOU H., LI Y., LIU X., WANG X. An APAF-1·cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]