Abstract
The β-secretase BACE1 is one of the enzymes that contribute to the production of the Aβ peptide, in vitro and in vivo. JMV1195 was previously shown to inhibit BACE activity in vitro but was unable to block cellular BACE activity. We have designed a new permeable inhibitor, JMV2764 that corresponds to a derivative of JMV1195 to which a penetratin sequence had been added at its N-terminus. We have assessed the ability of JMV2764 to affect BACE1 activity in vitro, and to modify Aβ production in various cell systems.
Endogenous β-secretase or BACE1 activities were monitored in vitro by means of two distinct fluorimetric substrates in HEK293 extracts of cells expressing either wild-type βAPP, Swedish mutated βAPP or SPA4CT constructs. Aβ40 recovery was monitored by immunoprecipitation and Western blot analysis.
JMV2764 and JMV1195 inhibited endogenous β-secretase activity of HEK293 cellular homogenates with IC50s of 0.8 and 6.6 μM, respectively. Interestingly, JMV2764 also inhibited β-secretase activity after preincubation with intact cells while JMV1195 was inactive, indicating that unlike JMV1195, JMV2764 could penetrate into the cells.
JMV2764 but not JMV1195 also prevented Aβ production by HEK293 cells overexpressing wild-type and Swedish-mutated βAPP. However, JMV2764 was unable to affect Aβ production from cells expressing SPA4CT, a βAPP-derived sequence that does not need β-secretase to produce Aβ.
Altogether, we have designed a new cell-permeable BACE1 inhibitor that allows to envision to prevent Aβ production in vivo. Work is in progress to assess the potential of these compounds to prevent Aβ production in transgenic mice overproducing Aβ.
Keywords: BACE, inhibitors, Aβ production, statine, penetratin, HEK293 cells, wild-type βAPP, Swedish mutated βAPP, SPA4CT
Introduction
Although the exact etiology of Alzheimer's disease remains a matter of discussion, several lines of both anatomical, genetic and cell biology evidences have suggested that the Aβ peptide that accumulates as disease progresses plays a major role in neurodegenerative process (Hardy & Higgins, 1992). Aβ is one of the proteolytic products of βAPP generated by subsequent cleavages by β- and γ-secretases (Checler, 1995). Therefore, the simplest and most straightforward strategy aimed at slowing or preventing Alzheimer's disease would be to block these secretases.
At first sight, targeting the β-secretase appears more innocuous than interfering with γ-secretase pathway. First, a rapidly growing set of data has indicated that affecting expression or activity of various proteins of the presenilin-dependent γ-secretase complex leads to lethality, in embryos (Shen et al., 1997; Wong et al., 1997; Qian et al., 1998) or drastically alters various vital functions at the adulthood in conditional knockout mice lacking presenilins (Beglopoulos et al., 2004; Saura et al., 2004). By contrast, soon after the identification of the β-secretase referred to as BACE1, memapsin 2, or ASP2 (Hussain et al., 1999; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999; Lin et al., 2000), it was shown that abolition of β-secretase expression was, if not totally innocuous, by far less drastic than altering γ-secretase activity. Thus, mice in which β-site APP cleaving enzyme (BACE) has been knocked out are viable and fertile (Luo et al., 2001; Roberds et al., 2001). Most important with respect to Alzheimer's disease, BACE depletion abolishes Aβ production by mice engineered to overproduce the peptide (Cai et al., 2001; Luo et al., 2001).
We have recently described JMV1195, one of a series of peptidomimetic statine-derived compounds able to inhibit BACE (Andrau et al., 2003). However, JMV1195 that appears potent and rather selective in vitro was unable to inhibit cellular β-secretase (Andrau et al., 2003). Here we report on a new inhibitor, JMV2764 that corresponds to an analog of JMV1195 to which a penetratin sequence had been incorporated at the N-terminus. We show that JMV2764 inhibits cellular BACE and prevents Aβ production from several cell systems expressing wild-type or Swedish-mutated βAPP, indicating that the inhibitor penetrates into the cells and reaches its cellular target.
Methods
Chemical reagents
Boc- and Fmoc-amino acids, HBTU and resins were purchased from Senn Chemicals International (Gentilly, France) or Advanced ChemTech (Louisville, U.S.A.). Reagents and solvents for the solid-phase synthesis were obtained from Acros (Noisy-le-Grand, France) or Sigma-Aldrich Fine Chemicals (Saint Quentin Fallavier, France) and were used without additional purification. All other chemicals were of the purest grade available.
Synthesis of the chimerical inhibitor penetratin-ahx-JMV1195 (JMV2764)
The chimerical inhibitor JMV2764 (Ac-RQIKIWFQNRR-Nle-KWKK-ahx-EVN-AHPPA-AEF-NH2) is composed, from the N-terminus to the C-terminus, of a blocking acetyl group, the penetratin sequence (in which the Met residue has been replaced by Nle), a 6-aminohexanoyl residue spacer and the JMV inhibitor sequence containing AHPPA, a phenylalanine-derived statine residue. AHPPA was synthesized as its Boc-protected derivative following a published procedure (Jouin & Castro, 1987). JMV1195 was manually assembled stepwise on an MBHA resin using Boc chemistry. Couplings starting from JMV1195 resin were performed in DMF using HBTU in the presence of diisopropylethylamine on a Pioneer PerSeptive Biosystems automatic synthesizer, then the synthesis was continued using Fmoc chemistry. The peptide was blocked at its N-terminus by an acetyl group using acetic anhydride in dichloromethane. Deprotection and cleavage from the resin were carried out by treatment with HF/anisole (9/1). The crude peptide was purified by reverse-phase HPLC on a C18 column (Deltapack Waters 40 × 100 mm) by means of a linear gradient of 22–45% acetonitrile in 0.1% aqueous trifluoroacetic acid over 25 min (flow rate 28 ml min−1). Its purity and identity were assessed by reverse-phase HPLC and electrospray mass spectrometry (experimental mass, 3263.4±0.2; calculated mass, 3263.6).
HEK293 cell culture, transfections and Western blot analyses
HEK293 cells were stably transfected with DAC30 (Eurogentec) containing 2 μg of pcDNA3 vector encoding SPA4CT (Dyrks et al., 1993). Positive clones were identified by Western blot by means of BR188 polyclonal antibody that recognizes the C-terminus of βAPP. Cells overexpressing wild-type βAPP, Swedish-mutated βAPP and BACE1 have been previously described (Chevallier et al., 1997; Andrau et al., 2003). Western blot analyses were carried out as previously detailed (Andrau et al., 2003).
Fluorimetric assays
Hydrolyses of JMV2236 or a BACE commercial substrate (Mca-SEVNLDAEFRK(Dnp)RRNH2, R&D System, Oxon, U.K.) were monitored in cell extracts as previously detailed, in absence or in the presence of JMV1195, JMV2764 or a commercial inhibitor (KTEEISEVN-(statine) VAEF-OH, Enzyme System Product, Aurora, U.S.A.). At the end of incubation, fluorescence was recorded at 320 and 420 nm as excitation and emission wavelengths, respectively. When inhibitors were examined on plated cells, cells were preincubated for 1 h at 37°C then lysed and assayed as above, in the absence or in the presence of various concentrations of JMV1195. BACE activity was considered as the JMV1195-sensitive hydrolysing activity.
Cells treatment with inhibitors and detection of Aβ
Stably transfected wild-type βAPP, Swedish-mutated βAPP or SPA4CT-expressing HEK293 cells were allowed to secrete Aβ for 6 h in the absence or in the presence of JMV1195 or JMV2764 and with phosphoramidon in order to prevent degradation of secreted Aβ. Media were collected, diluted in 1/10th RIPA 10 × buffer and incubated overnight with a 200-fold dilution of FCA3340 (Barelli et al., 1997). Aβ was immunoprecipitated, monitored by Tris/tricine gels, Western blotted and revealed with WO2 (The Genetics Company, Schlieren, Switzerland) as primary monoclonal antibody as described previously (Ancolio et al., 1999).
Results
Effect of JMV2764 on endogenous β-secretase and overexpressed BACE1 activities
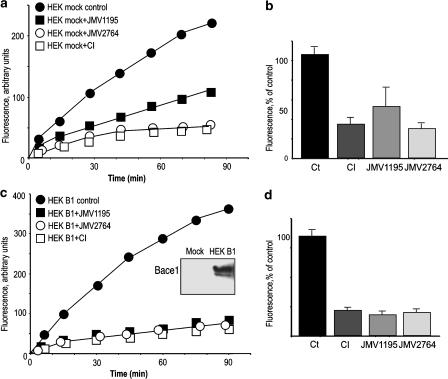
We previously reported on a series of new statine-derived sequences that dose-dependently inhibit BACE1 in stably transfected HEK293 cell extracts (Andrau et al., 2003). Although these inhibitors appeared relatively potent (IC50 in the micromolar range) and specific, they were unable to affect intracellular β-secretase activity, indicating that they were likely poorly permeable and therefore, unable to reach their intracellular target. We therefore designed a novel inhibitor, namely JMV2764 corresponding to the JMV1195 sequence to which a penetratin sequence had been incorporated at the N-terminus (Figure 1). Total endogenous β-secretase-like activity present in HEK293 cells was measured by following the hydrolysis of a previously characterized quenched fluorimetric substrate JMV2236 (Andrau et al., 2003). Figure 2a shows that JMV2764 time-dependently blocked endogenous β-secretase activity. JMV2764 appeared as potent as a commercial inhibitor (CI) and slightly more potent than JMV1195 (Figure 2a, b).
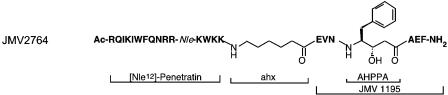
Figure 1.
Structure of JMV2764. AHPPA, phenylalanine-derived statine residue; Ahx, 6-aminohexanoyl; Nle, norleucine.
Figure 2.
Effect of JMV1195 and JMV2764 on JMV2236-hydrolyzing activity by endogenous β-secretase or BACE1. Kinetics of β-secretase activities were measured with 10 μM of JMV2236 in the absence (control) or in the presence of 10 μM of JMV1195, JMV2764 or a commercial inhibitor (CI) with protein homogenates from HEK293 cells harbouring endogenous β-secretase (a) or overexpressed BACE1 (c). In panel c (inset) BACE1-like immunoreactivity in the indicated cell line was detected by means of anti-1D4 antibodies. In panels b and d, histograms compare the extent of inhibition of JMV2236-hydrolyzing activities triggered by 10 μM of indicated inhibitor after 30 min incubation with Mock- (b) or BACE1 (d)-transfected HEK293 cell extracts. Bars are the means±s.e.m. of four independent experiments.
We previously established HEK293 overexpressing BACE1 (Figure 2c, inset). Figure 2c shows that JMV2764, JMV1195 and CI are equipotent in blocking BACE1 activity. The remaining JMV2236-hydrolysing activity could likely be accounted for nonspecific cleavage triggered by peptidases/proteases unrelated to β-secretase.
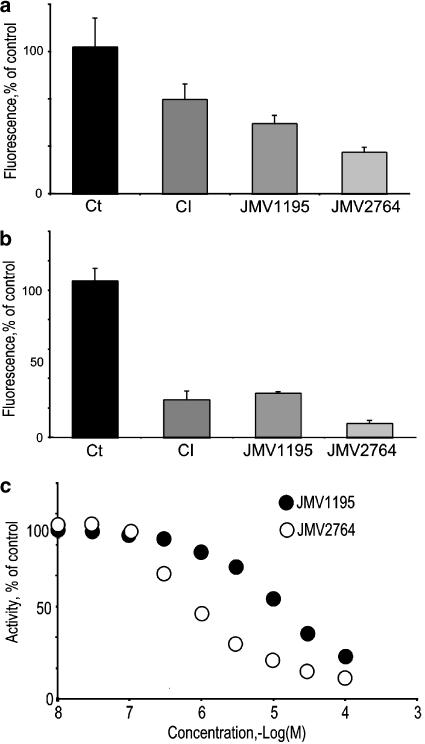
We have used a commercial substrate of β-secretase to further confirm the inhibitory potency of JMV2764 on endogenous β-secretase and BACE1 activities in HEK293 cell extracts. JMV2764 was clearly more potent on endogenous β-secretase-like activity (Figure 3a) than CI and JMV1195. The relatively low inhibitory potency of CI confirmed our previous study showing that the commercial substrate of β-secretase was less specific than JMV2236 (Andrau et al., 2003). When the commercial substrate was used with homogenates of cells overexpressing BACE1 (Figure 3b), the extent of inhibition triggered by all inhibitors was more important, although JMV2764 remained the more potent. Full dose–response curves (Figure 3c) indicate that JMV2764 inhibits BACE1 with an IC50 of about 0.8 μM while JMV1195 displays an IC50 value of about 6 μM, in agreement with our previous study (Andrau et al., 2003; 3 μM).
Figure 3.
Effect of JMV1195 and JMV2764 on the β-secretase hydrolysis of a commercial substrate. Histograms compare the extent of inhibition elicited by 10 μM of indicated inhibitor on the hydrolysis of a commercial β-secretase substrate (CS) by Mock- (a) or BACE1 (b)-transfected HEK293 cell extracts. Bars are the means±s.e.m. of four independent experiments. (c) Full dose–response curve inhibition by JMV1195 and JMV2764 of CS hydrolysis by mock-transfected cell extracts.
JMV2764 but not JMV1195 inhibits BACE1 activity in intact cells
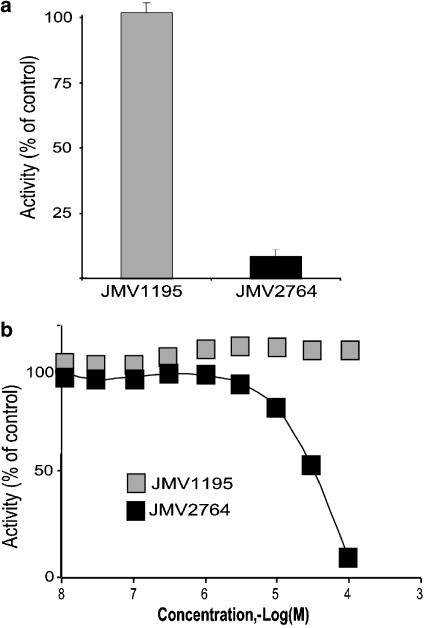
We have examined whether JMV2764 could inhibit intracellular β-secretase activity after preincubation with intact HEK293 cells overexpressing BACE1. JMV2764 fully (Figure 4a) and dose-dependently (Figure 4b) inhibited BACE1-hydrolysing activity of the commercial substrate while JMV1195 was totally inactive. These data show that the penetratin peptide of JMV2764 has likely enhanced the ability of JMV1195 to enter cells and to reach its intracellular target. However, the IC50 observed was about 30 times lower than the one exhibited on cell extracts, indicating that JMV2764 permeability could not be complete or that JMV2764 could not be fully catabolically stable in HEK293 cells.
Figure 4.
Effect of JMV1195 and JMV2764 on intracellular β-secretase activity. BACE1-expressing cells were incubated for 1 h at 37°C with 100 μM (a) or various concentrations (b) of JMV1195 or JMV2764. Then cells were harvested, lysed and activity was measured with the β-secretase commercial substrate as described in Methods. Bars are the means±s.e.m. of four experiments.
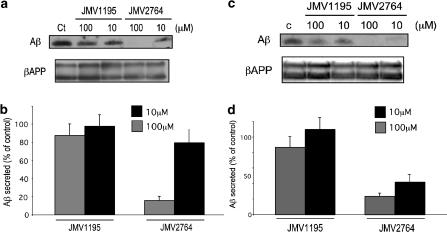
JMV2764 but not JMV1195 inhibits Aβ production by wild-type- and Swedish-mutated βAPP-expressing HEK293 cells
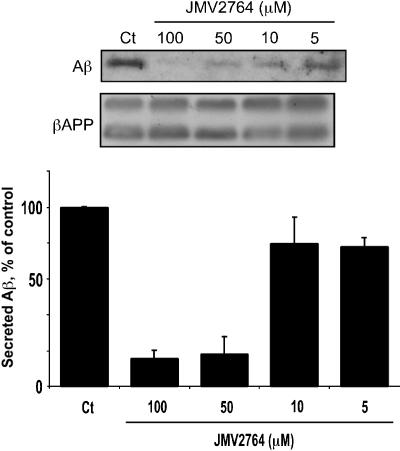
JMV2764-elicited inhibition of Aβ production was dose-dependent (Figure 5), with a half-maximal effect elicited between 10 and 50 μM of JMV2764, in agreement with the IC50 of 30 μM measured with the β-secretase commercial fluorimetric substrate (see Figure 4). Figure 6a and b show that a 100 μM concentration of JMV2764 fully prevents Aβ production by wild-type βAPP-expressing HEK293 cells while JMV1195 remained inactive at this concentration. Interestingly, JMV2764 appeared more potent on Aβ production by Swedish-mutated βAPP than on Aβ generated by the wild-type βAPP-expressing cells (Figure 6c, d). Thus, more than 50% of inhibition was achieved at a 10 μM concentration of JMV2764 (Figure 6d).
Figure 5.
Effect of JMV2764 on Aβ production by wild-type βAPP expressing cells. Wild-type βAPP-expressing HEK293 cells were incubated for 6 h at 37°C with various concentrations of JMV2764. Then Aβ40 was immunoprecipitated with FCA3340 and detected by Western blot with WO2 as described in Methods (a). Cells were extracted and assayed for βAPP-like immunoreactivity with 10D5 as described in Methods. Bars in panel b represent the densitometric analysis of Aβ40 immunoreactivity expressed as the control production obtained in absence of inhibitor and are the means±s.e.m. of five experiments.
Figure 6.
Comparison of JMV2764 and JMV1195 on Aβ production by wild-type and Swedish-mutated βAPP-expressing cells. Wild-type (a,b) and Swedish-mutated (c,d) βAPP-expressing HEK293 cells were incubated for 6 h at 37°C with 10 or 100 μM of JMV1195 or JMV2764. Then Aβ40 was immunoprecipitated with FCA3340 and detected by Western blot with WO2 as described in Methods (a,c). Cells were extracted and assayed for βAPP-like immunoreactivity with 10D5 as described in Methods. Bars in panels b and d represent the densitometric analysis of Aβ40 immunoreactivity expressed as the control Aβ production obtained in absence of inhibitor and are the means±s.e.m. of four experiments.
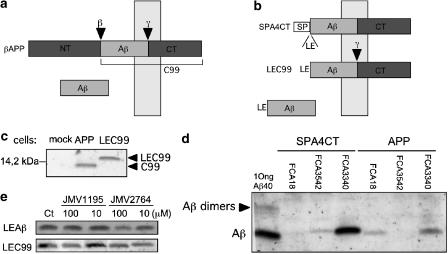
JMV2764 does not inhibit Aβ production by SPA4CT-expressing HEK293 cells
SPA4CT construction is particularly convenient to study the specificity of JMV2764 on β-secretase-associated Aβ production. Thus, unlike for βAPP, SPA4CT generates Aβ after the unique action of γ-secretase (Figure 7a, b). After the action of a signal peptidase, Aβ harboring a N-terminal extension of two amino acids is released via the constitutive secretory pathway as is genuine Aβ produced from βAPP (Dyrks et al., 1993). We have established HEK293 cells overexpressing SPA4CT (Figure 7c). As expected, SPA4CT-expressing cells produce both Aβ-like species ending at 40th and, to a lesser extent at 42nd amino-acid residue (Figure 7d). However, these Aβ-related species were not labelled by FCA18, a polyclonal antibody with restricted specificity for the free Asp1 residue of Aβs (Barelli et al., 1997). This indicates that the Aβ40/42 peptides released by SPA4CT-expressing cells were likely bearing the Leu-Glu (LE) extension precluding FCA18 recognition. Interestingly, JMV2764 was unable to prevent Aβ production by SPA4CT-HEK293 cells, Figure 7e demonstrating that, indeed, the inhibitor targeted β-secretase but did not interfere with γ-secretase pathway.
Figure 7.
JMV2764 does not affect Aβ40 production by SPA4CT-expressing HEK293 cells. Aβ production from βAPP derives from subsequent cleavages by β- and γ-secretases (a) while a single cleavage by γ-secretase on SPA4CT releases an Aβ bearing a Leu-Glu N-terminal extension (LEAβ) (b). Stably transfected cells expressing βAPP produce a β-secretase-derived C99 fragment of slightly lower molecular weight than LEC99 (c). Aβ40 and Aβ42 species derived from SPA4CT-expressing cells were detected by FCA3340 and FCA3542, respectively, but not detected by a anti-N-terminal antibody (FCA18; Barelli et al., 1997) that recognizes only Asp1 residue of Aβ (d). Neither JMV1195 nor JMV2764 affect Aβ40 recovery from SPA4CT-expressing cells (e).
Discussion
As far as the amyloidogenic cascade hypothesis of Alzheimer's disease is concerned (Hardy & Higgins, 1992), secretases, the enzymes that act on βAPP to release Aβ peptides, appear as key contributors of AD aetiology (Suh & Checler, 2002). Several lines of evidence suggest BACE1 as the primary target for anti-Alzheimer therapeutic strategies. First, BACE1 activity increases with aging (Fukumoto et al., 2004) and in Alzheimer's disease (Fukumoto et al., 2002; Holsinger et al., 2002; Yang et al., 2003; Li et al., 2004). Furthermore, downregulation of BACE1 activity by an antisense approach leads to drastic reduction of Aβ production in primary cortical neurons (Kao et al., 2004). This observation perfectly agrees with previous studies showing that mice devoid of BACE1 do not produce Aβ (Cai et al., 2001; Luo et al., 2001; Roberds et al., 2001). More importantly, BACE deficiency allows to rescue memory deficits and cholinergic dysfunction in an ‘Alzheimerized' mice overexpressing βAPP (Ohno et al., 2004).
Of most importance was the observation that mice invalidated for their endogenous BACE1 content were healthy and fertile, even at the adulthood (Roberds et al., 2001; Luo et al., 2003). This apparently normal phenotype indicates that either BACE is specific for βAPP and does not target other proteins bearing vital functions or that other proteases substitute for BACE1 function unrelated to Aβ production. Thus, recent studies showed that BACE1 hydrolyses P-selectin glycoprotein ligand-1 (Lichtenthaler et al., 2003) and that α2,6-sialyltransferase is a substrate of BACE1 both in vitro (Kitazume et al., 2001; 2003) and in vivo (Kitazume et al., 2004). Furthermore, deep investigations of neurotransmission in mice devoid of or overexpressing BACE1 indicate that the enzyme could participate in serotoninergic neurotransmission but this phenotype was not accompanied by altered fertility or increased morbidity (Harrison et al., 2003). Altogether, it appears that inhibiting BACE1 dramatically reduces Aβ load without any serious side effects. As a corollary, the design of highly specific, potent and bioavailable BACE1 inhibitors is likely the most promising track for fighting Alzheimer's disease.
The design of β-secretase inhibitors has been rather difficult but has been facilitated by the recent determination of the X-ray crystal structure of BACE complexed with an inhibitor (Hong et al., 2000). Thus, the tri-dimensional structure of BACE has allowed delineating the nature of the binding domains underlying BACE interaction with its ligands. Described inhibitors could be gathered into two main families (for reviews see Roggo, 2002; Vassar, 2002; John et al., 2003). First, substrate-based inhibitors designed as peptidomimetic BACE inhibitors and second, nonpeptidomimetic inhibitors, the discovery of which usually necessitates tedious random wide screening of numerous compounds. Peptidomimetic BACE inhibitors are more rationally designed. However, in vivo, their structural nature usually implies at least two main drawbacks: their relatively poor catabolic stability and their low bioavailability after systemic administration due to weak blood–brain barrier permeability.
Although several potent BACE1 inhibitors have been described, it should be noted that their potency to inhibit Aβ production in cell systems has not been often reported. Thus, several studies have reported on the in vitro inhibitory potency of compounds on purified recombinant BACE (Hu et al., 2003) or soluble truncated form of BACE (Brady et al., 2004). Other studies used a fusion protein containing maltose binding protein in-frame with the 125 C-terminal residues of βAPP as BACE substrate (Hom et al., 2003; 2004). In these assays, the rather high affinity/potency reported for BACE should be considered cautiously and likely does not reflect the in vivo genuine efficiency. This is supported by an interesting study showing that a series of pentapeptide mimetics inhibits BACE in vitro with affinities in the nanomolar range while the same compounds prevent Aβ production by Swedish-mutated βAPP-expressing HEK293 cells with IC50s ranging between 1 and 50 μM (Lamar et al., 2004). JMV2764 inhibits Aβ production by cells expressing Swedish-mutated βAPP with an IC50 below 10 μM and therefore compares favorably with other compounds assayed in the same cell system.
It should be noted that JMV2764 is more potent on Aβ production in cells expressing Swedish-mutated βAPP than wild-type βAPP (IC50<10 versus 30 μM, respectively). Previous data have indicated that Aβ production from wild-type and Swedish-mutated βAPP occurs in distinct cell compartments (Checler, 1995). It is therefore conceivable that JMV2764, that is not fully permeable, could reach distinct cell compartments with variable efficiency.
Compound JMV2764 illustrates the possibility of designing BACE inhibitors able to penetrate cells and reach its intracellular target. Whether JMV2764 could alter in vivo Aβ production in animals overproducing Aβ is currently under study in our laboratory.
Acknowledgments
S.L.J. is supported by Aventis Pharma. This work was supported by the French Ministère de le Recherche, the Centre National de la Recherche Scientifique and by an EU contract LSHM-CT-2003-503330 (APOPIS).
Abbreviations
- AHPPA
(3S,4S)-4-amino-3-hydroxy-5-phenylpentanoic acid
- ahx
6-aminohexanoic acid
- BACE
β-site APP cleaving enzyme
- Boc
tertiobutyloxycarbonyl
- Fmoc
N-(9-fluorenyl)methoxycarbonyl
- HBTU
N-[(1H-benzotriazol-1-yloxy)(dimethylamino-methylene)]-N-methylmethanaminium hexafluorophosphate
- MBHA
methylbenzhydrylamine
References
- ANCOLIO K., DUMANCHIN C., BARELLI H., WARTER J.M., BRICE A., CAMPION D., FRÉBOURG T., CHECLER F. Unusual phenotypic alteration of β amyloid precursor protein (βAPP) maturation by a new Val->Met βAPP-770 mutation responsible for probable early-onset Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4119–4124. doi: 10.1073/pnas.96.7.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRAU D., DUMANCHIN-NJOCK C., AYRAL E., VIZZAVONA J., FARZAN M., BOISBRUN M., FULCRAND P., HERNANDEZ J.F., MARTINEZ J., LEFRANC-JULLIEN S., CHECLER F. BACE1 and BACE2 expressing human cells: characterization of βAPP-derived catabolites, design of a novel fluorimetric assay and identification of new in vitro inhibitors. J. Biol. Chem. 2003;278:25859–25866. doi: 10.1074/jbc.M302622200. [DOI] [PubMed] [Google Scholar]
- BARELLI H., LEBEAU A., VIZZAVONA J., DELAERE P., CHEVALLIER N., DROUOT C., MARAMBAUD P., ANCOLIO K., BUXBAUM J.D., KHORKOVA O., HEROUX J., SAHASRABUDHE S., MARTINEZ J., WARTER J.-M., MOHR M., CHECLER F. Characterization of new polyclonal antibodies specific for 40 and 42 aminoacid-long amyloid β peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol. Med. 1997;3:695–707. [PMC free article] [PubMed] [Google Scholar]
- BEGLOPOULOS V., SUN X., SAURA C.A., LEMERE C.A., KIM R.D., SHEN J. Reduced β-amyloid production and increased inflammatory response in presenilin conditional knockout mice. J. Biol. Chem. 2004;279:46566–46572. doi: 10.1074/jbc.M409544200. [DOI] [PubMed] [Google Scholar]
- BRADY S.F., SINGH S., CROUTHAMEL M.-C., HOLLOWAY M.K., COBURN C.A., GARSKY V.M., BOGUSKY M., PENNINGTON M.W., VACCA J.P., HAZUDA D., LAI M.-T. Rational design and synthesis of selective BACE inhibitors. Bioorg. Med. Chem. Lett. 2004;14:601–604. doi: 10.1016/j.bmcl.2003.11.061. [DOI] [PubMed] [Google Scholar]
- CAI H., WANG Y., MCCARTHY D., WEN H., BORCHELT D.R., PRICE D.L., WONG P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- CHECLER F. Processing of the β-amyloid precursor protein and its regulation in Alzheimer's disease. J. Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- CHEVALLIER N., JIRACEK J., VINCENT B., BAUR C.P., SPILLANTINI M.G., GOEDERT M., DIVE V., CHECLER F. Examination of the role of endopeptidase 3.4.24.15 in Aβ secretion by human transfected cells. Br. J. Pharmacol. 1997;121:556–562. doi: 10.1038/sj.bjp.0701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYRKS T., DYRKS E., MÖNNING U., URMONEIT B., TURNER J., BEYREUTHER K. Generation of βA4 from the amyloid protein precursor and fragments thereof. FEBS Lett. 1993;335:89–93. doi: 10.1016/0014-5793(93)80446-2. [DOI] [PubMed] [Google Scholar]
- FUKUMOTO H., CHEUNG B.S., HYMAN B.T., IRIZARRY M.C. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- FUKUMOTO H., ROSENE D.L., MOSS M.B., RAJU S., HYMAN B.T., IRIZARRY M.C. β-Secretase activity increases with aging in human monkey, and mouse brain. Am. J. Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY J.A., HIGGINS G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- HARRISON S.M., HARPER A.J., HAWKINS J., DUDDY G., GRAU E., PUGH P.L., WINTER P.H., SHILLIAM C.S., HUGHES Z.A., DAWSON L.A., GONZALEZ M.I., UPTON N., PANGALOS M.N., DINGWALL C. BACE1 (β-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol. Cell. Neurosci. 2003;24:646–655. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- HOLSINGER R.M.D., MCLEAN C.A., BEYREUTHER K., MASTERS C.L., EVIN G. Increased expression of the amyloid precursor β-secretase in Alzheimer's disease. Ann. Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- HOM R.K., FANG L.Y., MAMO S., TUNG J.S., GUINN A.C., WALKER D.E., DAVIS D.L., GAILUNAS A.F., THORSETT E.D., SINHA S., KNOPS J.E., JEWETT N.E., ANDERSON J.P., JOHN V. Design and synthesis of statine-based cell-permeable peptidomimetic inhibitors of human β-secretase. J. Med. Chem. 2003;46:1799–1802. doi: 10.1021/jm025619l. [DOI] [PubMed] [Google Scholar]
- HOM R.K., GAILUNAS A.F., MAMO S., FANG L.Y., TUNG J.S., WALKER D.E., DAVIS D.L., THORSETT E.D., JEWETT N.E., MOON J.B., JOHN V. Design and synthesis of hydroxyethylene-based peptidomimetic inhibitors of human β-secretase. J. Med. Chem. 2004;47:158–164. doi: 10.1021/jm0304008. [DOI] [PubMed] [Google Scholar]
- HONG L., KOELSCH G., LIN X., WU S., TERZYAN S., GHOSH A.K., ZHANG X.C., TANG J. Structure of the protease domain of memapsin 2 (β-secretase) complexed with inhibitor. Science. 2000;290:150–153. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- HU J., CWI C.L., SMILEY D.L., TIMM D., ERICKSON J.A., MCGEE J.E., YANG H.-C., MENDEL D., MAY P.C., SHAPIRO M., MCCARTHY J.R. Design and synthesis of statine-containing BACE inhibitors. Bioorg. Med. Chem. Lett. 2003;13:4335–4339. doi: 10.1016/j.bmcl.2003.09.037. [DOI] [PubMed] [Google Scholar]
- HUSSAIN I., POWELL D., HOWLETT D.R., TEW D.G., MEEK T.D., CHAPMAN C., GLOGER I.S., MURPHY K.E., SOUTHAN C.D., RYAN D.M., SMITH T.S., SIMMONS D.L., WALSH F.S., DINGWALL C., CHRISTIE G. Identification of a novel aspartic protease (Asp2) as β-secretase. Mol. Cell. Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- JOHN V., BECK J.P., BIENKOWSKI M.J., SINHA S., HEINRICKSON R.L. Human β-secretase (BACE) and BACE inhibitors. J. Med. Chem. 2003;46:4625–4630. doi: 10.1021/jm030247h. [DOI] [PubMed] [Google Scholar]
- JOUIN P., CASTRO B. Stereospecific synthesis of N-protected statine and its analogs via chiral tetramic acid. J. Chem. Soc. Perkin Trans. 1987;1:1177–1182. [Google Scholar]
- KAO S.-C., KRICHEVSKY A.M., KOSIK K.S., TSAI L.-H. BACE1 suppression by RNA interference in primary cortical neurons. J. Biol. Chem. 2004;279:1942–1949. doi: 10.1074/jbc.M309219200. [DOI] [PubMed] [Google Scholar]
- KITAZUME S., NAKAGAWA K., OKA R., TACHIDA Y., OGAWA K., LUO Y., CITRON M., SHITARA H., TAYA C., YONEKAWA H., PAULSON J.C., MIYOSHI E., TANIGUCHI N., HASHIMOTO Y.In vivo cleavage of α2,6-sialyltransferase by Alzheimer's β-secretase J. Biol. Chem. 2004. in press [DOI] [PubMed]
- KITAZUME S., TACHIDA Y., OKA R., KOTANI N., OGAWA K., SUZUKI M., DOHMAE N., TAKIO K., SAIDO T.C., HASHIMOTO Y. Characterization of α2,6-sialyltransferase cleavage by Alzheimer's β-secretase (BACE1) J. Biol. Chem. 2003;278:14865–14871. doi: 10.1074/jbc.M206262200. [DOI] [PubMed] [Google Scholar]
- KITAZUME S., TACHIDA Y., OKA R., SHIROTANI K., SAIDO T.C., HASHIMOTO Y. Alzheimer's β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMAR J., HU J., BUENO A.B., YANG H.-C., GUO D., COPP J.D., MCGEE J., GITTER B., TIMM D., MAY P.C., MCCARTHY J., CHEN S.-H. Phe*-Ala-based pentapeptide mimetics are BACE inhibitors: P2 and P3 SAR. Bioorg. Med. Chem. Lett. 2004;14:239–243. doi: 10.1016/j.bmcl.2003.09.084. [DOI] [PubMed] [Google Scholar]
- LI R., LINDHOLM K., YANG L.-B., YUE X., CITRON M., YAN R., BEACH T., SUE L., SABBAGH M., CAI H., WONG P., PRICE D., SHEN Y. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer's disease patients. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3622–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTENTHALER S.F., DOMINGUEZ D.I., WESTMEYER G.G., REISS K., HAASS C., SAFTIG P., DE STROOPER B., SEED B. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE. J. Biol. Chem. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- LIN X., KOELSCH G., WU S., DOWNS D., DASHTI A., TANG J. Human aspartyl protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO Y., BOLON B., DAMORE M.A., FITZPATRICK D., LIU H., ZHANG J., YAN Q., VASSAR R., CITRON M. BACE1 (β-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol. Dis. 2003;14:81–88. doi: 10.1016/s0969-9961(03)00104-9. [DOI] [PubMed] [Google Scholar]
- LUO Y., BOLON B., KAHN S., BENNETT B.D., BABU-KHAN S., DENIS P., FAN W., KHA H., ZHANG J., GONG Y., MARTIN L., LOUIS J.C., YAN Q., RICHARDS W.G., CITRON M., VASSAR R. Mice deficient in BACE1, the Alzheimer's β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- OHNO M., SAMETSKY E.A., YOUNKIN L.H., OAKLEY H., YOUNKIN S.G., CITRON M., VASSAR R., DISTERHOFT J.F. BACE1 deficicency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's Disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- QIAN S., JIANG P., GUAN X., SING G., TRUMBAUER S.M., YU H., CHEN H.Y., VAN DER PLOEG L.H.T., ZHENG H. Mutant human presenilin 1 protects presenilin-1 null mouse against embryonic lethality and elevates Aβ1-42/43 expression. Neuron. 1998;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- ROBERDS S.L., ANDERSON J., BASI G., BIENKOWSKI M.J., BRANSTETTER D.G., CHEN K.S., FREEDMAN S.B., FRIGON N.L., GAMES D., HU K., JOHNSON-WOOD K., KAPPENMAN K.E., KAWABE T.T., KOLA I., KUEHN R., LEE M., LIU W., MOTTER R., NICHOLS N.F., POWER M., ROBERTSON D.W., SCHENK D., SCHOOR M., SHOPP G.M., SHUCK M.E., SINHA S., SVENSSON K.A., TATSUNO G., TINTRUP H., WIJSMAN J., WRIGHT S., MCCONLOGUE L. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- ROGGO S. Inhibition of BACE, a promising approach to Alzheimer's disease therapy. Curr. Top. Med. Chem. 2002;2:359–370. doi: 10.2174/1568026024607490. [DOI] [PubMed] [Google Scholar]
- SAURA C.A., CHOI S.-Y., BEGLOPOULOS V., MALKANI S., ZHANG D., RAO B.S.S., CHATTARJI S., KELLEHER R.J., III, KANDEL E.R., DUFF K., KIRKWOOD A., SHEN J. Loss of presenilin function causes impairements of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- SHEN J., BRONSON R.T., CHEN D.F., XIA W., SELKOE D., TONEGAWA S. Skeletal and CNS defects in presenilin-1- deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- SINHA S., ANDERSON J.P., BARBOUR R., BASI G.S., CACCAVELLO R., DAVIS D., DOAN M., DOVEY H.F., FRIGON N., HONG J., JACOBSON-CROAK K., JEWETT N., KEIM P., KNOPS J., LIEBERBURG I., POWER M., TAN H., TATSUNO G., TUNG J., SCHENK D., SEUBERT P., SUOMENSAARI S.M., WANG S., WALKER D., ZHAO J., MCCONLOGUE L., JOHN V. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- SUH Y.-H., CHECLER F. Amyloid precursor protein, presenilins and α-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimers' disease. Pharmacol. Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- VASSAR R. β-secretase (BACE) as a drug target for Alzheimer's disease. Adv. Drug Delivery Rev. 2002;54:1589–1602. doi: 10.1016/s0169-409x(02)00157-6. [DOI] [PubMed] [Google Scholar]
- VASSAR R., BENNETT B.D., BABU-KHAN S., KHAN S., MENDIAZ E.A., DENIS P., TEPLOW D.B., ROSS S., AMARANTE P., LOELOFF R., LUO Y., FISHER S., FULLER J., EDENSON S., LILE J., JAROSINSKI M.A., BIERE A.L., CURRAN E., BURGESS T., LOUIS J.-C., COLLINS F., TREANOR J., ROGERS G., CITRON M. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- WONG P.C., ZHENG H., CHEN H., BECHER M.W., SIRINATHSINGHJI D.J.S., TRUMBAUER M.E., CHEN H.Y., PRICE D.L., VAN DER PLOEG L.H.T., SISODIA S.S. Presenilin 1 is required for Notch1 and DII 1 expression in the paraaxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- YAN R., BIENKOWSKI M.J., SHUCK M.E., MIAO H., TORY M.C., PAULEY A.M., BRASHLER J.R., STRATMAN N.C., MATHEWS W.R., BUHL A.E., CARTER D.B., TOMASSELLI A.G., PARODI L.A., HEINRIKSON R.L., GURNEY M.E. Membrane-anchored aspartyl protease with Alzheimer's disease β-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- YANG L.-B., LINDHOLM K., XIA R., CITRON M., XIA W., YANG X.-L., BEACH T., SUE L., WONG P.C., PRICE D.L., LI R., SHEN Y. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]