Abstract
The nuclear translocation of transcription factors may be a critical factor in the intracellular pathway involved in ischaemia/reperfusion (I/R) injury. Here, we examined whether NF-κB and AP-1 participated in the cascade of events leading to TNF-α production, neutrophil recruitment, tissue injury and lethality following intestinal I/R.
The superior mesenteric artery (SMA) of mice was made ischaemic for 60 min followed by 30 min of reperfusion. The effects of NF-κB and AP-1 were studied by the administration of the thioredoxin inhibitor, MOL-294 (methyl 4-hydroxy-4-(8-methyl-1,3-dioxo-2-phenyl-2,3,5,8-tetrahydro-1H-[1,2,4]triazolo[1,2-a]pyridazin-5-yl)but-2-ynoate), and the AP-1 inhibitor, PNRI-299 (N-benzyl-2-(3-cyanophenyl)-1,3,7-trioxo-2,3,7,8-tetrahydro-1H-[1,2,4]triazolo[1,2-a]pyridazine-5-carboxamide). After I/R, there was increase of translocation of NF-κB, but not of AP-1, in the intestine and lungs, as assessed by a gel shift assay.
Treatment with MOL-294 inhibited the increase in vascular permeability, neutrophil accumulation, hemorrhage and proinflammatory cytokine levels, induced by intestinal I/R injury in the intestine. In the lungs, MOL-294 partially inhibited edema formation, TNF-α production, but did not alter neutrophil recruitment.
Treatment with MOL-294 inhibited reperfusion-associated lethality, an effect likely to be secondary to the inhibition of systemic TNF-α levels. PNRI-299 had no effects on the inflammatory changes or lethality induced by I/R injury.
Our results point to an important role for NF-κB in triggering endogenous proinflammatory networks during intestinal I/R injury. Inhibition of NF-κB prevents tissue injury and lethality, and this was associated with inhibition of TNF-α production and decrease in neutrophil recruitment.
Keywords: Reperfusion injury, NF-κB, AP-1, TNF-α, IL-10
Introduction
A major goal in the treatment of ischaemia of a vascular territory is to restore blood flow to normal values, that is, to ‘reperfuse' the ischaemic vascular bed (Carden & Granger, 2000). However, reperfusion of ischaemic tissues is associated with local and systemic leukocyte activation and trafficking, endothelial barrier dysfunction in postcapillary venules, enhanced production of inflammatory mediators and great lethality (Lefer & Lefer, 1996; Granger, 1999; Carden & Granger, 2000, Souza et al., 2000a, 2000b, 2001, 2003a). The recruitment of neutrophils, production of TNF-α and release of free radicals are considered major events involved in the injury following reperfusion of an ischaemic tissue (Gilmont et al., 1996; Cornejo et al., 1997; Willerson 1997; Souza et al., 2001, 2002).
The transcription factors NF-κB and AP-1 are induced in response to oxidative stress and a wide range of stimuli including proinflammatory cytokines such as TNF-α (Beg et al., 1993; Funakoshi et al., 2001; Chen et al., 2003; Takada et al., 2004). On the other hand, NF-κB and AP-1 can induce proinflammatory gene expression including cytokines, adhesion molecules and chemokines (Barnes & Karin, 1997; Ghosh et al., 1998). The contribution of these transcription factors to the inflammatory response has been demonstrated in several models of acute severe inflammation. A few studies have evaluated the potential contribution of AP-1 and NF-κB to ischaemia and reperfusion (I/R) injury using genetic (Chen et al., 2003; Fan et al., 2004) and pharmacological strategies (Lefler et al., 2002; Pye et al., 2003; Zingarelli et al., 2003; Zou et al., 2003). However, most studies have used nonspecific inhibitors of transcription factor translocation and few have attempted to compare the relative contribution of AP-1 and NF-κB to I/R injury.
The present study was carried out to evaluate the translocation and relative contribution of AP-1 and NF-κB in a model of intestinal I/R injury in mice. To this end, we assessed the effects of MOL-294 (methyl 4-hydroxy-4-(8-methyl-1,3-dioxo-2-phenyl-2,3,5,8-tetrahydro-1H-[1,2,4]triazolo[1,2-a]pyridazin-5-yl)but-2-ynoate) on the local, remote and systemic inflammatory changes after ischaemia of the superior mesenteric artery (SMA) in mice. MOL-294 is an inhibitor of thioredoxin, an oxireductase known to translocate from the cytosol to the nucleus under a variety of stress-inducing stimuli and to regulate the expression of the AP-1 family of genes through redox effector factor-1 (Xanthoudakis & Curran, 1992; Hirota et al., 1997) and the NF-κB family directly (Qin et al., 1996). Thioredoxin inhibitors do not appear to have an effect on other transcription factors (Misra-Press et al., 2002). As MOL-294 has been shown to inhibit AP-1-dependent transcriptional activity at a concentration similar to that known to inhibit NF-κB-dependent transcriptional activity (Misra-Press et al., 2002), we also tested the effects of a selective inhibitor of AP-1, PNRI-299 (Nguyen et al., 2003). PNRI-299 (N-benzyl-2-(3-cyanophenyl)-1,3,7-trioxo-2,3,7,8-tetrahydro-1H-[1,2,4]triazolo[1,2-a]pyridazine-5-carboxamide) has been shown to react specifically with Ref-1 and inhibit AP-1 transcription (Nguyen et al., 2003).
Methods
Animals
Male C57/BL6 mice (8–10 weeks) obtained from the Bioscience Unit of Instituto de Ciências Biológicas were housed under standard conditions and had free access to commercial chow and water. All procedures described here had prior approval from the local animal ethics committee.
Ischaemia and reperfusion
Mice were anesthetized with urethane (1400 mg kg−1, intraperitoneally (i.p.)) and laparotomy was performed. The SMA was isolated and ischaemia was induced by totally occluding the SMA for 60 min. For measuring the percentage of surviving mice, reperfusion was re-established, and mice were monitored for indicated time periods. For the other parameters, reperfusion was allowed to occur for 30 min (I60R30) when mice were killed. This time of reperfusion (30 min) was chosen based on the presence of significant tissue injury without unduly high mortality rates (Souza et al., 2003b). Sham-operated animals were used as controls. MOL-294 (3–30 mg kg−1), PNRI-299 (3 or 10 mg kg−1) or vehicle were administered (intravenously (i.v.)) 5 min before reperfusion. The doses selected were based upon IC50 values in cell-based assays and preliminary bioavailability data in mice (M. Kahn, unpublished results).
Preparation of nuclear extracts and band shift assay
Electrophoretic mobility shift assay (EMSA) was carried out essentially as described (Bonjardim, 1997). Nuclear extracts were obtained from powdered intestine or lung and prepared as described by Dignam et al. (1983). Protein concentration was determined by the Bio-Rad assay. Nuclear extracts (10 μg) were preincubated with 1.2 μl poly-(dI-dC) (Amersham Biosciences, U.K.; 5.4 mg ml−1) at room temperature for 10 min, followed by the addition of 1.25 μg BSA, 0.125 μg of Escherichia coli DNA, 0.25 μg yeast tRNA, 2% Ficoll 400 and 0.32 ng labeled probe (8.0 × 104 c.p.m.). The reactions were incubated at room temperature for 15 min and then analyzed by 6% PAGE. The 5′ 32P-end-labeled double-stranded probes (only one strand is shown) corresponding to both consensus binding site of the NF-κB and AP-1 are as follows: NF-κB – 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ or AP-1 – 5′-CGC TTG ATG ACT CAG CCG GAA-3′. Heterologous competitions assays were performed with a 100-fold molar excess of cold oligonucleotide corresponding to: c-fos SRE: – 5′-GATGTCCATATTAGGACATC-3′.
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability, as described previously (Souza et al., 2000b). The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue per μg per 100 mg of the tissue.
Myeloperoxidase concentrations
The extent of neutrophil accumulation in the intestine and the flushed right lung tissue was measured by assaying myeloperoxidase activity, as described previously (Souza et al., 2002). Results were expressed as the total number of neutrophils by comparing the O.D. of tissue supernatant with the O.D. of casein-elicited murine peritoneal neutrophils processed in the same way.
Measurement of hemoglobin concentrations
The determination of hemoglobin concentrations in the tissue was used as an index of tissue hemorrhage. After washing and perfusing the intestines to remove excess blood in the intravascular space, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's color reagent according to instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 min at 3000 × g and filtered using 0.2 μm filters. The resulting solution was read using an ELISA plate reader at 520 nm and compared against a standard curve of hemoglobin.
Measurement of cytokine/chemokine concentrations in the serum, intestine and lungs
The concentration of TNF-α, IL-10, CXCL1-3 (KC) and CCL2 (MCP-1) in samples was measured in the serum and tissue of animals using commercially available antibodies and according to the procedures supplied by the manufacturer (R&D Systems, Minneapolis, U.S.A.). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analyzed at a 1 : 3 dilution in phosphate-buffered saline (PBS). In all, 100 mg of duodenum or lung of sham-operated and reperfused animals were homogenized in 1 ml of PBS (0.4 M NaCl and 10 mM of NaPO4) containing antiproteases (0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged of 10 min at 3000 × g and the supernatant immediately used for ELISA assays at a 1:3 dilution in PBS.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, hexadecyltrimethylammonium bromide. MOL-294 and PNRI-299 were synthesized by Dr M. McMillan at the Institute for Chemical Genomics. Details of the syntheses will be published in due course. MOL-294 was dissolved in DMSO solution to 0.5%, while PNRI-299 was dissolved in DMSO solution to 0.2%. Both drugs were administrated i.v. 10 min before reperfusion.
Statistical analysis
Results are shown as means±s.e.m. The percent inhibition was calculated by subtracting the background values obtained in sham-operated animals. Differences were compared by using analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc analysis. Results with a P<0.05 were considered significant.
Results
Translocation of NF-κB and AP-1 after I/R injury
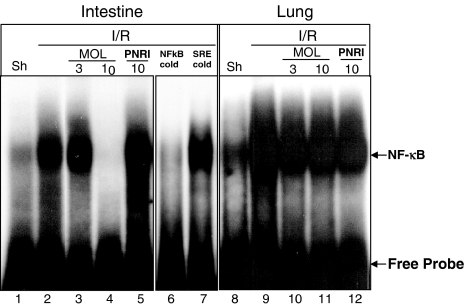
The translocation of the transcription factors NF-κB and AP-1 to the nucleus in the intestine or lung after 30 min of reperfusion was determined by EMSAs of tissue nuclear extracts. In the tissue of sham-operated mice, there was basal NF-κB and AP-1 translocation. I/R induced significant increase of NF-κB translocation (Figure 1), but there was no detectable increase in the translocation of AP-1 (data not shown). Next, we assessed whether treatment with MOL-294 or PNRI-299 was capable of inhibiting the reperfusion-induced NF-κB translocation. MOL-294 dose-dependently inhibited NF-κB translocation in the intestine, but not in the lung. Maximal inhibition was observed at 10 mg kg−1 (Figure 1), with no further inhibition at the dose of 30 mg kg−1 (data not shown). PNRI-299 had no significant effect on the translocation of NF-κB (Figure 1).
Figure 1.
Translocation of NF-κB to the nucleus determined by EMSAs of tissue nuclear extracts. Translocation of NF-κB in the intestine (lanes 1–7) and lungs (lanes 8–12) after sham operation (Sh) or following ischaemia (60 min) and reperfusion (30 min) injury of the SMA (I/R). MOL-294 ((lanes 3, 4 and 10, 11) and PNRI-299 (lanes 5 and 12) effects in NF-κB translocation. Also shown is the translocation of NF-κB after the addition of 100-fold molar excess of cold NF-κB oligonucleotide (NF-κB cold) and heterologous ccompetition with a 100-fold molar excess of cold SRE oligonucleotide (SRE cold). Result shown is representative of at least three experiments with each tissue.
The specificity of the DNA–protein complex formed was assessed by preincubating the nuclear extracts with 100-fold molar excess of unlabeled oligonucleotides before the addition of the labeled oligo NF-κB. The DNA–protein interaction was completely blocked by competing with the related oligo, but was not affected by molar excess of unrelated oligo, confirming the specificity of these interactions (Figure 1).
Effects of MOL-294 in local, remote and systemic inflammatory response induced by I/R injury
The next experiments were designed to investigate the effects of MOL-294, an inhibitor of NF-κB and AP-1, in a model of reperfusion injury and, hence, the putative role of these transcription factors in the system. Treatment with MOL-294 at the end of the ischaemic period and immediately before reperfusion dose-dependently inhibited both the increase in vascular permeability and the recruitment of neutrophils in the intestine following reperfusion of the ischaemic SMA (Figure 2a and c). Treatment with MOL-294 also abolished the intestinal increase of hemoglobin, a marker of tissue hemorrhage (Figure 2e). In the lungs, an organ remote from the site of original injury, MOL-294 partially prevented the increase in vascular permeability but had little effect on the reperfusion-induced neutrophil influx (Figure 2b and d).
Figure 2.
Effects of the treatment with MOL-294 or PNRI-299 on the increase in vascular permeability, recruitment of neutrophils and hemorrhage in the intestine and lung following ischaemia (60 min) and reperfusion (30 min) injury of the SMA. Changes in vascular permeability in the (a) intestine and (b) lungs were assessed by evaluating the extravasation of Evans blue dye. Neutrophil recruitment in the (c) intestine and (d) lungs was assessed by evaluating tissue levels of myeloperoxidase. Hemorrhage was evaluated by hemoglobin content in the intestine (e). MOL-294 (3–30 mg kg−1) or PNRI-299 (10 mg kg−1) was given i.v. 5 min prior to reperfusion and control animals received saline (vehicle). Results are shown as μg Evans blue, as the number of neutrophils or μg hemoglobin per 100 mg of the tissue and are the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared to sham-operated animals; #P<0.05 when compared to vehicle I/R animals.
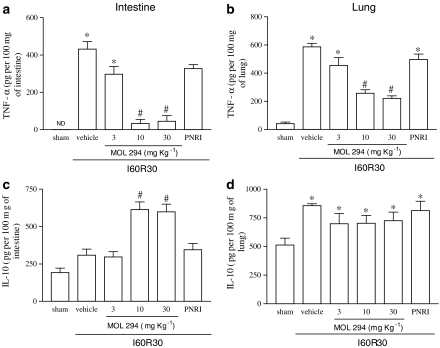
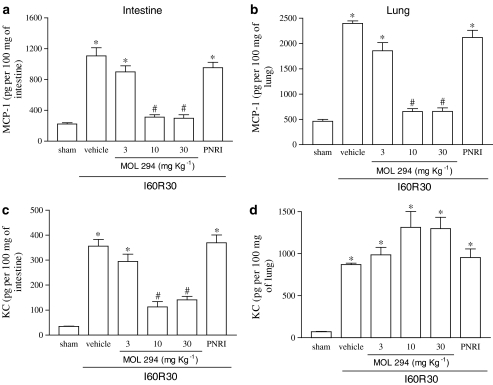
Postischaemic treatment with MOL-294 significantly inhibited the reperfusion-induced elevations in TNF-α concentrations in the intestine and lungs (Figure 3a and b). Interestingly, treatment with MOL-294 was accompanied by an enhancement in the concentration of IL-10 following reperfusion injury in the intestine, but not in the lung (Figure 3c and d). MOL-294 also prevented the reperfusion-induced elevations in the concentration of CCL2 in both organs (Figure 4a and b). In agreement with the inhibition of neutrophil influx in the intestine but not in the lungs, MOL-294 prevented the reperfusion-induced increase in the concentration of the neutrophil active chemokine CXCL1-3 in the intestine (Figure 4c), and actually partially enhanced the production of CXCL1-3 in the lungs (Figure 4d).
Figure 3.
Effects of the treatment with MOL-294 or PNRI-299 on the concentrations of TNF-α and IL-10 in the intestine and lung following ischaemia (60 min) and reperfusion (30 min) of the SMA. The concentration of TNF-α (a and b) and IL-10 (c and d) were assessed in the intestine (a and c) and lung (b and d) by using specific ELISA. MOL-294 (3–30 mg kg−1) or PNRI-299 (10 mg kg−1) was given i.v. 5 min prior to reperfusion and the control animals received saline (vehicle). Results are shown as pg TNF-α or IL-10 per 100 mg of the tissue and are the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared to sham-operated animals; #P<0.05 when compared to I/R animals.
Figure 4.
Effects of the treatment with MOL-294 or PNRI-299 on the concentrations of MCP-1 and KC in the intestine and lung following ischaemia (60 min) and reperfusion (30 min) of the SMA. The concentration of MCP-1 (a and b) and KC (c and d) were assessed in the intestine (a and c) and lung (b and d) by using specific ELISA. MOL-294 (3–30 mg kg−1) or PNRI-299 (10 mg kg−1) was given i.v. 5 min prior to reperfusion and the control animals received saline (vehicle). Results are shown as pg MCP-1 or KC per 100 mg of the tissue and are the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared to sham-operated animals; #P<0.05 when compared to I/R animals.
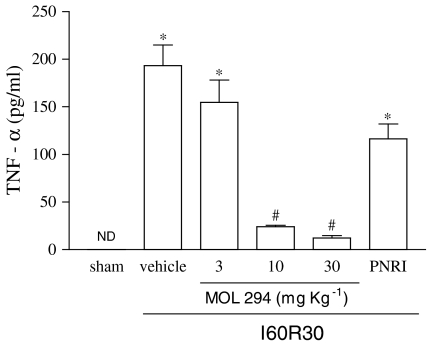
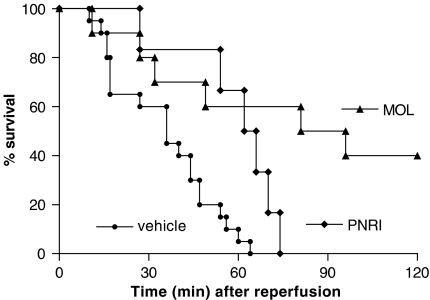
Our previous studies have shown that severe reperfusion injury is accompanied by significant TNF-α-dependent lethality (Souza et al., 2001, 2002). In the present series of experiments, reperfusion of the ischaemic SMA resulted in 100% lethality by 60 min of reperfusion (Figure 5). Treatment with MOL-294 delayed and partially prevented lethality (Figure 6). At 120 min after reperfusion, 40% of MOL-294 animals were still alive. The latter result is in accordance with the ability of MOL-294 to significantly prevent the reperfusion-induced increase in systemic TNF-α concentration (compare Figures 5 and 6). Of note, the doses of 10 and 30 mg kg−1 of MOL-294 resulted in very similar inhibition in all parameters evaluated.
Figure 5.
Effects of the treatment with MOL-294 or PNRI-299 on the serum concentrations of TNF-α following ischaemia (60 min) and reperfusion (30 min) of the SMA. TNF-α was measured using specific ELISA. MOL-294 (3–30 mg kg−1) or PNRI-299 (10 mg kg−1) was given i.v. 5 min prior to reperfusion and the control animals received saline (vehicle). Results are shown as pg TNF-α per ml of serum and are the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared to sham-operated animals; #P<0.05 when compared to severe I/R animals.
Figure 6.
Effects of the treatment with MOL-294 or PNRI-299 on the lethality following ischaemia (60 min) and reperfusion (30 min) of the SMA. Survival was monitored as indicated and survivors were killed after 120 min. MOL-294 (10 mg kg−1) or PNRI-299 (10 mg kg−1) was given i.v. 5 min prior to reperfusion and the control animals received saline (vehicle).
Effects of PNRI-299 in local, remote and systemic inflammatory response induced by I/R injury
In contrast to the effects of MOL-294, pretreatment with PNRI-299 had little effect on the inflammatory response that follows intestinal I/R, as assessed by vascular permeability (Figure 2a and c), neutrophil recruitment (Figure 2b and d) or hemoglobin content (Figure 2e). PNRI-299 also had little effect on the reperfusion-induced increase in cytokine (Figure 3) or chemokine (Figure 4) concentration in the tissues. PNRI-299 failed to alter the reperfusion-induced increase in IL-10 concentrations (Figure 3). Overall, the maximal inhibition attained by PNRI-299 was around 20% of the levels found in vehicle-treated animals. Furthermore, the concentration of systemic TNF-α (Figure 5) and reperfusion-associated lethality (Figure 6) were only marginally modified by PNRI-299 treatment. Indeed, there was only a slight delay but no prevention of lethality in PNRI-299-treated mice (Figure 6).
Discussion
The reperfusion of the ischaemic SMA evokes a molecular and cellular response within the tissues that includes release of TNF-α and other proinflammatory cytokines, release of reactive oxygen species and recruitment of neutrophils. Together, the latter events may lead to the activation of transcription factors such as NF-κB and AP-1. NF-κB and AP-1 activation may lead to the coordinated expression of many genes that encode proteins involved in mediator synthesis and the further amplification and perpetuation of the inflammatory response (Ghosh & Karin, 2002). Consequently, transcription factors, such as NF-κB and AP-1, are obvious targets for anti-inflammatory treatment. Pharmacological agents that inhibit NF-κB include glucocorticoids (Almawi & Melemedjian, 2002), antioxidants (Kunsch et al., 2004), certain cyclooxygenase inhibitors (Tegeder et al., 2001), proteasome and calpain inhibitors (Lee & Burckart, 1998) and inhibitors of IκB-α phosphorylation (Pierce et al., 1997). Most of the inhibitors cited previously lack specificity. Here, we show that MOL-294, a potent nonpeptide inhibitor of NF-κB and AP-1, based upon a β-strand template that binds to and inhibits the cellular redox protein thioredoxin (Misra-Press et al., 2002), is an effective inhibitor of the injury and lethality that occurs after intestinal I/R.
Our initial experiments demonstrated that there was significant NF-κB translocation in the lungs and intestines after intestinal I/R injury and, interestingly, MOL-294 treatment was able to inhibit NF-κB translocation in the intestine but not in the lung. Other study demonstrated that there was activation of NF-κB after reperfusion injury in several organs including the liver (Takeuchi et al., 2004), intestine (Chen et al., 2003; Zou et al., 2003), heart (Pye et al., 2003) and kidney (Pompermayer et al., 2005). In contrast to the observed NF-κB translocation, we failed to detect increase in AP-1 translocation both in the lungs and in the intestine. The latter results differ from those of Yeh and co-workers (2000), who demonstrated significant AP-1 translocation after mesenteric I/R injury. One possibility to explain the discrepancies between these data and ours may be the different I/R times used in the experiments. Whereas we assessed AP-1 translocation after 30 min (as lethality occurred shortly thereafter), Yeh and co-workers evaluated AP-1 translocation 1–12 h after reperfusion. Thus, it is apparent that there is a preferential translocation of NF-κB over AP-1 in the early stages after severe intestinal reperfusion injury.
Administration of MOL-294, at the end of the ischaemic period and just prior to reperfusion, thus mimicking closely the clinical situation, effectively reduced vascular permeability, neutrophil recruitment and hemorrhage in the intestine. It has been suggested that superoxide and TNF-α mediate gut I/R-induced E-selectin expression via an NF-κB-dependent mechanism and this upregulation of E-selectin contributes significantly to I/R-induced neutrophil recruitment (Russell et al., 2000). As the local influx of neutrophils is a determinant in the development of reperfusion injury (Ma et al., 1993; Lefer et al., 1996; Omata et al., 1997; Ritter et al., 1998; Souza et al., 2000a, 2000b; Onai et al., 2003), the capacity of MOL-294 to modulate the recruitment of neutrophils may underlie the beneficial effects of the drug in our model. MOL-294 failed to alter neutrophil recruitment and only partially inhibited Evans blue extravasation in the lung. The latter results are consistent with the inability of MOL-294 to prevent NF-κB translocation in the lungs. Moreover, there was no inhibition of lung parameters even after MOL-294 was used at a supramaximal dose of 30 mg kg−1, suggesting that there was sufficient drug to attain adequate inhibition of NF-κB translocation. The lower effects of the drug in the lung is in agreement with other studies, suggesting that different signal transduction pathways may be important to establish lung injury that follows I/R including PKC (Fujita et al., 2004), c-Jun NH2-terminal kinase (Ishii et al., 2004) and p38 MAPK (Khadaroo et al., 2003). In addition to preventing intestinal injury and partially preventing pulmonary injury, MOL-294 prevented the release of TNF-α and CCL2 in both the intestine and lungs. The inability of MOL-294 to prevent neutrophil influx while partially preventing TNF-α production may suggest that TNF-α is not relevant for neutrophil influx to the lung. Alternatively, a greater inhibition of TNF-α production in the lung would be necessary for the inhibition of neutrophil influx to that organ to occur. The latter possibility is in agreement with other studies of our group (Souza et al., 2001, 2002). The lack of inhibition of CXCL1-3 in the lung was consistent with the lack of effect of MOL-294 on neutrophil recruitment to that organ. Thus, the demonstrated ability of MOL-294 to modulate both neutrophil influx and cytokine/chemokine production could be contributing to the beneficial effects of this drug.
In addition to abolishing the increase on tissue concentrations of TNF-α, MOL-294 prevented the increase in the concentration of TNF-α in serum. Similarly, Chen et al. (2003) demonstrated that inhibition of NF-κB activation in intestinal epithelial cells prevented the increase in systemic TNF-α concentrations. As concentrations of TNF-α in serum, but not in the tissue, appear to be the best correlate of lethality in our system (Souza et al., 2001, 2002), the latter results are consistent with the ability of MOL-294 to prevent lethality. There is much hypotension immediately after reperfusion of the ischaemic mesenteric artery in rats (Souza et al., 2000a). Whether MOL-294 had an effect on such cardiovascular parameter was not investigated here, but may also have contributed to the protective effects of the drug. Importantly, this is the first study to demonstrate the capacity of an NF-κB inhibitor to delay and prevent lethality in a model of I/R injury.
We have previously shown that IL-10 is a major protective endogenous cytokine during I/R injury (Souza et al., 2003a) and several strategies that protect against reperfusion injury such as platelet-activating factor receptor or bradykinin receptor antagonists and IL-1β treatment, are associated with an enhancement of IL-10 production (Souza et al., 2003b, 2003c, 2004a). The IL-10 produced does appear to have a protective effect, at least in the case of reperfused animals treated with IL-1β (Souza et al., 2003a, 2004b). Treatment with MOL-294 was also accompanied by reperfusion-induced enhancement in the concentrations of IL-10 in the intestine. We have not investigated here whether the IL-10 produced was important for the protective effects of MOL-294, but it is noteworthy that the drug only induced IL-10 elevation in the intestine but not in the lung. As mentioned above, the drug was more effective in preventing injury to the former tissue.
As MOL-294 has been shown to inhibit both AP-1 and NF-κB activity, we also assessed the effects of PNRI-299, a selective inhibitor of AP-1 transcription (Nguyen et al., 2003). It was important to examine the role of AP-1 in our system as at least one study has shown cardioprotection with the reduction of the DNA binding of AP-1 (Zingarelli et al., 2004). However, and consistently, with the lack of an increase in the translocation of AP-1 at 30 min after reperfusion, PNRI-299 had little effect on the inflammatory response or lethality induced by intestinal I/R. Thus, whereas it is apparent that AP-1 may be translocated in models of less severe intestinal I/R injury (Yeh et al., 2000) and inhibition of AP-1 function may be protective in models of cardiac I/R injury, our results do suggest that AP-1 translocation does not have a primordial role in severe intestinal I/R injury.
In conclusion, our results demonstrate that there is an early activation of NF-κB following reperfusion of the ischaemic SMA and that blockade of the activation of this transcription factor with MOL-294 is accompanied by the prevention of tissue injury and lethality. Inhibition of NF-κB activation by MOL-294, a thioredoxin inhibitor, is accompanied by diminished neutrophil influx and TNF-α production, both important mediators of reperfusion injury. This is one of the first studies to compare the translocation and functional relevance of AP-1 and NF-κB translocation to I/R injury. Thus, treatment with inhibitors of NF-κB translocation or function, such as MOL-294, may be effective coadjuvants in the treatment of the injuries that follow reperfusion of an ischaemic vascular territory.
Acknowledgments
We are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo as Pesquisas do Estado de Minas Gerais (FAPEMIG) for financial support.
Abbreviations
- EMSA
electrophoretic mobility shift assay
- I/R
ischaemia and reperfusion
- KI
kalicrein unit
- SMA
superior mesenteric artery
References
- ALMAWI W.Y., MELEMEDJIAN O.K. Negative regulation of nuclear factor-kappaB activation and function by glucocorticoids. J. Mol. Endocrinol. 2002;28:69–78. doi: 10.1677/jme.0.0280069. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BEG A.A., FINCO T.S., NANTERMET P.V., BALDWIN A.S., JR Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol. Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONJARDIM C.A. A mutant cell line partially responsive to both IFN-alpha and IFN-gamma. Braz. J. Med. Biol. Res. 1997;30:41–50. doi: 10.1590/s0100-879x1997000100007. [DOI] [PubMed] [Google Scholar]
- CARDEN D.L., GRANGER D.N. Pathophysiology of ischaemia–reperfusion injury. J. Pathol. 2000;90:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- CHEN L.W., EGAN L., LI Z.W., GRETEN F.R., KAGNOFF M.F., KARIN M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischaemia–reperfusion. Nat. Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- CORNEJO C.J., WINN R.K., HARLAN J.M. Anti-adhesion therapy. Adv. Pharmacol. 1997;39:99–142. doi: 10.1016/s1054-3589(08)60070-8. [DOI] [PubMed] [Google Scholar]
- DIGNAM J.D., LEBOVITZ R.M., ROEDER R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN C., LI Q., ZHANG Y., LIU X., LUO M., ABBOTT D., ZHOU W., ENGELHARDT J.F. IkappaBalpha and IkappaBbeta possess injury context-specific functions that uniquely influence hepatic NF-kappaB induction and inflammation. J. Clin. Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA T., ASAI T., ANDRASSY M., STERN D.M., PINSKY D.J., ZOU Y.S., OKADA M., NAKA Y., SCHMIDT A.M., YAN S.F. PKCbeta regulates ischaemia/reperfusion injury in the lung. J. Clin. Invest. 2004;113:1615–1623. doi: 10.1172/JCI19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNAKOSHI M., SONODA Y., TAGO K., TOMINAGA S., KASAHARA T. Differential involvement of p38 mitogen-activated protein kinase and phosphatidyl inositol 3-kinase in the IL-1-mediated NF-kappa B and AP-1 activation. Int. Immunopharmacol. 2001;1:595–604. doi: 10.1016/s1567-5769(00)00035-7. [DOI] [PubMed] [Google Scholar]
- GILMONT R.R., DARDANO A., ENGLE J.S., ADAMSON B.S., WELSH M.J., LI T., REMICK D.G., SMITH D.J., JR, REES R.S. TNF-alpha potentiates oxidant and reperfusion-induced endothelial cell injury. J. Surg. Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- GHOSH S., KARIN M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- GHOSH S., MAY M.J., KOPP E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N. Ischaemia–reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- HIROTA K., MATSUI M., IWATA S., NISHIYAMA A., MORI K., YODOI J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII M., SUZUKI Y., TAKESHITA K., MIYAO N., KUDO H., HIRAOKA R., NISHIO K., SATO N., NAOKI K., AOKI T., YAMAGUCHI K. Inhibition of c-Jun NH2-terminal kinase activity improves ischaemia/reperfusion injury in rat lungs. J. Immunol. 2004;172:2569–2577. doi: 10.4049/jimmunol.172.4.2569. [DOI] [PubMed] [Google Scholar]
- KHADAROO R.G., PARODO J., POWERS K.A., PAPIA G., MARSHALL J.C., KAPUS A., ROTSTEIN O.D. Oxidant-induced priming of the macrophage involves activation of p38 mitogen-activated protein kinase through an Src-dependent pathway. Surgery. 2003;134:242–246. doi: 10.1067/msy.2003.228. [DOI] [PubMed] [Google Scholar]
- KUNSCH C., LUCHOOMUN J., GREY J.Y., OLLIFF L.K., SAINT L.B., ARRENDALE R.F., WASSERMAN M.A., SAXENA U., MEDFORD R.M. Selective inhibition of endothelial and monocyte redox-sensitive genes by AGI-1067: a novel antioxidant and anti-inflammatory agent. J. Pharmacol. Exp. Ther. 2004;308:820–829. doi: 10.1124/jpet.103.059733. [DOI] [PubMed] [Google Scholar]
- LEE J.I., BURCKART G.J. Nuclear factor kappa B: important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998;38:981–993. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia–reperfusion. Cardiovasc. Res. 1996;32:743–751. [PubMed] [Google Scholar]
- LEFER D.J., FLYNN D.M., BUDA A.J. Effects of a monoclonal antibody directed against P-selectin after myocardial ischaemia and reperfusion. Am. J. Physiol. 1996;270:H88–H98. doi: 10.1152/ajpheart.1996.270.1.H88. [DOI] [PubMed] [Google Scholar]
- LEFLER S.R., LILLE S.T., HUEMER G., TUCKER R., MURRAY T., SCHOELLER T., MULLIGAN D.C. Activation time course of activator protein-1 and effect of proline dithiocarbamate during ischaemia–reperfusion in rat skeletal muscle. Ann. Plast. Surg. 2002;49:654–659. doi: 10.1097/00000637-200212000-00016. [DOI] [PubMed] [Google Scholar]
- MA X.L., WEYRICH A.S., LEFER D.J., BUERKE M., ALBERTINE K.H., KISHIMOTO T.K., LEFER A.M. Monoclonal antibody to L-selectin attenuates neutrophil accumulation and protects ischaemic reperfused cat myocardium. Circulation. 1993;88:649–658. doi: 10.1161/01.cir.88.2.649. [DOI] [PubMed] [Google Scholar]
- MISRA-PRESS A., MCMILLAN M., CUDABACK E., QABAR M., RUAN F., NGUYEN M., VAISAR T., NAKANISHI H., KAHN M. Identification of a novel inhibitor of the NF-κB pathway. Curr. Med. Chem.- Anti-inflammatory Anti-Allergy Agents. 2002;1:29–39. [Google Scholar]
- NGUYEN C., TEO J.L., MATSUDA A., EGUCHI M., CHI E.Y., HENDERSON W.R., JR, KAHN M. Chemogenomic identification of Ref-1/AP-1 as a therapeutic target for asthma. Proc. Natl. Acad. Sci U.S.A. 2003;100:1169–1173. doi: 10.1073/pnas.0437889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMATA M., MATSUI N., INOMATA N., OHNO T. Protective effects of polysaccharide fucoidin on myocardial ischaemia–reperfusion injury in rats. J. Cardiovasc. Pharmacol. 1997;30:717–724. doi: 10.1097/00005344-199712000-00003. [DOI] [PubMed] [Google Scholar]
- ONAI Y., SUZUKI J., NISHIWAKI Y., GOTOH R., BERENS K., DIXON R., YOSHIDA M., ITO H., ISOBE M. Blockade of cell adhesion by a small molecule selectin antagonist attenuates myocardial ischaemia/reperfusion injury. Eur. J. Pharmacol. 2003;481:217–225. doi: 10.1016/j.ejphar.2003.09.040. [DOI] [PubMed] [Google Scholar]
- PIERCE J.W., SCHOENLEBER R., JESMOK G., BEST J., MOORE S.A., COLLINS T., GERRITSEN M.E. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- POMPERMAYER K., SOUZA D.G., LARA G.G., SILVEIRA K.D., CASSALI G.D., ANDRADE A.A., BONJARDIM C.A., PASSAGLIO K.T., ASSREUY J., CUNHA F.Q., VIEIRA M.A.R., TEIXEIRA M.M.The ATP-sensitive potassium channel blocker glibendamide prevents renal ischemia and reperfusion injury in rats Kidney Int 2005(in press) [DOI] [PubMed]
- PYE J., ARDESHIRPOUR F., MCCAIN A., BELLINGER D.A., MERRICKS E., ADAMS J., ELLIOTT P.J., PIEN C., FISCHER T.H., BALDWIN A.S., JR, NICHOLS T.C. Proteasome inhibition ablates activation of NF-kappa B in myocardial reperfusion and reduces reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H919–H926. doi: 10.1152/ajpheart.00851.2002. [DOI] [PubMed] [Google Scholar]
- QIN J., CLORE G.M., GRONENBORN A.M. Ionization equilibria for side-chain carboxyl groups in oxidized and reduced human thioredoxin and in the complex with its target peptide from the transcription factor NF kappa B. Biochemistry. 1996;35:7–13. doi: 10.1021/bi952299h. [DOI] [PubMed] [Google Scholar]
- RITTER L.S., COPELAND J.G., MCDONAGH P.F. Fucoidin reduces coronary microvascular leukocyte accumulation early in reperfusion. Ann. Thorac. Surg. 1998;66:2063–2072. doi: 10.1016/s0003-4975(98)00823-6. [DOI] [PubMed] [Google Scholar]
- RUSSELL J., EPSTEIN C.J., GRISHAM M.B., ALEXANDER J.S., YEH K.Y., GRANGER D.N. Regulation of E-selectin expression in postischaemic intestinal microvasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G878–G885. doi: 10.1152/ajpgi.2000.278.6.G878. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000a;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischaemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000b;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., GUABIRABA R., PINHO V., BRISTOW A., POOLE S., TEIXEIRA M.M. IL-1-driven endogenous IL-10 production protects against the systemic and local acute inflammatory response following intestinal reperfusion injury. J. Immunol. 2003a;170:4759–4766. doi: 10.4049/jimmunol.170.9.4759. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., LOMEZ E.S., PINHO V., PESQUERO J.B., BADER M., PESQUERO J.L., TEIXEIRA M.M. Role of bradykinin B2 and B1 receptors in the local, remote, and systemic inflammatory responses that follow intestinal ischaemia and reperfusion injury. J. Immunol. 2004a;172:2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., PINHO V., PESQUERO J.L., LOMEZ E.S., POOLE S., JULIANO L., CORREA A., JR, DE A., CASTRO M.S., TEIXEIRA M.M. Role of the bradykinin B2 receptor for the local and systemic inflammatory response that follows severe reperfusion injury. Br. J. Pharmacol. 2003c;139:129–139. doi: 10.1038/sj.bjp.0705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., PINHO V., SOARES A.C., SHIMIZU T., ISHII S., TEIXEIRA M.M. Role of PAF receptors during intestinal ischaemia and reperfusion injury. A comparative study between PAF receptor-deficient mice and PAF receptor antagonist treatment. Br. J. Pharmacol. 2003b;139:733–740. doi: 10.1038/sj.bjp.0705296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., SOARES A.C., PINHO V., TORLONI H., REIS L.F., TEIXEIRA M.M., DIAS A.A. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischaemia and reperfusion injury. Am. J. Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., VIEIRA A.T., SOARES A.C., PINHO V., NICOLI J.R., VIEIRA L.Q., TEIXEIRA M.M. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J. Immunol. 2004b;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- TAKADA Y., SINGH S., AGGARWAL B.B. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI D., YOSHIDOME H., KATO A., ITO H., KIMURA F., SHIMIZU H., OHTSUKA M., MORITA Y., MIYAZAKI M. Interleukin 18 causes hepatic ischaemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- TEGEDER I., NIEDERBERGER E., ISRAR E., GUHRING H., BRUNE K., EUCHENHOFER C., GROSCH S., GEISSLINGER G. Inhibition of NF-kappaB and AP-1 activation by R- and S-flurbiprofen. FASEB J. 2001;15:595–597. doi: 10.1096/fasebj.15.3.595. [DOI] [PubMed] [Google Scholar]
- XANTHOUDAKIS S., CURRAN T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINGARELLI B., HAKE P.W., O'CONNOR M., DENENBERG A., WONG H.R., KONG S., ARONOW B.J. Differential regulation of activator protein-1 and heat shock factor-1 in myocardial ischaemia and reperfusion injury: role of poly(ADP-ribose) polymerase-1. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1408–H1415. doi: 10.1152/ajpheart.00953.2003. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., SHEEHAN M., WONG H.R. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit. Care Med. 2003;31:S105–S111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- ZOU L., ATTUWAYBI B., KONE B.C. Effects of NF-kappa B inhibition on mesenteric ischaemia–reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G713–G721. doi: 10.1152/ajpgi.00431.2002. [DOI] [PubMed] [Google Scholar]
- WILLERSON J.T. Pharmacologic approaches to reperfusion injury. Adv. Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]
- YEH K.Y., YEH M., GLASS J., GRANGER D.N. Rapid activation of NF-kappaB and AP-1 and target gene expression in postischaemic rat intestine. Gastroenterology. 2000;118:525–534. doi: 10.1016/s0016-5085(00)70258-7. [DOI] [PubMed] [Google Scholar]