Abstract
The present study was designed to examine the functional role of membrane phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) in the regulation of the muscarinic K+ channel (IK,ACh) by extracellular ATP and adenosine in guinea-pig atrial myocytes, using the whole-cell patch-clamp method.
Bath application of ATP in micromolar concentrations typically evoked a transient activation of IK,ACh; a rapid activation phase was consistently followed by a progressive decline even to the baseline level despite the continued presence of ATP. This progressive decline of IK,ACh was significantly attenuated either by blockade of phospholipase C (PLC) with compound 48/80 (100 μM) or by addition of PtdIns(4,5)P2 (50 μM) to the cell inside, suggesting that depletion of membrane PtdIns(4,5)P2 via PLC activation is mainly, if not totally, responsible for the progressive decline of IK,ACh during the presence of ATP.
When atrial myocytes were exposed to wortmannin (50 μM) following ATP (50 μM) application to impair the resynthesis of PtdIns(4,5)P2, the activation of IK,ACh evoked by subsequently applied ATP (50 μM) was greatly reduced. Activation of IK,ACh by adenosine (100 μM) was partially reduced by pretreatment of atrial myocytes with ATP (100 μM) and was largely abolished by a further addition of wortmannin (50 μM) in the presence of ATP (100 μM). These results support the view that the activation of IK,ACh by ATP and adenosine depends on membrane PtdIns(4,5)P2 that is subject to reduction by extracellular ATP.
The present study thus provides functional evidence to suggest that extracellular ATP activates PLC and thereby depletes membrane PtdIns(4,5)P2 that is critically involved in the activation process of IK,ACh by its agonists ATP and adenosine in guinea-pig atrial myocytes.
Keywords: IK,ACh; PtdIns(4,5)P2; ATP; P2Y receptor; wortmannin; PtdIns 4-kinase
Introduction
The muscarinic K+ channel (IK,ACh) is primarily expressed in atria and conductive tissue of the heart and plays an important role in mediating negative inotropic, chronotropic and dromotropic responses to the vagal neurotransmitter acetylcholine (ACh) in the heart. In addition to ACh, IK,ACh is activated by several neurotransmitters and extracellular signaling molecules, such as adenosine (Belardinelli & Isenberg, 1983; Kurachi et al., 1986), somatostatin (Lewis & Clapham, 1989), endothelins (ETs) (Kim, 1991; Ono et al., 1994; Yamaguchi et al., 1997), sphingosine-1-phosphate (Bünemann et al., 1995) and ATP (Friel & Bean, 1990; Matsuura et al., 1996; Hara & Nakaya, 1997). Previous studies have provided evidence to support the view that the membrane receptor for each of these agonists is directly coupled to the channel proteins through a pertussis toxin (PTX)-sensitive heterotrimeric G protein GK; thus, the activation of IK,ACh typically arises through a membrane-delimited pathway (Soejima & Noma, 1984; Breitwieser & Szabo, 1985; Pfaffinger et al., 1985; Kurachi et al., 1986).
On the other hand, it was reported that IK,ACh is substantially inhibited by several agonists acting on membrane receptors that are assumed to activate a Gq-phospholipase C (PLC) signaling pathway. These include methoxamine (Braun et al., 1992), ET-1, ET-3 (Yamaguchi et al., 1997) and ATP (Matsuura & Ehara, 1996; Hara & Nakaya, 1997). It is well known that activation of PLC cleaves membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) into two second messengers, namely, inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG), which respectively releases Ca2+ from intracellular stores and activates protein kinase C (PKC). However, these studies have suggested that the inhibition of IK,ACh by methoxamine and ATP is mediated through the activation of their respective receptors (α1-adrenergic and P2Y receptors, respectively) and a PTX-insensitive G protein, but not through the activation of PKC or an elevation of intracellular Ca2+. Hilgemann & Ball (1996) revealed for the first time that membrane PtdIns(4,5)P2 exerts a direct modulatory action on some kinds of ionic channels and transporters in cardiac cell membrane. For example, PtdIns(4,5)P2 acts as an important cofactor for the activation of both native IK,ACh and heterologously expressed GIRK1/GIRK4 channels (Huang et al., 1998; Sui et al., 1998; Ho & Murrell-Lagnado, 1999; Logothetis & Zhang, 1999; Zhang et al., 1999; Bender et al., 2002), the molecular constituents of atrial IK,ACh (Krapivinsky et al., 1995; Silverman et al., 1996).
The activation of IK,ACh during exposure to extracellular ATP is characteristically transient, with a complete abolishment of current activation within ∼1–2 min, despite the continued presence of the agonist, which has been accounted for by dual actions of extracellular ATP via two distinct types of G proteins, as mentioned above (Matsuura & Ehara, 1996; Matsuura et al., 1996; Hara & Nakaya, 1997). Although the signaling molecule that acts downstream of a PTX-insensitive G protein to mediate the inhibitory action of extracellular ATP has yet to be fully elucidated, it may be expected that depletion of membrane PtdIns(4,5)P2 via PLC activation is involved in this process. Accordingly, the objective of the present study was to examine the functional role of membrane PtdIns(4,5)P2 in the regulation of IK,ACh by its agonists ATP and adenosine in guinea-pig atrial myocytes. Our results strongly suggest that the characteristic progressive decline of IK,ACh in the presence of extracellular ATP involves activation of PLC and concomitant depletion of membrane PtdIns(4,5)P2 and that the activation of IK,ACh by ATP and adenosine is critically dependent on membrane PtdIns(4,5)P2 that is subject to depletion by extracellular ATP-induced activation of PLC.
Methods
Isolation of atrial myocytes
All protocols were approved by the institution's Animal Care and Use Committee and were performed in compliance with The Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85-23, revised 1996). Single atrial myocytes were enzymatically dissociated from the heart of adult Hartley guinea-pigs using a retrograde Langendorff perfusion method as described previously (Powell et al., 1980; Ding et al., 2004).
Solutions and chemicals
Normal Tyrode solution contained (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose and 5.0 HEPES, and pH was adjusted to 7.4 with NaOH. Whole-cell IK,ACh was measured during superfusion of atrial myocytes with normal Tyrode solution containing 0.4 μM nisoldipine (a generous gift from Bayer, Germany) to block the L-type Ca2+ channel (ICa,L). Agents added to the external solutions included ATP (disodium salt; Sigma Chemical Co., St Louis, MO, U.S.A.), adenosine (sodium salt; Sigma) and wortmannin (Sigma). The control pipette solution contained (in mM): 70 potassium aspartate, 50 KCl, 10 KH2PO4, 1 MgSO4, 3 Na2-ATP (Sigma), 0.1 Li2-GTP (Roche Diagnostics GmbH, Mannheim, Germany), 5 EGTA and 5 HEPES, and pH adjusted to 7.2 with KOH. The amount of KOH required for titration, measured in several experiments, was found to average 24 mM. Therefore, the total K+ concentration in the control pipette solution was 154 mM, and the equilibrium potential for K+ (EK) under the present experimental conditions was calculated to be −88.5 mV with the Nernst equation. The concentration of free Ca2+ and Mg2+ in the pipette solution was estimated to be approximately 1.5 × 10−10 M (pCa=9.8) and 3.7 × 10−5 M (pMg=4.4), respectively (Fabiato & Fabiato, 1979; Tsien & Rink, 1980). PtdIns(4,5)P2 (Calbiochem, San Diego, CA, U.S.A.) and compound 48/80 (Sigma) were added to the pipette solution and were allowed to dialyze into the myocyte for 15–20 min before the start of whole-cell current measurements. Wortmannin and compound 48/80 were prepared as a 100-mM stock solution in DMSO. ATP and adenosine were directly dissolved in the extracellular solution. PtdIns(4,5)P2 was directly dissolved in the control pipette solution at a concentration of 50 μM with a 30-min sonication on ice.
Whole-cell patch-clamp technique and data analysis
Isolated atrial myocytes were voltage-clamped using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) with a patch-clamp amplifier (CEZ-2400, Nihon Kohden, Tokyo, Japan). Borosilicate glass electrodes had a resistance of 2.0–3.0 MΩ when filled with pipette solutions. Membrane currents were measured at a holding potential of −40 mV or during the voltage ramp protocol (dV dt−1=0.4 V s−1), which consisted of ascending (depolarizing) phase from the holding potential (−40 mV) to +50 mV followed by a descending (hyperpolarizing) phase to −130 mV. The current–voltage (I–V) relationship was determined during the descending phase. The activation of the delayed rectifier K+ channel (IK) composed of the rapid and slow components (IKr and IKs, respectively) was expected to be minimal during the rapid voltage ramp (0.4 V s−1) used in the present study. All experiments were performed at 36±1°C.
Current and voltage signals were stored on digital audiotape using a PCM data recorder (RD-120TE, TEAC, Tokyo, Japan) for subsequent computer analysis (PC98RL, NEC, Tokyo, Japan). Data values given in the text and in figures with error bars represent mean±s.e.m., and n indicates the number of myocytes studied. Statistical comparisons between two groups were made using Student's unpaired t-test. Comparisons among multiple (n⩾3) groups were performed by analysis of variance (single f-factor). Where significant f-values were obtained, pairwise comparisons of means were carried out using Tukey's multiple means comparison test. P<0.05 was taken as the limit of significance. In the figures, periods of exposure to various reagents are denoted by horizontal bars over the current traces.
Results
A transient nature of IK,ACh activation during exposure to ATP
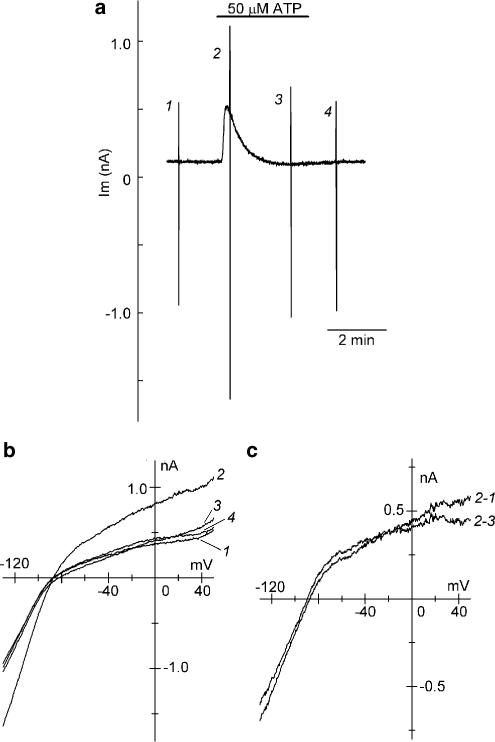
Figure 1 shows a representative example of the effect of extracellular ATP (50 μM) on whole-cell currents measured at a holding potential of −40 mV and during the voltage-ramp protocol in a guinea-pig atrial myocyte. Addition of 50 μM ATP to the bath evoked a rapid outward shift of the holding current, which then progressively declined to a steady level close to the baseline level despite the continued presence of the agonist (Figure 1a), consistent with previous observations in the same cell types (Matsuura & Ehara, 1996; Hara & Nakaya, 1997). Figure 1b illustrates superimposed I–V relationships, recorded using the voltage ramps between +50 and −130 mV, under control conditions (1), shortly (2) and ∼2.5 min (3) after ATP application, and after withdrawal of the ATP (4). To characterize the membrane currents that are associated with the initial outward shift of the holding current and its subsequent decline during exposure to ATP, difference currents were obtained by digital subtraction of the corresponding current records. The membrane current that underlies the outward shift of the holding current, obtained by digitally subtracting the current record under control condition from that measured at the time point of the maximal outward shift of the holding current (Figure 1c, 2-1), has an inwardly rectifying I–V relationship with a mean reversal potential of −87.5±2.5 mV (n=6), which is very close to the estimated EK (−88.5 mV). It was previously shown that pretreatment of atrial myocytes with PTX totally prevents the activation of the membrane current associated with the outward shift of the holding current at −40 mV during exposure to ATP (Matsuura et al., 1996). Taken together, it can be confirmed that extracellular ATP initially activates IK,ACh in guinea-pig atrial myocytes.
Figure 1.
Effect of bath application of ATP on whole-cell currents in guinea-pig atrial myocytes. (a) Chart record of whole-cell current (Im) recorded at a holding potential of −40 mV and during voltage ramps between +50 and −130 mV at 0.4 V s−1 (see Methods). Rapid deflections represent changes in membrane current in response to the voltage ramps applied before (1), and during exposure to 50 μM ATP (2 and 3), and after washout of the ATP (4). (b) Superimposed I–V relationships obtained using the voltage ramps applied at the time points indicated by numerals (1–4) in (a). (c) Superimposed I–V relationships for the difference currents obtained by digital subtraction of current records as indicated.
The membrane current responsible for the subsequent decline of the outward holding current (Figure 1c, 2-3), determined by digital subtraction of current trace ∼2.5 min after ATP application from that shortly after ATP application, was also characterized by the presence of inward rectification and the reversal potential (−86.4±2.8 mV, n=6) near EK, and its I–V relationship was nearly superimposable with that of the membrane current activated by extracellular ATP (Figure 1c, 2-1). Based on these observations, it seems most likely that IK,ACh was initially activated and was subsequently inhibited (reduced) during exposure to ATP, causing a characteristic transient activation of IK,ACh in guinea-pig atrial myocytes.
It was previously demonstrated in guinea-pig atrial myocytes that extracellular ATP activates IK,ACh by a membrane-delimited pathway involving a PTX-sensitive G protein GK (Matsuura et al., 1996), analogous to the activation mechanism by ACh and adenosine (Breitwieser & Szabo, 1985; Pfaffinger et al., 1985; Kurachi et al., 1986). In subsequent experiments, the signal transduction mechanism mediating the inhibitory effect of extracellular ATP on IK,ACh was examined. Based on the analysis of membrane currents in atrial myocytes during exposure to ATP (Figure 1), the initial outward shift of the holding current and its subsequent decline during exposure to ATP can be assumed to respectively reflect the degree of activation and inhibition of IK,ACh by extracellular ATP. The inhibitory action of ATP on IK,ACh activated by ATP per se was therefore quantitatively evaluated by normalizing the amplitude of the subsequent decline of outward holding current with reference to the initial outward shift during exposure to ATP (% decrease).
Involvement of PLC-induced PtdIns(4,5)P2 depletion in the progressive decline of IK,ACh activation during exposure to ATP
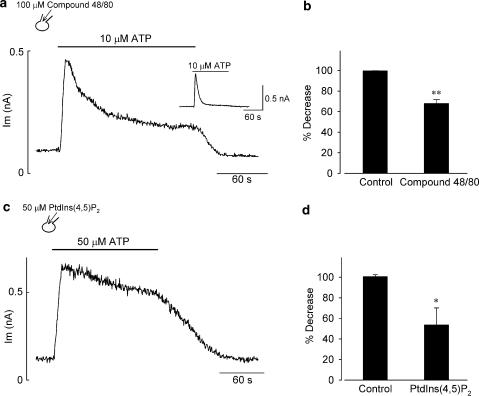
It was demonstrated in guinea-pig ventricular myocytes that micromolar concentrations of extracellular ATP stimulates a P2Y receptor-coupled Gq-PLC signaling pathway and thereby depletes plasma membrane PtdIns(4,5)P2 that is required for maintaining the activity of the ATP-sensitive K+ channels (Oketani et al., 2002). We therefore tested the possible involvement of PLC activation and subsequent PtdIns(4,5)P2 depletion in the progressive decline of IK,ACh during exposure to ATP. A commonly used PLC inhibitor U-73122 was reported to block IK,ACh in cardiac atrial myocytes, independently of its action on PLC (Cho et al., 2001b). Since compound 48/80 was shown to be effective at inhibiting PLC activity following P2Y stimulation by extracellular ATP in guinea-pig cardiac myocytes (Bronner et al., 1987; Oketani et al., 2002), we evaluated the role of PLC action using this reagent. In the atrial myocyte dialyzed with compound 48/80 (100 μM), bath application of 10 μM ATP rapidly shifted the holding current level (at −40 mV) in the outward direction, which was then followed by a gradual decline to a steady level that was substantially more outward relative to the baseline level (Figure 2a). The degree of IK,ACh decrease during exposure to 10 μM ATP was significantly reduced by the addition of 100 μM compound 48/80, compared with that in control conditions (67.9±3.8%, n=5 vs 99.6±0.1%, n=4; P<0.01; Figure 2b), which supports the view that the inhibitory action of ATP on IK,ACh involves PLC activation.
Figure 2.
Effect of PLC inhibition or PtdIns(4,5)P2 addition on the progressive decline of IK,ACh during exposure to ATP. (a, c) Time course of changes in the holding current level at −40 mV during ∼2.5 min exposure to ATP at concentrations of 10 μM (a) and 50 μM (c) in atrial myocyte dialyzed with a pipette solution containing either 100 μM compound 48/80 (a) or 50 μM PtdIns(4,5)P2 (c). The myocyte was stimulated with extracellular ATP 15–20 min after rupture of the patch membrane (establishment of whole-cell mode) with each reagent. (b, d) Percentage decrease of IK,ACh during exposure to ATP at 10 μM (b) and 50 μM (d) in myocytes dialyzed with control pipette solution (Control) and pipette solution supplemented with either 100 μM compound 48/80 (b) or 50 μM PtdIns(4,5)P2 (d). Percentage decrease was determined at the current levels measured at the end of ∼2.5 min exposure to ATP. Inset in (a) shows a representative time course of IK,ACh (at −40 mV) during exposure to 10 μM ATP, recorded from an atrial myocyte dialyzed with a control pipette solution. *P<0.05 and **P<0.01 compared with control value (Student's unpaired t-test).
Previous whole-cell patch-clamp experiments have proved that exogenously applied PtdIns(4,5)P2 through a recording pipette can effectively modulate the function of ionic channels assumed to be dependent on membrane PtdIns(4,5)P2, probably after being fused to the plasma membrane (Rohács et al., 1999; Bian et al., 2001; Meyer et al., 2001; Ford et al., 2003; Ding et al., 2004). We therefore examined the effect of intracellular dialysis with 50 μM PtdIns(4,5)P2 on IK,ACh response to ATP. As demonstrated in Figure 2c, the decline of the outward holding current associated with the reduction of IK,ACh activation during exposure to ATP was greatly attenuated in PtdIns(4,5)P2-loaded atrial myocytes. In a total of four myocytes, the degree of IK,ACh decrease during exposure to 50 μM ATP was markedly attenuated by dialyzing atrial myocytes with 50 μM PtdIns(4,5)P2, compared with that in control (53.7±16.3%, n=4 vs 100.7±1.7%, n=4; P<0.05; Figure 2d). These observations are consistent with the view that the ATP-induced activation of PLC and resultant depletion of membrane PtdIns(4,5)P2 contribute at least partly to the progressive reduction of IK,ACh.
Functional role of membrane PtdIns(4,5)P2 that is subject to reduction by extracellular ATP in the activation of IK,ACh by ATP and adenosine
We further evaluated the functional role of membrane PtdIns(4,5)P2 that is subject to reduction by extracellular ATP in the activation of IK,ACh. It is generally accepted that micromolar concentrations of wortmannin blocks phosphatidylinositol 4-kinase (PtdIns 4-kinase) as well as phosphatidylinositol 3-kinase (PtdIns 3-kinase) and thereby impairs the replenishment of PtdIns(4,5)P2 following the PLC-coupled receptor-mediated depletion in various cell types (Nakanishi et al., 1995; Meyers & Cantley, 1997; Willars et al., 1998) including cardiac myocytes (Xie et al., 1999). In contrast, nanomolar concentrations of wortmannin selectively inhibits PtdIns 3-kinase (Nakanishi et al., 1995) and is therefore expected to have little, if any, significant effect on recovery from receptor-mediated depletion of membrane PtdIns(4,5)P2.
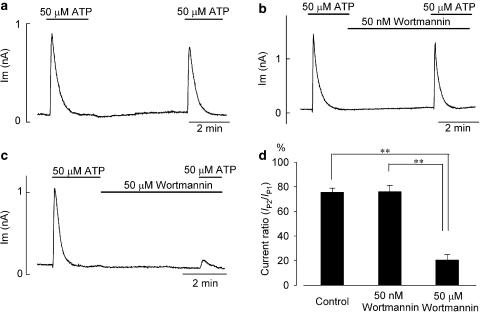
As demonstrated in Figure 3a–c, 50 μM ATP was applied (for ∼2 min) twice to the same myocyte, with a washout period of ∼4 min. The peak amplitude of IK,ACh activated by the second application of ATP (IP2) was 75.8±3.1% (n=7) of that activated by the first application of ATP (IP1) under control conditions (Figure 3a, d). Thus, the IK,ACh response to 50 μM ATP was largely, if not totally, recovered from possible desensitization during ∼4 min washout period. When 50 μM wortmannin was added to the bath immediately after washout of the first application of ATP, the ratio of IK,ACh activation during the first and second applications of ATP (IP2/IP1=20.6±4.4%, n=4; Figure 3c, d) became significantly (P<0.01) reduced, when compared with that in control (IP2/IP1=75.8±3.1%, n=7; Figure 3a, d). However, addition of 50 nM wortmannin during an ∼4-min washout period had no significant effect on the current ratio (IP2/IP1=76.1±5.1%, n=7; Figure 3b, d), when compared with that in control. A significant (P<0.01) difference was also observed between the actions of 50 nM and 50 μM of wortmannin on the current ratio (Figure 3d), which suggests that replenishment of membrane PtdIns(4,5)P2 via the action of PtdIns 4-kinase takes place during ∼4-min washout period and thereby plays an essential role in contributing to the activation of IK,ACh during the second application of ATP. Taken together with the results shown in Figure 2, these findings again suggest that bath application of 50 μM ATP for a period of ∼2 min is accompanied by substantial depletion of membrane PtdIns(4,5)P2 (via PLC activation) required for the activation of IK,ACh by ATP per se in guinea-pig atrial myocytes.
Figure 3.
Effect of wortmannin on the activation of IK,ACh by extracellular ATP. (a) An atrial myocyte was stimulated with twin-pulse applications of 50 μM ATP with an interval of ∼4 min, as indicated by horizontal bars. (b, c) Following the first stimulation with 50 μM ATP, the atrial myocyte was then exposed to wortmannin at a concentration of 50 nM (b) or 50 μM (c) for ∼4 min and was again stimulated with 50 μM ATP, as indicated. (d) Summary data for current ratio of IP2/IP1, obtained by normalizing the peak amplitude of IK,ACh during the second application of ATP (IP2) with reference to that during the first ATP application (IP1), expressed as percentage. Asterisks represent P-values according to Tukey's multiple means comparison test (**P<0.01). There was no significant difference between the control and wortmannin (50 nM) groups, but the difference was significant (P<0.01) when the wortmannin (50 μM) group was compared with either the control or wortmannin (50 nM) group.
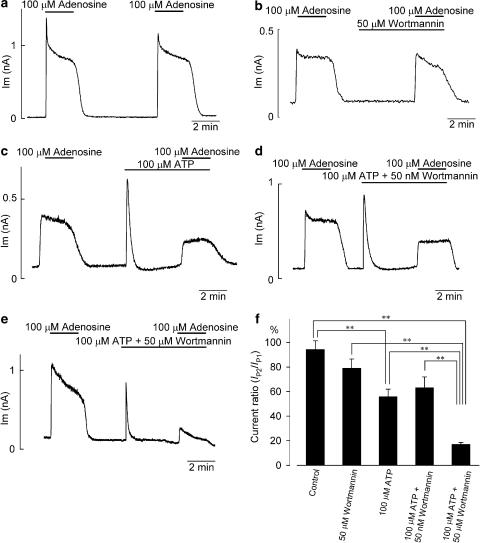
We then evaluated the functional role of membrane PtdIns(4,5)P2 that is subject to depletion by extracellular ATP in the activation of IK,ACh by adenosine. As demonstrated in Figure 4, atrial myocytes were stimulated by twin-pulse applications of 100 μM adenosine for 2 min with an interval of ∼6–8 min, during which time atrial myocytes were exposed to ATP and/or wortmannin to modify PtdIns(4,5)P2 levels in the plasma membrane. The significance of these reagents was evaluated by normalizing the peak amplitude of IK,ACh during the second application of adenosine with reference to that during the first adenosine application (IP2/IP1). Without ATP and wortmannin during ∼6-min washout period (Figure 4a), an almost similar magnitude of IK,ACh was activated by the first and second applications of adenosine (IP2/IP1=94.2±7.1%, n=4; Figure 4f). This result suggests that a period of ∼6 min is long enough to allow a full recovery from the short-term desensitization evoked by A1 receptor stimulation with 100 μM adenosine. When atrial myocytes were pretreated for 4 min with 50 μM wortmannin before the second application of adenosine (Figure 4b), the value for the current ratio of IP2/IP1 was 79.1±7.3% (n=6, Figure 4f), which was slightly but insignificantly (P=0.174) smaller compared with the current ratio under control conditions. This finding is consistent with a previous study that showed that wortmannin (100 μM) alone did not significantly affect the activation of IK,ACh by ACh (10 μM) in mouse atrial myocytes (Cho et al., 2001a) and suggests the following two points. Firstly, the inhibition of PtdIns 4-kinase alone for a period of 4 min does not result in depletion of membrane PtdIns(4,5)P2 associated with reduction of IK,ACh activation. Secondly, stimulation of adenosine receptors, which are predominantly of the A1 subtype in atrial cell membrane, does not effectively activate a Gq-PLC signaling pathway leading to depletion of membrane PtdIns(4,5)P2 and inhibition of IK,ACh (Chen & Bache, 2003).
Figure 4.
Effect of pretreatment with wortmannin and/or ATP on adenosine-evoked IK,ACh. (a) Adenosine (100 μM) was twice added to the same myocyte with an interval of ∼6 min, which elicited IK,ACh of almost similar magnitude. (b) Following the first stimulation with 100 μM adenosine, an atrial myocyte was exposed to 50 μM wortmannin for ∼4 min and subsequently stimulated with 100 μM adenosine. (c–e) The atrial myocytes were exposed to 100 μM ATP (c), 100 μM ATP plus 50 nM wortmannin (d) or 100 μM ATP plus 50 μM wortmannin (e) for ∼4 min before the second application of 100 μM Ado. (f) Current ratio of IP2/IP1, obtained by normalizing the peak amplitude of IK,ACh during the second application of 100 μM adenosine (IP2) with reference to that during the first adenosine application (IP1), from the experimental protocols shown in (a–e). Asterisks represent P-values according to Tukey's multiple means comparison test (**P<0.01). Note that there was no significant difference in the current ratio (IP2/IP1) between the ATP alone and the ATP plus wortmannin (50 nM) application groups, but the difference was highly significant (P<0.01) when the ATP plus wortmannin (50 μM) application group was compared with either the ATP alone or ATP plus wortmannin (50 nM) application group (f).
On the other hand, when an atrial myocyte was exposed to 100 μM ATP for ∼4 min before the second application of adenosine (Figure 4c), the current ratio of IP2/IP1 became significantly (P<0.01) reduced compared with that in control (55.9±6.2%, n=8 vs 94.2±7.1%, n=4; Figure 4f). This reduction of the current ratio by the presence of ATP was further significantly (P<0.01) potentiated by the concomitant presence of 50 μM wortmannin (IP2/IP1=17.1±1.5%, n=5; Figure 4e, f), but was not appreciably affected by 50 nM wortmannin (IP2/IP1=63.3±8.7%, n=3; Figure 4d, f). These results may be interpreted to suggest that resynthesis of PtdIns(4,5)P2 mediated by PtdIns 4-kinase takes place even during exposure to ATP, which is effective at activating IK,ACh in atrial myocytes (Figure 4c).
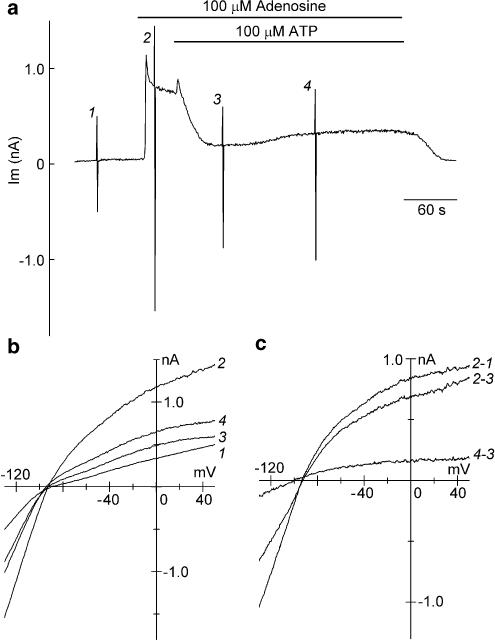
It has previously been demonstrated in guinea-pig atrial myocytes that extracellular ATP in micromolar concentrations substantially inhibits IK,ACh preactivated by ACh and adenosine (Matsuura & Ehara, 1996; Hara & Nakaya, 1997). In the experiment demonstrated in Figure 5, the inhibitory action of extracellular ATP on IK,ACh preactivated by adenosine was characterized. Bath application of 100 μM adenosine evoked a rapid outward shift of the holding current by activating IK,ACh (Figure 5a), which had an inwardly rectifying I–V relationship with mean reversal potential of −86.3±2.4 mV (n=6; Figure 5c, 2–1), a value very close to the estimated EK (−88.5 mV). Subsequent application of 100 μM ATP in the presence of adenosine initially increased the outward holding current and then decreased it to near baseline levels within 1 min (Figure 5a). The initial outward shift of the holding current appears to be due to a further activation of IK,ACh by extracellular ATP (Hara & Nakaya, 1997). The membrane currents inhibited by ATP, obtained by an appropriate digital subtraction of the corresponding current traces, also had an inwardly rectifying I–V relationship with a mean reversal potential of −86.1±2.6 mV (n=6; Figure 5c, 2–3), thus confirming that extracellular ATP inhibited the adenosine-activated IK,ACh.
Figure 5.
Inhibition of adenosine-evoked IK,ACh by extracellular ATP. (a) Chart records of whole-cell currents (Im) recorded at a holding potential of −40 mV and during voltage ramps applied before (1), and during exposure to 100 μM adenosine (2), and after further addition of 100 μM ATP (3 and 4). The rapid deflections in the current recording reflect the imposition of voltage-ramp protocols. (b) Superimposed I–V relationships measured during the voltage ramps applied at the points indicated by numerals (1–4) in (a). (c) Superimposed I–V relationships for the difference currents obtained by digital subtraction of current records as indicated.
The degree of ATP inhibition of the adenosine-activated IK,ACh was evaluated by normalizing the amplitude of IK,ACh (at −40 mV) inhibited by ATP (obtained by subtracting the holding current level during exposure to ATP from that immediately before ATP application) with reference to that activated by adenosine (obtained by subtracting the current level under control conditions from that immediately before ATP application). In a total of six myocytes, ATP at 100 μM inhibited the adenosine (100 μM)-activated IK,ACh by 91.2±10.4%, which is similar to the degree of inhibition of IK,ACh activated by 100 μM ATP per se (100.8±2.4%, n=6, data not shown; refer to Figure 2b, d). It was frequently found (four out of six myocytes) that, during exposure to ATP with adenosine, the holding current level (at −40 mV) slightly but gradually shifted in the outward direction after reaching a peak inward shift (Figure 5a), due to the reactivation of IK,ACh, as evidenced by the inwardly rectifying I–V relationship with a reversal potential of −91.2±4.4 mV (n=4, Figure 5c, 4–3). The ATP-evoked inhibition of adenosine-activated IK,ACh was thus found to be gradually attenuated even in the presence of ATP. The concomitant addition of 50 μM wortmannin together with ATP was confirmed to consistently abolish such a gradual attenuation of the ATP-evoked inhibition of IK,ACh preactivated by adenosine (data not shown).
Discussion
The activation of IK,ACh by micromolar concentrations of extracellular ATP is characterized by its complete transient nature, arising from total abolishment of the current activation within ∼1–2 min despite the continued presence of the agonist (Figure 1). A rapid activation phase of IK,ACh by extracellular ATP was previously shown to arise from stimulation of a P2Y receptor, leading to a membrane-delimited, GK-mediated channel activation (Matsuura et al., 1996; Hara & Nakaya, 1997). The present study confirms that the subsequent progressive decline of IK,ACh activation in the presence of extracellular ATP is significantly attenuated either by blocking the PLC activity with compound 48/80 or by exogenously adding PtdIns(4,5)P2 to the cell interior (Figure 2). Taken together with the previous reports showing that extracellular ATP markedly inhibits IK,ACh preactivated by ACh, by stimulating P2Y receptor coupled to a PTX-insensitive G protein (presumably Gq) (Matsuura & Ehara, 1996; Hara & Nakaya, 1997), these findings strongly suggest that P2Y receptor-mediated reduction of membrane PtdIns(4,5)P2 via G protein-PLC activation is involved in the characteristic progressive decline of IK,ACh during exposure to ATP. Molecular biological studies have indicated that electrostatic interaction of PtdIns(4,5)P2 with positively charged amino acid residues in the proximal C terminus of GIRK channels is required for gating by βγ subunits of GK (Huang et al., 1998; Sui et al., 1998; Zhang et al., 1999).
The present study found that the progressive decline of IK,ACh during exposure to 50 μM ATP can be substantially (∼50%, Figure 2c, d) but not fully prevented by loading PtdIns(4,5)P2 to the cell inside through a recording pipette. As judged from the similarity of the activation mechanism of IK,ACh by ACh and ATP (Soejima & Noma, 1984; Breitwieser & Szabo, 1985; Pfaffinger et al., 1985; Kurachi et al., 1986; Matsuura et al., 1996; Hara & Nakaya, 1997), it is possible that, in addition to PtdIns(4,5)P2 hydrolysis, some of cellular and molecular mechanisms that are associated with the acute (fast) desensitization of the ACh-activated IK,ACh, such as the nucleotide exchange and hydrolysis cycle of G proteins (Chuang et al., 1998), may also be involved in the progressive decline of the ATP-induced IK,ACh. Thus, further investigations will be necessary to fully elucidate the mechanism of the progressive decay of IK,ACh in the presence of ATP in atrial myocytes.
The present study also assesses the functional consequence of the PLC-induced PtdIns(4,5)P2 depletion and its resynthesis mediated via PtdIns 4-kinase, in the activation of IK,ACh in atrial myocytes. It has been reported that wortmannin at micromolar concentrations, but not at nanomolar concentrations, potently inhibits PtdIns 4-kinase that catalyzes phosphorylation of phosphatidylinositol (PtdIns) to phosphatidylinositol 4-phosphate (PtdIns(4)P), and thereby disrupts synthesis of PtdIns(4,5)P2 in plasma membrane (Nakanishi et al., 1995). The present experiments demonstrate that the activation of IK,ACh by the second application of ATP was not appreciably affected by pretreatment of 50 nM wortmannin, but was largely abolished by that of 50 μM wortmannin (Figure 3). Similarly, the activation of IK,ACh by adenosine was partly reduced by pretreatment of either ATP or ATP plus 50 nM wortmannin, but was almost totally abolished by the concomitant presence of ATP plus 50 μM wortmannin (Figure 4). The present study thus provides evidence to suggest that the activation of IK,ACh by its agonists ATP and adenosine is critically dependent on membrane PtdIns(4,5)P2 that is subject to depletion by extracellular ATP.
The present experiments also demonstrate that whereas extracellular ATP at 100 μM almost completely (91.2±10.4%, n=6; Figure 5) inhibits IK,ACh preactivated by 100 μM adenosine within ∼1 min, pretreatment of atrial myocytes with the same concentration of ATP for 4 min only partially (44.1±6.2%, n=8; Figure 4c, f) reduces the activation of IK,ACh by the same concentration of adenosine. However, the adenosine-evoked activation of IK,ACh can be largely (82.9±1.5%, n=5; Figure 4e, f) abolished by further adding 50 μM wortmannin during exposure to ATP. It has been demonstrated in SH-SY5Y human neuroblastoma cells that stimulation of M3-muscarinic receptor with carbachol (1 mM) rapidly decreases membrane PtdIns(4,5)P2 level via PLC activation to ∼25% of control level within 1 min (Willars et al., 1998). This study further shows that when 10 μM wortmannin is added to inhibit PtdIns 4-kinase together with carbachol, membrane PtdIns(4,5)P2 level following a 10-min stimulation of M3-muscarinic receptor is further decreased below the levels seen in the presence of carbachol alone, suggesting that the resynthesis of PtdIns(4,5)P2 does take place via PtdIns 4-kinase during the time period of M3-muscarinic receptor stimulation. The significant difference in the degree of ATP inhibition of the adenosine-evoked IK,ACh in the absence and presence of 50 μM wortmannin (Figure 4c, e) is therefore likely to be due to the occurrence of PtdIns(4,5)P2 replenishment by PtdIns 4-kinase during the agonist stimulation. The present observation that the ATP inhibition of IK,ACh preactivated by adenosine is not sustained, but is somewhat attenuated in a wortmannin (50 μM)-sensitive manner during the presence of ATP (Figure 5) might also reflect the occurrence of PtdIns(4,5)P2 resynthesis accompanied by reactivation of IK,ACh even during the PLC stimulation by ATP.
In recent years, it was demonstrated in mouse atrial myocytes that membrane PtdIns(4,5)P2 depletion caused by the PLC-coupled M1 (or M3/M5) muscarinic receptors does not lead to the inhibition of IK,ACh, whereas reduction of PtdIns(4,5)P2 evoked by α1-adrenergic receptors (through PLC activation) does significantly inhibit IK,ACh (Cho et al., 2002). This observation strongly suggests that the IK,ACh proteins are preferentially co-localized with α1-adrenergic receptor but not with M1 (or M3/M5) muscarinic receptor in mouse atrial myocytes. Extracellular ATP (100 μM) consistently evokes an almost full inhibition of IK,ACh preactivated by ACh (96.3±8.1% inhibition, n=5; see Matsuura & Ehara, 1996) and adenosine (91.2±10.4% inhibition, n=6; Figure 5), while ET-1 (10–100 nM) and phenylephrine (100 μM) maximally inhibit IK,ACh preactivated by M2 receptor stimulation by ∼80% (Yamaguchi et al., 1997; Meyer et al., 2001). ATP thus appears to be one of the most potent agonists to activate PLC and thereby affect the level of membrane PtdIns(4,5)P2 closely related to the regulation of IK,ACh in atrial myocytes.
It is interesting to note that the activation of IK,ACh by ET-1 and ET-3 is almost completely transient despite the continued presence of the agonists in guinea-pig atrial myocytes (Yamaguchi et al., 1997). It has been suggested that while ET-1 and ET-3 directly activate IK,ACh via GK (Kim, 1991; Ono et al., 1994), ET-1 also evokes phosphoinositide hydrolysis presumably by activating a Gq-PLC pathway in cardiac myocytes (Hattori et al., 1993). Thus, it is likely that bath application of both ATP and ETs simultaneously activates two distinct signaling pathways in the plasma membrane to exert opposite actions on IK,ACh in atrial myocytes, namely, an activation of GK leading to membrane-delimited channel openings and the stimulation of PLC (presumably via Gq) which depletes membrane PtdIns(4,5)P2 and thereby causes channel closure. In general, the agonist-mediated regulation of ion channel through a direct G protein interaction takes place much more rapidly than that involving the metabolic reaction via the activation of effector enzyme of G protein, such as adenylyl cyclase (AC) and PLC (Osterrider et al., 1981; Yatani & Brown, 1989). Consistent with this prediction, activation of IK,ACh by ATP and adenosine reaches its peak response within several seconds, whereas the inhibitory action of ATP on IK,ACh takes place over several tens of seconds (see Figure 1a, 3a–c and 4c–e). It is therefore highly probable that, during exposure of atrial myocytes to ATP or ETs, IK,ACh is rapidly activated through a membrane-delimited pathway, which is subsequently inhibited primarily by depletion of membrane PtdIns(4,5)P2 that gradually occurs through the PLC-mediated hydrolysis. In recent years, experimental evidence has been presented to indicate that ET-1 potently inhibits IK,ACh preactivated by ACh by depleting membrane PtdIns(4,5)P2 through a stimulation of ETA receptor in rat atrial myocytes (Meyer et al., 2001).
Acknowledgments
We thank Drs Mariko Omatsu-Kanbe and Futoshi Toyoda (Shiga University of Medical Science) for invaluable discussions. This study was supported by Grant-in-Aid for Scientific Research (Nos. 14370225 to M.H. and 15590184 to H.M.) from Japan Society for the Promotion of Science.
Abbreviations
- AC
adenylyl cyclase
- Ach
acetylcholine
- ATP
adenosine triphosphate
- DAG
diacylglycerol
- ET-1
endothelin-1
- ET-3
endothelin-3
- ETs
endothelins
- GIRK
G protein-gated inwardly rectifying K+ channel
- ICa,L
L-type Ca2+ channel
- IK
delayed rectifier K+ channel
- IK,ACh
muscarinic K+ channel
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- PLC
phospholipase C
- PtdIns
phosphatidylinositol
- PtdIns(4)P
phosphatidylinositol 4-phosphate
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PtdIns 3-kinase
phosphatidylinositol 3-kinase
- PtdIns 4-kinase
phosphatidylinositol 4-kinase
- PTX
pertussis toxin
References
- BELARDINELLI L., ISENBERG G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am. J. Physiol. 1983;244:H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- BENDER K., WELLNER-KIENITZ M.-C., POTT L. Transfection of a phosphatidyl-4-phosphate 5-kinase gene into rat atrial myocytes removes inhibition of GIRK current by endothelin and α-adrenergic agonists. FEBS Lett. 2002;529:356–360. doi: 10.1016/s0014-5793(02)03426-9. [DOI] [PubMed] [Google Scholar]
- BIAN J., CUI J., MCDONALD T.V. HERG K+ channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ. Res. 2001;89:1168–1176. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- BRAUN A.P., FEDIDA D., GILES W.R. Activation of α1-aderenoceptors modulates the inwardly rectifying potassium currents of mammalian atrial myocytes. Pflügers Arch. 1992;421:431–439. doi: 10.1007/BF00370253. [DOI] [PubMed] [Google Scholar]
- BREITWIESER G.E., SZABO G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- BRONNER C., WIGGINS C., MONTE D., MARKI F., CAPRON A., LANDRY Y., FRANSON R.C. Compound 48/80 is a potent inhibitor of phospholipase C and a dual modulator of phospholipase A2 from human platelet. Biochim. Biophys. Acta. 1987;920:301–305. doi: 10.1016/0005-2760(87)90108-1. [DOI] [PubMed] [Google Scholar]
- BÜNEMANN M., BRANDTS B., MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H., POTT L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J. Physiol. (London) 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y., BACHE R.J. Adenosine: a modulator of the cardiac response to stress. Circ. Res. 2003;93:691–693. doi: 10.1161/01.RES.0000097920.18551.36. [DOI] [PubMed] [Google Scholar]
- CHO H., HWANG J.Y., KIM D., SHIN H.S., KIM Y., EARM Y.E., HO W.K. Acetylcholine-induced phosphatidylinositol 4,5-bisphosphate depletion does not cause short-term desensitization of G protein-gated inwardly rectifying K+ current in mouse atrial myocytes. J. Biol. Chem. 2002;277:27742–27747. doi: 10.1074/jbc.M203660200. [DOI] [PubMed] [Google Scholar]
- CHO H., NAM G.-B., LEE S.H., EARM Y.E., HO W.K. Phosphatidylinositol 4,5-bisphosphate is acting as a signal molecule in α1-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J. Biol. Chem. 2001a;276:159–164. doi: 10.1074/jbc.M004826200. [DOI] [PubMed] [Google Scholar]
- CHO H., YOUM J.B., RYU S.Y., EARM Y.E., HO W.K. Inhibition of acetylcholine-activated K+ currents by U73122 is mediated by the inhibition of PIP2-channel interaction. Br. J. Pharmacol. 2001b;134:1066–1072. doi: 10.1038/sj.bjp.0704347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUANG H.-H., YU M., JAN Y.N., JAN L.Y. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING W.G., TOYODA F., MATSUURA H. Regulation of cardiac IKs potassium current by membrane phosphatidylinositol 4,5-bisphophate. J. Biol. Chem. 2004;279:50726–50734. doi: 10.1074/jbc.M409374200. [DOI] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- FORD C.P., STEMKOWSKI P.L., LIGHT P.E., SMITH P.A. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J. Neurosci. 2003;23:4931–4941. doi: 10.1523/JNEUROSCI.23-12-04931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEL D.D., BEAN B.P. Dual control by ATP and acetylcholine of inwardly rectifying K+ channels in bovine atrial cells. Pflügers Arch. 1990;415:651–657. doi: 10.1007/BF02584001. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARA Y., NAKAYA H. Dual effects of extracellular ATP on the muscarinic acetylcholine receptor-operated K+ current in guinea-pig atrial cells. Eur. J. Pharmacol. 1997;134:295–303. doi: 10.1016/s0014-2999(97)00088-5. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., NAKAYA H., NISHIHIRA J., KANNO M. A dual-component positive inotropic effect of endothelin-1 in guinea pig left atria: a role of protein kinase C. J. Pharmacol. Exp. Ther. 1993;266:1202–1212. [PubMed] [Google Scholar]
- HILGEMANN D.W., BALL R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- HO I.H., MURRELL-LAGNADO R.D. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J. Physiol. (London) 1999;520:645–651. doi: 10.1111/j.1469-7793.1999.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C.-L., FENG S., HILGEMANN D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- KIM D. Endothelin activation of an inwardly rectifying K+ current in atrial cells. Circ. Res. 1991;69:250–255. doi: 10.1161/01.res.69.1.250. [DOI] [PubMed] [Google Scholar]
- KRAPIVINSKY G., GORDON E.A., WICKMAN K., VELIMIROVIC B., KRAPIVINSKY L., CLAPHAM D.E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- KURACHI Y., NAKAJIMA T., SUGIMOTO T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflügers Arch. 1986;407:264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- LEWIS D.L., CLAPHAM D.E. Somatostatin activates an inwardly rectifying K+ channel in neonatal rat atrial cells. Pflügers Arch. 1989;414:492–494. doi: 10.1007/BF00585062. [DOI] [PubMed] [Google Scholar]
- LOGOTHETIS D.E., ZHANG H. Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5-bisphosphate. J. Physiol. (London) 1999;520:630. doi: 10.1111/j.1469-7793.1999.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUURA H., EHARA T. Modulation of the muscarinic K+ channel by P2-purinoceptors in guinea-pig atrial myocytes. J. Physiol. (London) 1996;497:379–393. doi: 10.1113/jphysiol.1996.sp021775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUURA H., SAKAGUCHI M., TSURUHARA Y., EHARA T. Activation of the muscarinic K+ channel by P2-purinoceptors via pertussis toxin-sensitive G proteins in guinea-pig atrial cells. J. Physiol. (London) 1996;490:659–671. doi: 10.1113/jphysiol.1996.sp021175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER T., WELLNER-KIENITZ M.-C., BIEWALD A., BENDER K., EICKEL A., POTT L. Depletion of phosphatidylinositol 4,5-bisphosphate by activation of phospholipase C-coupled receptors causes slow inhibition but not desensitization of G protein-gated inward rectifier K+ current in atrial myocytes. J. Biol. Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- MEYERS R., CANTLEY L.C. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J. Biol. Chem. 1997;272:4384–4390. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S., CATT K.J., BALLA T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKETANI N., KAKEI M., ICHINARI K., OKAMURA M., MIYAMURA A., NAKAZAKI M., ITO S., TEI C. Regulation of KATP channels by P2Y purinoceptors coupled to PIP2 metabolism in guinea pig ventricular cells. Am. J. Physiol. 2002;282:H757–H765. doi: 10.1152/ajpheart.00246.2001. [DOI] [PubMed] [Google Scholar]
- ONO K., TSUJIMOTO G., SAKAMOTO A., ETO K., MASAKI T., OZAKI Y., SATAKE M. Endothelin-A receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994;370:301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- OSTERRIDER W., YANG Q.F., TRAUTWEIN W. The time course of the muscarinic response to ionophoretic acetylcholine application to the S-A node of the rabbit heart. Pflügers Arch. 1981;389:283–291. doi: 10.1007/BF00584791. [DOI] [PubMed] [Google Scholar]
- PFAFFINGER P.J., MARTIN J.M., HUNTER D.D., NATHANSON N.M., HILLE B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- POWELL T., TERRAR D.A., TWIST V.W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J. Physiol. (London) 1980;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHÁCS T., CHEN J., PRESTWICH G.D., LOGOTHETIS D.E. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J. Biol. Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- SILVERMAN S.K., LESTER H.A., DOUGHERTY D.A. Subunit stoichiometry of a heteromultimeric G protein-coupled inward-rectifier K+ channel. J. Biol. Chem. 1996;271:30524–30528. doi: 10.1074/jbc.271.48.30524. [DOI] [PubMed] [Google Scholar]
- SOEJIMA M., NOMA A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflügers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- SUI J.L., PETIT-JACQUES J., LOGOTHETIS D.E. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSIEN R.Y., RINK T.J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim. Biophys. Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- WILLARS G.B., NAHORSKI S.R., CHALLISS R.A.J. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J. Biol. Chem. 1998;273:5037–5046. doi: 10.1074/jbc.273.9.5037. [DOI] [PubMed] [Google Scholar]
- XIE L.H., HORIE M., TAKANO M. Phospholipase C-linked receptors regulate the ATP-sensitive potassium channel by means of phosphatidylinositol 4,5-bisphosphate metabolism. Proc. Natl. Acad. Sci. U.S.A. 1999;96:15292–15297. doi: 10.1073/pnas.96.26.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI H., SAKAMOTO N., WATANABE Y., SAITO T., MASUDA Y., NAKAYA H. Dual effects of endothelins on the muscarinic K+ current in guinea pig atrial cells. Am. J. Physiol. 1997;273:H1745–H1753. doi: 10.1152/ajpheart.1997.273.4.H1745. [DOI] [PubMed] [Google Scholar]
- YATANI A., BROWN A.M. Rapid β-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989;245:71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- ZHANG H., HE C., YAN X., MIRSHAHI T., LOGOTHETIS D.E. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]