Abstract
Marijuana's appetite-increasing effects have long been known. Recent research suggests that the CB1 cannabinoid receptor antagonist SR141716A may suppress appetite. This study represents a further, systematic investigation of the role of CB1 cannabinoid receptors in the pharmacological effects of cannabinoids on food intake.
Mice were food-restricted for 24 h and then allowed access to their regular rodent chow for 1 h. Whereas the CB1 antagonist SR141716A dose-dependently decreased food consumption at doses that did not affect motor activity, Δ9-tetrahydrocannabinol (Δ9-THC) increased food consumption at doses that had no effect on motor activity. O-3259 and O-3257, structural analogs of SR141716A, produced effects similar to those of the parent compound.
Amphetamine (a known anorectic) and diazepam (a benzodiazepine and CNS depressant) decreased food consumption, but only at doses that also increased or decreased motor activity, respectively. The CB2 cannabinoid receptor antagonist SR144528 and the nonpsychoactive cannabinoid cannabidiol did not affect food intake nor activity.
SR141716A decreased feeding in wild-type mice, but lacked pharmacological activity in CB1 knockout mice; however, basal food intake was lower in CB1 knockout mice. Amphetamine decreased feeding in both mouse genotypes.
These results suggest that SR141716A may affect the actions of endogenous cannabinoids in regulating appetite or that it may have effects of its own aside from antagonism of cannabinoid effects (e.g., decreased feeding behavior and locomotor stimulation). In either case, these results strongly suggest that CB1 receptors may play a role in regulation of feeding behavior.
Keywords: Cannabinoids, feeding, SR141716A
Introduction
Obesity has been identified as one of the top 10 global health problems by the World Health Organization and may soon exceed smoking as the primary cause of preventable death in the U.S. (Mokdad et al., 2004). One important contributor to obesity is increased caloric intake without concomitant increase in activity. In humans and other mammals, appetite regulation is a complex physiological process that involves interactions among many neuromodulatory systems (e.g., Chiesi et al., 2001). Leptin, a key regulator of feeding behavior, is found mainly in white adipose tissue and is released into circulation on feeding. Upon reaching the hypothalamus, leptin binds to receptors on neurons that ultimately link to neuromodulatory pathways that release anorexigenic peptides, pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript, or orexigenic peptides, neuropeptide Y and agouti-related protein. Interestingly, in this regard, it has been reported that (a) levels of endocannabinoids (i.e., anandamide and/or 2-arachidonoyl glycerol (2-AG)) are under the negative control of the hormone leptin and (b) obese mice or rats with congenitally disrupted leptin signaling show higher amounts of endocannabinoids in the hypothalamus (Di Marzo et al., 2001). Further, endocannabinoid levels in the limbic forebrain and hypothalamus are increased in hungry rats and return to basal levels when rats are satiated (Kirkham et al., 2002).
These findings and other recent evidence suggest that the endogenous cannabinoid system may also play a role in appetite regulation (for a review see Cota et al., 2003a). Pharmacological manipulation of this system through exogenous administration of cannabinoid agonists, such as Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive substituent of the marijuana plant, revealed increased food intake in humans and rodents (Williams et al., 1998; Koch, 2001; Hart et al., 2002). In contrast, SR141716A, the prototypic antagonist of CB1 cannabinoid receptors, decreased food intake (Rowland et al., 2001; McLaughlin et al., 2003) and is presently undergoing Phase III clinical trials as an appetite suppressant. The present study represents a further, systematic investigation of the role of CB1 cannabinoid receptors in the pharmacological effects of cannabinoids on food intake and supports the hypothesis that the endogenous cannabinoid system is involved in appetite regulation.
Methods
Subjects
All compounds were tested in adult male ICR mice (25–32 g), obtained from Harlan (Dublin, VA, U.S.A.), that were housed in groups of five. SR141716A and amphetamine were also tested in male and female CB1 knockout (CB1−/−) and wild-type (CB1+/+) mice, bred on a C57BL/6 background, as described previously (Zimmer et al., 1999). These mice were derived from breeding pairs of heterozygotes (obtained from A. Zimmer, National Institute of Mental Health, Bethesda, MD, U.S.A.) and were born at Virginia Commonwealth University. All animals were kept in a temperature-controlled (20–22°C) environment with a 12-h light–dark cycle (lights on at 7 a.m.). For the feeding experiments, each ICR mouse was tested with each dose of a single drug, presented in randomized Latin square order. CB1 knockout and wild-type mice were tested with all doses of SR141716A and then, with all doses of amphetamine, presented in randomized order. Separate mice were used for testing each dose of each compound in the locomotor activity studies. The studies reported in this manuscript were carried out in accordance with the guidelines published in the guide for the care and use of laboratory animals (National Research Council, 1996).
Apparatus
The weight of food pellets was measured with a Mettler AT261 Delta Range scale (Toledo, OH, U.S.A.) at 0.01 mg accuracy. Assessment of spontaneous activity in mice occurred in standard activity chambers interfaced with a Digiscan Animal Activity Monitor (Omnitech Electronics, Inc., Columbus, OH, U.S.A.).
Procedure
Feeding trials normally occurred on Tuesdays and Fridays between 12:00 and 14:00 h. At 24 h before the start of a feeding trial, all food was removed from the home cages of mice to be tested. The next day mice were transported to the laboratory at least 1 h before the beginning of the feeding trial. They were injected with the test compound at the specified pre-session injection interval. Subsequently, they were placed in a clear plastic cage with thick brown paper lining the bottom and allowed access to a pre-measured amount of their regular lab chow. At the end of 1 h, mice were removed from the test cage and placed back into their home cage. The amount of food left in the test cage, including crumbs, was measured, and amount consumed was calculated. Mice received no more than two feeding trials per week, separated by at least 72 h.
Locomotor trials occurred in a different laboratory. For these tests, mice were injected intraperitoneally (i.p.) at the same pre-session injection interval as for the feeding trial and were placed in individual activity chambers and spontaneous activity was measured for 10 min. Activity was measured as the total number of interruptions of 16 photocell beams per chamber during the 10-min test and expressed as % inhibition of activity of the vehicle group.
Drugs
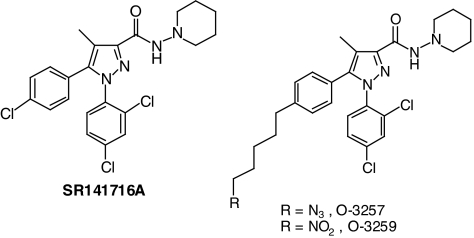
Δ9-THC, SR141716A, SR144528, and cannabidiol were obtained from the National Institute on Drug Abuse (Rockville, MD, U.S.A.). They were suspended in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ, U.S.A.), and saline in a ratio of 1 : 1 : 18. The two pyrazole analogs, O-3257 and O-3259 (Figure 1), were synthesized in our lab (Organix, Inc., Woburn, MA, U.S.A.) and were also prepared in 1 : 1 : 18 vehicle. Diazepam (Elkin-Sinn, Cherry Hill, NJ, U.S.A.) was purchased commercially in a concentration of 5 mg ml−1. A vehicle of ethanol : propylene glycol : distilled water in a 1 : 4 : 5 volume ratio was used to dilute this stock concentration to lower doses. D-Amphetamine (NIDA) was dissolved in physiological saline. All drugs were administered to the mice i.p. at a volume of 0.1 ml for every 10 g body weight. Pre-session injection times were based on our previous experience with these compounds, were identical for the feeding and locomotor studies, and were as follows: 60 min for SR141716A, O-3257, O-3259, and SR144528; 30 min for Δ9-THC and cannabidiol; and 15 min for amphetamine and diazepam.
Figure 1.
Chemical structures of SR141716A, O-3257, and O-3259.
Statistical analysis
For each dose–effect curve in the feeding study, a repeated-measures analysis of variance (ANOVA) was used to analyze the food intake (in grams). For the locomotor activity experiments, separate independent sample ANOVAs were used to analyze the activity across doses for each compound. Tukey post hoc tests (α=0.05) were used to specify differences revealed by significant ANOVAs.
Results
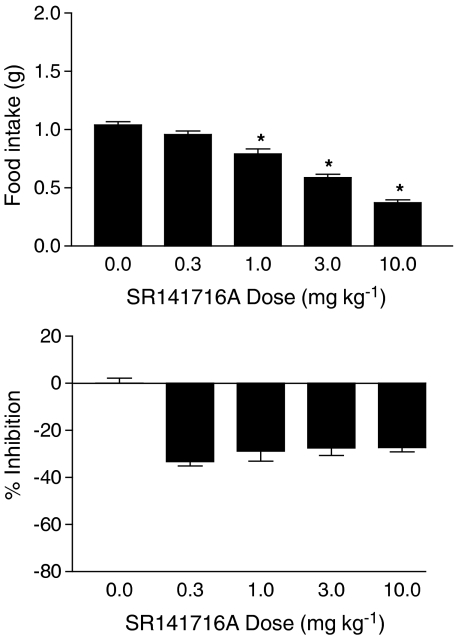
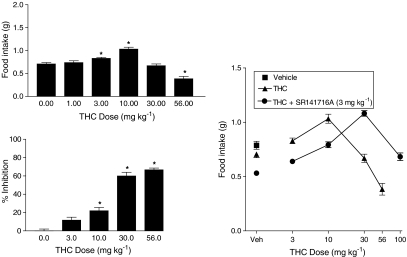
Figure 2 shows the effects of SR141716A on food intake (top panel) and % inhibition of locomotor activity (bottom panel). SR141716A produced a significant dose-dependent decrease in food intake. Although some minimal stimulation of locomotor activity was observed following administration of SR141716A, this effect was not significant, nor was it dose-dependent. In contrast, Δ9-THC significantly and dose-dependently increased food intake at doses up to 10 mg kg−1 (Figure 3, top panel), but significantly decreased food intake at a higher dose of 56 mg kg−1. At doses of 10 mg kg−1 and higher, Δ9-THC significantly decreased locomotor activity (Figure 3, bottom left panel). When administered in combination with Δ9-THC, 3 mg kg−1 SR141716A shifted the Δ9-THC dose–effect curve for food intake to the right (Figure 3, right panel), suggesting that SR141716A was acting as an antagonist in this model.
Figure 2.
Effects of SR141716A on food intake (top panel) and % inhibition of locomotor activity (bottom panel). Bars represent the mean (±s.e.m.) of data from the same 10 mice at each dose for food intake and data from five separate mice at each dose for assessment of locomotor activity. * indicates significant difference from vehicle control (P<0.05). (Note: On all graphs that illustrate % inhibition, negative numbers represent stimulation of locomotor activity.)
Figure 3.
Effects of Δ9-THC on food intake (top panel) and % inhibition of locomotor activity (bottom left panel). Also shown are the effects of combination of 3 mg kg−1 SR141716A and different doses of Δ9-THC (bottom right panel). Bars represent the mean (±s.e.m.) of data from the same 9–10 mice across doses for food intake (but different mice for each Δ9-THC dose–effect curve) and data from six separate mice at each dose of each drug for assessment of locomotor activity. * indicates significant difference from vehicle control (P<0.05).
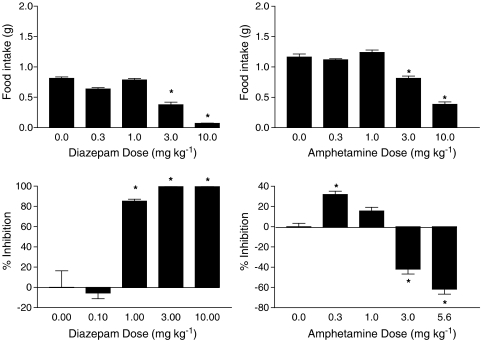
Figure 4 shows the results of tests with diazepam (left panels) and amphetamine (right panels). Although both drugs significantly decreased food intake at higher doses (top panels), these decreases were accompanied by significant changes in locomotor activity (bottom panels). Diazepam produced substantial inhibition of locomotor activity at a dose of 1 mg kg−1 and almost complete elimination of activity at doses of 3 and 10 mg kg−1. In contrast, amphetamine significantly stimulated locomotor activity at higher doses.
Figure 4.
Effects of diazepam (left panels) and amphetamine (right panels) on food intake (top panels) and % inhibition of locomotor activity (bottom panels). Bars represent the mean (±s.e.m.) of data from the same 10 mice across doses for food intake (but different mice for each drug) and data from 5–6 separate mice at each dose of each drug for assessment of locomotor activity. * indicates significant difference from vehicle control (P<0.05).
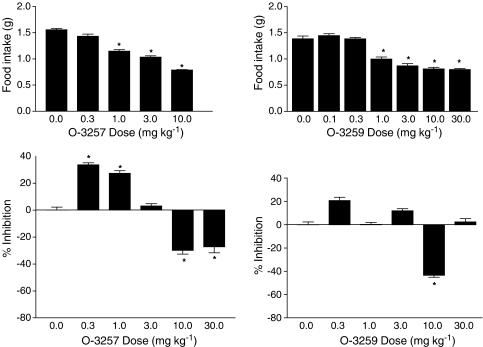
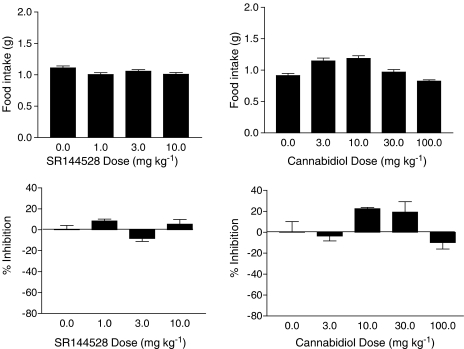
O-3257 and O-3259, two structural analogs of SR141716A, also significantly decreased food intake in this model (Figure 5, top panels); however, unlike diazepam and amphetamine, both compounds did so at one or more doses that did not significantly compromise locomotor activity. O-3257 had a biphasic effect on locomotor activity (Figure 5, bottom left panel), significantly decreasing the activity at lower doses and significantly increasing the activity at higher doses. Overall, O-3259 had little effect on locomotor activity (Figure 5, bottom right panel), except at a dose of 10 mg kg−1 where it significantly stimulated activity. In contrast with these results, tests with the CB2 antagonist SR144528 and the nonpsychoactive cannabinoid cannabidiol resulted in no significant effects on food intake (Figure 6, top panels) or on locomotor activity (Figure 6, bottom panel).
Figure 5.
Effects of two structural analogs of SR141716A, O-3257 (left panels) and O-3259 (right panels), on food intake (top panels) and % inhibition of locomotor activity (bottom panels). Bars represent the mean (±s.e.m.) of data from the same 10 mice across doses for food intake (but different mice for each analog) and data from 4–5 separate mice at each dose of each analog for assessment of locomotor activity. * indicates significant difference from vehicle control (P<0.05).
Figure 6.
Effects of the CB2 antagonist SR144528 (left panels) and cannabidiol (right panels) on food intake (top panels) and % inhibition of locomotor activity (bottom panels). Bars represent the mean (±s.e.m.) of data from the same 10 mice across doses for food intake (but different mice for each drug) and data from five separate mice at each dose of each drug for assessment of locomotor activity. * indicates significant difference from vehicle control (P<0.05).
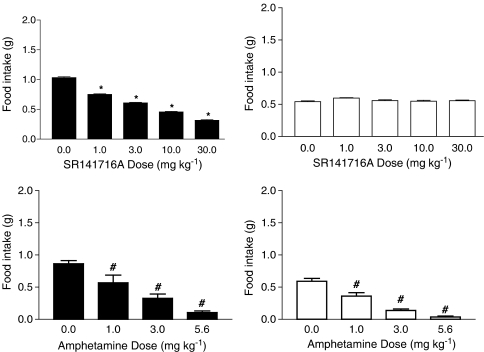
Figure 7 shows the effects of SR141716A (top panels) and amphetamine (bottom panels) in wild-type and CB1 knockout mice (left and right panels, respectively). In C57BL/6 wild-type mice, SR141716A and amphetamine produced significant decreases in food intake similar to those produced by these drugs in ICR mice. Two major differences in these patterns were observed with CB1 knockout mice. The first difference was that baseline food intake under vehicle conditions was significantly decreased for CB1 knockout mice as compared to that of wild-type mice. The second difference was that SR141716A did not have any effect on food intake in CB1 knockout mice. In contrast, amphetamine's effect on food intake was similar in both mouse strains.
Figure 7.
Effects of SR141716A (top panels) and amphetamine (bottom panels) in wild-type (left panels) and CB1 knockout (right panels) mice. Bars represent the mean (±s.e.m.) of data from the same six CB1 knockout and six wild-type mice across all doses of both drugs. For each test drug, a main effect of genotype was found (P<0.05), indicating that basal food intake for CB1 knockout mice was decreased compared to that of wild-type mice. * indicates significant difference from vehicle control (P<0.05). # indicates significant difference from vehicle control (main effect of amphetamine dose) (P<0.05).
Discussion
The results of the present study show that SR141716A and two of its pyrazole analogs (O-3257 and O-3259) dose-dependently decreased food intake in moderately food-restricted mice over a dose range that had no significant effect on locomotor activity. The binding affinities of O-3257 and O-3259 for the CB1 cannabinoid receptor are similar to that of SR141716A (Ki=1.98±0.36 (Rinaldi-Carmona et al., 1994) as compared to Ki=2.2±0.17 for O-3257 and Ki=1.2±0.05 for O-3259 (B.R. Martin and J.L. Wiley, unpublished observations)), as is the minimal effective dose for reduced food intake for all three pyrazoles (1 mg kg−1). Consistent with these results, a third pyrazole analog of SR141716A, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), has also been shown to decrease feeding in rats (McLaughlin et al., 2003) and in two mouse models of obesity, but not in lean mice controls that were not food restricted (Zhou & Shearman, 2004). SR141716A itself has been found to decrease food intake in deprived and non-deprived rats (Rowland et al., 2001; McLaughlin et al., 2003; De Vry et al., 2004; Verty et al., 2004), in wild-type mice (Di Marzo et al., 2001; Poncelet et al., 2003), in mouse pups (Fride et al., 2001; 2003), in lean and obese (fa/fa) Zucker rats (Vickers et al., 2003), and in a diet-induced obesity model in mice (Ravinet-Trillou et al., 2003). It also decreased the intake of other oral reinforcers such as sucrose and ethanol (Arnone et al., 1997; Higgs et al., 2003). Further, the anorectic effect was selective for the CB1 cannabinoid receptor antagonist SR141716A and its analogs, as administration of the CB2 cannabinoid receptor antagonist SR144528 did not change food intake. Together, these results strongly suggest that SR141716A and other pyrazole-based cannabinoids decrease feeding via their interaction with CB1 cannabinoid receptors, either through antagonism of the effects of increased endocannabinoids in the brains of hungry rats or through production of opposing effects to those of endocannabinoids (i.e., inverse agonism). An action on peripheral CB1 cannabinoid receptors may also be involved. Gomez et al. (2002) reported that anandamide levels were increased in the small intestine in food-deprived rats (as compared to basal levels during satiation). Further, they reported that systemic, but not central, administration of SR141716A reduced food intake in these rats. Destruction of the sensory neurons enervating the gut resulted in failure to observe SR141716A-induced decreases and cannabinoid agonist-induced increases in feeding. While these results seem to suggest that a primary peripheral effect of SR141716A on CB1 cannabinoid receptors might be responsible for its hypophagic effect, SR141716A action on brain CB1 cannabinoid receptors cannot be discounted. For example, Kirkham et al. (2002) found that site-selective, bilateral administration of 2-AG into the nucleus accumbens shell produced increased feeding that was reversed by SR141716A. Failure to observe an anorectic effect of centrally administered SR141716A in the Gomez et al. (2002) study may have resulted from lack of diffusion to relevant brain areas after i.c.v. injection.
The similarity of results with structurally distinct cannabinoid antagonists suggest that food intake may be regulated at least in part by antagonist effects on endocannabinoid modulation of appetite, a hypothesis that has received additional indirect support from studies that have reported leptin-induced regulation of anandamide levels in the hypothalamus (Di Marzo et al., 2001), SR141716A-induced decreases in leptin (Ravinet-Trillou et al., 2003), increased endocannabinoid levels in limbic forebrain and hypothalamus of hungry rats (Kirkham et al., 2002; Hanus et al., 2003), and SR141716A antagonism of increases in food intake produced by exogenously administered anandamide and 2-AG (Williams & Kirkham, 1999; Kirkham et al., 2002). Our finding that the baseline food intake of CB1 knockout mice was significantly reduced as compared with wild-type mice is also consistent with this hypothesis. Further reduction of food intake was observed when CB1 knockout mice were injected with amphetamine, but not with SR141716A, suggesting that the response of these mice to a noncannabinoid anorectic drug resembles that of wild-type mice. These results are consistent with previous results in which Di Marzo et al. (2001) found that food-restricted CB1 knockout mice showed decreased baseline feeding behavior when they were maintained on a reverse light–dark cycle, but showed no further decrease when SR141716A was administered. In another study, Cota et al. (2003b) reported that a reduction of caloric intake in non-restricted CB1 knockout mice occurred across development. The decreased food intake in these mice was accompanied by alterations in neuropeptides in the hypothalamus that are involved in appetite regulation. Interestingly, CB1 knockout mice were not sensitive to overeating induced by exogenous administration of neuropeptide Y, as were wild-type mice (Poncelet et al., 2003).
In contrast with SR141716A and its analogs, the cannabinoid agonist Δ9-THC increased food intake. Anecdotal reports and historical accounts suggest that the stimulatory effects of marijuana on appetite in humans have long been known (e.g., Kalant, 2001). Indeed, oral formulations of Δ9-THC are currently used therapeutically to treat cachexia in cancer and AIDS patients (Beal et al., 1995; Jatoi et al., 2002). In rodents, Δ9-THC and Δ8-THC also increased food intake (present study; Williams et al., 1998; Koch, 2001; Williams & Kirkham, 2002; Avraham et al., 2004). Further, this effect was selective; that is, the dose range at which Δ9-THC produced this effect did not entirely overlap that at which it decreased locomotor activity. In contrast, cannabidiol, a nonpsychoactive cannabinoid with little affinity for the CB1 cannabinoid receptor (Ki=2283±453; Thomas et al., 1998), failed to alter either food intake or locomotor activity at doses that were up to 10-fold greater than those used with Δ9-THC, suggesting activation of CB1 cannabinoid receptors is necessary to increase food intake and suppress locomotion. This hypothesis is strengthened by our observation that SR141716A produced a rightward shift in the Δ9-THC dose–effect function.
In summary, the results of the present study strongly suggest that cannabinoid regulation of appetite occurs via interaction with CB1 cannabinoid receptors. Although not entirely conclusive, evidence available to date suggests that the appetite suppressive effects of SR141716A and other cannabinoid antagonists occurred as the result of antagonism of the action of endogenous cannabinoids at CB1 cannabinoid receptors in the brain and/or periphery rather than through inverse agonism at these receptors.
Acknowledgments
This research was supported by NIDA grants DA-03672, DA-09789, and DA-08904. During the time this study was conducted, J.J.B. was on internship at VCU from the University of the West of England, Bristol, U.K. D.C.L. is a student at the Virginia Union University, Richmond, Virginia, and is enrolled in the Minority Access to Research Careers (MARC) Program, supported by NIGMS T34 grant GM-08503. We thank Ramona Winckler and Rhys Davies for technical assistance.
Abbreviations
- 2-AG
2-arachidonyl glycerol
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- Δ9-THC
Δ9-tetrahydrocannabinol
References
- ARNONE M., MARUANI J., CHAPERON F., THIEBOT M.H., PONCELET M., SOUBRIE P., LE FUR G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- AVRAHAM Y., BEN-SHUSHAN D., BREUER A., ZOLOTAREV O., OKON A., FINK N., KATZ V., BERRY E.M. Very low doses of Δ8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacol. Biochem. Behav. 2004;77:675–684. doi: 10.1016/j.pbb.2004.01.015. [DOI] [PubMed] [Google Scholar]
- BEAL J.E., OLSON R., LAUBENSTEIN L., MORALES J.O., BELLMAN P., YANGCO B., LEFKOWITZ L., PLASSE T.F., SHEPARD K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manage. 1995;10:89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- CHIESI M., HUPPERTZ C., HOFBAUER K.G. Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol. Sci. 2001;22:247–254. doi: 10.1016/s0165-6147(00)01664-3. [DOI] [PubMed] [Google Scholar]
- COTA D., MARSICANO G., LUTZ B., VICENNATI V., STALLA G.K., PASQUALI R., PAGOTTO U. Endogenous cannabinoid system as a modulator of food intake. Int. J. Obes. 2003a;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- COTA D., MARSICANO G., TSCHOP M., GRUBLER Y., FLACHSKAMM C., SCHUBERT M., AUER D., YASSOURIDIS A., THONE-REINEKE C., ORTMANN S., TOMASSONI F., CERVINO C., NISOLI E., LINTHORST A.C.E., PASQUALI R., LUTZ B., STALLA G.K., PAGOTTO U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003b;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRY J., SCHREIBER R., ECKEL G., JENTZSCH K.R. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur. J. Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., GOPARAJU S.K., WANG L., LIU J., BATKAI S., JARAI Z., FEZZA F., MIURA G.I., PALMITER R.D., AUGIURA T., KUNOS G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- FRIDE E., FOOX A., ROSENBERG E., FAIGENBOIM M., COHEN V., BARDA L., BLAU H., MECHOULAM R. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: evidence for a ‘CB3' receptor. Eur. J. Pharmacol. 2003;461:27–34. doi: 10.1016/s0014-2999(03)01295-0. [DOI] [PubMed] [Google Scholar]
- FRIDE E., GINZBURG Y., BREUER A., BISOGNO T., DI MARZO V., MECHOULAM R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001;419:207–214. doi: 10.1016/s0014-2999(01)00953-0. [DOI] [PubMed] [Google Scholar]
- GOMEZ R., NAVARRO M., FERRER B., TRIGO J.M., BILBAO A., DEL ARCO I., CIPPITELLI A., NAVA F., PIOMELLI D., RODRIGUEZ DE FONSECA F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANUS L., AVRAHAM Y., BEN-SHUSHAN D., ZOLOTAREV O., BERRY E.M., MECHOULAM R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003;983:144–151. doi: 10.1016/s0006-8993(03)03046-4. [DOI] [PubMed] [Google Scholar]
- HART C.L., WARD A.S., HANEY M., COMER S.D., FOLTIN R.W., FISCHMAN M.W. Comparison of smoked marijuana and oral Δ9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- HIGGS S., WILLIAMS C.M., KIRKHAM T.C. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after Δ9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology. 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- JATOI A., WINDSCHITL H.E., LOPRINZI C.L., SLOAN J.A., DAKHIL S.R., MAILLIARD J.A., PUNDALEEKA S., KARDINAL C.G., FITCH T.R., KROOK J.E., NOVOTNY P.J., CHRISTENSEN B. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J. Clin. Oncol. 2002;20:567–573. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- KALANT H. Medicinal use of cannabis: history and current status. Pain Res. Manage. 2001;6:80–91. doi: 10.1155/2001/469629. [DOI] [PubMed] [Google Scholar]
- KIRKHAM T.C., WILLIAMS C.M., FEZZA F., DI MARZO V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH J.E. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol. Biochem. Behav. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN P.J., WINSTON K., SWEZEY L., WISNIECKI A., ABERMAN J., TARDIF D.J., BETZ A.J., ISHIWARI K., MAKRIYANNIS A., SALAMONE J.D. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav. Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- MOKDAD A.H., MARKS J.S., STROUP D.F., GERBERDING J.L. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- NATIONAL RESEARCH COUNCIL . Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- PONCELET M., MARUANI J., CALASSI R., SOUBRIE P. Overeating, alcohol and sucrose consumption descrease in CB1 receptor deleted mice. Neurosci. Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- RAVINET-TRILLOU C., ARNONE M., DELGORGE C., GONALONS N., KEANE P., MAFFRAND J.P., SOUBRIE P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR1417116A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- ROWLAND N.E., MUKHERJEE M., ROBERTSON K. Effects of the cannabinoid receptor antagonist SR141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology. 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- THOMAS B.F., GILLIAM A.F., BURCH D.F., ROCHE M.J., SELTZMAN H.H. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J. Pharmacol. Exp. Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- VERTY A.N.A., MCGREGOR I.S., MALLET P.E. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci. Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- VICKERS S.P., WEBSTER L.J., WYATT A., DOURISH C.T., KENNETT G.A. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.M., KIRKHAM T.C. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.M., KIRKHAM T.C. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol. Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.M., ROGERS P.J., KIRKHAM T.C. Hyperphagia in pre-fed rats following oral Δ9-THC. Physiol. Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- ZHOU D., SHEARMAN L.P. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol. Biochem. Behav. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., HOHMANN A.G., HERKENHAM M., BONNER T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]