Abstract
Uniaxial cyclic stretch leads to an upregulation of cyclooxygenase (COX)-2 through increases in the intracellular Ca2+ concentration via the stretch-activated (SA) channel and following nuclear factor kappa B (NF-κB) activation in human fibroblasts. However, the signaling mechanism as to how the elevated Ca2+ activates NF-κB is unknown. In this study, we examined the involvement of reactive oxygen species (ROS) as an intermediate signal, which links the elevated Ca2+ with NF-κB activation.
4-Hydroxy-2-nonenal (HNE) was produced and modified IκB peaking at 2 min. The phosphorylation of IκB peaked at 8 min. HNE modification and IκB phosphorylation, NF-κB translocation to the nucleus, and following COX-2 production were inhibited by extracellular Ca2+ removal or Gd3+ application, as well as by the antioxidants. The stretch-induced Ca2+ increase was inhibited by extracellular Ca2+ removal, or Gd3+ application.
IκB kinase (IKK) activity peaked at 4 min, which was inhibited by extracellular Ca2+ removal, Gd3+ or the antioxidants. IKK was also HNE-modified and, similarly to IκB, peaked at 2 min. IKK under static conditions was activated by exogenously applied HNE at a relatively low dose (1 μM), while it was inhibited at higher concentrations, suggesting that HNE could be one of the candidate signals in the stretch-induced NF-κB activation.
The present study suggests that the NF-κB activation by cyclic stretch is mediated by the following signal cascade: SA channel activation → intracellular Ca2+ increase → production of ROS → activation of IKK → phosphorylation of IκB → NF-κB translocation to the nucleus.
Keywords: Cyclic stretch, fibroblast, calcium, reactive oxygen species, 4-hydroxy-2-nonenal, IκB kinase, NF-κB, antioxidants
Introduction
Mechanical stimuli to cells elicit a variety of biochemical and morphologic reactions in many cell types (Sumpio & Widmann, 1990; Awolesi et al., 1995; Suzuki et al., 1997). Sometimes, they are closely related to pathogenesis. For example, repetitive mechanical stimuli trigger inflammation such as tendonitis, bursitis, and fascitis by increasing prostaglandin (PG) E2 production (Ngan et al., 1988; Vandenburgh et al., 1990; Almekinders et al., 1993).
It has been reported that PGE2 production (Vandenburgh et al., 1990) and cyclooxygenase (COX) activity (Vandenburgh et al., 1995) in skeletal muscle cells will be promoted 4–6 h after the onset of mechanical stretch. COX is a key regulatory enzyme that transforms arachidonate and other 20-carbon polyunsaturated fatty acids into endoperoxides (eventually into PGs). COX exists in at least two isoforms: COX-1 and COX-2 (Mitchell et al., 1995). COX-2 expression is induced by inflammatory stimuli, hormones and mitogens, such as transforming growth factor β (TGF-β) (Sumitani et al., 1989; Jackson et al., 1993; Roy et al., 1996), interleukin-1 (IL-1) (Hla & Neilson, 1992; Jackson et al., 1993; Roy et al., 1996), and tumor necrosis factor α (TNF-α) (Yamamoto et al., 1995). We previously reported that uniaxial cyclic stretch (Kato et al., 1998) promoted COX-2, but not COX-1, protein expression in human lung fibroblasts. We also found that uniaxial cyclic stretch increased intracellular Ca2+ concentration via stretch-activated (SA) channel activation, leading to nuclear factor kappa B (NF-κB) activation followed by COX-2 expression (Inoh et al., 2002).
However, little is known about the intermediate signaling mechanism between the SA channel and NF-κB activation. In unstimulated cells, NF-κB dimers exist in an inactive form by binding with the inhibitory protein, IκB. When cells are stimulated by phorbol esters (Sen & Baltimore, 1986), TNF-α (Griffin et al., 1989; Beg et al., 1993; Baeuerle & Henkel, 1994; Siebenlist et al., 1994; DiDonato et al., 1995; Baldwin, 1996; Kurokouchi et al., 1998), IL-1 (Freimuth et al., 1989; Osborn et al., 1989; Beg et al., 1993; DiDonato et al., 1995), UV light (Stein et al., 1989a, 1989b), hypoxia (Schmedtje et al., 1997), or lipopolysaccharide (Cordle et al., 1993), IκB will be released from NF-κB dimer and the freed dimers will be translocated into the nucleus.
Reactive oxygen species (ROS) have been proposed as messengers that lead to NF-κB activation (Schreck et al., 1991), and it is now widely acknowledged that NF-κB is an oxidative stress-responsive transcription factor in eucaryotic cells (Schreck et al., 1992). Intracellular ROS have been shown to initiate a tyrosine kinase cascade resulting in activation of the IκB kinases (IKKs) and subsequent dissociation and degradation of IκB (Barchowsky et al., 1995; Kamata & Hirata, 1999). It is also increasingly recognized that many cellular signaling pathways are oxidation-sensitive. Recently, it was reported that an intracellular Ca2+ increase augmented ROS production, leading to NF-κB and STAT-3 activations in mitochondria (Gong et al., 2001). It was also shown that mechanical stretch against cells activates IKK, which phosphorylates IκB, leading to release of IκB from NF-κB dimers and their translocation to the nucleus (Kobayashi et al., 2003).
Collectively, it is highly likely that mechanically induced ROS production will activate IKKs leading to NF-κB activation. The aim of the present study was to prove this hypothesis.
Methods
Cell preparation
Human lung fibroblasts (TIG-1) were obtained from the Japanese Cancer Research Resource Bank (JCRB0501, Tokyo, Japan). We transferred the cells (23–28 passage) onto a 10 cm2 fibronectin-coated elastic silicone chamber at a density of 5 × 104 cell cm−2, which were subcultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU ml−1 penicillin G sodium, and 100 μg ml−1 streptomycin sulfate. The silicone chamber had a 200 μm thick transparent bottom and 5 mm thick sidewalls to prevent narrowing of its bottom center, as described previously (Suzuki et al., 1997; Kato et al., 1998; Naruse et al., 1998a, 1998b, 1998c; Inoh et al., 2002). The silicone chambers were attached to a stretching apparatus, which was driven by a computer-controlled stepping motor (NS-100, Scholar Tech, Inc., Osaka, Japan).
Application of mechanical stretch
The cells were allowed to attach to the chamber bottom for 12 h in DMEM supplemented with 10% FBS, and were incubated for 12 h in modified Eagle's medium (MEM) supplemented with 1% FBS to starve the cells. After the cells were incubated for 30 min in a solution containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM glucose, and 10 mM N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulphonic acid) (HEPES), pH 7.40 (standard external solution, S.E.S.), we applied uniaxial sinusoidal stretch (120% peak-to-peak, at 1 Hz) at 37°C, 5% CO2.
Immunoprecipitation and immunoblotting
After the cyclic stretch application, the cells were washed with ice-cold PBS and lysed in immunoprecipitation (IP)-kinase buffer (50 mM HEPES (pH 8.0), 150 mM NaCl, 25 mM EGTA, 1 mM EDTA, 0.1% Tween 20, and 10% glycerol) containing a cocktail of protease inhibitors (20 μg ml–1 soybean trypsin inhibitor, 2 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, and 100 μg ml−1 phenylmethylsulfonyl fluoride) and phosphatase inhibitors (50 mM NaF and 0.1 mM Na3VO4). The cell lysates were centrifuged at 15,000 r.p.m. for 10 min. Polyclonal anti-IκBα antibody (2 μl) (Santa Cruz, CA, U.S.A.) was added and samples were incubated at 4°C for 1 h with gentle agitation. Then, 20 μl of protein G-Sepharose was added and samples were incubated at 4°C for 1 h with gentle agitation. The resulting immunoprecipitates were washed three times with IP-kinase buffer. The immunoprecipitates were lysed with a sample (1 ×) buffer and separated by 12.5% SDS–polyacrylamide gel electrophoresis (PAGE). The proteins were transferred electrophoretically onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, U.S.A.). The membranes were blocked with Block Ace (Dainihon Seiyaku, Osaka, Japan) and subsequently probed with anti-phospho-IκBα (Ser-32) (Santa Cruz, CA, U.S.A.) or anti-4-hydroxy-2-nonenal (HNE) (JaICA, Shizuoka, Japan) antibodies in PBS containing 5% BSA for 1 h. We detected antibody–antigen complexes using horseradish peroxidase-conjugated goat anti-mouse IgG (1 : 1000 dilution). Immunoreactivity was determined using the ECL plus chemiluminescence reaction (Amersham, Buckinghamshire, U.K.).
IP and in vitro kinase assay
After the cyclic stretch application, the cells were washed with ice-cold PBS and lysed in IP-kinase buffer (50 mM HEPES (pH 8.0), 150 mM NaCl, 25 mM EGTA, 1 mM EDTA, 0.1% Tween 20, and 10% glycerol) containing a cocktail of protease inhibitors (20 μg ml−1 soybean trypsin inhibitor, 2 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, and 100 μg ml−1 phenylmethylsulfonyl fluoride) and phosphatase inhibitors (50 mM NaF and 0.1 mM Na3VO4). The cell lysates were centrifuged at 15,000 r.p.m. for 10 min. Polyclonal anti-IKKα antibody (2 μl) (Santa Cruz, CA, U.S.A.) was added and samples were incubated at 4°C for 1 h with gentle agitation. Then, 20 μl of protein G-Sepharose was added and samples were incubated at 4°C for 1 h with gentle agitation. The resulting immunoprecipitates were washed three times with IP-kinase buffer. The kinase activity was determined at 30°C for 30 min in 30 μl of reaction buffer containing 50 mM HEPES (pH 8.0), 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol (DTT), 10 μM β-glycerophosphate, 1 mM NaF, 0.1 mM PMSF, and 20 μM Na3VO4. Reaction products were separated by 12.5% SDS–PAGE. The proteins were transferred electrophoretically onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, U.S.A.). The membranes were blocked with Block Ace (Dainihon Seiyaku, Osaka, Japan) and subsequently were probed with anti-phospho-IκBα (Ser-32) or anti-IκBα antibodies (1 : 1000 dilution, Santa Cruz, CA, U.S.A.) in PBS containing 5% BSA. The antibody–antigen complexes were detected using horseradish peroxidase-conjugated goat anti-mouse IgG or anti-rabbit IgG (1 : 1000 dilution). Immunoreactivity was determined using the ECL plus chemiluminescence reaction (Amersham, Buckinghamshire, U.K.).
Measurement of cytosolic calcium
The intracellular concentration of free calcium ions ([Ca2+]i) was measured as follows: fibroblasts on silicone membranes were incubated with the fluorescent calcium indicator fura-2 (2-(6-(bis(carboxymethyl)amino)-5-(2-(2-(bis(carboxymethyl)amino)-5-methylphenoxy)ethoxy)-2-benzofuranyl)-5-oxazolecarboxylic acid-, methyl ester; Molecular Probes, Eugene, Oregon, U.S.A.) for 45 min and another 30 min in a solution containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM glucose, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.40), as previously described (Naruse et al., 1998c). [Ca2+]i was measured by the fura-2 method using a fluorescence microscope system (ARUGAS/HiSCA, Hamamatsu Photonics K.K., Hamamatsu, Japan) with a 20 × objective (Zeiss, Fluor 20) as previously described (Naruse et al., 1998c). Fluorescence ratio (R) was calculated from the following equation: R=(F340−B340)/(F380−B380), where F340 and F380 are the emission intensities at 510 nm excited at 340 and 380 nm, respectively, and B340 and B380 are the corresponding autofluorescence values.
Immunostaining and fluorescence microscopy
After the cyclic stretch application, the cells were washed three times with ice-cold PBS and fixed in 4% paraformaldehyde in PBS at room temperature for 20 min. The cells were incubated with 0.2% Triton X-100 in PBS for 3 min and were blocked with Block Ace at 37°C for 30 min. After being washed with PBS, the cells were incubated with anti-RelA antibodies (Santa Cruz, CA, U.S.A.) in PBS at room temperature for 1 h. Then, the cells were washed three times with 0.02% Triton X-100 in PBS and were incubated with fluorophore-labeled rabbit anti-goat IgG (H+L) antibodies (Alexa Fluor 488, Molecular Probes, Eugene, OR, U.S.A.) in PBS at room temperature for 30 min. The immunostaining was observed under an epifluorescence microscope (20 × objective lens, Olympus, Tokyo, Japan).
RNA extraction and RT–PCR analysis
After the stretch application, total RNA was extracted from stretched cells using an RNeasy Mini Kit (Qiagen, Cologne, Germany). The samples were subjected to first-strand synthesis using an oligo (dT) primer and reverse transcriptase (Superscript II (Invitrogen, Carlsbad, CA, U.S.A.)). The PCR was performed in a reaction volume of 20 μl containing 250 nM dNTP, 50 pM of each specific primer, and 2.5 U Taq polymerase. The primers used were as follows (Kato et al., 1998): human COX-1: 5′-TGC CCA GCT CCT GGC CCG CCG CTT-3′ (sense) (303 bp), 5′-GTG CAT CAA CAC AGG CGC CTC TTC-3′ (antisense); human COX-2: 5′-TTC AAA TGA GAT TGT GGG AAA ATT GCT- (305 bp) 3′ (sense), 5′-AGA TCA TCT CTG CCT GAG TAT CTT-3′ (antisense); human glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-TTC ATT GAC CTC AAC TAC AT-3′ (sense) (469 bp), 5′-GAG GGG CCA TCC ACA GTC TT-3′ (antisense).
We amplified GAPDH, a constitutively expressed gene, by PCR and used the expression as an internal control. The specificity of the amplified products for each primer was ensured by the sizes of the products. We then performed 33 cycles of amplification for COX-1, COX-2, and GAPDH following the recommended procedure by Perkin–Elmer (120 s at 95°C, 30 s at 60°C, 1 min at 72°C). After combining 8 μl of the solution containing PCR products with 2 μl loading buffer, the mixed solutions were electrophoresed on a 1.5% agarose gel in the presence of 0.5 pg ml−1 ethidium bromide. Agarose gel images were converted into video images with a CCD camera (Fotodyne, Hartland, WI, U.S.A.). All of the video images were analyzed using an NIH image 1.59 system from Wayne Rasband (National Institutes of Health, Bethesda, MD, U.S.A.). The serial concentration of RT products of COX-1 and COX-2 was tested to conform to the linearity of amplification for each PCR product. A semilogarithmic plot of pixel values from the video images versus cycle number showed that amplification was exponential between cycles 32 and 40 and then reached a plateau. Thus, we used 33 cycles of PCR amplification for further experiments (Kato et al., 1998).
Chemicals
Gadolinium III chloride hexahydrate was purchased from Aldrich Chemical (St Louis, MO, U.S.A.), and on arrival, it was dissolved in distilled water at 1 M and stored at −80°C. Since Gd3+ is unstable, the concentrated Gd3+ was first diluted in distilled water at a concentration of 10 mM water and then diluted in S.E.S to the desired concentration (20 μM), just before use. The removal of extracellular Ca2+ was achieved with 5 mM EGTA. Human plasma fibronectin was purified according to the method of Regnault et al. 1988). N-acetyl-L-cysteine (NAC), α-tocopherol, MG-132, and A23187 were purchased from Calbiochem (San Diego, CA, U.S.A.). HNE was purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). Other chemicals used were of special grade.
Statistics
All values are expressed as mean±standard error (s.e.). Analysis of variance with subsequent Student's t-test was used to determine significant differences in multiple comparisons. P<0.05 was considered significant.
Results
Stretch-dependent phosphorylation of IκB
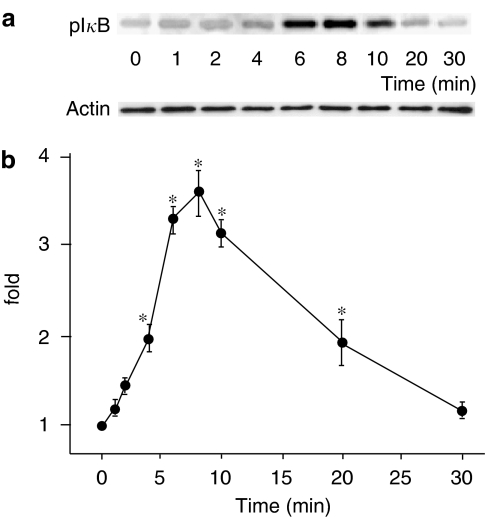
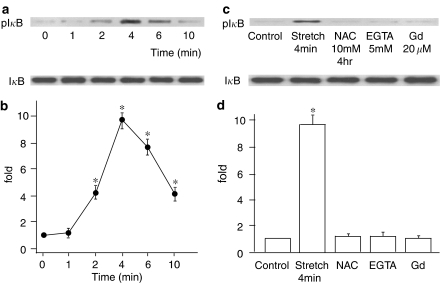
First, we investigated whether uniaxial cyclic stretch would induce IκB phosphorylation, which is a crucial step to NF-κB activation. IκB phosphorylation was examined with IP of IκB, followed by immunoblotting using antibodies against phosphorylated IκB. Figure 1a shows the time course of a typical immunoblot stained with an anti-phospho-IκB Ab (upper) and an anti-actin Ab as a control (lower). Figure 1b shows the time course of IκB phosphorylation normalized by the amount of actin. IκB phosphorylation was considerable 4 min after the onset of cyclic stretch, peaked at 8 min, and returned to the basal level within 30 min.
Figure 1.
(a) Representative immunoblot stained with anti-phospho-IκB Ab (upper) and with actin Ab (lower). (b) Time course of IκB phosphorylation in response to cyclic stretch normalized with the amount of actin. Results are presented as mean±s.e.m. *P<0.05 compared to 0 min.
Stretch-dependent HNE-modification of IκB
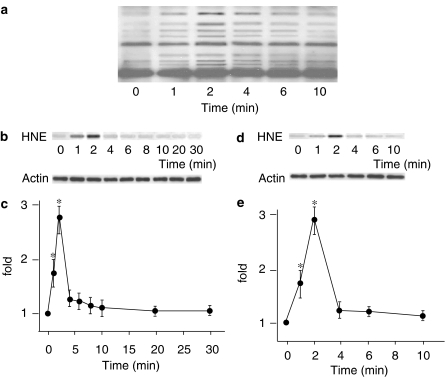
To examine intracellular oxidative stress during stretch, we measured HNE-modification of proteins. Intracellular HNE-modification of proteins in response to uniaxial cyclic stretch was evaluated by immunoblotting using anti-HNE Ab. Immediately after the onset of cyclic stretch, intensively stained bands were detected, suggesting that cyclic stretch rapidly produced oxidative stress to form HNE-modified proteins (Figure 2a). We then measured HNE-modification of IκB with IP using antibodies against IκB, followed by immunoblotting using anti-HNE Ab. Figure 2b shows a typical immunoblot stained with anti-HNE Ab (upper) and anti-actin Ab (lower). Figure 2c shows the time course of HNE-modification of IκB. HNE-modification of IκB became significant 1 min after the onset of cyclic stretch, peaked at 2 min, and returned to the basal level within 4 min. This HNE-modification was not affected by the proteasome inhibitor MG-132 (10 μM), and had quickly returned to the basal level (Figure 2d and e), suggesting that degradation of HNE-modified IκB is not proteasome-dependent but probably caused by intracellular reductive reactions.
Figure 2.
(a) Representative immunoblot stained with anti-4-hydroxy-2-nonenal (HNE) Ab of TIG-1 whole-cell lysate. (b) Representative immunoblot stained with HNE Ab (upper) and with actin Ab (lower). (c) Time course of the formation of HNE-modified IκB in response to cyclic stretch normalized with the amount of actin. Results are presented as mean±s.e.m. *P<0.05 compared to 0 min. (d) Representative immunoblot stained with anti-HNE Ab (upper) and with actin Ab (lower). (e) Effect of MG-132 (10 μM) on the formation of HNE-modified IκB.
Effects of antioxidants on the stretch-induced phosphorylation of IκB and HNE-modified IκB formation
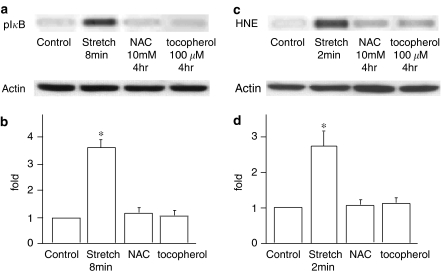
HNE-modification of IκB was observed prior to its phosphorylation (Figures 1 and 2), suggesting that increases in intracellular oxidative stress are upstream of IκB phosphorylation. To confirm this, we investigated the effect of antioxidants on the phosphorylation and HNE-modification of IκB. After pretreatment of cells with NAC, a precursor of glutathione (GSH), and α-tocopherol, a fat-soluble antioxidant that acts as a radical scavenger around the biomembrane, the phosphorylation and HNE-modification of IκB were measured by immunoblotting (Figure 3). As expected, phosphorylation and HNE-modification of IκB were significantly inhibited by these antioxidants, strongly suggesting that an increase in intracellular oxidative stress is upstream of phosphorylation and HNE-modification of IκB.
Figure 3.
(a) Representative immunoblot stained with anti-phospho-IκB Ab (upper) and with actin Ab (lower). (b) Effects of NAC and α-tocopherol on IκB phosphorylation. Results are presented as mean±s.e.m. *P<0.05 compared to control. (c) Representative immunoblot stained with anti-4-hydroxy-2-nonenal (HNE) Ab (upper) and with actin Ab (lower). (d) Effects of NAC and α-tocopherol on HNE-modification of IκB. Results are presented as mean±s.e.m. P<0.05 compared to control.
Involvement of SA channels in the stretch-induced phosphorylation and HNE-modification of IκB
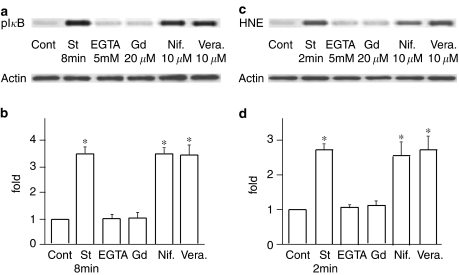
The next question may be what signal would be upstream of the stretch-induced increase in oxidative stress. Considering that ROS production is dependent on the level of intracellular Ca2+ concentration and that activation of Ca2+ permeable SA channels would be the earliest event in the stretch-induced cell responses, it is natural to speculate that SA channel activation and following intracellular Ca2+ increases would be the upstream events. We had previously investigated the involvement of SA channels in various stretch-induced responses such as intracellular Ca2+ mobilization, morphological changes, and enzyme activation (Naruse & Sokabe, 1993; Naruse et al., 1998a, 1998b, 1998c). From these studies, we found that extracellular Ca2+ depletion and Gd3+ application are highly useful in determining the involvement of SA channels in reactions, and used the same protocol in this study. When cells were cyclically stretched in a Ca2+-free solution, phosphorylation and HNE-modification of IκB were significantly inhibited (Figure 4). Similarly, 20 μM of Gd3+ inhibited the stretch-induced phosphorylation and HNE-modification of IκB. To exclude the possibility of the involvement of classical voltage-dependent Ca2+ channels, we examined the effects of Ca2+ blockers like nifedipine (10 μM) or verapamil (10 μM). The phosphorylation and HNE-modification of IκB were not inhibited by these blockers, strongly suggesting that Ca2+ influx, through SA channels and following intracellular Ca2+ increases, plays a crucial role upstream for phosphorylation and HNE-modification of IκB.
Figure 4.
(a) Representative immunoblot stained with anti-phospho-IκB Ab (upper) and with actin Ab (lower). (b) Effects of EGTA, Gd3+, nifedipine and verapamil on IκB phosphorylation. Results are presented as mean±s.e.m. *P<0.05 compared to control. (c) Representative immunoblot stained with anti-4-hydroxy-2-nonenal (HNE) Ab (upper) and with actin Ab (lower). (d) Effects of EGTA, Gd3+, nifedipine and verapamil on HNE-modification of IκB. Results are presented as mean±s.e.m. *P<0.05 compared to control.
Measurement of stretch-induced [Ca2+]i increases
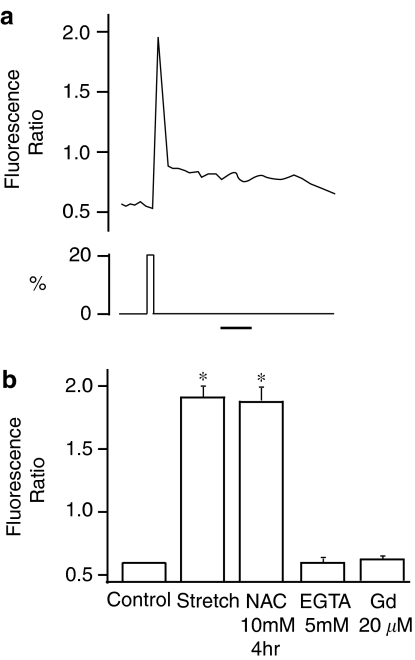
To confirm that mechanical stretch actually increases [Ca2+]i in TIG-1 fibroblasts, we measured [Ca2+]i by fluorescence intensity ratiometry using fura-2 AM as an indicator. Intracellular Ca2+ concentrations were measured while cells were stretched for 3 s. As shown in Figure 5a, a single stretch elicited a transient increase in [Ca2+]i, which declined to the initial basal [Ca2+]i level within a few minutes. This increase in [Ca2+]i was inhibited by the removal of extracellular Ca2+ or by an application of 20 μM Gd3+, but not by NAC (Figure 5b), suggesting that the stretch-induced [Ca2+]i increase is upstream of ROS production.
Figure 5.
(a) Fibroblasts in an elastic silicone chamber were subjected to a single stretch and the change in [Ca2+]i was measured. The trace is a representative of five different experiments. The bar indicates 1 min. (b) Effects of NAC, EGTA and Gd3+ on fluorescence ratio. Results are presented as mean±s.e.m. *P<0.05 compared to control.
To further confirm that intracellular Ca2+ increase is essential in the following increases in oxidative stress, we examined the effects of calcium ionophore (A23187) on the HNE-modification of IκB. When 10 μM A23187, which nonphysiologically increases intracellular Ca2+ concentration, was applied to human lung fibroblast cells, HNE-modification of IκB was enhanced, which was significantly inhibited by pretreatment of cells with antioxidants (Figure 6).
Figure 6.
Representative immunoblot stained with anti-4-hydroxy-2-nonenal (HNE) Ab (upper) and with actin Ab (lower). When calcium ionophore (A23187) was applied to human lung fibroblast cells, HNE-modification of IκB was enhanced, which was significantly inhibited by pretreatment of cells with NAC or α-tocopherol.
Stretch, ROS, and Ca2+-dependent activation of IKKs
Recent reports showed that IκB proteins are specifically phosphorylated by IKKs, leading to IκB ubiquitination and degradation at proteasomes (DiDonato et al., 1996; Karin, 1999). To determine whether cyclic stretch activates IKKs to promote the NF-κB signaling pathway, we examined the time course of the stretch-dependent activation of IKKs using an in vitro kinase assay. The results demonstrated that IKKs were activated 2 min after the onset of cyclic stretch, peaked at 4 min, and gradually decreased (Figure 7a and b). Next, we investigated the effect of antioxidants on the activation of IKKs. Pretreatment of cells with NAC caused the activation of IKKs to be significantly inhibited (Figure 7c and d), indicating that the increase in intracellular oxidative stress caused by cyclic stretch is upstream of IKKs activation. The stretch-induced IKKs activation was also inhibited by extracellular Ca2+ removal or 20 μM Gd3+ application (Figure 7c and d).
Figure 7.
(a) Representative immunoblot probed with anti-phospho-IκB Ab (upper) and with anti-IκBα Ab (lower). (b) The relative kinase activation of IκB kinase (IKK) at various time points normalized to IκBα content. Results are presented as mean±s.e.m. *P<0.05 compared to 0 min. (c) Representative immunoblot probed with anti-phospho-IκB Ab (upper) and with anti-IκBα Ab (lower). (d) Effects of NAC, EGTA and Gd3+ on the relative kinase activation of IKK. Results are presented as mean±s.e.m. *P<0.05 compared to control.
Effects of HNE on IKKs activation
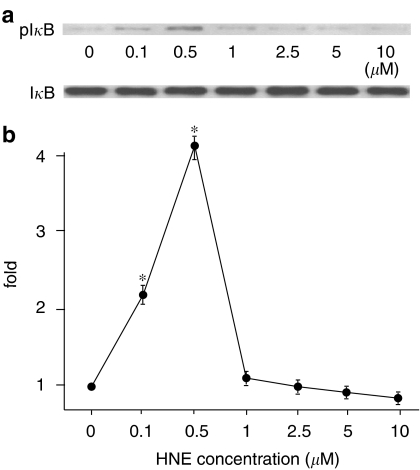
As shown in Figure 2, many proteins were HNE-modified by the cyclic stretch. IKKs were also HNE-modified and peaked at 2 min, which was prevented by NAC, as in the case of IκB. However, several studies have reported that exogenously applied HNE inhibits IKKs activity, which seems inconsistent with our hypothesis that stretch-induced ROS production would activate IKKs leading to an activation of NF-κB. To address this question, we conducted an experiment on the effects of exogenously applied various concentrations of HNE on IKK activity. We obtained interesting results, which appears to resolve the above-mentioned discrepancy. Namely, HNE modulated IKK activity, in a dose-dependent manner, has a biphasic mode. In a low concentration range, less than 1 μM, HNE activated IKK activity, which supports our conclusion in the present study. In contrast, in concentrations higher than 2.5 μM, HNE inhibited IKK activity, which confirms the previously reported inhibitory effects of HNE on IKK activity (Figure 8).
Figure 8.
(a) Representative immunoblot probed with anti-phospho-IκB Ab (upper) and with anti-IκBα Ab (lower). (b) The relative kinase activation of IκB kinase (IKK) at various HNE concentrations normalized to IκBα content. Results are presented as mean±s.e.m. *P<0.05 compared to 0 μM.
Requirement of ROS in stretch-induced NF-κB import and COX-2 expression
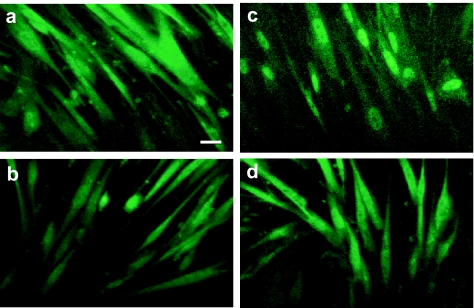
We investigated the involvement of intracellular oxidative stress in the stretch-induced translocation of NF-κB to the nucleus. The immunostaining of Rel A, which is an NF-κB subunit, showed diffuse staining in the cytoplasm in nonstretched cells (Figure 9a), and in stretched cells for 2 min (Figure 9b). Cells that were exposed to uniaxial cyclic stretch for 6 min showed strong nucleus staining in their nucleus, indicating the stretch-induced translocation of NF-κB to the nucleus (Figure 9c). However, cells that had been pretreated with NAC showed diffuse staining in the cytoplasm, which was similar to that in nonstretched cells (Figure 9d). These results strongly suggest that increased intracellular oxidative stress plays a crucial role in the stretch-induced translocation of NF-κB to the nucleus.
Figure 9.
(a) Immunostaining of the NF-κB subunit RelA demonstrated diffuse staining in the cytoplasm of nonstretched cells. (b) The cells exposed to 20% of uniaxial cyclic stretch at 1 Hz for 2 min showed diffuse staining in the cytoplasm. (c) The cells exposed to 20% of uniaxial cyclic stretch at 1 Hz for 6 min showed strong staining in the nucleus. (d) Even subjected to cyclic stretch, cells pretreated with NAC (10 mM, 4 h) showed diffuse staining of NF-κB in the cytoplasm, the same as in nonstretched cells. White bars indicate 10 μm.
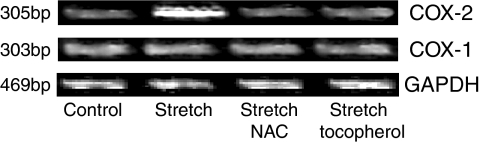
Our previous study showed that COX-2 mRNA level increased in response to uniaxial cyclic stretch, whereas, the levels of COX-1 mRNA and GAPDH mRNA did not change (Kato et al., 1998). To confirm if the increased intracellular oxidative stress is required for the expression of these genes, we investigated the effect of antioxidants such as NAC and α-tocopherol. When cells were cyclically stretched in the presence of NAC and α-tocopherol, the stretch-induced expression of COX-2 mRNA was significantly inhibited, while the levels of COX-1 mRNA and GAPDH mRNA did not change (Figure 10).
Figure 10.
RT–PCR analysis of COX-1, COX-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression during stretch in the presence of antioxidants. When cells were mechanically stretched in the presence of NAC (10 mM, 4 h) and α-tocopherol (100 μM, 4 h), stretch-induced expression of COX-2 mRNA was significantly inhibited, while the levels of COX-1 mRNA and GAPDH mRNA did not change.
Discussion
The present study suggests that the NF-κB activation by uniaxial cyclic stretch in human fibroblast cells is mediated by the following signal cascade: SA channel activation → intracellular Ca2+ increase → production of ROS → activation of IKKs → phosphorylation of IκB → NF-κB translocation to the nucleus.
Recent studies have revealed that mechanical stretch of cells activates NF-κB (Granet et al., 2001; Wang et al., 2001). Our previous study showed that uniaxial cyclic stretch in human lung fibroblasts activated SA channels followed by the translocation of NF-κB to the nucleus, leading to an increase in the COX-2, but not COX-1, expression level (Inoh et al., 2002). However, the signal cascade that links SA channel activation to NF-κB translocation has remained unknown. Gong et al. (2001) reported that the intracellular Ca2+ signal promoted ROS production followed by the translocation of NF-κB to the nucleus. Another study showed that NF-κB is activated by γ-radiation and UV-C radiation via IκB phosphorylation (Li & Karin, 1998). Taking these observations, we speculated that ROS production by intracellular Ca2+ increases caused by SA channel activation would induce IκB phosphorylation, leading to the translocation of NF-κB to the nucleus.
ROS induces membrane lipid peroxidation, initiating a free radical chain reaction. In this process, aldehydes are produced as final products. Of these, HNE, an α,β-unsaturated aldehyde, produced almost exclusively from phospholipid-bound arachidonic acid, has been demonstrated to be a reliable index of ROS-induced lipid peroxidation as indicated by its cytopathologic effects (Esterbauer et al., 1991) and by immunohistochemical staining for HNE-modified proteins (Toyokuni et al., 1994). We have demonstrated here that mechanical stretching of TIG-1 cells elicited proteinic HNE-modification, which was used as the major marker for the intracellular oxidative stress. Results showed that HNE-modification of many proteins including IκB and IKKs was detected at 1 min and peaked at 2 min after the onset of stretch followed by IκB phosphorylation initiation, which peaked at 4 and 8 min. This indicates that in a very short time, uniaxial cyclic stretch increases intracellular oxidative stress. Proteasome inhibitors did not prolong the decline of HNE-modification of IκB, suggesting that the short life of stretch-induced HNE modification is caused by intracellular reductive reactions. IKK activity started to increase 2 min after the onset of stretch reaching its peak at 4 min, which was inhibited by antioxidants. Taken together the time courses in biochemistry and pharmacology, we can reasonably propose the signal cascade: SA channel activation → intracellular Ca2+ increase → production of ROS → activation of IKKs → phosphorylation of IκB → NF-κB translocation to the nucleus.
We do not know whether ROS directly activates IKK. As there was a 2 min delay in the peak time (4 min) of IKK activation compared to that (2 min) of HNE modification, it is possible that some kinases upstream of IKK would be activated by ROS. There are several studies suggesting that IKK is activated by various kinases such as IRAK, TAK1, and NIK (Ninomiya-Tsuji et al., 1999; Sakurai et al., 1999; Jiang et al., 2002; Takaesu et al., 2003). On the other hand, HNE, which was used as a marker for ROS production, may contribute to the stretch-induced IKK activation. Our experiment, in which HNE was added exogenously, revealed that a relatively low dose (<1 μM) of HNE activated IKK. As H2O2 has been known to regulate IKK activity (Kamata et al., 2002), a certain redox control by HNE may play a role in IKK activation. In contrast, many groups have shown that a relatively high dose (>5 μM) of HNE added exogenously blocks IKK activation and IκB phosphorylation, and that HNE modifies IKK (Ji et al., 2001; Donath et al., 2002; Luckey et al., 2002). This apparent contradiction may be explained by the biphasic action of exogenously applied HNE as presented in Figure 8 in which a low dose of HNE activates IKK, while a higher dose inhibits IKK. Which ROS species is the major signal in the stretch-dependent NF-κB activation and which molecule is its main target remains to be determined.
Stretch-induced HNE-modification and phosphorylation of IκB and following NF-κB activation were nearly perfectly inhibited by extracellular Ca2+ depletion and Gd3+ application, but not by the classic Ca2+ channel blockers, nifedipine and verapamil. These results indicate that activation of putative Ca2+-permeable SA channels and following intracellular Ca2+ increases are indispensable for the cyclic stretch-induced NF-κB activation in human lung fibroblasts. Considering the immediate increase in [Ca2+]i in response to stretch and its resistance against antioxidants, SA channel-mediated [Ca2+]i increases should be the most upstream signaling mechanism followed by ROS production. To examine whether increases in [Ca2+]i solely can promote HNE-modification of IκB, we administered the Ca2+ ionophore A23187 to elicit a large amount of increase in [Ca2+]i. It was found that A23187 significantly promoted the HNE-modification of IκB, which was inhibited by antioxidants. This result indicates that the [Ca2+]i increase itself promotes ROS production. Dolmetsch et al. (1997) provided a report supporting our interpretation on the role of [Ca2+]i increase in ROS production. They demonstrated that the amplitude and duration of calcium signals in B lymphocytes control differential activations of proinflammatory transcriptional regulators, NF-κB, c-Jun N-terminal kinase, and nuclear factor of activated T cells (NFAT), and that NF-κB is selectively activated by a large Ca2+ transient. They also showed, in human lung fibroblast cells, that A23187 augmented HNE-modification of IκB, which was sensitive to antioxidants.
Mechanical stretch enhances the PGE2 production, which is known to increase in inflammation induced by repetitive motion such as tendonitis, bursitis, and fascitis, in fibroblast (Ngan et al., 1988; Almekinders et al., 1993) and skeletal muscle cells (Vandenburgh et al., 1990). We previously reported that in human lung fibroblasts, COX-2 expression is upregulated by uniaxial cyclic stretch via SA channel activation (Kato et al., 1998), and that COX-2 expression depends on NF-κB activation (Inoh et al., 2002). The latter is consistent with the fact that the human COX-2 promoter region contains binding sites for the transcription factor NF-κB (Yang et al., 1997). NF-κB activation induces COX-2 expression, which is the first rate-limiting enzyme during PGE2 synthesis in inflammatory cases.
In the present study, we showed that Ca2+-dependent increase in oxidative stress and the following IκB phosphorylation are involved in the SA channel-dependent NF-κB activation pathway.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (#13480216 to MS), Object-Oriented research (#15086207 to MS), and Creative Scientific Research (#16GS0308 to MS) from the Ministry of Education Science Sports and Culture, and a grant from Japan Space Forum (to MS).
Abbreviations
- [Ca2+]i
intracellular concentration of free calcium ions
- GSH
glutathione
- HNE
4-hydroxy-2-nonenal
- IKK
IκB kinase
- IL-1
interleukin-1
- NAC
N-acetyl-L-cysteine
- PG
prostaglandin
- ROS
reactive oxygen species
- SA channel
stretch-activated channel
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor α
References
- ALMEKINDERS L.C., BANES A.J., BALLENGER C.A. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. [PubMed] [Google Scholar]
- AWOLESI M.A., SESSA W.C., SUMPIO B.E. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J. Clin. Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAEUERLE P.A., HENKEL T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- BARCHOWSKY A., MUNRO S.R., MORANA S.J., VINCENTI M.P., TREADWELL M. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am. J. Physiol. 1995;269:L829–L836. doi: 10.1152/ajplung.1995.269.6.L829. [DOI] [PubMed] [Google Scholar]
- BEG A.A., FINCO T.S., NANTERMET P.V., BALDWIN A.S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol. Cell. Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORDLE S.R., DONALD R., READ M.A., HAWIGER J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-kappa B proteins in human monocytic THP-1 cells. J. Biol. Chem. 1993;268:11803–11810. [PubMed] [Google Scholar]
- DIDONATO J., MERCURIO F., ROSETTE C., WU-LI J., SUYANG H., GHOSH S., KARIN M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIDONATO J.A., MERCURIO F., KARIN M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol. Cell. Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLMETSCH R.E., LEWIS R.S., GOODNOW C.C., HEALY J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- DONATH B., FISCHER C., PAGE S., PREBECK S., JILG N., WEBER M., DA COSTA C., NEUMEIER D., MIETHKE T., BRAND K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- ESTERBAUER H., SCHAUR R.J., ZOLLNER H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- FREIMUTH W.W., DEPPER J.M., NABEL G.J. Regulation of the IL-2 receptor alpha-gene. Interaction of a kappa B binding protein with cell-specific transcription factors. J. Immunol. 1989;143:3064–3068. [PubMed] [Google Scholar]
- GONG G., WARIS G., TANVEER R., SIDDIQUI A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANET C., BOUTAHAR N., VICO L., ALEXANDRE C., LAFAGE-PROUST M.H. MAPK and SRC-kinases control EGR-1 and NF-kappa B inductions by changes in mechanical environment in osteoblasts. Biochem. Biophys. Res. Commun. 2001;284:622–631. doi: 10.1006/bbrc.2001.5023. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G.E., LEUNG K., FOLKS T.M., KUNKEL S., NABEL G.J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989;339:70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- HLA T., NEILSON K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOH H., ISHIGURO N., SAWAZAKI S., AMMA H., MIYAZU M., IWATA H., SOKABE M., NARUSE K. Uni-axial cyclic stretch induces the activation of transcription factor nuclear factor kappaB in human fibroblast cells. FASEB J. 2002;16:405–407. doi: 10.1096/fj.01-0354fje. [DOI] [PubMed] [Google Scholar]
- JACKSON B.A., GOLDSTEIN R.H., ROY R., COZZANI M., TAYLOR L., POLGAR P. Effects of transforming growth factor beta and interleukin-1 beta on expression of cyclooxygenase 1 and 2 and phospholipase A2 mRNA in lung fibroblasts and endothelial cells in culture. Biochem. Biophys. Res. Commun. 1993;197:1465–1474. doi: 10.1006/bbrc.1993.2642. [DOI] [PubMed] [Google Scholar]
- JI C., KOZAK K.R., MARNETT L.J. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- JIANG Z., NINOMIYA-TSUJI J., QIAN Y., MATSUMOTO K., LI X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA H., HIRATA H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- KAMATA H., MANABE T., KAKUTA J., OKA S., HIRATA H. Multiple redox regulation of the cellular signaling system linked to AP-1 and NFkappaB: effects of N-acetylcysteine and H2O2 on the receptor tyrosine kinases, the MAP kinase cascade, and IkappaB kinases. Ann. N. Y. Acad. Sci. 2002;973:419–422. doi: 10.1111/j.1749-6632.2002.tb04675.x. [DOI] [PubMed] [Google Scholar]
- KARIN M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J. Biol. Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- KATO T., ISHIGURO N., IWATA H., KOJIMA T., ITO T., NARUSE K. Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem. Biophys. Res. Commun. 1998;244:615–619. doi: 10.1006/bbrc.1998.8335. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., NAGINO M., KOMATSU S., NARUSE K., NIMURA Y., NAKANISHI M., SOKABE M. Stretch-induced IL-6 secretion from endothelial cells requires NF-kappaB activation. Biochem. Biophys. Res. Commun. 2003;308:306–312. doi: 10.1016/s0006-291x(03)01362-7. [DOI] [PubMed] [Google Scholar]
- KUROKOUCHI K., KAMBE F., YASUKAWA K., IZUMI R., ISHIGURO N., IWATA H., SEO H. TNF-alpha increases expression of IL-6 and ICAM-1 genes through activation of NF-kappaB in osteoblast-like ROS17/2.8 cells. J. Bone Miner. Res. 1998;13:1290–1299. doi: 10.1359/jbmr.1998.13.8.1290. [DOI] [PubMed] [Google Scholar]
- LI N., KARIN M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCKEY S.W., TAYLOR M., SAMPEY B.P., SCHEINMAN R.I., PETERSEN D.R. 4-Hydroxynonenal decreases interleukin-6 expression and protein production in primary rat Kupffer cells by inhibiting nuclear factor-kappaB activation. J. Pharmacol. Exp. Ther. 2002;302:296–303. doi: 10.1124/jpet.102.033522. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., LARKIN S., WILLIAMS T.J. Cyclooxygenase-2: regulation and relevance in inflammation. Biochem. Pharmacol. 1995;50:1535–1542. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- NARUSE K., SAI X., YOKOYAMA N., SOKABE M. Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via SA channel activation. FEBS Lett. 1998a;441:111–115. doi: 10.1016/s0014-5793(98)01528-2. [DOI] [PubMed] [Google Scholar]
- NARUSE K., SOKABE M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am. J. Physiol. 1993;264:C1037–C1044. doi: 10.1152/ajpcell.1993.264.4.C1037. [DOI] [PubMed] [Google Scholar]
- NARUSE K., YAMADA T., SAI X.R., HAMAGUCHI M., SOKABE M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene. 1998b;17:455–463. doi: 10.1038/sj.onc.1201950. [DOI] [PubMed] [Google Scholar]
- NARUSE K., YAMADA T., SOKABE M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am. J. Physiol. 1998c;274:H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- NGAN P.W., CROCK B., VARGHESE J., LANESE R., SHANFELD J., DAVIDOVITCH Z. Immunohistochemical assessment of the effect of chemical and mechanical stimuli on cAMP and prostaglandin E levels in human gingival fibroblasts in vitro. Arch. Oral Biol. 1988;33:163–174. doi: 10.1016/0003-9969(88)90041-6. [DOI] [PubMed] [Google Scholar]
- NINOMIYA-TSUJI J., KISHIMOTO K., HIYAMA A., INOUE J., CAO Z., MATSUMOTO K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- OSBORN L., KUNKEL S., NABEL G.J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGNAULT V., RIVAT C., STOLTZ J.F. Affinity purification of human plasma fibronectin on immobilized gelatin. J. Chromatogr. 1988;432:93–102. doi: 10.1016/s0378-4347(00)80636-2. [DOI] [PubMed] [Google Scholar]
- ROY R., POLGAR P., WANG Y., GOLDSTEIN R.H., TAYLOR L., KAGAN H.M. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: interactions among TGF-beta, IL-1 beta, and prostaglandin E. J. Cell Biochem. 1996;62:411–417. doi: 10.1002/(SICI)1097-4644(199609)62:3%3C411::AID-JCB11%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- SAKURAI H., MIYOSHI H., TORIUMI W., SUGITA T. Functional interactions of transforming growth factor beta-activated kinase 1 with IkappaB kinases to stimulate NF-kappaB activation. J. Biol. Chem. 1999;274:10641–10648. doi: 10.1074/jbc.274.15.10641. [DOI] [PubMed] [Google Scholar]
- SCHMEDTJE J.F., Jr, JI Y.S., LIU W.L., DUBOIS R.N., RUNGE M.S. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- SCHRECK R., ALBERMANN K., BAEUERLE P.A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic. Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- SCHRECK R., RIEBER P., BAEUERLE P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN R., BALTIMORE D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- SIEBENLIST U., FRANZOSO G., BROWN K. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- STEIN B., KRAMER M., RAHMSDORF H.J., PONTA H., HERRLICH P. UV-induced transcription from the human immunodeficiency virus type 1 (HIV-1) long terminal repeat and UV-induced secretion of an extracellular factor that induces HIV-1 transcription in nonirradiated cells. J. Virol. 1989a;63:4540–4544. doi: 10.1128/jvi.63.11.4540-4544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN B., RAHMSDORF H.J., STEFFEN A., LITFIN M., HERRLICH P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol. Cell. Biol. 1989b;9:5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMITANI K., KAWATA T., YOSHIMOTO T., YAMAMOTO S., KUMEGAWA M. Fatty acid cyclooxygenase activity stimulated by transforming growth factor-beta in mouse osteoblastic cells (MC3T3-E1) Arch. Biochem. Biophys. 1989;270:588–595. doi: 10.1016/0003-9861(89)90541-9. [DOI] [PubMed] [Google Scholar]
- SUMPIO B.E., WIDMANN M.D.Enhanced production of endothelium-derived contracting factor by endothelial cells subjected to pulsatile stretch Surgery 1990108277–281.; discussion 281–282 [PubMed] [Google Scholar]
- SUZUKI M., NARUSE K., ASANO Y., OKAMOTO T., NISHIKIMI N., SAKURAI T., NIMURA Y., SOKABE M. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem. Biophys. Res. Commun. 1997;239:372–376. doi: 10.1006/bbrc.1997.7364. [DOI] [PubMed] [Google Scholar]
- TAKAESU G., SURABHI R.M., PARK K.J., NINOMIYA-TSUJI J., MATSUMOTO K., GAYNOR R.B. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J. Mol. Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- TOYOKUNI S., UCHIDA K., OKAMOTO K., HATTORI-NAKAKUKI Y., HIAI H., STADTMAN E.R. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDENBURGH H.H., HATFALUDY S., SOHAR I., SHANSKY J. Stretch-induced prostaglandins and protein turnover in cultured skeletal muscle. Am. J. Physiol. 1990;259:C232–C240. doi: 10.1152/ajpcell.1990.259.2.C232. [DOI] [PubMed] [Google Scholar]
- VANDENBURGH H.H., SHANSKY J., SOLERSSI R., CHROMIAK J. Mechanical stimulation of skeletal muscle increases prostaglandin F2 alpha production, cyclooxygenase activity, and cell growth by a pertussis toxin sensitive mechanism. J. Cell. Physiol. 1995;163:285–294. doi: 10.1002/jcp.1041630209. [DOI] [PubMed] [Google Scholar]
- WANG D.S., PROFFIT D., TSAO P.S. Mechanotransduction of endothelial oxidative stress induced by cyclic strain. Endothelium. 2001;8:283–291. doi: 10.3109/10623320109090806. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO K., ARAKAWA T., UEDA N., YAMAMOTO S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- YANG X., HOU F., TAYLOR L., POLGAR P. Characterization of human cyclooxygenase 2 gene promoter localization of a TGF-beta response element. Biochim. Biophys. Acta. 1997;1350:287–292. doi: 10.1016/s0167-4781(96)00225-4. [DOI] [PubMed] [Google Scholar]