Abstract
Substance P (SP) is deeply involved in lung pathophysiology and plays a key role in the modulation of inflammatory-immune processes. We previously demonstrated that SP activates guinea-pig alveolar macrophages (AMs) and human monocytes, but a careful examination of its effects on human AMs is still scarce.
This study was undertaken to establish the role of SP in human AM isolated from healthy smokers and non-smokers, by evaluating the presence of tachykinin NK1 receptors (NK-1R) and SP's ability to induce superoxide anion (O2−) production and cytokine release, as well as activation of the nuclear factor-κB (NF-κB) pathway.
By Western blot analysis and immunofluorescence, we demonstrate that authentic NK-1R are present on human AMs, a three-fold enhanced expression being observed in healthy smokers. These NK-1R are functional, as SP and NK1 agonists dose-dependently induce O2− production and cytokine release. In AMs from healthy smokers, SP evokes an enhanced respiratory burst and a significantly increased release of tumor necrosis factor-α as compared to healthy non-smokers, but has inconsistent effects on IL-10 release. The NK1 selective antagonist CP 96,345 ((2S,3S)-cis-2-diphenylmethyl-N[(2-methoxyphenyl)-methyl]-1-azabicyclo-octan-3-amine)) competitively antagonized SP-induced effects.
SP activates the transcription factor NF-κB, a three-fold increased nuclear translocation being observed in AMs from healthy smokers. This effect is receptor-mediated, as it is reproduced by the NK1 selective agonist [Sar9Met(O2)11]SP and reverted by CP 96,345.
These results clearly indicate that human AMs possess functional NK-1R on their surface, which are upregulated in healthy smokers, providing new insights on the mechanisms involved in tobacco smoke toxicity.
Keywords: Substance P, human alveolar macrophages, NK1 receptor, NF-κB activation, respiratory burst, cytokine release, TNF-α; IL-1β; IL-10
Introduction
The neuropeptide substance P (SP), a member of the tachykinin receptor family, is involved in many physiological processes, including nociception, vasodilation, exocrine and endocrine gland secretion, smooth muscle contraction, cell proliferation, and largely contributes to the local control of the immune responses (Severini et al., 2002). It induces lymphocyte proliferation (Payan et al., 1983), enhances immunoglobulin production by cloned B lymphoma cells (Pascual et al., 1991), degranulates rat mast cells (Mousli et al., 1989), modulates eosinophil and neutrophil activity (Brunelleschi et al., 1991; Iwamoto et al., 1993), stimulates human peripheral monocytes to produce inflammatory cytokines including IL-1, IL-6, IL-12 and tumor necrosis factor-α (TNF-α) (Lotz et al., 1988; Lavagno et al., 2001).
By using natural tachykinins and selective receptor agonists and antagonists, we previously demonstrated that guinea-pig alveolar macrophages (AMs) possess NK1 receptors (NK-1R) and NK2 receptors, their stimulation leading to superoxide anion (O2−) production and eicosanoid release, and that ovalbumin-sensitized AMs demonstrate an enhanced responsiveness to NK-2R stimulation (Brunelleschi et al., 1990, 1992). We also showed that SP, as well as neurokinin A (NKA) and the selective NK2 receptor agonist [β-Ala8]-NKA(4–10), induces O2− production in AMs obtained from patients with active sarcoidosis (Brunelleschi et al., 1996).
The biological responses to SP are mediated by the G protein-coupled tachykinin NK-1R, although SP can also bind, with lower affinity, NK2 and NK3 tachykinin receptors (Severini et al., 2002; Pennefather et al., 2004). The presence of NK1 receptors (NK-1R) on monocyte/macrophages has been demonstrated by evaluating the effects of selective receptor agonists and antagonists on functional parameters (for example, Brunelleschi et al., 1990, 1992, 1998) and/or by molecular biology and protein chemistry techniques. RT–PCR and in situ hybridization have been used to identify NK-1R mRNA expression in monocytes and macrophages (Ho et al., 1997; Germonpre et al., 1999), but little is known about NK1 expression at the protein level. Marriott & Bost (2000) and Simeonidis et al. (2003) demonstrated the presence of NK-1R protein in murine peritoneal macrophages and THP-1 cells, respectively, but, to our knowledge, nobody has investigated this possibility in human AMs. Recent evidence indicates that NK-1R gene expression in THP-1 cells is increased after exposure to IL-1β and TNF-α: this effect is mediated by the transcription factor NF-κB, which binds to the promoter region of the NK-1R gene and so regulates its expression (Simeonidis et al., 2003).
In resting cells, nuclear factor-κB (NF-κB) is retained in the cytoplasm through an association with inhibitory proteins of the IκB family, which mask the nuclear localization signal (Baldwin, 1996). Upon stimulation, IκBα is phosphorylated, ubiquitinylated and degraded, thus allowing NF-κB to translocate to the nucleus. Once NF-κB enters the nucleus, it binds to the promoter region of various genes and induces their transcription (Baldwin, 1996). SP specifically activates NF-κB pathway in cells of the monocyte/macrophage lineage, for example, human astrocytoma cells, murine peritoneal macrophages and dendritic cells (Lieb et al., 1997; Marriott et al., 2000), but no information are available concerning human AMs.
The present study was undertaken to establish the role of tachykinin NK-1R in human AMs isolated from healthy smokers and non-smokers. We demonstrate the presence of authentic NK-1R, as determined by Western blot analysis and immunofluorescence, and indicate that the NK-1R expressed in human AM are functional, as demonstrated by the ability of SP to evoke O2− production and cytokine release. We also present direct evidence that SP activates the transcription factor NF-κB pathway, so providing new insights on the mechanisms involved in neuropeptidergic control of AM responsiveness.
Methods
Study population
This study and the research protocol were approved by the local Ethical Committee. A total of 25 individuals, 15 male and 10 female subjects, aged between 28 and 76 years, 13 smokers and 12 non-smokers, were studied. The characteristics and smoking history of the study population are presented in Table 1, Results section. None of the subjects received medical therapy at the time of the study. Broncho-alveolar lavage (BAL) was mainly performed for diagnostic purposes to have a further validation/confirmation of the suspected disease; healthy subjects were individuals who had no history of cardiopulmonary disease or other chronic disease, no diagnosed lung diseases and were not on medication. In a few cases, the attribution of a ‘healthy' subject to the category was done after the BAL procedure.
Table 1.
Study population
| Subject | Sex (F or M) | Age (years) | Smoker | Number of cigarette day−1 | Years on smoke |
|---|---|---|---|---|---|
| 1 | F | 51 | Yes | 20 | 25 |
| 2 | M | 54 | Yes | 12 | 20 |
| 3 | F | 43 | No | — | — |
| 4 | M | 63 | Yes | 15 | 40 |
| 5 | M | 50 | No | — | — |
| 6 | F | 28 | Yes | 10 | 10 |
| 7 | M | 43 | Yes | 30 | 18 |
| 8 | M | 50 | No | — | — |
| 9 | M | 46 | No | — | — |
| 10 | F | 29 | No | — | — |
| 11 | M | 37 | Yes | 20 | 15 |
| 12 | F | 35 | No | — | — |
| 13 | M | 69 | No | — | — |
| 14 | F | 56 | Yes | 25 | 32 |
| 15 | M | 48 | No | — | — |
| 16 | M | 62 | Yes | 16 | 34 |
| 17 | F | 70 | No | — | — |
| 18 | F | 45 | Yes | 20 | 15 |
| 19 | M | 39 | No | — | — |
| 20 | M | 45 | Yes | 35 | 20 |
| 21 | M | 55 | No | — | — |
| 22 | F | 76 | No | — | — |
| 23 | F | 33 | Yes | 12 | 8 |
| 24 | M | 67 | Yes | 10 | 30 |
| 25 | M | 58 | Yes | 12 | 33 |
Isolation of human AMs from BAL
AMs were isolated from BAL as described (Brunelleschi et al., 1996). After informed consent was obtained from each patient and pretreatment with parenteral atropine sulphate (0.5 mg), airways were anaesthetized with 2% lidocaine. A fiberoptic bronchoscope was advanced and wedged into the middle lobe under direct visualization. Lavage was carried out with 140–200 ml of prewarmed (37°C) sterile saline solution in 20-ml aliquots with immediate gentle vacuum (syringe) aspiration after each injection. The fluid so obtained was filtered through two layers of sterile surgical gauze and centrifuged (400 × g, 30 min). The whole BAL pellet was washed twice in phosphate-buffered salt solution (PBS), resuspended in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS), 2 mM glutamine, 10 mM Hepes, 50 μg ml−1 streptomycin and 5 U ml−1 penicillin, and plated in six-well tissue culture plates (35 mm diameter; Costar, U.K.). After 2 h at 37°C in humidified 5% CO2 atmosphere, nonadherent cells (mainly lymphocytes) were gently removed and AMs were used for the experiments. Total cell count and viability evaluation (Trypan blue dye exclusion test, always >98%) were performed on a Burker haemocytometer. Differential cell count was carried out on Diff-Quick (Don Baxter)-stained cytospin smears, counting at least 400 cells. The adherent cell population was >99% AM. Phenotypical analysis was carried out on cytocentrifuge (Cytospin, U.K.; 500 r.p.m., 10 min) slides by employing leukocyte-specific monoclonal antibodies for CD68, CD14 and HLA-DR (from Becton Dickinson, U.K.).
O2− production in AMs
Adherent AMs (0.4–1 × 106 cells plate−1) were washed twice with PBS, incubated in RPMI 1640 medium (without phenol red, no antibiotics and no FCS) and challenged with increasing concentrations of tachykinins for 30 min. SP is the major endogenous ligand for NK-1R, [Sar9Met(O2)11]SP and Pro9SP are selective NK1 agonists. In the experiments with the NK-1R antagonists CP 96,345, GR82334 ([D-Pro9(spiro-gamma-lactam)Leu10,Trp11]physalaemin(1-11)) and GR71251 (([Pro9(spiro-gamma-lactam) Leu10,Trp11]SP)), AMs were preincubated for 15 min with these drugs and then challenged with tachykinins. The effects of tachykinins were compared with those evoked by phorbol 12-mirystate 13-acetate (PMA), a standard stimulus acting as a direct protein kinase C activator. The O2− production was evaluated by the superoxide dismutase (SOD)-inhibitable cytochrome c reduction, the absorbance changes being recorded at 550 nm in a Beckman DU 650 spectrophotometer. O2− production was expressed as nmol cytochrome c reduced/106 cells/30 min, using an extinction coefficient of 21.1 mM (Brunelleschi et al., 2001). To avoid interference with spectrophotometrical recordings of O2− production, AMs were incubated with RPMI 1640 without phenol red. Experiments were performed in duplicate or triplicate; control values (e.g., basal O2− production in the absence of stimuli) were subtracted from all determinations.
Release of TNF-α and other cytokines from AMs
Adherent AMs were challenged with the selected stimuli (SP, NK1 selective agonists, PMA) for 24 h at 37°C to ensure maximal cytokine release. Supernatants were collected and stored at −20°C. TNF-α, IL-1β and IL-10 (the latter was evaluated as the most important anti-inflammatory cytokine) in the samples were measured using enzyme-linked immunoassay kit (Pelikine Compact™ human ELISA kit). The measurements were performed according to the manufacturer's instructions. The minimum detectable concentrations of human TNF-α, IL-1β and IL-10 were 1.4, 1.5 and 1.3 pg ml−1, respectively. No crossreactivity was observed with any other known cytokine. Control values (e.g., cytokine release from untreated, unstimulated cells) were subtracted from all determinations. Results are expressed in pg ml−1.
Immunofluorescence for NK-1R in AMs
Human AMs were cultured onto gelatin-coated glass slides. The cells were fixed in ice-cold 4% paraformaldahyde (20 min), washed twice with PBS, permeabilized with 0.5% Triton X-100 (15 min, 25°C), washed twice with PBS, and blocked with 10% FCS, 2% BSA, 1% glycine, 0.5% Triton X-100 in PBS (1 h, 25°C). The cells were then incubated in the presence of a rabbit antibody directed against the human NK-1R (Santa Cruz Biotechnology, U.S.A.) at a dilution of 1 : 120 in PBS overnight at 4°C. After washing, FITC-conjugated anti-rabbit immunoglobulins (1 : 30) (Dako Cytomation, Milan, Italy) were added (2 h, 25°C). After another washing with PBS, nuclear staining was performed using Hoechst 33258 (0.8 μg ml−1, 1 h, 37°C) (Sigma-Aldrich, Milan, Italy). Fluorescence was visualized using 100-fold magnification.
Western blotting of the tachykinin NK-1R in AMs
Subconfluent AMs (10 × 106 cells) were washed twice with ice-cold PBS and lysed with 1 ml RIPA buffer (1% Triton X-100, 1% sodium deoxycolate, 0.1% SDS, 50 mM Hepes pH 7.4, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM NaF) containing 1 mM Na3VO4 and protease inhibitors (10 μg ml−1 aprotinin, 10 μg ml−1 pepstatin, 50 μg ml−1 leupeptin, 1 mM phenylmethylsulphonyl fluoride-PMSF). Cells were placed on ice for 20 min and scraped. Cell lysates were sonicated on ice four times for 5 s each, cleared by centrifugation at 15,000 × g for 10 min at 4°C and the supernatants (cell lysates) transferred into a new tube. If necessary, cell lysates were stored at −80°C. About 30 μg total extracts were separated on 8–10% SDS–PAGE and transferred to nitrocellulose filters (Protran, Perkin-Elmer Life Sciences, Boston, MA U.S.A.). Nonspecific binding sites on membrane were blocked at room temperature for 1 h in TBS-5% BSA nitrocellulose filters. Filters were probed with a commercial anti-human NK-1R antibody (NK-1R (H-83): sc-15323, Santa Cruz Biotechnology, U.S.A.; a rabbit polyclonal antibody mapping at the C-terminus of the human NK1 receptor) (1 : 200 in TBS-5% BSA) for 2 h at room temperature. Proteins were visualized by using ECL Western blotting detection reagents (Perkin-Elmer Life Science, Boston, MA, U.S.A.). Quantification of Western blots was performed by densitometry using ‘Quantity One, 1-D Analysis' software (Bio-Rad, U.S.A.) and expressed as intensity data units.
Evaluation of NF-κB activation
The activation of NF-κB induced by SP, NK1 agonists or PMA was evaluated by measuring the nuclear migration (by electrophoretic mobility shift assay (EMSA)) as well as the nuclear content of p50 and p65 subunits (by ELISA and Western blot). The methods we used are detailed below.
Preparation of nuclear and cytosolic cellular fractions
After challenge with stimuli, AMs (about 5 × 106 cells) were washed with ice-cold PBS, scraped and centrifuged at 1000 × g for 5 min at 4°C. The cell pellet was resuspended in 300 μl of lysis buffer (10 mM Hepes, pH 7.6, 60 mM KCl, 1 mM EDTA, 1 mM PMSF, 1 mM dithiothreitol, 10 μl ml−1 protease cocktail inhibitors) and incubated on ice for 15 min. At the end of this incubation, 20 μl of 10% NP-40 was added and the tube vortexed for 10 s. After centrifugation at 13,000 × g for 1 min at 4°C, supernatants (cytosolic fractions) were collected and stored at −80°C, whereas the pellets were further processed to obtain nuclear extracts. The pellets were resuspended in extraction buffer (20 mM Tris–HCl, pH 8, 420 mM NaCl, 1.5 mM MgCl2, 0.5 mM PMSF, 0.2 mM EDTA, 10 μl ml−1 protease cocktail inhibitors, glycerol 25% v v−1) and incubated for 30 min at 4°C. Nuclear proteins were isolated by centrifugation at 13,000 × g for 15 min. The supernatant was aliquoted and stored at −80°C until used for EMSA or p50/p60 ELISA assays. Protein concentrations were determined by using a protein assay (Bio-Rad, U.S.A.).
EMSA of NF-κB
Nuclear extracts (5 μg) were incubated with 2 μg poly (dI-dC) and the γ [32P]ATP-labelled oligonucleotide probe (100,000–150,000 c.p.m.; Promega) in binding buffer (50% glycerol, 10 mM Tris–HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 1 mM dithiothreitol) in a final volume of 20 μl for 30 min at room temperature. The NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was obtained from Promega. The nucleotide–protein complex was separated on a 5% polyacrylamide gel in 0.5 × TBE buffer (100 mM Tris–HCl, 100 μM boric acid, 2 mM EDTA) at 150 V on ice. The gel was dried and radioactive bands were detected by autoradiography.
p50 and p65/RelA assays
Nuclear extracts were prepared as described above and evaluated for the presence of p50 and p65/RelA subunits using Trans AM™ NF-κB p50 Chemi and NF-κB p65 Chemi Transcription Factor Assay kits (Active Motif Europe, Belgium), according to the manufacturer's instructions. Briefly, an equal amount (1 μg) of nuclear lysate was added to incubation wells precoated with an oligonucleotide containing the NF-κB consensus site (5′-GGGACTTTCC-3′) sequence, the active form of NF-κB contained in the cell extract specifically binding to this oligonucleotide. Sometimes, the cytosolic content of both subunits was also measured. These assay kits specifically detected bound NF-κB p65 or p50 subunits in human extracts; activities of p50 and p65 were measured by a Rosys Anthos Lucy 1 luminometer and results are expressed as RLU (relative luminescence unit). In some cases, the nuclear extracts (5 μg protein, for each sample) were also used to evaluate p50 and p65 subunits by Western blot. For these assays, commercial antibodies (anti-NF-κB p50: ab 7971 and anti-NF-κB p65: ab 7970) were obtained from Abcam (U.K.). Nuclear extracts were challenged for 2 h at room temperature with the antibody at a final concentration of 1 μg ml−1.
Drugs and analytical reagents
Substance P, selective NK1 agonists and NK1 antagonists were obtained from Neo-System (Strasbourg, France). The anti-NK-1R-specific antibody (NK-1R (H-83): sc-15323) was from Santa Cruz Biotechnology (U.S.A.). The anti-NF-κB p50 and anti-NF-κB p65 antibodies were obtained from Abcam (U.K.). PBS, RPMI 1640 (with or without phenol red), BSA, glutamine, Hepes, streptomycin, penicillin, PMA, ethanol, SOD, cytochrome c, Na-deoxycholate, NaCl, EDTA, protease cocktail inhibitors (aprotinin 0.3 μM, bestatin 130 μM, leupeptin 1 μM), bromophenol blue, glycine, glycerol, methanol and Tween 20 were obtained from Sigma (Milwaukee, WI, U.S.A.). Poly(dI-dC) were obtained from Pharmacia (Uppsala, Sweden). Triton X-100 and β-mercaptoethanol were from Fluka (Buchs, Switzerland); PMSF was from Promega (Madison, WI, U.S.A.). SDS and DMSO were from Merck (Darmstadt, Germany). BCA Protein Assay Reagent kit was from Pierce (Rockford, IL, U.S.A.). Nitrocellulose filters (Hybond) and the enhanced chemiluminescence system were from Amersham (Buckinghamshire, U.K.). Tissue-culture plates were purchased from Costar Ltd (Buckinghamshire, U.K.). All cell culture reagents, with the exception of fetal bovine serum, were endotoxin-free according to details provided by the manufacturer. Fetal bovine serum (lot 40G3410 K, containing <10 EU ml−1) was from Life Technologies Inc. (Rockville, U.S.A.). TNF-α, IL-1β and IL-10 immunoassay kit was obtained from CLB/Sanquin, Central Laboratory of the Netherlands Red Cross (Netherlands). Gel shift assay Core system and all the reagents for NF-κB EMSA were from Promega Corporation (St Louis, CA, U.S.A.).
Data and statistical analysis
Data are mean±s.e.m. of duplicate determinations of ‘n' independent experiments. Concentration–response curves for SP and NK1 agonists were constructed and EC50 values were interpolated from curves of best-fit. When required, statistical evaluation was performed by Student's t test.
Results
Study population, BAL and phenotype of AMs
In all, 25 individuals, 15 male and 10 female subjects (mean age=50.2±2.7 years; mean age of male and female subjects: 55±3.7 and 44.5±4.3 years, respectively, P=0.07), were studied. A total of 13 (eight male and five female subjects) were smokers and 12 (seven male and five female subjects) were non-smokers; mean age of smokers (49.4±3.3 years; n=13) and non-smokers (50.8±4 years, n=12) being very similar. The characteristics and smoking history of the study population are listed in Table 1. None of the subjects received medical therapy at the time of the study. Total and differential cell counts in BAL and phenotype of AMs from smokers and non-smokers are presented in Table 2. As expected, a significant (P<0.05) increase in the total cell number in BAL (with no significant differences in differential cell counts) was observed in smokers as compared to non-smokers. The great majority of AMs (96±1%) in healthy smokers was CD68+ and a high percentage (86±1 and 66±3%, respectively) of AM expressed also HLA-DR and CD14. As known, CD68 expression is related to the presence of AM involved in the oxidative burst, CD14 expression is related to cytokine production by LPS receptor, whereas HLA-DR is related to antigen presentation. The expression CD14 and CD68 was significantly (P<0.05) higher in AMs collected from healthy smokers as compared to healthy non-smokers (Table 2).
Table 2.
Total and differential cell count in BAL and AM phenotype
| Subjects | Total cell ml−1 BAL | AMS (%) | Lympho (%) | PMNs (%) | CD68+ (%) | HLA-DR+ (%) | CD14+ (%) |
|---|---|---|---|---|---|---|---|
| Smokers (n=13) | 390.000±6.000 | 90.8±1.9 | 8.2±2 | 1±0.5 | 96±1 | 86±1 | 66±3 |
| Non-smokers (n=12) | 139.000±5.100* | 90.6±1 | 8.6±1 | 0.2±0.1 | 83±1* | 84±2 | 50±2* |
Data are given as total cell number ml−1 BAL and percentage of total cell population (differential) in BAL. AMs=alveolar macrophages; Lympho=alveolar lymphocytes; PMNs=alveolar neutrophils. The AM phenotype was evaluated by measuring CD68, CD14 and HLA-DR: positive cells are expressed as percentage of total AMs.
Denotes P<0.05 vs smokers.
NK-1R expression in human AMs
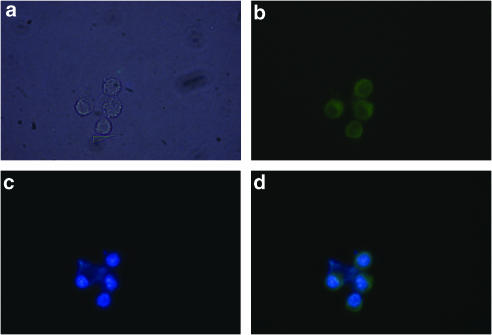
We examined the expression pattern of the NK-1R gene products in human AMs. We collected AMs from healthy smokers and healthy non-smokers undergoing BAL for diagnostic procedures, after their informed consent. First, we performed immunofluorescence assays in AMs isolated from healthy non-smokers and observed the expression of the NK-1R protein localized primarily at the cell surface (Figure 1). A phase contrast photomicrograph of AM is shown in Figure 1a. Immunofluorescence with anti-NK-1R antibody and FITC-conjugated anti-rabbit antibody reveals a green colour (Figure 1b), nuclear staining with Hoechst 33258 (0.8 μg ml−1, 30 min, 37°C) is depicted in blue (Figure 1c), and merge of both visualizes NK-1R on AM surface (Figure 1d).
Figure 1.
Immunocytochemical analysis of the expression and location of NK-1R in human AMs. (a) Phase contrast. (b) Immunofluorescence of anti-NK-1R polyclonal Ab followed by FITC-conjugated anti-rabbit immunoglubulins (green). (c) Nuclear staining with Hoechst 33258 (blue). (d) Merge of B and C. Fluorescence was visualized by a vertical fluorescence microscopy (100-fold magnification; Eclipse E600, Nikon, Tokyo, Japan).
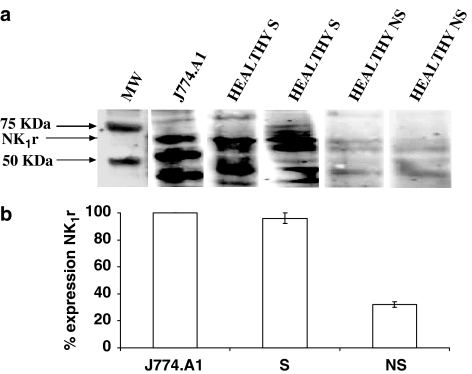
We next performed Western blot in AMs isolated from healthy smokers and non-smokers to evaluate NK-1R expression at the protein level (Figure 2). AMs were obtained from four healthy smokers (subjects 1, 2, 14 and 20) and four healthy non-smokers (subjects 3, 5, 10 and 21); the experiments were performed separately (by using the same protein amount, 30 μg, for each individual assay) and were always compared with the NK-1R expression in the positive control, the cultured J774.A1 cells (a murine macrophage cell line). As shown in Figure 2a, Western blot analysis of J774.A1 cells, AMs from two healthy smokers (subjects 1 and 2) and two non-smokers (subjects 3 and 5), probed with the polyclonal anti-NK-1R antibody, reveals three prominent bands of 68, 53 and 42 kDa, respectively (Figure 2a). According to manufacturer's instructions, the commercial polyclonal antibody we used detected a protein of about 68 kDa. As known, NK-1R possess different sites for acetylation and phosphorylation and may be present as truncated forms (Li et al., 1997; Page & Bell, 2002; Caberlotto et al., 2003). In our experiments, we always observed a positive band at 68 kDa (as indicated by the manufacturer) and two bands of 53 and 42 kDa (Figure 2a). Such observations are in keeping with previous reports in which other authors, by using noncommercial monoclonal NK-1R antibodies, detected a 46 kDa protein in human antral tissue (Smith et al., 2000), a molecular mass band of 53 kDa in the monocyte/macrophage THP-1 cells (Simeonidis et al., 2003) or a 42 kDa protein in murine peritoneal macrophages and murine microglia (Marriott & Bost, 2000; Rasley et al., 2002).
Figure 2.
NK-1R expression in human AMs from healthy smokers (S) and non-smokers (NS). In (a), Western blot analysis of NK-1R in AM extracts of two smokers and two non-smokers. The blots are assembled from different single experiments in which AMs from S or NS have been evaluated. As a positive control for the presence of NK-1R, the macrophage cell line J774.A1 was used (for clarity, only one blot of J774.A1 cells, representative of seven others, is shown). The same protein amount (30 μg) was used in each experiment with AMs from smokers and non-smokers. An arrowhead indicates the 68-kDa band corresponding to the receptor. The migration of protein standards of known sizes is shown on the left. In (b), quantitative evaluation of NK-1R expression by densitometry. Intensity of the specific band of NK-1R in the macrophage J774.A1 cells amounted to 8710±250 intensity units (means±s.e.m. of eight experiments) and was taken as 100%. Results are expressed as % expression of the positive control; mean+s.e.m. of four experiments for S and NS.
Interestingly, densitometric evaluation of NK-1R expression revealed that AMs collected from healthy smokers demonstrated a >3-fold increase as compared to AMs isolated from healthy non-smokers (Figure 2b). The intensity of the specific band of 68 kDa in the positive control, the J774.A1 cell line, amounted to 8710±250 intensity units (n=8) and was taken as 100%. NK-1R expression in AMs from healthy non-smokers was 32±1.5% (n=4), whereas NK-1R expression in AMs from healthy smokers amounted to 96±4% (n=4).
NK-1R are functional in human AMs: O2− production and cytokine release
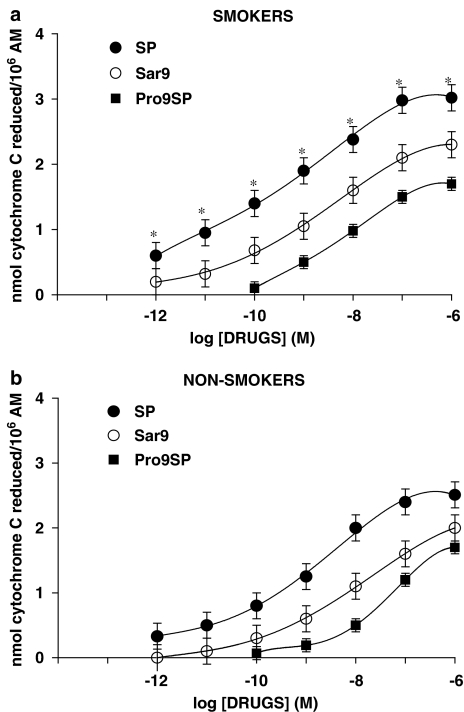
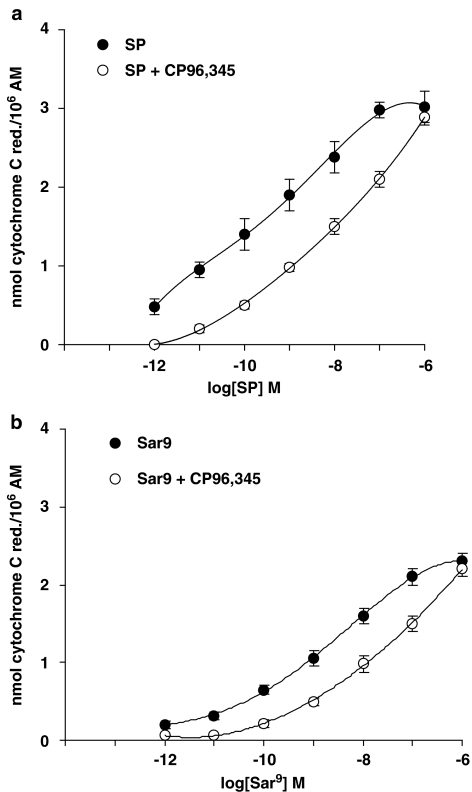
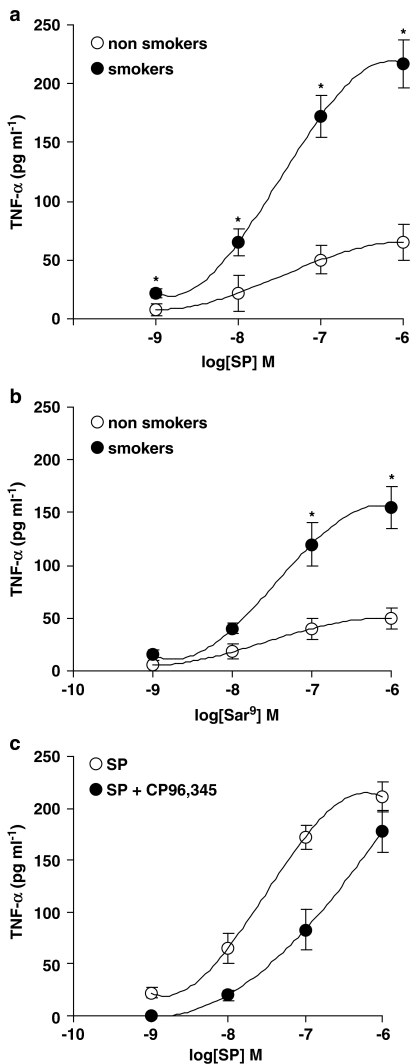
Basal values (O2− production from unstimulated AMs) in smokers and non-smokers were 13.5±2 (n=6) and 2.2±0.4 (n=5; P<0.01) nmol cytochrome c reduced/106 AMs, respectively. These values were subtracted from those obtained after tachykinins or PMA challenge to obtain the net O2− production. PMA, used at 10−7 M (a near maximal concentration), produced 23.5±2 (n=6) and 17±0.6 (n=5; P<0.05) nmol cytochrome c reduced/106 AMs in smokers and non-smokers, respectively. In the concentration range 10−12–10−6 M, SP dose-dependently evoked O2− production in AMs from both smokers and non-smokers, higher production being observed in smokers (Figure 3). As depicted in Figure 3, maximal activation by SP was observed at 10−6 M, EC50s being 0.25 nM in smokers and 1 nM in non-smokers. In the presence of a cocktail of inhibitors (thiorphan, captopril and bestatin, all at 1 μM) of tachykinin degrading enzymes, SP effects were significantly enhanced (data not shown). The metabolically stable NK-1R agonist [Sar9Met(O2)11]SP, although less potent than SP, evoked a significant respiratory burst in AMs collected from both smokers and non-smokers, EC50s being about 3 nM in smokers and 10 nM in non-smokers (Figure 3). Pro9SP, the other NK1 selective agonist we used, acted dose-dependently, although less active and potent than SP or [Sar9Met(O2)11]SP (Figure 3). EC50s for Pro9SP were about 10 nM in smokers and 30 nM in non-smokers (Figure 3). The nonpeptide NK1 selective antagonist CP 96,345 at 1 nM competitively antagonized the effects of SP and NK1 selective agonists: the dose–response curve for SP was shifted to the right about two orders of magnitude (Figure 4a) as were those for [Sar9Met(O2)11]SP (about 1.5-fold; Figure 4b) and Pro9SP (one order of magnitude; not shown). GR82334, a reversible NK1 antagonist devoid of histamine-releasing properties in rat mast cells (Guo et al., 1995), and GR71251, a selective NK1 antagonist that possesses GABA-releasing actions in rat spinal cord (Hagan et al., 1990), at 1 μM competitively antagonized the effects of SP and NK1 selective agonists in AM (data not shown).
Figure 3.
NK-1R agonists evoke O2− production in human AMs isolated from healthy smokers (a) and non-smokers (b). Cells were challenged with increasing concentrations of SP, [Sar9Met(O2)11]SP and Pro9SP for 30 min. Results are means±s.e.m. of five to six experiments in duplicate. *P<0.05 vs non-smokers.
Figure 4.
The NK1 selective antagonist CP96,345 competitively antagonizes SP-induced O2− production (a) and [Sar9Met(O2)11]SP-induced O2− production (b) in AMs from healthy smokers. Cells were preincubated with CP 96,345 at 1 nM for 15 min and then challenged with the NK1 agonists for further 30 min. Results are means±s.e.m. of four experiments in duplicate.
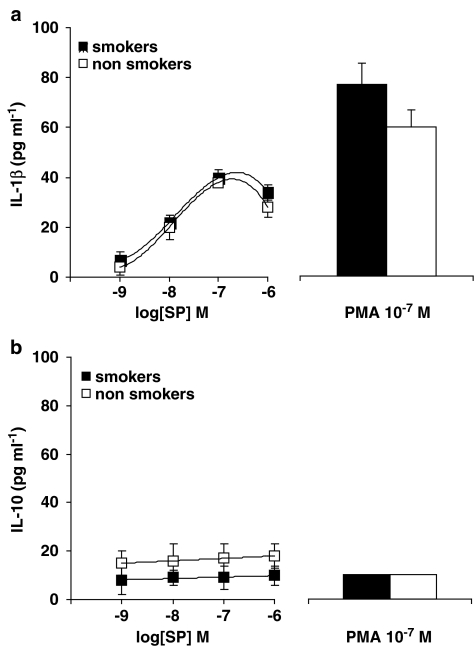
We also evaluated the release of proinflammatory cytokines, namely TNF-α (the most abundant cytokine in AMs) and IL-1β, as well as IL-10 release (the most relevant anti-inflammatory cytokine in AMs), after challenge with tachykinins or PMA. Basal values (that is the release from unstimulated AM) were subtracted from all determinations and are listed in Table 3. As shown in Figure 5, SP (Figure 5a) and the NK1 agonist [Sar9Met(O2)11]SP (Figure 5b) dose-dependently induced TNF-α release from AMs, a significantly higher effect being observed in AMs from healthy smokers as compared to non-smokers (Figure 5a and b; P<0.01). CP 96,345, tested at 1 nM, competitively antagonized SP-induced TNF-α release in smokers' AMs (Figure 5c), so confirming that this effect is mediated by NK-1R activation. Also PMA, at 10−7 M, induced a higher TNF-α release in AMs from smokers (535±60 pg ml−1; n=8) as compared to non-smokers (136±20 pg ml−1; n=6; P<0.05). By evaluating IL-1β production from human AMs, we observed that SP acted dose-dependently, maximal release (about 40 pg ml−1) being detected at 0.1–1 μM, with no major difference between smokers and non-smokers (Figure 6a). PMA, at 10−7 M, also released similar amounts of IL-1β in both smokers and non-smokers (Figure 6a). SP, in a dose-independent way, released very small amounts of the anti-inflammatory cytokine IL-10 (Figure 6b) by human AMs, a higher but not significant release being observed in non-smokers. As depicted in Figure 6, PMA 10−7 M did not stimulate IL-10 secretion, while inducing IL-1β release from human AMs.
Table 3.
Basal release of cytokines in AMs collected from healthy smokers and non-smokers
| Cytokine | Smokers (n=6) | Non-smokers (n=6) |
|---|---|---|
| TNF-α (pg ml−1) | 42±8* | 11±2 |
| IL-1β (pg ml−1) | 15±4 | 10±3 |
| IL-10 (pg ml−1) | 13±5 | 15±7 |
Values are means±s.e.m. of experiments in triplicate.
P<0.05 vs non-smokers.
Figure 5.
NK-1R stimulation induces TNF-α release in human AMs isolated from healthy smokers and healthy non-smokers. SP-induced TNF-α release in (a); [Sar9Met (O2)11]SP-induced release in (b); reversal by CP 96,345 1 nM of SP-induced release in smokers in (c). Data are means±s.e.m. of five to six experiments in duplicate. *P<0.01 vs non-smokers. See text for further details.
Figure 6.
Effects of SP on IL-1β release (a) and IL-10 release (b) in human AMs isolated from healthy smokers and healthy non-smokers. PMA-induced release is shown for comparison. Data are means±s.e.m. of five to six experiments in duplicate.
SP and NK-1R agonists induce NF-κB activation
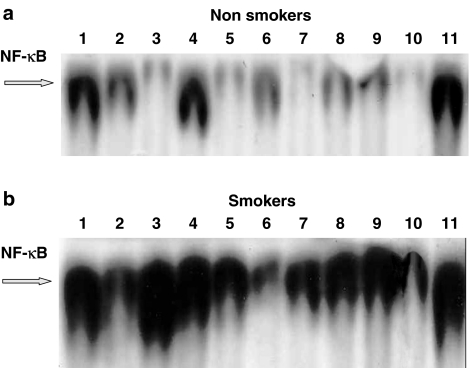
Previous observations indicate that, in different cell models, SP can induce the activation of the transcription factor NF-κB. We checked this hypothesis in human AMs by first evaluating the nuclear translocation of NF-κB by EMSA and, to ensure a better quantitative evaluation, the amounts of translocated p50 and p65 subunits by an ELISA kit. As known, although different NF-κB forms have been described, the p50–p65 heterodimer is the predominant species in many cell types (Baldwin, 1996). As a positive control for the detection of NF-κB activation, human AMs were stimulated by PMA, as this agent has previously been demonstrated to induce NF-κB nuclear translocation in human monocytes (Lavagno et al., 2004). To investigate the time- and dose-dependent effects of SP, AMs from healthy non-smokers (Figure 7a) and smokers (Figure 7b) were challenged with two different concentrations of SP (10−8 and 10−6 M) for different times (1–2 h). As reported in Figure 7, SP-induced NF-κB activation, just detectable after 1 h, was maximal at 2 h and had about the same intensity as PMA (Figure 7). Interestingly, AMs obtained from healthy smokers (Figure 7b) demonstrated a constitutively (control, unstimulated AMs, lane 7) enhanced nuclear translocation of the transcription factor NF-κB as compared to AMs isolated from non-smokers (Figure 7a, lane 7). Accordingly, PMA- and SP-induced nuclear translocation was higher in AMs from smokers (Figure 7, lanes 1–2: PMA10−6 M, 2 and 1 h challenge, respectively; lanes 3–6: SP 10−6 and 10−8 M, 1 or 2 h challenge) as compared to non-smokers. The NK-1R antagonist CP96,345 ((2S,3S)-cis-2-diphenylmethyl-N[(2-methoxyphenyl) -methyl]-1-azabicyclo-octan-3-amine)), here evaluated at 1 μM, while not affecting per se PMA-stimulated translocation (Figure 7, lane 11) and basal constitutive activity (lane 10), potently reduced SP-induced effects (lanes 8 and 9), so confirming that SP-induced NF-κB nuclear translocation in human AMs is a receptor-mediated effect. Moreover, the NK1 antagonist seemed to be less effective in reducing SP-induced nuclear translocation in smokers, as compared to smokers. The autoradiographs presented in Figure 7 (which are representative of two other additional experiments) are relative to AMs from smokers and non-smokers, which were obtained and contemporarily processed on the same day; the two gels presented as Figure 7a (non-smokers) and Figure 7b (smokers) were run concomitantly. Competition experiments performed with 100-fold excess unlabelled NF-κB sequence demonstrated the specificity of the induced NF-κB/DNA binding complex (not shown).
Figure 7.
SP induces NF-κB activation in human AMs from healthy non-smokers (a) and healthy smokers (b) in a time- and dose-dependent manner. AMs were stimulated with SP (10−6 and 10−8 M) or PMA 10−6 M for 1 or 2 h, in the presence or absence of CP 96,345. The NK-1R antagonist was evaluated at 1 μM and preincubated for 15 min. Nuclear extracts (5 μg) were prepared and assayed for NF-κB activity by EMSA (see text for further details). In (a) (non-smoker): lane 1=PMA 2 h; lane 2=PMA 1 h; lane 3=SP 10−6 M 1 h; lane 4=SP 10−6 M 2 h; lane 5=SP 10−8 M 1 h; lane 6=SP 10−8 M 2 h; lane 7=control, unstimulated AM; lane 8=CP 96,345 + SP 10−8 M 2 h; lane 9=CP 96.345 + SP 10−6 M 2 h; lane 10=CP 96,345 alone; lane 11=CP 96,345 + PMA 2 h. In (b) (smoker): lane 1=PMA 2 h; lane 2=PMA 1 h; lane 3=SP 10−6 M 2 h; lane 4=SP 10−6 M 1 h; lane 5=SP 10−8 M 2 h; lane 6=SP 10−8 M 1 h; lane 7=control, unstimulated AM; lane 8=CP 96,345 + SP 10−8 M 2 h; lane 9=CP 96.345 + SP 10−6 M 2 h; lane 10=CP 96,345 alone; lane 11=CP 96,345 + PMA 2 h. This experiment was performed three times with similar results.
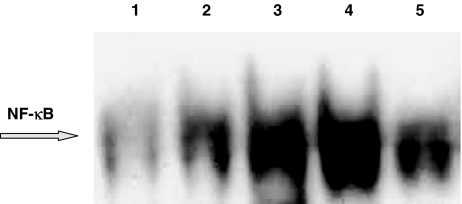
In AMs collected from healthy smokers, SP effects are reproduced, to about the same intensity, by the NK1 agonist [Sar9Met(O2)11]SP, evaluated at 10−8 and 10−6 M (Figure 8). The antagonist CP 96,345 potently reduced the effects of the NK1 agonist, so confirming that NF-κB nuclear translocation is a receptor-mediated effect (Figure 8).
Figure 8.
The NK1 selective agonist [Sar9Met(O2)11]SP induces NF-κB activation in human AMs from healthy smokers and its effects are reduced by the NK-1R antagonist CP 96,345. AMs were stimulated with [Sar9Met(O2)11]SP 10−8 or 10−6 M for 2 h, in the presence or absence of CP 96,345 at 1 μM. The effects of SP 10−6 M are shown for comparison. Nuclear extracts (5 μg) were prepared and assayed for NF-κB activity by EMSA (see text for further details). Lane 1=control, unstimulated AM; lane 2=Sar9 10−8 M; lane 3=Sar9 10−6 M; lane 4=SP 10−6 M; lane 5=CP 96,345 + Sar9 10−6 M. This experiment was performed three times with similar results.
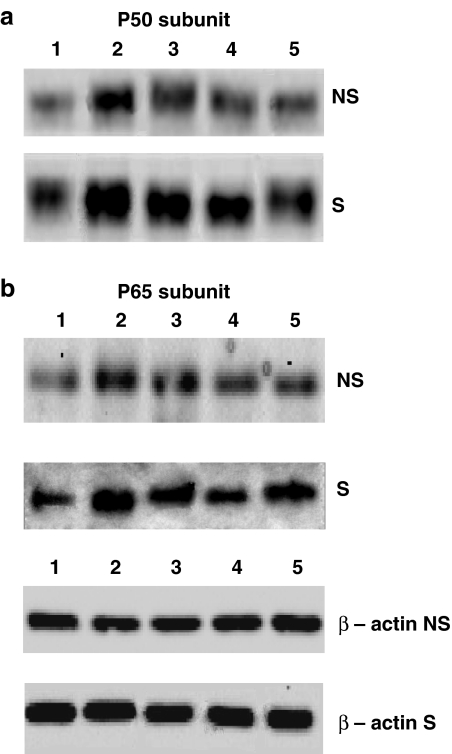
We also performed Western blot experiments to evaluate the nuclear translocation of p50 and p65 subunits of the NF-κB complex in AMs from both smokers and non-smokers. As depicted in Figure 9 (a: p50 subunit and b: p65 subunit), PMA, SP and the NK1 agonist [Sar9Met(O2)11]SP induced the translocation of both subunits, an increased p50 translocation being observed in smokers (Figure 9).
Figure 9.
Western blots of p50 and p65 subunits in AMs from both non-smokers (NS) and smokers (S). The nuclear translocation of p50 is reported in (a); the translocation of p65 is reported in (b). Beta-actin is shown for comparison. Lane 1=control; lane 2=PMA 10−6 M; lane 3=SP 10−6 M; lane 4=SP 10−8 M; lane 5=Sar9 10−6 M.
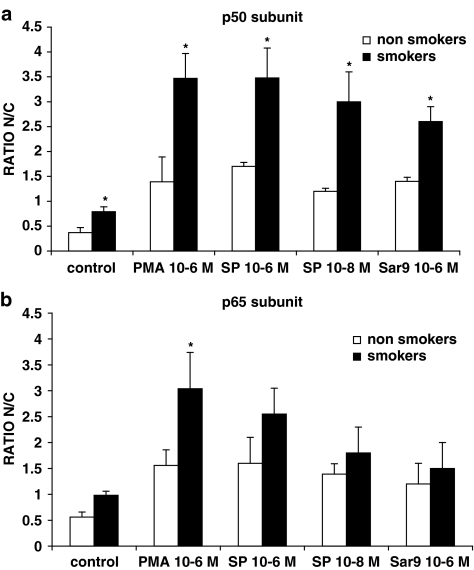
To ensure a better quantitative evaluation, we also assessed the translocation of p65 subunit and p50 subunit in AMs from both smokers and non-smokers, by using a commercially available ELISA kit. As depicted in Figure 10, SP dose-dependently induced p50 translocation (Figure 10a) and p65 translocation (Figure 10b) in AMs, a significantly (P<0.05) enhanced effect being observed in smokers especially for the p50 subunit. The NK1 agonist [Sar9Met(O2)11]SP, here evaluated at 1 μM, also increased p50 and p65 translocation, AMs from smokers depicting a significant enhanced p50 translocation. PMA-induced translocation is shown for comparison (Figure 10). CP 96,345 at 10−6 M significantly reduced SP-induced nuclear p50 and p65 translocation (not shown). Results are expressed as the nuclear/cytosolic ratio, which is the ratio between the amount of p50 (or p65) in nuclear extracts and cytosolic extracts.
Figure 10.
NK-1R stimulation induces the translocation of p50 (a) and p65 (b) subunits in human AMs from healthy smokers and healthy non-smokers. PMA-induced translocation is shown for comparison. AMs were challenged for 2 h with the stimuli; nuclear and cytosolic extracts were prepared and evaluated for their content in p50 and p65 subunits. Results are expressed as the nuclear/cytosolic ratio (ratio N/C) for both p50 and p65 subunits. Data are means±s.e.m. of five experiments in duplicate. *P<0.05 vs non-smokers.
Discussion
These results demonstrate that human AMs, isolated from both healthy smokers and non-smokers, possess functional NK-1R. In fact, we here report the presence of NK-1R protein (by Western blotting) as well as the ability of SP, the endogenous NK-1R ligand, to evoke O2− production and cytokine release in AMs, as well as to induce activation of the transcription factor NF-κB. These effects are all receptor-mediated, as they are reproduced by NK1 selective agonists and reverted by NK-1R antagonists.
NK-1R expression has been evaluated by different authors in different cell types, by using RT–PCR technology, mainly. By this approach, human monocytes and monocyte-derived macrophages were shown to express SP and NK-1R, besides producing and releasing SP (Ho et al., 1997). Moreover, an NK-1R antagonist downregulated SP mRNA expression in monocyte-derived macrophages (Lai et al., 2002a). Lai et al. (2002b) quantified SP mRNA in different cells by real time RT–PCR and reported a large variability in the level of transcripts in monocyte-derived macrophages, the number of SP mRNA copies/μg total mRNA detected in preparations from four different donors ranging from 949 to 113,388 (Lai et al., 2002b). Therefore, given the demonstrated large variability in SP transcripts (Lai et al., 2002b) and depending on the number of collected AMs for the evaluation of all the other parameters (respiratory burst, cytokine release, NF-κB activation), in our AMs preparations, we evaluated NK-1R expression at the protein level, only. By using a noncommercial monoclonal anti-NK-1R antibody (raised in chicken against the final 15 amino acids at the C-terminus of the rat NK-1R), Smith et al. (2000) demonstrated the presence of NK-1R in human antrum and detected both a positive band at 46 kDa and a larger molecular mass band of 110 kDa, representing the glycosylated form of the NK-1R. Two NK-1R isoforms that differ in the length of the cytoplasmic carboxyl-terminus have been reported (Fong et al., 1992; Mantyh et al., 1996; Li et al., 1997): in the rat, the full-length and the truncated receptor presented molecular weights of the receptor proteins of about 80 and 50 kDa, respectively, the deglycosylated receptors being 46-kDa and 37-kDa, respectively (Li et al., 1997). The truncated receptor, at variance from the full-length one, did not undergo rapid and long-lasting desensitization (Li et al., 1997); cells possessing the short NK-1R isoform would, therefore, be expected to have a prolonged responsiveness. By means of noncommercial NK-1R antibodies, a 42 kDa protein was detected in murine peritoneal macrophages and microglia (Marriott & Bost, 2000; Rasley et al., 2002), whereas a 53 kDa protein was demonstrated in the THP-1 cells (Simeonidis et al., 2003). Given the heterogeneity of NK-1R and the fact that we used a commercial polyclonal anti-NK-1R antibody, we detected three prominent bands of 68 (as indicated by the manufacturer), 53 and 42 kDa in human AMs, in accordance to what observed by others in cells of the monocyte/macrophage lineage. Interestingly, AMs from healthy smokers demonstrated a >3-fold increase in NK-1R expression as compared to healthy non-smokers: these results are in keeping with previous data indicating the key role for SP and NK-1R activation in tobacco-induced lung toxicity in both animals and humans (Dusser et al., 1989; Tomaki et al., 1995; Wu & Lee, 1999) and further extend observations from Bai et al. (1995), who detected an increased NK-1R expression in lung biopsies from smokers.
Furthermore, we have determined the functional nature of these NK-1R by demonstrating the ability of SP and selective NK1 agonists to induce O2− production and cytokine release from human AMs. These observations extend our previous data in both human and guinea-pig AMs (Brunelleschi et al., 1990, 1992, 1996), demonstrating an enhanced responsiveness to tachykinins in AMs isolated from healthy smokers. By evaluating the respiratory burst, we measured a significantly increased O2− production when AMs from smokers were challenged with SP or selective NK1 agonists, EC50 s being 0.25 nM in smokers and 1 nM in non-smokers for SP and 3 nM in smokers and 10 nM in non-smokers for [Sar9Met(O2)11]SP. To further confirm the receptor nature of these effects, the non-peptide NK1 antagonist CP 96,345 at 1 nM shifted to the right the concentration–response curve for both endogenous and synthetic NK1 ligands.
TNF-α, IL-1β, IL-2 and IL-6 are frequently encountered proinflammatory cytokines, which are involved in a variety of immunological functions as well as interaction with different target cells. TNF-α is secreted by monocyte/macrophages mainly and, besides exerting cytotoxic activity to tumor cells, has a key role in chronic inflammation. TNF-α induces the expression of, and enhances cellular responsiveness to, other cytokines and growth factors, and affect signal transduction pathways leading to proliferation. SP and NK1 selective agonists dose-dependently induce TNF-α release from human AMs, a more than doubled significant TNF-α release (P<0.01) being observed in AMs from healthy smokers. CP 96,345 at 1 nM competitively antagonize these effects, further confirming the receptor-mediated nature of SP-induced effects in human AMs. In the concentration range 1 nM–1 μM, SP also induces IL-1β release from AMs, no significant differences being observed between smokers and non-smokers. This result was somewhat unexpected also considering the fact (see below) that SP and NK-1R agonists activate the transcription factor NF-κB. As known, the regulation of TNF-α and IL-1β production is largely NF-κB-dependent, although evidence exists that TNF-α and other cytokines can also be induced through NF-κB-independent pathways (Bondeson et al., 1999; Andreakos et al., 2004). We have no definitive explanation for SP-induced IL-1β release being similar in smokers and non-smokers; however, PMA, too, although activating NF-κB and inducing cytokine release, did not release an enhanced amount of IL-1β in smokers. Moreover, AMs isolated from smokers and challenged with LPS released significantly decreased amounts of IL-1β as compared to non-smokers (Brown et al., 1989; Yamaguchi et al., 1989). Brown et al. (1989) concluded that ‘there is a defect in release but not production of IL-1β from the AMs of chronic smokers'. In addition, SP exerts inconsistent effects on IL-10 release, in keeping with previous observations on peripheral blood mononuclear cells from healthy donors (Kim et al., 2003).
An important component controlling the synthesis of many cytokines and other proinflammatory gene products is the transcriptional activator NF-κB (reviewed in Baldwin, 1996). Five related mammalian gene products participate in NF-κB functions (p50/NF-κB1, p52/NF-κB2, p65/Rel A, c-Rel and RelB), the predominant species in many cell types being a p50-p65 heterodimer. As known, the transcription factor NF-κB regulates the expression of many proinflammatory genes, including those of TNF-α and IL-1β, and, in turn, these inflammatory cytokines are potent inducers of NF-κB activation (Baldwin, 1996).
SP specifically activates NF-κB in cells of the monocyte/macrophage lineage, for example, human astrocytoma cells, murine peritoneal macrophages and dendritic cells (Lieb et al., 1997; Marriott et al., 2000), but no information are available concerning human AM. We originally report here that activation of NK-1R by SP or [Sar9Met(O2)11]SP dose-dependently stimulates nuclear translocation of NF-κB, as evaluated by EMSA. This effect is reverted by the NK1 antagonist CP 96,345. Interestingly, the entity of the effect is similar to the PMA-induced one, so indicating SP as a potent activator of this transcription factor in human AMs. Interestingly, SP induced a three-fold increase (as evaluated by densitometry) in NF-κB nuclear translocation in AMs isolated from healthy smokers as compared to non-smokers. Consistent with previous reports in which human AMs were used (Carter et al., 1998; Farver et al., 1998), a constitutive expression of NF-κB in the nucleus of unstimulated AMs was always observed in our experiments. By using specific NF-κB DNA-binding sequences for IL-6, IL-8 and TNF-α promoters, Carter et al. (1998) demonstrated that different NF-κB complexes are generated in AMs from healthy volunteers and that specific NF-κB complexes are used for the transcription of these cytokine genes. They found that both p50 and p65 proteins bound to the IL-6 sequence, whereas a p50 protein bound to the TNF sequence and a p65 protein bound to the IL-8 sequence (Carter et al., 1998).
By Western blot assays and ELISA kits, we have detected both p65 and p50 subunits in human AMs. In our experiments, the p50 subunit seems to be the most abundant one in AMs from both smokers and non-smokers, being more efficiently translocated in smokers. In fact, unstimulated AMs from healthy smokers presented a more than doubled nuclear translocation of p50 (but about the same for p65) as compared to AMs from non-smokers. When AMs were challenged by PMA or NK-1R agonists, a further enhanced nuclear translocation of NF-κB subunits was observed: SP and [Sar9Met(O2)11]SP were particularly effective on p50 translocation (about three-fold) and their effects were significantly enhanced in AMs from smokers. PMA, too, was very potent (more than three-fold) in inducing p50 translocation and also significantly stimulated (although to a lesser degree) p65 translocation in healthy smokers. On the contrary, SP and [Sar9Met(O2)11]SP potentiated p65 nuclear translocation, but no significant variations were observed between smokers and non-smokers. This observation deserves further investigations but, in our opinion, is in keeping with the data demonstrating p50 as the major NF-κB subunit for the transcription of TNF-α gene (Carter et al., 1998). In fact, among the cytokines we evaluated, TNF-α is the one released to greater amounts by SP and NK1 agonists, whereas inconsistent effect on IL-10 release are observed. As known, IL-10 exerts anti-inflammatory effects and inhibits NF-κB activation in LPS-stimulated human AMs (Raychaudhuri et al., 2000). Moreover, according to the fact that reactive oxygen intermediates (in particular hydrogen peroxide) have been proposed as second messenger molecules in the activation pathway of NF-κB and that antioxidants usually inhibit NF-κB activation (Lieb et al., 1997), the demonstrated SP ability to induce oxy-radical production could play a role in SP-induced translocation of the transcription factor. To our knowledge, this is the first paper that describes NK-1R expression and activation in human AMs as a whole, although other different reports have investigated, in monocyte/macrophages of different origin, some of the points we focused here. In conclusion, this paper indicates SP as a potent contributor for tobacco smoke toxicity.
Acknowledgments
We thank Pfizer (Italy) for the kind gift of the NK-1R antagonist CP 96,345. We also are indebted with Drs Donato Colangelo and Luigia Grazia Fresu (School of Medicine, Novara, Italy) for helpful discussion. Research described in this article was supported by Philip Morris U.S.A. Inc., by Philip Morris International and by MIUR ex-60% 2003 grants.
Abbreviations
- AMs
alveolar macrophages
- CP96,345
(2S,3S)-cis-2-diphenylmethyl-N[(2-methoxyphenyl)-methyl]-1-azabicyclo-octan-3-amine)
- GR82334
([D-Pro9(spiro-gamma-lactam)Leu10,Trp11]physalaemin(1–11))
- GR71251
([Pro9(spiro-gamma-lactam) Leu10,Trp11]SP)
- NF-κB
nuclear factor-κB
- NK-1R
NK1 receptor
- O2−
superoxide anion
- PMA
phorbol 12-myristate 13-acetate
- SP
substance P
References
- ANDREAKOS E., SACRE S.M., SMITH C., LUNDBERG A., KIRIAKIDIS S., STONEHAUSE T., MONACO C., FELDMANN M., FOXWELL B.M. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103:2229–2237. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- BAI T.R., ZHOU D., WEIR T., WALKER B., HEGELE R., HAYASHI S., MCKAY K., BONDY G.P., FONG T. Substance P (NK-1)- and neurokinin A (NK-2)-receptor gene expression in inflammatory airway disease. Am. J. Physiol. 1995;269:L309–L317. doi: 10.1152/ajplung.1995.269.3.L309. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- BONDESON J., BROWNE K.A., BRENNAN F.M., FOXWELL B.M., FELDMANN M. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J. Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- BROWN G.P., IWAMOTO G.K., MONICK M.M., HUNNINGHAKE G.W. Cigarette smoking decreases interleukin 1 release by human alveolar macrophages. Am. J. Physiol. 1989;256:C260–C264. doi: 10.1152/ajpcell.1989.256.2.C260. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., BORDIN G., COLANGELO D., VIANO I. Tachykinin receptors on human monocytes: their involvement in rheumatoid arthritis. Neuropeptides. 1998;32:215–223. doi: 10.1016/s0143-4179(98)90040-3. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., GUIDOTTO S., VIANO I., FANTOZZI R., POZZI E., GHIO P., ALBERA C. Tachykinin activation of human alveolar macrophages in tobacco smoke and sarcoidosis: a phenotypical and functional study. Neuropeptides. 1996;30:456–464. doi: 10.1016/s0143-4179(96)90010-4. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., PARENTI A., CENI E., GIOTTI A., FANTOZZI R. Enhanced responsiveness of ovalbumin-sensitized guinea-pig alveolar macrophages to tachykinins. Br. J. Pharmacol. 1992;107:964–969. doi: 10.1111/j.1476-5381.1992.tb13392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNELLESCHI S., PENENGO L., LAVAGNO L., SANTORO C., COLANGELO D., VIANO I., GAUDINO G. Macrophage stimulating protein (MSP) evokes superoxide anion production by human macrophages of different origin. Br. J. Pharmacol. 2001;134:1285–1295. doi: 10.1038/sj.bjp.0704356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNELLESCHI S., TARLI S., GIOTTI A., FANTOZZI R. Priming effects of mammalian tachykinins on human neutrophils. Life Sci. 1991;48:PL1–PL5. doi: 10.1016/0024-3205(91)90416-9. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., VANNI L., LEDDA F., GIOTTI A., MAGGI C.A., FANTOZZI R. Tachykinins activate guinea-pig alveolar macrophages: involvement of NK1 and NK2 receptors. Br. J. Pharmacol. 1990;100:417–420. doi: 10.1111/j.1476-5381.1990.tb15821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABERLOTTO L., HURD Y.L., MURDOCK P., WAHLIN J.P., MELOTTO S., CORSI M., CARLETTI R. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur. J. Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- CARTER A.B., MONICK M.M., HUNNINGHAKE G.W. Lipopolysaccharide-induced NF-κB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am. J. Respir. Cell. Mol. Biol. 1998;18:384–391. doi: 10.1165/ajrcmb.18.3.2972. [DOI] [PubMed] [Google Scholar]
- DUSSER D.J., DJOKIC T.D., BORSON D.B., NADEL J.A. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J. Clin. Invest. 1989;84:900–906. doi: 10.1172/JCI114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARVER C.F., RAYCHAUDHURI B., BUHROW L.T., CONNORS M.J., THOMASSEN M.J. Constitutive NF-κB levels in human alveolar macrophages from normal volunteers. Cytokine. 1998;10:868–871. doi: 10.1006/cyto.1998.0373. [DOI] [PubMed] [Google Scholar]
- FONG T.M., ANDERSON S.A., YU H., HUANG R.R., STRADER C.D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- GERMONPRE P.R., BULLOCK G.R., LAMBRECHT B.N., VAN DE VELDE V., LUYTEN W.H., JOOS G., PAUWELS R.A. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur. Respir. J. 1999;14:776–782. doi: 10.1034/j.1399-3003.1999.14d08.x. [DOI] [PubMed] [Google Scholar]
- GUO J.Z., YOSHIOKA K., ZHAO F.Y., HOSOKI R., MAEHARA T., YANAGISAWA M., HAGAN R.M., OTSUKA M. Pharmacological characterization of GR82334, a tachykinin NK1 receptor antagonist, in the isolated spinal cord of the neonatal rat. Eur. J. Pharmacol. 1995;281:49–54. doi: 10.1016/0014-2999(95)00228-d. [DOI] [PubMed] [Google Scholar]
- HAGAN R.M., IRELAND S.J., JORDAN C.C., BERESFORD I.J.M., STEPHENS-SMITH M.L., EWAN G., WARD P. GR71251, a novel, potent and highly selective antagonist at neurokinin-1 receptors. Br. J. Pharmacol. 1990;99:62P. [Google Scholar]
- HO W.Z., LAI J.P., ZHU X.H., UVAYDOVA M., DOUGLAS S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- IWAMOTO I., NAKAGAWA N., YAMAZAKI H., KIMURA A., TOMIOKA H., YOSHIDA S. Mechanism for substance P-induced activation of human neutrophils and eosinophils. Regul. Pept. 1993;46:228–230. doi: 10.1016/0167-0115(93)90042-7. [DOI] [PubMed] [Google Scholar]
- KIM K.H., PARK K.C., CHUNG J.H., CHOI H.R. The effect of substance P on peripheral blood mononuclear cells in patients with atopic dermatitis. J. Dermat. Sci. 2003;32:115–124. doi: 10.1016/s0923-1811(03)00070-7. [DOI] [PubMed] [Google Scholar]
- LAI J.P., DOUGLAS S.D., SHAHEEN F., PLEASURE D.E., HO W.Z. Quantification of Substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin. Diagn. Lab. Immunol. 2002b;9:138–143. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI J.P., HO W.Z., YANG J.H., WANG X., SONG L., DOUGLAS S.D. A non-peptide substance P antagonist down-regulates SP mRNA expression in human mononuclear phagocytes. J. Neuroimmunol. 2002a;128:101–108. doi: 10.1016/s0165-5728(02)00164-9. [DOI] [PubMed] [Google Scholar]
- LAVAGNO L., BORDIN G., COLANGELO D., VIANO I., BRUNELLESCHI S. Tachykinin activation of human monocytes from patients with rheumatoid arthritis: in vitro and ex-vivo effects of cyclosporin A. Neuropeptides. 2001;35:92–99. doi: 10.1054/npep.2001.0850. [DOI] [PubMed] [Google Scholar]
- LAVAGNO L., GUNELLA G., BARDELLI C., SPINA S., FRESU L.G., VIANO I., BRUNELLESCHI S. Anti-inflammatory drugs and tumor necrosis factor-α production in monocytes: role of transcription factor NF-κB and implication in rheumatoid arthritis therapy. Eur. J. Pharmacol. 2004;501:199–208. doi: 10.1016/j.ejphar.2004.07.101. [DOI] [PubMed] [Google Scholar]
- LI H., LEEMAN S.E., SLACK B.E., HAUSER G., SALTSMAN W.S., KRAUSE J.E., BLUSZTAJN J.K., BOYD N.D. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEB K., FIEBICH B.L., BERGER M., BAUER J., SCHULZE-OSTHOFF K. The neuropeptide substance P activates transcription factor NF-κB and κB-dependent gene expression in human astrocytoma cells. J. Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- LOTZ M., VAUGHAN J.H., CARSON D.A. Effects of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- MANTYH P.W., ROGERS S.D., GHILARDI J.R., MAGGIO J.E., MANTYH C.R., VIGNA S.R. Differential expression of two isoforms of the neurokinin-1 (substance P) receptor in vivo. Brain Res. 1996;719:8–13. doi: 10.1016/0006-8993(96)00050-9. [DOI] [PubMed] [Google Scholar]
- MARRIOTT I., BOST K.L. IL-4 and IFN-γ up-regulate substance P receptor expression in murine peritoneal macrophages. J. Immunol. 2000;165:182–191. doi: 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- MARRIOTT I., MASON M.J., ELHOFY A., BOST K.L. Substance P activates NF-κB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J. Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BRONNER C., BUEB J.L., TSCHIHART E., GIES J.P., LANDRY Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J. Pharmac. Exp. Ther. 1989;250:329–335. [PubMed] [Google Scholar]
- PAGE N.M., BELL N.J. The human tachykinin NK1 (short form) and tachykinin NK4 receptor; a reappraisal. Eur. J. Pharmacol. 2002;437:27–30. doi: 10.1016/s0014-2999(02)01278-5. [DOI] [PubMed] [Google Scholar]
- PASCUAL D.W., XU-AMANO J.C., KIYONO H., MCGHEE J.R., BOST K.L. Substance P acts directly upon cloned B lymphoma cells to enhance IgA and IgM production. J. Immunol. 1991;146:2130–2136. [PubMed] [Google Scholar]
- PAYAN D.G., BREWSTER J.D., GOETZL E.J. Specific stimulation of human T lymphocytes by substance P. J. Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- PENNEFATHER J.N., LECCI A., CANDENAS M.L., PATAK E., PINTO F.M., MAGGI C.A. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- RASLEY A., BOST K.L., OLSON J.K., MILLER S.D., MARRIOTT I. Expression of functional NK-1 receptors in murine microglia. Glia. 2002;37:258–267. doi: 10.1002/glia.10034. [DOI] [PubMed] [Google Scholar]
- RAYCHAUDHURI B., FISHER C.J., FARVER C.F., MALUR A., DRAZBA J., KAVURU M.S., THOMASSEN M.J. Interleukin 10 (IL-10)-mediated inhibition of inflammatory cytokine production by human alveolar macrophages. Cytokine. 2000;12:1348–1355. doi: 10.1006/cyto.2000.0721. [DOI] [PubMed] [Google Scholar]
- SEVERINI C., IMPROTA G., FALCONIERI-ERSPAMER G., SALVADORI S., ERSPAMER V. The tachykinin peptide family. Pharmacol. Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- SIMEONIDIS S., CASTAGLIUOLO I., PAN A., LIU J.L., WANG C.C., MYKONIATIS A., PASHA A., VALENICK L., SOUGIOULTZIS S., ZHAO D., POTHOULAKIS C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-κB site on its promoter. Proc. Natn. Acad. Sci. U.S.A. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH V.C., SAGOT M.A., WONG H., BUCHAN A.M.J. Cellular expression of the neurokinin 1 receptor in the human antrum. J. Autonom. Nerv. Syst. 2000;79:165–172. doi: 10.1016/s0165-1838(99)00092-2. [DOI] [PubMed] [Google Scholar]
- TOMAKI M., ICHINOSE M., MIURA M., HIRAYAMA Y., YAMAUCHI H., NAKAJIMA N., SHIRATO K. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 1995;151:613–617. doi: 10.1164/ajrccm.151.3.7533601. [DOI] [PubMed] [Google Scholar]
- WU Z.X., LEE L.Y. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea-pigs: role of tachykinins. J. Appl. Physiol. 1999;87:1621–1628. doi: 10.1152/jappl.1999.87.5.1621. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI E., OKAZAKI N., ITOH A., ABE S., KAWAKAMI Y., OKUYAMA H. Interleukin 1 production by alveolar macrophages is decreased in smokers. Am. Rev. Respir. Dis. 1989;140:397–402. doi: 10.1164/ajrccm/140.2.397. [DOI] [PubMed] [Google Scholar]