Abstract
We have previously demonstrated that nitric oxide (NO) triggers CD34+-derived megakaryocyte apoptosis. We here show that prostacyclin (PGI2) inhibits PAPA/NO-induced megakaryocyte death detected by fluorescent microscopy and flow cytometry.
The cAMP-specific phosphodiesterase inhibitor, Ro 20-1724, and the permeable analog dibutyryl-cAMP also delayed apoptosis. PGI2 effect was fully prevented when adenylyl cyclase activity was suppressed by SQ 22536, and partially reversed by the permeable protein kinase A inhibitor PKI 14-22 amide. ELISA showed that while both PGI2 and NO alone or synergistically raised cAMP, only NO was able to increase intracellular cGMP levels.
Treatment of megakaryocytes with PGI2 abolished both basal and NO-raised cGMP levels. Addition of 8-pCPT-cGMP or activation of soluble guanylyl cyclase by BAY 41-2272 induced cell death in a concentration-dependent manner, and ODQ, an inhibitor of guanylyl cyclase, prevented both PAPA/NO- or BAY 41-2272-induced apoptosis. Specific cGMP phosphodiesterase inhibition by Zaprinast or suppression of adenylyl cyclase by SQ 22536 enhanced the PAPA/NO proapoptotic effect.
PGI2 completely inhibited NO-mediated generation and the increased activity of the cleaved form of caspase-3.
In conclusion, our results demonstrate that contrary to their well-known direct and synergistic inhibitory effects on platelets, PGI2 and NO regulate opposite megakaryocyte survival responses through a delicate balance between intracellular cyclic nucleotide levels and caspase-3 activity control.
Keywords: PGI2, NO, cAMP, cGMP, caspase
Introduction
The role of nitric oxide (NO) and prostacyclin (PGI2) in platelets has been extensively examined by in vitro and in vivo studies (Radomski & Moncada, 1993; Vane & Botting, 1995). In contrast, their effects on the platelet progenitor cell, the megakaryocyte, as well as in megakaryocytopoiesis are less known.
Concerning NO, Battinelli & Loscalzo (2000) and Battinelli et al. (2001) described that NO induces apoptosis of megakaryocytic cell lines and promotes platelet formation. Using CD34+-derived megakaryocytes, we have demonstrated that not only mature cells but also progenitors are susceptible to undergo programmed cellular death when exposed to NO (Schattner et al., 2001).
Regarding PGI2, the presence of its membrane receptor (IP) on megakaryocytes has been demonstrated previously; it was observed that IP appears in immature megakaryocytes and cell maturation accompanies enhancement of its expression (Sasaki et al., 1997). The functional role of IP during the different stages of human megakaryocyte maturation is not known.
Cytoprotection is a PGI2 biological property described soon after its discovery (Konturek et al., 1981; Bursch et al., 1989; Divald et al., 1990). However, in these studies, cell damage was attributed to cell necrosis. More recently, several reports showed that it not only protects cells from necrosis but also exerts antiapoptotic activity (Kroll et al., 1998; Meyer zu Vilsendorf et al., 2001; Cutler et al., 2003). Interestingly, depending on the cell type and/or PGI2 receptor interaction (cell surface IP or intracellular peroxisome proliferator-activated receptors (PPARs)), PGI2 exerts anti- or proapoptotic effects. Hatae et al. (2001) using embryonic kidney 293 cells demonstrated that while extracellular PGI2 exerted antiapoptotic effects through IP receptors, endogenous PGI2 triggered proapoptotic mechanisms through PPAR. In contrast, Cutler et al. (2003) demonstrated that stromal production of PGI2 promotes survival of colonocytes through PPAR δ activation.
Considering that both NO and PGI2 are released in the bone marrow milieu in the present study, we evaluated the potential cytoprotective effect of PGI2 in NO-mediated megakaryocyte apoptosis. We found that PGI2 prevents NO-induced megakaryocyte-programmed cellular death. This function is related not only to the recognized ability of PGI2 to increase cAMP levels but also to its capacity of interfering with NO-triggered death signaling pathways such as increases in cGMP and caspase-3 activation.
Methods
Materials
Myristoylated protein kinase A inhibitor 14–22 amide (PKI), adenylyl cyclase inhibitor SQ 22536, cGMP-specific phosphodiesterase inhibitor Zaprinast, guanylyl cyclase inhibitor 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) and caspase colorimetric substrate acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA) were purchased from Calbiochem (San Diego, CA, U.S.A.). BAY 41-2272 was a gift from Bayer HealthCare (Leverkusen, Germany). Sp-8-(4-chlorophenylthio) guanosine-3′,5′-cyclic monophosphate (8-pCPT-cGMP) was from Biomol International LP (Plymouth Meeting, PA, U.S.A.). Ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA), human α-thrombin, hirudin, ethylenediaminetetraacetic acid (EDTA), a specific phosphodiesterase (PDE) IV inhibitor, 4-(3-butoxy-4-methoxybenzyl) imidazolidin-2-one (Ro 20-1724) and N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (Dib-cAMP) were from Sigma Chemical Co. (St Louis, MO, U.S.A.). 1-propanamine,3-(2-hydroxy-2-nitroso-1-propylhydrazino) (PAPA/NO) and PGI2 (sodium salt) were from Cayman Chemical (Ann Arbor, MI, U.S.A.). Thrombopoietin (TPO) was from Peprotech (Veracruz, Mexico). Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against CD34 (Clone 581), glycoprotein (GP) IIb (CD41), P-selectin (CD62P), phycoerythrin (PE)-conjugated anti-CD41, anti-GPIb (CD42b) and FITC- or PE-conjugated isotypes were purchased from Beckman Coulter (Miami, FL, U.S.A.). Mini-MACS, goat-anti-mouse magnetic microbeads and human CD34 progenitor cell isolation kit were from Miltenyi Biotec (Bergisch Gladbach, Germany). Direct cAMP and cGMP kits were from Assay Designs (Ann Arbor, MI, U.S.A.). von Willebrand factor (VWF) kit was from Research Diagnostics Inc. (Flanders, NJ, U.S.A.). Anti-cleaved (activated) caspase-3 (Asp175) antibody was purchased from Cell Signaling Technology Inc. (Beverly, MA, U.S.A.). Rabbit IgG was from Sigma Chemical Co. Swine FITC-conjugated anti-rabbit immunoglobulins and anti-CD61 (Y2/51) were from Dako A/S (Glostrup, Denmark).
Stock solutions of BAY 41-2272, Zaprinast and ODQ were prepared in DMSO. Ro 20-1724 was solubilized in ethanol. PGI2 was dissolved in a basic phosphate-buffered saline (PBS, pH 10), PAPA/NO in NaOH (0.01 M) and TPO in Tris (10 mM, pH 8.0). All other drugs were dissolved in MilliQ water. Further dilutions of all reagents were carried out in Iscove's modified Dulbecco's medium (IMDM). When drugs were dissolved in DMSO or ethanol, its concentration never exceeded 0.04%. This DMSO or ethanol concentration did not elicit megakaryocyte apoptosis.
Isolation of CD34+ cells
Umbilical cord blood was collected during normal full-term deliveries with informed consent of the mother and used within 24 h. After collection, samples were diluted one-third in PBS and centrifuged. The upper phase containing platelets was removed. Low-density mononuclear cells were prepared by centrifugation of the remaining blood over a Ficoll Hypaque (1.077 g (cm3)−1) gradient. Cells collected from the interface were washed and resuspended in PBS containing EDTA (2 mM) (PBS/EDTA) and human albumin (0.5% (w v−1)). CD34+ cells were purified using a magnetic cell-sorting system (Miltenyi Biotec) in accordance with the manufacturer's recommendations. The purity of the CD34+-enriched population was determined by immunolabeling the cells with FITC-conjugated anti-CD34 monoclonal antibody that reacted with an epitope other than the antibody used for separation. After two Mini-MACS column separations, the purity of cell suspension was determined by flow cytometry and ranged typically between 95 and 99% for CD34+.
Megakaryocyte in vitro expansion and purification
Freshly isolated CD34+ cells (5 × 104 ml−1) were cultured in a six-well plate at 37°C in a humidified atmosphere with 5% CO2 as described previously (Sanz et al., 2001) with some modifications. Briefly, CD34+ cells were cultured in IMDM containing glutamine (2 mM), human serum (5%) obtained by recalcification of citrated platelet-free plasma, TPO (25 ng ml−1) and antibiotics (growth medium). At day 6, cell density was readjusted to 2.5 × 105 ml−1 with fresh growth medium. After 4–6 days, flow cytometry studies showed that more than 75% of cells were megakaryocytes (CD41+) presenting maturation markers such as: (1) increased CD41 intensity (mean fluorescence intensity expressed as arbitrary units: 205±26, 1191±198, 1755±266, 4058±118 at days 3, 6, 9 and 12 of culture, n=4), (2) CD42b expression (72±5% of the megakaryocyte population determined by two-color flow cytometry analysis, n=5) and (3) P-selectin expression and VWF release after α-thrombin stimulation (Figure 6). In addition, cultures showed proplatelet-bearing megakaryocytes. Apoptosis was induced without changing the growth medium, at 37°C in a humidified atmosphere with 5% CO2.
Figure 6.
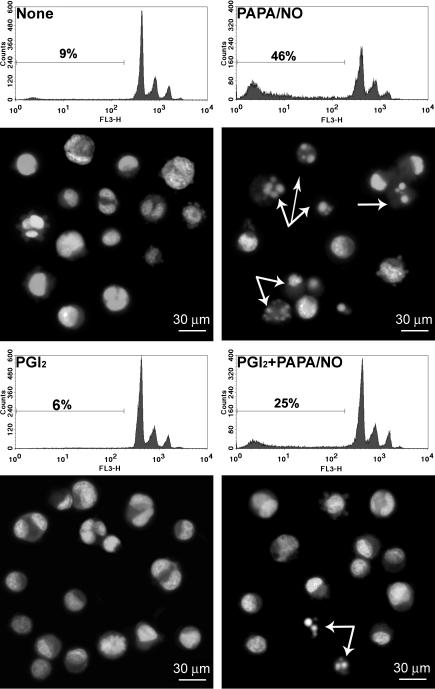
Inhibition of caspase-3 cleavage by cAMP raising agents. Megakaryocytes were treated for 1 min with PGI2 (3 μM) and further incubated for 5 h with PAPA/NO (100 μM). Activated caspase-3 was then detected by flow cytometry. Histograms show isotype (gray area) or activated caspase-3-stained cells (black line) and are representative of three similar experiments performed in duplicate.
In selected experiments and for cyclic nucleotide measurement, megakaryocytes were further purified by immunomagnetical positive selection using anti-CD61 antibody (10 min on ice) and goat-anti-mouse magnetic microbeads (20 min, 4°C) (Schmitz et al., 1994). After Mini-MACS column separation, the purity of the final cell suspension was determined by flow cytometry and was typically between 90 and 95%. Cell viability was greater than 90%.
Quantitation of cellular apoptosis and viability by fluorescence microscopy
Cells (5 × 105 ml−1) were labeled with a mixture of the fluorescent DNA-binding dyes acridine orange (100 μg ml−1) to determine the percentage of cells that had undergone apoptosis and ethidium bromide (100 μg ml−1) to differentiate between viable and nonviable cells. With this method, nonapoptotic cell nuclei show structural variations in fluorescence intensity that reflect the distribution of euchromatin and heterochromatin. In contrast, apoptotic nuclei exhibit highly condensed chromatin uniformly stained by acridine orange (Coligan et al., 1994). To assess the percentage of cells showing morphologic features of apoptosis, at least 300 cells were scored in each experiment.
Quantitation of megakaryocyte apoptosis by propidium iodide staining and flow cytometry
Megakaryocyte population displaying a hypodiploid DNA peak was determined as described previously (Schattner et al., 2001). Briefly, cells (1 × 106 ml−1) were labeled with FITC-conjugated anti-CD41 or isotype-matched IgG and fixed in 0.5% paraformaldehyde. DNA was then stained by cell incubation for 20 min at 4°C with 400 μl of a solution containing propidium iodide (50 μg ml−1 in 0.1% sodium citrate) and 0.1% Triton X-100 for cell permeabilization. This was followed by RNA digestion. The red fluorescence of propidium iodide in individual nuclei of the CD41+ population was measured by flow cytometry.
Analysis of caspase-3 activation by flow cytometry
Cells (1 × 106 ml−1) were first labeled with PE-conjugated anti-CD41 or isotype-matched IgG and then fixed and permeabilized using Fix & Perm kit (Beckman Coulter), following the manufacturer's instructions. After labeling with a polyclonal antibody specific for the activated form of caspase-3 for 2 h at room temperature, cells were washed and incubated with secondary FITC-conjugated swine anti-rabbit immunoglobulins for 30 min at room temperature. As nonspecific binding control, anti-cleaved caspase-3 antibody was replaced by a similar concentration of rabbit IgG. The percentage of activated caspase-3-expressing cells in the CD41+ population was analyzed by two-color flow cytometry excluding debris and platelets on the basis of their low light-scatter properties.
Ac-DEVD-pNA cleavage assay
Megakaryocytes (1 × 106 ml−1) were incubated for 5 h with PAPA/NO or PGI2+PAPA/NO. Then, cells were washed twice with PBS/EDTA and lysed with lysis buffer (50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10 mM EGTA, 10 μM digitonin, 0.5 mM phenylmethylsulfonyl fluoride, 1.5 μM aprotinin, 14.6 μM pepstatin and 63.9 μM benzamidine). Lysates were collected, clarified by centrifugation and caspase-3-like activity in the supernatants was measured 2 h after substrate addition by spectrophotometry. Substrate alone was used as blank and subtracted from each sample. The caspase-catalyzed release of pNA was monitored at 405 nm in a microtiter plate reader and the cleavage activity was expressed as pNA absorbance units (A405) per 106 cells.
cAMP and cGMP measurement
Purified megakaryocytes (5 × 105 ml−1) were stimulated with PGI2, PAPA/NO or the combination of both drugs for 15 min at 37°C. Triton X-100 (1%) in 0.1 N HCl was used as lysis buffer. Acetylated intracellular cAMP or cGMP levels were measured in cell lysates using a commercial enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions.
Megakaryocyte VWF release and P-selectin expression measurements
Megakaryocytes (1 × 106 ml−1) were centrifuged in PBS/EDTA for 10 min at 200 × g. Cells were then resuspended in IMDM containing saturating concentrations of FITC-conjugated anti-CD62P or isotype-matched IgG in the presence or absence of PAPA/NO or PGI2. Cells were stimulated with α-thrombin for 3 min followed by the addition of hirudin. After 20 min, cells were immediately analyzed by flow cytometry. In VWF experiments, after centrifugation, cells were resuspended in IMDM in the presence or absence of PAPA/NO or PGI2. After α-thrombin stimulation, the amount of VWF released was measured in supernatants by ELISA. Results are expressed as the percentage of VWF released by α-thrombin stimulation (VWF values were extrapolated from a standard curve plotted from serial dilutions of normal pooled plasma, assuming a 10 μg ml−1 VWF concentration).
Statistical analysis
Data are expressed as means±s.e.m. and were analyzed by one-way analysis of variance (ANOVA), followed by the Newman–Keuls procedure to determine significant differences between groups. P-values lower than 0.05 were considered statistically significant.
Results
PGI2 reversion of NO-induced megakaryocyte apoptosis
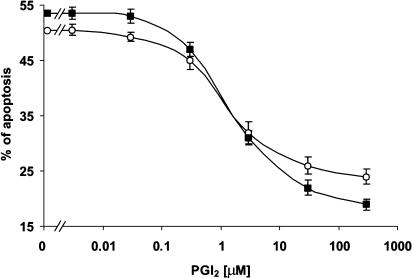
The addition of PAPA/NO to megakaryocyte cultures triggered apoptosis (ED50=98±4 μM). Figures 1 and 2 show that preincubation of cells with PGI2 significantly reduced the NO-induced megakaryocyte apoptosis in a concentration-dependent manner. In contrast, no significant antiapoptotic effect was observed when PGI2 was added after the NO donor (Table 1).
Figure 1.
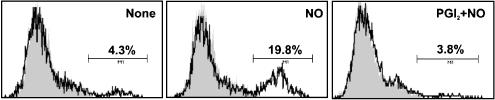
PGI2 protects megakaryocytes from NO-induced apoptosis. PGI2 was added 1 min before PAPA/NO (100 μM) and megakaryocyte apoptosis was evaluated 18 h later by fluorescent microscopy (open circles) or flow cytometry (solid squares) (n=5). All experiments were performed in duplicate.
Figure 2.
Microscope and flow cytometry analysis of apoptosis. PGI2 (3 μM) was added 1 min before PAPA/NO (100 μM). Data shown are representative of five independent experiments performed in duplicate and arrows indicate apoptotic cells.
Table 1.
Loss of PGI2 antiapoptotic effect when added after PAPA/NO
| Treatments | Apoptosis (%) |
|---|---|
| None | 16±3 |
| PAPA/NO | 42±4* |
| PGI2 1 min+PAPA/NO | 23±3# |
| PAPA/NO 15 min+PGI2 | 42±5¶ |
Megakaryocytes were treated as indicated, and 18 h later, the percentage of apoptosis was determined by flow cytometry (n=3).
P<0.05 vs none.
P<0.05 vs PAPA/NO alone.
P<0.05 vs PGI2 1 min+PAPA/NO alone.
Although we have used megakaryocyte cultures with at least 75% of CD41+ cells and apoptosis was evaluated in this cell population by using double stain flow cytometry, some experiments were performed using highly purified megakaryocytes (⩾98%). Under these conditions, PGI2 (3 μM) showed a similar pattern of apoptosis inhibition (18, 56 and 30% of apoptotic cells, in control, PAPA/NO and PGI2+PAPA/NO respectively, n=2).
Role of cAMP in PGI2 antiapoptotic activity
The IP-receptor-mediated PGI2 signaling pathway involves activation of adenylyl cyclase followed by an increase in intracellular cAMP levels (Best et al., 1977). In order to examine whether cAMP was involved in the prevention of apoptosis mediated by PGI2, we used a cAMP-specific PDE inhibitor Ro 20-1724 and the permeable analog Dib-cAMP. Like PGI2, both drugs significantly reduced NO-induced apoptosis (Table 2). Moreover, preincubation (5 min) with the adenylyl cyclase inhibitor SQ 22536 (500 μM) abolished PGI2 cytoprotection (SQ 22536 12±1%, NO 50±2%, PGI2+NO 24±1% and SQ 22536+PGI2+NO 48±1% of apoptosis, n=3). As expected, treatment with PGI2 significantly raised cAMP when compared to untreated cells. In a lesser degree, PAPA/NO elicited the same response, and a higher cAMP increase was observed when megakaryocytes were treated with both drugs suggesting a synergistic effect (Table 3). In order to evaluate whether cAMP increases mediated by PAPA/NO serve as a self-regulatory mechanism of NO-induced apoptosis, the NO proapoptotic effect was evaluated in the presence of SQ 22536. Figure 3 shows that blocking of adenylyl cyclase enhanced megakaryocyte sensitivity towards PAPA/NO cytotoxic effect.
Table 2.
cAMP elevating drugs prevent megakaryocyte apoptosis
| Apoptosis (%) | ||
|---|---|---|
| Treatments | None | PAPA/NO |
| None | 14±2 | 51±4* |
| PGI2 | 12±1 | 25±2# |
| Ro 20-1724 | 14±1 | 27±2# |
| Dib-cAMP | 14±2 | 24±2# |
Megakaryocytes were incubated in the absence (none) or presence of PAPA/NO (100 μM). PGI2 (3 μM), Dib-cAMP (100 μM) or Ro 20-1724 (10 μM) were added 1, 1 and 30 min, respectively, before PAPA/NO. After 18 h, the percentage of apoptosis was determined by flow cytometry (n=4 in duplicate).
P<0.05 vs none
P<0.05 vs PAPA/NO alone.
Table 3.
Regulation of intracellular cAMP and cGMP levels
| Treatments | cAMP (nM) | cGMP (nM) |
|---|---|---|
| None | 0.29±0.01 | 0.10±0.01 |
| PAPA/NO | 0.49±0.01* | 4.03±0.09* |
| PGI2 | 2.68±0.08* | Nondetectable |
| PGI2+PAPA/NO | 5.01±0.08¶,# | 0.60±0.02# |
Acetylated cAMP or cGMP in megakaryocyte lysates were determined 15 min post treatment with PGI2 (3 μM), PAPA/NO (100 μM) or the combination of both drugs (PGI2 was added 1 min before NO) (n=4 in duplicate).
P<0.05 vs none.
P<0.05 vs PGI2 alone.
P<0.05 vs PAPA/NO. The detection limit of the cGMP assay was 0.025 nM.
Figure 3.
Role of cAMP in NO-induced apoptosis. Megakaryocytes were preincubated in the presence (white bars) or absence (black bars) of SQ 22536 (500 μM) for 5 min before NO donor. Apoptosis was evaluated by detection of hypodiploid nuclei in the CD41+ population 18 h later (n=5 in duplicate). *P<0.05 vs none; #P<0.05 vs NO; &P<0.05 vs SQ 22536 alone.
Role of PKA on PGI2-mediated cytoprotection
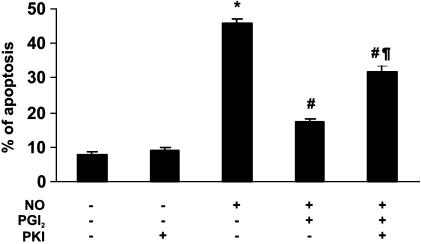
To investigate whether the cytoprotective effects of cAMP involved PKA activation, megakaryocytes were incubated with PKI (a PKA inhibitor). The antiapoptotic effect of PGI2 was significantly reduced, although not completely suppressed, suggesting that, at least in part, activation of PKA is a downstream signal in the cAMP cascade that mediates the PGI2 cytoprotective effect (Figure 4).
Figure 4.
Role of PKA in cytoprotection mediated by PGI2. Megakaryocytes were preincubated for 30 min with PKI (100 nM) before PGI2 (3 μM) and NO donor (100 μM PAPA/NO). Apoptosis was evaluated by detection of hypodiploid nuclei in the CD41+ population 18 h later (n=4 in duplicate). *P<0.05 vs none; #P<0.05 vs NO; ¶P<0.05 vs PGI2+NO.
Regulation of cGMP megakaryocyte levels by NO and PGI2
As the main pathway of intracellular NO signaling involves an increase in cGMP (Loscalzo, 2001), we also evaluated PGI2 intracellular cGMP regulation. While the NO donor increased cGMP, unexpectedly, PGI2 markedly diminished both basal and NO-mediated increase in cGMP (Table 3). These findings point out that not only an increase in cAMP but also an abolition of NO-induced cGMP raised levels are involved in PGI2 antiapoptotic effect.
cGMP, a mediator of NO-induced megakaryocyte apoptosis
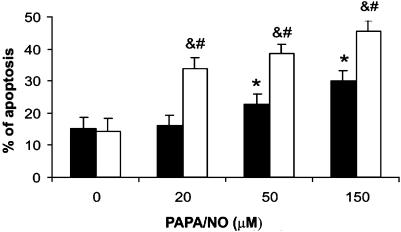
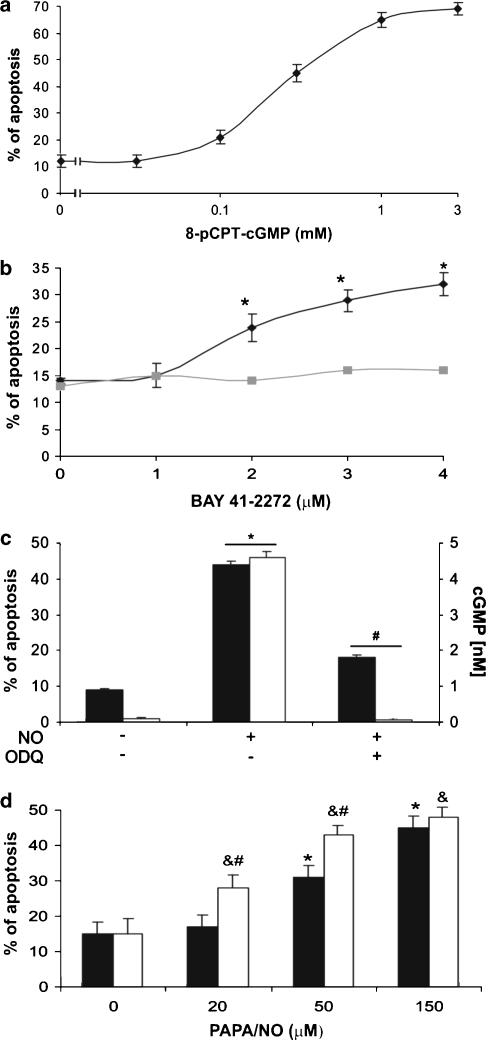
In our previous study, we reported that NO-induced megakaryocyte apoptosis seemed unrelated to cGMP because treatment with 8-Br-cGMP (3–6 mM) failed to trigger megakaryocyte apoptosis (Schattner et al., 2001). Since, in the present study, reversion of NO-induced apoptosis by PGI2 correlated with marked cGMP downregulation, we re-examined the role of this nucleotide on megakaryocyte death, to find that other cGMP analog, 8-pCPT-cGMP, increased the percentage of apoptosis in a concentration-dependent manner with an EC50=230 μM (Figure 5a). When the activity of soluble guanylyl cyclase was suppressed by ODQ, treatment of megakaryocytes with PAPA/NO was markedly reduced (Figure 5c). To further substantiate the possible involvement of cGMP in the control of cell viability, we tested the effect of BAY 41-2272, a direct activator of guanylyl cyclase (Stasch et al., 2001). Figure 5b shows that BAY 41-2272 triggered apoptosis in a concentration-dependent manner and it was completely abrogated by pretreatment of megakaryocytes with ODQ. On the other hand, Zaprinast (a cGMP-PDE inhibitor) was capable of increasing the apoptotic effect of PAPA/NO (Figure 5d).
Figure 5.
cGMP-mediated megakaryocyte apoptosis. (a) Megakaryocytes were treated with 8-pCPT-cGMP (n=3 in duplicate). (b) Cells were stimulated with BAY 41-2272 (n=5). ODQ (500 μM) was added 30 min before stimulation (n=3 in duplicate). (c) Apoptosis (black bars) was induced by PAPA/NO (100 μM), and acetylated cGMP (white bars) in megakaryocyte lysates was measured 15 min after NO addition. ODQ (500 μM) was added 30 min before the NO donor (n=5). (d) Cells were preincubated in the presence (white bars) or absence (black bars) of Zaprinast (40 μM) for 5 min before PAPA/NO and apoptosis was measured by flow cytometry (n=6). *P<0.05 vs none; #P<0.05 vs PAPA/NO; &P<0.05 vs Zaprinast alone.
Caspase-3 activation in NO-mediated cellular death
To further analyze the molecular mechanism involved in the PGI2 antiapoptotic effect and considering that it was recently demonstrated that senescent and apoptotic megakaryocytes show high levels of activated caspase-3 (De Botton et al., 2002), we next evaluated its expression in our experimental model. Maximal induction of caspase-3 was seen 5 h after NO-induced apoptosis (control 4.0±0.5%; NO 18.4±1.0% of positive cells, n=3, P<0.05) and was abolished by PGI2 (PGI2+NO 3.6±0.6% of positive cells, n=3; P<0.05 vs NO alone) (Figure 6). Considering that immunodetection of caspase-3 active fragment not necessarily imply an evidence of its enzymatic function, we next measured caspase-3 activity. As shown in Table 4, NO-treated megakaryocytes revealed more than four-fold increase in functional caspase-3 activity, whereas PGI2 completely abolished it.
Table 4.
Regulation of caspase-3 activity
| Treatments |
Ac-DEVD-pNA cleavage (A405/106 cells) |
|---|---|
| None | 0.056±0.012 |
| PAPA/NO | 0.240±0.023* |
| PGI2+PAPA/NO | 0.076±0.018 |
Megakaryocytes were exposed to drugs for 5 h and caspase-3 activity was determined by colorimetric detection of Ac-DEVD-pNA cleavage (n=3).
P<0.01 vs none or PGI2+PAPA/NO.
PGI2 and NO inhibit megakaryocyte activation responses
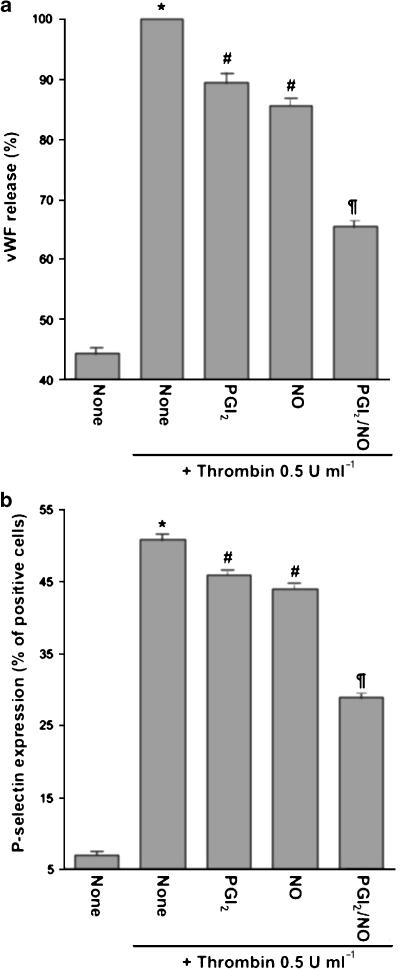
It is widely known that NO and PGI2 synergistically inhibit platelet activation through impairments in Ca2+ mobilization (Schwarz et al., 2001). To analyze the effect of these mediators on non-nuclear-mediated megakaryocyte responses, we studied PGI2 and NO regulation of α-thrombin-stimulated VWF release or P-selectin externalization. Unlike their opposite control of cell survival, both drugs independently and synergistically decreased both activation responses either in platelets (data not shown) or megakaryocytes (Figure 7).
Figure 7.
Regulation of α-thrombin-stimulated VWF release or P-selectin expression. Megakaryocytes were incubated with PGI2 (3 μM) and/or PAPA/NO (100 μM) for 1 min and α-thrombin was then added as indicated. Incubation was continued for 30 min and supernatants (a) or cells (b) collected for VWF assay or P-selectin detection, respectively (n=5 in duplicate). *P<0.05 vs none; #P<0.05 vs α-thrombin alone; ¶P<0.05 vs PGI2+α-thrombin and NO+α-thrombin.
Discussion
The present study shows that PGI2 protects megakaryocytes from NO-induced apoptosis. Our data showing that an increase in cAMP by different agents significantly inhibited NO-triggered megakaryocyte death and that PGI2 effect was completely suppressed by adenylyl cyclase blockade strongly indicate cAMP as the mediator of PGI2 antiapoptotic activity. Moreover, partial suppression of PGI2 effect by PKA inhibition suggests PKA as one of the downstream mediators in this cAMP signaling transduction pathway. However, we cannot rule out that other molecules could be involved. NO-induced apoptosis was not inhibited when PGI2 was added after the NO donor, indicating that cAMP is no longer effective once the apoptotic process had started.
Although pro- and antiapoptotic effects of cAMP have been previously described on other cells (Mcconkey & Orrenius, 1996), to the best of our knowledge, this is the first report showing that cAMP inhibits NO-induced megakaryocyte apoptosis.
The role of NO in the induction of the apoptotic cell death program has been extensively studied; however, the underlying mechanisms accounting for NO-induced cell toxicity are controversial. Rabkin & Kong (2000) demonstrated that H2O2 is involved in sodium nitroprusside-induced cardiotoxicity. Peroxinitrite formation has also been considered as a possible mediator of NO-induced neutrophil apoptosis (Ward et al., 2000). However, other groups reported that NO and cGMP analogs increase apoptosis in several cell types (Pollman et al., 1996; Loweth et al., 1997; Wu et al., 1997; Chiche et al., 1998; Suenobu et al., 1999; Kaminski et al., 2004).
Surprisingly, we found that PGI2 negatively regulated NO-mediated increase of cGMP levels. We also observed that while a guanylyl cyclase activator (BAY 41-2272) or a cGMP analog triggered megakaryocyte apoptosis, Zaprinast (a cGMP-specific PDE inhibitor) enhanced PAPA/NO-induced cell death. In addition, inhibition of guanylyl cyclase completely suppressed BAY 41-2272-mediated megakaryocyte death. All together, these results indicate that increases in cGMP levels exert a proapoptotic effect and that this second messenger is a major mediator of PAPA/NO death mechanism. However, since cellular death induced by this NO donor was not completely suppressed by ODQ, we do not rule out that other radical species (e.g. superoxide, peroxinitrite) could also be involved in the PAPA/NO cytotoxic effect.
The observation that elevation of cGMP levels triggers megakaryocyte-programmed cellular death also suggests that PGI2 antiapoptotic activity is associated with both phenomena: cAMP upregulation and cGMP downregulation. Furthermore, as in resting platelets (Jang et al., 2002), in unstimulated megakaryocytes, cAMP levels were greater than those of cGMP. While NO exposure reversed this relationship, pretreatment of cells with PGI2 maintained cAMP levels higher than cGMP. These data point out that the cAMP/cGMP ratio may be critical to determine the cell survival/death decision. In fact, the finding that both PGI2 and the NO donor increase cAMP, but only NO triggers apoptosis, could be explained by the fact that NO-mediated cGMP increases exceeded those of cAMP. The results showing that blockade of adenylyl cyclase sensitized megakaryocytes for PAPA/NO effects reinforce the notion that the NO-mediated cAMP increases represent a negative feedback control of the proapoptotic NO effects.
The ability of NO to increase cAMP as well as that of PGI2 to decrease cGMP levels indicates a crosstalk between cyclic nucleotides. The concept that in cells of megakaryocytic lineage, cAMP and cGMP downstream signaling events may interfere with each other was previously demonstrated in platelets (Maurice & Haslam, 1990; Grunberg et al., 1995). These studies showed that NO-mediated platelet inhibition involves not only cGMP but also cAMP increases due to PDE3A activity inhibition by cGMP. Herein, we demonstrated that a similar cAMP increase by NO occurs in megakaryocytes, although the mechanism has not yet been investigated. cGMP decreases by PGI2 have not been reported. However, recent in vitro studies show that PDE5 (specific for cGMP hydrolysis) can be phosphorylated by cGMP-PK and by cAMP-PK, thus increasing its catalytic activity and providing physiological negative feedback regulation of intracellular cGMP levels (Corbin et al., 2000). The possibility that similar PDE activation accounts for PGI2-mediated downregulation of cGMP deserves further investigation.
Our findings demonstrating that cAMP and cGMP exert opposite cellular actions in megakaryocytes are in sharp contrast with the well-known synergistic inhibitory effect of these second messengers on platelet activation (Radomski et al., 1987; Schwarz et al., 2001). However, we also showed that α-thrombin-mediated megakaryocyte activation (VWF release and P-selectin expression) was inhibited directly and synergistically by NO and PGI2. As apoptosis includes nuclear events, it is not unlikely that, besides calcium signaling pathway, downstream cyclic nucleotide signals in megakaryocytes include differential regulation of proteins involved in cell cycle, so that in anucleated platelets, these effects would be lacking. Interestingly, using another model, Wu et al. (1997) have previously described a similar cAMP and cGMP opposing roles in the modulation of cardiac myocyte growth and survival. It is important to note that in our experiments, concentrations of NO donor required to trigger apoptosis were higher than those generally used to exert other NO activities such as inhibition of platelet aggregation or vascular relaxation (Moro et al., 1996). However, this observation is in agreement with other studies performed not only in megakaryocytes (Battinelli & Loscalzo, 2000) but also in other cell types (Wu et al., 1997; Jarry et al., 2004; Velardez et al., 2004). It could be speculated that in vivo, these differences in NO effects are related to eNOS or iNOS involvement in NO generation.
Proteases of the caspase family represent the central executioners of the apoptotic process. Recent evidence has shown that in vitro grown megakaryocytes exhibit activation of caspase-3 and -9 during their terminal stages of maturation (De Botton et al., 2002). We found that while NO-induced apoptosis increased the percentage of positive cells for the active form of caspase-3 cells and its activity, PGI2 completely prevented both effects. Our results showing caspase-3 involvement in NO-mediated megakaryocyte apoptosis are in contrast to earlier publications suggesting that although NO induces Meg-01 apoptosis, it inhibits the protease activity possibly by S-nitrosylation of the caspase active site (Battinelli & Loscalzo, 2000). However, this effect was not observed in other cell types (Kim et al., 2003; Chae et al., 2004). A possible explanation for the differences between our findings and those from Battinelli's group could be the megakaryocytic source employed in each study (immortalized vs CD34+-derived megakaryocytes).
Developing megakaryocytes are distributed within two main compartments of the bone marrow: the osteoclastic niche (immature progenitors) and the vascular niche (mature megakaryocytes) (de Sauvage et al., 1996). The influence of the bone marrow microenvironment on megakaryocyte development and survival was recently examined by Avecilla et al. (2004). They have demonstrated that stromal cell-derived factor-1 (SDF-1) and fibroblast growth factor-4 (FGF-4) allow localization of megakaryocyte progenitors to the bone marrow vascular niche, promoting survival, maturation and platelet release independently of TPO. A very interesting feature of this study is that megakaryocytes and bone marrow endothelium interaction is required since both chemokines alone had no major effects. Moreover, because adhesion and transendothelial migration mediated by FGF-4 and SDF-1, respectively, were only partially blocked when adhesion molecules were inhibited, they suggested that other molecules released by vascular endothelium or extracellular matrix components may support chemokine-mediated effects. As we have shown that NO kills not only mature but also megakaryocyte progenitors and CD34+ cells (Schattner et al., 2001), NO apoptotic activity at early stages of megakaryocytopoiesis, in the osteoclastic niche, should be effectively controlled to avoid thrombocytopenia. In early megakaryocytopoiesis stages, when PGI2 surface receptor is absent (Sasaki et al., 1997), it may be speculated that the TPO survival signal would be predominant. However, when megakaryocytes are mobilized to the vascular niche, close contact with the marrow sinus endothelial barrier would allow maximal exposure to PGI2, which might contribute to the SDF-1- and FGF-4-mediated megakaryocyte survival. Interestingly, it has been demonstrated that SDF-1 triggers PGI2 release (Molino et al., 2000). Whether PGI2 regulates megakaryocyte maturation and platelet formation is currently under investigation in our laboratory. Although our findings provide new concepts for understanding megakaryocyte survival/death decision, additional experiments are required to determine the in vivo physiopathological relevance of these processes.
In summary, we provide in vitro evidence that cGMP is a key mediator of NO-induced megakaryocyte-programmed cellular death and that caspase-3 activation is a downstream effector. We also found that PGI2 prevents NO-induced megakaryocyte death not only by increasing intracellular cAMP levels but also through its ability to antagonize NO-triggered cGMP raises and caspase-3 activation. In contrast to the well-known inhibitory synergistic effect in platelets, this is the first report describing that, in their precursors, NO and PGI2 regulate opposite responses.
Acknowledgments
We are grateful to Dr Salvador Moncada for helpful discussions. This work was supported by grants from CONICET (PIP 733/98) (M.S.), National Agency of Scientific and Technological Support (PICT 8875) (M.A.L., M.S.), Antorchas Foundation (M.S.), René Baron Foundation (M.A.L.) and Ministry of Health ‘Ramón Carrillo-Arturo Oñativia' (M.S.).
Abbreviations
- 8-pCPT-cGMP
Sp-8-(4-chlorophenylthio) guanosine-3′,5′-cyclic monophosphate
- Ac-DEVD-pNA
acetyl-Asp-Glu-Val-Asp-p-nitroanilide
- Dib-cAMP
dibutyryladenosine 3′,5′-cyclic monophosphate
- FGF-4
fibroblast growth factor-4
- GP
glycoprotein
- IP
surface prostacyclin receptor
- NO
nitric oxide
- ODQ
1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one
- PAPA/NO
1-propanamine,3-(2-hydroxy-2-nitroso-1-propylhydrazino)
- PDE
phosphodiesterase
- PGI2
prostacyclin
- PKI
myristoylated protein kinase A inhibitor
- PPAR
peroxisome proliferator-activated receptor
- SDF-1
stromal cell-derived factor-1
- TPO
thrombopoietin
- VWF
von Willebrand factor
References
- AVECILLA S.T., HATTORI K., HEISSIG B., TEJADA R., LIAO F., SHIDO K., JIN D.K., DIAS S., ZHANG F., HARTMAN T.E., HACKETT N.R., CRYSTAL R.G., WITTE L., HICKLIN D.J., BOHLEN P., EATON D., LYDEN D., DE SAUVAGE F., RAFII S.Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis Nat. Med. 20041064–71.(Epub 21 Dec 2003) [DOI] [PubMed] [Google Scholar]
- BATTINELLI E., LOSCALZO J. Nitric oxide induces apoptosis in megakaryocytic cell lines. Blood. 2000;95:3451–3459. [PubMed] [Google Scholar]
- BATTINELLI E., WILLOUGHBY S.R., FOXALL T., VALERI C.R., LOSCALZO J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEST L.C., MARTIN T.J., RUSSELL R.G., PRESTON F.E. Prostacyclin increases cyclic AMP levels and adenylate cyclase activity in platelets. Nature. 1977;267:850–852. doi: 10.1038/267850a0. [DOI] [PubMed] [Google Scholar]
- BURSCH W., TAPER H.S., SOMER M.P., MEYER S., PUTZ B., SCHULTE-HERMANN R. Histochemical and biochemical studies on the effect of the prostacyclin derivative iloprost on CCl4-induced lipid peroxidation in rat liver and its significance for hepatoprotection. Hepatology. 1989;9:830–838. doi: 10.1002/hep.1840090607. [DOI] [PubMed] [Google Scholar]
- CHAE I.H., PARK K.W., KIM H.S., OH B.H. Nitric oxide-induced apoptosis is mediated by Bax/Bcl-2 gene expression, transition of cytochrome c, and activation of caspase-3 in rat vascular smooth muscle cells. Clin. Chim. Acta. 2004;341:83–91. doi: 10.1016/j.cccn.2003.11.009. [DOI] [PubMed] [Google Scholar]
- CHICHE J.D., SCHLUTSMEYER S.M., BLOCH D.B., DE LA MONTE S.M., ROBERTS J.D., JR, FILIPPOV G., JANSSENS S.P., ROSENZWEIG A., BLOCH K.D. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J. Biol. Chem. 1998;273:34263–34271. doi: 10.1074/jbc.273.51.34263. [DOI] [PubMed] [Google Scholar]
- COLIGAN J., KRUIBEEK A., MARGULIES D., SHEVACH E., STROBER W.Morphological and biochemical assays of apoptosis Current Protocols in Immunology 1994New York: John Wiley & Sons Inc; 3.17eds. Coligan, J., Kruisbeer, A., Margulies, D., Shevach, E. & Strober, W. pp [Google Scholar]
- CORBIN J.D., TURKO I.V., BEASLEY A., FRANCIS S.H. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 2000;267:2760–2767. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- CUTLER N.S., GRAVES-DEAL R., LAFLEUR B.J., GAO Z., BOMAN B.M., WHITEHEAD R.H., TERRY E., MORROW J.D., COFFEY R.J. Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer Res. 2003;63:1748–1751. [PubMed] [Google Scholar]
- DE BOTTON S., SABRI S., DAUGAS E., ZERMATI Y., GUIDOTTI J.E., HERMINE O., KROEMER G., VAINCHENKER W., DEBILI N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- DE SAUVAGE F.J., CARVER-MOORE K., LUOH S.M., RYAN A., DOWD M., EATON D.L., MOORE M.W. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J. Exp. Med. 1996;183:651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIVALD A., JENEY A., NAGY J.O., TIMAR F., LAPIS K. Modification of the inhibitory effects of CCl4 on phospholipid and protein biosynthesis by prostacyclin. Biochem. Pharmacol. 1990;40:1477–1483. doi: 10.1016/0006-2952(90)90443-o. [DOI] [PubMed] [Google Scholar]
- GRUNBERG B., NEGRESCU E., SIESS W. Synergistic phosphorylation of platelet rap1B by SIN-1 and iloprost. Eur. J. Pharmacol. 1995;288:329–333. doi: 10.1016/0922-4106(95)90045-4. [DOI] [PubMed] [Google Scholar]
- HATAE T., WADA M., YOKOYAMA C., SHIMONISHI M., TANABE T. Prostacyclin-dependent apoptosis mediated by PPAR delta. J. Biol. Chem. 2001;276:46260–46267. doi: 10.1074/jbc.M107180200. [DOI] [PubMed] [Google Scholar]
- JANG E.K., AZZAM J.E., DICKINSON N.T., DAVIDSON M.M., HASLAM R.J. Roles for both cyclic GMP and cyclic AMP in the inhibition of collagen-induced platelet aggregation by nitroprusside. Br. J. Haematol. 2002;117:664–675. doi: 10.1046/j.1365-2141.2002.03479.x. [DOI] [PubMed] [Google Scholar]
- JARRY A., CHARRIER L., BOU-HANNA C., DEVILDER M.C., CRUSSAIRE V., DENIS M.G., VALLETTE G., LABOISSE C.L. Position in cell cycle controls the sensitivity of colon cancer cells to nitric oxide-dependent programmed cell death. Cancer Res. 2004;64:4227–4234. doi: 10.1158/0008-5472.CAN-04-0254. [DOI] [PubMed] [Google Scholar]
- KAMINSKI A., GAO H., MORGAN N.G. Involvement of the cGMP signalling pathway in the regulation of viability in insulin-secreting BRIN-BD11 cells. FEBS Lett. 2004;559:118–124. doi: 10.1016/S0014-5793(04)00048-1. [DOI] [PubMed] [Google Scholar]
- KIM S.J., HWANG S.G., KIM I.C., CHUN J.S.Actin cytoskeletal architecture regulates nitric oxide-induced apoptosis, dedifferentiation, and cyclooxygenase-2 expression in articular chondrocytes via mitogen-activated protein kinase and protein kinase C pathways J. Biol. Chem. 200327842448–42456.(Epub 7 Aug 2003) [DOI] [PubMed] [Google Scholar]
- KONTUREK S.J., BRZOZOWSKI T., PIASTUCKI I., DEMBINSKI A., RADECKI T., DEMBINSKA-KIEC A., ZMUDA A., GREGORY H. Role of mucosal prostaglandins and DNA synthesis in gastric cytoprotection by luminal epidermal growth factor. Gut. 1981;22:927–932. doi: 10.1136/gut.22.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROLL B., KUNZ S., TU N., SCHWARZ L.R. Inhibition of transforming growth factor-beta1 and UV light-induced apoptosis by prostanoids in primary cultures of rat hepatocytes. Toxicol. Appl. Pharmacol. 1998;152:240–250. doi: 10.1006/taap.1998.8513. [DOI] [PubMed] [Google Scholar]
- LOSCALZO J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- LOWETH A.C., WILLIAMS G.T., SCARPELLO J.H., MORGAN N.G. Evidence for the involvement of cGMP and protein kinase G in nitric oxide-induced apoptosis in the pancreatic B-cell line, HIT-T15. FEBS Lett. 1997;400:285–288. doi: 10.1016/s0014-5793(96)01392-0. [DOI] [PubMed] [Google Scholar]
- MAURICE D.H., HASLAM R.J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol. Pharmacol. 1990;37:671–681. [PubMed] [Google Scholar]
- MCCONKEY D.J., ORRENIUS S. Signal transduction pathways in apoptosis. Stem Cells. 1996;14:619–631. doi: 10.1002/stem.140619. [DOI] [PubMed] [Google Scholar]
- MEYER ZU VILSENDORF A., LINK C., JORNS A., NAGEL E., KOHL J. Preconditioning with the prostacyclin analog epoprostenol and cobra venom factor prevents reperfusion injury and hyperacute rejection in discordant liver xenotransplantation. Xenotransplantation. 2001;8:41–47. doi: 10.1034/j.1399-3089.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- MOLINO M., WOOLKALIS M.J., PREVOST N., PRATICO D., BARNATHAN E.S., TARABOLETTI G., HAGGARTY B.S., HESSELGESSER J., HORUK R., HOXIE J.A., BRASS L.F. CXCR4 on human endothelial cells can serve as both a mediator of biological responses and as a receptor for HIV-2. Biochim. Biophys. Acta. 2000;1500:227–240. doi: 10.1016/s0925-4439(99)00110-6. [DOI] [PubMed] [Google Scholar]
- MORO M.A., RUSSEL R.J., CELLEK S., LIZASOAIN I., SU Y., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLMAN M.J., YAMADA T., HORIUCHI M., GIBBONS G.H. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ. Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- RABKIN S.W., KONG J.Y. Nitroprusside induces cardiomyocyte death: interaction with hydrogen peroxide. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H3089–H3100. doi: 10.1152/ajpheart.2000.279.6.H3089. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., MONCADA S. The biological and pharmacological role of nitric oxide in platelet function. Adv. Exp. Med. Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987;92:181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANZ C., BENET I., RICHARD C., BADIA B., ANDREU E.J., PROSPER F., FERNANDEZ-LUNA J.L. Antiapoptotic protein Bcl-x(L) is up-regulated during megakaryocytic differentiation of CD34(+) progenitors but is absent from senescent megakaryocytes. Exp. Hematol. 2001;29:728–735. doi: 10.1016/s0301-472x(01)00635-x. [DOI] [PubMed] [Google Scholar]
- SASAKI Y., TAKAHASHI T., TANAKA I., NAKAMURA K., OKUNO Y., NAKAGAWA O., NARUMIYA S., NAKAO K. Expression of prostacyclin receptor in human megakaryocytes. Blood. 1997;90:1039–1046. [PubMed] [Google Scholar]
- SCHATTNER M., POZNER R.G., ENGELBERGER I., GOROSTIZAGA A., MAUGERI N., GOMEZ R., PASQUALINI A., TORRES O., LAZZARI M.A. Effect of nitric oxide on megakaryocyte growth induced by thrombopoietin. J. Lab. Clin. Med. 2001;137:261–269. doi: 10.1067/mlc.2001.113659. [DOI] [PubMed] [Google Scholar]
- SCHMITZ B., RADBRUCH A., KUMMEL T., WICKENHAUSER C., KORB H., HANSMANN M.L., THIELE J., FISCHER R. Magnetic activated cell sorting (MACS) – a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. Eur. J. Haematol. 1994;52:267–275. doi: 10.1111/j.1600-0609.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- SCHWARZ U.R., WALTER U., EIGENTHALER M. Taming platelets with cyclic nucleotides. Biochem. Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- STASCH J.P., BECKER E.M., ALONSO-ALIJA C., APELER H., DEMBOWSKY K., FEURER A., GERZER R., MINUTH T., PERZBORN E., PLEISS U., SCHRODER H., SCHROEDER W., STAHL E., STEINKE W., STRAUB A., SCHRAMM M. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- SUENOBU N., SHICHIRI M., IWASHINA M., MARUMO F., HIRATA Y. Natriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 1999;19:140–146. doi: 10.1161/01.atv.19.1.140. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. Pharmacodynamic profile of prostacyclin. Am. J. Cardiol. 1995;75:3A–10A. doi: 10.1016/s0002-9149(99)80377-4. [DOI] [PubMed] [Google Scholar]
- VELARDEZ M.O., POLIANDRI A.H., CABILLA J.P., BODO C.C., MACHIAVELLI L.I., DUVILANSKI B.H.Long-term treatment of anterior pituitary cells with nitric oxide induces programmed cell death Endocrinology 20041452064–2070.(Epub 30 Dec 2003) [DOI] [PubMed] [Google Scholar]
- WARD C., WONG T.H., MURRAY J., RAHMAN I., HASLETT C., CHILVERS E.R., ROSSI A.G. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem. Pharmacol. 2000;59:305–314. doi: 10.1016/s0006-2952(99)00329-9. [DOI] [PubMed] [Google Scholar]
- WU C.F., BISHOPRIC N.H., PRATT R.E. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J. Biol. Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]