Abstract
The development of selective inhibitors of cyclooxygenase-2 (COX-2) was based on the concept that this enzyme played little, if any, role in modulating the ability of the gastrointestinal (GI) tract to resist and respond to injury. There is now overwhelming evidence that this is far from true. Indeed, COX-2 mediates several of the most important components of ‘mucosal defense', contributes significantly to the resolution of GI inflammation and plays a crucial role in regulating ulcer healing. COX-2 also contributes to long-term changes in GI function after bouts of inflammation.
Keywords: Cyclooxygenase, prostaglandin, ulcer, inflammation, lipoxin, nonsteroidal anti-inflammatory drugs, mucosal defense
Introduction
‘Never ignore a gut feeling, but never believe that it is enough'—Kermit (‘The Muppets')
The gastrointestinal (GI) tract is a dynamic environment that serves primarily as an entry site to the body for nutrients, while restricting the uptake of harmful antigens, toxins and microbes. Pivotal to this role is the ability to respond appropriately to potentially damaging luminal agents, a process referred to as ‘mucosal defense'. Failure of mucosal defense can lead to sustained mucosal tissue injury and chronic inflammation, and in extreme cases, to systemic infection.
Over a decade ago, cyclooxygenase-2 (COX-2) was cloned and described as an inducible enzyme involved in the generation of potent lipid mediators (i.e., prostaglandins (PGs)) during inflammation. Today, medications designed to selectively inhibit the activity of this enzyme are widely prescribed for the treatment of osteoarthritis and rheumatoid arthritis, as well as for acute pain. Selective inhibition of the COX-2 isotype with ‘coxibs', it was proposed, would achieve anti-inflammatory and analgesic effects comparable to those of conventional nonsteroidal anti-inflammatory drugs (NSAIDs), without causing damage in the GI tract. However, it is now clear that, at best, coxibs cause significant ulceration at about half the rate of conventional NSAIDs (Bombardier et al., 2000), while also exhibiting significant toxicity in the renal and cardiovascular systems (Cheng & Harris, 2004). The cardiovascular toxicity of rofecoxib, first suggested as early as 1998 (Mitchell & Evans, 1998), was cited as the reason for its withdrawal from world markets on September 30, 2004. Reports of increased cardiovascular complications in trials of rofecoxib, celecoxib, etoricoxib, paracoxib and valdecoxib support the notion that this toxicity is a feature of the entire class of selective COX-2 inhibitors (Bombardier et al., 2000; Crofford et al., 2000; Mukherjee et al., 2001; The Pink Sheet, 2001; Ott et al., 2003; Mamdani et al., 2004). The advent of the selective COX-2 inhibitor has stimulated considerable research into the role of this enzyme in mucosal defense and the associated impact on human health and disease. Here, we review some recent findings on COX-2 in the context of GI mucosal defense with a focus on cellular and molecular mechanisms, as well as some genetic considerations.
COX-2 and resistance to damage by luminal irritants
The lining of the GI tract is exposed regularly to a wide range of potentially damaging substances, including those that we ingest (alcohol, aspirin, etc.) and endogenous secretions (acid, bile salts). Perhaps most notable about the GI mucosa is not simply its ability to resist damage by these substances but also the impressive reparative capacity when damage is produced. PGs, particularly PGE2 and PGI2, play a very important role in modulating GI mucosa defense and repair (Wallace & Granger, 1996; Wallace & Ma, 2001). Among the many important effects of these PGs, with respect to mucosal defense, are stimulation of mucus and bicarbonate secretion, and maintenance of mucosal blood flow. Thus, inhibition of PG synthesis by NSAIDs is one of the primary mechanisms through which this class of drugs produces injury in the GI tract (Wallace, 1997). NSAIDs reduce mucus and bicarbonate secretion, as well as reduce mucosal blood flow. These drugs also trigger an increase in adhesion of leukocytes (most notably neutrophils) to the vascular endothelium in the GI microcirculation, which has been shown to be an early and critical event in the pathogenesis of NSAID-induced mucosal ulceration (Wallace, 1997).
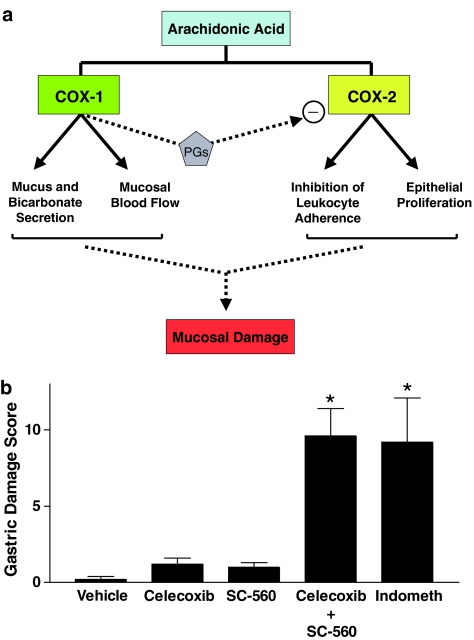
Early studies of selective COX-2 inhibitors in healthy rats and mice confirmed the predictions of their manufacturers that these drugs would not produce gastric injury. Since mice with targeted disruption of COX-2 did not develop spontaneous gastric lesions (Morham et al., 1995), the production of the PGs that mediate gastric mucosal defense, at least under normal conditions, was attributed to COX-1 activity. Surprisingly, COX-1 knockout mice also did not spontaneously develop gastric lesions, despite negligible gastric PG synthesis, but were susceptible to lesion formation when given an NSAID (Langenbach et al., 1995). Other studies demonstrated that suppression of gastric COX-1 activity in the rat with a selective inhibitor (SC-560) failed to elicit gastric damage (Wallace et al., 2000). When both COX-1 and COX-2 were inhibited, as would occur with conventional NSAIDs, gastric damage was elicited (Wallace et al., 2000). A similar situation was observed in models of NSAID-induced small intestinal injury; that is, selective inhibition of COX-1 or COX-2 did not result in injury, but suppression of both isoforms of COX led to significant damage (Tanaka et al., 2002). It can therefore be concluded that PGs derived from both COX-1 and COX-2 contribute to mucosal defense. Indeed, there is good evidence that the two COX isoforms may influence different components of mucosal defense: suppression of COX-1 accounts for the reduction in mucosal blood flow that is observed following NSAID administration, while suppression of COX-2 accounts for the increase in leukocyte adherence to vascular endothelium that is observed following NSAID administration (Wallace et al., 2000) (Figure 1).
Figure 1.
Contributions of COX-1 and COX-2 to GI mucosal defense. Panel a: PGs derived from the two COX isoforms influence different aspects of mucosal defense. COX-1 appears to produce the PGs that regulate mucosal blood flow and epithelial secretion of mucus and bicarbonate, while PGs from COX-2 influence epithelial proliferation and endothelial-leukocyte adherence. PGs from COX-1 also tonically suppress COX-2 activity in the GI tract. COX-2 is rapidly upregulated when COX-1 is inhibited, when the GI mucosa is exposed to a potentially damaging agent or when mucosal injury occurs. Suppression of COX-2 activity in these circumstances leads to enhanced mucosal injury and delayed repair. Panel b: Selective suppression of COX-1 (with SC-560) or of COX-2 (with celecoxib) does not result in mucosal injury in healthy rats, while suppression of both isoforms of COX leads to such damage. Indomethacin is a nonselective inhibitor of COX-1 and COX-2. This figure was constructed using previously published data (Wallace et al., 2000).
The studies described above involved administration of COX inhibitors to healthy animals, in which the only ‘challenge' of the mucosa was the COX inhibitor itself. We are only beginning to understand the contribution of COX-2 to the ability of the GI mucosa to resist and respond to luminal insults. For example, exposure of the rat stomach to 70% ethanol results in the formation of hemorrhagic lesions. Exposure of the rat stomach to a ‘mild irritant', such as 20% ethanol, results in a marked reduction in damage induced by subsequent exposure to 70% ethanol. This phenomenon has been termed ‘adaptive cytoprotection' (Robert et al., 1979). Pretreatment with anti-inflammatory doses of selective COX-2 inhibitors (NS-398, DFU, L-745,337) led to inhibition of the adaptive cytoprotective response (Gretzer et al., 2001). Another circumstance in which COX-2 appears to play a role in mucosal resistance to injury is that in which the stomach is subjected to a period of ischemia followed by reperfusion. In a rat model of ischemia–reperfusion, hemorrhagic lesions consistently form in the stomach, particularly if acid is present in the lumen during the period of ischemia. Ischemia–reperfusion leads to a marked upregulation of COX-2 expression in the stomach, and treatment with a selective COX-2 inhibitor prior to the period of ischemia resulted in a significant worsening of gastric injury (Maricic et al., 1999). Also, inhibition of the upregulation of COX-2 expression, through prior administration of a glucocorticoid, resulted in a significant exacerbation of ischemia–reperfusion-induced gastric damage (Maricic et al., 1999). As ischemia–reperfusion injury is primarily a neutrophil-driven response (Hernandez et al., 1987), it is possible that the ability of selective COX-2 inhibitors to increase leukocyte adherence to the vascular endothelium (Muscará et al., 2000) contributed to the production of injury in the stomach in this setting.
Emerging evidence indicates that COX-2 plays an expanded role in modulating resistance to luminal irritants when other mediators of mucosal defense are pharmacologically or genetically depressed. For example, nitric oxide is an important mediator of many components of mucosal defense (Wallace & Miller, 2000). Studies in the rat have demonstrated that when nitric oxide synthesis is inhibited, administration of selective COX-2 inhibitors results in significant gastric damage (Ehrlich et al., 2004). Sensory afferent nerves also contribute significantly to the ability of the GI mucosa to resist injury, mainly by regulating mucosal blood flow. When sensory afferent nerves are chemically ablated, administration of selective COX-2 inhibitors results in formation of hemorrhagic lesions (Ehrlich et al., 2004). Thus, at the molecular level, mucosal resistance to luminal irritants involves significant cross-talk among COX-2-derived signals and other endogenous signalling pathways.
A major focus of the research on this enzyme in the context of mucosal injury and defense in the upper GI tract is largely a consequence of manufacturers' claims that selective COX-2 inhibitors did not cause ulceration. However, there is convincing evidence for physiological and beneficial roles for COX-2 in maintenance of mucosal integrity in more distal parts of the GI tract. For example, COX-2 has been suggested to be an essential factor in immune tolerance. Newberry et al. (1999, 2001) reported that lamina propria stromal cells constitutively express COX-2 and produce PGE2 via this enzyme in a continuous manner. Given the known immunomodulatory effects of PGE2, these authors suggested that COX-2 contributes to ongoing downregulation of the intestinal immune response. Further evidence of COX-2 involvement in maintenance of mucosal integrity was the report that COX-2 knockout mice spontaneously developed peritonitis, presumably related to deterioration of intestinal barrier function (Morham et al., 1995). These consequences of either chronic suppression of COX-2 activity or absence of COX-2 expression may be related to a role for products of this enzyme in mediating GI epithelial proliferation (Sawaoka et al., 1997; Erickson et al., 1999). No doubt, further studies will delineate the molecular mechanisms behind beneficial roles of COX-2 in maintenance of mucosal integrity during responses to luminal irritants along the GI tract.
Dynamic regulation of GI COX-2 expression
The observation that COX-2 expression is low in the GI tract of healthy humans and animals (Kargman et al., 1996; Davies et al., 1997; Zimmerman et al., 1998) contributed to the notion that COX-2 played little, if any, role in mediating physiological events in the GI tract. However, as outlined in the section above, it is now clear that COX-2 expression is dynamic and required for maintenance of homeostasis. At the transcriptional level, COX-2 is an early-response gene to several stimuli. For example, significant induction of COX-2 in the rat stomach was detected as early as 1 h after administration of aspirin or indomethacin (Davies et al., 1997). The observation that induction of COX-2 could be prevented by concomitant administration of a PG suggested that diminished mucosal PG levels were responsible for triggering the upregulation of COX-2 expression (Davies et al., 1997). Indeed, Tanaka et al. (2002) observed that selective inhibition of COX-1 in the rat intestine led to rapid upregulation of COX-2 expression. Significant upregulation of COX-2 in the rat stomach has also been observed within 40 min of oral challenge with acid (Sawaoka et al., 1997). Taken together, these data suggest that rapid COX-2 induction is a common response to luminal irritation aimed at enhancing mucosal resistance to injury and at priming the mucosa to be prepared for repair in the event that injury does occur.
The specific role of COX-2 in resolution of inflammation is discussed later in this article. Studies in a rat model of colitis indicate that COX-2 also contributes to long-term changes in colonic function after resolution of colitis. As colitis resolves, COX-2 expression increases significantly, and, in parallel, there is increased synthesis of the downstream product PGD2. The elevated COX-2 expression and activity contributes to impaired epithelial secretion and increased bacterial translocation (Zamuner et al., 2003). Thus, selective inhibition of COX-2 causes a rapid normalization of colonocyte function and of epithelial barrier function. The prolonged increase in COX-2 expression and PGD2 synthesis following a bout of colitis might also contribute significantly to an increased susceptibility of the post-colitis rats to colon cancer (Zamuner & Wallace, 2004), mimicking the human situation in which patients with ulcerative colitis are at much greater risk of developing colon cancer (Itzkowitz & Yio, 2004). Selective COX-2 inhibitors have been suggested to have some utility for chemoprevention of colon cancer (Mann & DuBois, 2004). Thus, the dynamic nature of COX-2 expression is an important determinant in GI homeostasis and susceptibility to disease.
Lipoxin A4 (LXA4): a gastroprotective lipid
Concomitant administration of aspirin and selective COX-2 inhibitors results in the production of significantly more gastric damage than with either drug alone. This synergistic interaction has been observed in rodent (Fiorucci et al., 2002; Souza et al., 2003; Wallace et al., 2004) and human studies (Fiorucci et al., 2003b; Laine et al., 2004). An initial interpretation of these findings was that combined inhibition of COX-1 and COX-2 would produce more gastric damage because PGs from both isoforms of COX contribute to mucosal defense (Wallace et al., 2000). However, it now seems that products of arachidonic acid metabolism other than PGs may hold the key. In the presence of aspirin, COX-2 is acetylated and the enzyme is no longer able to catalyze the conversion of arachidonic acid to PGs. However, acetylated COX-2 can still metabolize arachidonic acid to 15-R-hydroxyeicosatetraenoic acid (15-R-HETE), which can be converted via 5-lipoxygenase to 15-epi-LXA4 (or ‘aspirin-triggered lipoxin' (ATL)) (Serhan, 1994). Like its epimer, ATL is a potent inhibitor of various neutrophil functions and exhibits a wide range of anti-inflammatory effects, as well as effects that stimulate resolution of inflammation (Serhan, 1994; Devchand et al., 2003).
The aspirin-dependent switch in COX-2 activity, leading to elevated levels of ATL, does occur in the stomach (Fiorucci et al., 2002). Co-administration of a selective COX-2 inhibitor with aspirin blocks the elevated formation of ATL, confirming that COX-2 activity is required for its synthesis. This is associated with a significant increase in the severity of gastric damage (Fiorucci et al., 2002; Wallace et al., 2004). LXA4 has potent protective effects in the stomach, being capable of significantly reducing the extent of aspirin-induced gastric damage in the rat when administered parenterally in the low nanomolar range (Fiorucci et al., 2002). Moreover, blockade of ALX, the G-protein-coupled LXA4 receptor, with peptide antagonists was found to significantly exacerbate aspirin-induced gastric damage, in a manner similar to the effects of selective COX-2 inhibition (Fiorucci et al., 2002).
COX-2-derived ATL may also mediate, at least in part, the well-recognized ability of the stomach to adapt to repeated exposure to aspirin. In humans and animals, many studies have demonstrated that the gastric mucosa also becomes progressively more resistant to injury during chronic ingestion of aspirin (Wallace, 1997). In parallel with this increased resistance to damage, there is marked upregulation of COX-2 expression in the gastric mucosa, and increased generation of ATL (Fiorucci et al., 2003a). Inhibition of ATL generation, with a COX-2 inhibitor, reversed this adaptive response, returning the susceptibility of the stomach to injury to its basal level (Fiorucci et al., 2003a). The studies above identify the aspirin-triggered LXA4 pathway as a protective mechanism involved in gastric resistance to damage caused by synthetic COX modulators like aspirin.
The studies described above have focussed on understanding the role of aspirin-triggered LXA4 derived from the acetylated-COX-2 pathway as a lipid mediator of gastroprotection. Both15-R-LXA4 and 15-S-LXA4 can trigger downstream signalling effects to the nucleus via circuits that have significant overlap (Qiu et al., 2001). It is important to remember that these two isomers can also be derived via mechanisms independent of aspirin and COX-2 (Claria et al., 1996).
COX-2 in the inflamed GI tract
Inflammation is a key element of mucosal defense. It is aimed at limiting entry of foreign material and microbes to the systemic circulation, as well as facilitating the repair of damaged tissue. Dysregulated inflammation can itself cause significant damage to host tissue. For instance, a dysregulated inflammatory response is thought to contribute to ulcer formation associated with use of NSAIDs, infection with Helicobacter pylori and in IBD. Resolution of inflammation is therefore a crucial process for restoring homeostasis, and one in which COX-2 plays a key role.
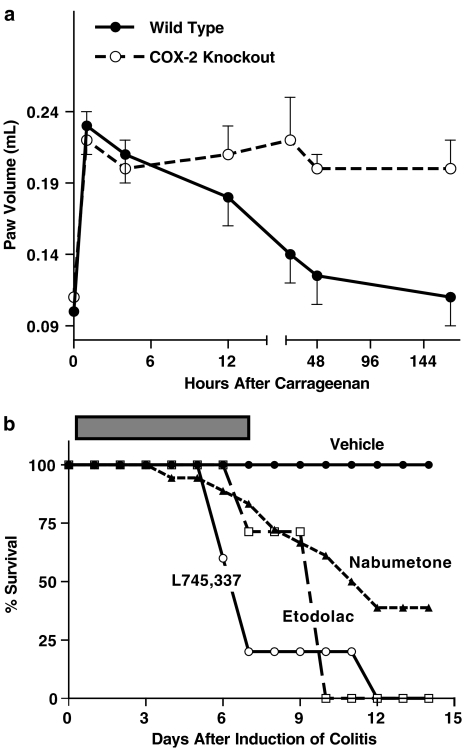
The importance of COX-2-derived PGs in resolving peripheral inflammation was evident from studies we performed using COX-2-deficient mice (Figure 2a). When carrageenan was injected into the hindpaw of normal mice, significant inflammation was induced, including significant edema formation, and it had resolved within 24–48 h. When carrageenan was injected into the hindpaw of COX-2-deficient mice, a similar acute inflammatory response was observed. However, even 7 days later the inflammatory response, including granulocyte infiltration and edema, was still evident (Wallace et al., 1998). Thus, in the absence of COX-2, the acute inflammatory response to carrageenan was dysregulated. This important role of COX-2 in resolution of inflammation has been observed in a range of inflammatory models. For example, Gilroy et al. (1999) demonstrated a key role for COX-2-derived PGs, in particular PGD2 and its metabolites, in the resolution of inflammation in a pleurisy model in rats. These metabolites appeared to produce the resolution via induction of apoptosis of infiltrating neutrophils and macrophages (Gilroy et al., 2003).
Figure 2.
COX-2 is crucial in resolution of inflammation. Panel a shows the change in paw volume in wild-type and COX-2-deficient mice of a 1-week period after subplantar injection of carrageenan. Note that the edema resolves in the wild-type mice, but not in the COX-2-deficient mice (Wallace et al., 1998). Panel b shows survival of rats after induction of colitis with trinitrobenzene sulfonic acid. The rats were treated with vehicle or a COX-inhibitor twice daily for 1 week after induction of colitis (Reuter et al., 1996). Equieffective anti-inflammatory doses of the COX inhibitors were used. The rank order of selectivity for COX-2 is L745,337>etodolac>nabumetone.
An anti-inflammatory role of COX-2 has been observed in studies of the colon. There is a rapid and substantial upregulation of COX-2 after induction of colitis in rats. This is accompanied by a sharp increase in colonic generation of PGD2, which has a dampening effect on granulocyte infiltration (Ajuebor et al., 2000). Consistent with these observations, treatment with selective COX-2 inhibitors during the early phase of experimental colitis leads to enhancement of granulocyte infiltration and, if continued for several days, to further penetration of ulcers deeper into the wall of the bowel and, eventually, to perforation and death (Figure 2b) (Reuter et al., 1996). Mice deficient in COX-2 (or in COX-1) were similarly shown to be more susceptible to chemically induced colitis (Morteau et al., 2000). There are also clinical reports of NSAIDs exacerbating colitis (Bonner, 2001; Matuk et al., 2004), in some cases with such events described as occurring with ‘high incidence' (Matuk et al., 2004).
At least one-half of the world's population has ongoing gastric inflammation, attributable to colonization of the stomach by H. pylori. This infection is associated with significantly elevated expression of COX-2 in the stomach (Tatsuguchi et al., 2000). Moreover, while acute administration of selective inhibitors of COX-2 to H. pylori-negative individuals does not result in mucosal injury, the same treatment of individuals with H. pylori-associated gastritis resulted in significant damage (Takahashi et al., 2000). Thus, there is an increased contribution of COX-2 to mucosal defense in a setting of H. pylori-associated inflammation. This has also been demonstrated in a rat model of gastric inflammation (Souza et al., 2003). Interestingly, the inflamed stomach was found to have a greater capacity to produce LXA4 in response to aspirin administration, and an increased resistance to aspirin-induced damage (Souza et al., 2003).
COX-2 and ulcer healing
Damage to the GI epithelium likely occurs on a daily basis, but true ‘ulcers' (which penetrate deeper than the mucosal layer) only rarely develop. Superficial damage to the mucosa can be healed in a few hours or days. When damage penetrates into the submucosa and muscularis, repair can take several weeks or months, and involves formation of granulation tissue at the ulcer base, formation of new blood vessels (angiogenesis) and and re-establishment of the glandular architecture. As outlined above, COX-2 expression in the normal stomach is low. However, at the margins of ulcers, COX-2 expression is very strong (Mizuno et al., 1997). It is at the margins of ulcers that epithelial proliferation primarily occurs, which is critical for re-establishment of glands. COX-2 is also strongly expressed in endothelial cells in the ulcer bed (Mizuno et al., 1997), which is the site of new vessel growth. Perhaps not surprisingly, selective COX-2 inhibitors, like conventional NSAIDs that also suppress COX-2 activity, significantly delay gastric ulcer healing (Mizuno et al., 1997; Schmassmann et al., 1998; Halter et al., 2001; Ma et al., 2002; Perini et al., 2003). Both selective and nonselective NSAIDs inhibit angiogenesis through direct effects on endothelial cells. Jones et al. (1999) demonstrated that this action involved inhibition of mitogen-activated protein kinase (ERK2) activity and interference with ERK nuclear translocation. This process occurred independently of protein kinase C and was partially PG-dependent and partially PG-independent.
The inhibition of ulcer healing associated with inhibition of COX-2 activity may be in part related to effects on serum levels of growth factors that regulate angiogenesis. Growth factors released from platelets and contained within serum can profoundly affect ulcer healing (Ma et al., 2001). When gastric ulcers are induced in rats, a shift in the serum and platelet levels of growth factors occurs such that the balance between pro- and antiangiogenic factors is tilted in favour of promotion of angiogenesis, thereby assisting ulcer healing (Ma et al., 2001). When rats with pre-established gastric ulcers were treated with a selective COX-2 inhibitor (celecoxib) or a conventional NSAID (flurbiprofen), the balance of pro- and antiangiogenic factors in serum was altered in the opposite direction, favoring inhibition of angiogenesis (Ma et al., 2002). Moreover, both celecoxib and flurbiprofen significantly inhibited ulcer repair in this model.
Genetic considerations
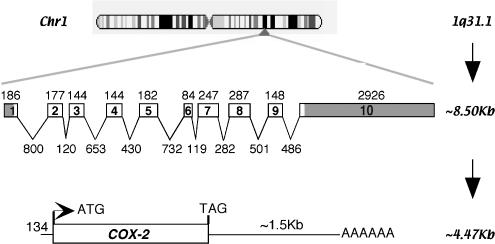
The human COX-2 gene is located on Chromosome 1 at q31.1 and is organized in 10 exons and introns (Figure 3; http://www.ensembl.org/Homo_sapiens/). Mutation databases report at least 35 single-nucleotide polymorphisms (SNPs) in both coding and noncoding (5′-upstream, introns and 3′-untranslated) regions (http://www.ncbi.nlm.nih.gov/projects/SNP/). When translated, the 16 SNPs in the coding region result in both synonymous and nonsynonymous changes but, to date, no phenotypes, GI-related or otherwise, have been associated with these hCOX-2 polymorphisms. Based on genetic ablation experiments in mice, one could speculate that, in the adult, the lack of phenotypic mutations in COX-2 is because a fully functional COX-2 is required for fetal implantation and embryonic development. However, rigorous analyses of larger population data sets might still reveal SNP-associated phenotypes.
Figure 3.
COX-2 gene. Human COX-2 is located on Chromosome 1 at q31.1. The transcript ∼8.5 kb consists of 10 introns and exons. Splicing results in a ∼4.47 kb mRNA, including a long 3′-untranslated region. This hCOX-2 message codes for a 604 amino-acid protein.
Processing of the ∼8.5 kb hCOX-2 transcript results in a spliced message of ∼4.47 kb (Figure 3a; Appleby et al., 1994). Particularly noteworthy are the short 5′- and long 3′-untranslated regions (O'Banion et al., 1992). Control of COX-2 expression is in part mediated via sequences in the ∼1.5 kb 3′-untranslated region. For example, studies using gamma irradiation on HT-29 colon cancer epithelial cells have deduced that control of COX-2 translation can be mediated by two AU-rich sequences in the first 60 nucleotides of the 3′-untranslated region. The molecular mechanism involves interaction of the AU-rich sequences with a RNA-binding protein previously identified for its editing activity at CUG sequences (CUGBP), to increase mRNA stability and inhibit translation of COX-2 (Mukhopadhyay et al., 2003). This dynamic, antagonistic relationship between COX-2 and CUGBP2 is relevant in intestinal epithelial crypt cells, as demonstrated by recent studies examining endotoxin-induced protection against radiation damage using mouse loss-of-function models for COX-1 and COX-2 (Murmu et al., 2004).
Human COX-2 is a 604 amino-acid heme protein that localizes to the nuclear membrane. Better understanding of the function of COX-2, including its role in GI mucosal defense, may be gained by studies of lower vertebrates. COX-2 is highly conserved in vertebrates, with recent reports of a zebrafish COX-2 with 67% identity to hCOX-2, as compared to 84% identity between COX-2 of rodents and hCOX-2 (Grosser et al., 2002). Comparison of COX-2 primary sequences from human (Hla & Neilson, 1992), rat (Kennedy et al., 1993), mouse (Dewitt et al., 1990) and zebrafish (Grosser et al., 2002) show that the greatest variability lies in the N- and C-terminals of the protein, while the highest conservation is in the epidermal growth factor and catalytic domains. Specific amino acids associated with post-translational modification (glycosylation sites at Asn53, 130, 396 and 580), heme coordination (His193 and 374) and catalytic residues (Arg106, Tyr341 and Tyr371) are conserved from human to zebrafish. Notably, the aspirin-acetylation site (Ser516) is also conserved (Lecomte et al., 1994). Moreover, several fish species have been characterized for their ability to generate and respond to LXs (Rowley, 1991). In particular, LXs have been shown to modulate migration of fish leukocytes, as they do in mammals. Thus, in the future, comparative studies in fish might be a useful approach to better understanding the role of COX-2 in human health and disease.
Conclusions
It is somewhat ironic that the development of selective COX-2 inhibitors, whose raison d'être was to reduce inflammation but spare the GI tract of injury, resulted in a series of discoveries that strongly suggest a crucial role of COX-2 in GI mucosal defense and repair. While expressed in low levels in the healthy GI tract, COX-2 nonetheless contributes significantly to mucosal immunity and to the ability of the mucosa to resist injury induced by luminal irritants. The COX-2 gene rapidly responds to stress, and the downstream products of this enzyme are potent lipid mediators that enhance resistance to injury and regulate dynamics of both inflammation and resolution. Emerging evidence implicates COX-2 in mediating some of the long-term consequences of inflammation in the GI tract, including the generation of symptoms in conditions such as irritable bowel syndrome, and the predisposition to cancer in individuals with colitis.
Acknowledgments
Dr Wallace is an Alberta Heritage Foundation for Medical Research Senior Scientist, is supported by research grants from the Canadian Institutes of Health Research and holds a Canada Research Chair in Inflammation Research.
Abbreviations
- ATL
aspirin-triggered lipoxin
- COX
cyclooxygenase
- GI
gastrointestinal
- HETE
hydroxyeicosatetraenoic acid
- IBD
inflammatory bowel disease
- LX
lipoxin
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- SNP
single-nucleotide polymorphism
References
- AJUEBOR M.N., SINGH A., WALLACE J.L. Cyclooxygenase-2-derived prostaglandin D2 is an early anti-inflammatory signal in experimental colitis. Am. J. Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- APPLEBY S.B., RISTIMAKI A., NEILSON K., NARKO K., HLA T. Structure of the human cyclo-oxygenase-2 gene. Biochem. J. 1994;302:723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMBARDIER C., LAINE L., REICIN A., SHAPIRO D., BURGOS-VARGAS R., DAVIS B., DAY R., FERRAZ M.B., HAWKEY C.J., HOCHBERG M.C., KVIEN T.K., SCHNITZER T.J. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N. Engl. J. Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- BONNER G.F. Exacerbation of inflammatory bowel disease associated with use of celecoxib. Am. J. Gastroenterol. 2001;96:1306–1308. doi: 10.1111/j.1572-0241.2001.03730.x. [DOI] [PubMed] [Google Scholar]
- CHENG H.F., HARRIS R.C. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- CLARIA J., LEE M.H., SERHAN C.N. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)–neutrophil interactions and are potent inhibitors of cell proliferation. Mol. Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- CROFFORD L.J., OATES J.C., MCCUNE W.J., GUPTA S., KAPLAN M.J., CATELLA-LAWSON F., MORROW J.D., MCDONAGH K.T., SCHMAIER A.H. Thrombosis in patients with connective tissue diseases treated with specific cyclooxygenase 2 inhibitors. A report of four cases. Arthritis Rheum. 2000;43:1891–1896. doi: 10.1002/1529-0131(200008)43:8<1891::AID-ANR28>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- DAVIES N.M., SHARKEY K.A., ASFAHA S., MACNAUGHTON W.K., WALLACE J.L. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment. Pharmacol. Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- DEVCHAND P.R., ARITA M., HONG S., BANNENBERG G., MOUSSIGNAC R.L., GRONERT K., SERHAN C. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- DEWITT D.L., EL-HARITH E.A., KRAEMER S.A., ANDREWS M.J., YAO E.F., ARMSTRONG R.L., SMITH W.L. The aspirin and heme-binding sites of ovine and murine prostaglandin endoperoxide synthases. J. Biol. Chem. 1990;265:5192–5198. [PubMed] [Google Scholar]
- EHRLICH K., SICKING C., RESPONDEK M., PESKAR B.M. Interaction of cyclooxygenase isoenzymes, nitric oxide, and afferent neurons in gastric mucosal defence in rats. J. Pharmacol. Exp. Ther. 2004;308:277–283. doi: 10.1124/jpet.103.057752. [DOI] [PubMed] [Google Scholar]
- ERICKSON B.A., LONGO W.E., PANESAR N., MAZUSKI J.E., KAMINSKI D.L. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J. Surg. Res. 1999;81:101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., DE LIMA O.M., JR, MENCARELLI A., PALAZZETTI B., DISTRUTTI E., MCKNIGHT W., DICAY M., MA L., ROMANO M., MORELLI A., WALLACE J.L. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., DISTRUTTI E., DE LIMA O.M., ROMANO M., MENCARELLI A., BARBANTI M., PALAZZINI E., MORELLI A., WALLACE J.L. Relative contribution of acetylated cyclo-oxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. FASEB J. 2003a;17:1171–1173. doi: 10.1096/fj.02-0777fje. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., WALLACE J.L., SARDINA M., ROMANO M., DEL SOLDATO P., MORELLI A. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc. Natl. Acad. Sci. U.S.A. 2003b;100:10937–10941. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., MCMASTER S., SAWATZKY D.A., WILLOUGHBY D.A., LAWRENCE T. Inducible cyclooxygenase-derived 15-deoxy-Δ12−14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. FASEB J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., WILLIS D., CHIVERS J., PAUL-CLARK M.J., WILLOUGHBY D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- GRETZER B., MARICIC N., RESPONDEK M., SCHULIGOI R., PESKAR B.M. Effects of specific inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the rat stomach with normal mucosa and after acid challenge. Br. J. Pharmacol. 2001;132:1565–1573. doi: 10.1038/sj.bjp.0703955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSER T., YUSUFF S., CHESKIS E., PACK M.A., FITZGERALD G.A. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8418–8423. doi: 10.1073/pnas.112217799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALTER F., TARNAWSKI A.S., SCHMASSMANN A., PESKAR B.M. Cyclooxygenase 2 – implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49:443–453. doi: 10.1136/gut.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ L.A., GRISHAM M.B., TWOHIG B., ARFORS K.E., HARLAN J.M., GRANGER D.N. Role of neutrophils in ischemia–reperfusion-induced microvascular injury. Am. J. Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- HLA T., NEILSON K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZKOWITZ S.H., YIO X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- JONES M.K., WANG H., PESKAR B.M., LEVIN E., ITANI R.M., SARFEH I.J., TARNAWSKI A.S. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- KARGMAN S., CHARLESON S., CARTWRIGHT M., FRANK J., RIENDEAU D., MANCINI J., EVANS J., O'NEILL G. Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111:445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- KENNEDY B.P., CHAN C.C., CULP S.A., CROMLISH W.A. Cloning and expression of rat prostaglandin endoperoxide synthase (cyclooxygenase)-2 cDNA. Biochem. Biophys. Res. Commun. 1993;197:494–500. doi: 10.1006/bbrc.1993.2506. [DOI] [PubMed] [Google Scholar]
- LAINE L., MALLER E.S., YU C., QUAN H., SIMON T. Ulcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double-blind trial. Gastroenterology. 2004;127:395–402. doi: 10.1053/j.gastro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- LANGENBACH R., MORHAM S.G., TIANO H.F., LOFTIN C.D., GHANAYEM B.I., CHULADA P.C., MAHLER J.F., LEE C.A., GOULDING E.H., KLUCKMAN K.D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- LECOMTE M., LANEUVILLE O., JI C., DEWITT D.L., SMITH W.L. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J. Biol. Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- MA L., DEL SOLDATO P., WALLACE J.L. Divergent effects of new cyclooxygenase inhibitors on gastric ulcer healing: shifting the angiogenic balance. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13243–13247. doi: 10.1073/pnas.202392199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA L., ELLIOTT S.N., CIRINO G., BURET A., IGNARRO L.J., WALLACE J.L. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6470–6475. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMDANI M., JUURLINK D.N., LEE D.S., ROCHON P.A., KOPP A., NAGLIE G., AUSTIN P.C., LAUPACIS A., STUKEL T.A. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet. 2004;363:1751–1756. doi: 10.1016/S0140-6736(04)16299-5. [DOI] [PubMed] [Google Scholar]
- MANN J.R., DUBOIS R.N. Cyclooxygenase-2 and gastrointestinal cancer. Cancer J. 2004;10:145–152. doi: 10.1097/00130404-200405000-00001. [DOI] [PubMed] [Google Scholar]
- MARICIC N., EHRLICH K., GRETZER B., SCHULIGOI R., RESPONDEK M., PESKAR B.M. Selective cyclo-oxygenase-2 inhibitors aggravate ischaemia–reperfusion injury in the rat stomach. Br. J. Pharmacol. 1999;128:1659–1666. doi: 10.1038/sj.bjp.0702966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATUK R., CRAWFORD J., ABREU M.T., TARGAN S.R., VASILIAUSKAS E.A., PAPADAKIS K.A. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2004;10:352–356. doi: 10.1097/00054725-200407000-00005. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., EVANS T.W. Cyclooxygenase-2 as a therapeutic target. Inflamm. Res. 1998;47 Suppl 2:S88–S92. doi: 10.1007/s000110050287. [DOI] [PubMed] [Google Scholar]
- MIZUNO H., SAKAMOTO C., MATSUDA K., WADA K., UCHIDA T., NOGUCHI H., AKAMATSU T., KASUGA M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- MORHAM S.G., LANGENBACH R., LOFTIN C.D., TIANO H.F., VOULOUMANOS N., JENNETTE J.C., MAHLER J.F., KLUCKMAN K.D., LEDFORD A., LEE C.A. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- MORTEAU O., MORHAM S.G., SELLON R., DIELEMAN L.A., LANGENBACH R., SMITHIES O., SARTOR R.B. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHERJEE D., NISSEN S.E., TOPOL E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY D., HOUCHEN C.W., KENNEDY S., DIECKGRAEFE B.K., ANANT S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol. Cell. 2003;11:113–126. doi: 10.1016/s1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- MURMU N., JUNG J., MUKHOPADHYAY D., HOUCHEN C.W., RIEHL T.E., STENSON W.F., MORRISON A.R., ARUMUGAM T., DIECKGRAEFE B.K., ANANT S. Dynamic antagonism between RNA-binding protein CUGBP2 and cyclooxygenase-2-mediated prostaglandin E2 in radiation damage. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13873–13878. doi: 10.1073/pnas.0406066101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- MUSCARÁ M.N., VERGNOLLE N., LOVREN F., TRIGGLE D.R., ELLIOTT S.N., ASFAHA S., WALLACE J.L. Selective cyclooxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br. J. Pharmacol. 2000;129:1423–1430. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWBERRY R.D., MCDONOUGH J.S., STENSON W.F., LORENZ R.G. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune response. J. Immunol. 2001;166:4465–4472. doi: 10.4049/jimmunol.166.7.4465. [DOI] [PubMed] [Google Scholar]
- NEWBERRY R.D., STENSON W.F., LORENZ R.G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat. Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- O'BANION M.K., WINN V.D., YOUNG D.A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTT E., NUSSMEIER N.A., DUKE P.C., FENECK R.O., ALSTON R.P., SNABES M.C., HUBBARD R.C., HSU P.H., SAIDMAN L.J., MANGANO D.T. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2003;125:1481–1492. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- PERINI R.F., MA L., WALLACE J.L. Mucosal repair and COX-2 inhibition. Curr. Pharm. Res. 2003;9:2207–2211. doi: 10.2174/1381612033454027. [DOI] [PubMed] [Google Scholar]
- QIU F.H., DEVCHAND P.R., WADA K., SERHAN C.N. Aspirin-triggered lipoxin A4 and lipoxin A4 up-regulate transcriptional corepressor NAB1 in human neutrophils. FASEB J. 2001;15:2736–2738. doi: 10.1096/fj.01-0576fje. [DOI] [PubMed] [Google Scholar]
- REUTER B.K., ASFAHA S., BURET A., SHARKEY K.A., WALLACE J.L. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J. Clin. Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERT A., NEZAMIS J.E., LANCASTER C., HANCHAR A.J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- ROWLEY A.F. Lipoxin formation in fish leucocytes. Biochim. Biophys. Acta. 1991;1084:303–306. doi: 10.1016/0005-2760(91)90073-q. [DOI] [PubMed] [Google Scholar]
- SAWAOKA H., TSUJI S., TSUJII M., GUNAWAN E.S., NAKAMA A., TAKEI Y., NAGANO K., MATSUI H., KAWANO S., HORI M. Expression of the cyclooxygenase-2 gene in gastric epithelium. J. Clin. Gastroenterol. 1997;25 Suppl 1:S105–S110. doi: 10.1097/00004836-199700001-00018. [DOI] [PubMed] [Google Scholar]
- SCHMASSMANN A., PESKAR B.M., STETTLER C., NETZER P., STROFF T., FLOGERZI B., HALTER F. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br. J. Pharmacol. 1998;123:795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN C.N. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- SOUZA M.H., DE LIMA O.M., ZAMUNER S.R., FIORUCCI S., WALLACE J.L. Gastritis increases resistance to aspirin-induced mucosal injury via COX-2-mediated lipoxin synthesis. Am J Physiol. 2003;285:G54–G61. doi: 10.1152/ajpgi.00525.2002. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI S., FUJITA T., YAMAMOTO A. Role of cyclooxygenase-2 in Helicobacter pylori-induced gastritis in Mongolian gerbils. Am. J. Physiol. 2000;279:G791–G798. doi: 10.1152/ajpgi.2000.279.4.G791. [DOI] [PubMed] [Google Scholar]
- TANAKA A., HASE S., MIYAZAWA T., TAKEUCHI K. Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J. Pharm. Exp. Ther. 2002;300:754–761. doi: 10.1124/jpet.300.3.754. [DOI] [PubMed] [Google Scholar]
- TATSUGUCHI A., SAKAMOTO C., WADA K., AKAMATSU T., TSUKUI T., MIYAKE K., FUTAGAMI S., KISHIDA T., FUKUDA Y., YAMANAKA N., KOBAYASHI M. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000;46:782–789. doi: 10.1136/gut.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THE PINK SHEET Merck COX-2 cardiovascular safety studies will enrol 30,000 subjects; data will address ‘lingering concerns'. 2001;63:51. [Google Scholar]
- WALLACE J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., BAK A., MCKNIGHT W., ASFAHA S., SHARKEY K.A., MACNAUGHTON W.K. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., GRANGER D.N. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MA L. Inflammatory mediators in gastrointestinal defence and injury. Exp. Biol. Med. 2001;226:1003–1115. doi: 10.1177/153537020122601107. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., REUTER B.K., VERGNOLLE N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MILLER M.J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., ZAMUNER S.R., MCKNIGHT W., DICAY M., MENCARELLI A., DEL SOLDATO P., FIORUCCI S. Aspirin, but not NO-releasing aspirin (NCX-4016), interacts with selective COX-2 inhibitors to aggravate gastric damage and inflammation. Am. J. Physiol. 2004;286:G76–G81. doi: 10.1152/ajpgi.00295.2003. [DOI] [PubMed] [Google Scholar]
- ZAMUNER S.R., WALLACE J.L.Post-inflammatory colonic dysfunction: role of COX-2 and H-PGD synthase in pre-disposition to colon cancer Can. J. Gastroenterol. 200418755(abstract) [Google Scholar]
- ZAMUNER S.R., WARRIER N., BURET A.G., MACNAUGHTON W.K., WALLACE J.L. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720. doi: 10.1136/gut.52.12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN K.C., SARBIA M., SCHROR K., WEBER A.A. Constitutive cyclooxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol. Pharmacol. 1998;54:536–540. doi: 10.1124/mol.54.3.536. [DOI] [PubMed] [Google Scholar]