Abstract
Urocortin is a vasodilator peptide related to corticotrophin-releasing factor, which may protect endothelial function during coronary ischemia–reperfusion (I–R). The aim of this study was to study the mechanisms of this protective effect.
Hearts from Sprague–Dawley rats were isolated and perfused at constant flow and then exposed to 15 min global zero-flow ischemia, followed by 15 min reperfusion. The relaxation to acetylcholine (10 nM–10 μM) was recorded after pre-constriction of the coronary vasculature with U46619 (100–300 nM) in ischemic–reperfused or time-control hearts.
After I–R, the coronary relaxation to acetylcholine was reduced and this reduction was attenuated by treatment with urocortin (10 pM), administered before ischemia and during reperfusion.
This urocortin-induced improvement of the relaxation to acetylcholine was not modified by tetraethylammonium (10 mM), blocker of Ca2+ dependent-potassium channels; glibenclamide (10 μM), blocker of KATP channels; Nw-nitro-L-arginine methyl ester (L-NAME, 100 μM), blocker of nitric oxide synthesis; or meclofenamate (10 μM), blocker of cyclooxygenase, but it was abolished by chelerythrine (3 μM), blocker of protein kinase C (PKC).
These results suggest that urocortin may protect coronary endothelial function during I–R by activation of PKC.
Keywords: Urocortin, coronary circulation, ischemia–reperfusion, endothelium, protein kinase C
Introduction
Urocortin is a 40 amino-acid peptide which has a high degree of structural homology with the peptide corticotrophin-releasing factor (CRF), and has marked cardiovascular effects (Vaughan et al., 1995), mainly through binding to the corticotrophin-releasing factor receptor 2 subtype (CRF-R2) (Bale et al., 2000). In the heart, exogenous urocortin increases heart rate and cardiac output, and also produces coronary vasodilation (Parkes et al., 1997). In addition, exogenous urocortin may protect myocardial cells during coronary ischemia, as it increases the survival of cultured cardiac cells exposed to simulated ischemia (Brar et al., 1999), and reduces the infarct area in the ischemic and reperfused rat heart (Brar et al., 2000). Several mechanisms may be involved in this protective effect of urocortin in the myocardial cells (see Huang et al., 2004) such as activation of mitogen-activated protein kinases (MAPK) (Schulman et al., 2002), of the phosphatidylinositol 3-OH kinase (PI3K) (Brar et al., 2002), of phospholipase A2 (Lawrence et al., 2003), or activation of mitochondrial ATP-sensitive potassium channels (KATP) (Lawrence et al., 2002). It has been demonstrated that urocortin mRNA and protein are produced in the heart (Nishikimi et al., 2000), and its production may be increased in cardiac cells exposed to ischemia (Brar et al., 1999; Scarabelli et al., 2004). Therefore, urocortin may act as an endogenous protective substance in the myocardium after ischemia–reperfusion (I–R).
I–R may also produce coronary endothelial dysfunction as it reduces endothelium-dependent coronary vasodilatation (Ku, 1982; Van Benthuysen et al., 1987; Kim et al., 1992), and this dysfunction may contribute to the adverse cardiac effects of I–R. Substances which protect cardiac function during I–R may also protect endothelial function (Laude et al., 2001), as it has been demonstrated for adenosine (Maczewski & Beresewicz, 1998), heparin (Kouretas et al., 1998) or calcitonin gene-related peptide (Zhou et al., 1999). The mechanisms of this protection in the endothelial cells may be similar to or different from those operating in myocardial cells. In a previous study from our laboratory (García-Villalón et al., 2004) we have found that urocortin may also be protective of endothelial function, as low concentrations of this peptide improved the coronary relaxation to acetylcholine in perfused rat hearts after I–R. The aim of this study was to further study the mechanisms of this protective action of urocortin on the endothelium. The present work was performed in the heart from rats perfused according to the Langendorff procedure, which is an experimental model frequently used for the study of I–R (Sutherland & Hearse, 2000).
Methods
Animals
In this study, 87 male Sprague–Dawley rats (weight 300–350 g) were used. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and has been conducted in compliance with applicable laws and regulations. Use of animals has been approved by the Institution's Animal Care and Use Committee.
Preparation of isolated hearts
The hearts were isolated from rats after anesthesia with pentobarbital sodium (40 mg kg−1) and injection of heparin (1000 UI). After obtaining the hearts, the ascending aorta was cannulated, and retrograde perfusion of the heart with Krebs–Henseleit buffer (mM NaCl, 115; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11) was initiated in a nonrecirculating Langendorff heart perfusion apparatus, at a constant flow of 11–15 ml min−1 to reach a basal perfusion pressure of approximately 70 mmHg, and the hearts were paced with an electrical stimulator at a rate of 240 beats min−1, which was similar to the baseline heart rate of isolated hearts. Perfusion coronary pressure was measured by a lateral connection in the perfusion cannula, and left intraventricular pressure with a latex balloon inflated to a diastolic pressure of 5–10 mmHg, both connected to Statham transducers, and were recorded in a Grass model 7 polygraph.
Experimental procedures
After a 15 min equilibration period with perfusion at constant flow, the hearts were exposed to 15 min of global zero-flow ischemia, followed by reperfusion at the same flow rate as before ischemia. After reperfusion for 15 min, the coronary vascular bed was pre-constricted by adding U46619 (100–300 nM) to the perfusion buffer, and then the coronary relaxation to acetylcholine (10 nM–10 μM) was recorded. These durations of ischemia and reperfusion were chosen because in a previous study (García-Villalón et al., 2004) it was found that they impaired specifically endothelium-dependent relaxation. Time-control hearts were perfused during the same total time (45 min) at constant flow before the concentration–response curve to acetylcholine. Acetylcholine was delivered with an infusion pump at a constant rate of 1 : 100 of the coronary perfusion flow, just proximal to the aortic cannula during 1 min. The volume infused was 0.1–0.15 ml. Each concentration of acetylcholine was infused after vascular tone had recovered from the previous concentration. Only one concentration–response curve to acetylcholine was performed in each heart to avoid tachyphylaxis. As the flow was maintained constant, increases in perfusion pressure were considered as constrictions, and reductions in perfusion pressure as relaxations, of the coronary vasculature.
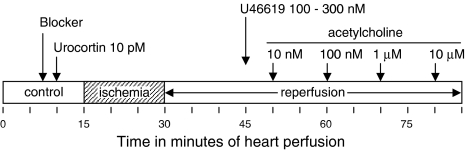
The relaxation to acetylcholine was recorded in perfused hearts under control conditions, in hearts after I–R, and in hearts after I–R pretreated with urocortin (10 pM). In this case, urocortin was added to the perfusion solution 5 min before the ischemia period and was present during reperfusion and during acetylcholine infusion. Also, to analyze the mechanisms of urocortin action during I–R, the effects of this peptide were assessed in the presence of the following blockers: tetraethylammonium (TEA, 10 mM); blocker of Ca2+-dependent potassium channels; glibenclamide (10 μM), blocker of KATP channels; Nw-nitro-L-arginine methyl ester (L-NAME, 100 μM), blocker of nitric oxide synthesis; meclofenamate (10 μM), blocker of cyclooxygenase; and chelerythrine (3 μM), blocker of PKC. In these experiments, first the blocker was added to the perfusion solution, then urocortin was also added, I–R performed, and the relaxation to acetylcholine recorded after I–R (Figure 1). In the case of L-NAME, this inhibitor was removed from the solution before recording the response to acetylcholine.
Figure 1.
Experimental protocol followed in the present study. The arrow marked ‘Blocker' indicates the start of the infusion of one of the following compounds – TEA (10 mM), glibenclamide (10 μM), L-NAME (100 μM), meclofenamate (10 μM), or chelerythrine (3 μM).
Materials
Substances used were: Urocortin (rat), 9,11-dideoxy-1a,9a-epoxymethanoprostaglandin F2α (U46619), acetylcholine chloride, tetraethylammonium chloride, glibenclamide, L-NAME, meclofenamic acid sodium salt (meclofenamate) and chelerythrine chloride, all from Sigma.
Data analysis
The coronary relaxation is expressed as percentage of the contraction (active tone) induced with U46619, and calculated as means±s.e.m., from at least five hearts per condition. The actual numbers for each condition are shown in the text or in the figure legends, as appropriate. The coronary responses in different experimental conditions were compared by ANOVA, followed by Bonferroni or by Dunnet test, as appropriate, to analyze what comparisons were statistically significant.
Results
The hearts were equilibrated at a basal perfusion pressure of 70±3 mmHg (n=11), and this basal pressure was not modified by addition of urocortin (10 pM), or after I–R (67±2 mmHg; n=8). However, systolic intraventricular pressure was reduced after I–R (74±3 vs 53±3 mmHg, P<0.001). Pretreatment with urocortin during I–R (n=8) did not modify perfusion pressure (63±5 mmHg) or systolic intraventricular pressure (51±3 mmHg) compared with untreated ischemic–reperfused hearts.
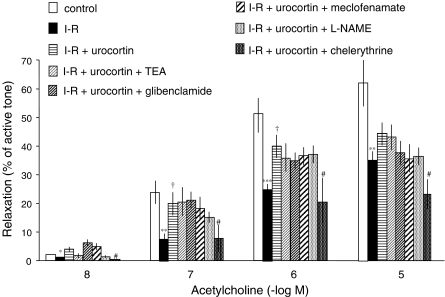
Addition of U46619 after I–R or in time-control hearts increased the perfusion pressure (74±7 mmHg above basal in time controls), and this increase was comparable in every experimental condition. In the hearts pre-constricted with U46619, acetylcholine (10 nM–10 μM) produced concentration-dependent coronary vascular relaxation, which was less after I–R in hearts untreated with urocortin than in control condition, but in the ischemic–reperfused hearts treated with urocortin, the relaxation to acetylcholine was greater than in ischemic–reperfused hearts untreated with urocortin, although it remained less than that observed under control conditions (Figure 2).
Figure 2.
Coronary vascular relaxation to acetylcholine (10 nM–10 μM) in rat perfused hearts after pre-constriction of coronary vasculature with U46619 (100–300 nM), in control conditions (n=11), after untreated I–R (n=6), after I–R treated with urocortin alone (10 pM) (n=8), urocortin plus TEA (10 mM) (n=5), urocortin plus glibenclamide (10 μM) (n=5), urocortin plus L-NAME (100 μM) (n=6), urocortin plus meclofenamate (10 μM) (n=5), and urocortin plus chelerythrine (3 μM) (n=6). Data shown are means±s.e.m. *,**, ***Significant difference with control (*P<0.05; **P<0.01; ***P<0.001), †Significant difference compared with I–R nontreated (P<0.05), both by Bonferroni test, and #significant difference by Dunnett test, compared with I–R pretreated only with urocortin (P<0.05).
In hearts subjected to I–R and treated with TEA (10 mM), glibenclamide (10 μM), L-NAME (100 μM) or meclofenamate (10 μM), together with urocortin, the relaxation to acetylcholine was similar to that after I–R treated only with urocortin (Figure 2).
In the ischemic–reperfused hearts treated with chelerythrine (3 μM) and urocortin, the relaxation to acetylcholine was lower than in those treated only with urocortin (Figure 2), and it was similar to that in untreated ischemic–reperfused hearts.
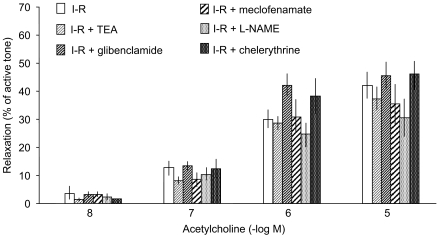
In hearts subjected to I–R but untreated with urocortin, TEA (10 mM), glibenclamide (10 μM), L-NAME (100 μM), meclofenamate (10 μM), or chelerythrine (3 μM) did not modify the relaxation to acetylcholine (Figure 3).
Figure 3.
Coronary vascular relaxation to acetylcholine (10 nM–10 μM) in rat perfused hearts after pre-constriction of coronary vasculature with U46619 (100–300 nM), after I–R in the absence (n=6) and in the presence of TEA (10 mM) (n=5), glibenclamide (10 μM) (n=9), L-NAME (100 μM) (n=5), meclofenamate (10 μM) (n=5) and chelerythrine (3 μM) (n=5). Data shown are means±s.e.m.
Discussion
In this study, 15 min of global zero-flow ischemia followed by 15 min of reperfusion did not modify coronary vascular resistance, as coronary perfusion pressure was not changed. This contrasts with other studies showing increases in coronary vascular resistance after I–R, which is known as the ‘non-reflow' phenomenon (Kloner et al., 1974). This discrepancy may be due to the relatively short duration of ischemia in the present study (15 min). However, a reduction in myocardial function, as indicated by lower systolic intraventricular pressure, was observed. I–R may alter not only myocardial function but also the function of coronary endothelium (Laude et al., 2001), and this latter phenomenon was also observed in the present study as the coronary relaxation to acetylcholine was reduced in this condition. In a previous study from our laboratory using the same experimental model of I–R (García-Villalón et al., 2004), we have found that the coronary relaxation to a endothelium-independent agent (sodium nitroprusside) is not modified, suggesting that the reduction in the acetylcholine response found in the present study is due to specific impairment of the endothelial function.
The results of the present study suggest that the impairment of endothelial function during I–R may be attenuated by treatment with urocortin, as the reduced relaxation to acetylcholine after this condition was partly reversed by this peptide, confirming previous studies from our laboratory (García-Villalón et al., 2004). It is remarkable that this protective effect of urocortin was observed at a low concentration (10 pM), which produced no change in coronary vascular tone. This concentration of urocortin is higher than the concentration present in plasma of humans under normal conditions (1 pM, Watanabe et al., 1999), but as urocortin may be produced in the myocardial tissue (Nishikimi et al., 2000), it may reach, locally, a concentration higher than in plasma, especially during myocardial ischemia, when the production of urocortin is increased (Brar et al., 1999). Therefore, urocortin may act as an endogenous protective factor of the heart during ischemia. These protective effects of urocortin may act on the endothelial function (García-Villalón et al., 2004; present results) as well as on the myocardial function (Brar et al., 1999; 2000) during I–R.
Several substances may protect the myocardial function and/or the coronary endothelial cells during I–R. This protective phenomenon has been subjected to intense study because of its pathophysiological and therapeutic implications. However, underlying mechanisms have not been completely elucidated, and factors such as nitric oxide or prostacyclin, different protein kinases and activation of potassium channels have been implicated. The mechanisms of this protection in myocardial cells may coincide in part with those for the protection of endothelial function during I–R, and may be partially different in both cellular types.
Activation of mitochondrial KATP channels may also be involved in the protective effects of urocortin on myocardial cells, as inhibitors of these channels blocked urocortin-induced protection in rat cardiomyocytes (Lawrence et al., 2002; Gordon et al., 2003). Also, the direct vasodilating effect of urocortin may be mediated, in part, by potassium channels of the Ca2+-sensitive subtype, because TEA, an inhibitor of these channels, reduced the vasodilation to urocortin in rat coronary arteries (Huang et al., 2003) and in human saphenous veins (Sanz et al., 2002). Therefore, urocortin may activate ATP-sensitive and Ca2+-sensitive potassium channels in cardiac myocytes and in vascular smooth muscle cells, respectively. However, we found that neither glibenclamide nor TEA modified the urocortin-induced improvement of the coronary endothelial function, suggesting that these channels do not seem to be involved in the protective effect of this peptide in endothelial cells during I–R.
Likewise, nitric oxide may participate in the relaxation by urocortin of rat aorta (Miki et al., 2004) and renal (Sanz et al., 2003) and coronary (Huang et al., 2002) arteries, and vasodilator prostanoids may also participate in the vasodilation by this peptide in perfused rat hearts (Terui et al., 2001). These vasodilatory factors, however, may not be involved in the protective endothelial effect of urocortin during I–R, because we observed that this effect was not modified by L-NAME or meclofenamate.
The protective effect of urocortin on the endothelial cells during I–R may be mediated by activation of PKC, as an inhibitor of this enzyme, chelerythrine, abolished the improvement of endothelial function by urocortin. This partly agrees with the study of Gordon et al. (2003), who found that the protection by urocortin in adult rat cardiomyocytes was attenuated by chelerythrine. Also, our results partly agree with those of Lawrence et al. (2004), who showed involvement of PKC epsilon in the prevention of mitochondrial damage during I–R. However, in the studies of Gordon et al. (2003) and Lawrence et al. (2004), KATP channels were also involved, a phenomenon that was not observed in the present study. It may be supposed that urocortin action may share some mechanisms, but not others, in endothelial and in myocardial cells.
In conclusion, the present results suggest that relatively low concentrations of urocortin may protect endothelial function in the coronary circulation against deleterious effects of I–R through activation of PKC. Urocortin might act as an endogenous protective factor in the heart during coronary I–R, and identification of its protective mechanisms on endothelial function may help to prevent the coronary vascular dysregulation that frequently occurs after heart ischemia.
Acknowledgments
We are indebted to María Ester Martínez and Hortensia Fernández-Lomana for technical assistance. This work was supported, in part, by MCyT (BFI2002-01852) and MCyT (BSA 2001-0158).
Abbreviations
- CRF
corticotrophin-releasing factor
- KATP
ATP-sensitive potassium channels
- L-NAME
Nw-nitro-L-arginine methyl ester
- PKC
protein kinase C
- TEA
tetraethylammonium
References
- BALE T.L., CONTARINO A., SMITH G.W., CHAN R., GOLD L.H., SAWCHENKO P.E., KOOB G.F., VALE W.W., LEE K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- BRAR B.K., STEPHANOU A., OKOSI A., LAWRENCE K.M., KNIGHT R.A., MARBER M.S., LATCHMAN D.S. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol. Cell. Endocrinol. 1999;158:55–63. doi: 10.1016/s0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- BRAR B.K., JONASSEN A.K., STEPHANOU A., SANTILLI G., RAILSON J., KNIGHT R.A., YELLON D.M., LATCHMAN D.S. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J. Biol. Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- BRAR B.K., STEPHANOU A., KNIGHT R., LATCHMAN D.S. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J. Mol. Cell. Cardiol. 2002;34:483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- GARCÍA-VILLALÓN A.L., SANZ E., MONGE L., FERNÁNDEZ N., CLIMENT B., DIÉGUEZ G. Urocortin protects coronary endotelial function during ischemia–reperfusion: a brief communication. Exp. Biol. Med. 2004;229:118–120. doi: 10.1177/153537020422900114. [DOI] [PubMed] [Google Scholar]
- GORDON J.M., DUSTING G.J., WOODMAN O.L., RITCHIE R.H. Cardioprotective action of CRF peptide urocortin against simulated ischemia in rat adult cardiomyocites. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H330–H336. doi: 10.1152/ajpheart.01121.2001. [DOI] [PubMed] [Google Scholar]
- HUANG Y., CHAN F.L., LAU C.W., TSANG S.Y., HE G.W., CHEN Z.Y., YAO X. Urocortin-induced endothelium-dependent relaxation of rat coronary artery: role of nitric oxide and K+ channels. Br. J. Pharmacol. 2002;135:1467–1476. doi: 10.1038/sj.bjp.0704587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Y., CHAN F.L., LAU C.W., TSANG S.Y., CHEN Z.Y., HE G.W., YAO X. Roles of cyclic AMP and Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc. Res. 2003;57:824–833. doi: 10.1016/s0008-6363(02)00773-3. [DOI] [PubMed] [Google Scholar]
- HUANG Y., YAO X.Q., LAU C.W., CHAN Y.C., TSANG S.Y., CHAN F.L. Urocortin and cardiovascular protection. Acta Pharmacol. Sin. 2004;25:257–265. [PubMed] [Google Scholar]
- KIM Y.D., FOMSGAARD J.S., HEIM K.F., RAMWELL P.W., THOMAS G., KAGAN E., MOORE S.P., COUGHLIN S.S., KUWAHARA M., ANALOUEI A., MYERS A.K. Brief ischemia–reperfusion induces stunning of endothelium in canine coronary artery. Circulation. 1992;85:1473–1482. doi: 10.1161/01.cir.85.4.1473. [DOI] [PubMed] [Google Scholar]
- KLONER R.A., GANOTE C.E., JENNINGS R.B. The ‘no-reflow' phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOURETAS P.C., KIM Y.D., CAHILL P.A., MYERS A.K., TO L.N., WANG Y.N., WALLACE R.B., KRON I.L., HANNAN R.L. Heparin preserves nitric oxide activity in coronary endothelium during ischemia–reperfusion injury. Ann. Thorac. Surg. 1998;66:1210–1215. doi: 10.1016/s0003-4975(98)00811-x. [DOI] [PubMed] [Google Scholar]
- KU D.D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218:576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- LAUDE K., THUILLEZ C., RICHARD V. Coronary endothelial dysfunction after ischemia and reperfusion: a new therapeutic target. Braz. J. Med. Biol. Res. 2001;34:1–7. doi: 10.1590/s0100-879x2001000100001. [DOI] [PubMed] [Google Scholar]
- LAWRENCE K.M., CHANALARIS A., SCARABELLI T., HUBANK M., PASINI E., TOWNSEND P.A., COMINI L., FERRARI R., TINKER A., STEPHANOU A., KNIGHT R.A., LATCHMAN D.S. K(ATP) channel gene expression is induced by urocortin and mediates its cardioprotective effect. Circulation. 2002;106:1556–1562. doi: 10.1161/01.cir.0000028424.02525.ae. [DOI] [PubMed] [Google Scholar]
- LAWRENCE K.M., SCARABELLI T.M., TURTLE L., CHANALARIS A., TOWNSEND P.A., CARROLL C.J., HUBANK M., STEPHANOU A., KNIGHT R.A., LATCHMAN D.S. Urocortin protects cardiac myocytes from ischemia/reperfusion injury by attenuating calcium-insensitive phospholipase A2 gene expression. FASEB J. 2003;17:2313–2315. doi: 10.1096/fj.02-0832fje. [DOI] [PubMed] [Google Scholar]
- LAWRENCE K.M., TOWNSEND P.A., DAVIDSON S.M., CARROLL C.J., EATON S., HUBANK M., KNIGHT R.A., STEPHANOU A., LATCHMAN D.S. The cardioprotective effect of urocortin during ischaemia/reperfusion involves the prevention of mitochondrial damage. Biochem. Biophys. Res. Commun. 2004;321:479–486. doi: 10.1016/j.bbrc.2004.06.170. [DOI] [PubMed] [Google Scholar]
- MACZEWSKI M., BERESEWICZ A. The role of adenosine and ATP-sensitive potassium channels in the protection afforded by ischemic preconditioning against the post-ischemic endothelial dysfunction in guinea-pig hearts. J. Mol. Cell. Cardiol. 1998;30:1735–1747. doi: 10.1006/jmcc.1998.0736. [DOI] [PubMed] [Google Scholar]
- MIKI I., SEYA K., MOTOMURA S., FURUKAWA K. Role of corticotropin-releasing factor receptor type 2beta in urocortin-induced vasodilation of rat aortas. J. Pharmacol. Sci. 2004;96:170–176. doi: 10.1254/jphs.fp0040364. [DOI] [PubMed] [Google Scholar]
- NISHIKIMI T., MIYATA A., HORIO T., YOSHIHARA F., NAGAYA N., TAKISHITA S., YUTANI C., MATSUO H., MATSUOKA H., KANGAWA K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am. J. Physiol. Heart. Circ. Physiol. 2000;279:H3031–H3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- PARKES D.G., VAUGHAN J., RIVIER J., VALE W., MAY C.N. Cardiac inotropic actions of urocortin in conscious sheep. Am. J. Physiol. 1997;272:H2115–H2122. doi: 10.1152/ajpheart.1997.272.5.H2115. [DOI] [PubMed] [Google Scholar]
- SANZ E., MONGE L., FERNÁNDEZ N., MARTÍNEZ M.A., MARTÍNEZ-LEÓN J.B., DIÉGUEZ G., GARCÍA-VILLALÓN A.L. Relaxation by urocortin of human saphenous veins. Br. J. Pharmacol. 2002;136:90–94. doi: 10.1038/sj.bjp.0704670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANZ E., FERNÁNDEZ N., MONGE L., CLIMENT B., DIÉGUEZ G., GARCÍA-VILLALÓN A.L. Relaxation by urocortin of rat renal arteries: effects of diabetes in males and females. Cardiovasc. Res. 2003;58:706–711. doi: 10.1016/s0008-6363(03)00293-1. [DOI] [PubMed] [Google Scholar]
- SCARABELLI T.M., PASINI E., FERRARI G., FERRARI M., STEPHANOU A., LAWRENCE K., TOWNSEND P., CHEN-SCARABELLI C., GITTI G., SARAVOLATZ L., LATCHMAN D., KNIGHT R.A., GARDIN J.M. Warm blood cardioplegic arrest induces mitochondrial-mediated cardiomyocyte apoptosis associated with increased urocortin expression in viable cells. J. Thorac. Cardiovasc. Surg. 2004;128:364–371. doi: 10.1016/j.jtcvs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- SCHULMAN D., LATCHMAN D.S., YELLON D.M. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H1481–H1488. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND F.J., HEARSE D.J. The isolated blood and perfusion fluid perfused heart. Pharmacol. Res. 2000;41:613–627. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- TERUI K., HIGASHIYAMA A., HORIBA N., FURUKAWA K.I., MOTOMURA S., SUDA T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J. Endocrinol. 2001;169:177–183. doi: 10.1677/joe.0.1690177. [DOI] [PubMed] [Google Scholar]
- VAN BENTHUYSEN K.M., MCMURTRY I.F., HORWITZ L.D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J. Clin. Invest. 1987;79:265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN J., DONALDSON C., BITTENCOURT J., PERRIN M.H., LEWIS K., SUTTON S., CHAN R., TURNBULL A.V., LOVEJOY D., RIVIER C., RIVIER J., SAWCHENKO P., VALE W. Urocortin, a mammalian neuropeptide related to fish urotensin 1 and corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- WATANABE F., OKI Y., OZAWA M., MASUZAWA M., IWABUCHI M., YOSHIMI T., NISHIGUCHI T., IINO K., SASANO H. Urocortin in human placenta and maternal plasma. Peptides. 1999;20:205–209. doi: 10.1016/s0196-9781(98)00175-2. [DOI] [PubMed] [Google Scholar]
- ZHOU F.-W., LI Y.-J., LU R., DENG H.-W. Protection of calcitonin gene-related peptide-mediated preconditioning against coronary endothelial dysfunction induced by reperfusion in the isolated rat heart. Life Sci. 1999;64:1091–1097. doi: 10.1016/s0024-3205(99)00037-5. [DOI] [PubMed] [Google Scholar]