Abstract
Although the pathogenesis of scleroderma is not fully understood, activation of connective-tissue-type mast cells (CTMCs) has been implicated in various fibrotic diseases.
Our previous study showed that the number of CTMCs was markedly increased during fibrous proliferation in the skin of a scleroderma model, namely tight-skin (Tsk) mice. Because mast cells express numerous bioactive factors, such as cytokines, growth factors, proteases, and others, it is crucial to identify the primary factors that may be involved in the pathogenesis of scleroderma. Our previous study also showed that a CTMC-specific protease, chymase-4, was selectively upregulated in accordance with the development of skin fibrosis in Tsk mice.
To further elucidate the role of chymase secreted from CTMCs, we evaluated the therapeutic effects of a synthetic chymase-specific inhibitor, SUN-C8257, on the development of skin fibrosis in Tsk mice. SUN-C8257 (50 mg kg−1 day−1) was administered via intraperitoneal injection in 13-week-old Tsk mice for a period of 2 weeks.
Treatment with SUN-C8257 significantly reduced chymase activity by 43% and the chymase-4 mRNA level by 47%, and also decreased the thickness of the subcutaneous fibrous layer of Tsk mice by 42% compared with that of Tsk mice injected with vehicle.
Furthermore, immunohistochemical analysis revealed that transforming growth factor (TGF)-beta1 staining in the fibrous layer of Tsk skin was markedly reduced by the treatment with SUN-C8257. This chymase inhibitor may prevent the chymase-dependent pathway that activates the latent TGF-beta1 in fibrous tissue, and may exhibit beneficial effects that inhibit the development of fibrosis.
In conclusion, our results strongly support the assumption that CTMC-derived chymase may play a key role in the pathogenesis of scleroderma.
Keywords: Chymase inhibitor, fibrosis, mast cell, scleroderma, serine protease, TGF-beta1
Introduction
Scleroderma, also called progressive systemic sclerosis, is a multisystem disorder that is characterized by fibrosis and massive deposition of an extracellular matrix in the skin and various organs. Scleroderma has a worldwide distribution and affects all races. The prevalence of scleroderma in U.S.A. is estimated to be 276 cases per million (Mayes et al., 2003). Although the pathogenesis of scleroderma is not understood completely, numerous lines of evidence suggest the involvement of mast cells in the process of skin fibrosis. In the early stage of scleroderma patients, the number of mast cells in the clinically involved skin is greater than that in controls (Hawkins et al., 1985; Nishioka et al., 1987). Mast cells in rapid progressive scleroderma have been shown to be activated with a remarkable degree of degranulation (Claman, 1989). Seibold et al. (1990) indicated that activation and proliferation of mast cells preceded the onset of clinical fibrosis. Moreover, a clinical trial showed that treatment with ketotifen, which inhibits degranulation of mast cells, prevented the progression of scleroderma (Gruber & Kaufman, 1990). On the basis of these observations, it is postulated that mast cells play a major role in the clinical progression of skin changes in scleroderma (Levi-Schaffer, 1995).
Mast cell activation has been recognized in scleroderma in mice. The tight-skin (Tsk) mouse, a genetic mouse model for human scleroderma, shows widespread disorder of connective tissues. The spontaneous mutation causing Tsk phenotype is located on chromosome 2 (Green et al., 1976). In the skin, Tsk mice develop the genetically linked fibrosis involving dermis and subcutaneous loose connective tissue (Green et al., 1976; Menton et al., 1978; Jimenez & Christner, 1994). Previous studies suggested that mast cells are also involved in the pathogenesis of skin fibrosis in Tsk mice. The total number of mast cells and the proportion of degranulating mast cells in cutaneous tissue of Tsk mice were both significantly increased compared to control mice (Walker et al., 1985). Mast cell involvement in the pathogenesis of Tsk mice was further investigated with mast cell-deficient mutant mice carrying the Tsk locus generated by selective interbreeding between Tsk and w/wv mice (Everett et al., 1995). That report showed that the increase in the number of skin mast cells was correlated with development of fibrosis in old Tsk mice. Treatment with an antiallergic drug, cromolyn, which prevents mast cell degranulation, also inhibits the development of skin fibrosis in Tsk mice (Walker et al., 1987). These findings suggest a causal relationship between skin fibrosis and mast cell activation in Tsk mice, as well as in patients with scleroderma. However, the precise role of mast cells in the pathogenesis of scleroderma still remains unclear. Because mast cells express numerous bioactive factors such as cytokines, growth factors, proteases, and others, it is crucial to identify the primary factors that induce fibroblast activation and deposition of the extracellular matrix.
Two types of mast cells, connective-tissue-type mast cells (CTMCs) and mucosal-type mast cells, have been recognized based on the differences in their localization, ultrastructural morphology, and content of neutral proteases. The subset of mast cells that is increased in number and shown to be in an activated state in the involved tissues is mostly the CTMC subset as judged from its granular ultrastructure and positive staining for heparin (Claman, 1993). Chymase is a protease that is exclusively expressed by mast cells. Unlike humans, mice have more than one chymase isoform. Among these isoforms, mouse chymase-4 (usually called mouse mast cell protease-4, MMCP-4) is specifically expressed by mature CTMCs (Huang et al., 1991; Springman & Serafin, 1995). Therefore, mouse chymase-4 can be regarded as the functional equivalent to human chymase. Our previous study showed that number of CTMCs and the chymase activity were increased in the skin of Tsk mice. Moreover, the mRNA level of chymase-4, but not other isoforms such as chymase-2 and chymase-5, was elevated during the development of skin fibrosis (Kakizoe et al., 2001). These findings suggest that the chymase-4 liberated from CTMCs is one of the important factors in the promotion of fibrosis in the skin of Tsk mice. To clarify the precise causality between skin fibrosis and upregulated chymase expression, an experiment with a specific inhibitor for chymase is highly required. Recently, a nonpeptide chymase-specific inhibitor, SUN-C8257, was newly synthesized (Fukami et al., 2000). In the present study, the functional role of chymase in the pathogenesis of scleroderma was further investigated by treatment with a specific inhibitor for chymase.
Methods
Chymase inhibitor
The nonpeptide chymase inhibitor 3-[(3-amino-4-carboxy)phenylsulfonyl]-7-chloroquinazoline 2,4(1H,3H)-dione (SUN-C8257) was provided by Suntory Ltd (Osaka, Japan). The IC50 values for human chymase, mouse chymase-4, and human chymotrypsin were 0.31, 0.89, and 23 μM, respectively (Fukami et al., 2000; Watanabe et al., 2002). The IC50 value for both bovine trypsin and human leukocyte elastase was greater than 100 μM (Fukami et al., 2000; Watanabe et al., 2002).
Animal preparation and drug administration
Tsk mice (C57BL/6-Tsk+/+pa) and control mice (C57BL/6J) were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). Our previous study showed that the subcutaneous fibrous layer in Tsk mice at the age of 5 weeks was already significantly thicker than that in control mice. Thickening of the fibrous layer in Tsk mice was markedly accelerated between 10 and 20 weeks of age (Kakizoe et al., 2001). Therefore, the age of 13 weeks was selected as the starting point for the administration of the chymase inhibitor. A previous report showed that intraperitoneal injection of SUN-C8257 for 6 weeks inhibited the increase of dermal mast cell counts in dermatitis tissues induced by 2,4-dinitrofluorobenzene in a dose-dependent manner, and that 50 mg kg−1 dose (intraperitoneal, once a day) was sufficiently effective (Tomimori et al., 2002). The same dose was used for the present study. Five male Tsk mice and six control C57BL/6J mice were treated with intraperitoneal injections of SUN-C8257 (50 mg kg−1 day−1, once a day) for 2 weeks without any apparent side effects. Separate sets of five Tsk and six control C57BL/6J mice received injections containing only the vehicle during the same period. After the animals were killed by administration of an overdose of pentobarbital, dorsal skins from the interscapular region were collected for histological analysis and for determinations of chymase activity and for the mRNA level of chymase-4. This study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals approved by the Committee of Shimane University School of Medicine.
Histological analysis
Using a light microscope, morphologic characteristics of skin sections from Tsk mice were compared with those from control mice. The number of mast cells in the dermis and subcutaneous fibrous layer was counted on toluidine blue-stained sections. Mast cell numbers were determined as the number of cells per square millimeter, as described previously (Kakizoe et al., 2001). The thickness of the subcutaneous fibrous layer was measured with an image-analyzing system (Image Pro, Media Cybernetics, Silver Spring, MD, U.S.A.) using Azan-stained sections. The thickness of the subcutaneous fibrous layer from 10 representative sites was averaged and used as the index of fibrous proliferation. For detection of chymase and transforming growth factor (TGF)-beta1 in paraffin sections of the skin, polyclonal rabbit antibodies against chymase (kindly provided by Dr G.H. Caughey; Fang et al., 1999) and against TGF-beta1 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) were used. Paraffin sections were treated overnight at 4°C with primary antibodies against chymase (1 : 10,000 dilution) or TGF-beta1 (1 : 500 dilution), or equal amounts of nonimmune serum (control for the specificity of antibodies). The antibodies were then detected with a DAKO ENVISION kit (Dakocytomation, Tokyo, Japan) by the standard method and visualized with DAB substrate (Dakocytomation) for TGF-beta1 or with TrueBlue™ peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD, U.S.A.) for chymase.
Measurement of chymase activity
Chymase was extracted from the dorsal skin and its activity was measured as described previously (Nishikori et al., 1998). Briefly, skin specimens were minced and homogenized in 10 volumes of 20 mM sodium phosphate buffer (pH 7.4). The homogenate was then twice centrifuged at 20,000 × g for 30 min and the supernatant liquid was discarded. The resulting pellet was resuspended and homogenized in five volumes of high ionic-strength buffer consisting of 10 mM sodium phosphate buffer (pH 7.4), 2 M KCl, and 0.1% polyoxyethylene octylphenyl ether. The homogenate was agitated overnight at 4°C, and then centrifuged at 20,000 × g for 30 min. The final supernatant liquid was used as the chymase extract. Chymase activity (mU per mg of protein) was estimated as the hydrolysis rate of suc-Phe-Pro-Phe-p-nitroanilide (BACHEM Feinchemikalen, Bubendorf, Switzerland) at 37°C. This chromogenic substrate, however, may be cleaved not only by chymase but also by other peptidases that were possibly carried over into the final tissue extract. Thus, nonspecific peptidases may interfere with the accurate measurement of chymase activity. A solution for this problem is as follows. Aprotinin inhibits the activities of most serine-class proteases except chymase, whereas chymostatin completely inhibits chymase activity. Therefore, the net chymase activity was determined by subtracting the hydrolysis activity in the presence of both chymostatin and aprotinin from that in the presence of aprotinin alone.
Quantitative reverse transcriptase–polymerase chain reaction (RT–PCR) analysis
The mRNA level of chymase-4 was measured by the quantitative RT–PCR method, as described previously (Shiota et al, 1997, 1998; Kakizoe et al., 2001). Briefly, the PCR primers for chymase-4 were selected according to the chymase-4 cDNA sequence (sense primer: 5′-ACCACTGAGAGAGGGTTCACAGC-3′; antisense primer: 5′-GAAGACTCTGATGCACGCAGGTC)-3′. Competitive RT–PCR was performed in 100 μl of PCR buffer containing 2 μl of the RT reaction mixture, 25 pmol of the sense and antisense primers, 100 μM each deoxynucleotide, 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 2.5 U Taq DNA polymerase, and chymase-4 competitor DNA. The PCR products for target chymase-4 and the competitor were 654 and 490 bp, respectively. The amplification conditions for chymase-4 were 94°C for 1 min, 63°C for 1 min, and 72°C for 1 min over 45 cycles. The integrated density of PCR products was measured as described previously (Kakizoe et al., 2001).
Statistical analysis
All data are expressed as means±s.e.m. When a single treatment group was compared with a control group, statistical analysis was performed using Student's t-test. Multiple comparisons were made by analysis of variance followed by the post hoc Bonferroni/Dunn's test. Differences were considered significant at a level of P<0.05.
Results
Effect of chymase inhibitor on fibrous proliferation and mast cell numbers of the skin in Tsk mice
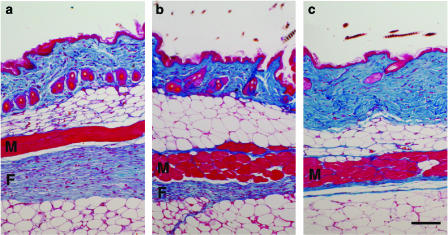
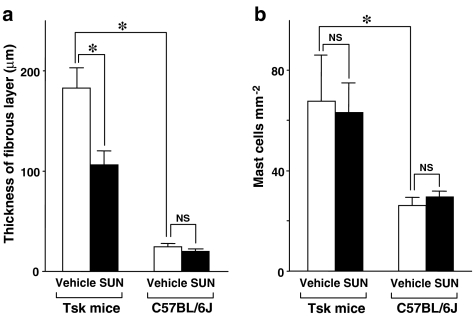
In 15-week-old mice, microscopy revealed a remarkable thickening of the subcutaneous fibrous layer beneath panniculus carnosus muscle in the dorsal skin in vehicle-treated Tsk mice (Figure 1a), whereas the fibrous layer in control C57BL/6J mice was much thinner (Figure 1c). Specifically, the thickness of the fibrous layer from vehicle-treated Tsk mice was 7.5 times higher than that in vehicle-treated control C57BL/6J mice (Figure 2a). SUN-C8257 markedly reduced the thickness of subcutaneous fibrous layer in Tsk mice by 42% (P<0.05), compared with that in vehicle-treated Tsk mice (Figures 1b and 2a). In contrast, SUN-C8257 showed no effect on the fibrous layer in the control mice (Figure 2a). In contrast to the fibrous layer in Tsk mice, microscopy revealed no significant pathological abnormalities in the panniculus carnosus muscle layer in Tsk mice. There was no statistically significant difference in the thickness of the panniculus carnosus muscle layer and the dermis between Tsk mice and control C57BL/6J mice, nor between the vehicle-treated mice and the SUN-C8257-treated mice (data not shown). Thus, SUN-C8257 was shown to be specifically effective in reducing the thickness of the subcutaneous fibrous layer in Tsk mice. The number of mast cells in the skin of the vehicle-treated mice was 2.6 times higher than that in the skin from vehicle-treated C57BL/6J mice acting as a control. However, treatment with SUN-C8257 for 2 weeks produced no effect on the number of mast cells in the skin of Tsk mice or in the control C57BL/6J mice (Figure 2b).
Figure 1.
Effect of a chymase inhibitor on the skin fibrous proliferation in Tsk and control C57B/6J mice. Skin fibrosis was analyzed with Azan-stained sections from a vehicle-treated Tsk mouse (a), SUN-C8257-treated Tsk mouse (b), and vehicle-treated control C57BL/6J (c). The blue color staining indicates the presence of collagen. In vehicle-treated Tsk mouse, thick blue-stained fibrous layer was detected beneath panniculus carnosus muscle layer (a), whereas fibrous layer in SUN-C8257-treated Tsk mouse was thinner than that in vehicle-treated Tsk mouse (b). The fibrous layer in control C57BL/6J mice was obviously thin (c). F: fibrous layer, M: panniculus carnosus muscle layer. Scale bar=100 μm.
Figure 2.
Effect of a chymase inhibitor on the skin fibrous proliferation and the number of skin mast cells in Tsk and control C57B/6J mice. Thickness of the subcutaneous fibrous layer (a) and the number of skin mast cells (b) of Tsk and control C57BL/6J mice were measured after 2 weeks treatment with vehicle or SUN-C8257. White bars indicate the data of vehicle-treated mice (Vehicle) and black bars indicate the data of mice treated with SUN-C8257 (SUN). Vertical bars represent s.e.m. *P<0.05. NS=not significant.
Effect of chymase inhibitor on chymase activity and chymase-4 mRNA level
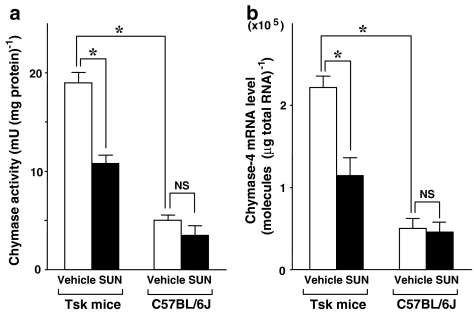
The chymase activity and mRNA level of chymase-4 in the dorsal skin of vehicle-treated Tsk mice were 3.8 and 4.4 times higher, respectively, as compared to vehicle-treated control C57BL/6J mice (Figure 3a and b). However, the mast cell count in the vehicle-treated control Tsk mice was 2.6 times higher than that in the skin of vehicle-treated control C57BL/6J mice (Figure 2b). These results suggest that the reason for the increase in chymase activity and the chymase mRNA level in the skin of vehicle-treated Tsk mice cannot be simply explained by the increase in the number of mast cells in the skin of Tsk mice. The function of each mast cell may also be activated in the skin of Tsk mice. SUN-C8257 treatment significantly inhibited the skin chymase activity (by 43%) and mRNA levels of chymase-4 of Tsk mice (by 47%, P<0.05) compared with those of vehicle-treated Tsk mice (Figure 3a and b). However, in our present study, the number of mast cells was not changed by treatment with SUN-C8257 for 2 weeks (Figure 2b). These results suggest that short-term treatment with chymase inhibitor may be effective for stabilizing each mast cell function and in inhibiting chymase gene expression. In contrast, SUN-C8257 showed no significant effect on chymase activity nor on the mRNA level of chymase-4 in the dorsal skin of the control C57BL/6J mice (Figure 3a and b). Although the reason for the negative effect of SUN-C8257 on normal C57BL/6J mice is unclear, SUN-C8257 may be more effective on the disordered skin tissue in Tsk mice where mast cells are activated.
Figure 3.
Effect of a chymase inhibitor on chymase activity and chymase-4 mRNA level in the skin tissues of Tsk and control C57B/6J mice. Chymase activity (a) and chymase-4 mRNA level (b) of Tsk and control C57BL/6J mice were measured after 2 weeks treatment with vehicle or SUN-C8257. White bars indicate the data of vehicle-treated mice (Vehicle) and black bars indicate the data of mice treated with SUN-C8257 (SUN). Vertical bars represent s.e.m. *P<0.05. NS=not significant.
Immunohistochemical localization of TGF-beta1 and chymase
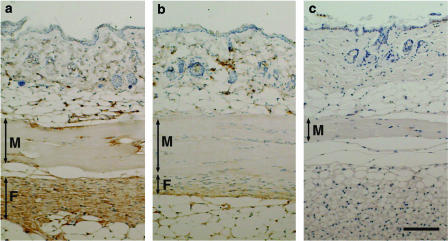
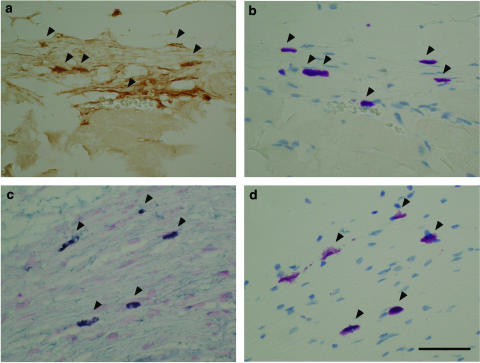
Immunohistochemical analysis of the skin of vehicle-treated Tsk mice revealed strong staining of TGF-beta1 in fibroblasts and extracellular matrix in the proliferative fibrous layer beneath panniculus carnosus muscle layer, and weak TGF-beta1 staining in endothelial cells, fibroblasts, and the extracellular matrix in the dermis (Figure 4a). However, staining of TGF-beta1 in the fibrous layer of the skin of Tsk mice treated with SUN C8257 was very weak compared to that of vehicle-treated Tsk mice (Figure 4a and b). TGF-beta1 production in the fibrous layer of Tsk mice was suppressed by the treatment with SUN C8257. In the proliferative fibrous layer of vehicle-treated Tsk mice, strong TGF-beta1 staining was also detected in mast cells (Figure 5a), which were identified by toluidine blue (Figure 5b). In the proliferative fibrous layer of the vehicle-treated Tsk mice, strong staining of chymase was also found in the mast cells (Figure 5c), which were identified by toluidine blue (Figure 5d). CTMCs in the fibrous layer were one of the major sources for TGF-beta1 production.
Figure 4.
Effect of a chymase inhibitor on immunohistochemical staining of TGF-beta1. Histological cross-sections of the skin tissues from vehicle-treated Tsk mouse (a), SUN-C8257-treated Tsk mouse (b), and vehicle-treated control C57BL/6J mouse (c) were incubated with antibody against TGF-beta1, and then the tissue-bound antibody was detected with the DAKO ENVISION kit and DAB substrate. Sections were then counterstained with hematoxylin. The brown color staining indicates the presence of TGF-beta1. F: fibrous layer; M: panniculus carnosus muscle layer. Scale bar=100 μm.
Figure 5.
Immunohistochemical localization of TGF-beta1 and chymase. Histological cross-sections of skin tissues from vehicle-treated Tsk mouse were incubated with polyclonal antibody against TGF-beta1 or chymase. The antibody against TGF-beta1 was detected with the DAKO ENVISION kit and DAB substrate. The brown color staining indicates the presence of TGF-beta1 (a). To identify mast cells, adjacent section to that in part (a) was stained with toluidine blue. On reacting with toluidine blue, mast cells showed metachromasia, which is in red-purple color (b). The antibody against chymase was detected with the DAKO ENVISION kit and TrueBlue peroxidase substrate. Sections were then counterstained with eosin. Positive staining of chymase was revealed by dark blue color (c). To identify mast cells, adjacent section to that in part (c) was stained with toluidine blue. Mast cells were stained with red-purple color (d). Arrows point to mast cells. Scale bar=50 μm.
Discussion
The present study demonstrated for the first time that a newly synthesized specific inhibitor for chymase, SUN-C8257, decreased the chymase activity and suppressed the gene expression of chymase-4 (CTMC-specific chymase) and furthermore it effectively attenuated fibrous proliferation in the skin of Tsk mice. These results strongly support our assumption that CTMC-derived chymase may play a key role in the development of fibrosis in the Tsk mice.
Chymase is a chymotrypsin-like serine protease and is most likely involved in the immune and inflammatory responses by hydrolyzing a variety of substrates including extracellular matrix (Vartio et al., 1981; Banovac & de Forteza, 1992), vasoactive substances (Reilly et al., 1982; Caughey et al., 1988; Wypij et al., 1992), cytokines (Mizutani et al., 1991; Longley et al., 1997), metalloproteases (Saarinen et al., 1994), and lipoproteins (Paananen & Kovanen, 1994). These properties of chymase may be relevant to the mechanisms of tissue remodeling. Although its precise in vivo pathophysiological role and the actual substrate for chymase have yet to be determined, recent observations have focused on the profibrotic function of chymase through activation of latent TGF-beta1 (Taipale et al., 1995), which plays a key role in the regulation of extracellular matrix gene expression in tissues. Since TGF-beta1 is expressed in most tissues in a latent form, the activation of latent TGF-beta1 by chymase is especially crucial in local tissues. Overexpressed chymase in connective tissues is believed to also exaggerate the destruction of extracellular matrix, and furthermore, the excessive amount of growth factors such as TGF-beta1, which bind to the extracellular matrix, would be liberated by chymase in a bioactive free form. A recent study has shown that mast cells also produce a latent form of TGF-beta1, which is immediately converted by cosecreted chymase into active form of TFG-beta1 (Lindstedt et al., 2001). In one study, immunohistochemical analysis of the skin from scleroderma patients showed that TGF-beta was localized in fibroblasts, endothelial cells, and extracellular matrix; however, no significant staining of TGF-beta was detected in the skin from normal subjects (Gabrielli et al., 1993). In addition, expression of TGF-beta receptors was higher in scleroderma fibroblasts than in normal fibroblasts (Kawakami et al., 1998). These findings suggest that activation of TGF-beta signaling system may be a crucial factor in the pathogenesis of scleroderma. Our immunohistochemical analysis also revealed that strong staining of TGF-beta1 was localized to fibroblasts and extracellular matrix in the fibrous layer of skin of Tsk mice. Interestingly, mast cells located in the fibrous layer are also one of the major sources of TGF-beta1 in this same model. An important finding in the present study is that the immunostaining of TGF-beta1 in the fibrous layer was clearly reduced by treatment with the chymase inhibitor. Chymase inhibitors may prevent the chymase-dependent pathway that activates latent TGF-beta1 in the fibrous layer, thereby exhibiting beneficial effects by inhibiting the development of fibrosis.
Another important profibrotic factor produced by chymase is angiotensin (ANG) II. When chymase was upregulated in cardiomyopathic hearts with extensive fibrosis and mechanically injured arteries yielding intimal and adventitial fibrosis, chymase-dependent ANG II formation in those tissues was significantly activated (Shiota et al., 1993, 1997, 1998, 1999). Mouse chymase-4 is also known to be highly efficient in converting ANG I to ANG II (Caughey et al., 2000). Thus, it can be presumed that a chymase inhibitor may exhibit its beneficial effect, at least in part, by reducing chymase-generated ANG II in scleroderma tissues.
The chymase inhibitor SUN-C8257 is a potential tool for specifically inhibiting the enzymatic activity of chymase. However, a chymase inhibitor may have additional effects on mast cell function and the gene regulatory mechanism of chymase. One of the important findings in our present study is that mouse chymase-4 gene expression in the skin of Tsk mice was significantly inhibited by SUN-C8527. SUN-C8257 has also been shown to reduce chymase gene expression in the failing heart and to prevent cardiac fibrosis (Matsumoto et al., 2003). Although the precise gene regulatory mechanism of chymase is yet unknown, degranulation of mast cells may trigger the onset of gene expression of chymase. A recent study showed that the gene expression of chymase and also of TGF-beta1 were upregulated after mast cell degranulation induced by compound 48/80 (Lindstedt et al., 2001). Interestingly, chymase has been reported to induce degranulation of mast cells (Schick et al., 1984). Furthermore, another recent study clearly showed that a selective chymase inhibitor stabilized the human skin mast cells and reduced the histamine release by up to 80% (He et al., 1999). These results suggest that chymase may trigger further activation of mast cells and induction of mast cell-derived factors including chymase itself. Chymase inhibitors may regulate mast cell function by inhibiting the mechanism for chymase-induced activation of mast cells.
The number of mast cells in tissues depends on infiltration of progenitor cells from circulation and also on the rate of their proliferation and survival in the tissues. The stem cell factor (SCF)/c-kit signaling system is one of the most important factors in the regulation of the number of mast cells in local tissues. Chymase was shown to cleave the membrane-bound SCF and produce bioactive soluble SCF (Longley et al., 1997). Although the functional difference between membrane-bound SCF and soluble SCF in vivo is unknown, recent reports showed that treatment with the chymase inhibitor SUN-C8257 for 5–6 weeks reduced the number of mast cells in dermatitis tissues (Tomimori et al., 2002; Watanabe et al., 2002). These results suggest that chymase may regulate the mechanism of mast cell accumulation in local tissues by producing soluble SCF from membrane-bound SCF that is associated with surrounding cells such as fibroblasts and keratinocytes (Tomimori et al., 2002). However, the present study showed no effect of SUN-C8527 on the number of skin mast cells in Tsk mice, although the number of skin mast cells was significantly higher in vehicle-treated Tsk mice than in the control C57BL/6J mice.
Mast cells are known to survive for an extensive period of time once they migrate into local tissues and differentiate into mature cells. The half-life of the newly formed mast cells has been estimated to be approximately 40 days (Enerback & Lowhagen, 1979). In our study, Tsk mice were treated with a chymase inhibitor for only 2 weeks. Long-term treatment may be necessary to reduce the number of mast cells in local tissues. Nonetheless, our study clearly showed that short-term treatment with the chymase inhibitor SUN-C8527 sufficiently inhibited gene expression of chymase-4 and the development of fibrosis in the skin of Tsk mice, although the number of mast cells was not changed. These results suggest that short-term treatment with a chymase inhibitor may be effective in stabilizing the various mast cell functions and in inhibiting chymase gene expression. Further experimentation is necessary to clarify whether or not fibrous proliferation in Tsk mice is suppressed further when the number of mast cells is also decreased by long-term treatment with a chymase inhibitor.
In conclusion, treatment of Tsk mice with a new synthetic inhibitor of chymase, SUN-C8257, attenuated fibrous proliferation in the Tsk skin. As a specific chymase inhibitor, SUN-C8257 was not only effective in suppressing the enzymatic activity of chymase in the skin tissues of Tsk mice, but it also showed additional beneficial effects. Further studies with chymase inhibitors are warranted to uncover the true roles of chymase in vivo and to confirm the significant involvement of mast cells in the pathogenesis of fibrotic disorders.
Acknowledgments
We thank Dr G.H. Caughey, University of California at San Francisco, for kindly providing the antibody against chymase. This work was supported in part by a Grant-in-Aid from Japan Society for the Promotion of Science No. 16590193 (to N. Shiota in 2004).
Abbreviations
- CTMC
connective-tissue-type mast cell
- SCF
stem cell factor
- TGF
transforming growth factor
- Tsk
tight-skin
References
- BANOVAC K., DE FORTEZA R. The effect of mast cell chymase on extracellular matrix: studies in autoimmune thyroiditis and in cultured thyroid cells. Int. Arch. Allergy Immunol. 1992;99:141–149. doi: 10.1159/000236348. [DOI] [PubMed] [Google Scholar]
- CAUGHEY G.H., LEIDIG F., VIRO N.F., NADEL J.A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J. Pharmacol. Exp. Ther. 1988;244:133–137. [PubMed] [Google Scholar]
- CAUGHEY G.H., RAYMOND W.W., WOLTERS P.J. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim. Biophys. Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- CLAMAN H.N. Mast cell changes in a case of rapidly progressive scleroderma – ultrastructural analysis. J. Invest. Dermatol. 1989;92:290–295. doi: 10.1111/1523-1747.ep12276876. [DOI] [PubMed] [Google Scholar]
- CLAMAN H.N.Mast cells and fibrosis: hints from graft-versus-host disease and scleroderma The Mast Cell in Health and Disease. Lung Biology in Health and Disease 1993New York: Marcel Dekker; 653–667.eds. Kaliner, M.A. & Metcalfe, D.D. Vol. 62, pp [Google Scholar]
- ENERBACK L., LOWHAGEN G.B. Long term increase of mucosal mast cells in the rat induced by administration of compound 48/80. Cell Tissue Res. 1979;198:209–215. doi: 10.1007/BF00232005. [DOI] [PubMed] [Google Scholar]
- EVERETT E.T., PABLOS J.L., HARLEY R.A., LEROY E.C., NORRIS J.S. The role of mast cells in the development of skin fibrosis in tight-skin mutant mice. Comp. Biochem. Physiol. A. 1995;110:159–165. doi: 10.1016/0300-9629(94)00127-f. [DOI] [PubMed] [Google Scholar]
- FANG K.C., WOLTERS P.J., STEINHOFF M., BIDGOL A., BLOUNT J.L., CAUGHEY G.H. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J. Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- FUKAMI H., IMAJO S., ITO A., KAKUTANI S., SHIBATA H., SUMIDA M., TANAKA T., NIWATA S., SAITOH M., KISO Y., MIYAZAKI M., OKUNISHI H., URATA H., ARAKAWA K. Substituted 3-phenylsulfonylquinazoline-2,4-dione derivatives as novel nonpeptide inhibitors of human heart chymase. Drug Des. Discov. 2000;17:69–84. [PubMed] [Google Scholar]
- GABRIELLI A., DI LORETO C., TABORRO R., CANDELA M., SAMBO P., NITTI C., DANIELI M.G., DELUSTRO F., DASCH J.R., DANIELI G. Immunohistochemical localization of intracellular and extracellular associated TGF beta in the skin of patients with systemic sclerosis (scleroderma) and primary Raynaud's phenomenon. Clin. Immunol. Immunopathol. 1993;68:340–349. doi: 10.1006/clin.1993.1136. [DOI] [PubMed] [Google Scholar]
- GREEN M.C., SWEET H.O., BUNKER L.E. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am. J. Pathol. 1976;82:493–512. [PMC free article] [PubMed] [Google Scholar]
- GRUBER B.L., KAUFMAN L.D. Ketotifen-induced remission in progressive early diffuse scleroderma: evidence for the role of mast cells in disease pathogenesis. Am. J. Med. 1990;89:392–395. doi: 10.1016/0002-9343(90)90360-p. [DOI] [PubMed] [Google Scholar]
- HAWKINS R.A., CLAMAN H.N., CLARK R.A., STEIGERWALD J.C. Increased dermal mast cell populations in progressive systemic sclerosis: a link in chronic fibrosis. Ann. Intern. Med. 1985;102:182–186. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- HE S., GACA M.D., MCEUEN A.R., WALLS A.F. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J. Pharmacol. Exp. Ther. 1999;291:517–523. [PubMed] [Google Scholar]
- HUANG R.Y., BLOM T., HELLMAN L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur. J. Immunol. 1991;21:1611–1621. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- JIMENEZ S.A., CHRISTNER P. Animal models of systemic sclerosis. Clin. Dermatol. 1994;12:425–436. doi: 10.1016/0738-081x(94)90295-x. [DOI] [PubMed] [Google Scholar]
- KAKIZOE E., SHIOTA N., TANABE Y., SHIMOURA K., KOBAYASHI Y., OKUNISHI H. Isoform-selective upregulation of mast cell chymase in the development of skin fibrosis in scleroderma model mice. J. Invest. Dermatol. 2001;116:118–123. doi: 10.1046/j.1523-1747.2001.00165.x. [DOI] [PubMed] [Google Scholar]
- KAWAKAMI T., IHN H., XU W., SMITH E., LEROY C., TROJANOWSKA M. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J. Invest, Dermatol. 1998;110:47–51. doi: 10.1046/j.1523-1747.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- LEVI-SCHAFFER F.Mast cell/fibroblast interactions in health and disease Human Basophils and Mast Cells: Biological Aspects Chemical Immunology 1995Basel: Karger; 165–185.ed. Marone, G. Vol. 61, pp [Google Scholar]
- LINDSTEDT K.A., WANG Y., SHIOTA N., SAARINEN J., HYYTIAINEN M., KOKKONEN J.O., KESKI-OJA J., KOVANEN P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- LONGLEY B.J., TYRRELL L., MA Y., WILLIAMS D.A., HALABAN R., LANGLEY K., LU H.S., SCHECHTER N.M. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO T., WADA A., TSUTAMOTO T., OHNISHI M., ISONO T., KINOSHITA M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- MAYES M.D., LACEY J.V., JR, BEEBE-DIMMER J., GILLESPIE B.W., COOPER B., LAING T.J., SCHOTTENFELD D. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- MENTON D.N., HESS R.A., LICHTENSTEIN J.R., EISEN A. The structure and tensile properties of the skin of tight-skin (Tsk) mutant mice. J. Invest. Dermatol. 1978;70:4–10. doi: 10.1111/1523-1747.ep12543353. [DOI] [PubMed] [Google Scholar]
- MIZUTANI H., SCHECHTER N., LAZARUS G., BLACK R.A., KUPPER T.S. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J. Exp. Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKORI Y., KAKIZOE E., KOBAYASHI Y., SHIMOURA K., OKUNISHI H., DEKIO S. Skin mast cell promotion of matrix remodeling in burn wound healing in mice: relevance of chymase. Arch. Dermatol. Res. 1998;290:553–560. doi: 10.1007/s004030050351. [DOI] [PubMed] [Google Scholar]
- NISHIOKA K., KOBAYASHI Y., KATAYAMA I., TAKIJIRI C. Mast cell numbers in diffuse scleroderma. Arch. Dermatol. 1987;123:205–208. [PubMed] [Google Scholar]
- PAANANEN K., KOVANEN P.T. Proteolysis and fusion of low density lipoprotein particles independently strengthen their binding to exocytosed mast cell granules. J. Biol. Chem. 1994;269:2023–2031. [PubMed] [Google Scholar]
- REILLY C.F., TEWKSBURY D.A., SCHECHTER N.M., TRAVIS J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J. Biol. Chem. 1982;257:8619–8622. [PubMed] [Google Scholar]
- SAARINEN J., KALKKINEN N., WELGUS H.G., KOVANEN P.T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J. Biol. Chem. 1994;269:18134–18140. [PubMed] [Google Scholar]
- SCHICK B., AUSTEN K.F., SCHWARTZ L.B. Activation of rat serosal mast cells by chymase, an endogenous secretory granule protease. J. Immunol. 1984;132:2571–2577. [PubMed] [Google Scholar]
- SEIBOLD J.R., GIORNO R.C., CLAMAN H.N. Dermal mast cell degranulation in systemic sclerosis. Arthritis Rheum. 1990;33:1702–1709. doi: 10.1002/art.1780331114. [DOI] [PubMed] [Google Scholar]
- SHIOTA N., FUKAMIZU A., OKUNISHI H., TAKAI S., MURAKAMI K., MIYAZAKI M. Cloning of the gene and cDNA for hamster chymase 2, and expression of chymase 1, chymase 2 and angiotensin-converting enzyme in the terminal stage of cardiomyopathic hearts. Biochem. J. 1998;333:417–424. doi: 10.1042/bj3330417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIOTA N., FUKAMIZU A., TAKAI S., OKUNISHI H., MURAKAMI K., MIYAZAKI M. Activation of angiotensin II-forming chymase in the cardiomyopathic hamster heart. J. Hypertens. 1997;15:431–440. doi: 10.1097/00004872-199715040-00014. [DOI] [PubMed] [Google Scholar]
- SHIOTA N., OKUNISHI H., FUKAMIZU A., SAKONJO H., KIKUMORI M., NISHIMURA T., NAKAGAWA T., MURAKAMI K., MIYAZAKI M. Activation of two angiotensin-generating systems in the balloon-injured artery. FEBS Lett. 1993;323:239–242. doi: 10.1016/0014-5793(93)81348-4. [DOI] [PubMed] [Google Scholar]
- SHIOTA N., OKUNISHI H., TAKAI S., MIKOSHIBA I., SAKONJO H., SHIBATA N., MIYAZAKI M. Tranilast suppresses vascular chymase expression and neointima formation in balloon-injured dog carotid artery. Circulation. 1999;99:1084–1090. doi: 10.1161/01.cir.99.8.1084. [DOI] [PubMed] [Google Scholar]
- SPRINGMAN E.B., SERAFIN W.E.Secretory endo- and exopeptidases of mouse mast cells: structure, genetics, and regulation of expression Mast Cell Proteases in Immunology and Biology. Clinical Allergy and Immunology 1995New York: Marcel Dekker; 169–201.ed. Caughey, G.H. Vol. 6, pp [Google Scholar]
- TAIPALE J., LOHI J., SAARINEN J., KOVANEN P.T., KESKI-OJA J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J. Biol. Chem. 1995;270:4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- TOMIMORI Y., MUTO T., FUKAMI H., SAITO K., HORIKAWA C., TSURUOKA N., YAMASHIRO K., SAITO M., SUGIURA N., SUMIDA M., KAKUTANI S., FUKUDA Y. Mast cell chymase regulates dermal mast cell number in mice. Biochem. Biophys. Res. Commun. 2002;290:1478–1482. doi: 10.1006/bbrc.2002.6365. [DOI] [PubMed] [Google Scholar]
- VARTIO T., SEPPA H., VAHERI A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J. Biol. Chem. 1981;256:471–477. [PubMed] [Google Scholar]
- WALKER M., HARLEY R., MAIZE J., DELUSTRO F., LEROY E.C. Mast cells and their degranulation in the Tsk mouse model of scleroderma. Proc. Soc. Exp. Biol. Med. 1985;180:323–328. doi: 10.3181/00379727-180-42183. [DOI] [PubMed] [Google Scholar]
- WALKER M.A., HARLEY R.A., LEROY E.C. Inhibition of fibrosis in TSK mice by blocking mast cell degranulation. J. Rheumatol. 1987;14:299–301. [PubMed] [Google Scholar]
- WATANABE N., TOMIMORI Y., SAITO K., MIURA K., WADA A., TSUDZUKI M., FUKUDA Y. Chymase inhibitor improves dermatitis in NC/Nga mice. Int. Arch. Allergy Immunol. 2002;128:229–234. doi: 10.1159/000064256. [DOI] [PubMed] [Google Scholar]
- WYPIJ D.M., NICHOLS J.S., NOVAK P.J., STACY D.L., BERMAN J., WISEMAN J.S. Role of mast cell chymase in the extracellular processing of big-endothelin-1 to endothelin-1 in the perfused rat lung. Biochem. Pharmacol. 1992;43:845–853. doi: 10.1016/0006-2952(92)90252-e. [DOI] [PubMed] [Google Scholar]