Abstract
Bisindolylmaleimide inhibitors of protein kinase C (PKC), such as GF109203X and Ro31-8220, have been used to investigate the roles of PKC isoforms in many cellular processes in cardiac myocytes, but these agents may also inhibit p90RSK activity.
In in vitro kinase assays utilising 50 μM [ATP], GF109203X and Ro31-8220 inhibited p90RSK isoforms (IC50 values for inhibition of RSK1, RSK2 and RSK3, respectively, were 610, 310 and 120 nM for GF109203X, and 200, 36 and 5 nM for Ro31-8220) as well as classical and novel PKC isoforms (IC50 values for inhibition of PKCα and PKCɛ, respectively, were 8 and 12 nM for GF109203X, and 4 and 8 nM for Ro31-8220).
At physiological [ATP] (5 mM), both GF109203X and Ro31-8220 exhibited reduced potency as inhibitors of RSK2, PKCα and PKCɛ (IC50 values of 7400, 310 and 170 nM, respectively, for GF109203X, and 930, 150 and 140 nM, respectively, for Ro31-8220), with the latter agent retaining its relatively greater potency.
To determine the effects of GF109203X and Ro31-8220 on p90RSK activity in cultured adult rat ventricular myocytes (ARVM), phosphorylation of the eukaryotic elongation factor 2 kinase (eEF2K) at Ser366, a known p90RSK target, was used as the index of such activity. Adenoviral expression of a constitutively active form of mitogen-activated protein kinase (MAPK) or extracellular signal-regulated kinase (ERK) kinase 1 (MEK1) was used to induce PKC-independent p90RSK activation and downstream phosphorylation of eEF2K.
eEF2K phosphorylation was abolished by U0126 (1 μM), a selective inhibitor of MEK1, and was significantly reduced by GF109203X at ⩾3 μM and by Ro31-8220 at ⩾1 μM. At 1 μM, both agents inhibited PMA-induced PKC activity in ARVM.
These data show that GF109203X and Ro31-8220 inhibit various isoforms of PKC and p90RSK in vitro and in intact ARVM, with the former agent exhibiting relatively greater selectivity for PKC.
Keywords: Protein kinase inhibitor, protein kinase C, 90 kDa ribosomal S6 kinase, bisindolylmaleimides, eukaryotic elongation factor-2 kinase, adenovirus, myocytes
Introduction
Bisindolylmaleimide inhibitors of protein kinase C (PKC), such as GF109203X (bisindolylmaleimide I; originally described by Toullec et al., 1991) and Ro31-8220 (bisindolylmaleimide IX; originally described by Davis et al., 1989), have been used extensively as ‘specific' inhibitors of mixed PKC isoforms, in order to delineate PKC functions in multiple systems. The use of these inhibitors, particularly GF109203X, in myocardial tissue and cells has implicated PKC-mediated signalling events in the regulation of physiological functions, such as contraction (Pi & Walker, 2000; Szokodi et al., 2002; von Lewinski et al., 2003) and protein synthesis (Ponicke et al., 1999), as well as a variety of pathophysiological processes, such as myocyte hypertrophy (Mullan et al., 1997; Ponicke et al., 1999; Ruf et al., 2002) and ischaemic cell death (Kitakaze et al., 1996; Chen et al., 1999; Inagaki et al., 2000; Fryer et al., 2001). In addition, GF109203X and Ro31-8220 have been used to investigate the roles of PKC in the regulation of sarcolemmal ion-transporting proteins, such as K+ (Hu et al., 1996; Wang et al., 2001a), Ca2+ (Woo & Lee, 1999; Hu et al., 2000) and Cl− (Middleton & Harvey, 1998; Duan et al., 1999) channels and the Na+/K+ pump (Jo et al., 2000). These inhibitors have also been utilised by this and other laboratories to explore the involvement of PKC isoforms in the stimulation of the sarcolemmal Na+/H+ exchanger (NHE1) by diverse stimuli, such as adrenergic (Pucéat et al., 1993; Snabaitis et al., 2000), thrombin (Yasutake et al., 1996), angiotensin (Gunasegaram et al., 1999) and opioid (Bian et al., 2000) receptor agonists, anaesthetic agents (Kanaya et al., 2001) and oxidative stress (Snabaitis et al., 2002). As an alternative to pharmacological PKC inhibition, some investigators have utilised PKC downregulation by prolonged phorbol ester stimulation (Clerk et al., 1998; Wang et al., 2001c). However, this method requires an extended period (atleast 24 h) of such stimulation and is likely to be accompanied by compensatory changes that would not occur with acute pharmacological inhibition of PKC.
Contrary to the assumption that bisindolylmaleimides are specific inhibitors of PKC, it has been known for some time that, in vitro, GF109203X and Ro31-8220 also inhibit the activity of the RSK2 isoform of the 90 kDa ribosomal S6 kinase (p90RSK) family with comparable potency (Alessi, 1997). Nonspecific inhibition of p90RSK isoform(s) at PKC-inhibitory concentrations would complicate the interpretation of data obtained with bisindolylmaleimide inhibitors. This would be of particular concern when investigating the roles of PKC isoforms in (patho)physiological processes, such as the regulation of NHE1 activity (Takahashi et al., 1999; Snabaitis et al., 2000; Haworth et al., 2003) and protein synthesis (Wang, 2001b; Wang & Proud, 2002) and the pathogenesis of heart failure (Takeishi et al., 2002; Aker et al., 2004)), where p90RSK activity is also likely to play a role. Nevertheless, the relative effects of GF109203X and Ro31-8220 on the activities of the different isoforms of p90RSK versus PKC in vitro and in cardiac myocytes are unknown, and it may not be appropriate to extrapolate from in vitro findings with RSK2 to the intact cell or organ. In this context, a previous study from our laboratory has indicated that, in contrast to in vitro findings (Alessi, 1997), GF109203X and Ro31-8220 do not inhibit the 70 kDa ribosomal S6 kinase (p70S6K) in intact adult rat ventricular myocytes (ARVM) (Roberts et al., 2004).
In this study, we determined: (1) the relative in vitro potencies of GF109203X and Ro31-8220 as inhibitors of recombinant p90RSK isoforms RSK1, RSK2 and RSK3 versus recombinant PKC isoforms PKCα and PKCɛ; (2) the in vitro selectivity of these bisindolylmaleimide inhibitors for recombinant PKC isoforms versus recombinant RSK2, the predominant p90RSK isoform in myocardium, at a physiological concentration of ATP; (3) the concentration-dependent effects of GF109203X and Ro31-8220 on the total cellular activities of native p90RSK versus PKC isoforms expressed in intact ARVM.
Methods
This investigation was performed in accordance with the Home Office ‘Guidance on the Operation of the Animals (Scientific Procedures) Act 1986', published by Her Majesty's Stationery Office, London, U.K.
Synthesis and purification of recombinant proteins
Bacterial expression vectors encoding GST-NHE1 and GST-MARCKS (pGEX-KG and pGEX-2 T, respectively) were transformed into the BL21 strain of Escherichia coli. Cultures were grown to the sub-log phase and induced for 3 h at 37°C in 0.5 mM isopropyl-β-D-thioglactopyranoside. Cells were then harvested, resuspended in phosphate-buffered saline (PBS) containing 1% v v−1 Triton X-100 (PBS-T), sonicated on ice, and centrifuged at 12,000 r.p.m. for 30 min at 4°C. The fusion protein, contained in the supernatant, was passed through a pre-packed glutathione-sepharose 4B column (PharmaciaBiotech) at 4°C. Bound fusion protein was then washed extensively with PBS-T before being eluted from the column using reduced glutathione (5 mM) in Tris-HCl (50 mM) at pH 8.0. The eluted sample was concentrated using a Centriplus 30 kDa filter device (Millipore) until sample volume was approximately 500 μl. Protein concentration of the sample was then determined using a Bradford assay, and the presence of the pertinent fusion protein was confirmed by Coomassie blue staining after protein separation by sodium dodecylsulphate–polyacrylamide gel electrophoresis (SDS–PAGE).
Determination of kinase activity in vitro
GST-NHE1 and GST-MARCKS were used as substrates for recombinant human p90RSK isoforms (RSK1, RSK2, RSK3) and recombinant human PKCα and PKCɛ, respectively, in in vitro kinase assays. Serial dilutions of GF109203X and Ro31-8220 (1 nM–10 μM) and dilutions of all other reagents were performed in kinase assay buffer (Tris-base 1 M, MgCl2 1 M, DTT 1 M; pH 7.6). Aliquots (40 μl) of the assay mixture (containing protein kinase (0.1 U ml−1), protein kinase substrate (2 μM), and either GF109203X or Ro31-8220 (1 nM–10 μM) were stored on ice. The phosphorylation reaction was started by the addition of 10 μl kinase assay buffer containing ATP (50 μM or 5 mM). In experiments using PKCα or PKCɛ, phosphatidylserine (50 μg ml−1), diacylglycerol (5 μg ml−1) and CaCl2 (100 μM) were also present during the reaction. The aliquots were incubated for 15 min at 37°C before the reaction was stopped by the addition of SDS–PAGE sample buffer (glycerol 20% v v−1, β-mercaptoethanol 3% v v−1, sodium dodecylsulphate 6% w v−1, Tris-HCl 187.5 mM, bromophenol blue 0.1 mg ml−1; pH 6.8) and samples were stored at −20°C for subsequent Western immunoblot analysis.
Phosphorylation status of protein kinase substrates was determined through Western immunoblot analysis using phosphospecific antibodies recognising the sequence RXRXX(pS), to detect p90RSK-induced phosphorylation of GST-NHE1, or raised against a peptide comprising pS152/pS156 of MARCKS, to detect PKC-induced phosphorylation of GST-MARCKS. Equal substrate loading was confirmed through Western immunoblot analysis using an antibody recognising GST. Each experiment was repeated 3–6 times.
Western immunoblot analysis
Protein samples in SDS–PAGE sample buffer (20 μl of reaction mix from in vitro kinase assays; 20 μl of cellular extract (approximately 20 μg protein) from cultured ARVM) were subjected to SDS–PAGE (8 or 12%) and transferred to PVDF membranes. The membranes were incubated for 2 h, at room temperature, in Tris-buffered saline (TBS) containing 0.1% v v−1 Tween20 (TBS-T) and 10% w v−1 non-fat dry milk. They were then washed several times in TBS-T and incubated with primary antibody (in TBS-T containing 1% w v−1 nonfat dry milk) overnight at 4°C on an orbital shaker. Following three 10-min washes in TBS-T, the membranes were incubated with horseradish peroxidase-coupled anti-rabbit IgG secondary antibody for 1 h at room temperature. Membranes were then washed three times (3 × 15 min) in TBS-T, before incubation with enhanced chemiluminescence reagent. Bands were visualised by exposure of the membranes to X-ray film, digitised by optical scanning and quantified using NIH Image 1.62.
Isolation of ARVM
ARVM were isolated from the hearts of adult (250–300 g body weight) male Wistar rats (B&K Universal, Hull, U.K.) by enzymatic digestion, as described previously (Snabaitis et al., 2005). In brief, rats were anaesthetised with sodium pentobarbitone (100 mg kg−1 i.p.) and hearts were excised and perfused (37°C) in the Langendorff mode for three sequential periods, as follows: (1) with modified Tyrode's solution (in mM: NaCl 130, KCl 5.4, MgCl2 1.4, NaH2PO4 0.4, taurine 20, creatinine 10, HEPES 10, and glucose 10; adjusted to pH 7.3 at 37°C with NaOH) containing 0.75 mM CaCl2, for 5 min, (2) with nominally calcium-free modified Tyrode's solution containing 0.1 mM EGTA, for 4 min, and (3) with modified Tyrode's solution containing 0.1 mM CaCl2 and collagenase (Worthington type II, 0.75 mg ml−1), for 8 min. All solutions were gassed with 100% O2 and coronary flow rate was maintained at 12 ml min−1. After the perfusion protocol, the ventricles were removed and chopped into several pieces in modified Tyrode's solution containing 0.1 mM CaCl2 and 0.75 mg ml−1 collagenase. The tissue fragments were then gently agitated and bubbled with 100% O2 for 5 min to facilitate cell dispersion before being allowed to settle by gravity. The calcium concentration of modified Tyrode's solution was increased in two steps to 1 mM and the cells kept at room temperature for subsequent culture.
Short-term culture and adenoviral infection of ARVM
ARVM were washed in M199 medium (Invitrogen, Paisley, U.K.) with added penicillin (100 i.u. ml−1) and streptomycin (100 i.u. ml−1). The cell suspension was centrifuged at 100 × g for 2 min to pellet the myocytes, which were then resuspended in modified M199 (mM199) medium (M199 medium with added penicillin (100 i.u. ml−1), streptomycin (100 i.u. ml−1), L-carnitine (2 mM), creatine (5 mM) and taurine (5 mM)). To each well of a laminated six-well culture plate, 2 ml of cell suspension was added and the plates were maintained in a 5% CO2 incubator at 37°C. After 2 h of pre-plating, the medium was aspirated, leaving only adherent cells, and 2 ml of fresh, pre-warmed mM199 medium was added.
Adenoviral infection of cultured myocytes was performed after the initial 2 h pre-plating step. The number of rod-shaped cells in a field of 1 mm2 (as defined by an eye-piece graticule) was counted in several wells and used to estimate the number of cells per well. Myocytes were exposed to adenovirus encoding constitutively active MEK1 (caMEK1) at a multiplicity of infection (MOI) of 0–1000 plaque forming units (PFU)/cell for 1 h at 37°C, before the medium containing residual virus was removed by aspiration and replaced with fresh, pre-warmed (37°C) mM199 medium. Experiments were performed 42 h after adenoviral infection.
Determination of cellular kinase activity in ARVM
The phosphorylation status of S366 in eEF2K, the site targeted by p90RSK (Wang et al., 2001b), was determined through Western immunoblotting with a phosphospecific antibody and was used as the index of cellular p90RSK activity. To activate native p90RSK isoforms in a PKC-independent manner, ARVM were infected with adenovirus encoding caMEK1 and treated with either a kinase inhibitor (U0126, Rapamycin, GF109203X or Ro31-8220) or vehicle, before being lysed in SDS–PAGE sample buffer for subsequent Western immunoblot analysis.
Adenoviral expression of caMEK1 was determined through Western immunoblotting with an MEK1 antibody. Downstream activation of ERK1/2 was assessed by determining the phosphorylation status of the T and Y residues within their regulatory T–X–Y motif, by Western immunoblotting with a dual phosphospecific antibody. Similarly, the activation of p90RSK and the phosphorylation status of eEF2 were assessed by Western immunoblotting with phosphospecific antibodies that recognise phosphorylated S381 or T56, respectively. To confirm equal protein loading throughout, parallel Western immunoblots were probed with an antibody that recognises total ERK2. To determine PKC activity in ARVM, the phosphorylation status of the PKC substrate PKD was assessed by Western immunoblotting with a phosphospecific antibody that recognises phosphorylated S916.
Experimental protocols
For the determination of drug effects on p90RSK activity, ARVM were infected with adenovirus encoding caMEK1 (50 PFU cell−1). At 42 h after infection, cells were incubated with a kinase inhibitor (U0126 (1 μM), rapamycin (100 nM), GF109203X (1–10 μM), Ro31-8220 (1–10 μM)) or vehicle (DMSO) for 4 h, prior to the addition of SDS–PAGE sample buffer. For the determination of drug effects on PKC activity, ARVM were pretreated with a kinase inhibitor (GF109203X (1 μM) or Ro31-8220 (1 μM)) or vehicle (DMSO) for 15 min, prior to a 5-min stimulation with 30 nM PMA or vehicle (ethanol) and subsequent lysis in SDS–PAGE sample buffer. Each experiment was repeated 3–6 times.
Data analysis
All data are expressed as mean±s.e.m. Dose–response curves and IC50 values were obtained by nonlinear regression analysis of in vitro phosphorylation data, using GraphPad Prism 4 software. Data on in vivo phosphorylation (arbitrary units) or the relative change in phosphorylation (%) were subjected to ANOVA; further analysis was performed using Dunnett's test (to compare each treatment group with a single control) or Student–Newman–Keuls test (for multiple comparisons). P<0.05 was considered significant.
Materials
Recombinant active human PKC and p90RSK isoforms were from Upstate Biotechnology. Plasmids encoding glutathione S-transferase (GST)-linked fusion proteins comprising the full-length myristoylated alanine-rich C-kinase substrate (MARCKS) protein (GST-MARCKS) or amino acids 625–747 of NHE1 (GST-NHE1) were kind gifts from Dr T. Herget (Johannes Gutenberg University, Germany) (Herget & Rozengurt, 1994) and Dr B. Berk (University of Rochester, U.S.A.) (Takahashi et al., 1997), respectively. Adenovirus encoding a constitutively active form of mitogen-activated protein kinase (MAPK) or extracellular signal-regulated kinase (ERK) kinase 1 (MEK1), the upstream activator of ERK1/2, was a kind gift from Dr J. Molkentin (Cincinnati Children's Hospital Medical Centre, U.S.A.) (Bueno et al., 2000). GF109203X, Ro31-8220, U0126 (an inhibitor of MEK1) and rapamycin (an inhibitor of the mammalian target of rapamycin (mTOR)) were from Merck Biosciences, Nottingham, U.K. and were dissolved in DMSO to prepare stock solutions. Final vehicle (DMSO) concentration was ⩽0.1% in any experiment and this was included in relevant control solutions. Antibodies detecting phosphorylated forms of eukaryotic elongation factor-2 (eEF2), eEF2 kinase (eEF2K), ERK1/2, p90RSK, protein kinase D (PKD), NHE-1 and MARCKS, and antibody detecting total MEK1 were from Cell Signalling Technology, Hertfordshire, U.K. Antibody detecting total ERK2 was from Santa Cruz Biotechnology, California, U.S.A. and antibody detecting GST was from Amersham Biosciences, Buckinghamshire, U.K.
Results
Effects of bisindolylmaleimides on PKC and p90RSK isoform activity in vitro
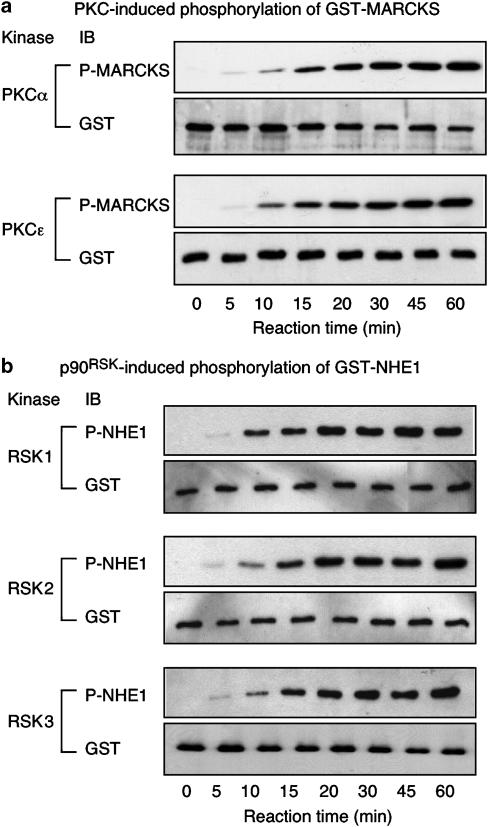
Recombinant human PKC isoforms PKCα and PKCɛ induced a time-dependent phosphorylation of MARCKS, with the reaction reaching saturation after approximately 45 min under our conditions (Figure 1a). Similarly, recombinant human p90RSK isoforms RSK1, RSK2 and RSK3 induced a time-dependent phosphorylation of the fusion protein comprising NHE1 amino acids 625–747, with maximum phosphorylation occurring after approximately 30 min (Figure 1b). On the basis that, with extended reaction times, even a reduced kinase activity would produce complete phosphorylation of the available substrate, a 15-min reaction time, which produced substantial but submaximal substrate phosphorylation, was selected for use in subsequent in vitro kinase activity assays designed to determine the inhibitory effects of bisindolylmaleimides on PKC and p90RSK isoform activities.
Figure 1.
Time-dependent phosphorylation of (a) GST-MARCKS by the PKC isoforms PKCα and PKCɛ and (b) GST-NHE1 by the p90RSK isoforms RSK1, RSK2 and RSK3. Recombinant human PKCα and PKCɛ were incubated with GST-MARCKS for 0–60 min at 37°C, prior to addition of SDS–PAGE sample buffer and Western immunoblot analysis with an antibody recognising pS152/pS156 of MARCKS. Similarly, recombinant human RSK1, RSK2 and RSK3 were incubated with GST-NHE1 for 0–60 min at 37°C, prior to addition of SDS–PAGE sample buffer and Western immunoblot analysis with an antibody recognising the RXRXX(pS) motif in GST-NHE1. An antibody recognising GST was used to confirm the presence of equal amounts of substrate. Autoradiograms representative of three experiments.
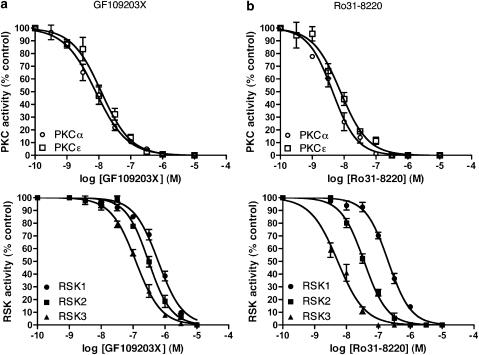
As expected, at a low ATP concentration (50 μM), GF109203X and Ro31-8220 inhibited both PKCα and PKCɛ with high potency, with no apparent isoform selectivity (Figure 2a and b, top panels; Table 1). Both bisindolylmaleimides also inhibited all three p90RSK isoforms, in a dose-dependent manner (Figure 2a and b, bottom panels; Table 1). GF109203X exhibited a rank order of potency against p90RSK isoforms of RSK3>RSK2>RSK1, with approximately two- to five-fold differences in IC50 values for different isoforms (Table 1). Ro31-8220 exhibited the same rank order of potency as GF109203X but a greater degree of selectivity between p90RSKisoforms, with approximately six- to 40-fold differences in IC50 values for RSK1, RSK2 and RSK3 (Table 1).
Figure 2.
Concentration-dependent inhibition of PKC and p90RSK isoforms in vitro by (a) GF109203X and (b) Ro31-8220, in the presence of low [ATP] (50 μM). Recombinant human PKCα and PKCɛ were incubated with GST-MARCKS and either GF109203X or Ro31-8220 (1 nM–10 μM) for 15 min at 37°C. Reactions were stopped in SDS–PAGE sample buffer and samples underwent Western immunoblot analysis with an antibody recognising pS152/pS156 of MARCKS (top panels). Similarly, recombinant human RSK1, RSK2 and RSK3 were incubated with GST-NHE1 and either GF109203X or Ro31-8220 (1 nM–10 μM) for 15 min at 37°C. Reactions were stopped in SDS–PAGE sample buffer and samples underwent Western immunoblot analysis with an antibody recognising the RXRXX(pS) motif in GST-NHE1 (bottom panels). n=4 experiments.
Table 1.
Inhibitory potencies of GF109203X and Ro31-8220
| IC50 (nM) | ||||
|---|---|---|---|---|
| 50 μM [ATP] | 5 mM [ATP] | |||
| Kinase | GF109203X | Ro31-8220 | GF109203X | Ro31-8220 |
| PKCα | 8 | 4 | 310 | 150 |
| PKCɛ | 12 | 8 | 170 | 140 |
| RSK1 | 610 | 200 | — | — |
| RSK2 | 310 | 36 | 7400 | 930 |
| RSK3 | 120 | 5 | — | — |
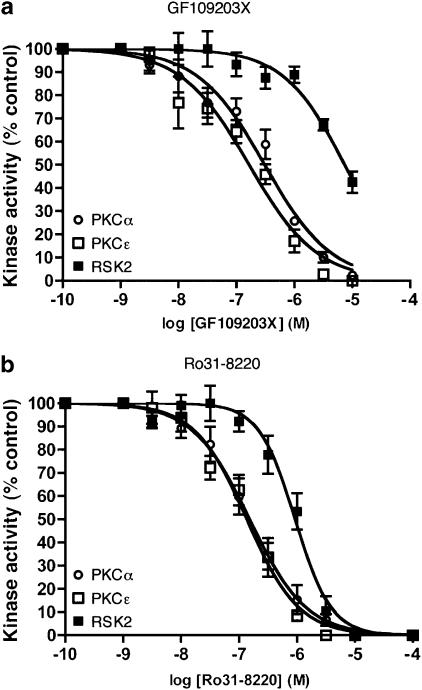
We also determined the in vitro potency of GF109203X and Ro31-8220 as inhibitors of PKCα, PKCɛ and RSK2 (the predominant p90RSK isoform expressed in myocardium (Wagner, 2004)) at an ATP concentration (5 mM) that is akin to the estimated intracellular ATP concentration in ARVM (Allue et al., 1996). At this ATP concentration, the inhibitory potencies of both bisindolylmaleimides against all three kinases were markedly reduced (Figure 3a and b; Table 1). Interestingly, GF109203X exhibited greater selectivity for the PKC isoforms, with the IC50 value for RSK2 inhibition being 24- and 43-fold greater than the corresponding values for PKCα and PKCɛ inhibition, respectively (Table 1). In comparison, for Ro31-8220, the IC50 value for RSK2 inhibition was only six-fold greater than those for PKCα and PKCɛ inhibition (Table 1).
Figure 3.
Concentration-dependent inhibition of PKC isoforms and RSK2 in vitro by (a) GF109203X and (b) Ro31-8220, in the presence of physiological [ATP] (5 mM). Recombinant human PKCα and PKCɛ were incubated with GST-MARCKS and either GF109203X or Ro31-8220 (1 nM–10 μM) for 15 min at 37°C. Reactions were stopped in SDS–PAGE sample buffer and samples underwent Western immunoblot analysis with an antibody recognising pS152/pS156 of MARCKS. Similarly, recombinant human RSK2 was incubated with GST-NHE1 and either GF109203X or Ro31-8220 (1 nM–10 μM) for 15 min at 37°C. Reactions were stopped in SDS–PAGE sample buffer and samples underwent Western immunoblot analysis with an antibody recognising the RXRXX(pS) motif in GST-NHE1. n=4 experiments.
p90RSK activation in intact ARVM by adenoviral expression of caMEK1
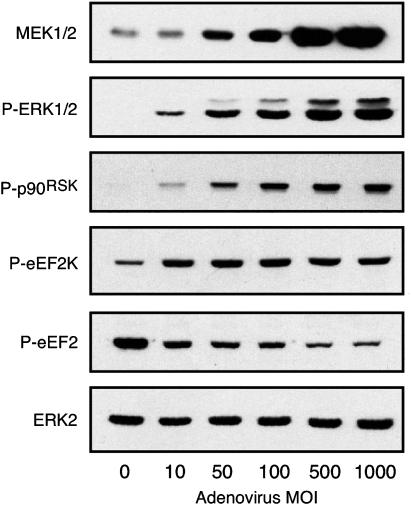
Infection of ARVM with adenovirus encoding caMEK1 resulted in an MOI-dependent increase in MEK1 expression (Figure 4). Constitutive activity of the heterologously expressed protein gave rise to an MOI-dependent increase in the phosphorylation status of the downstream components of this canonical MAPK pathway. Thus, increased phosphorylation of ERK1/2, p90RSK and eEF2K was detectable at the lowest MOI of 10 PFU cell−1 and was statistically significant at an MOI of 50 PFU cell−1 (Figure 4). Since phosphorylation of eEF2K results in its inactivation (Wang et al., 2001b), heterologous expression of caMEK1 also resulted in an MOI-dependent reduction in the phosphorylation of eEF2, the eEF2K substrate (Figure 4).
Figure 4.
Activation of the ERK/p90RSK pathway by adenoviral expression of caMEK1. ARVM were maintained in culture for 42 h, following a 1 h infection with adenovirus encoding caMEK1 at MOI of 10–1000 PFU cell−1. Cellular protein samples were subsequently subjected to Western immunoblot analysis for expression of MEK1 and phosphorylated forms of ERK1/2 (P-ERK1/2), p90RSK(P-p90RSK), eEF2K (P-eEF2K) and eEF2 (P-eEF2). Total ERK2 expression is also shown to illustrate equal protein loading. Autoradiograms representative of six experiments.
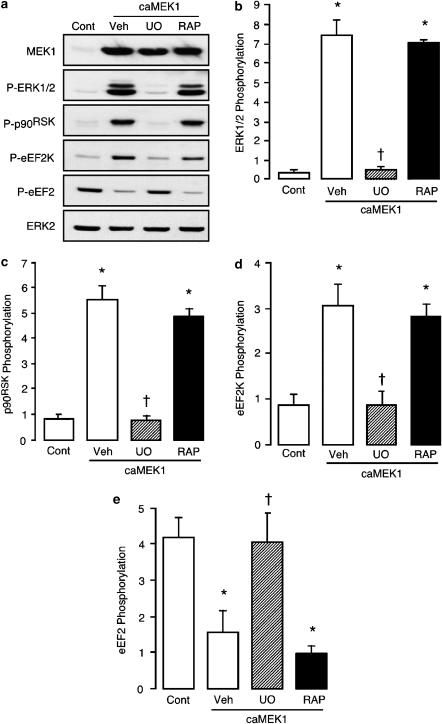
It is known that p70S6K, as well as p90RSK, may phosphorylate eEF2K at a common site that is detected by the phosphospecific antibody used (Wang et al., 2001b). Therefore, we next determined whether the increased phosphorylation of eEF2K that arises from heterologous expression of caMEK1 occurs solely through MEK1-mediated activation of p90RSK, or whether mTOR-mediated activation of p70S6K also plays a role. As shown in Figure 5, increased phosphorylation of eEF2K in ARVM infected with the caMEK1 adenovirus (50 PFU cell−1) was inhibited by the MEK inhibitor U0126, which also inhibited the increased phosphorylation of ERK1/2 and p90RSK and the decreased phosphorylation of eEF2. In contrast, the mTOR inhibitor rapamycin had no effect on these cellular responses to heterologous expression of caMEK1, including the increase in eEF2K phosphorylation (Figure 5). Consistent with a recent report (Wang & Proud, 2002), these data indicate that, in ARVM with heterologous expression of caMEK1, increased eEF2K phosphorylation occurs principally through p90RSKactivity. Thus, in this setting, the phosphorylation status of eEF2K may be used as an index of such activity.
Figure 5.
Effects of U0126 and rapamycin on the phosphorylation status of ERK1/2, p90RSK, eEF2K and eEF2 following adenovirus-mediated expression of caMEK1. ARVM were maintained in culture for 42 h, following a 1 h infection with empty virus (Cont) or adenovirus encoding caMEK1, both at an MOI of 50 PFU cell−1. ARVM were then exposed to vehicle (Veh), 1 μM U0126 (UO) or 100 nM rapamycin (RAP) for 4 h, before being lysed in SDS–PAGE sample buffer for subsequent Western immunoblot analysis. (a) Representative Western immunoblots showing the expression of MEK1 and phosphorylated forms of ERK1/2 (P-ERK1/2), p90RSK(P-p90RSK), eEF2K (P-eEF2K) and eEF2 (P-eEF2). Total ERK2 expression is also shown to illustrate equal protein loading. Quantitative data (panels b–e) illustrate the phosphorylation status of (b) ERK1/2, (c) p90RSK, (d) eEF2K and (e) eEF2, *P<0.05 versus Cont, †P<0.05 versus Veh (n=6).
Effects of bisindolylmaleimides on p90RSK activity in intact ARVM
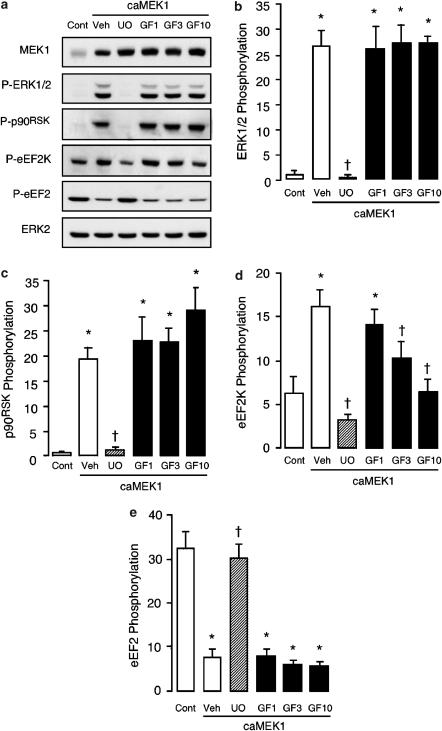
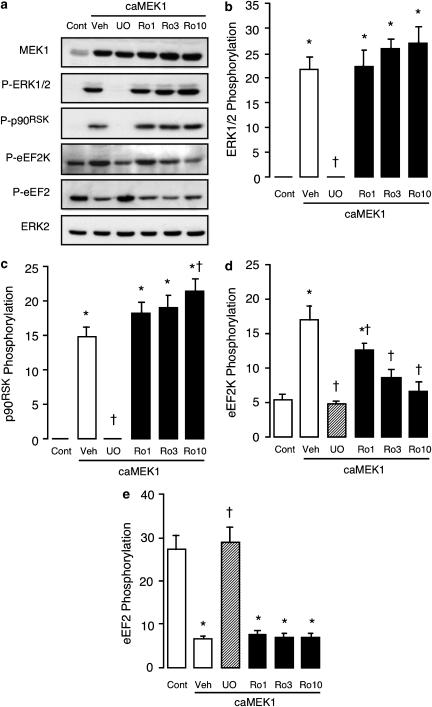
At 1–10 μM, GF109203X had no significant effect on the increases in ERK1/2 or p90RSK phosphorylation induced by heterologous expression of caMEK1, while U0126 (used as a positive control) again inhibited these responses (Figure 6, panels a–c), as well as the increase in eEF2K phosphorylation (Figure 6, panel d) and the decrease in eEF2 phosphorylation (Figure 6, panel e). At concentrations ⩾3 μM, GF109203X also significantly inhibited the increase in eEF2K phosphorylation, such that with the 10 μM concentration eEF2K phosphorylation was no longer significantly different from that in uninfected control cells (Figure 6, panel d). Although the increase in eEF2K phosphorylation was abolished by 10 μM GF109203X, a parallel inhibition of the reduction in eEF2 phosphorylation was not observed (Figure 6, panel e). Over the same concentration range, Ro31-8220 was similarly ineffective against the changes in the phosphorylation status of ERK1/2, p90RSK and eEF2 that arose from the heterologous expression of caMEK1 (Figure 7, panels a–c, e). However, like GF109203X, Ro31-8220 caused a concentration-dependent reduction in eEF2K phosphorylation (Figure 7, panel d). Indeed, Ro31-8220 was considerably more potent than GF109203X as an inhibitor of eEF2K phosphorylation, such that even the lowest concentration of 1 μM produced a significant reduction in the response to the heterologous expression of caMEK1 (Figure 7, panel d). These data suggest that GF109203X and Ro31-8220 both significantly inhibit p90RSK activity in intact ARVM, at concentrations ⩾3 and ⩾1 μM, respectively.
Figure 6.
Effects of GF109203X on the phosphorylation status of ERK1/2, p90RSK, eEF2K and eEF2 following adenoviral expression of caMEK1. ARVM were maintained in culture for 42 h, following a 1 h infection with empty virus (Cont) or adenovirus encoding caMEK1, both at an MOI of 50 PFU cell−1. ARVM were then exposed to vehicle (Veh), 1 μM U0126 (UO) or 1–10 μM GF109203X (GF) for 4 h, before being lysed in SDS–PAGE sample buffer for subsequent Western immunoblot analysis. (a) Representative Western immunoblots showing the expression of MEK1 and phosphorylated forms of ERK1/2 (P-ERK1/2), p90RSK(P-p90RSK), eEF2K (P-eEF2K) and eEF2 (P-eEF2). Total ERK2 expression is also shown to illustrate equal protein loading. Quantitative data (panels b–e) illustrate the phosphorylation status of (b) ERK1/2, (c) p90RSK, (d) eEF2K and (e) eEF2, *P<0.05 versus Cont, †P<0.05 versus Veh (n=6).
Figure 7.
Effects of Ro31-8220 on the phosphorylation status of ERK1/2, p90RSK, eEF2K and eEF2 following adenoviral expression of caMEK1. ARVM were maintained in culture for 42 h, following a 1 h infection with empty virus (Cont) or adenovirus encoding caMEK1, both at an MOI of 50 PFU cell−1. ARVM were then exposed to vehicle (Veh), 1 μM U0126 (UO) or 1–10 μM Ro31-8220 (Ro) for 4 h, before being lysed in SDS–PAGE sample buffer for subsequent Western immunoblot analysis. (a) Representative Western immunoblots showing the expression of MEK1 and phosphorylated forms of ERK1/2 (P-ERK1/2), p90RSK(P-p90RSK), eEF2K (P-eEF2K) and eEF2 (P-eEF2). Total ERK2 expression is also shown to illustrate equal protein loading. Quantitative data (panels b–e) illustrate the phosphorylation status of (b) ERK1/2, (c) p90RSK, (d) eEF2K and (e) eEF2, *P<0.05 versus Cont, †P<0.05 versus Veh (n=6).
Effects of bisindolylmaleimides on PKC activity in intact ARVM
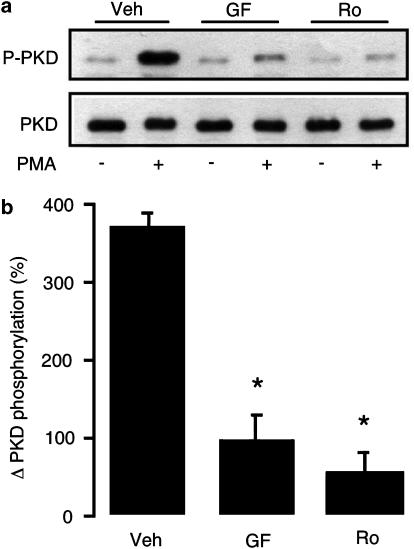
To obtain an indication of the relative selectivity of GF109203X and Ro31-8220 for native PKC versus p90RSK isoforms in intact ARVM, we also determined the effects of these inhibitors on cellular PKC activity. The activation status of the downstream PKC substrate PKD, determined by Western immunoblotting using a phosphospecific antibody (Haworth et al., 2000), was used as the index of cellular PKC activity. Stimulation of cellular PKC activity by PMA produced a significant increase in PKD phosphorylation (Figure 8). The PMA-induced increase in PKD phosphorylation was significantly reduced by a 1 μM concentration of either GF109203X or Ro31-8220 (Figure 8), indicating that cellular PKC activity is inhibited by both agents, even at the lowest concentration used in the present study.
Figure 8.
Effects of GF019203X and Ro31-8220 on the phosphorylation status of PKD following exposure to PMA. ARVM were treated with vehicle (Veh), 1 μM GF109203X (GF) or 1 μM Ro31-8220 (Ro) for 15 min, prior to a 5-min exposure to vehicle or 30 nM PMA, lysis in SDS–PAGE sample buffer and subsequent Western immunoblot analysis. (a) Representative Western immunoblots showing the expression of phosphorylated and total PKD. (b) Quantitative data illustrating the PMA-induced increase in the phosphorylation status of PKD. *P<0.05 versus Veh (n=3).
Discussion
Our study demonstrates, for the first time to our knowledge, that all three p90RSK isoforms (RSK1, RSK2 and RSK3) are inhibited by GF109203X and Ro31-8220 in vitro, and that, even at a physiological ATP concentration, both bisindolylmaleimides retain their inhibitory effects on RSK2 (the predominant p90RSK isoform in cardiac myocytes (Wagner, 2004)). Furthermore, the study provides novel data which indicate that GF109203X and Ro31-8220 significantly inhibit p90RSK activity in the intact ARVM at concentrations ⩾3 and ⩾1 μM, respectively.
Our in vitro data extend the observations of Alessi (1997), who reported the inhibitory effects of GF109203X and Ro31-8220 on the activities of mixed PKC isoforms purified from rat brain and RSK2 (also known as MAPKAP kinase-1β) purified from rabbit skeletal muscle. In the present work, the effects of these agents on the activities of representative recombinant human PKC isoforms from the classical and novel PKC subfamilies (PKCα and PKCɛ, respectively), as well as the recombinant human p90RSK isoforms RSK1, RSK2 and RSK3 were determined. Additionally, the impact of increasing the ATP concentration to a physiological level on the inhibitory potencies of these agents against the selected PKC isoforms and RSK2 was examined. All three p90RSK family isoforms studied were inhibited by both GF109203X and Ro31-8220 with similar relative selectivity. However, Ro31-8220 demonstrated greater potency than GF109203X against all three p90RSK isoforms. Previous data have demonstrated that both GF109203X and Ro31-8220 inhibit mixed PKC isoforms and RSK2 with near equipotency (reported IC50 values for inhibition of mixed PKCs and RSK2 were 30 and 50 nM, respectively, for GF109203X and 5 and 3 nM, respectively, for Ro31-8220 (Alessi, 1997)). The present data demonstrate that GF109203X exhibits approximately 30-fold selectivity for PKCα and PKCɛ when compared to RSK2, with IC50 values for PKC isoform inhibition (8–12 nM) close to that described in the original study describing this inhibitor (14 nM) (Toullec et al., 1991). The selectivity of GF109203X for PKC isoforms was retained at an elevated ATP concentration of 5 mM, which is akin to that estimated in ventricular myocytes (Allue et al., 1996). Ro31-8220 exhibited considerably lower (approximately six-fold) selectivity for PKC isoforms over RSK2. Interestingly, this difference in the relative selectivity of the two inhibitors arose from a less potent inhibition of RSK2 by GF109203X when compared to Ro31-8220, since both bisindolylmaleimides inhibited PKC isoforms with comparable potency.
Previous evidence that bisindolylmaleimide PKC inhibitors have nonspecific inhibitory effects on the activity of a number of kinases has relied on in vitro kinase assays (Alessi, 1997; Davies et al., 2000). More recently, a proteomic screen has identified a number of cellular targets for bisindolylmaleimides (Brehmer et al., 2004), consistent with potential nonspecific effects also in the intact cell. Nevertheless, our previous work has drawn into question the validity of extrapolating data from in vitro kinase assays to the intact cellular environment, by demonstrating that GF109203X and Ro31-8220 do not inhibit p70S6K in the intact ARVM (Roberts et al., 2004). Unlike the contrasting effects of the bisindolylmaleimide inhibitors on p70S6K activity in vitro versus in the intact ARVM, their potent inhibition of p90RSK activity in vitro was reflected by their inhibition of such activity also in the cellular environment. Thus, both GF109203X and Ro31-8220 showed significant inhibition of p90RSK activity in intact ARVM, at concentrations ⩾3 and ⩾1 μM, respectively. These data suggest that p90RSK inhibition may contribute to the functional effects of bisindolylmaleimides in ARVM and potentially in other cell types. Importantly, nonspecific inhibition of cellular p90RSK activity by the two PKC inhibitors may require re-evaluation of some data from previous studies that used these agents as pharmacological tools, particularly to investigate the roles of PKC isoforms in (patho)physiological processes where p90RSK activity is likely, or indeed known, to play a key regulatory role. Of particular interest is the use of these inhibitors to implicate PKC isoforms in the stimulation of NHE1 activity by a variety of stimuli in ARVM (Yasutake et al., 1996; Gunasegaram et al., 1999; Snabaitis et al., 2000; Snabaitis et al., 2002).
Experiments that have used GF109203X and inhibitors of the ERK/p90RSK pathway (e.g. PD98059 and U0126) to study the signalling mechanisms through which thrombin (Yasutake et al., 1996), angiotensin II (AT1) (Gunasegaram et al., 1999) and α1A-adrenergic (Snabaitis et al., 2000) receptors stimulate NHE1 activity in ARVM have led to the conclusion that PKC and p90RSK participate in two parallel and necessary pathways that facilitate such a response (Avkiran & Haworth, 2003). However, in the light of the present data, it is possible that the common inhibitory effects of GF109203X and PD98059/U0126 could have arisen from the inhibition of a contiguous signalling pathway at proximal and distal points, by targeting ERK and therefore p90RSK activation (PD98059/U0126) or p90RSK activity (GF109203X). Notably, previous studies in our laboratory that have used GF109203X as a PKC inhibitor have employed concentrations ⩽1 μM (Yasutake et al., 1996; Gunasegaram et al., 1999; Snabaitis et al., 2000; Snabaitis et al., 2002), which did not inhibit cellular p90RSK activity significantly in the present study (Figure 6). Thus, nonspecific inhibition of p90RSK is unlikely to have been primarily responsible for the inhibitory effects of GF109203X on the stimulation of NHE1 activity by the pertinent GPCR agonists in intact ARVM. In addition, other studies in noncardiac cell types have used alternative tools to manipulate cellular PKC activity, and have supported a role for PKC and/or specific PKC isoforms in NHE1 regulation (Chen & Wu, 1995; Maly et al., 2002). Nevertheless, further investigation, using complementary techniques, is required to confirm the involvement of PKC isoforms in the regulation of NHE1 activity in ARVM, particularly since, unlike p90RSK (Takahashi et al., 1999), PKC isoforms do not appear to directly phosphorylate the regulatory C-terminal domain of NHE1 (Fliegel et al., 1992).
The discrepancy between the lack of inhibition of p70S6K (Roberts et al., 2004) and the significant inhibition of p90RSK (present study) by GF109203X and Ro31-8220 in intact ARVM, despite the potent inhibition of both kinases by the two compounds in vitro (Alessi, 1997; Davies et al., 2000), can potentially be explained through differences in the intracellular localisation and/or regulation of the pertinent proteins. Bisindolylmaleimide inhibitors exert their actions through competitive inhibition at the ATP-binding site within the catalytic domains of PKC isoforms (Toullec et al., 1991), and it is assumed that GF109203X and Ro31-8220 act in a similar manner to inhibit the catalytic activity of p70S6K and p90RSK (Alessi, 1997). It is possible that there is variable access of bisindolylmaleimides to the catalytic domains of the two kinases in the intact ARVM, due to differences in their localisation and/or regulation. Although both kinases are predominantly cytosolic in localisation under basal conditions in other cell types (Pullen & Thomas, 1997; Frödin & Gammeltoft, 1999), there are currently no data describing the cellular localisation of either p70S6K or p90RSK in ARVM. Furthermore, it is possible that the kinases occupy distinct subcellular compartments in the activated state, allowing differential access to pharmacological inhibitors. Indeed, activated p70S6K has been shown to exist in a large multi-protein complex (Hannan et al., 2003), which may restrict inhibitor access to the kinase. Such differences could lead to greater access and preferential binding of GF109203X and Ro31-8220 to p90RSK compared with p70S6K, resulting in more potent inhibition of the former in the intact cell.
eEF2 is a cytoplasmic protein that catalyses the movement of the ribosome along mRNA during translation (which is essential for the extension of the polypeptide chain) and is regulated through phosphorylation by eEF2K (Ryazanov et al., 1997). eEF2K itself is negatively regulated by the upstream kinases p90RSK and p70S6K through phosphorylation at S366 (Wang et al., 2001b), such that activation of either pathway results in the inhibition of eEF2K activity and the consequent attenuation of eEF2 phosphorylation (Wang et al., 2001b). Interestingly, in the present study, the attenuation of eEF2 phosphorylation following heterologous expression of caMEK1 was not inhibited by GF109203X or Ro31-8220, despite the significant attenuation of eEF2K phosphorylation by both agents. In contrast, the inhibition of ERK activation by U0126 abolished both the increase in eEF2K phosphorylation and the decrease in eEF2 phosphorylation that arose from the heterologous expression of caMEK1, as previously shown (Wang & Proud, 2002). The discrepant effects of bisindolylmaleimides and the consistent effects of U0126 on the phosphorylation status of eEF2K versus eEF2 may indicate the existence of MEK/ERK-dependent but p90RSK-independent mechanisms that regulate eEF2 phosphorylation in ARVM. Thus, in addition to the pharmacological characterisation of GF109203X and Ro31-8220, our study provides novel mechanistic information, which is of potential relevance to the regulation of protein synthesis in ARVM and thus warrants further investigation.
In conclusion, our data show that GF109203X and Ro31-8220 are potent inhibitors of p90RSK activity in vitro and target all three p90RSK isoforms tested (RSK1, RSK2 and RSK3). Our data also show that both GF109203X and Ro31-8220 significantly inhibit p90RSK activity in the intact ARVM, at concentrations ⩾3 and ⩾1 μM, respectively. Although GF109203X appears to be more selective than Ro31-8220 as a PKC inhibitor, both in vitro and in intact ARVM, care must still be taken in selecting an appropriate concentration of either GF109203X or Ro31-8220 and in interpreting data arising from the use of these agents in cellular systems such as ARVM, due to their potential to inhibit p90RSK as well as PKC activity.
Acknowledgments
This study was funded by a Prize Studentship (R010217) from the Charitable Foundation of Guy's and St Thomas' Hospitals. RSH is the holder of a British Heart Foundation Intermediate Research Fellowship (FS/02/001/13240). We thank Miss Semjidmaa Dashnyam, who is funded by a Medical Research Council Co-operative Group Core Grant (G0001112), for her expert help in the preparation of ARVM.
Abbreviations
- ARVM
adult rat ventricular myocytes
- eEF2
eukaryotic elongation factor-2
- eEF2K
eEF2 kinase
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- MARCKS
myristoylated alanine-rich C-kinase substrate
- MEK1
MAPK or ERK kinase 1
- p90RSK
90 kDa ribosomal S6 kinase
- RSK
90 kDa ribosomal S6 kinase isoform
References
- AKER S., SNABAITIS A.K., KONIETZKA I., VAN DE SAND A., BONGLER K., AVKIRAN M., HEUSCH G., SCHULZ R. Inhibition of the Na+/H+ exchanger attenuates the deterioration of ventricular function during pacing-induced heart failure in rabbits. Cardiovasc. Res. 2004;63:273–282. doi: 10.1016/j.cardiores.2004.04.014. [DOI] [PubMed] [Google Scholar]
- ALESSI D.R. The protein kinase C inhibitors Ro 318220 and GF109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997;402:121–123. doi: 10.1016/s0014-5793(96)01510-4. [DOI] [PubMed] [Google Scholar]
- ALLUE I., GANDELMAN O., DEMENTIEVA E., UGAROVA N., COBBOLD P. Evidence for rapid consumption of millimolar concentrations of cytoplasmic ATP during rigor-contracture of metabolically compromised single cardiomyocytes. Biochem. J. 1996;319 Part 2:463–469. doi: 10.1042/bj3190463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVKIRAN M., HAWORTH R.S. Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance. Cardiovasc. Res. 2003;57:942–952. doi: 10.1016/s0008-6363(02)00782-4. [DOI] [PubMed] [Google Scholar]
- BIAN J.S., PEI J.M., CHEUNG C.S., ZHANG W.M., WONG T.M. κ-opioid receptor stimulation induces arrhythmia in the isolated rat heart via the protein kinase C/Na+–H+exchange pathway. J. Mol. Cell. Cardiol. 2000;32:1415–1427. doi: 10.1006/jmcc.2000.1175. [DOI] [PubMed] [Google Scholar]
- BREHMER D., GODL K., ZECH B., WISSING J., DAUB H. Proteome-wide identification of cellular targets affected by bisindolylmaleimide-type protein kinase C inhibitors. Mol. Cell. Proteomics. 2004;3:490–500. doi: 10.1074/mcp.M300139-MCP200. [DOI] [PubMed] [Google Scholar]
- BUENO O.F., DE WINDT L.J., TYMITZ K.M., WITT S.A., KIMBALL T.R., KLEVITSKY R., HEWETT T.E., JONES S.P., LEFER D.J., PENG C.F., KITSIS R.N., MOLKENTIN J.D. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C.C., WU M.L. Protein kinase C isoform δ is involved in the stimulation of the Na+–H+ exchanger in C6 glioma cells. Mol. Pharmacol. 1995;48:995–1003. [PubMed] [Google Scholar]
- CHEN C.-H., GRAY M.O., MOCHLY-ROSEN D. Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: role of epsilon protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12784–12789. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLERK A., MICHAEL A., SUGDEN P.H. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem. J. 1998;333:581–589. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS P.D., HILL C.H., KEECH E., LAWTON G., NIXON J.S., SEDGWICK A.D., WADSWORTH J., WESTMACOTT D., WILKINSON S.E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989;259:61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- DUAN D., YE L., BRITTON F., MILLER L.J., YAMAZAKI J., HOROWITZ B., HUME J.R. Purinoceptor-coupled Cl− channels in mouse heart: a novel, alternative pathway for CFTR regulation. J. Physiol. 1999;521 Part 1:43–56. doi: 10.1111/j.1469-7793.1999.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLIEGEL L., WALSH M.P., SINGH D., WONG C., BARR A. Phosphorylation of the C-terminal domain of the Na+/H+ exchanger by Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 1992;282:139–145. doi: 10.1042/bj2820139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRÖDIN M., GAMMELTOFT S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., WANG Y., HSU A.K., GROSS G.J. Essential activation of PKC-δ in opioid-initiated cardioprotection. Am. J. Physiol. 2001;280:H1346–H1353. doi: 10.1152/ajpheart.2001.280.3.H1346. [DOI] [PubMed] [Google Scholar]
- GUNASEGARAM S., HAWORTH R.A., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT1versus AT2 receptors. Circ. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- HANNAN K.M., THOMAS G., PEARSON R.B. Activation of S6K1 (p70 ribosomal protein S6 kinase 1) requires an initial calcium-dependent priming event involving formation of a high-molecular-mass signalling complex. Biochem. J. 2003;370:469–477. doi: 10.1042/BJ20021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWORTH R.S., GOSS M.W., ROZENGURT E., AVKIRAN M. Expression and activity of protein kinase D/protein kinase Cμ in myocardium: evidence for α1-adrenergic receptor- and protein kinase C-mediated regulation. J. Mol. Cell. Cardiol. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- HAWORTH R.S., MCCANN C., SNABAITIS A.K., ROBERTS N.A., AVKIRAN M. Stimulation of the plasma membrane Na+/H+ exchanger NHE1 by sustained intracellular acidosis. Evidence for a novel mechanism mediated by the ERK pathway. J. Biol. Chem. 2003;278:31676–31684. doi: 10.1074/jbc.M304400200. [DOI] [PubMed] [Google Scholar]
- HERGET T., ROZENGURT E. Bombesin, endothelin and platelet-derived growth factor induce rapid translocation of the myristoylated alanine-rich C-kinase substrate in Swiss 3T3 cells. Eur. J. Biochem. 1994;225:539–548. doi: 10.1111/j.1432-1033.1994.00539.x. [DOI] [PubMed] [Google Scholar]
- HU K., DUAN D., LI G.R., NATTEL S. Protein kinase C activates ATP-sensitive K+ current in human and rabbit ventricular myocytes. Circ. Res. 1996;78:492–498. doi: 10.1161/01.res.78.3.492. [DOI] [PubMed] [Google Scholar]
- HU K., MOCHLY-ROSEN D., BOUTJDIR M. Evidence for functional role of ɛPKC isozyme in the regulation of cardiac Ca2+ channels. Am. J. Physiol. 2000;279:H2658–H2664. doi: 10.1152/ajpheart.2000.279.6.H2658. [DOI] [PubMed] [Google Scholar]
- INAGAKI K., KIHARA Y., HAYASHIDA W., IZUMI T., IWANAGA Y., YONEDA T., TAKEUCHI Y., SUYAMA K., MUSO E., SASAYAMA S. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of delta-isoform of protein kinase C in rat ventricular myocardium. Circulation. 2000;101:797–804. doi: 10.1161/01.cir.101.7.797. [DOI] [PubMed] [Google Scholar]
- JO S.H., CHO C.H., CHAE S.W., LEE C.O. Role of protein kinase C in α1-adrenergic regulation of aNai in guinea pig ventricular myocytes. Am. J. Physiol. 2000;279:H1661–H1668. doi: 10.1152/ajpheart.2000.279.4.H1661. [DOI] [PubMed] [Google Scholar]
- KANAYA N., MURRAY P.A., DAMRON D.S. Propofol increases myofilament Ca2+ sensitivity and intracellular pH via activation of Na+–H+ exchange in rat ventricular myocytes. Anesthesiology. 2001;94:1096–1104. doi: 10.1097/00000542-200106000-00026. [DOI] [PubMed] [Google Scholar]
- KITAKAZE M., NODE K., MINAMINO T., KOMAMURA K., FUNAYA H., SHINOZAKI Y., CHUJO M., MORI H., INOUE M., HORI M., KAMADA T. Role of activation of protein kinase C in the infarct size-limiting effect of ischemic preconditioning through activation of ecto-5′-nucleotidase. Circulation. 1996;93:781–791. doi: 10.1161/01.cir.93.4.781. [DOI] [PubMed] [Google Scholar]
- MALY K., STRESE K., KAMPFER S., UEBERALL F., BAIER G., GHAFFARI-TABRIZI N., GRUNICKE H.H., LEITGES M. Critical role of protein kinase C α and calcium in growth factor induced activation of the Na+/H+ exchanger NHE1. FEBS Lett. 2002;521:205–210. doi: 10.1016/s0014-5793(02)02867-3. [DOI] [PubMed] [Google Scholar]
- MIDDLETON L.M., HARVEY R.D. PKC regulation of cardiac CFTR Cl− channel function in guinea pig ventricular myocytes. Am. J. Physiol. 1998;275:C293–C302. doi: 10.1152/ajpcell.1998.275.1.C293. [DOI] [PubMed] [Google Scholar]
- MULLAN D.M., BELL D., KELSO E.J., MCDERMOTT B.J. Involvement of endothelin ETA and ETB receptors in the hypertrophic effects of ET-1 in rabbit ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 1997;29:350–359. doi: 10.1097/00005344-199703000-00008. [DOI] [PubMed] [Google Scholar]
- PI Y., WALKER J.W. Diacylglycerol and fatty acids synergistically increase cardiomyocyte contraction via activation of PKC. Am. J. Physiol. 2000;279:H26–H34. doi: 10.1152/ajpheart.2000.279.1.H26. [DOI] [PubMed] [Google Scholar]
- PONICKE K., HEINROTH-HOFFMANN I., BECKER K., OSTEN B., BRODDE O.E. Gq/11-coupled receptors and protein synthesis in rat cardiomyocytes: role of Gi-proteins and protein kinase C-isozymes. Naunyn-Schmiedebergs' Arch. Pharmacol. 1999;360:301–308. doi: 10.1007/s002109900077. [DOI] [PubMed] [Google Scholar]
- PUCÉAT M., CLEMENT-CHOMIENNE O., TERZIC A., VASSORT G. α1-adrenoceptor and purinoceptor agonists modulate Na–H antiport in single cardiac cells. Am. J. Physiol. 1993;264:H310–H319. doi: 10.1152/ajpheart.1993.264.2.H310. [DOI] [PubMed] [Google Scholar]
- PULLEN N., THOMAS G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- ROBERTS N.A., MARBER M.S., AVKIRAN M. Specificity of action of bisindolylmaleimide protein kinase C inhibitors: do they inhibit the 70 kDa ribosomal S6 kinase in cardiac myocytes. Biochem. Pharmacol. 2004;68:1923–1928. doi: 10.1016/j.bcp.2004.07.040. [DOI] [PubMed] [Google Scholar]
- RUF S., PIPER M., SCHLUTER K.D. Specific role for the extracellular signal-regulated kinase pathway in angiotensin II- but not phenylephrine-induced cardiac hypertrophy in vitro. Pflugers Arch. 2002;443:483–490. doi: 10.1007/s004240100710. [DOI] [PubMed] [Google Scholar]
- RYAZANOV A.G., WARD M.D., MENDOLA C.E., PAVUR K.S., DOROVKOV M.V., WIEDMANN M., ERDJUMENT-BROMAGE H., TEMPST P., PARMER T.G., PROSTKO C.R., GERMINO F.J., HAIT W.N. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4884–4889. doi: 10.1073/pnas.94.10.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNABAITIS A.K., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na+/H+ exchange by hydrogen peroxide in adult rat ventricular myocytes. Cardiovasc. Res. 2002;53:470–480. doi: 10.1016/s0008-6363(01)00464-3. [DOI] [PubMed] [Google Scholar]
- SNABAITIS A.K., MUTENDORF A., WIELAND T., AVKIRAN M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of Gq/11, Gi and G12/13 proteins in signalling by α1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell. Signal. 2005;17:655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- SNABAITIS A.K., YOKOYAMA H., AVKIRAN M. Roles of mitogen-activated protein kinases and protein kinase C in α1A-adreoreceptor-mediated stimulation of the sarcolemmal Na+–H+ exchanger. Circ. Res. 2000;86:214–220. doi: 10.1161/01.res.86.2.214. [DOI] [PubMed] [Google Scholar]
- SZOKODI I., TAVI P., FOLDES G., VOUTILAINEN-MYLLYLA S., ILVES M., TOKOLA H., PIKKARAINEN S., PIUHOLA J., RYSA J., TOTH M., RUSKOAHO H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002;91:434–440. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI E., ABE J., BERK B.C. Angiotensin II stimulates p90rsk in vascular smooth muscle cells. A potential Na+–H+ exchanger kinase. Circ. Res. 1997;81:268–273. doi: 10.1161/01.res.81.2.268. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI E., ABE J., GALLIS B., AEBERSOLD R., SPRING D.J., KREBS E.G., BERK B.C. p90RSK is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J. Biol. Chem. 1999;274:20206–20214. doi: 10.1074/jbc.274.29.20206. [DOI] [PubMed] [Google Scholar]
- TAKEISHI Y., HUANG Q., ABE J., CHE W., LEE J.D., KAWAKATSU H., HOIT B.D., BERK B.C., WALSH R.A. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc. Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., COSTE H., BELLEVERGUE P., GRAND-PERRET T., AJAKANE M., BAUDET V., BOISSIN P., BOURSIER E., LORIOLLE F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- VON LEWINSKI D., VOSS K., HULSMANN S., KOGLER H., PIESKE B. Insulin-like growth factor-1 exerts Ca2+-dependent positive inotropic effects in failing human myocardium. Circ. Res. 2003;92:169–176. doi: 10.1161/01.res.0000051885.70159.12. [DOI] [PubMed] [Google Scholar]
- WAGNER C. Myocardial expression and NHE1 kinase activity of p90RSK isoforms. J. Mol. Cell. Cardiol. 2004;36:713. [Google Scholar]
- WANG H., YANG B., ZHANG Y., HAN H., WANG J., SHI H., WANG Z. Different subtypes of α1-adrenoceptor modulate different K+ currents via different signaling pathways in canine ventricular myocytes. J. Biol. Chem. 2001a;276:40811–40816. doi: 10.1074/jbc.M105572200. [DOI] [PubMed] [Google Scholar]
- WANG L., PROUD C.G. Regulation of the phosphorylation of elongation factor 2 by MEK- dependent signalling in adult rat cardiomyocytes. FEBS Lett. 2002;531:285–289. doi: 10.1016/s0014-5793(02)03536-6. [DOI] [PubMed] [Google Scholar]
- WANG X., LI W., WILLIAMS M., TERADA N., ALESSI D.R., PROUD C.G. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. EMBO J. 2001b;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., TAKASHI E., XU M., AYUB A., ASHRAF M. Downregulation of protein kinase C inhibits activation of mitochondrial KATP channels by diazoxide. Circulation. 2001c;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- WOO S.H., LEE C.O. Effects of endothelin-1 on Ca2+ signaling in guinea-pig ventricular myocytes: role of protein kinase C. J. Mol. Cell. Cardiol. 1999;31:631–643. doi: 10.1006/jmcc.1998.0899. [DOI] [PubMed] [Google Scholar]
- YASUTAKE M., HAWORTH R.S., KING A., AVKIRAN M. Thrombin activates the sarcolemmal Na+–H+ exchanger. Evidence for a receptor-mediated mechanism involving protein kinase C. Circ. Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]