Abstract
Cannabinoid (CB) receptor agonists have potential utility as anti-inflammatory drugs for the treatment of many disease conditions. In the present study, we investigated the effects of the synthetic CB2 ligand, JWH-133 on the production of interleukins (ILs), IL-12 and IL-10 by lipopolyssacharide (LPS) or Theiler's virus (TMEV)-activated macrophages.
JWH-133 evoked a concentration-related inhibition (10 nM–5 μM) of LPS/IFN-γ induced IL-12p40 release. The effect of JWH-133 (100 nM) was significantly blocked by the CB2 antagonist SR-144528 (1 μM). Macrophages infected with TMEV increased IL-12p40 production and activation of CB2 receptors by JWH-133 (100 nM) inhibited it.
The inhibitory effect of JWH-133 (100 nM) on IL-12p40 production may involve extracellular-regulated kinase (ERK1/2) signaling: (i) JWH-133 induced a greater and sustained activation of ERK1/2 kinase in comparison with the level of activation observed following LPS; (ii) the inhibition of ERK1/2 by the specific inhibitor PD98059 increased LPS-induced IL-12p40 production in the presence or absence of JWH-133 suggesting a negative regulation of ERK pathway on IL-12p40 biosynthesis.
Activation of CB2 receptors by JWH-133 (10 nM–5 μM) enhanced IL-10 release by LPS/IFN-γ-activated macrophages and addition of SR144558 (1 μM) totally blocked the effect of JWH (100 nM).
Inhibition of ERK by PD98059 significantly suppressed IL-10 production by LPS-activated macrophages. Endogenous IL-10 plays a modulatory role in IL-12 production. Blocking IL-10 with neutralizing antibody resulted in increased IL-12p40 secretion by LPS-activated macrophages in the absence or presence of JWH-133. In contrast, the addition of exogenous mIL-10 reduced the secretion of IL-12p40 in response to LPS.
Keywords: Cannabinoids, CB2 receptors, IL-12p40, IL-10, ERK1/2, macrophages
Introduction
The effects of both synthetic and endogenous cannabinoids (CBs) upon the immune system have acquired a great interest during the last years, because of their possible usefulness in chronic immune inflammatory diseases. However, the role of CB receptors and the molecular mechanisms involved in the modulation of immune reactivity by CBs remain unclear. Two CB G protein-coupled receptors have been cloned until now, CB1 receptors (Matsuda et al., 1990) expressed primarily by neurons, and CB2 receptors (Munro et al., 1993) expressed primarily by immune cells. Besides, there is evidence supporting the presence of yet uncloned CB receptors mainly on the basis of pharmacological activity of CBs in CB1 and CB2 receptor-deficient mice (Jarai et al., 1999; Di Marzo et al., 2000; Breivogel et al., 2001). In addition, two endocannabinoid ligands have been identified and characterized: anandamide (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995). Another recently identified endocannabinoid includes N-arachidonoyl-dopamine (NADA), which binds to the vanilloid subfamily member 1 (TRPV1) and CB1 receptor and which shows anti-inflammatory and immunosuppressive activities (Sancho et al., 2004).
Macrophages are activated early in response to immune challenge and are major players in both innate and adaptive immunity. The finding that a CB2 synthetic agonist elicited benefits in a viral model of multiple sclerosis (MS), in terms of motor improvement, down-inflammation and remyelination (Arévalo-Martín et al., 2003), prompted us to investigate the immunodulatory actions of the synthetic CB2 ligand, JWH-133 in bacterial endotoxin (LPS) or Theiler's virus (TMEV)-activated macrophages. IL-12 is a heterodimeric cytokine composed of two disulfide-linked glycosylated chains of 40 kDa (p40) and 35 kDa (p35) encoded by two distinct genes (Gubler et al., 1991). IL-12 is produced primarily by monocytes, macrophages and dendritic cells in response to bacterial or intracellular pathogens (D'Andrea et al., 1993) or upon interaction with activated T cells (Shu et al., 1995). It also induces the development of Th1 responses and has an important role in maintaining the balance between Th1 and Th2 responses in vivo (Trinchieri, 2003). IL-12 has been implicated in the pathogenesis of infectious, inflammatory and autoimmune diseases such as MS. A relationship between cannabinoids and T-helper cell biasing has been established by several lines of evidence (Klein et al., 2003). In vivo experiments showed that THC injection suppressed the development of Th1 cell-mediated immunity to Legionella pneumophila infection, with decreased IFN-γ and IL-12 levels in the serum (Klein et al., 2000). However, the cellular targets, the specific contribution of CB receptors and the molecular mechanisms involved in these effects remain unknown. Cells of the macrophage lineage seem to express CB1 receptors at low level and, mainly CB2 receptors (Carlisle et al., 2002; Walter et al., 2003). In this report, we show that JWH-133 inhibits IL-12p40 production and enhances IL-10 release by activated macrophages through a CB2 receptor-mediated pathway. In this study, we also focused on the role of extracellular signal-regulated kinase (ERK1/2) in IL-12p40 and IL-10 regulation by the activation of CB2 receptors. Finally, we suggest a contribution of endogenous IL-10 in mediating CB-induced downregulation of IL-12p40 production.
Methods
Animals
Balb/c and SJL/J mice, susceptible to TMEV-induced demyelination development (TMEV-IDD), from our in-house colony (Cajal Institute, Madrid, Spain) were used. Animals were housed in cages with filter tops in a laminar flow hood and maintained on food and water ad libitum in a 12-h dark–light cycle. Handling of animals was performed in compliance with the guidelines of animal care set by the European Union (86/609/EEC).
Macrophage cultures
Peritoneal macrophages were harvested by peritoneal lavage with Hanks Balanced Salt Solution (HBSS; Gibco BRL, Life Tech. Ltd, Germany) 3 days after intraperitoneal (i.p.) injection of mice with 2 ml of 5% thioglycollate broth (Sigma, Spain). Cells were centrifuged at 800 r.p.m., 10 min at room temperature (RT) and resuspended in Gey's red cells lysis buffer. After 20 min of incubation at RT, cells were centrifuged and resuspended in fresh Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% inactivated fetal bovine serum (FBS, Gibco BRL, Life Tech. Ltd, Germany), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Gibco BRL, Life Tech. Ltd, Germany). Cells were seeded in 12-well plates (0.5 × 106 cells/well) and incubated overnight at 37°C in a humified atmosphere containing 5% CO2. At 1 h prior to stimulation, nonadherent cells were removed by washing twice with DMEM and macrophages were resuspended in fresh culture medium supplemented with antibiotics and 5% FBS. At 5 min previous to LPS/IFN-γ exposure, JWH-133 (10 nM, 100 nM, 1 μM and 5 μM) and the CB1 and CB2 receptor antagonists SR141716A and SR144528, respectively, were added at a dose of 1 μM. LPS and IFN-γ were diluted in DMEM, and added to each well at a final concentration of 50 ng ml−1 and 100 U ml−1, respectively. JWH-133 stock solution was prepared in DMSO and aliquots (1 mM) were diluted in PBS and 1% DMSO. Control cells were cultured with the relevant amounts of DMSO. Cells stimulated were incubated 18 h at 37°C in a humidified atmosphere with 5% CO2. After this time, cells were harvested for protein measurement, and supernatants collected for cytokine determination. Trypan blue dye exclusion testing or the 3,4,5-dimethylthiazol 2-5-diphenyltetrazolium bromide thiazol blue test indicated that the cannabinoid-related compounds at the highest concentrations used (5 μM) did not affect macrophage cell viability. In the experiments involving ERK 1/2 inhibition, cells were preincubated with the inhibitor of MEK1, PD98059 (10 μM) for 30 min in the presence or absence of JWH-133 (100 nM), and then stimulated with LPS 50 ng ml−1 for 18 h. In a set of experiments, murine neutralizing IL-10 antibody or exogenous murine IL-10 (10 ng ml−1 was added to macrophage cultures before LPS stimulation for 18 h in order to collect cell supernatants to measure IL-12p40.
Murine macrophage cell line RAW 264.7
The murine macrophage cell line RAW 264.7 was purchased from Center of Biological Sciences (CIB, CSIC, Madrid, Spain). Low passage number cells were grown in DMEM medium supplemented with 2 mM glutamine, 100U ml−1 penicillin, 100 μg ml−1 streptomycin and 10% FBS.
Infection of macrophage cultures
The Daniel's (DA) strain of TMEV was plaque purified on BHK-21 cells, and the virus titers were determined by standard plaque assay on BHK-21 cells. Cell cultures were infected with the DA strain of TMEV at a multiplicity of infection (MOI) of 5-plaque format units (PFU) per cell. Cell cultures were washed twice to remove serum components and 0.25 ml of appropriately diluted virus stock solution was added to each well. After adsorption of the virus for 2 h at 37°C cells, we added 0.75 ml of new medium containing only 2% FBS. The supernatants from infected and noninfected cultures were collected in parallel after 24 h postinfection for cytokines determination.
Western blot analysis
Cells were lysed in 200 μl of Tris-buffered saline (TBS) pH 7.6 containing 10% glycerol, 1% Nonidet P-40, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 50 μg ml−1 leupeptin, 10 μg ml−1, aprotinin, 5 mM benzamidine, 1 mM sodium orthovanadate, 2 mM NaF and 5 mM DTT. Whole-cell lysates were mixed with 5 × Laemmli sample buffer and boiled for 5 min. Equal amounts of protein (30 μg) were resolved in 10% SDS-polyacrylamide gel electrophoresis and electroblotted to nitrocellulose at 4°C. The membrane blots were blocked for 1 h with 5% dry milk in TBS containing 0.1% Tween-20 and then incubated with primary antibodies, at a dilution of 1:1000 (phosphospecific and total ERK1/2 from New England Biolabs, Beverly, MA, U.S.A.). Then, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence using an ECL Western Blotting Detection Kit (Amersham Pharmacia Biotech). The blots were stripped using a 2% SDS and 0.7% β-mercaptoethanol solution in 62.5 mM Tris buffer, pH 6.8 and reprobed.
Measurement of IL-10 and IL-12p40 production by ELISA
IL-10 content in macrophage culture supernatants was measured by solid phase sandwich ELISA, using a monoclonal antibody specific for mIL-10 (Diaclon, CA, U.S.A.). The minimum detectable dose of mIL-10 was less than 7 pg ml−1 and the intra- and inter-coefficient of variation were 4.4±0.5% and 8.9±0.9% respectively. Levels of IL-12p40 in macrophage supernatants were quantified using specific ELISA kits purchased from Biosource Int (CA, U.S.A.), according to the manufacturer's instructions. The assay detected >2 pg ml−1 and the intra- and inter-assay coefficients of variations were between 3.3–4.5 and 5.6–6.4%, respectively.
Drugs
LPS was from Escherichia coli (Serotype 026:B6, Sigma, Spain), IFN-γ was from PeproTech (London U.K.). JWH-133 was purchased from Tocris Cookson Ltd (U.K.). SR141716A (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazol-carboxamide and SR144528 (N-[1S)-endo-1,3,3,-trimethylbicyclo [2.2.1]heptan-2-yil-5-(4-chloro-3methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were a gift from Sanofi Recherche (Montpellier, France). The MEK inhibitor PD98059 was obtained from New England Biolabs (Beverly, MA, U.S.A). Neutralizing mAb to IL-10 and murine IL–10 were from Genzyme (Cambridge, U.K.).
Statistics
Results are presented as means±s.e.m. of at least three experiments performed with different cell preparations. Analysis of variance followed by the Tukey test for multiple comparison were used to determine statistical significance (95%; P<0.05).
Results
Effects of the activation of CB2 receptors by the agonist JWH-133 on the production of IL-12p40 by activated macrophages
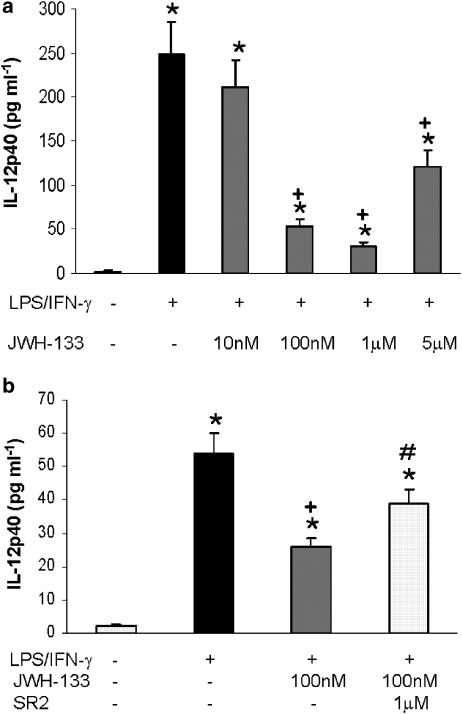
THC administration in mice subjected to immunological challenge has been shown to drive a reduction in Th1 immunity. This process has been suggested to be induced by downregulation of IL-12 through activation of both CB1 and CB2 receptors (Klein et al., 2000). IL-12 is a cytokine primarily produced by monocytes and macrophages, and plays an essential role in the development of cell-mediated immunity. Bioactive IL-12 (IL-12p70) is a heterodimer formed by p35 and p40 subunit, and here, we examined the effect of JWH-133 on the production of IL-12 subunit p40, which is secreted by LPS/IFN-γ stimulated macrophages. To evaluate this, we measured IL-12p40 levels in the supernatants of LPS/IFN-γ stimulated macrophage cultures in the presence or absence of the selective CB2 agonist JWH-133. Cells were preincubated with different doses of JWH-133 or vehicle for 5 min, before activation with LPS/IFN-γ for 18 h, and tested for IL-12p40 levels in cell supernatants. JWH-133 inhibited LPS/IFN-γ induced IL-12 production in a dose-dependent manner (Figure 1a), but the higher dose used (5 μM) was less effective than the dose of 100 nM, suggesting an inverted U-shaped dose effect. In Figure 1b it is shown that the effect of JWH-133 (100 nM) on IL-12 production was significantly blocked by the CB2 antagonist SR144528 (Figure 1b), while the addition of the CB1 antagonist SR141716A did not modify IL-12 levels (data not shown). In all cases, the results obtained using the two CB antagonists alone in normal or activated macrophages were not significantly different from those obtained in the absence of antagonists. Interestingly, the infection of macrophages from SJL/J mice with TMEV also elicited the biosynthesis of IL-12p40 (non-infected: non detectable; TMEV: 119.20±12.3 pg ml−1; P<0.001), and the treatment with the CB2 agonist, JWH-133 (100 nM) significantly reduced its release to cell medium culture (TMEV:130.1±10.6 pg ml−1; TMEV+JWH-133: 23.66±4.8 pg ml−1; P<0.001). This effect was also significantly antagonized by the CB2 antagonist SR 144528 (IL-12 levels: 77.5±8.1 pg ml−1; P<0.001).
Figure 1.
JWH-133 inhibits LPS-induced IL-12 p40 production by murine macrophages. (a) Macrophages (0.5 × 106 cells/well) were pretreated with JWH-133 at concentrations ranging from 0 to 5 μM for 5 min before LPS (50 ng ml−1)/IFN-γ (100 U ml−1) stimulation for 18 h following which cell supernatants were harvested and analyzed for IL-12p40 production. The results shown are the mean±s.e.m. of three independent experiments in triplicate. Statistics: *P<0.001 vs control; +P<0.001 vs LPS/IFN-γ. (b) Macrophages were pretreated with the CB2 antagonist SR144558 for 30 min before CB2 activation with JWH-133 (100 nM) and then subjected to LPS (50 ng ml−1)/IFN-γ (100 U ml−1) stimulation for 18 h, following which supernatants were collected and analyzed for IL-12p40 production. The results shown are the mean±s.e.m. of three independent experiments performed in triplicate. Statistics: *P<0.001 vs control; +P<0.001 vs LPS/IFN-γ; #P<0.01 vs LPS/IFN-γ plus JWH-133.
ERK1/2 activation is associated with JWH-133-mediated IL-12p40 inhibition by LPS-activated macrophages
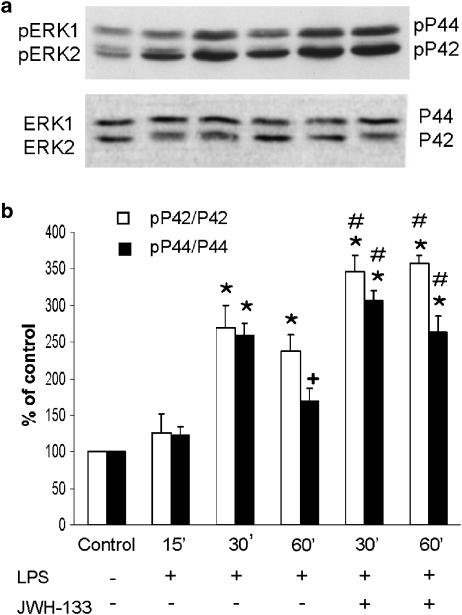
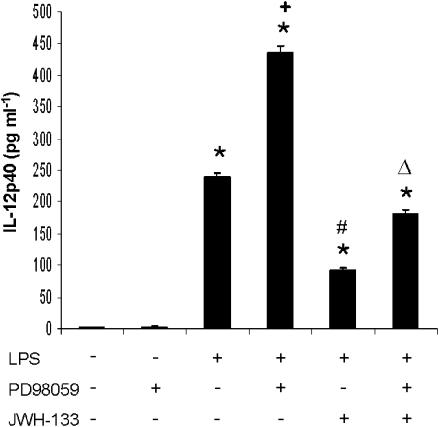
The molecular mechanisms underlying regulation of IL-12 production in macrophages are not fully understood. It has been suggested that MAPKs regulate IL-12 production in APC cells (Feng et al., 1999; Utsugi et al., 2003). To elucidate the mechanisms involved in the effects of JWH-133 on IL-12p40 production by LPS-stimulated murine macrophage cell line (RAW 264.7), we assessed the activation state of ERK1/2, after LPS treatment in the absence or presence of JWH-133. As expected, the level of phosphorylated ERK 1/2 was significantly increased by LPS, with a peak at 30 min, whereas at 60 min it showed a tendency to diminish (Figure 2a). The treatment with JWH-133 significantly enhanced the levels of phosphorylated ERK1/2 at 30 min in comparison with those obtained after LPS alone and this significant activation was maintained at 60 min (Figure 2b). This sustained activation of ERK1/2 may be involved in the decreased production of IL-12p40 accordingly to results showing that ERK activation negatively regulates the production of IL-12p40 in activated APC cells (Yanagawa et al., 2002). Therefore, the increased and sustained ERK1/2 following JWH-133 treatment may be, in part, responsible for the inhibition of IL-12p40 in LPS-activated macrophages. To confirm the role of ERK1/2 MAP kinases, we analyzed IL-12p40 production in LPS-stimulated macrophages treated with the specific inhibitor of MEK, PD98059. The pharmacological inhibition of ERK1/2 MAP kinase by PD98059 (10 μM) significantly enhanced the production of IL-12p40 by LPS-stimulated macrophages in the absence or presence of JWH-133 (Figure 3). These data point to ERK1/2 MAP kinase as a possible mediator of the inhibitory action of CB2 activation on IL-12p40 production.
Figure 2.
Time course of ERK1/2 activation by LPS in presence or absence of JWH-133 (100 nM). Macrophage cell line RAW 264.7 cultures (0.5 × 106 cells/well) were pretreated or not with JWH-133 (100 nM) 5 min before LPS (1 μg ml−1) stimulation for 30 and 60 min. (a) Whole-cell lysates (proteins 30 μg) were prepared and subjected to SDS–PAGE followed by Western blot analysis using antiphospho-ERK1/2 kinase (dilution 1 : 1000) as described in Methods. To control for equal loading, membranes were stripped and reprobed with antibody recognizing total ERK1/2 kinase (dilution 1 : 1000). The experiments were repeated at least three times with similar results. Bands were visualized by the ECL method. It is shown as a representative Western blot. (b) Densitometric analysis showing the means±s.e.m. of three independent experiments of pP42/p42 and pP44/p44 band densities. *P<0.001 vs control; +P<0.02 vs control; #P<0.001 vs LPS (30 and 60 min).
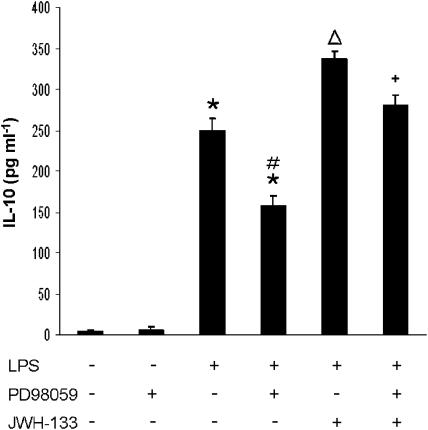
Figure 3.
Increased production of IL-12 after inhibition of ERK1/2 signaling. Murine macrophages (0.5 × 106 cells/well) were pretreated with PD98059 (10 μM) 1 h before LPS (50 ng ml−1), in the absence or presence of JWH-133 (100 nM) and the supernatants were harvested after 24 h and analyzed by ELISA for IL-12p40 production. The results shown are the mean±s.e.m. of three independent experiments performed in triplicate. Statistics: *P<0.001 vs control; +P<0.001 vs LPS; #P<0.001 vs LPS; ΔP<0.01 vs LPS+JWH-133.
Effects of the activation of CB2 receptors by the agonist JWH-133 on the production of IL-10 by activated macrophages. Role of ERK1/2
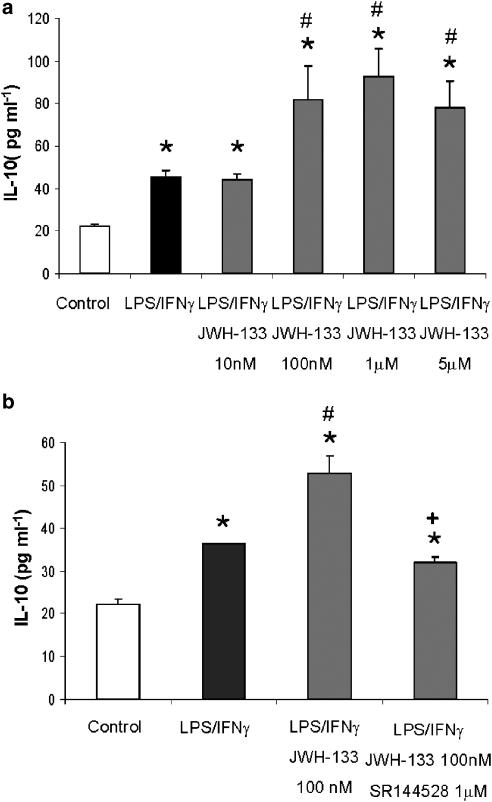
In mouse models of endotoxemia, it has been reported that CBs exert modulatory effects on cytokine responses, including the anti-inflammatory cytokine IL-10 (Smith et al., 2000) and suggested a role for the CB1 receptor in this modulation. Thus, to study whether activation of CB2 receptor affects IL-10 production by LPS/IFN-γ-activated macrophages, we performed a set of experiments in the presence or absence of JWH-133. In Figure 4a, it is shown that after treatment with LPS/IFN-γ, IL-10 production was increased in murine macrophages, and that JWH-133 at doses of 100 nM, 1 μM and 5 μM significantly enhanced the release of IL-10 as indicated by the levels of this cytokine in cell supernatants. In addition, by using the CB2 antagonist SR144528, we confirmed the involvement of CB2 receptors in the modulatory action of JWH-133 on IL-10 production (Figure 4b), as the pretreatment with SR144528 completely abolished the enhancement of IL-10 release induced by JWH-133 at the dose of 100 nM. To determine whether inhibition of ERK1/2 is also effective in modifying IL-10 responses to LPS, we used the specific inhibitor PD98059 (10 μM).The inhibitor added to LPS-activated macrophages significantly decreased IL-10 production in the presence or absence of JWH-133, when compared with their corresponding controls (Figure 5).
Figure 4.
JWH-133 enhances LPS-induced IL-10 production by murine macrophages. (a) Macrophages (0.5 × 106 cells/well) were pretreated with JWH-133 at concentrations ranging from 0 to 5 μM for 30 min before LPS (50 ng ml−1)/IFN-γ (100 U ml−1) stimulation for 18 h, following which cell supernatants were harvested and analyzed for IL-10 production. The results shown are the mean±s.e.m. of three independent experiments perfomred in triplicate. Statistics: *P<0.001 vs control; #P<0.001 vs LPS/IFN-γ. (b) Macrophages were pretreated with the CB2 antagonist SR144558, prior CB2 activation with JWH-133 (100 nM) and then subjected to LPS stimulation for 18 h, following which supernatants were collected and analyzed for IL-10 production. The results shown are the mean±s.e.m. of three independent performed experiments in triplicate. Statistics: *P<0.001 vs control; #P<0.01 vs LPS/IFN-γ; +P<0.001 vs LPS/IFN-γ+JWH-133.
Figure 5.
Decreased production of IL-10 after inhibition of ERK1/2 signaling. Murine macrophages (0.5 × 106 cells/well) were pretreated with PD98059 (10 μM) 1 h before LPS (50 ng ml−1), in the absence or presence of JWH-133 (100 nM) and the supernatants were harvested after 24 h and analyzed by ELISA for IL-10 production. The results shown are the mean±s.e.m. of three independent experiments performed in triplicate. Statistics: *P<0.001 vs control; +P<0.001 vs LPS; #P<0.001 vs LPS; ΔP<LPS+JWH-133.
Endogenous IL-10 is involved in the suppressive effect of JWH-133 on the production of IL-12p40 by activated macrophages
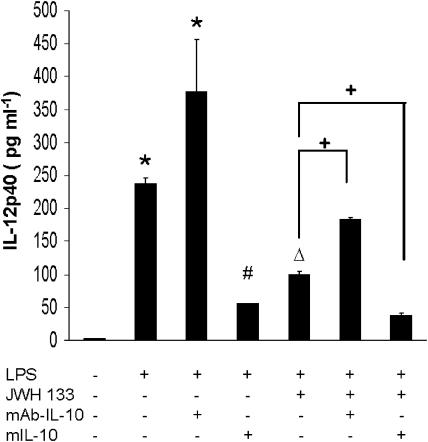
Since IL-10 is a potent biological inhibitor of IL-12 synthesis by activated macrophages (D'Andrea et al., 1993), we assessed whether stimulated endogenous synthesis of IL-10 by JWH-133 may contribute to the observed decreases on IL-12p40. To this end, we examined the effects of the addition of IL-10 neutralizing antibody (mIL-10Ab) to stimulated macrophages in order to avoid negative feedback regulation of IL-12p40. The results showed that mIL-10Ab resulted in a higher production of IL-12p40 in comparison with the levels obtained in cells stimulated with LPS alone (Figure 6). Indeed, the addition of exogenous murine IL-10 (10 ng ml−1 exerted the opposite effect by blocking IL-12p40 secretion in a significant manner (P<0.001; Figure 6). Following the activation of CB2 receptors by JWH-133, the blockade of endogenous IL-10 induced a significantly (P<0.001) diminished response to the CB, consistent with the proposed regulatory role of IL-10 upon IL-12p40 synthesis. By contrast, the addition of murine IL-10 (10 ng ml−1 increased the degree of IL-12p40 inhibition evoked by JWH-133 (P<0.001). Therefore, these data strongly suggested that the increase in IL-10 production induced by JWH-133 contributed to the downregulation of IL-12p40 biosynthesis.
Figure 6.
Endogenous IL-10 inhibits IL-12p40 production by stimulated macrophages. Murine macrophages (0.5 × 106 cells/well) were pretreated with neutralizing antibody (mAb-IL-10) or added exogenous murine IL-10 (10 ng ml−1) before LPS (50 ng ml−1) activation and supernatants were collected after 24 h for assessing IL-12p40 release. Results are the means±s.e.m. of three independent experiments performed in triplicate. *P<0.001 vs control; #P<0.001 vs LPS; ΔP<0.02 vs LPS; +P<0.05 vs LPS+JWH-133.
Discussion
CB receptor agonists have potential utility as anti-inflammatory drugs for the treatment of many disease conditions. We have previously shown the efficacy of the CB2 agonist JWH-015 in diminishing macrophage/microglia reactivity and inducing recovery of motor function in a viral (TMEV) model of MS (Arévalo-Martín et al., 2003). These effects may be justified based on the influence of CBs on the motor pathways together with their immunomodulatory activity. Although numerous reports indicate that CBs alter the functional activities of immune cells (Berdyshev, 2000; Klein et al., 2003), the cellular basis for these CB actions remain unclear. The results presented in this study show that the activation of CB2 receptors by JWH-133 evoked a concentration-related inhibition of LPS/IFN-γ-induced IL-12p40 release from murine macrophages. IL-12p40 inhibition by JWH-133 (100 nM) was significantly antagonized by SR144528, confirming the involvement of CB2 receptors. Other groups have reported that THC inhibited the production of IL-12, as detected in serum of mice subjected to L. pneumoniae infection, but this effect appeared to involve both type of receptors, CB1 and CB2 receptors (Klein et al., 2000). Later studies using activated peripheral blood T-cell cultures showed that treatment with THC inhibited cell proliferation and the generation of Th1 cytokines, including IL-12, by CB2-mediated mechanisms (Yuan et al., 2002). IL-12 plays a central role as a link between the innate and adaptive immunity, and polarizes the immune system toward a Th-1 response required for protection against intracellular microorganisms. The role of IL-12 in the protective and pathological processes during viral infections depends on the type of virus, but macrophages from susceptible strain of mice (SJL/J), which develop demyelinating disease after TMEV infection (TMEV-IDD), showed increased levels of IL-12p40 when infected with the virus. Under these conditions, the application of JWH-133 abolishes the generation of IL-12 by TMEV-infected macrophages. This is of special interest in TMEV-IDD, since infiltrating macrophages and microglia are the viral reservoir and constitute the main APCs in the CNS (Martinat et al., 2002). IL-12 is critical for the induction of clonal expansion and differentiation of TMEV-specific MHC class II-restricted effector delayed-type hypersensitivity DTH (Th1) cells (Pope et al., 1998; Olson et al., 2001). Therefore, blunting IL-12 production by macrophages following activation of CB2 receptors may have important implications for the pathogenesis of TMEV-IDD. In this regard, previous studies demonstrated that treatment with anti-IL-12 shifted Th1–Th2 balance to Th2 dominance and led to a significant attenuation of symptomatology and demyelination in TMEV-infected mice (Inoue et al., 1998).
The results of our study also showed that ERK1/2 MAP kinases play a negative regulatory role in the production of IL-12p40 by LPS-stimulated macrophages. The MEK 1 inhibitor that selectively targets the ERK MAP kinase signaling cascade promoted IL-12p40 production, a finding consistent with earlier reports (Wittmann et al., 2002; Yanagawa et al., 2002; Tang et al., 2004). Moreover, activation of CB2 receptors by JWH-133 induced a greater and sustained activation of ERK1/2 MAP kinase for up to at least 60 min, perhaps contributing to the downregulation of IL-12p40. In addition, we find that JWH-133 enhanced the release of IL-10 by LPS/IFN-γ-stimulated macrophages and this effect was blocked by the CB2 antagonist SR144558. Since inhibition of ERK by PD98059 significantly suppressed IL-10 and exogenous IL-10 reversed the upregulated production of IL-12 induced by PD98059, it is suggested a unidirectional negative autocrine regulation of IL-12 by IL-10, as well as that activation of ERK involves the differential production of IL-10 and IL-12 by activated macrophages. Thus, the regulation of differential production of IL-10 and IL-12 may play an important role for macrophages in priming Th1 or Th2 in the immune responses. In vivo experiments have shown that other CB agonists, WIN 55212-2 and HU-210, decreased IL-12 and increased levels of IL-10 in the serum of LPS-treated mice through a CB1 receptor action (Smith et al., 2000). However, the mechanisms by which the CB agonists produced their modulatory effects on LPS-induced serum cytokines are unknown as well as the cellular source of these cytokines, since cytokine alterations may be secondary to changes in cellular targets other than immune cells. According to other studies (Yi et al., 2002), our data show that endogenous IL-10 acts as a crucial factor for maintenance of the balance of appropriate macrophage responses to LPS by limiting the production of synthesis of IL-12. We also provided evidence that the negative regulation of IL-12 by endogenous IL-10 may be a relevant mechanism involved in the suppressive effect of JWH-133 upon IL-12 production. It is conceivable that the cytokine profile reported here in macrophages may occur during induction of an immune response as a consequence of activation of CB2 receptors. Direct effects of CB2 agonists targeting macrophages revealed a reduction in the production of IL-1β and TNF-α following activation with LPS/IFN-γ (Klegeris et al., 2003) and a decrease of neurotoxicity of culture supernatants. In addition, activation of CB2 receptors also decreases the expression of MHC class II antigens by activated macrophages (unpublished results). The overall actions due to activation of CB2 receptors in cells of macrophage lineage may prevent the generation of a Th-1 immune response affecting the required immunity to combact a particular pathogen or, alternatively, reduce inflammation/pathology associated with certain chronic disease states, such as MS.
In summary, the results of this study show that (i) activation of CB2 receptors inhibits IL-12p40 production and enhances IL-10 biosynthesis by activated macrophages, (ii) JWH-133 may exert its inhibitory effect on IL-12p40 production by a greater and sustained activation of ERK1/2 MAP kinase, (iii) pharmacological inhibition of ERK promotes IL-12p40 production and reduces IL-10 by activated macrophages, (iv) increased endogenous IL-10 secretion may also contribute to this inhibition by acting in an autocrine way. These results suggest that CB2 agonists might be useful for chronic inflammatory diseases therapies. Additional research is being performed to establish the possible role of endocannabinoids in the regulation of immunity in normal and pathological conditions.
Acknowledgments
We gratefully appreciate Dr M. Rodriguez (Department of Immunology and Neurology, Mayo Clinic/Foundation, Rochester, MN, U.S.A.) for kindly providing Theiler's virus strain. This work was supported by grants from the MCYT (SAF-2001/1246 and SAF 2004-00416).
Abbreviations
- APCs
antigen presenting cells
- CBs
cannabinoids
- CB1 receptor
type I CB receptor
- CB2 receptor
type 2 CB receptor
- DTH
delayed-type hypersensivity
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- IFN-γ
interferon-gamma
- IL-10
interleukin-10
- IL-12
interleukin-12
- LPS
lipopolyssacharide
- MAP kinase
mitogen-activated protein kinase
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- MOI
multiplicity of infection
- THC
tetrahydrocannabinol
- Th1
T helper 1
- Th2
T helper 2
- TMEV
Theiler's virus
- TMEV-IDD
Theiler's virus induced demyelinating disease
References
- ARÉVALO-MARTÍN A., VELA J.M., MOLINA-HOLGADO E., BORRELL J., GUAZA C. Therapeutic actions of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERDYSHEV E.V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein–coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- CARLISLE S.J., MARCIANO-CABRAL F., STAAB A., LUDWICK C., CABRAL C.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- D'ANDREA A.M., ASTE-AMEZAGA N.M., VALIANTE X., MA M., KUBIN M., TRINCHERI G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon gamma production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C.S., TAO Q., BRIDGEN D.T., RAZDAN R.K., ZIMMER A.M., ZIMMER A., MARTIN B.R. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- FENG G., GOODRIDGE H.S., HARNETT M.M., WEI X., NIKOLAEV A.V., HIGSON A.P., LIEW F. Extracellular signal-related (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolyssacharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophages IL-12 production by targeting ERK MAP kinase. J. Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- GUBLER U., CHUA O., SCHOENHAUT C., DWYER M., MC COMAS V., MOTIKA R., NABAVI N., WOLITZY G., QUINN P.M., FAMILETTI P.C. Coexpression of two distinct genes is required to generate secrete bioactive cytotoxic lymphocyte maduration factor. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4143. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE A., KOH C., YAMAZAKI M., YAHIHOZAWA H., ICHIKAWA M., YAGITA H., KIM B.S. Suppressive effect on Theiler's murine encephalomyelitis virus-induced demyelinating disease by the administration of anti-IL-12 antibody. J. Immunol. 1998;161:5586–5593. [PubMed] [Google Scholar]
- JARAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid induced mesenteric vasodilation through an endothelial site distint from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEGERIS A., BISSONNETTE C.J., MCGEER P.L. Reduction of human monocyte neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN T.W., NEWTON C., NAKACHI N., FRIEDMAN H. Delta 9 tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J. Immunol. 2000;164:6461–6466. doi: 10.4049/jimmunol.164.12.6461. [DOI] [PubMed] [Google Scholar]
- KLEIN T.W., NEWTON C., LARSEN K., LU L., PERKINS I., NONG L., FRIEDMAN H. The cannabinoid system and immune modulation. J. Leukocyte Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- MARTINAT C., MENA I., BRAHIC M. Theiler's virus infection of primary cultures of bone marrow-derived monocytes/macrophages. J. Virol. 2002;76:12823–12833. doi: 10.1128/JVI.76.24.12823-12833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTAIN M.J., YOUNG A.G., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWEE R.G., GRIFFIN G., BAYEWITCH M., BARG M., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- OLSON J.K., GIRVIN A.M., MILLER S.D. Direct activation of innate and antigen presentating functions of microglia following infection with Theiler's virus. J. Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J.G., VANDERLUGT C.L., RAHBE S.M., LIPTON H.L., MILLER S.D. Characterization of and functional antigen presentation by central nervous system mononuclear cells from mice infected with Theiler's murine encephalomyelitis virus. J. Virol. 1998;72:7762–7771. doi: 10.1128/jvi.72.10.7762-7771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHO R., MACHO A., DE LA VEGA L., CALZADO M.A., FIEBICH B.L., APPENDINO G., MUÑOZ E. Immunosuppressive activity of endovanilloids:N-Arachidonoyl-dopamine inhibits activation of the NF-kB, NFAT, and activator protein 1 signaling pathways. J. Immunol. 2004;172:2341–2351. doi: 10.4049/jimmunol.172.4.2341. [DOI] [PubMed] [Google Scholar]
- SHU U., KINIWA M., WU Y., MALISZEWSKI C., VEZZIO N., HAKIMI J., GATELY M., DELESPESSE G. Activated T cells induce IL-12 production by monocytes via CD40-CD40 ligand interactions. Eur. J. Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- SMITH S.R., TERMINELLI C., DENHARDT G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUKAGAWA A., NAKANE S., SHINODA A., ITOH K., YAMASHITA A., WAKU K. 2-Arachidonoyl-glycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- TANG N., LIMING T., MCCORMICK S., COOPER D., GHANNOUM M. Inhibition of monocyte interleukin-12 production by Candida albicansvia selective activation of ERK mitogen-activated protein kinase. Infect. Immun. 2004;72:2512–2513. doi: 10.1128/IAI.72.5.2513-2520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRINCHIERI G. Interleukin-12 and the regulation of innate resistence and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- UTSUGI M., DOBASHI K., ISHIZUKA T., ENDOU K., HAMURO J., MURATA Y., NAKAZAWA T., MORI M. C-Jun N-terminal kinases negatively regulates lipopolysaccaride-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production. J. Immunol. 2003;171:628–635. doi: 10.4049/jimmunol.171.2.628. [DOI] [PubMed] [Google Scholar]
- WALTER L., FRANKLIN A., WITTING A., WADE C., XIE Y., KUNOS G., MACKIE K., STELLA N. Nonsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITTMANN M., KIENLIN P., MOMMERT S., KAPP A., WERFEL T. Suppression of IL-12 production by soluble CD40 ligand: evidence for involvement of the p42/44 mitogen-activated protein kinase pathway. J. Immunol. 2002;168:3793–3880. doi: 10.4049/jimmunol.168.8.3793. [DOI] [PubMed] [Google Scholar]
- YANAGAWA Y., IJIMA K., IWABUCHI K., ONOÉ K. Activation of extracellular signal-related kinase by TNF-alpha controls the maturation and function of murine dendritic cells. J. Leukoc. Biol. 2002;71:125–132. [PubMed] [Google Scholar]
- YUAN M., KIERTSCHER S.M., CHENG Q., ZOUMALAN R., TASHKIN D.P., ROTH M.D. Delta 9 tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- YI A.K., YOON J.G., YEO S.J., HONG S.G., ENGLISH B.K., KRIEG A.M. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J. Immunol. 2002;168:4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]