Abstract
Neuroprotection has been reported after either activation or blockade of the group I metabotropic glutamate receptor subtype 5 (mGluR5). However, some recent evidence suggests that protection provided by mGluR5 antagonists may reflect their ability to inhibit N-methyl-D-aspartate (NMDA) receptor activity.
Here, in both rat and mouse cortical neurons, we compare the neuroprotective actions of two mGluR5 antagonists: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), which has been commonly used and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP), a more recently developed compound believed to have greater mGluR5 selectivity. We have previously shown that MPEP directly reduces single-channel NMDA receptor open time at the same concentrations (20 μM or greater) that show neuroprotection, whereas MPEP antagonizes mGluR5 agonist ((RS)-2-chloro-5-hydroxyphenylglycine (CHPG))-induced changes in inositol phosphates (IP) at concentrations as low as 0.2 μM.
In the present studies, MTEP significantly inhibited CHPG-mediated IP hydrolysis at concentrations as low as 0.02 μM. In contrast to MPEP, which significantly reduced glutamate- or NMDA-mediated cell death in primary rat neuronal cultures at a concentration of 20 μM, small neuroprotective effects were observed with MTEP only at a concentration of 200 μM. Neither MPEP- nor MTEP-mediated mGluR5 inhibition had any effect on etoposide-induced apoptotic cell death. In rat cortical neurons, the neuroprotective effects of MTEP at very high concentrations, like those of MPEP, reflect ability to directly reduce NMDA receptor peak and steady-state currents.
We also compared the effects of MPEP and MTEP in primary cortical neuronal cultures from parental and mGluR5 knockout mice. Both agents were neuroprotective, at high concentrations in normal as well as in the knockout cultures. In contrast to rat cortical neurons, neither MPEP nor MTEP appears to directly alter NMDA receptor activity.
Combined, these studies support the conclusion that MTEP has greater mGluR5 selectivity than MPEP, and that neuroprotection provided by either antagonist in neuronal cultures does not reflect inhibition of mGluR5 receptors.
Keywords: mGluR5, NMDA receptors, MTEP, MPEP, neuroprotection
Introduction
Metabotropic glutamate receptors (mGluRs) are known to modulate ionotropic glutamate receptors (iGluRs), various calcium and potassium channels, and neurotransmitter release (Anwyl, 1999). Because many of these systems are involved in the pathophysiology of acute CNS injury, drugs that activate or inhibit mGluR have been examined with regard to post-traumatic or postischemic cell death (Bruno et al., 1998; Lea & Faden, 2003b). The mGluRs have been categorized into three groups, on the basis of structure, signal transduction mechanisms, and pharmacological sensitivities (Conn & Pin, 1997; Schoepp et al., 1999). Group I mGluRs include mGluR1 and mGluR5. Activation of mGluR1 appears to exacerbate necrotic cell death after trauma or ischemia, in part by activating N-methyl-D-aspartate (NMDA) receptors (Allen et al., 2000; 2001). However, the role of mGluR5 has been more controversial because of the lack of truly selective antagonists. Several purportedly selective mGluR5 antagonists have been developed, such as 2-methyl-6-(phenylethynyl)-pyridine (MPEP) (Gasparini et al., 1999) and (E)-2-methyl-6-(2-phenylethenyl)-pyridine (SIB-1893) (Varney et al., 1999). Although both compounds show some neuroprotective activity, such actions occur only at concentrations that inhibit NMDA receptors and are substantially higher than that required to inhibit agonist-induced phosphoinositide (PI) hydrolysis (O'Leary et al., 2000; Movsesyan et al., 2001a). These findings are consistent with a prior study using antisense oligonucleotides (ODN) directed against mGluR1 or mGluR5 receptors; at concentrations that equally reduced agonist-induced IP3, only inhibition of mGluR1 was neuroprotective (Mukhin et al., 1996).
Recently, a noncompetitive mGluR5-specific antagonist was developed. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) appears to have greater selectivity at mGluR5 than other antagonists (Cosford et al., 2003a). In vivo and in vitro characterization of MTEP (Anderson et al., 2002; 2003; Cosford et al., 2003a) indicates that it is highly selective for mGluR5 over mGluR1, has no effect on other mGluR subtypes, and has fewer off target effects than MPEP (Cosford et al., 2003a). In the present studies, we compared the selectivity and neuroprotective actions of MPEP and MTEP against glutamate-, NMDA-, and etoposide-induced toxicity in primary rat cortical neuronal cultures. We also compared the actions of these compounds in cortical neuronal cultures derived from mGluR5-deficient mice and parental (mGluR5 positive) animals.
Methods
Drugs
The following drugs were obtained from Tocris Cookson (St Louis, MO, U.S.A.): selective mGluR5 agonist (RS)-2-chloro-5-hydroxyphenylglycine (CHPG); selective mGluR5 antagonist MPEP; L-type calcium channel blocker 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 2-methyloxyethyl 1-methylethyl ester (Nimodipine); NMDA receptor agonist NMDA; NMDA receptor antagonist (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate/dizocilpine (MK 801). The reversible sodium channel blocker tetrodotoxin (TTX) and glutamate were obtained from Sigma (St Louis, MO, U.S.A.). The mGluR5 antagonist MTEP was a gift from Merck Research Laboratories (Rahway, NJ, U.S.A.). All drugs were prepared and stored according to the manufacturer's guidelines. Selection of doses used for CHPG and MPEP was based on published EC50 and IC50 values (Schoepp et al., 1999; Brodkin et al., 2002; Cosford et al., 2003b), and previous experience with these compounds (O'Leary et al., 2000; Movsesyan et al., 2001a).
Cell cultures
Cortical neuronal cultures (CN) were derived from rat (Taconic, Germantown, NY, U.S.A.) or mouse (in-house breeding colony established from animals purchased from Jackson Labs) embryonic cortices as described previously (Faden et al., 2001; Movsesyan et al., 2001a; Yakovlev et al., 2001a). Briefly, cortices from 17- to 18-day-old Sprague–Dawley rat embryos, or 15- to 16-day-old B6;129-Gprc1etm1Rod (knockout) or B6;129SF2/J (control) mouse embryos, were cleaned from their meninges and blood vessels in Krebs–Ringers bicarbonate buffer containing 0.3% bovine serum albumin (BSA, Gibco, Gaithersburg, MD, U.S.A.). Cortices were then minced and dissociated in the same buffer with 1800 U ml−1 trypsin (Sigma, St Louis, MO, U.S.A.) at 37°C for 20 min. Following the addition of 200 U ml−1 DNase I (Sigma) and 3600 U ml−1 soybean trypsin inhibitor (Sigma) to the suspension, cells were triturated through a 5 ml pipette. After the tissue settled for 5–10 min, the supernatant was collected and the remaining tissue pellet was retriturated as before. The combined supernatants were then centrifuged through a 4% BSA layer. The cell pellet was resuspended in neuronal seeding medium (NSM), which consisted of Neurobasal Medium (Gibco), supplemented with 1.1% 100 × antibiotic–antimycotic solution (Biofluids, Rockville, MD, U.S.A.), 25 μM Na-glutamate, 0.5 mM L-glutamine, and 2% B27 supplement (Gibco). Cells were seeded at 5 × 105 cells ml−1 onto 96-well tissue culture plates (Corning, Corning, NY, U.S.A.) precoated with poly-D-lysine or at a density of 2.5 × 105 cells ml−1 on poly-D-lysine-coated, 12 mm round glass coverslips (Fisher Scientific, Pittsburgh, PA, U.S.A.) placed in four- or 24-well plates. In all cases, 10 μg ml−1 of poly-D-lysine (70–150 kDa, Sigma; prepared in sterile water) was used for precoating. On day 4 in vitro, feeding medium (NSM without Na-glutamate and B27 supplement) in 1 : 2 proportion was added to cultures. Subsequent feedings were performed until cells were used. All experiments were performed at 7 or 14 days in vitro (DIV). Analysis of cultures indicates that the percentage of neuronal cells in rat cultures (using neuron-specific enolase; Yakovlev et al., 2001b) is 91±5% and the percentage of GFAP-positive cells in mouse cultures (using GFAP-positive staining) is 7.3±1.7%, similar to other studies (Moldrich et al., 2001). Immunostaining was performed as described previously (Yakovlev et al., 2001b) using anti-GFAP antibody (Chemicon, Temecula, CA, U.S.A.).
Phosphoinositide hydrolysis
PI hydrolysis was measured in cortical neuronal cultures at 7 DIV as described previously (Mukhin et al., 1996; Movsesyan et al., 2001a) with minor modifications. Cortical neuronal cells cultured in 96-well plates were incubated overnight with myo-[3H]inositol (22.3 Ci mmol−1, NEN, Boston, MA, U.S.A.) at 1.5 μCi well−1. Cells were washed twice with Locke's buffer and incubated at 37°C in the same buffer for 20 min, in the presence or absence of the mGluR5 antagonist MTEP (0.02–200 μM). Subsequently, the mGluR5 agonist CHPG (1 mM) was added to the cultures together with 20 mM LiCl and incubation was continued for an additional 40 min. Thereafter, incubation buffer was aspirated and inositol phosphates (IP) were extracted by 0.1 M HCl containing 2 mM CaCl2 and transferred to columns with AG 1-X8 anion-exchange resin (Bio-Rad, Hercules, CA, U.S.A.). After separation according to the method of Berridge et al. (1982), accumulated [3H]IP were measured using a liquid scintillation counter LS 6500 (Beckman Instruments, Fullerton, CA, U.S.A.).
Glutamate- and NMDA-induced injury
Either 150 μM Na-glutamate (Sigma) or 150 μM NMDA (Tocris), dissolved in NBM, was administered to cell cultures 20 min after the addition of mGluR5 antagonists. Control cultures (sister cultures from the same 96-well plate) received the same volume of the vehicle alone.
Induction of apoptosis in cell cultures
Apoptosis was induced in cultured neuronal cells by incubation with 50 μM etoposide dissolved in relevant serum-free medium with or without MPEP or MTEP. Etoposide was administered to cultures 20 min after the application of MPEP or MTEP. In all experiments, control cultures received the same volume of vehicle alone.
Cell viability assays
Cell viability was measured by LDH release assay (Sinensky et al., 1995) using the CytoTox-96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI, U.S.A.) according to the manufacturer's protocol. Relative absorbance was measured at 490 nm using a Multiscan Ascent microplate reader (Labsystems Inc., Helsinki, Finland). Alternatively, cell viability was assessed by retention and de-esterification of calcein AM as described previously (Movsesyan et al., 2001b). Calcein AM fluorescence was measured using a CytoFluor II fluorometer (PerSeptive Biosystems, Framingham, MA, U.S.A.) at 485 nm excitation and 560 nm emission wavelengths.
Electrophysiology
At the time of recording, coverslips with cells were transferred to a recording chamber on the stage of an inverted microscope (Zeiss Axiovert 135) undergoing continuous perfusion with phenol red, calcium, and magnesium-free Hank's balanced salt solution (Invitrogen Corporation, Grand Island, NY, U.S.A.), pH 7.2, supplemented with 1 mM CaCl2 and 20 μM glycine. A small piece of 1 mm diameter platinum wire was used to weigh the coverslip down to the bottom of the recording chamber. Electrophysiological recordings were performed at room temperature (20–22°C). Electrodes were pulled from Wiretrol II capillary glass (Drummond Scientific Company, Broomall, PA, U.S.A.) in three stages on a horizontal pipette puller (Mecanex S.A., Switzerland). Typical pipette resistance was 4–8 MΩ. The recording pipette contained (mM) 145 K-gluconate, 5 MgCl2, 11 ethylene glycol bis (β-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA), 5 Na-adenosine-5′-triphosphate (ATP), 0.2 guanosine-5′-triphosphate (GTP), and 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH 7.2 with KOH. Whole-cell recordings were performed with a patch-clamp amplifier (Axopatch 1D, Axon Instrument, Foster City, CA, U.S.A.). Cultured cortical neurons were voltage-clamped at a −60 mV holding potential. Access and input resistances were monitored intermittently during recordings by giving a 10 mV hyperpolarizing pulse. Drugs were applied to the cells via an SF-77B fast perfusion system (Harvard Apparatus) controlled by pClamp 8 Clampex software. The recording solution (see above) used for wash and for drug application was supplemented with 10 μM Nimodipine and 0.6 μM TTX. Electrophysiological recordings were both digitized and analyzed using pClamp 8 software (Axon Instruments).
Data analysis
Cell viability data were analyzed by ANOVA, followed by the Dunnett's test. A P-value less than 0.05 was considered to be statistically significant. Electrophysiological data were analyzed using Clampfit 8.0 (Axon Instruments), Excel (Microsoft Corp.), and Statview 5.0.1 (SAS Institute Inc.). All currents were measured at either the peak of the response or the first point at which average steady-state levels were obtained. Direct effects of MTEP on NMDA-evoked responses were determined by a paradigm that included (1) eliciting NMDA-evoked currents with 50 μM NMDA alone (control); (2) a 30 s washout period; and (3) eliciting NMDA-evoked currents with 50 μM NMDA in the presence of MTEP (20 or 200 μM). These evaluations were performed during a single recording period from an individual cortical neuron. Within a given neuron, responses were normalized to the response to 50 μM NMDA alone. The resultant percentages were averaged between multiple cells and presented as average±s.e.m. A probability of 0.05 was selected as the level of statistical significance for all data.
Results
MTEP blocks mGluR5 agonist-induced PI hydrolysis in vitro
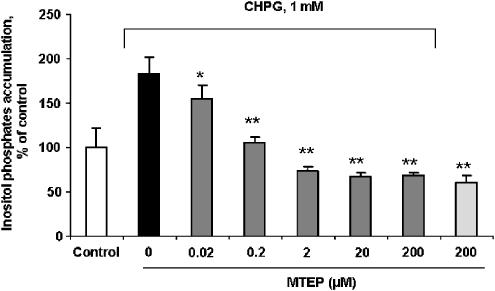
We tested the effect of MTEP on PI hydrolysis induced in cultured rat cortical neuronal cells by the selective mGluR5 agonist CHPG to confirm that MTEP acts as a PLC-coupled mGluR5 antagonist. Changes in levels of PI hydrolysis were assessed by measurement of IP accumulation in cortical neuronal cells. Pretreatment with MTEP fully blocked CHPG-induced IP accumulation at concentrations of 0.2 μM and higher (Figure 1). A smaller but significant reduction in the CHPG-induced PI hydrolysis was also observed at 0.02 μM MTEP, the lowest concentration of the antagonist examined.
Figure 1.
Treatment with MTEP blocks mGluR5 agonist-induced PI hydrolysis in rat cortical neuronal cultures. MTEP at indicated concentrations was administered to 7 DIV rat cortical neuronal cultures 20 min prior to stimulation with 1 mM of CHPG. PI hydrolysis was measured by IP accumulation within 40 min after addition of CHPG as described in Methods. Histograms represent IP levels as percentage of control±s.d.; n=6 cultures per condition. *P<0.05, **P<0.001 versus cultures treated with CHPG alone compared by ANOVA, followed by the Student–Newman–Keuls test.
Comparison of effects of MPEP and MTEP on neuronal cell viability in rat-derived cortical cultures
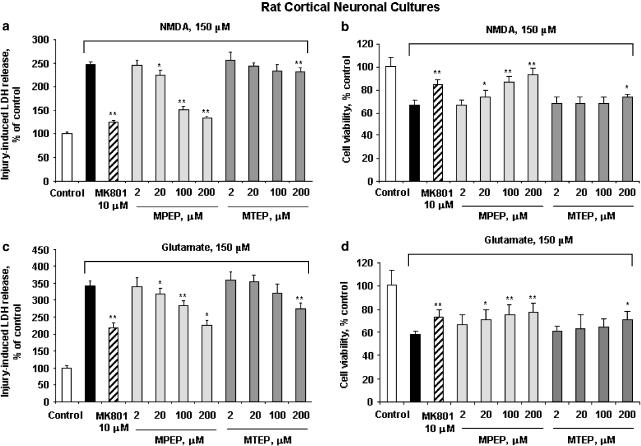
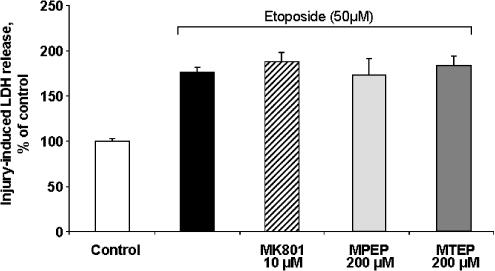
The effects of various concentrations of MTEP or MPEP were compared with regard to the viability of cultured rat cortical neuronal cells subjected to glutamate-, NMDA-, or etoposide-induced toxicity. The NMDA receptor noncompetitive antagonist MK801 ((5R,10S)-(+)5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine) showed significant neuroprotection against both NMDA- and glutamate-induced toxicity at 10 μM, as revealed by either LDH release or calcein AM assay (Figure 2), demonstrating significant NMDA receptor contribution to such in vitro excitotoxicity models. In glutamate- and NMDA-induced toxicity, pretreatment with MPEP showed significant neuroprotection at concentrations of 20 μM and higher, as revealed by either LDH release or calcein AM assay (Figure 2). In contrast, pretreatment with 2–100 μM MTEP had no effect. However, MTEP was somewhat protective at the highest dose used (200 μM; Figure 2). Both MPEP (100 and 200 μM) and MTEP (200 μM), although the latter was more variable, appeared to provide levels of neuroprotection comparable to 10 μM MK-801, suggesting that neuroprotection at these levels is due, in part, through modulation of NMDA receptor activity (Figure 2). In etoposide-induced apoptotic cell death, pretreatment with either 200 μM MPEP or MTEP had no effect, demonstrating that neuronal mGluR5 receptor inhibition does not protect against etoposide-induced apoptotic cell death (Figure 3).
Figure 2.
Comparison of effects of MTEP, MPEP, and MK801 on NMDA- or glutamate-induced cell death in 14 DIV rat cortical neuronal cultures. MPEP, MTEP, or MK801 at indicated concentrations was added to cultures 20 min prior to administration of NMDA (a, b) or Na-glutamate (c, d) (each at 150 μM). Cell viability was assessed by LDH release (a, c) or calcein AM assay (b, d) after 24 h of treatment. Histograms indicate LDH release or calcein AM fluorescence as percentage of that in intact controls±s.d.; n=8–16 cultures per condition. *P<0.05, **P<0.01 versus injured cultures as shown by ANOVA, followed by the Student–Newman–Keuls test.
Figure 3.
Comparison of effects of MTEP, MPEP, and MK801 on etoposide-induced apoptotic cell death in rat cortical neuronal cultures. MPEP, MTEP, or MK801 at indicated concentrations was added to cultures 20 min prior to administration of etoposide (50 μM). Cell viability was assessed by LDH release after 24 h of treatment. Histograms indicate LDH release as percentage of that in intact controls±s.d.; n=8–16 cultures per condition. *P<0.05, **P<0.01 versus injured cultures as shown by ANOVA, followed by the Student–Newman–Keuls test.
MTEP at high doses alters NMDA receptor activity in rat cortical neurons
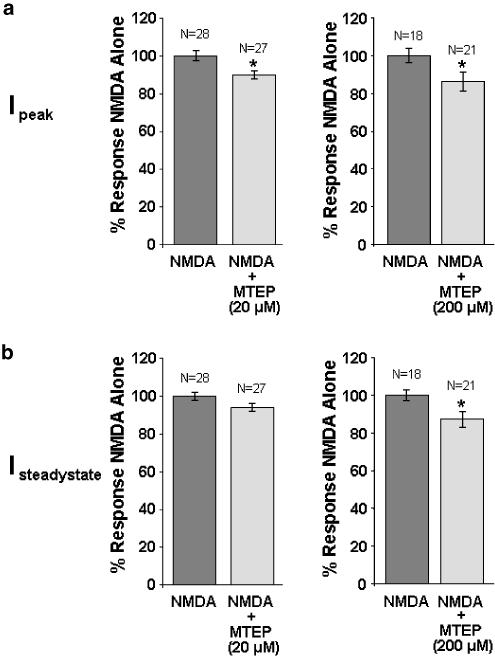
We previously demonstrated that the mGluR5 antagonist MPEP, at concentrations of 20 μM or greater, modulates NMDA receptor activity (O'Leary et al., 2000). Here we demonstrate that in 14 DIV rat cortical neurons, 20 μM MTEP directly reduced NMDA-evoked NMDA receptor peak current by 10%, but had no effect on steady-state currents (Figure 4). In contrast, 200 μM MTEP directly reduced both NMDA-evoked NMDA receptor peak and steady-state currents by 14 and 13%, respectively (Figure 4).
Figure 4.
Effects of MTEP on NMDA receptor activity in rat cortical neurons. (a) NMDA-evoked peak currents were directly reduced by both 20 and 200 μM MTEP. (b) NMDA-evoked steady-state currents were directly reduced by 200 but not 20 μM MTEP. Data are expressed as average±s.e.m. *P<0.05 versus NMDA alone compared by Student's two-tailed t-test.
Comparison of effects of MPEP and MTEP on neuronal cell viability in cortical cultures derived from mGluR5-positive and mGluR5 knockout mice
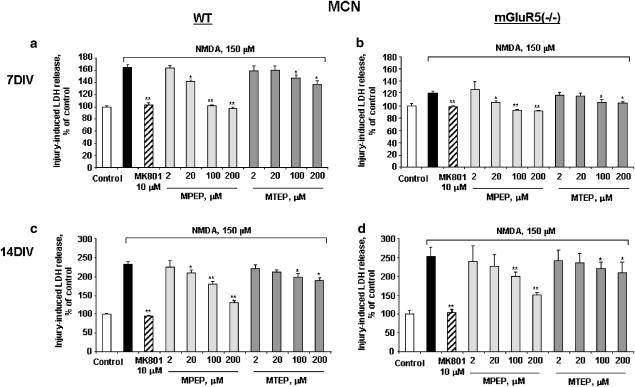
We used parental-line and mGluR5 knockout mouse-derived cortical neuronal cultures to test whether MPEP- or MTEP-mediated neuroprotection against NMDA-induced neurotoxicity occurs independent of mGluR5. The NMDA receptor noncompetitive antagonist MK801 showed significant neuroprotection against NMDA-induced toxicity in both parental-line and mGluR5 (−/−) neurons regardless of culture age. Pretreatment with 20–200 μM MPEP and 100 and 200 μM MTEP decreased NMDA-induced cell death in both 7 and 14 DIV parental-line neurons (Figure 5). Similarly, pretreatment with 20–200 μM MPEP and 100 and 200 μM MTEP decreased NMDA-induced cell death in 7 DIV mGluR5 (−/−) neurons (Figure 5). In both 7 DIV parental and knockout cultures, responses to 100 and 200 μM MPEP were similar to those obtained with MK-801, whereas responses in 14 DIV cultures were not as robust. In all paradigms, responses to MTEP were not as strong as either MK-801 or MPEP. In 14 DIV mGluR5 (−/−) neurons, 100 and 200 μM MPEP markedly decreased NMDA-induced cell death, whereas MTEP had relatively modest effect. Comparable results were obtained using calcein AM cell viability assay (data not shown).
Figure 5.
Comparison of effects of MTEP, MPEP, and MK801 on NMDA-induced cell death in wild-type (a, c) or mGluR5-deficient (b, d) mouse cortical neuronal cultures. MPEP, MTEP, or MK801 at indicated concentrations was added to cultures 20 min prior to administration of NMDA (150 μM). Cell death was measured by LDH release after 24 h of treatment. Histograms indicate LDH release as percentage of that in intact controls±s.d.; n=8–16 cultures per condition. *P<0.05, **P<0.01 versus injured cultures as shown by ANOVA, followed by the Student–Newman–Keuls test.
High-dose MPEP- and MTEP-mediated neuroprotection in mouse cortical neurons is not due to modulation of mGluR5 or NMDA receptor activity
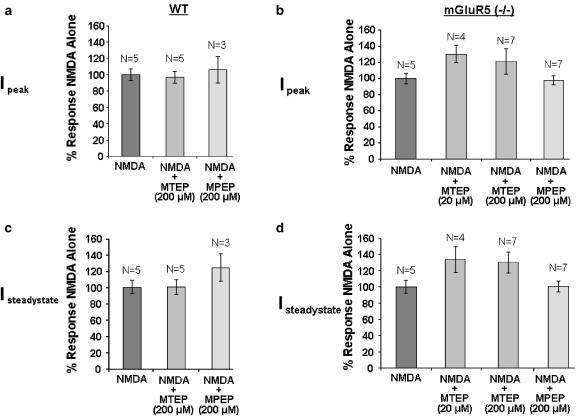
Here we demonstrate that in 14 DIV mGluR5 (+/+) mouse cortical neurons, neither 20 nor 200 μM MTEP has any effect on NMDA-evoked NMDA receptor peak current (Figure 6). In mGluR5 (−/−) mouse cortical cultures, both 20 and 200 μM MTEP did not induce statistically significant changes in either peak or steady-state current (Figure 6). Similarly, 200 μM MPEP produced no change in NMDA receptor activity in either mGluR5 (+/+) or mGluR5 (−/−) mouse cortical neurons (Figure 6).
Figure 6.
Effects of MPEP and MTEP on NMDA receptor activity in mouse cortical neurons. (a) In wild-type mouse cortical neurons, neither 200 μM MPEP nor MTEP had any effect on NMDA-evoked peak currents. (b) In mGluR5 (−/−) mouse cortical neurons, neither MTEP (20 and 200 μM) nor MPEP (200 μM) had direct effects on NMDA-evoked peak currents. (c) In wild-type mouse cortical neurons, neither 200 μM MPEP nor MTEP had any effect on NMDA-evoked steady-state currents. (d) In mGluR5 (−/−) mouse cortical neurons, neither MTEP (20 and 200 μM) nor MPEP (200 μM) had direct effects on NMDA-evoked steady-state currents. Data are expressed as average±s.e.m. *P<0.05 versus NMDA alone compared by ANOVA followed by Fisher's PLSD.
Discussion
Although the weight of experimental evidence indicates that activation of group I mGluRs contributes to post-traumatic or postischemic neuronal cell death (Bruno et al., 1995; 1999; Mukhin et al., 1996; Agrawal et al., 1998; Strasser et al., 1998; Allen et al., 2000; O'Leary et al., 2000; Bao et al., 2001; Faden et al., 2001a; Lyeth et al., 2001; Movsesyan et al., 2001a; Meli et al., 2002; Mills et al., 2002), the role of mGluR5 in cell death modulation has been limited by the availability of highly selective agonists or antagonists. However, there is increasing experimental support for the concept that mGluR5 activation can reduce apoptotic cell death (Copani et al., 1995; Allen et al., 1999; 2000). Whether mGluR5 antagonism is also neuroprotective has been more controversial, because the most-utilized compounds also antagonize NMDA receptor activity at the same concentrations that show neuroprotection in vitro (O'Leary et al., 2000; Movsesyan et al., 2001a).
Recently, a new mGluR5 antagonist (MTEP) was synthesized, in part to address such selectivity issues (Anderson et al., 2002; 2003; Cosford et al., 2003a). Here we confirm that MTEP reduces mGluR5 agonist-induced PI hydrolysis in rat cortical neurons at concentrations as low as 0.02 μM. Previously, it was shown that both unlabeled methoxymethyl-MTEP and the mGluR5 antagonist MPEP displaced [3H]methoxymethyl-MTEP binding with IC50 values in the low nanomolar range (Anderson et al., 2002).
We used glutamate-, NMDA-, and etoposide-induced toxicity to test mGluR5-mediated neuroprotection. The first two well-established in vitro models of neuronal injury produce significant excitotoxic cell death within 24 h (O'Leary et al., 2000; Movsesyan et al., 2001a), whereas etoposide-induced toxicity primarily produces apoptotic cell death (Allen et al., 2000; Movsesyan et al., 2001b).
In both rat- and mouse-derived cortical neurons, MPEP shows neuroprotection against glutamate- and/or NMDA-induced toxicity at concentrations as low as 20 μM. Although supported, in part, by previous studies in rat cortical neurons demonstrating that MPEP directly reduces NMDA receptor single-channel open time (O'Leary et al., 2000), we did not find direct MPEP-mediated reductions in NMDA receptor peak or steady-state current in either mGluR5 (+/+) or (−/−) mouse-derived cortical neurons. The mechanisms underlying such differences between rat and mouse cultures remain unknown, although the effects in rat cultures are quite small in magnitude. Nevertheless, the results from both rat and mouse cultures support MPEP-mediated neuroprotection via mechanisms other than through the mGluR5 receptor.
Compared to MPEP, MTEP only shows slight neuroprotection against NMDA-induced toxicity at 100 μM in mouse neurons and at 200 μM in rat neurons (Table 1). Similar to our previous studies using MPEP (O'Leary et al., 2000; Movsesyan et al., 2001a), here MTEP doses showing neuroprotection also directly inhibit NMDA-mediated current in rat neurons. In contrast, the neuroprotective effects of MTEP in mouse-derived cultures (Table 1) do not appear to be due to direct antagonist effects on NMDAR activity (Figure 6).
Table 1.
Summary of effects of MPEP and MTEP
| MPEP | MTEP | |
|---|---|---|
| Drug | 2-Methyl-6-(phenylethynyl)-pyridine | 3-[(2-Methyl-1, 3-thiazol-4-yl)ethynyl]pyridine |
| Receptor functional activity | mGluR5 (0.2–200 μM block of mGluR5 agonist (CHPG)–antagonist-induced PI hydrolysis) | mGluR5 (0.2–200 μM block of mGluR5 agonist (CHPG)–antagonist-induced PI hydrolysis) |
| In vitro neuroprotection | Lowest neuroprotective dose (μM) | Lowest neuroprotective dose (μM) |
| Glutamate (150 μM)-induced excitotoxicity | ||
| Rat cortical neurons | 20 | 200 |
| NMDA (150 μM)-induced excitotoxicity | ||
| Rat cortical neurons | 20 | 200 |
| Mouse cortical neurons | 20 | 100 |
| mGluR5 (−/−) cortical neurons | 20 | 100 |
| Etoposide(50 μM)-induced apoptosis | ||
| Rat cortical neurons | No effect (2–200 μM) | No effect (2–200 μM) |
Through the use of cortical neuronal cultures derived from rat and mGluR5 (+/+) and (−/−) mice, we demonstrate that off target effects, in part, underlie both MPEP- and MTEP-mediated neuroprotection against NMDA toxicity. Despite differences between rat and mouse culture responses, our findings are consistent with studies demonstrating fewer off target MTEP-mediated effects, as compared to MPEP, such as minimal inhibition of NMDA/glycine-evoked increases in recombinant human NR1A/2B receptor-mediated intracellular calcium (MTEP: 19% at 300 μM; MPEP: IC50=18 μM) (Cosford et al., 2003a, 2003b). Collectively, our findings indicate that blocking neuronal mGluR5 (i.e. without confounding effects of mGluR5-expressing glia) (Lea et al., 2002; 2003a; Lea & Faden, 2003b) is not protective against glutamate receptor-mediated cell death, and that use of high-dose concentrations of these drugs can lead to neuroprotection through mechanisms not associated with mGluR5 modulation.
Acknowledgments
We thank Merck Research Laboratories (Rahway, NJ, U.S.A.) for kindly providing MTEP for this study. We also thank Ms Elvira Dabaghyan and Ms Lioudmila Zoubak for excellent technical assistance in preparation of cell cultures and cell viability assays. This study was supported by an NIH Grant R01NS37313 and a cooperative research agreement Department of Defense Grant (DAMD17-99-2-9007).
Abbreviations
- CHPG
(RS)-2-chloro-5-hydroxyphenylglycine
- DIV
day in vitro
- IP
inositol phosphates
- mGluRs
metabotropic glutamate receptors
- MK801
(5R,10S)-(+)5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine
- NMDA
N-methyl-D-aspartate
- NSM
neuronal seeding medium
- PI
phosphoinositide
- SIB-1893
(E)-2-methyl-6-(2-phenylethenyl)-pyridine
References
- AGRAWAL S.K., THERIAULT E., FEHLINGS M.G. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J. Neurotrauma. 1998;15:929–941. doi: 10.1089/neu.1998.15.929. [DOI] [PubMed] [Google Scholar]
- ALLEN J.W., ELDADAH B.A., FADEN A.I. Beta-amyloid-induced apoptosis of cerebellar granule cells and cortical neurons: exacerbation by selective inhibition of group I metabotropic glutamate receptors. Neuropharmacology. 1999;38:1243–1252. doi: 10.1016/s0028-3908(99)00044-1. [DOI] [PubMed] [Google Scholar]
- ALLEN J.W., KNOBLACH S.M., FADEN A.I. Activation of group I metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro. Cell Death Differ. 2000;7:470–476. doi: 10.1038/sj.cdd.4400678. [DOI] [PubMed] [Google Scholar]
- ALLEN J.W., VICINI S., FADEN A.I. Exacerbation of neuronal cell death by activation of group I metabotropic glutamate receptors: role of NMDA receptors and arachidonic acid release. Exp. Neurol. 2001;169:449–460. doi: 10.1006/exnr.2001.7672. [DOI] [PubMed] [Google Scholar]
- ANDERSON J.J., BRADBURY M.J., GIRACELLO D.R., CHAPMAN D.F., HOLTZ G., ROPPE J., KING C., COSFORD N.D., VARNEY M.A. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur. J. Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- ANDERSON J.J., RAO S.P., ROWE B., GIRACELLO D.R., HOLTZ G., CHAPMAN D.F., TEHRANI L., BRADBURY M.J., COSFORD N.D., VARNEY M.A. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J. Pharmacol. Exp. Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- ANWYL R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- BAO W.L., WILLIAMS A.J., FADEN A.I., TORTELLA F.C. Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res. 2001;922:173–179. doi: 10.1016/s0006-8993(01)03062-1. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., DOWNES C.P., HANLEY M.R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem. J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODKIN J., BRADBURY M., BUSSE C., WARREN N., BRISTOW L.J., VARNEY M.A. Reduced stress-induced hyperthermia in mGluR5 knockout mice. Eur. J. Neurosci. 2002;16:2241–2244. doi: 10.1046/j.1460-9568.2002.02294.x. [DOI] [PubMed] [Google Scholar]
- BRUNO V., BATTAGLIA G., COPANI A., CASABONA G., STORTO M., DI GIORGI GEREVINI V., NGOMBA R., NICOLETTI F. Metabotropic glutamate receptors and neurodegeneration. Prog. Brain Res. 1998;116:209–221. doi: 10.1016/s0079-6123(08)60439-2. [DOI] [PubMed] [Google Scholar]
- BRUNO V., BATTAGLIA G., KINGSTON A., O'NEILL M.J., CATANIA M.V., DI GREZIA R., NICOLETTI F. Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist, (+)-2-methyl-4 carboxyphenylglycine ( LY367385): comparison with LY357366, a broader spectrum antagonist with equal affinity for mGlu1a and mGlu5 receptors. Neuropharmacology. 1999;38:199–207. doi: 10.1016/s0028-3908(98)00159-2. [DOI] [PubMed] [Google Scholar]
- BRUNO V., COPANI A., KNOPFEL T., KUHN R., CASABONA G., DELL'ALBANI P., CONDORELLI D.F., NICOLETTI F. Activation of metabotropic glutamate receptors coupled to inositol phospholipid hydrolysis amplifies NMDA-induced neuronal degeneration in cultured cortical cells. Neuropharmacology. 1995;34:1089–1098. doi: 10.1016/0028-3908(95)00077-j. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- COPANI A., BRUNO V., BATTAGLIA G., LEANZA G., PELLITTERI R., RUSSO A., STANZANI S., NICOLETTI F. Activation of metabotropic glutamate receptors protects cultured neurons against apoptosis induced by beta-amyloid peptide. Mol. Pharmacol. 1995;47:890–897. [PubMed] [Google Scholar]
- COSFORD N.D., ROPPE J., TEHRANI L., SCHWEIGER E.J., SEIDERS T.J., CHAUDARY A., RAO S., VARNEY M.A. [3H]-methoxymethyl-MTEP and [3H]-methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg. Med. Chem. Lett. 2003a;13:351–354. doi: 10.1016/s0960-894x(02)00997-6. [DOI] [PubMed] [Google Scholar]
- COSFORD N.D., TEHRANI L., ROPPE J., SCHWEIGER E., SMITH N.D., ANDERSON J., BRISTOW L., BRODKIN J., JIANG X., MCDONALD I., RAO S., WASHBURN M., VARNEY M.A. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003b;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- FADEN A.I., O'LEARY D.M., FAN L., BAO W., MULLINS P.G., MOVSESYAN V.A. Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improves outcome after brain trauma. Exp. Neurol. 2001;167:435–444. doi: 10.1006/exnr.2000.7577. [DOI] [PubMed] [Google Scholar]
- GASPARINI F., LINGENHOHL K., STOEHR N., FLOR P.J., HEINRICH M., VRANESIC I., BIOLLAZ M., ALLGEIER H., HECKENDORN R., URWYLER S., VARNEY M.A., JOHNSON E.C., HESS S.D., RAO S.P., SACAAN A.I., SANTORI E.M., VELICELEBI G., KUHN R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- LEA P.M., IV, CUSTER S.J., VICINI S., FADEN A.I. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol. Biochem. Behav. 2002;73:287–298. doi: 10.1016/s0091-3057(02)00825-0. [DOI] [PubMed] [Google Scholar]
- LEA P.M., IV, CUSTER S.J., STOICA B.A., FADEN A.I. Modulation of stretch-induced enhancement of neuronal NMDA receptor current by mGluR1 depends upon presence of glia. J. Neurotrauma. 2003a;20:1233–1249. doi: 10.1089/089771503770802907. [DOI] [PubMed] [Google Scholar]
- LEA P.M., IV, FADEN A.I. Modulation of metabotropic glutamate receptors as potential treatment for acute and chronic neurodegenerative disorders. Drug News Perspect. 2003b;16:513–522. doi: 10.1358/dnp.2003.16.8.829350. [DOI] [PubMed] [Google Scholar]
- LYETH B.G., GONG Q.Z., SHIELDS S., MUIZELAAR J.P., BERMAN R.F. Group i metabotropic glutamate antagonist reduces acute neuronal degeneration and behavioral deficits after traumatic brain injury in rats. Exp. Neurol. 2001;169:191–199. doi: 10.1006/exnr.2001.7643. [DOI] [PubMed] [Google Scholar]
- MELI E., PICCA R., ATTUCCI S., COZZI A., PERUGINELLI F., MORONI F., PELLEGRINI-GIAMPIETRO D.E. Activation of mGlu1 but not mGlu5 metabotropic glutamate receptors contributes to postischemic neuronal injury in vitro and in vivo. Pharmacol. Biochem. Behav. 2002;73:439–446. doi: 10.1016/s0091-3057(02)00834-1. [DOI] [PubMed] [Google Scholar]
- MILLS C.D., JOHNSON K.M., HULSEBOSCH C.E. Group I metabotropic glutamate receptors in spinal cord injury: roles in neuroprotection and the development of chronic central pain. J. Neurotrauma. 2002;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- MOLDRICH R.X., GIARDINA S.F., BEART P.M. Group II mGlu receptor agonists fail to protect against various neurotoxic insults induced in murine cortical, striatal and cerebellar granular pure neuronal cultures. Neuropharmacology. 2001;41:19–31. doi: 10.1016/s0028-3908(01)00045-4. [DOI] [PubMed] [Google Scholar]
- MOVSESYAN V.A., O'LEARY D.M., FAN L., BAO W., MULLINS P.G., KNOBLACH S.M., FADEN A.I. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl- 6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 2001a;296:41–47. [PubMed] [Google Scholar]
- MOVSESYAN V.A., YAKOVLEV A.G., FAN L., FADEN A.I. Effect of serine protease inhibitors on posttraumatic brain injury and neuronal apoptosis. Exp. Neurol. 2001b;167:366–375. doi: 10.1006/exnr.2000.7567. [DOI] [PubMed] [Google Scholar]
- MUKHIN A., FAN L., FADEN A.I. Activation of metabotropic glutamate receptor subtype mGluR1 contributes to post-traumatic neuronal injury. J. Neurosci. 1996;16:6012–6020. doi: 10.1523/JNEUROSCI.16-19-06012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'LEARY D.M., MOVSESYAN V., VICINI S., FADEN A.I. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br. J. Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEPP D.D., JANE D.E., MONN J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- SINENSKY M.C., LEISER A.L., BABICH H. Oxidative stress aspects of the cytotoxicity of carbamide peroxide: in vitro studies. Toxicol. Lett. 1995;75:101–109. doi: 10.1016/0378-4274(94)03168-7. [DOI] [PubMed] [Google Scholar]
- STRASSER U., LOBNER D., BEHRENS M.M., CANZONIERO L.M., CHOI D.W. Antagonists for group I mGluRs attenuate excitotoxic neuronal death in cortical cultures. Eur. J. Neurosci. 1998;10:2848–2855. doi: 10.1111/j.1460-9568.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- VARNEY M.A., COSFORD N.D., JACHEC C., RAO S.P., SACAAN A., LIN F.F., BLEICHER L., SANTORI E.M., FLOR P.J., ALLGEIER H., GASPARINI F., KUHN R., HESS S.D., VELICELEBI G., JOHNSON E.C. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5. J. Pharmacol. Exp. Ther. 1999;290:170–181. [PubMed] [Google Scholar]
- YAKOVLEV A.G., DI X., MOVSESYAN V., MULLINS P.G., WANG G., BOULARES H., ZHANG J., XU M., FADEN A.I. Presence of DNA fragmentation and lack of neuroprotective effect in DFF45 knockout mice subjected to traumatic brain injury. Mol. Med. 2001a;7:205–216. [PMC free article] [PubMed] [Google Scholar]
- YAKOVLEV A.G., OTA K., WANG G., MOVSESYAN V., BAO W.L., YOSHIHARA K., FADEN A.I. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J. Neurosci. 2001b;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]