Abstract
We investigated ethanol inhibition of the rat P2X4 receptor and the contribution of the three histidine residues in the extracellular loop of this receptor to ethanol inhibition of receptor function, using site-directed mutagenesis and electrophysiological characterization of recombinant receptors.
In the wild-type receptor, 50, 200 and 500 mM ethanol increasingly shifted the ATP concentration–response curve to the right in a parallel manner, increasing the EC50 value without affecting Emax. However, 750 or 900 mM ethanol did not produce a further increase in the EC50 value of the ATP concentration–response curve, suggesting that this inhibition is not competitive.
The P2X4 receptor mutations H140A and H286A did not significantly alter ethanol inhibition of ATP-activated current. By contrast, the mutation H241A changed the mechanism by which ethanol inhibits receptor function; viz., ethanol inhibition was not associated with an increased EC50 value of the ATP concentration–response curve, instead, ethanol decreased the maximal response to ATP without affecting the EC50 value of the ATP concentration–response curve.
Ethanol inhibition of the H241A mutant was voltage independent between −60 and +20 mV and ethanol did not alter the reversal potential of ATP-activated current. In addition, ethanol decreased the desensitization rate of the H241A-mediated current.
The purinoceptor antagonists, suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), did not alter the magnitude of ethanol inhibition of ATP-activated current in the H241A mutant.
The results suggest that ethanol inhibits the wild-type rat P2X4 receptor by an allosteric action to increase the EC50 value of the ATP concentration–response curve, the P2X4 receptor mutation H241A alters the mechanism by which ethanol inhibits P2X4 receptor function, and ethanol and PPADS or suramin appear to inhibit H241A-mutated receptors at independent sites.
Keywords: P2X, P2X4 receptor, histidine, mutation, ethanol, competitive inhibition, noncompetitive inhibition, suramin, PPADS
Introduction
Extracellular ATP is thought to be an important excitatory mediator in the nervous system. ATP mediates excitatory synaptic transmission in tissue culture of guinea-pig autonomic ganglion neurons (Evans et al., 1992; Silinsky et al., 1992), in rat spinal cord (Bardoni et al., 1997), and in hippocampus (Pankratov et al., 1998), medial habenula (Edwards et al., 1992), locus coeruleus (Nieber et al., 1997) and neocortex (Pankratov et al., 2002) of rat brain. ATP also enhances the release of glutamate from primary sensory afferents in the spinal cord of hamster (Li & Perl, 1995), cultured rat sensory neuron synapses (Gu & MacDermott, 1997), rat spinal cord dorsal horn neurons (Nakatsuka & Gu, 2001) and rat brain stem (Khakh & Henderson, 1998); γ-amino-n-butyric acid (GABA) from cultured rat spinal cord dorsal horn neurons (Hugel & Schlichter, 2000); noradrenaline from cultured rat sympathetic neurons (Boehm, 1999); and acetylcholine from rat motor nerve terminals (Salgado et al., 2000). In addition, a number of neuronal types are excited by the extracellular application of ATP, including sensory, sympathetic and parasympathetic neurons, as well as a number of central neurons (Ralevic & Burnstock, 1998; North, 2002). These actions of extracellular ATP in neurons result from its activation of P2X receptors, a class of ligand-gated membrane ion channels (Ralevic & Burnstock, 1998; North, 2002).
To date, seven P2X receptor subunits, designated P2X1–P2X7, have been identified (Ralevic & Burnstock, 1998; North, 2002). These subunits have been found to be widely distributed in the central nervous system, including cerebral cortex, hippocampus, thalamus, hypothalamus, midbrain, cerebellum and spinal cord, and in sensory and autonomic ganglia in the peripheral nervous system (Collo et al., 1996; Ralevic & Burnstock, 1998; North, 2002). All of these subunits are thought to consist of two transmembrane domains, a large extracellular loop, and intracellular amino- and carboxy-terminals (Ralevic & Burnstock, 1998; North, 2002). When expressed in Xenopus oocytes or cell lines, each of these subunits can form homomeric ATP-gated cation channels, except for P2X6, which shows a very low expression efficacy (Collo et al., 1996; Ralevic & Burnstock, 1998; North, 2002). Recent studies have revealed a number of amino-acid residues that are involved in regulating receptor function such as ATP binding, agonist-induced channel gating, deactivation, desensitization, resensitization, antagonist sensitivity, and modulation of receptor function by pH and trace metals (Ralevic & Burnstock, 1998; North, 2002; Coddou et al., 2003; Li et al., 2004; Xiong et al., 2004a, 2004b; Zemkova et al., 2004). For example, mutation of histidines 140, 241 and 286, in the extracellular loop, to alanines (H140A, H241A and H286A) in the rat P2X4 receptor produces slight to moderate changes in the EC50 value of the ATP concentration–response curves (Coddou et al., 2003; Xiong et al., 2004a, 2004b), and the H241A mutation facilitates opening of the receptor-channel (Xiong et al., 2004a). In addition, histidine mutation H241A in the rat P2X4 receptor also produces receptors that are sensitive to the purinoceptor antagonists, suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (Xiong et al., 2004b).
Previous studies have revealed that P2X receptors in bullfrog dorsal root ganglion (DRG) neurons (Li et al., 1993, 1998; Weight et al., 1999) and freshly isolated adult rat hippocampal CA1 neurons (Li et al., 2000), as well as recombinant rat P2X4 receptors expressed in Xenopus oocytes (Xiong et al., 2000; Davies et al., 2002), are inhibited by pharmacological concentrations of ethanol. In all of these cases, ethanol inhibits the function of the P2X receptors by shifting the ATP concentration–response curve to the right in a parallel manner, increasing the EC50 value without affecting Emax of the ATP concentration–response curve. In bullfrog DRG neurons, ethanol has been found to inhibit the P2X receptor function by an allosteric action to decrease apparent agonist affinity rather than by a competitive mechanism (Li et al., 1998). Whether there is a common mechanism of ethanol inhibition among P2X receptors has not been determined. In the present study, we investigated ethanol inhibition of rat P2X4 receptors and the possibility that the three histidine residues in the extracellular loop of these receptor may be involved in ethanol inhibition of receptor function. These studies used site-directed mutagenesis and electrophysiological characterization of recombinant rat P2X4 receptors expressed in Xenopus oocytes and in human embryonic kidney (HEK) 293 cells.
Methods
DNA site-directed mutagenesis
A P2X4 receptor cDNA clone from rat superior cervical ganglion (SCG) was kindly provided by Dr Gary Buell (Serono Pharmaceutical Research Institute, Geneva, Switzerland). Site-directed mutagenesis of H140, H241 and H286 in the rat P2X4 cDNA was performed using the Quikchange kit (Stratagene, Inc., La Jolla, CA, U.S.A.) and the following sets of primers were used to alter each histidine to alanine: H140 forward 5′ GGC TCC GTG GAC ACC GCA AGC AGT GGA GTT G 3′ and reverse 5′ CAA CTC CAC TGC TTG CGG TGT CCA CGG AGC C 3′; H241 forward 5′ GTG GGG GAC GCG GGA GCA AGC TTC CAG GAG ATG G 3′ and reverse 5′ CCA TCT CCT GGA AGC TTG CTC CCG CGT CCC CCA C 3′; and H286 forward 5′ GGA CAC CCG GGA CCT GGA AGC AAA TGT GTC TCC TGG CTA C 3′ and reverse 5′ GTA GCC AGG AGA CAC ATT TGC TTC CAG GTC CCG GGT GTC C 3′. The underlined nucleotides indicate the codon for alanine. In all, 16 cycles of amplification by polymerase chain reaction catalysed by PFU DNA polymerase were performed with the following temperature protocol: strand separation 98°C, primer annealing 50°C and primer extension 68°C for 20 min. Next, the parental template was digested with DpnI, the mutated P2X4 receptor construct transformed into competent host cells, and the transformed cells plated on ampicillin-containing agar plates. Individual clones were grown in the Luria–Bertani medium; DNA was isolated and then sequenced to verify each mutation. Each mutant is referred to by the original amino acid (one letter code) followed by the residue number and the substituted amino acid (one letter code).
The care and use of animals in this study was approved by the Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism in accord with National Institutes of Health guidelines.
Expression of receptors in Xenopus oocytes and two-electrode voltage-clamp recording
The procedures used for the preparation of cRNA and the expression of receptors in Xenopus oocytes have been described previously (Xiong et al., 2000). Oocytes were recorded 2–5 days after RNA injection at room temperature using a Geneclamp Amplifier (Axon Instruments Inc., Foster City, CA, U.S.A.). Oocytes were placed in a recording chamber and impaled with two sharp microelectrodes filled with 3 M KCl (tip resistances: 0.5–1.5 MΩ). The oocytes were constantly superfused at the rate of ∼2.5 ml min−1 with modified Ringer solution containing (in mM): 96 NaCl, 2 KCl, 1.8 BaCl2, 1 MgCl2, 5 HEPES (pH 7.4; Ba2+ replaced Ca2+ to prevent the activation of the endogenous calcium-activated chloride current in these cells). Agonist and other chemical solutions were prepared in the bathing solution. Solutions of ATP, added as the Na+ salt, were prepared daily. The pH of solutions was readjusted after the addition of ATP and/or other chemical substances, as necessary. Solutions were delivered by gravity flow from a 0.5 mm silica tube connected to a 7-barrel manifold. Solutions were changed via solenoid valves; at least 4 min was allowed to elapse between agonist applications. The oocytes were voltage-clamped at −70 mV, except as indicated. Currents were recorded on a pen recorder (Model RS3400, Gould Inc., Valley View, OH, U.S.A.).
Expression of receptors in HEK 293 cells and whole-cell patch-clamp recording
HEK 293 cells (American Type Culture Collection, Manassas, VA, U.S.A.) were grown in minimal essential medium (Invitrogen, Carlsbad, CA, U.S.A.) supplemented with 10% horse serum (Hyclone, Ogden, UT, U.S.A.). The cells were transfected transiently with wild-type or mutant receptor cDNA according to the protocol in the Lipofectamine 2000 reagent kit (Invitrogen). To distinguish between transfected and untransfected cells, a green fluorescent protein-plasmid (pGreen Lantern, Invitrogen) was coexpressed with the P2X4 receptor. Successfully transfected cells were detected by exposing the cells to ultraviolet light and green fluorescent cells were studied.
Transfected cells were recorded 1–3 days after transfection in the whole-cell configuration (Hamill et al., 1981) at room temperature using an Axopatch 200B amplifier (Axon Instruments). The cells were continuously superfused with a solution containing (in mM): 140 NaCl, 5 KCl, 1.8 CaCl2, 1.2 MgCl2, 5 glucose, and 10 HEPES (pH 7.4 with NaOH, ∼340 mmol kg−1 with sucrose). Pipettes were pulled from borosilicate glass (TW-150F, World Precision Instruments, Sarasota, FL, U.S.A.) using a two-stage puller (Flaming-Brown P-87; Sutter Instruments, Novato, CA, U.S.A.) and had resistances of ∼5 MΩ when filled with pipette solution containing (in mM): 140 CsCl, 2 MgCl2, 10 EGTA, 10 HEPES (pH 7.2 with CsOH, ∼315 mmol kg−1 with sucrose). Cells were held at −60 mV. Data were acquired using pClamp8.0 software (Axon Instruments). Currents were filtered at 2 kHz and digitized at 10 kHz. Agonist and/or other chemical solutions were applied with a piezoelectric device (PZ-150 M; Burleigh Instruments, Fishers, NY, U.S.A.) through two-barrel theta glass tubing (TGC150, Warner Instruments, Hamden, CT, U.S.A.) that had been pulled to a tip diameter of ∼200 μm. The piezoelectric device was driven by TTL pulses from pClamp 8.0 software. Voltage applied to the piezoelectric device produced a rapid lateral displacement (∼50 μm) of the theta tubing to move the interface between test solutions. Solution exchange rate was estimated by detecting the junctional potential change of an open pipette tip induced by a 10-fold dilution of the external solution; the solution exchange time-constant ranged from 0.3 to 0.6 ms.
Drugs and chemicals
The drugs and chemicals used in these experiments were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, U.S.A.), except for the salts which were purchased from Mallinckrodt, Inc. (Paris, KY, U.S.A.).
Data analysis
Data were statistically compared using Student's t-test or analysis of variance (ANOVA), as noted. Statistical analysis of concentration–response data was performed using the nonlinear curve-fitting program ALLFIT (DeLean et al., 1978), which uses an ANOVA procedure. Values reported for concentrations yielding 50% of the maximal effect (EC50) or 50% inhibition (IC50) and the slope factor (n) are those obtained by fitting the data to the Hill equation of the following form:
where X and Y are concentration and response, respectively, and Emax is the maximal response.
Current amplitudes reported are peak values. Average values are expressed as mean±s.e.m. with n equal to the number of cells studied. The desensitization rate of ATP-activated current in whole-cell patch-clamp experiments was determined by fitting with Levenberg-Marquardt algorithms with the program Origin 6.0 (Microcal Software, Northampton, MA, U.S.A.).
Results
Ethanol inhibition of rat P2X4 receptors
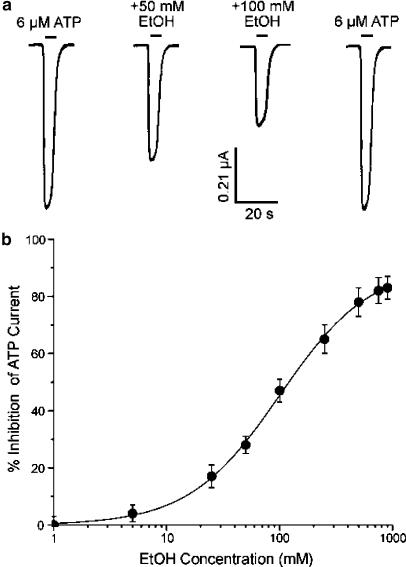
It has previously been reported that pharmacological concentrations of ethanol can inhibit ATP-activated current in Xenopus oocytes expressing rat P2X4 receptors (Xiong et al., 2000; Davies et al., 2002). Figure 1a shows that the amplitude of current activated by 6 μM ATP (producing 40% of the maximal effect) was markedly and reversibly decreased by 50 and 100 mM ethanol. On average, 50 and 100 mM ethanol decreased the amplitude of current activated by 6 μM ATP by 28±3% (n=9) and 47±4% (n=14), respectively. From the ethanol concentration–response curve in Figure 1b, the ethanol concentration that produced 50% inhibition (IC50), the slope factor (n) for ethanol inhibition of ATP-activated current and the maximal effect (Emax) are given in Table 1. Over the concentration range 1–900 mM, the application of ethanol alone did not activate detectable current in Xenopus oocytes expressing rat P2X4 receptors (n=9, data not shown). In addition, in uninjected oocytes, ATP at concentrations up to 500 μM did not evoke detectable current (n=7, data not shown).
Figure 1.
Effect of ethanol on ATP-activated current in wild-type rat P2X4 receptors expressed in Xenopus oocytes. (a) Current activated by the application of 6 μM ATP (producing 40% of the maximal effect) before, during and after the application of 50 and 100 mM ethanol (EtOH). Records are sequential current traces (from left to right) obtained from a single oocyte. Solid bar above each record indicates time of agonist application in the absence and presence of EtOH. (b) Graph plotting the average percentage inhibition of the amplitude of current activated by 6 μM ATP as a function of EtOH concentration. Each data point is the average of 4–14 cells; error bars indicate s.e.m. The curve shown is the best fit of the data to the equation in the Methods. Values obtained were: IC50=98.7±5.2 mM; n=1.1±0.1; Emax=93±7%.
Table 1.
Summary of data on the effect of histidine mutation on ethanol inhibition of ATP-activated current in rat P2X4 receptors expressed in Xenopus oocytes
| Parameter | Wild-type | H140A | H241A | H286A |
|---|---|---|---|---|
| EtOH IC50 (mM)a | 98.7±5.2 | 88±10.5 | 29.6±4.6** | 103.6±9.3 |
| EtOH slope factor | 1.1±0.1 | 1.1±0.2 | 1.0±0.1 | 1.1±0.2 |
| EtOH Emax | 93±7% | 93±6.7% | 100% | 95±6.1% |
| ATP EC50 (μM) | 8.7±0.9 | 14±1.8 | 0.6±1.6 | 15.4±2.1 |
| ATP EC50 with EtOH (μM)b | 18.9±0.7## | 23.9±3.4## | 0.67±0.12 | 28.4±3.5## |
| ATP slope factor | 1.1±0.1 | 1.1±0.1 | 1.1±0.2 | 1.2±0.2 |
| ATP slope factor with EtOHb | 1.1±0.2 | 1.2±0.1 | 1.2±0.1 | 1.3±0.2 |
| ATP Emaxc | 1.0 | 1.0 | 1.0 | 1.0 |
| ATP Emax with EtOHb | 1.0±0.04 | 1.0±0.05 | 0.33±0.03** | 1.0±0.05 |
An ATP concentration that was close to the EC40 value of each ATP concentration–response curve was used for the wild-type and each mutant receptor (6, 9, 0.6 and 10.2 μM for wild-type, H140A, H241A and H286A, respectively).
100 mM EtOH was used.
Corresponds to the current activated by 300 μM ATP.
P<0.01 vs wild-type and
P<0.01 vs ATP EC50 without EtOH in the same receptor. Values are expressed as mean peak current±s.e.m. (n=4–11 oocytes).
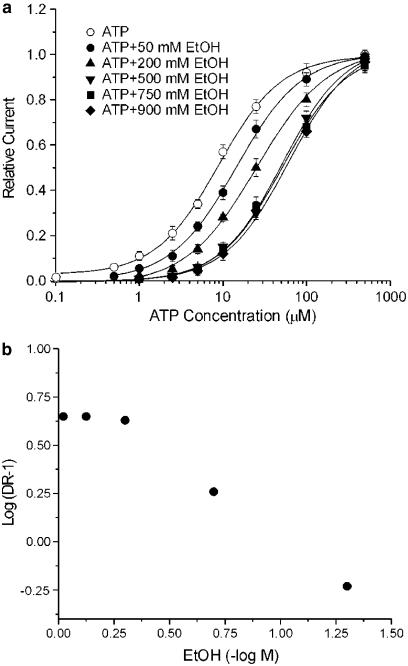
Ethanol has been reported to inhibit rat P2X4 receptor function by shifting the ATP concentration–response curve to the right in a parallel manner (Xiong et al., 2000). To assess further this rightward shift of the ATP concentration–response curve, we determined the effect of multiple concentrations of ethanol on the ATP concentration–response curve. We found that increasing the concentration of ethanol from 50 to 500 mM progressively shifted the ATP concentration–response curve to the right, increasing the EC50 value for ATP from 8.7±0.9 μM for 50 mM ethanol to 45.8±6.1 μM for 500 mM ethanol, without significantly changing the slope factor or Emax values of the curve (Figure 2a). The effect of ethanol on the EC50 value for ATP appeared to be maximal near 500 mM ethanol, as the EC50 values for ATP in the presence of 750 and 900 mM ethanol did not differ statistically from that in the presence of 500 mM ethanol (48.2±7.5 μM and 47.4±5.8 vs 45.8±6.1 μM, respectively; ANOVA, P>0.05). A Schild plot of the data (Figure 2b) showed a marked deviation from linearity, which precluded determination of a pA2 value for ethanol.
Figure 2.
Effect of various concentrations of ethanol on the ATP concentration–response curve in wild-type rat P2X4 receptors expressed in Xenopus oocytes. (a) Concentration–response curves for current activated by ATP in the absence and the presence of 50, 200, 500, 750 and 900 mM EtOH. Each data point is the average of 4–8 cells. The Emax, slope factor (n), and EC50 values (in μM) were, respectively: 1.0, 1.2±0.1 and 8.7±0.9 for the control curve; 1.0±0.04, 1.1±0.1 and 13.8±1.6 for 50 mM EtOH; 1.0±0.03, 1.0±0.1 and 24.4±3.0 for 200 mM EtOH; 1.0±0.04, 1.2±0.1 and 45.8±6.1 for 500 mM EtOH; 1.0±0.05, 1.1±0.1 and 48.2±7.5 for 750 mM EtOH; and 1.0±0.03, 1.2±0.1, and 47.4±5.8 for 900 mM EtOH. In this graph, the amplitude of currents activated by ATP in the presence of various concentrations of EtOH is plotted as fraction of the current activated by 500 μM ATP in the absence of EtOH; this was determined for each cell studied. Error bars not visible are smaller than the size of the symbols. (b) Graph plots dose ratio – 1 (DR-1) vs EtOH concentration for the data in (a). It was not possible to determine a pA2 value for EtOH due to the marked nonlinearity of the plot.
Effect of histidine mutation on ethanol inhibition
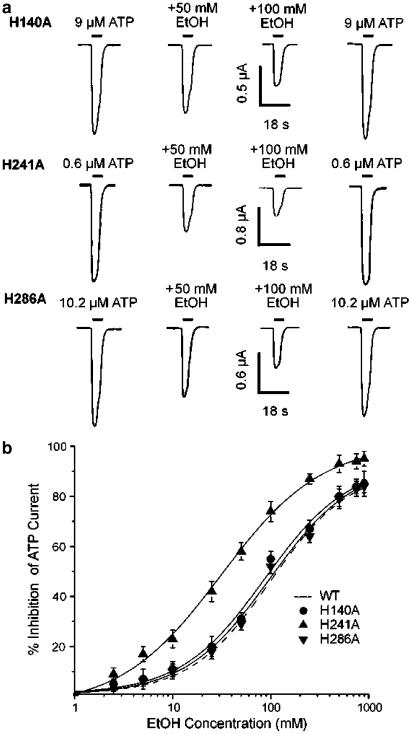
To investigate whether three histidine residues in the extracellular loop might be involved in the inhibition of P2X4 receptor function by ethanol, histidines 140, 241 and 286 were altered to alanines (H140A, H241A and H286A) and the effects of ethanol on these mutants were examined. Since ATP sensitivity was different in wild-type and mutated receptors and ethanol inhibition of ATP-activated current depends on ATP concentration (Xiong et al., 2000; 2004a), an ATP concentration that was close to the EC40 value of the ATP concentration–response curve was used in these experiments for the wild-type and each mutant receptor; the EC40 values were 6, 9, 0.6 and 10.2 μM for wild-type, H140A, H241A and H286A receptors, respectively. As shown in Figure 3a, the magnitude of inhibition of ATP-activated current by 50 and 100 mM ethanol in the H140A mutant appeared to be similar to the magnitude of inhibition of ATP-activated current in the H286A mutant. By contrast, 50 and 100 mM ethanol appeared to produce a greater inhibition of ATP-activated current in the H241A mutant. As shown in Figure 3b and Table 1, for the H140A and H286A mutants, the calculated values of IC50 for ethanol inhibition of ATP-activated current, the slope factor and the Emax are not significantly different from those in the wildtype receptor (ANOVA, P>0.05). However, for the H241A mutant, the IC50 value for ethanol inhibition of ATP-activated current is significantly lower than that of the wild-type receptor (Figure 3b, Table 1, ANOVA, P<0.01), whereas the slope factor and Emax values do not differ significantly from those of the wild-type receptor (P>0.05). Over the concentration range 1–900 mM, the application of ethanol alone did not activate detectable current in Xenopus oocytes expressing each of the three mutated rat P2X4 receptors (n=6 for each mutant, data not shown).
Figure 3.
Effect of histidine mutation on ethanol inhibition of ATP-activated current in rat P2X4 receptors expressed in Xenopus oocytes. (a) Records showing currents activated by 9, 0.6 and 10.2 μM ATP before, during and after the application of 50 and 100 mM EtOH in three oocytes expressing H140A, H241A or H286A mutated rat P2X4 receptors, as labelled. (b) Graph plotting average percentage inhibition of the amplitude of current activated by 9, 0.6 and 10.2 μM ATP as a function of EtOH concentration for H140A, H241A and H286A mutants. The dashed curve for the wild-type (WT) receptor is from Figure 1b and is shown for comparison. The magnitude of ethanol inhibition is calculated as percentage inhibition of ATP-activated current; this was determined for each cell studied. As ATP sensitivity was different in wild-type and mutated receptors, an ATP concentration that was close to the EC40 value of each ATP concentration–response curve was used for each mutant (9, 0.6 and 10.2 μM for H140A, H241A and H286A, respectively). Each data point is the average of 5–11 cells; error bars not visible are smaller than the size of the symbols. Values obtained were: IC50=88±10.5 mM; n=1.1±0.2, and Emax=93±6.7% for H140A; IC50=29.6±4.6 mM; n=1.0±0.1, and Emax=100% for H241A; IC50=103.6±9.3 mM; n=1.1±0.2, and Emax=95±6.1% for H286A.
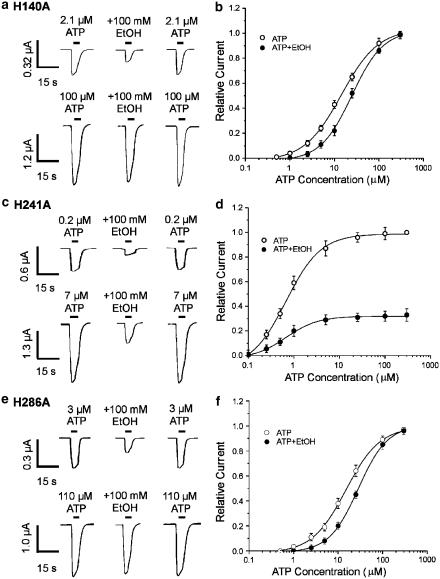
To investigate further the effect of histidine mutation on ethanol inhibition of rat P2X4 receptors, we examined whether the concentration of ATP affects ethanol inhibition in each of the three histidine-mutated receptors. Figures 4a, c and e illustrate the effect of two ATP concentrations that were close to the EC10 and EC90 values of the ATP concentration–response curve for each mutant (the respective EC10 and EC90 values were 2.1 and 100 μM for H140A, 0.2 and 7 μM for H241A, and 3 and 110 μM for H286A). As shown in Figure 4a and e, in both the H140A and H286A mutants, ethanol inhibition was overcome by increasing the ATP concentration to the EC90 value. In addition, for both the H140A and the H286A mutant, 100 mM ethanol shifted the ATP concentration–response curve to the right, significantly increasing the EC50 value for the ATP concentration–response curve (ANOVA, P<0.01), without significantly changing the slope (P>0.05) or the Emax value of ATP concentration–response curve (P>0.05) (Figure 4b, f, Table 1). By contrast, for the H241A mutant, ethanol inhibition was not overcome by increasing ATP concentration to the EC90 value (Figure 4c), and 100 mM ethanol reduced the Emax value of the ATP concentration–response curve by 67% (Figure 4d, Table 1), without significantly changing the slope (P>0.05) or the EC50 value of ATP concentration–response curve (Figure 4d, Table 1; P>0.05).
Figure 4.
Effect of ATP concentration on ethanol inhibition of ATP-activated current in the histidine-mutated rat P2X4 receptors expressed in Xenopus oocytes. (a) Records showing currents activated by 2.1 μM ATP (upper traces) and 100 μM ATP (lower traces), before, during and after the application of 100 mM EtOH in a single oocyte expressing the H140A mutant. (b) Graph plotting the amplitude of ATP-activated current in the presence of 100 mM EtOH relative to the amplitude of ATP-activated current in the absence of EtOH, as a function of ATP concentration. EtOH significantly increased the EC50 value for the ATP concentration–response curve from 14±1.8 μM in the absence of EtOH to 23.9±3.4 μM in the presence of 100 mM EtOH (ANOVA, P<0.01). (c) Records showing currents activated by 0.2 μM ATP (upper traces) and 7 μM ATP (lower traces), before, during and after the application of 100 mM EtOH in a single oocyte expressing the H241A mutant. (d) Graph plotting the amplitude of ATP-activated current in the presence of 100 mM EtOH relative to the amplitude of ATP-activated current in the absence of EtOH, as a function of ATP concentration. EtOH significantly decreased Emax of the ATP concentration–response curve by 67±1.1% (ANOVA, P<0.01), but did not significantly alter the EC50 value of the ATP concentration–response curve (P>0.05). (e) Records showing currents activated by 3 μM ATP (upper traces) and 110 μM ATP (lower traces), before, during and after the application of 100 mM EtOH in a single oocyte expressing the H286A mutant. (f) Graph plotting the amplitude of ATP-activated current in the presence of 100 mM EtOH relative to the amplitude of ATP-activated current in the absence of EtOH, as a function of ATP concentration. EtOH significantly increased the EC50 value for the ATP concentration–response curve from 15.4±2.1 μM in the absence of EtOH to 28.4±3.5 μM in the presence of 100 mM EtOH (ANOVA, P<0.01). In (b), (d) and (f), the amplitude of current activated by various concentrations of ATP in the absence and presence of EtOH is plotted as fraction of the current activated by 300 μM ATP in the absence of EtOH; this was determined for each cell studied. Each data point is the average current from 4 to 8 cells. As ATP sensitivity was different for each mutated receptor, ATP concentrations that were close to the EC10 and EC90 values of each ATP concentration–response curve were used in (a), (c) and (e) (2.1 and 100 μM for H140A, 0.2 and 7 μM for H241A, 3 and 110 μM for H284A, respectively).
Effect of membrane voltage on ethanol inhibition of ATP-activated current in H241A receptors
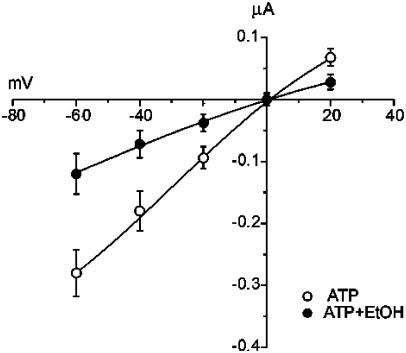
To examine whether the inhibition of ATP-gated channels by ethanol is voltage-dependent for the H241A mutant, the amplitude of current activated by 6 μM ATP was measured over a range of holding potentials in the absence and presence of 50 mM ethanol. As illustrated in Figure 5, ethanol did not change the reversal potential of ATP-activated current (0±3 mV in the absence vs −1±2 mV in the presence of 50 mM ethanol; Student's t-test, P>0.05, n=5).
Figure 5.
Effect of membrane potential on ethanol inhibition of ATP-activated current for the H241A mutant of rat P2X4 receptors expressed in Xenopus oocytes. Current–voltage relationship (I–V plot) showing the average amplitude of current activated by 6 μM ATP as a function of membrane potential, in the absence and presence of 50 mM EtOH (n=5). Note that EtOH did not alter the reversal potential of ATP-activated current (0±3 mV in the absence vs −1±2 mV in the presence of 50 mM ethanol; Student's t-test, P>0.05, n=5). The percentage inhibition of ATP-activated current by 50 mM EtOH was also not significantly different at the holding potentials shown (ANOVA, P>0.05, n=5).
In addition, the average inhibition of ATP-activated current by 50 mM ethanol did not differ significantly at membrane holding potentials between −60 and +20 mV (59±3, 58.2±3.5, 59.5±4.5 and 59±4% inhibition at −60, −40 −20 and +20 mV, respectively; ANOVA, P>0.05, n=5).
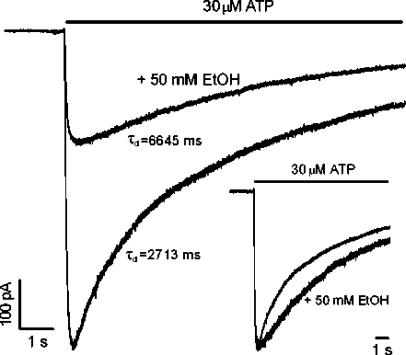
Effect of ethanol on receptor desensitization in H241A receptors
To assess whether ethanol inhibits ATP-activated current in the H241A mutant by enhancement of receptor desensitization, we studied the desensitization of current activated by ATP using a fast superfusion system, in the absence and presence of 50 mM ethanol in HEK 293 cells expressing the H241A mutant. In these experiments, 50 mM ethanol inhibited the current activated by 30 μM ATP by 59±9% (n=5); this value is similar to the percentage inhibition of ATP-activated current by 50 mM ethanol in Xenopus oocytes. In addition, the onset of ethanol inhibition was very fast and the recovery was complete without the presence of agonist. As shown in Figure 6, the rate of decay of current in the presence of 30 μM ATP was decreased, rather than increased, by the application of 50 mM ethanol. On average, the time-constants of desensitization of current activated by 30 μM ATP were increased significantly by 50 mM ethanol (7515±618 ms vs a control value of 2688±264 ms in the absence of ethanol; Student's t-test, P<0.01, n=5).
Figure 6.
Effect of ethanol on desensitization of ATP-activated current mediated by the H241A mutant of rat P2X4 receptors expressed in HEK 293 cells. Current traces illustrating the desensitization of current in the presence of 30 μM ATP in the absence and presence of 50 mM EtOH. The desensitization time-constant (τd) values are those obtained for the records shown. The inset shows the normalized currents. ATP was applied with a piezoelectric device (see Methods).
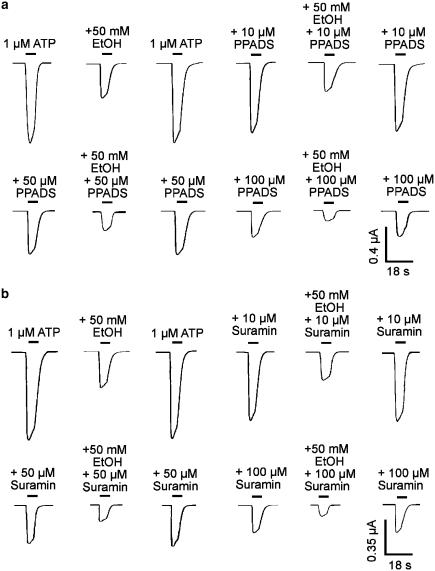
Effect of antagonists on ethanol inhibition of ATP-activated current in H241A receptors
To determine whether the antagonist action of PPADS or suramin interacts with inhibitory action of ethanol in the H241A mutant receptors, we investigated the action of ethanol in these receptors in the absence and presence of PPADS or suramin. Figure 7a shows currents activated by ATP in the absence and the presence of PPADS, and their inhibition by ethanol in an oocyte expressing the H241A mutant. PPADS, 10, 50 and 100 μM, inhibited ATP-activated current in a concentration–dependent manner, but had little apparent effect on ethanol inhibition. On average, 10, 50 and 100 μM PPADS inhibited ATP-activated current by 12±2, 49±5 and 69±4%, respectively, but did not significantly alter the percentage inhibition of ATP-activated current by 50 mM ethanol (57±5% in the absence of PPADS vs 56±4, 54±7 and 57±6% inhibition in the presence of 10, 50 and 100 μM PPADS; ANOVA, P>0.05, n=5–8). Similarly, 10, 50 and 100 μM suramin inhibited ATP-activated current in a concentration-dependent manner, but had little apparent effect on ethanol inhibition (Figure 7b). On average, 10, 50 and 100 μM suramin inhibited ATP-activated current by 19±3, 55±6 and 71±4%, respectively, but did not significantly alter the percentage inhibition of ATP-activated current by 50 mM ethanol (58±5% in the absence of suramin vs 57±6, 55±5 and 56±7% inhibition in the presence of 10, 50 and 100 μM suramin; ANOVA, P>0.05, n=5–7).
Figure 7.
Effect of purinoceptor antagonists on ethanol inhibition of ATP-activated current in the H241A mutant of rat P2X4 receptors expressed in Xenopus oocytes. (a) Records of current activated by 1 μM ATP and its inhibition by 50 mM EtOH in the absence and the presence of 10, 50 and 100 μM PPADS. All records are from the same cell. PPADS was preapplied for 6 min before the coapplication of ATP or ATP plus EtOH. (b) Records of current activated by 1 μM ATP and its inhibition by 50 mM EtOH in the absence and the presence of 10, 50 and 100 μM suramin. All records are from the same cell. Suramin was co-applied with ATP or ATP plus EtOH.
Discussion
In the P2X4 receptor cloned from rat SCG, there are three histidine residues located in the extracellular loop (Buell et al., 1996). It has been found that these three histidine residues are differentially involved in the regulation of rat P2X4 receptor function. Mutation of the rat P2X4 receptor histidine 241 to alanine (H241A) markedly decreased the EC50 value of the ATP concentration–response curve, whereas mutation of histidine 140 to alanine (H140A) or histidine 286 to alanine (H286A) slightly increased the EC50 value of the ATP concentration–response curve (Xiong et al., 2004a). In addition, the H241A mutation produced P2X4 receptors that were sensitive to the purinoceptor antagonists, suramin and PPADS. By contrast, the H140A or H286A mutation did not significantly affect the sensitivity of the P2X4 receptor to either suramin or PPADS (Xiong et al., 2004b). Moreover, H140A mutation, but not H241A or H286A mutation, diminished the inhibition of ATP-activated current by Cu2+ (Coddou et al., 2003). In the present study, we investigated the possible role of these histidine residues in the inhibition of P2X4 receptor function by ethanol.
Ethanol inhibition of wild-type P2X4 receptors
It has previously been reported that pharmacological concentrations of ethanol inhibit ATP-activated current in Xenopus oocytes expressing P2X4 receptors cloned from rat SCG by shifting the ATP concentration–response curve to the right in a parallel manner, increasing the EC50 value without affecting Emax (Xiong et al., 2000). Although this observation has the appearance of a competitive inhibition by ethanol at the agonist binding site, it could also result from an interaction of ethanol with an allosteric site on the receptor that results in a decrease in the apparent affinity of the receptor for ATP. The latter mechanism has been reported for GABAA receptor inhibition by inverse agonists at the benzodiazepine site (Kemp et al., 1987) and for inhibition of P2X receptors by Mg2+ (Li et al., 1997a), Zn2+ (Li et al., 1997b) and ethanol (Li et al., 1998). It should be noted that, in theory, for agonists with high intrinsic activity at ligand-gated membrane ion channels, changes in agonist efficacy might alter apparent agonist affinity (Stephenson, 1956; Colquhoun & Farrant, 1993). Such an action, however, seems unlikely in the present study because the available information indicates that ATP is not a particularly efficacious agonist at P2X receptors (Ding & Sachs, 1999) and P2X4 (Evans, 1996; Negulyaev & Markwardt, 2000). Theoretically, allosteric mechanisms of inhibition could be distinguished from a competitive mechanism of inhibition by the following approach. If ethanol acts competitively at the ATP binding site, increasing ethanol concentrations would be expected to continue to shift progressively the concentration–response curve to the right; whereas, if ethanol acts at an allosteric site, ethanol would be expected to cease to shift the concentration–response curve when its sites of action are saturated. Using this approach, we found in the present study that 50, 200 and 500 mM ethanol shifted the ATP concentration–response curve of the wild-type receptor to the right in a parallel manner. Increasing the concentration of ethanol to 750 or 900 mM, however, did not produce a further increase in the EC50 value of the ATP concentration–response curve, suggesting that the site of action of ethanol is saturated at concentrations >500 mM. A Schild analysis on these data (Figure 2b) showed a marked deviation from linearity, which precluded determination of a pA2 value for ethanol. Thus, despite the increases in the EC50 value produced by moderate to high concentrations of ethanol, ethanol appears to act in a manner that is noncompetitive with respect to the ATP concentration–response curve, perhaps by an allosteric action to decrease agonist affinity. This observation is consistent with a previous study in neurons in which ethanol produced a parallel rightward shift of the ATP concentration–response curve by a decrease in the affinity of the receptor for agonist via an action at an allosteric site (Li et al., 1998).
Effect of histidine mutation on ethanol inhibition
The present study also indicates that the histidine residues in the extracellular loop of the rat P2X4 receptor play different roles in the inhibition of this receptor by ethanol. For the H140A and H286A mutants, the IC50 values for ethanol inhibition of ATP-activated current were 88±10.5 and 103.6±9.3 mM, respectively; these values are not significantly different from the IC50 value of 98.7±5.2 mM for the wild-type receptor. As the ATP concentration used for H140A, H286A and wild-type receptors are all close to the EC40 value of the ATP concentration–response curve, this observation suggests that mutation of histidine 140 to alanine (H140A) or histidine 286 to alanine (H286A) did not significantly alter the ethanol sensitivity of the rat P2X4 receptor. In addition, 100 mM ethanol shifted the ATP concentration–response curve to the right in a parallel manner for both the H140A and the H286A mutants and the magnitudes of those shifts were similar to that of the wild-type receptor, suggesting that the mechanism by which ethanol inhibits the H140A and H286A mutants is not altered by these mutations. By contrast, for the H241A mutant, the IC50 value for ethanol inhibition of current activated by 0.6 μM ATP (an EC40 value) was 29.6±4.6 mM; this value is significantly lower than that of the wild-type receptor (98.7±5.2 mM). Furthermore, ethanol inhibition of the H241A mutant was not overcome by increasing ATP concentration. At a concentration of 100 mM, ethanol decreased the maximal response to ATP by 67% without significantly changing the EC50 value of the concentration–response curve, thus exhibiting a noncompetitive type inhibition.
Noncompetitive inhibition encompasses a range of mechanisms, including open-channel block (also referred to as uncompetitive inhibition) and enhancement of desensitization. Although a voltage-dependent ion channel block would not be expected because ethanol is not charged, ethanol might induce a conformational change in the channel protein that could result in voltage-dependence (Magleby & Stevens, 1972). Generally, evidence of agonist-dependence is required to establish a channel-block mechanism of inhibition using whole-cell recording. However, we did not expect to detect use-dependent inhibition by ethanol in the present study, because the affinity of ethanol for inhibition is very low compared to the affinity of ATP. An action of ethanol to increase receptor desensitization also seems unlikely in the present study, based upon the observation that ethanol decreased, rather than increased, the desensitization rate of ATP-activated current mediated by the H241A mutant. In addition, increasing receptor desensitization by elevating the ATP concentration did not alter the inhibitory effect of ethanol. Thus, ethanol appears to inhibit H241A receptor-channels by interacting with an allosteric site, although the identity of the site is, at present, not known. Interestingly, a similar type of noncompetitive mechanism has been reported for ethanol inhibition of NMDA receptor-channels in neurons (Wright et al., 1996; Peoples et al., 1997) and in recombinant NMDA receptors expressed in Xenopus oocytes (Masood et al., 1994). Although the allosteric site for ethanol action on NMDA receptors has not been identified (Peoples et al., 1997), single-channel recording has revealed that ethanol reduces the mean open channel lifetime and the frequency of opening of NMDA receptor-channels in neurons (Wright et al., 1996). Further work is needed to determine the precise mechanism by which ethanol inhibits H241A mutant rat P2X4 receptor-channels.
Role of the histidine 241 residue
Histidine 241, located in the extracellular loop of the rat P2X4 receptor, normally maintains a lower sensitivity to ATP, a relative insensitivity to the antagonists suramin and PPADS, and a mechanism of ethanol inhibition that involves a parallel shift to the right of the agonist concentration–response curve. Mutation of histidine 241 has been found to increase agonist sensitivity by altering channel gating rather than agonist binding (Xiong et al., 2004a). In a general model for ATP binding to P2X receptors, a region from amino acids 185–323 of the rat P2X4 receptor, which contains histidine 241, is predicted to have an alpha helix between two of the six beta pleated sheets (Freist et al., 1998). In view of this, it seems possible that mutation of histidine 241 may sufficiently alter the alpha helix that the change of receptor conformation results in altered agonist potency, a different mechanism of ethanol inhibition and a sensitivity to PPADS and suramin.
Functional implications
Of the known P2X receptors, the P2X4 subunit has been found to be the most abundant P2X receptor subunit in brain. Northern blot analysis and in situ hybridization studies have revealed extensive distribution of P2X4 mRNA in rat brain with particularly strong expression in the hippocampus, Purkinje cells of the cerebellum, and motoneurons of the spinal cord (Collo et al., 1996; Ralevic & Burnstock, 1998; North, 2002). It has been found that pharmacological concentrations of ethanol can inhibit ATP-activated current in freshly isolated adult rat hippocampal neurons (Li et al., 2000), suggesting that P2X receptors could be important effectors of ethanol action in the central nervous system. The present study suggests that ethanol inhibition of the rat P2X4 receptor may involve an allosteric mechanism. We have also identified a residue – histidine 241 – located in the extracellular loop of the rat P2X4 receptor that when mutated alters the mechanism by which ethanol inhibits receptor function. The results thus provide information about the mechanism of action of ethanol on a neurotransmitter-gated membrane ion channel and the influence of molecular structure on that action.
Acknowledgments
We thank Dr Gary Buell for providing the cDNA for the P2X4 subunit and Dr Robert W. Peoples for review of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- DRG
dorsal root ganglion
- EtOH
ethanol
- GABA
γ-amino-n-butyric acid
- HEK
human embryonic kidney
- NMDA
N-methyl-D-aspartate
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
- SCG
superior cervical ganglion
- s.e.m.
standard error of the mean
References
- BARDONI R., GOLDSTEIN P.A., LEE C.J., GU J.G., MACDERMOTT A.B. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHM S. ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J. Neurosci. 1999;19:737–746. doi: 10.1523/JNEUROSCI.19-02-00737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- CODDOU C., MORALES B., GONZÁLEZ J., GRAUSO M., GORDILLO F., BULL P., RASSENDREN F., HUIDOBRO-TORO J.P. Histidine 140 plays a key role in the inhibitory modulation of the P2X4 nucleotide receptor by copper but not zinc. J. Biol. Chem. 2003;278:36777–36785. doi: 10.1074/jbc.M305177200. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D., FARRANT M. The binding issue. Nature. 1993;366:510–511. doi: 10.1038/366510b0. [DOI] [PubMed] [Google Scholar]
- DAVIES D.L., MACHU T.K., GUO Y., ALKANA R.L. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol. Clin. Exp. Res. 2002;26:773–778. [PubMed] [Google Scholar]
- DELEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- DING S., SACHS F. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 1999;113:695–719. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS F.A., GIBB A.J., COLQUHOUN D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- EVANS R.J. Single channel properties of ATP-gated cation channels (P2X receptors) heterologously expressed in Chinese hamster ovary cells. Neurosci. Lett. 1996;212:212–214. doi: 10.1016/0304-3940(96)12804-4. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., DERKACH V., SURPRENANT A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- FREIST W., VERHEY J.F., STUHMER W., GAUSS D.H. ATP binding site of P2X channel proteins: structural similarities with class II aminoacyl-tRNA synthetases. FEBS Lett. 1998;434:61–65. doi: 10.1016/s0014-5793(98)00958-2. [DOI] [PubMed] [Google Scholar]
- GU J.G., MACDERMOTT A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HUGEL S., SCHLICHTER R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J. Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP J.A., MARSHALL G.R., WONG E.H.F., WOODRUFF G.N. The affinities, potencies and efficacies of some benzodiazepine-receptor agonists, antagonists and inverse-agonists at rat hippocampal GABAA-receptors. Br. J. Pharmacol. 1987;91:601–608. doi: 10.1111/j.1476-5381.1987.tb11253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., HENDERSON G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol. Pharmacol. 1998;54:372–378. doi: 10.1124/mol.54.2.372. [DOI] [PubMed] [Google Scholar]
- LI C., AGUAYO L., PEOPLES R.W., WEIGHT F.F. Ethanol inhibits a neuronal ATP-gated ion channel. Mol. Pharmacol. 1993;44:871–875. [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Mg2+ inhibition of ATP-activated current in rat nodose ganglion neurons: evidence that Mg2+ decreases the agonist affinity of the receptor. J. Neurophysiol. 1997a;77:3391–3395. doi: 10.1152/jn.1997.77.6.3391. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. J. Physiol. 1997b;505:641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br. J. Pharmacol. 1998;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., XIONG K., WEIGHT F.F. Ethanol inhibition of adenosine 5′-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci. Lett. 2000;295:77–80. doi: 10.1016/s0304-3940(00)01586-x. [DOI] [PubMed] [Google Scholar]
- LI J., PERL E.R. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J. Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z., MIGITA K., SAMWAYS D.S.K., VOIGT M.M., EGAN T.M. Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J. Neurosci. 2004;24:7378–7386. doi: 10.1523/JNEUROSCI.1423-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGLEBY K.L., STEVENS C.F. The effect of voltage on the time course of end-plate currents. J. Physiol. 1972;223:151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASOOD K., WU C., BRAUNEIS U., WEIGHT F.F. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol. Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- NAKATSUKA T., GU J.G. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J. Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEGULYAEV Y.A., MARKWARDT F. Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci. Lett. 2000;279:165–168. doi: 10.1016/s0304-3940(99)00976-3. [DOI] [PubMed] [Google Scholar]
- NIEBER K., POELCHEN W., ILLES P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br. J. Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- PANKRATOV Y., CASTRO E., MIRAS-PORTUGAL M.T., KRISHTAL O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur. J. Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- PANKRATOV Y., LALO U., KRISHTAL O., VERKHRATSKY A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J. Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEOPLES R.W., WRIGHT J.M., LOVINGER D.M., WEIGHT F.F. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurons: whole-cell patch-clamp analysis. Br. J. Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SALGADO A.I., CUNHA R.A., RIBEIRO J.A. Facilitation by P2 receptor activation of acetylcholine release from rat motor nerve terminals: interaction with presynaptic nicotinic receptors. Brain Res. 2000;877:245–250. doi: 10.1016/s0006-8993(00)02679-2. [DOI] [PubMed] [Google Scholar]
- SILINSKY E.M., GERZANICH V., VANNER S.M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br. J. Pharmacol. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON R.P. A modification of receptor theory. Br. J. Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGHT F.F., LI C., PEOPLES R.W. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochem. Int. 1999;35:143–152. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- WRIGHT J.M., PEOPLES R.W., WEIGHT F.F. Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res. 1996;738:249–256. doi: 10.1016/s0006-8993(96)00780-9. [DOI] [PubMed] [Google Scholar]
- XIONG K., LI C., WEIGHT F.F. Inhibition by ethanol of rat P2X4 receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG K., STEWART R.R., HU X.-Q., WERBY E., PEOPLES R.W., WEIGHT F.F., LI C. Role of extracellular histidines in agonist sensitivity of the rat P2X4 receptor. Neurosci. Lett. 2004a;365:195–199. doi: 10.1016/j.neulet.2004.04.078. [DOI] [PubMed] [Google Scholar]
- XIONG K., STEWART R.R., WEIGHT F.F., LI C. Role of extracellular histidines in antagonist sensitivity of P2X4 receptors. Neurosci. Lett. 2004b;367:197–200. doi: 10.1016/j.neulet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- ZEMKOVA H., HE M.-L., KOSHIMIZU T., STOJILKOVIC S.S. Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J. Neurosci. 2004;24:6968–6978. doi: 10.1523/JNEUROSCI.1471-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]