Abstract
We wanted to search for the mechanism(s) responsible for the brief pressor response induced by anandamide in urethane-anaesthetized rats.
The anandamide-induced pressor effect was not modified by the antagonists of cannabinoid CB1 and vanilloid TRPV1 receptors, SR 141716A (3 μmol kg−1) and capsazepine (1 μmol kg−1), respectively, by bilateral vagotomy and by pithing. Replacement of urethane by pentobarbitone virtually abolished the pressor effect of anandamide, both in pithed and vagotomized and in ‘intact' rats (i.e. not treated in this manner).
The pressor effect of anandamide was reduced by the nonselective TRPV family inhibitor ruthenium red (3 μmol kg−1) and by the blocker of L-type calcium channels nifedipine (1 μmol kg−1), both in pithed urethane-anaesthetized rats and in ‘intact' urethane-anaesthetized rats. The nonselective β-adrenoceptor antagonist propranolol (0.1 or 0.3 μmol kg−1) and the nonselective NMDA receptor antagonist MK-801 (1 μmol kg−1) diminished the anandamide-induced vasopressor response in ‘intact' but not in pithed rats. The inhibitory effect of propranolol in ‘intact' rats was mimicked by the β2-adrenoceptor antagonist ICI 118551 (1 μmol kg−1), but not by the β1-adrenoceptor antagonist CGP 20712 (1 μmol kg−1).
The present study revealed that two mechanisms may be responsible for the anandamide-induced pressor response in urethane-anaesthetized rats. The first involves the central nervous system (probably the medulla oblongata) and is sensitive to propranolol and MK-801. The second, which is located peripherally (most probably in blood vessels), is sensitive to nifedipine, ruthenium red and pentobarbitone and, hence, probably represents a Ca2+-dependent mode of action.
Keywords: Anandamide, cannabinoid receptors, TRPV1 receptors, RVLM, propranolol, ruthenium red, nifedipine, ICI 118551, MK-801, anaesthetized rats
Introduction
Anandamide, which was originally identified as the first endogenous cannabinoid receptor ligand (Devane et al., 1992), induces complex cardiovascular changes (for a review, see Hiley & Ford, 2004; Randall et al., 2004). In rats anaesthetized with urethane and in mice anaesthetized with pentobarbitone, its intravenous (i.v.) injection elicits a triphasic response. An initial short-lasting bradycardia and hypotension known as the Bezold–Jarisch reflex (phase I) is followed by a brief pressor response (phase II) and then by a more prolonged decrease in blood pressure (phase III; Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001; Pacher et al., 2004). Similar triphasic changes in cardiovascular parameters were also obtained after i.v. administration of the stable analogue of this compound, methanandamide, excluding the possibility that anandamide acts indirectly via its arachidonic acid metabolites (Malinowska et al., 2001). The initial bradycardia and depressor responses (phase I) have been demonstrated to be mediated via vanilloid TRPV1 receptors located on sensory vagal nerves in the heart. Thus, they were mimicked by capsaicin, an agonist, and strongly diminished by capsazepine and ruthenium red, antagonists of these receptors (Malinowska et al., 2001; Smith & McQueen, 2001). Moreover, they were also not observed in the presence of atropine or after vagotomy (Varga et al., 1996) or in TRPV1 knockout mice (Pacher et al., 2004). On the other hand, the prolonged hypotensive effect of anandamide (phase III) is due to an activation of cannabinoid CB1 receptors since it was blocked by their antagonist SR 141716A (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001) and it was absent in CB1 receptor knockout mice (Ledent et al., 1999). In addition, the involvement of TRPV1 receptors in this phase was also suggested (e.g. Li et al., 2003). However, the mechanism(s) underlying the brief pressor response to anandamide (phase II) still remain to be established (for a review, see Hiley & Ford, 2004; Randall et al., 2004).
The vasopressor response to anandamide was observed in urethane-anaesthetized and conscious normotensive and spontaneously hypertensive rats (Lake et al., 1997; Gardiner et al., 2002). On the other hand, it was induced only by a very high dose of this agonist in pentobarbitone-anaesthetized rats (Garcia et al., 2001). The brief pressor effect of anandamide was accompanied by constriction of the mesenteric, renal and hindquarters vascular beds in conscious rats (Gardiner et al., 2002) and was preceded by a transient rise in the activity of rostral ventrolateral medulla (RVLM) neurons and a brief rise in splanchic sympathetic nerve discharge in urethane-anaesthetized rats (Varga et al., 1996). In spite of these latter two findings, it is still believed that the anandamide-induced increase in blood pressure is not sympathetically mediated (e.g. for a review see Hiley & Ford, 2004; Randall et al., 2004) since (1) it was not modified after acute surgical transection of the spinal cord and (2) it was even slightly enhanced in the presence of the α-adrenoceptor antagonist phentolamine (Varga et al., 1995; 1996). Moreover, the lack of a modulatory influence of SR 141716A argues against the involvement of cannabinoid CB1 receptors in this response (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001). In contrast, in conscious normotensive rats and in spontaneously hypertensive (SHR) urethane-anaesthetized rats, the vasopressor response to anandamide was even enhanced in the presence of SR 141716A (Lake et al., 1997). However, the participation of vanilloid receptors in the pressor response of anandamide is possible since this effect was markedly reduced in pentobarbitone-anaesthetized vanilloid TRPV1 receptor knockout mice in comparison to their wild-type (TRPV1+/+) littermates (Pacher et al., 2004).
In view of these discrepancies, the aim of the present study was to search for the mechanism(s) responsible for the brief pressor response induced by anandamide.
Methods
Anaesthetized rats
All experiments were approved by the local Ethics Committee in Białystok (Poland). Male Wistar rats weighing 160–260 g were anaesthetized intraperitoneally (i.p.) with urethane (14 mmol kg−1) or pentobarbitone sodium (300 μmol kg−1). Some experiments were performed in adrenalectomized and some in bilaterally vagotomized animals. The trachea was cannulated. Diastolic blood pressure was measured in the right carotid artery via a transducer (ISOTEC; Hugo Sachs Elektronik, March-Hugstetten, Germany). Heart rate was measured by a rate-meter triggered from the pressure record. The left femoral vein was cannulated for i.v. injection of drugs administered in a volume of 0.5 ml kg−1. Body temperature was kept constant at about 37°C using a heating-pad (Bio-Sys-Tech, Białystok, Poland) and monitored by a rectal probe transducer (Physitemp BAT10, Clifton, U.S.A.). Since vasopressor/vasodepressor effects are more marked at a higher level of blood pressure (Malinowska & Schlicker, 1993), vasopressin (0.04–0.4 IU kg−1 min−1) was infused into the right femoral vein of pithed and/or ‘intact' animals (i.e. rats not treated in this manner) with a lower level of basal diastolic blood pressure (spontaneously or due to the influence of various experimental conditions or receptor antagonists (see Table 1)). Thus, diastolic blood pressure was raised to a level of about 60–70 mmHg. After 15–20 min of equilibration, during which the cardiovascular parameters were allowed to stabilize, experiments were performed.
Table 1.
Influence of various experimental conditions and receptor antagonists on changes in diastolic blood pressure (DBP) induced by anandamide (3 or 9 μmol kg−1). All results were compared to the anandamide-induced triphasic changes in blood pressure found in urethane-anaesthetized rats
| Experimental condition | Antagonist | Dose(s) (μmol kg−1) | Influence on DBP | ||
|---|---|---|---|---|---|
| Rats anaesthetized with | Phase I | Phase II | Phase III | ||
| Urethane | SR 141716Aa | 3 | NO | NO | Inhibition |
| Propranolol | 0.3 | NO | Inhibition | NO | |
| ICI 118551 | 1 | NO | Inhibition | NO | |
| CGP 20712 | 1 | NO | NO | NO | |
| Prazosinb | 1 | NE | NO | NO | |
| Rauwolscineb | 1 | NE | NO | NO | |
| Guanethidineb | 2 × 125 | NE | NO | Inhibition | |
| BIBP3226b | 3 | NO | NO | NO | |
| Suramin | 300 | NO | NO | NO | |
| Ruthenium red | 3 | Inhibitiona | Inhibition | NO | |
| Capsazepine | 1 | Inhibitiona | NO | NO | |
| Nifedipineb | 1 | NO | Inhibition | NO | |
| MK-801 | 1 | NO | Inhibition | NO | |
| Captoprilb | 10 | NE | NO | NO | |
| Losartanb | 10 | NO | NO | NE | |
| Ketanserin | 0.09 | NE | NO | NO | |
| Tezosentan | 10 | NO | NO | NO | |
| VP-antagonist | 0.01 | NO | NO | NO | |
| Urethane and vagotomized | None | 0 | Inhibition | NO | NO |
| Urethane and adrenalectomized | None | 0 | NO | NO | NO |
| Urethane, pithed and vagotomized | Noneb | 0 | Inhibition | NO | Inhibition |
| Pentobarbitone | None | 0 | Inhibition | Inhibition | NO |
| Pentobarbitone, pithed and vagotomized | Noneb | 0 | Inhibition | Inhibition | Inhibition |
All antagonists were injected i.v. with the exception of guanethidine, which was administered i.p. in two doses 14 and 1 h before the experiment.
Data from Malinowska et al. (2001).
Experiments performed under infusion of vasopressin.
VP-antagonist, d(CH2)5[Tyr(Me)2Arg8]-vasopressin.
NE=not evaluated because of too low hypotensive response (lower than 10%).
NO=no influence on the effect of anandamide.
Pithed rats
Rats were anaesthetized with urethane (14 mmol kg−1 i.p.) or with pentobarbitone sodium (300 μmol kg−1 i.p.), and then injected with atropine (2 μmol kg−1 i.p.). After cannulation of the trachea, the animals were pithed by inserting a stainless-steel rod (1.5 mm diameter and 190 mm length) through the orbit and foramen magnum into the vertebral canal and artificially ventilated with air (1 ml 100 g−1, 60 strokes min−1) using a respirator (7025 Rodent respirator; Hugo Sachs Elektronik, March-Hugstetten, Germany). Both vagal nerves were cut. Blood pressure, heart rate and body temperature were measured as above.
Experimental protocols
In order to construct dose–response curves, four increasing doses of anandamide (0.9–30 μmol kg−1) were injected to one rat with sufficient time to full recovery to the preinjection value. In separate series of experiments, rats, which were anaesthetized with urethane and either bilaterally adrenalectomized or treated with guanethidine (or their control groups, i.e., sham operated rats and animals treated with the solvent for guanethidine, respectively) obtained only one dose of anandamide (3 μmol kg−1). To examine the influence of different receptor antagonists on the changes in blood pressure induced by anandamide, the agonist under study was administered in a single dose (3 or 9 μmol kg−1) per animal. We started routinely with the lower dose of the agonist. If, however, the increase in blood pressure did not exceed at least 15% of the basal value (only in 8% of all experiments), the higher dose was injected to the same rat 10 min later. The same dose of the agonist was injected twice (S1 and S2) at an interval of 12, 15 or 20 min depending on the antagonist investigated. S1 was applied 10 min before, and S2 was administered 2, 5 or 10 min after, the antagonist under study or its solvent (for doses of each antagonist, see Table 1 and Figures 3, 4 and 5). Thus, S2 was injected 2 min after capsazepine, 5 min after propranolol, erythro-(±)-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol (ICI 118551), 2-hydroxy-5(2-((2-hydroxy-3-4(1-methyl-4-trifluoromethyl)-1H-imidazole-2-yl)-phenoxypropylamino)ethoxy)-benzamide monomethane sulphonate (CGP 20712), ruthenium red, nifedipine, suramin, MK-801, ketanserin, N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-D-arginine amide (BIBP 3226) or tezosentan and 10 min after d(CH2)5[Tyr(Me)2Arg8]-vasopressin, losartan, prazosin, rauwolscine or captopril. Guanethidine was injected twice, at a dose of 125 μmol kg−1 i.p. each, 14 and 1 h before the experiment.
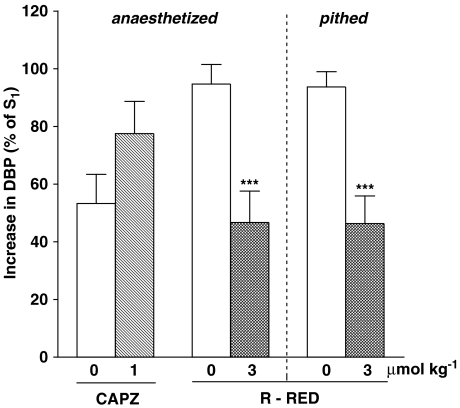
Figure 3.
Influence of capsazepine (CAPZ) and ruthenium red (R-RED) on the increase in diastolic blood pressure (DBP; phase II) induced by anandamide (3 or 9 μmol kg−1) in urethane-anaesthetized (otherwise ‘intact') or in urethane-anaesthetized, pithed and vagotomized rats. The agonist was injected twice (S1 and S2). S1 was given 10 min before antagonists. S2 was injected 2 min after capsazepine and 5 min after ruthenium red. S1 and S2 values were calculated as percentage of DBP before injection of the agonist and S2 was expressed as a percentage of S1. Means±s.e.m. of 3–17 rats. ***P<0.001 compared to the respective controls.
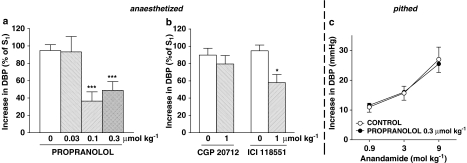
Figure 4.
Influence of propranolol (a, c), CGP 20712 and ICI 118551 (b) on the increase in diastolic blood pressure (DBP; phase II) induced by anandamide in urethane-anaesthetized (otherwise ‘intact') (a, b) or in urethane-anaesthetized, pithed and vagotomized rats (c). (a,b) Anandamide (3 or 9 μmol kg−1) was injected 10 min before (S1) and 5 min after administration of the antagonist (S2) or (c) three increasing doses of anandamide (0.9, 3 and 9 μmol kg−1) were injected to the same rat 5 min after administration of propranolol or its solvent (control). S1 and S2 values were calculated as percentage of DBP before injection of anandamide and S2 was expressed as a percentage of S1 (a, b). Means±s.e.m. of 4–17 rats. *P<0.05, ***P<0.001 compared to the respective controls.
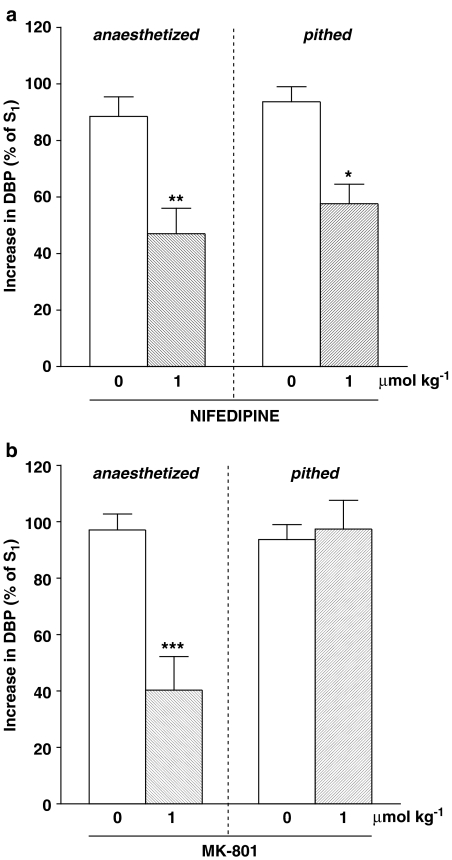
Figure 5.
Influence of nifedipine (a) or MK-801 (b) on the increase in diastolic blood pressure (DBP; phase II) induced by anandamide (3 or 9 μmol kg−1) in urethane-anaesthetized (otherwise ‘intact') or in urethane-anaesthetized, pithed and vagotomized rats. Anandamide was injected 10 min before (S1) and 5 min after (S2) administration of the antagonist. S1 and S2 values were calculated as percentage of DBP before injection of anandamide and S2 was expressed as a percentage of S1. Means±s.e.m. of 3–17 rats. *P<0.05, **P<0.01, ***P<0.001, compared to the respective controls.
Calculations and statistics
Results are given as means±s.e.m. (n=number of rats). In order to quantify the effects of antagonists on the anandamide-induced changes in blood pressure or heart rate, S1 and S2 values were calculated as percentage of the basal diastolic blood pressure or heart rate immediately before injection of that particular agonist dose. The final S2 results are expressed as a fraction or percentage of S1. These ratios were expressed as percentages of the corresponding ratios obtained from vehicle-treated animals. For comparison of mean values, the t-tests for paired and unpaired data were used. When two or more treatment groups were compared to the same control, one-way analysis of variance (ANOVA) followed by the Dunnett test was used. Differences were considered as significant when P<0.05.
Drugs
Anandamide, capsazepine, ICI 118551 (Tocris Cookson, Bristol, U.K.); BIBP 3226 (Thomae, Biberach, Germany), atropine sulphate, ruthenium red, prazosin hydrochloride, nifedipine, propranolol hydrochloride, CGP 20712, urethane, [Lys8]-vasopressin (Sigma, München, Germany); rauwolscine hydrochloride (Roth, Karlsruhe, Germany); MK-801 ((5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5,10-imine hydrogen), captopril (RBI, Natick, U.S.A.); guanethidine sulphate (CIBA-Geigy, Wehr, Germany); losartan (MSD Chibropharm, Haar, Germany); suramin hexasodium salt (Bayer, Wuppertal, Germany); d(CH2)5[Tyr(Me)2Arg8]-vasopressin (Bachem, Heidelberg, Germany); tezosentan (Actelion, Allschwil, Switzerland); ketanserin tartrate (Janssen, Beerse, Belgium); pentobarbitone sodium (Biowet, Puławy, Poland). Drugs were dissolved in saline with the following exceptions: capsazepine in a mixture of ethanol, Tween-80, DMSO and saline (1 : 1 : 1 : 9.5); prazosin in a mixture of HCl 0.01 M and DMSO (4 : 1); CGP 20712 in saline and DMSO (16 : 1); nifedipine in saline, DMSO and ethanol (8 : 1 : 1). Anandamide was purchased from Tocris Cookson as 10.1 mg ml−1 emulsion in soya water (1 : 4).
Results
Basal diastolic blood pressure and heart rate and effects of drugs on basal parameters
In control urethane-anaesthetized rats, basal diastolic blood pressure and heart rate was 65.0±1.5 mmHg and 382.2±9.8 beats min−1 (n=22), respectively. If experimental conditions including the presence of interacting drugs resulted in diastolic blood pressure lower than 60 mmHg, vasopressin (0.04–0.4 IU kg−1 min−1; for details, see Methods) was used to increase diastolic blood pressure to a level of 60–70 mmHg. Thus, basal cardiovascular values similar to those in control urethane-anaesthetized rats were also obtained in (1) adrenalectomized urethane-anaesthetized rats (n=6), (2) pithed and vagotomized urethane-anaesthetized rats (n=25), (3) pentobarbitone-anaesthetized rats (n=4) and (4) pithed and vagotomized pentobarbitone-anaesthetized rats (n=4). Bilateral vagotomy in urethane-anaesthetized rats did not affect blood pressure but tended to increase heart rate from 422.0±41.3 to 485.3±15.0 beats min−1 (n=3) 10 min later.
Vasopressin was used to compensate for the long-lasting decrease in blood pressure evoked by rauwolscine (1 μmol kg−1), prazosin (1 μmol kg−1), nifedipine (1 μmol kg−1), captopril (10 μmol kg−1), losartan (10 μmol kg−1), BIBP 3226 (3 μmol kg−1) and guanethidine (250 μmol kg−1 i.p.). Propranolol (0.3 μmol kg−1), CGP 20712 (1 μmol kg−1) and losartan (10 μmol kg−1) caused long-lasting decreases in heart rate; immediately before S2 (i.e. when anandamide was given for the second time) heart rate was lower by about 9% (P<0.01), 8% (P<0.01) and 10% (P<0.05), respectively, in comparison to the corresponding basal value before the first injection of anandamide (S1).
Cardiovascular responses to anandamide
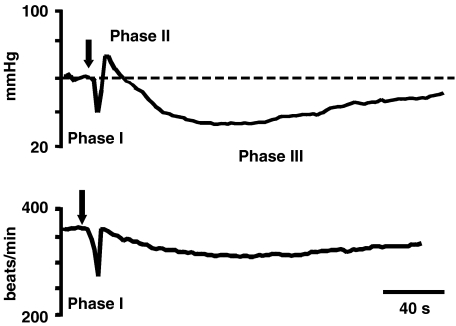
I.v. injection of anandamide 0.9–30 μmol kg−1 (corresponding to 0.3–10 mg kg−1) induced a triphasic change in blood pressure in urethane-anaesthetized rats (for typical traces see Figure 1). The initial pronounced, transient fall in blood pressure and bradycardia (phase I) was followed by a pressor response that lasted for about 30–60 s (phase II) and a subsequent hypotensive response that lasted for up to 10 min (phase III). The changes in blood pressure during phases I, II and III evoked by the first administration of a reliably effective dose (see Methods) of anandamide (S1 3 or 9 μmol kg−1) to control animals was −20.9±4.5, +28.1±2.0 and −25.7±2.6% of the basal value (n=22), respectively. These effects did not change when the same dose of the agonist was injected for a second time (S2) after 15 min (control condition; saline, the solvent for most receptor antagonists was given 5 min before S2). Accordingly, the respective S2/S1 ratios were close to unity (0.96±0.12; 0.97±0.05 and 0.97±0.07 (n=22) for phases I, II and III, respectively). Most solvents used in the present study failed to modify the changes in blood pressure induced by anandamide (data not shown). Only the solvent for capsazepine (see Methods) reduced phase II induced by the second administration of anandamide by about 40–50% (see below, Figure 3).
Figure 1.
Typical traces showing changes in diastolic blood pressure and heart rate (upper and lower panel, respectively) induced by intravenous injection of anandamide (3 μmol kg−1) to the urethane-anaesthetized rat. The arrow indicates the moment of drug application.
As mentioned above, during the anandamide-induced phase I, a rapid decrease in heart rate was observed; the S1 and S2/S1 values for this parameter under control conditions were 17.9±4.4% of the basal value and 1.02±0.13 (n=22), respectively.
Influence of various experimental conditions and different receptor antagonists on phase II
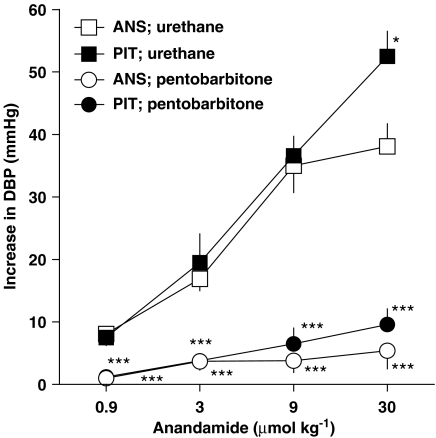
As shown in Figure 2, anandamide increased blood pressure in a dose-dependent manner (maximally by about 40 mmHg) in urethane-anaesthetized rats. Bilateral vagotomy did not affect the vasopressor response to this agonist (results not shown). In pithed and bilaterally vagotomized animals, treated with atropine (2 μmol kg−1 i.p.), the vasopressor response to the three lower doses of the agonist (0.9–9 μmol kg−1) did not differ from that obtained in anaesthetized rats which were not pithed, whereas the increase in blood pressure evoked by the highest anandamide dose (30 μmol kg−1) was about 15 mmHg higher in pithed rats (Figure 2). When pentobarbitone was used for anaesthesia instead of urethane, the anandamide-induced increase in blood pressure was extremely reduced both in pithed and anaesthetized rats (Figure 2). Unless stated otherwise, all further experiments were performed in urethane-anaesthetized rats that were not pithed.
Figure 2.
Increase in diastolic blood pressure (DBP; phase II) induced by anandamide in pithed and ‘intact' (i.e. not pithed) rats anaesthetized with urethane or with pentobarbitone. Pithed animals were also vagotomized. Four increasing doses of anandamide were injected to one rat with sufficient time to full recovery to the preinjection value. Means±s.e.m. of 3–7 rats. *P<0.05, and ***P<0.001 compared to urethane-anaesthetized rats. ANS – anaesthetized, otherwise ‘intact'; PIT – pithed, vagotomized and anaesthetized.
Injection of anandamide 3 or 9 μmol kg−1 was the standard condition (for further details see Methods), which was used to examine the mechanism underlying the pressor response in phase II (i.e. the main aim of this study). We found the vasopressor response to anandamide to be decreased by the nonselective vanilloid receptor antagonist ruthenium red (3 μmol kg−1; by about 50%), but not by the selective vanilloid TRPV1 receptor antagonist capsazepine (1 μmol kg−1) (Figure 3). As shown in Figure 4a, the nonselective β-adrenoceptor antagonist propranolol (0.1 and 0.3 μmol kg−1) inhibited the vasopressor response to anandamide by about 60 and 50%, respectively. This inhibitory effect was mimicked by the β2-adrenoceptor antagonist ICI 118551 (1 μmol kg−1), but did not occur with the β1-adrenoceptor antagonist CGP 20712 (1 μmol kg−1) (Figure 4b). Furthermore, the vasopressor response induced by anandamide was diminished by the antagonist of L-type calcium channels nifedipine (Figure 5a) and by the nonselective NMDA receptor antagonist MK-801 (Figure 5b; 1 μmol kg−1 each) by about 50 and 60%, respectively.
On the other hand, the vasopressor response to anandamide, which was 0.97±0.05 (S2/S1) in 22 controls, was not affected (P>0.05) by the following drugs (see Table 1 for doses): the α1- and α2-adrenoceptor antagonists prazosin and rauwolscine, the nonselective purinergic P2X/P2Y receptor antagonist suramin, the neuropeptide Y1 receptor antagonist BIBP 3226, the angiotensin converting enzyme inhibitor captopril, the angiotensin AT1 receptor antagonist losartan, the serotonin 5-HT2A receptor antagonist ketanserin, the nonselective endothelin ETA/ETB receptor antagonist tezosentan and the vasopressin V1a receptor antagonist d(CH2)5[Tyr(Me)2Arg8]-vasopressin. The S2/S1 ratios measured under these conditions ranged from 0.82±0.06 to 1.24±0.16 (n=3–6). Moreover, in animals treated with the adrenergic neurone blocker guanethidine (2 × 125 μmol kg−1 i.p. 14 and 1 h before the experiment), the vasopressor response to anandamide (3 μmol kg−1) did not differ from the control group (28.6±3.4 mmHg, n=6 and 31.9±4.1 mmHg, n=5, respectively). Furthermore, in adrenalectomized rats and in sham operated animals, anandamide (3 μmol kg−1) induced comparable increases in blood pressure by 24.9±4.4 (n=6) and 17.6±2.6 mmHg (n=4), respectively.
In addition, we studied in pithed and bilaterally vagotomized urethane-anaesthetized rats those antagonists that effectively reduced phase II in anaesthetized animals. Thus, in pithed rats, the vasopressor responses were diminished by ruthenium red 3 μmol kg−1 (Figure 3) and nifedipine 1 μmol kg−1 (Figure 5a) by about 50 and 40%, respectively. On the other hand, propranolol 0.3 μmol kg−1 (Figure 4c) and MK-801 1 μmol kg−1 (Figure 5b) failed to modify the increase in blood pressure evoked by anandamide.
Influence of various experimental conditions and different receptor antagonists on phases I and III
Table 1 summarizes the effects of all experimental conditions and different receptor antagonists on changes in blood pressure evoked by anandamide (phases I, II and III), including three series of experiments (effects of capsazepine, ruthenium red and SR 141716A) taken from Malinowska et al. (2001). In interaction experiments of anandamide with prazosin, rauwolscine, guanethidine, ketanserin, captopril or losartan, the decrease in blood pressure induced by anandamide in phase I or III was lower than 10% of the basal values and hence could not be reliably evaluated. In view of the main focus of the present study on the hypertensive response in phase II, which could be unequivocally evaluated, we decided not to further investigate the effects of these six drugs on phase I or III.
The anandamide-induced decrease in blood pressure in phase I in the urethane-anaesthetized rat was reduced by ruthenium red and capsazepine, as we already described (Malinowska et al., 2001). In our present paper, the anandamide (9 μmol kg−1)-elicited fall in blood pressure, which amounted to 29.5±3.3 mmHg in urethane-anaesthetized animals (n=7), was attenuated to 1.8±1.8 mmHg (***P<0.001; n=6), 8.2±2.6 mmHg (***P<0.001; n=5) and 2.8±1.9 mmHg (***P<0.001; n=6) by the three additional procedures: pithing, pentobarbitone and pentobarbitone plus pithing, respectively (Table 1). In addition, bilateral vagotomy decreased the vasodepressor effect of anandamide (3 μmol kg−1) from 10.0±2.2 to 2.7±1.5 mmHg (*P<0.05; n=3). The anandamide-induced fall in heart rate was attenuated by those procedures that also decreased blood pressure but was not affected by the remainder of the interacting drugs or experimental procedures shown in Table 1 (results not shown).
The decrease in blood pressure in phase III induced by anandamide 3 μmol kg−1 in the urethane-anaesthetized rat was reduced by SR 141716A (already described by Malinowska et al., 2001) and guanethidine from 10.3±2.0 to 3.1±2.1 mmHg (*P<0.05; n=6). In addition, pithing both in urethane and in pentobarbitone anaesthetized rats abolished phase III induced by the two lower doses of anandamide, 0.9 and 3 μmol kg−1, and tended to diminish the anandamide (9 μmol kg−1) evoked prolonged hypotension from 19.6±4.1 mmHg (n=7) in urethane-anesthetized animals to 9.7±1.7 mmHg (n=6) and 9.7±3.4 mmHg (n=6), respectively. All other conditions did not change phase III (Table 1). Anandamide failed to affect heart rate during phase III regardless of whether the drug was given alone or was examined under the influence of the experimental procedures listed in Table 1.
Discussion
The aim of the present study was to search for the mechanism(s) responsible for the brief pressor response induced by anandamide (phase II), which follows its short-lived hypotensive (phase I) and precedes its more prolonged hypotensive response (phase III) in urethane-anaesthetized rats. The mechanisms underlying phases I and III have been clarified in previous studies (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001; Pacher et al., 2004) and will be considered only marginally in the following paragraphs. Since vasopressor effects are more marked at a higher level of blood pressure (Malinowska & Schlicker, 1993) and some experimental procedures or receptor antagonists decreased diastolic blood pressure, vasopressin was infused to obtain a level of diastolic blood pressure of about 60–70 mmHg in all rats (including pithed animals). With the exception of those experiments in which dose–response curves were constructed anandamide, which does not interact with vasopressin V1a receptors (see below), was used at a dose of 3 or 9 μmol kg−1, which increased blood pressure by at least 15% of the respective basal value and was approximately equivalent to its EC50 value.
In our study on urethane-anaesthetized rats, the maximal anandamide-induced increase in blood pressure in phase II was by about 60% of the basal value and occurred at 30 μmol kg−1. Such a response was described earlier not only in urethane-anaesthetized (Varga et al., 1995; 1996; Lake et al., 1997; Malinowska et al., 2001) but also in conscious (Gardiner et al., 2002) rats. However, when pentobarbitone was used instead of urethane only a very high dose of 30 μmol kg−1 induced a measurable pressor response in rats (present study and Garcia et al., 2001) and a dose of 60 μmol kg−1 increased blood pressure to only about 120% of the basal value in mice (Pacher et al., 2004). Since the present study shows that the increase in blood pressure induced by anandamide was nearly abolished under pentobarbitone compared to urethane anaesthesia, subsequent experiments were performed under urethane anaesthesia.
Previously, it has been demonstrated that anandamide affects cardiovascular parameters via its influence on cannabinoid (phase III) or vanilloid receptors (phases I and III) (for a review see Hiley & Ford, 2004; Randall et al., 2004). Cannabinoid CB1 receptors do not contribute to the pressor effect (phase II) of anandamide since it was not counteracted by antagonists of these receptors, that is, SR 141716A in urethane-anaesthetized (e.g. Varga et al., 1995; 1996; Lake et al., 1997; Malinowska et al., 2001) and AM 251 in conscious rats (Gardiner et al., 2002). On the other hand, phase II elicited by anandamide in pentobarbitone-anaesthetized vanilloid TRPV1 receptor knockout mice was markedly reduced in comparison to their wild-type (TRPV1+/+) littermates (Pacher et al., 2004). Thus, in order to check the possible participation of vanilloid receptors in the pressor effect of anandamide, we applied the TRPV1 receptor antagonist capsazepine (Bevan et al., 1992) and ruthenium red, a channel blocker and inhibitor of vanilloid responses (Amann & Maggi, 1991). We found that the increase in blood pressure elicited by anandamide was diminished by ruthenium red but not changed by capsazepine. These results argue against the participation of the rat (in contrast to the mouse) vanilloid TRPV1 receptors, which should be blocked not only by ruthenium red but also by capsazepine (Gunthorpe et al., 2002), whereas the involvement of another TRPV receptor subtype (all of which are sensitive to ruthenium red; Alexander et al., 2004) cannot be excluded.
On the basis of these results, an action of anandamide via CB1 and TRPV1 receptor-independent mechanism(s) was considered. As will be discussed in detail below, the NMDA receptor antagonist MK-801 attenuated the second phase of the effect of anandamide. Due to the preferential location of NMDA receptors in the central nervous system (CNS), this finding points to a central site of action. In this context, one may wonder whether the increase in blood pressure elicited by anandamide represents a reflex response to the preceding rapid hypotension and bradycardia. The lack of influence of bilateral vagotomy on the vasopressor effect induced by anandamide rather argues against this hypothesis by excluding an involvement of the afferent and efferent vagal nerve fibres. Similarly, the anandamide-evoked hypertensive action was not modified in methylatropine-pretreated (Varga et al., 1995) or barodenervated (Varga et al., 1996) urethane-anaesthetized rats. Furthermore, our experiments on pithed rats and on animals pretreated with the adrenergic neurone blocker guanethidine are not compatible with an involvement of the sympathetic pathway in the effect of anandamide.
The possibility that anandamide interacts with peripheral non-CB1 and non-TRPV1 receptors in the cardiovascular system to produce vasoconstriction had to be considered as well. The lack of effect of prazosin, rauwolscine, suramin, BIBP 3226, ketanserin, d(CH2)5[Tyr(Me)2Arg8]-vasopressin and tezosentan, however, argues against the involvement of α1- and α2-adrenoceptors, P2X/P2Y, neuropeptide Y1, serotonin 5-HT2A, vasopressin V1a and endothelin receptors in the effect of anandamide. Nonetheless, anandamide may increase blood pressure due to a calcium-dependent effect in the cardiovascular system since nifedipine, an inhibitor of vascular L-type Ca2+ channels, markedly reduced its effect.
In the context of the putative involvement of peripheral receptors in the vasopressor effect of anandamide, a role of pre- and postsynaptic β-adrenoceptors also had to be considered. In fact, the increase in blood pressure evoked by anandamide was attenuated by the nonselective β-adrenoceptor antagonist propranolol. Further experiments with the β1- and β2-adrenoceptor antagonists CGP 20712 and ICI 118551 (the latter mimicked the effect of propranolol) showed that β2-adrenoceptors are involved. For this reason, it is unlikely that the effect of anandamide is related to a β-adrenoceptor-mediated positive inotropic effect or renin release since both mechanisms are mainly related to the activation of β1-adrenoceptors. Experiments with captopril and losartan, which block the converting enzyme forming angiotensin II from angiotensin I and angiotensin AT1 receptors, respectively, argue against the involvement of the renin–angiotensin system. An involvement of β2-adrenoceptors located in the vascular smooth muscle in the anandamide-induced rise in blood pressure can be excluded since their activation would lead to vasodilation. Presynaptic facilitatory β2-adrenoceptors (or other types of presynaptic facilitatory receptors) leading to an increased release of sympathetic neurotransmitters are also not a likely candidate in the effect of anandamide for two reasons. (i) The postsynaptic receptors for the three types of transmitters, that is, catecholamines, purines and neuropeptide Y, in the vascular wall could be already excluded as potential targets of the effect of anandamide (see above). (ii) Anandamide failed to increase the vasopressor response induced by electrical stimulation of the sympathetic outflow in pithed rats (results not shown). In this context, it is also not unexpected that the effect of anandamide was similar in adrenalectomized rats and in animals with intact adrenals.
In view of the exclusion of peripheral β2-adrenoceptors in the effect of anandamide, the possibility has to be considered that the β2-adrenoceptors are located in the CNS. This view is strengthened by experiments in the pithed rat preparation, in which anandamide still elicited an increase in blood pressure, which, however, was not counteracted by propranolol. Experiments in pithed rats also revealed that MK-801 no longer produced the diminution of anandamide's vasopressor effect observed in ‘intact' animals, supporting the idea that not only the propranolol but also the MK-801-sensitive site of action of anandamide are located in the CNS. How can these results integrated in a unifying hypothesis? Varga et al. (1996) demonstrated that the brief pressor effect of anandamide in urethane-anaesthetized rats was preceded by a transient rise in the activity of the RVLM neurones and a brief rise in splanchic sympathetic nerve discharge. Glutamate is the transmitter in vasomotor pathways in the CNS and activation of ionotropic glutamate receptors of the NMDA subtype in the RVLM (Pilowsky & Goodchild, 2002) and in the spinal cord (Garcia & Celuch, 2002) evokes an increase in blood pressure. Anandamide (in the presence of a cannabinoid receptor antagonist) has been shown to enhance the effects of NMDA receptor-stimulated responses in the CNS (Hampson et al., 1998), and this might explain why the NMDA receptor antagonist MK-801 attenuated the anandamide-induced increase in blood pressure. Furthermore, it was demonstrated that i.v. administration of propranolol decreases the release of glutamate in the RVLM of urethane-anaesthetized rats (Okuda et al., 1999).
However, this interpretation does not conform to the lack of influence on anandamide's effect by conditions or drugs blocking the sympathetic outflow at more distal sites. Thus, its effect was not modified by pithing or the adrenergic neurone blocker guanethidine (Table 1), and was rather increased by phentolamine, acting at the α-adrenoceptors in the vascular wall (Varga et al., 1996). Nevertheless, the lack of changes in the pressor effect of anandamide under these three conditions could result from the fact that they simultaneously abolish the (partially) overlapping phase I and/or phase III hypotensive responses.
In contrast to the effects of propranolol and MK-801, the inhibitory effects of nifedipine, ruthenium red and pentobarbitone on the vasopressor effect of anandamide were not diminished in pithed compared to ‘intact' rats. These findings are compatible with peripheral sites of action of anandamide, which appear at least as important as the central one. A direct vasoconstriction elicited by anandamide is, at first glance, difficult to understand since anandamide was shown to dilate isolated vessels by a variety of mechanisms (for a review see Hiley & Ford, 2004; Randall et al., 2004). However, in vivo a pressor effect of anandamide accompanied by constriction of mesenteric, renal and hindquarters vasculature was found in conscious rats (Gardiner et al., 2002). Moreover, in urethane-anaesthetized rats, the vascular resistance in the spleen was more than four times higher in the presence of anandamide and SR 141716A than under control conditions (Wagner et al., 2001). The vasoconstrictor effect of anandamide appears to be due to a Ca2+-dependent mechanism, as suggested by the abolition of the vasopressor effect of anandamide by nifedipine, ruthenium red and pentobarbitone. These three compounds may be assumed to decrease the availability of Ca2+ because not only nifedipine but also ruthenium red (Cibulsky & Sather, 1999) and pentobarbitone (Guertin & Hounsgaard, 1999) have been shown to block L-type Ca2+ channels, which are most plausibly located in the vascular smooth muscle. Although another location cannot be excluded on the basis of the present experiments, it is tempting to hypothesize that anandamide, by directly or indirectly influencing the L-type Ca2+ channel, increases Ca2+ influx in vascular smooth muscle, leading to vasoconstriction as basis for an increase in blood pressure.
Conclusions
The present study revealed that two mechanisms may be responsible for the anandamide-induced increase in blood pressure (phase II) in urethane-anaesthetized rats. The first of them involves the CNS (probably the RVLM) and is sensitive to propranolol and MK-801. The second one is located peripherally (most probably in the vasculature); it is sensitive to nifedipine, ruthenium red and pentobarbitone and, hence, probably represents a Ca2+-dependent mode of action.
Acknowledgments
This work was supported by the Medical University of Białystok (3-13737; 3-13898), by the Deutsche Forschungsgemeinschaft and by the Bundesministerium für Bildung und Forschung (POL 03/007). We are also indebted to the Alexander von Humboldt-Stiftung (Bonn, Germany) for generously providing some of the equipment. We wish to thank Actelion, MSD Chibropharm and Thomae for gifts of tezosentan, losartan and BIBP 3226, respectively.
Abbreviations
- BIBP 3226
N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-D-arginine amide
- CGP 20712
2-hydroxy-5(2-((2-hydroxy-3-4(1-methyl-4-trifluoromethyl)-1H-imidazole-2-yl)-phenoxy)propyl)amino)ethoxy)-benzamide monomethane sulphonate
- CNS
central nervous system
- ICI 118551
erythro-(±)-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol)
- MK-801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5,10-imine hydrogen
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rats
- SR 141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
References
- ALEXANDER S.P.H., MATHIE A., PETERS J.A.Transient receptor potential (TRP) cation channels Br. J. Pharmacol. 2004141S1In: Guide to Receptors and Channels. Ist edn., eds. Alexander, S.P.H., Mathie, A. & Peters, J.A.15082510 [Google Scholar]
- AMANN R., MAGGI C.A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S.J., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIBULSKY S.M., SATHER W.A. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J. Pharmacol. Exp. Ther. 1999;289:1447–1453. [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- GARCIA M.C., CELUCH S.M. Participation of nitric oxide and N-methyl-D-aspartic acid receptors in the pressor response to intrathecal injected noradrenaline at the spinal cord of the rat. Neurosci. Lett. 2002;329:125–128. doi: 10.1016/s0304-3940(02)00612-2. [DOI] [PubMed] [Google Scholar]
- GARCIA N., JR, JÁRAI Z., MIRISHAHI F., KUNOS G., SANYAL A.J. Systemic and portal hemodynamic effects of anandamide. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G14–G20. doi: 10.1152/ajpgi.2001.280.1.G14. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNET T. Complex regional haemodynamic effects of anandamide in conscious rats. Br. J. Pharmacol. 2002;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERTIN P.A., HOUNSGAARD J. Non-volatile general anaesthetics reduce spinal activity by suppressing plateau potentials. Neuroscience. 1999;88:353–358. doi: 10.1016/s0306-4522(98)00371-6. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE J.M., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- HAMPSON A.J., BORNHEIM L.M., SCANZIANI M., YOST C.S., GRAY A.T., HANSEN B.M., LEONOUDAKIS D.M., BICKLER P.E. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- HILEY C.R., FORD W.R. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol. Rev. Cambridge Philos. Soc. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., MARTIN B.R., KUNOS G., VARGA K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.-F., BESLOT F., BÖHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LI J., KAMINSKI N.E., WANG D.H. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., KWOLEK G., GÖTHERT M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Identification of endothelial H1, vascular H2 and cardiac presynaptic H3 receptors in the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:55–60. doi: 10.1007/BF00168772. [DOI] [PubMed] [Google Scholar]
- OKUDA N., KOHARA K., MIKAMI H., MORIGUCHI A., YAMADA K., HIGAKI J., OGIHARA T. Effect of propranolol on central neurotranmitter release in Wistar rats analysed by brain microdialysis. Clin. Exp. Pharmacol. Physiol. 1999;26:220–224. doi: 10.1046/j.1440-1681.1999.03018.x. [DOI] [PubMed] [Google Scholar]
- PACHER P., BÁTKAI S., KUNOS G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILOWSKY P.M., GOODCHILD A.K. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A., O'SULLIVAN S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P.J., McQUEEN D.S. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rats. Br. J. Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA K., LAKE K., MARTIN B.R., KUNOS G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- VARGA K., LAKE K.D., HUANGFU D., GUYENET P.G., KUNOS G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., JÁRAI Z., BÁTKAI S., KUNOS G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilatation mediated by cannabinoid CB1 receptors. Eur. J. Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]