Abstract
This study describes the effects of hypoxia on relaxing responses and cAMP production induced by the known vasodilator peptides: αCGRP, amylin (AMY) and adrenomedullin (AM) on isolated pig coronary arteries in vitro.
Hypoxic incubation increased the vasorelaxant effect of αCGRP (four-fold; P<0.05), AMY (3.2-fold; P<0.05), but not significantly for AM (two-fold; NS).
Whereas hypoxia had no effect on arterial cAMP levels, it significantly potentiated the production of cAMP stimulated of αCGRP and AMY, but not of AM.
The antagonist αCGRP8–37 also exerted an increased effect in hypoxia. The Schild plot-derived pKB values revealed an increase in the apparent affinity of the antagonist for the CGRP1 receptor from 7.0 to 7.2 under control conditions versus 8.0 in hypoxia.
Removal of endothelium, peptidase inhibitors, preincubation with the adenosine A2A receptor antagonist CSC (10−3 M), the ATP-sensitive K-channel inhibitor glibenclamide (10−5 M), the cyclooxygenase inhibitor indomethacin (10−3 M) or NG-monomethyl-L-arginine (10−4 M) had no effect on the αCGRP-induced vasorelaxation in hypoxia; neither did hypoxia influence the levels of CGRP and AM receptor mRNA.
We conclude that hypoxic incubation increases the relaxation and cAMP production induced by αCGRP and AMY in rings of porcine coronary arteries in vitro. A concomitant release of adenosine, a cyclooxygenase product, an endothelium-derived substance, activation of vascular ATP-sensitive K-channels, peptidase inhibitors or changes in CGRP and AM receptor mRNA cannot account for the changes observed in hypoxia. Moreover, αCGRP8–37 showed increased affinity at the CGRP1 receptor during hypoxia, possibly due to a conformational change at the CGRP1 receptor site.
Keywords: CGRP, adrenomedullin, amylin, calcitonin-like receptor, receptor-activity-modifying proteins, hypoxia, normoxia, hyperoxia
Introduction
The function of the heart depends critically on adequate oxygen supply. Imbalance between oxygen supply and the oxygen demand leads to myocardial hypoxia, one component of myocardial ischaemia, with its clinical manifestations angina pectoris/infarction or heart failure. Calcitonin gene-related peptide (CGRP), adrenomedullin (AM) and amylin (AMY) belong to the same family of vasorelaxant peptides and they probably play an important role in the regulation of coronary circulation under physiological and pathological conditions.
CGRP is widely distributed in the peripheral and central nervous systems and is released from cardiac unmyelinated sensory C-fibres (Franco-Cereceda et al., 1987). CGRP has been reported to gain potency in hypoxic conditions in sheep coronary arteries (Kwan et al., 1990) and CGRP release itself is also enhanced during hypoxia and at low pH levels in the guinea-pig myocardium (Franco-Cereceda et al., 1993). Furthermore, intravenously administered CGRP delays the onset of myocardial ischaemia during treadmill exercise testing in patients with chronic stable angina (Uren et al., 1993).
Unlike CGRP, AM is primarily produced by non-nervous tissue, especially vascular endothelium and smooth muscle cells (Sugo et al., 1994a, 1994b). Direct AM-induced vasorelaxation has been observed in a number of species and in different vascular beds, including the porcine coronary circulation (Kureishi et al., 1995; Hasbak et al., 2001). Hypoxia induces AM mRNA production in human coronary artery endothelial cells (Nakayama et al., 1999) and higher plasma AM levels are found in patients with myocardial infarction (Kobayashi et al., 1996), a condition where hypoxia is one essential component.
AMY is co-localised and co-released with insulin in the β pancreatic cells (Pittner et al., 1994) and is generally considered to be a glyco-regulatory peptide that inhibits the actions of insulin (Deems et al., 1991; Castle et al., 1998). But we have previously shown that AMY also induced a weak vasorelaxant effect probably via the CGRP1 receptor in both the human and the porcine coronary circulation (Hasbak et al., 2001, 2003).
On a molecular level, the CGRP1 receptor has been characterised and consists of the calcitonin-like receptor (CL receptor) (according to the guidelines from the International Union of Pharmacology CGRP nomenclature subcommittee (Poyner et al., 2002)) previously known as calcitonin receptor-like receptor (CRLR) and receptor-activity-modifying protein 1 (RAMP1) (McLatchie et al., 1998). RAMPs are required both for receptor trafficking and ligand binding. The association of CL receptor with either RAMP2 or RAMP3 produces AM receptors. Previously, we reported the presence of mRNA encoding the CL receptor and RAMPs in the porcine and human coronary arteries, indicating the presence of CGRP1 and AM receptors (Hasbak et al., 2001, 2003).
In the present study, investigations were performed at various oxygen concentrations to characterise the potential influence of hypoxia on CGRP-, AM- and AMY-induced vasodilatation and on the affinity of the CGRP receptor blocker αCGRP8−37 in porcine coronary arteries. The effects were determined by functional pharmacology using the myograph technique. Moreover, in order to characterise the responses in more detail, we investigated the alterations in intracellular cAMP, mRNA levels for the CGRP1 and AM receptors, the role of endothelium, ATP-sensitive K-channels (KATP channels), nitric oxide synthase (NOS), peptidase inhibitors and possible release of adenosine and vasodilator prostanoids in porcine intramyocardial arteries.
Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, DC, U.S.A.
Tissue preparation
Porcine hearts were obtained fresh from the local abattoir (Roskilde Slagteriskole, Roskilde, Denmark) and transported to our laboratory in ice-cold physiological salt solution (154 mM NaCl, DAK, Denmark). Intramural segments of the distal part of the left anterior descending (LAD) coronary artery were isolated from the heart under a microscope. The arteries, 0.4–0.6 mm in outer diameter, were cut into ring segments, 1 mm long. Arteries were used immediately for in vitro pharmacology.
Vasomotor responses
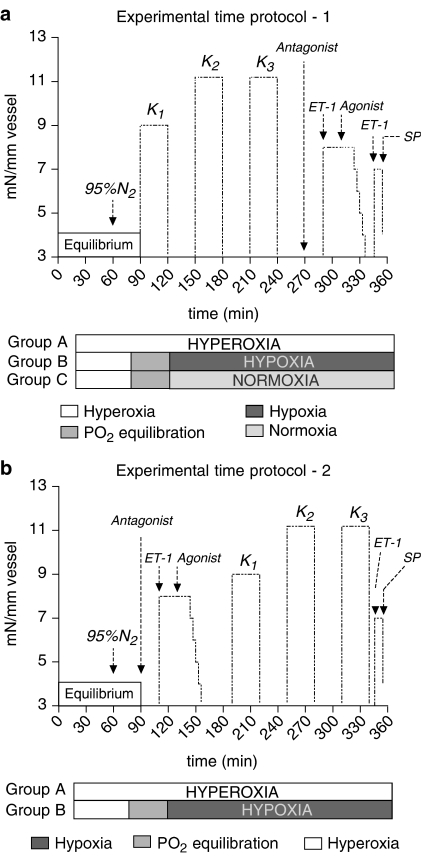
Each vessel segment was mounted in a temperature-controlled tissue bath (37°C) containing a buffer solution (mM): NaCl 119, NaHCO3 15, KCl 4.6, CaCl2 1.5, NaH2PO4 1.2, MgCl2 1.2 and glucose 5.5. Vessels with intact endothelium were confirmed by histological examination and substance P (10−6 M) evoked relaxation. Some artery segments were denuded of endothelium by inserting and rubbing the internal surface of the vessel segments with a human scalp hair (Prieto et al., 1991). Removal of endothelium was considered effective if substance P (10−6 M) no longer induced relaxation. To measure the isometric circular wall tension of the vessels, each segment was suspended between two L-shaped metal holders (0.1 mm in diameter) in a Myograph® (Model 700MO, J.P. Trading, Denmark). The vessels were stretched to their optimal lumen diameter in order to obtain the optimal condition for active tension development as previously described (Mulvany & Halpern, 1977), followed by an equilibration period of 1 h in 95% O2/5% CO2. The experimental time protocol is outlined in Figure 1a.
Figure 1.
(a, b) Two different experimental time protocols outlined with a common 60 min initial equilibration period with 95% O2/5% CO2 (hyperoxia), followed by gas mixture change to 95% N2/5% CO2 (hypoxia) in group B and 21% O2/5% CO2/74% N2 (normoxia) in group C, whereas group A continues with 95% O2/5% CO2 (hyperoxia). Arrows or abbreviations indicate the addition of KCl (K1–3), endothelin 1 (ET-1), agonist (αCGRP, AM or AMY), antagonist (αCGRP8–37) and substance P (SP).
The vessel segments were then tested in three settings: (A) hyperoxia/control was operationally defined as aeration with 95%O2/5%CO2, (B) hypoxia as aeration with 95% N2/5% CO2 (PO2<35 mmHg) and (C) normoxia as aeration with 21% O2/5% CO2/74% N2 (175 mmHg>PO2>125 mmHg). Oxygen tension was measured using an oxygen electrode and oxygen meter (Microelectrodes Inc., New Hampshire, U.S.A.) immersed in the organ bath. Aliquots of the bathing medium were removed by syringe, and PCO2 and pH were measured using a blood gas analyser (Radiometer, Denmark). Hypoxia in the bathing medium was reached in 22–27 min (n=10) after changing from 95% O2/5% CO2 to 95% N2/5% CO2, whereas normoxia was reached after 11–17 min (n=6) after switching gases. Thus after 30 min in (A) hyperoxia, (B) hypoxia or (C) normoxia, the vessels were exposed to a buffer solution high on KCl, obtained by supplementing the previously described buffer solution with KCl to a final concentration of 60 mM. Only vessel segments responding with a reproducible potassium-induced contraction after washout with normal buffer solution were used for further investigation. Maximally, three KCl exposures were allowed per vessel.
The αCGRP, AM and AMY were added in cumulative concentrations from 10−11 to 10−6 M every 3 min to vessel segments, which had been precontracted for 20 min with endothelin-1 (ET-1, 10−7.5 M). The contraction induced by ET-1 was set arbitrarily to 100% and used as an internal standard to which the relaxant response in the same vessel segment was compared.
When testing the effect of the receptor antagonist αCGRP8–37, this fragment was added 20 min prior to precontraction. Thus, the vessels were exposed to hypoxia for up to 4.5 h totally in this protocol (Figure 1a).
The incubation periods were changed in an additional protocol (Figure 1b) investigating the effects of αCGRP (10−10–10−7 M) and αCGRP8–37 (10−6 M). Thus, after 1 h of equilibration followed by hyperoxia/hypoxia exposure for 30 min, the vessels were exposed to αCGRP8–37/ET-1/αCGRP and subsequently by up to three KCl buffer exposures.
According to current publications, the release of vasodilatatory agents in coronary artery during hypoxia is a possibility. The interest centres on adenosine and vasodilator prostanoids. Activation of KATP channels, peptidase inhibitors and NOS are other possibilities. Thus, in separate studies adenosine (10−7–10−3 M added every 3 min) concentration–response curves were generated using porcine arteries bubbled with 95% O2/5% CO2 and precontracted with ET-1 as mentioned above. In subsequent experiments the antagonistic effects of a selective A2A adenosine receptor antagonist CSC (10−3 M), the KATP channel inhibitor glibenclamide (10−5 M), the cyclooxygenase inhibitor indomethacin (10−3 M), a mixture of peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon and thiorphan) (10−5 M each) and L-NMMA (10−4 M) were studied with αCGRP response under hyperoxia and hypoxia. All antagonists were added 20 min prior to precontraction.
To study if possible increase in sensitivity under hypoxic conditions can also be observed with isoprenaline (Iso) (10−11–10−5 M) (a drug acting through cAMP) and sodium nitroprusside (SNP) (10−10–10−5 M) (a NO donor acting through cGMP), concentration–response curves were generated using the above-mentioned protocol during hyperoxia and hypoxia.
Measurements of cyclic AMP
Each vessel segment was stabilised in the above-described buffer solution bubbled with either 95% O2/5% CO2 or 95% N2/5% CO2 for more than 60 min before the experiment. αCGRP (10−9–10−7 M), AM (10−8–10−6 M) and AMY (10−8–10−6 M) were added for exactly 60 s to vessels which had been precontracted for 20 min with ET-1 (10−8 M). Vessel segments were removed and immediately soaked in acidic ethanol (ethanol 96%; HCl, 1 M (100 : 1)) as previously described and stored at −20°C before each segment was homogenised in a glass tube by a glass pestle. Following centrifugation for 20 min at 2000 × g, the supernatant was isolated and evaporated under a steam of N2 at 50°C. Using the nonacetylation protocol, the cAMP content was assayed by a RIA kit (Amersham, Denmark).
Real-time quantitative RT–PCR
Segments of porcine coronary arteries were bubbled with either 95% O2/5% CO2 or 95% N2/5% CO2 in buffer solution for 4 h, time equivalent to the vasomotor response studies (n=4 in both groups). Total RNA was isolated from coronary artery tissue with the use of TRIzol® Reagent (Introvitrogen™) according to the instructions provided by the manufacturer. First-strand cDNA was synthesised from 0.3 μg total RNA in 20 μl reaction volume using random hexamer as primers. Real-time quantitative PCR for CL receptor, RAMP1 and RAMP2 was performed based on GeneAmp® SYBR Green I assays using a GeneAmp® 5700 sequence-detection system (PE Biosystems, Applied Biosystems, Sweden), with β-actin as endogenous controls to standardise the amount of sample RNA added to a reaction. Primers for CL receptor, RAMP1, RAMP2 and β-actin were designed as follows: CL receptor forward: 5′-TGGCCAC AAATCCTGTTAGTTG-3′; reverse: 5′-CAAACACGGCCACCACAATA-3′; RAMP1 forward: 5′-CATCAGGAGCTATAAAGACCTCTCAGA-3′ and reverse 5′-CTGGTGGACTCCCAGGAAGA-3′. RAMP2 forward: WGATCMACTTTGCCAACTGCT-3′ and reverse: 5′-TGATCATGGCCAGRAGYACATC-3′ and β-actin 5′-CGGCCAGGTCATCACCAT-3′ and 5′-CCACGTCGCACTTCATGATC-3′. Wobble IUPAC-IUB symbols (Cornish-Bowden, 1985) are used in the RAMP2 primers, thus W (A or T), M (A or C), R (A or G) and Y (C or T). SYBR Green I assays were performed on all selected genes with SYBR Green PCR Master Mix (PE Biosystems). Each tube contained a total volume of 50 μl and the following: 1 × SYBR Green PCR Master Mix, 300 nM forward and reverse primers, and 6 μl of first-strand cDNA equivalent to 30 ng of total RNA. The thermal cycling parameters for PCR was 50°C for 2 min, 10 min at 95°C and 40 cycles for 15 s at 95°C, and 1 min at 60°C. All experiments were performed in duplicate.
To prove that the cDNA of β-actin, CL receptor, RAMP1 and RAMP2 were amplified with the same efficacy during real-time PCR, a standard curve were made in which the CT values were plotted against cDNA concentration on the basis of the following equation: CT=[log(1+E)]−1 log(concentration), where CT is the number of PCR cycles performed in one sample at a specific point of time, and E is the amplification efficiency with an optimal value of 1.
Dissociation curves ran immediately after the real-time PCR run, and possible nonspecific amplification could thus be detected. Agarose gel electrophoretic analysis was used to verify that the amplified product corresponded to the size predicted for the amplified fragment.
Drugs
The human forms of the peptides αCGRP, αCGRP8–37, AM, AMY and ET-1 were obtained from Bachem AG, Bubendorf, Switzerland. All peptides were dissolved in distilled water. Human serum albumin (0.2%) was added to the final concentration of all reagents in the tissue bath. (1-4-Chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid (indomethacin), 1,3,7-trimethyl-8-(3-chlorostyryl)xanthine (CSC), 5-chloro-N-[4-(cyclohexylureido-sulphonyl)phenethyl]-2-methoxybenzamide (glibenclamide), adenosine, 9,11-dideoxy-11α, 9α-epoxymethano-prostaglandin F2α (U46619), the peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon and thiorphan), SNP dihydrate, isoprenaline hydrochloride, L-NMMA were obtained from Sigma, U.S.A.
Data analysis and statistics
The concentration–response curves for αCGRP, AM and AMY were analysed by iterative nonlinear regression analysis and the sensitivity to agonists expressed as pEC50 (−log of EC50; concentration of the agonist that produced 50% of the maximal response), using Graphpad Prism 4.00 (GraphPad software, U.S.A.). The relaxant responses of each peptide are expressed as a percentage of the contraction induced by ET-1 (10−7.5 M). Results are given as mean±s.e.m. (n), where n values represent the number of hearts from which arteries were isolated. Statistical evaluation was performed by means of one-way analysis of variance (ANOVA), followed by Dunnett's test (Winer, 1971) or by unpaired Student's t-test.
Where only a single concentration of antagonist was used, an apparent antagonist affinity was determined according to the Gaddum equation:
DR is the concentration ratio of the EC50 values in the presence and absence of the antagonist and [B] is the molar concentration of the antagonist. Where multiple concentrations of antagonists were used, pKB was evaluated by a Schild plot with slope constrained to −1, in the case that the unconstrained slope did not differ significantly from unity (Jenkinson, 1991; Jenkinson et al., 1995). P<0.05 was considered significant.
Results
The porcine coronary arteries were tested in three settings: (A) Hyperoxia resulted inPO2=678±30 mmHg, PCO2=33±3 mmHg and pH=7.4±0.05, n=12. (B) Hypoxia resulted in PO2=26±2 mmHg, PCO2=34±3 mmHg and pH=7.4±0.07, n=12. (C) Normoxia resulted in PO2=147±8 mmHg, PCO2=33±3 mmHg and pH=7.4±0.09, n=8.
Precontraction
U46619 (10−7–10−6 M), KCl (40–60 mM) and ET (10−7.5 M) were compared as precontracting agents at different oxygen supply. During hypoxic conditions U46619 (10−7–10−6 M) and KCl (40–60 mM) produced lower precontraction levels compared to hyperoxia. It also appears that the contraction produced by ET-1 was slightly reduced under hypoxic conditions compared to hyperoxia and normoxia, although not significant. However, the precontraction obtained 10 min after application of ET-1 (10−7.5 M) (8±1.2 mN mm−1 at 95% O2; n=10 and 6.9±1.1 mN mm−1 at 95% N2; n=10) was relatively stable and lasted for at least 1 h (6.5±1.5 mN mm−1 at 95% O2; n=10 and 5.8±1.4 mN mm−1 at 95% N2; n=10). To determine how much influence the level of precontraction had on the subsequent αCGRP concentration–response curves, different concentrations of ET-1 were tested during hyperoxia. Precontraction with ET-1 (10−7 M) induced 9.6±1.7 mN mm−1 (n=8), whereas ET-1 (10−8 M) induced 6.1±1.6 mN mm−1 (n=10). The subsequent αCGRP concentration–response curves generated from these experiments were almost identical with the ET-1 (10−7.5 M)/αCGRP curves (data not shown).
αCGRP-induced vasodilatation
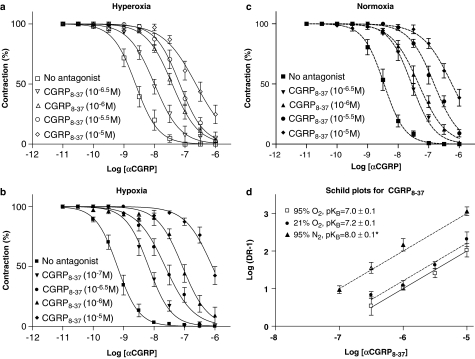
αCGRP induced concentration-dependent relaxation of the porcine coronary arteries. The results clearly indicate that the effect of αCGRP is enhanced under hypoxic conditions (pEC50=9.2±0.03, mean±s.e.m.) compared to hyperoxic (pEC50=8.6±0.1) and normoxic (pEC50=8.5±0.05) conditions (Figure 2a–c and Table 1). At 95, 21 and 0% oxygen supply, preincubation with αCGRP8–37 induced concentration-dependent (10−7–10−5 M) rightward shift of the αCGRP concentration–response curves (Figure 2a–c). The Schild-plot-derived pKB values increased significantly under hypoxia compared to hyperoxia and normoxia, indicating an approximately 10-fold affinity increase for αCGRP8–37 during hypoxia (Figure 2d). The concentration–response curves obtained in the endothelium-denuded group were almost identical compared to experiments with endothelium intact vessels (Table 1). The increased potency of αCGRP and αCGRP8–37 (10−6 M) under hypoxia was also observed in the experiments performed using the time protocol shown in Figure 1b. Thus, under hyperoxia the pEC50 was 8.5±0.1, mean±s.e.m. (n=6) and 7.6±0.1 (n=8) for αCGRP and αCGRP8–37 (10−6 M), respectively, whereas the pEC's50 in the hypoxia experiments were 9.0±0.1 (n=7) and 7.2±0.1 (n=6), respectively (concentration–response curves not shown), almost identical results compared to the data obtained using the time protocol shown in Figure 1a.
Figure 2.
(a–c) αCGRP (10−11–10−6 M) concentration–response curves under hyperoxia, hypoxia or normoxia in the presence or absence of αCGRP8–37 (10−7–10−5 M) in vessels with endothelium. Relaxant responses are given as percentage of precontraction induced by ET-1 (10−7.5 M). Points represent mean values and vertical lines indicate s.e.m. (d) Schild plots for αCGRP8–37 (10−7–10−5 M) tested with αCGRP as agonist in isolated porcine LAD coronary arteries during hyperoxia, normoxia or hypoxia. Each point represents mean values and vertical lines indicate s.e.m. *Significant difference between the pKB values obtained under hypoxia versus hyperoxia and normoxia.
Table 1.
pEC50 values of αCGRP, AM and AMY and the effect of the antagonist αCGRP8–37
| Agonist | Antagonist (log M) | Oxygen supply | Endothelium | n | pEC50 | Apparent pKB |
|---|---|---|---|---|---|---|
| αCGRP | — | 95% | + | 12 | 8.6±0.1 | — |
| αCGRP8–37 (−6.5) | 95% | + | 11 | 8.0±0.05a | — | |
| αCGRP8–37 (−6) | 95% | + | 10 | 7.6±0.03a | — | |
| αCGRP8–37 (−5.5) | 95% | + | 7 | 7.2±0.04a | — | |
| αCGRP8–37 (−5) | 95% | + | 9 | 6.6±0.06a | — | |
| — | 95% | − | 12 | 8.6±0.07 | — | |
| αCGRP8–37 (−6) | 95% | − | 11 | 7.5±0.05a | 7.1 (7.0–7.2) | |
| — | 21% | + | 8 | 8.5±0.05 | — | |
| αCGRP8–37 (−6.5) | 21% | + | 7 | 7.6±0.06a | — | |
| αCGRP8–37 (−6) | 21% | + | 7 | 7.3±0.08a | — | |
| αCGRP8–37 (−5.5) | 21% | + | 6 | 6.8±0.07a | — | |
| αCGRP8–37 (−5) | 21% | + | 8 | 6.2±0.06a | — | |
| — | 0% | + | 12 | 9.2±0.03b | — | |
| αCGRP8–37 (−7) | 0% | + | 10 | 8.2±0.03a | — | |
| αCGRP8–37 (−6.5) | 0% | + | 7 | 7.6±0.05a | — | |
| αCGRP8–37 (−6) | 0% | + | 13 | 7.1±0.05a | — | |
| αCGRP8–37 (−5) | 0% | + | 12 | 6.1±0.05a | — | |
| — | 0% | − | 11 | 9.1±0.04b | — | |
| αCGRP8–37 (−6) | 0% | − | 11 | 7.2±0.04a | 7.9 (7.8–8.0)b | |
| AM | − | 95% | + | 8 | 6.9±0.04 | — |
| αCGRP8–37 (−6.5) | 95% | + | 9 | 6.2±0.05a | 7.1 (7.0–7.2) | |
| — | 0% | + | 8 | 7.2±0.04 | — | |
| αCGRP8–37 (−6.5) | 0% | + | 9 | 6.0±0.05a | 7.6 (7.5–7.7)b | |
| AMY | — | 95% | + | 8 | 6.3±0.06 | — |
| αCGRP8–37 (−7) | 95% | + | 8 | 6.0±0.06a | 7.0 (6.7–7.2) | |
| — | 0% | + | 8 | 6.8±0.02b | — | |
| αCGRP8–37 (−7) | 0% | + | 8 | 5.9±0.11a | 7.9 (7.6–8.1)b |
Where only a single concentration of antagonist was used, apparent pKB values have been calculated for αCGRP8–37. Experiments without antagonists are shown with bold characters.
Significant difference between values compared with the above underlined pEC50 value.
Significant difference between the hyperoxia/normoxia and the hypoxia groups.
AM- and AMY-induced vasodilatation
As for αCGRP hypoxia altered the pEC50 values for AMY, whereas the AM response was not significantly changed (Figure 3a and b). Using αCGRP8–37 in 10−7 and 10−6.5 M, the apparent pKB values increased significantly during hypoxia compared with hyperoxic conditions for both peptides (Table 1).
Figure 3.
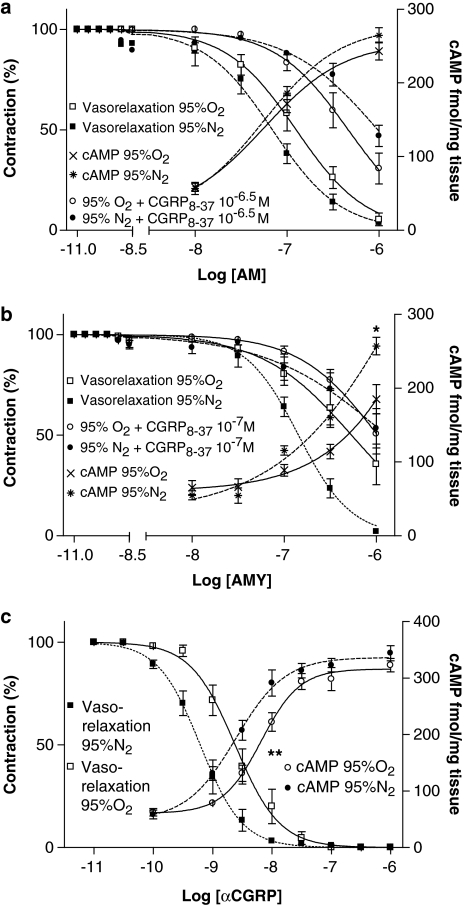
(a–c) cAMP production concentration–response curves during hyperoxia (95% O2/5% CO2) and hypoxia (95% N2/5% CO2) stimulated by AM (a), AMY (b) or αCGRP (c), illustrated together with the concentration–response curves for AM (a), AMY (b) or αCGRP (c) with or without αCGRP8–37. Relaxant responses are given as percentage of precontraction induced by ET-1 (10−7.5 M). Data are mean±s.e.m. (n=6 in the cAMP groups). *Significant difference between the AMY (10−6 M)-induced cAMP production in the hypoxia group versus the hyperoxia group. **Significant difference between the αCGRP-induced cAMP production (pEC50 values) in the hypoxia group versus the hyperoxia group. All other comparisons are made by EC50 values and appear in Table 1.
cAMP production
In control vessels without agonist the basal cAMP concentration was 65±9 fmol mg−1 tissue (n=6), during hyperoxia versus 55±6 fmol mg−1 tissue (n=6) during hypoxia (NS). Thus, whereas hypoxia by itself had no significant effect on arterial wall cAMP concentrations, these were significantly increased in presence of αCGRP. αCGRP also showed higher potency during hypoxia (pEC50=8.6±0.1 during hypoxia versus 8.2±0.1 during hyperoxia (Figure 3c)). The same effects are seen for AMY (10−6 M) (Figure 3b), while no significant changes in cAMP concentrations or potency are demonstrated for AM (Figure 3a).
Role of adenosine, KATP channels, vasodilator prostanoids, NOS and peptidase inhibitors
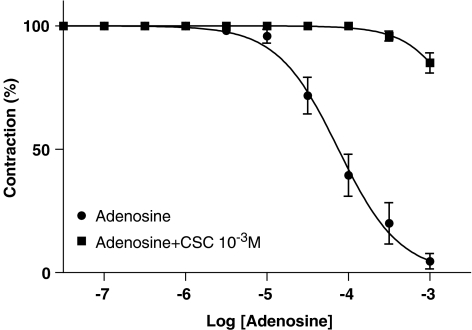
Adenosine induced concentration-dependent relaxation with pEC50 values of 4.1±0.1 (n=6) mean±s.e.m. (Figure 4). Preincubation with CSC (10−3 M, n=6) induced the expected pronounced rightward shift of the adenosine concentration–response curve.
Figure 4.
Adenosine concentration–response curves during hyperoxia with and without CSC (10−3 M). Relaxant responses are given as percentage of precontraction induced by ET-1 (10−7.5 M). Points represent mean values and vertical lines indicate s.e.m. (n=6 in each group).
In subsequent studies we found no significant alteration of the αCGRP concentration–response curves under hypoxia and hyperoxia (Figure 2a and b) despite preincubation with CSC (10−3 M, n=8), KATP channel inhibitor glibenclamide (10−5 M, n=7), the cyclooxygenase inhibitor indomethacin (10−3 M, n=8), L-NMMA (10−4 M, n=8) or peptidase inhibitors (n=8) (data not shown).
Iso- and SNP-induced vasodilatation
Both Iso and SNP induced concentration-dependent relaxation of the porcine coronary arteries. But, whereas the effect of Iso is enhanced under hypoxic conditions (pEC50=7.9±0.09; n=6 during hypoxia versus pEC50=7.1±0.1; n=6 during hyperoxia (P<0.05)), the SNP response (pEC50=6.2±0.12, n=5 during hypoxia versus pEC50=6.4±0.13, n=6 during hyperoxia (NS)) is not (data not shown).
Real time RT–PCR
The standard curve was straight for all primers employed, yielding slopes between 2.8 and 3.2, indicating replication efficiencies close to 1 (a replication efficiency of unity would yield a slope of 3.3). All standard curves regressed with correlations between 0.98 and 0.99, indicating a uniform PCR product independent of cDNA concentrations. Electrophoresis verified only one product for each primer pair at the expected size (data not shown).
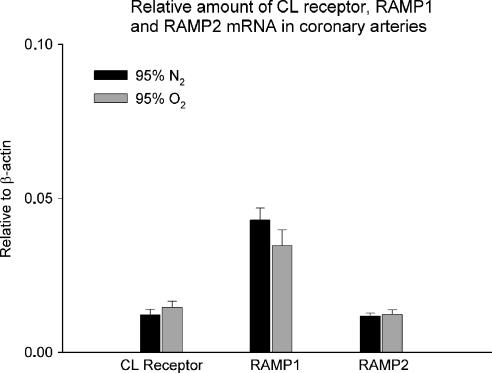
The β-actin was used as internal reference and relative amounts of mRNA for the CL receptor, RAMP1 and RAMP2 are presented in Figure 5. The mRNA for RAMP1 was higher than mRNA for CL receptor and RAMP2 in the porcine coronary arteries, but there was no difference between the hypoxic and hyperoxic conditions.
Figure 5.
Amount of CL receptor, RAMP1 and RAMP2 mRNA in porcine coronary arteries in buffer solution exposed to either 95% O2/ 5% CO2 or 95% N2/ 5% CO2 for 4 h and relative to β-actin mRNA levels. Data are mean±s.e.m. (n=6 in each group).
Discussion
This paper reports three new observations: (1) hypoxia results in increases in the potency of both αCGRP- and AMY- but not AM-induced vasodilatation, (2) increases in cAMP formation induced by αCGRP and AMY but not AM and (3) a 10-fold increase in affinity of αCGRP8–37 during hypoxic conditions.
Hypoxia strongly potentiates the vasorelaxant response of αCGRP and AMY, but does not alter the effect of AM significantly. In the early 1990s Kwan et al. (1990) described similar shift of the αCGRP concentration–response curve during hypoxia as compared to hyperoxia in sheep coronary arteries, but the phenomenon was not investigated further.
The increase in agonist potency during hypoxic conditions can be explained by one or more of the following possibilities alone or in combinations: (1) changes in signal transduction, (2) increase in number of receptors, (3) participation of another vasodilator agonist released from the vessel by hypoxia (e.g. adenosine), (4) peptidase inhibitors and/or (5) conformational changes in the receptor itself induced by hypoxia.
In the porcine coronary arteries, CGRP uses an NO- and endothelium-independent pathway, which correlates closely with a rise in intracellular cAMP (Kageyama et al., 1993; Yoshimoto et al., 1998; Wisskirchen et al., 1999). Whereas hypoxia has no effect on arterial cAMP levels in the present study, it significantly potentiates the production of cAMP stimulated by αCGRP and AMY, while AM shows no change in cAMP production. Comparing the estimated pEC50 values obtained from the concentration–response curves (Table 1 and Figure 3c), hypoxia increases the vasorelaxation of αCGRP four-fold and the cAMP production two-fold. Since the enhancement in the effect of CGRP is reflected by an increase in CGRP-induced accumulation of cAMP, it seems probable that the changes occur at the receptor level or anyway before cAMP production. Probably the mechanisms reside in the smooth muscle cells since endothelium denudation has no effect on the αCGRP-induced vasorelaxation.
Quantitative real-time PCR shows that acute hypoxia does not increase expression of mRNAs for CGRP or AM receptors, which is not unexpected, since others (Moller et al., 2002) have found that induction requires a time span of at least 7 h. However, since recruitment of receptor proteins within the applied time frame from intracellular compartments may be a possibility, Western blotting or immunofluorescence experiments could have been relevant. But so far no specific antibodies are commercially available for the porcine CL receptor or RAMPs. Interestingly, higher levels of RAMP1 mRNA are detected in porcine coronary artery compared to both RAMP2 and CL receptor mRNA. Knowing that RAMP1 is dominant over RAMP2 in binding the CL receptor (Buhlmann et al., 1999), it is likely that the formation of CGRP1 receptors is more common than AM receptors in porcine coronary arteries.
It is thought that compromised ATP synthesis during hypoxia or increased myocardial workload results in the release of adenosine from myocardial cells and dilates coronary arteries, helping to match coronary blood flow with metabolic demand (Berne, 1980; Mubagwa et al., 1996). Adenosine release from the arteries may therefore potentially be involved in the increased potency of CGRP during hypoxia. Although four adenosine receptor subtypes (i.e. A1, A2A, A2B and A3) have been cloned from various tissues, the vasodilatation elicited by adenosine in porcine coronary arteries has been shown predominantly to be induced by the A2A receptor (Lew & Kao, 1999; Hein et al., 2001). But, even though the commercially available A2A receptor antagonist CSC (10−3 M) induces potent adenosine blocking, it has no effect on the increased CGRP potency during hypoxia. The endothelium, KATP-channels and vasodilator prostanoids have also been suggested as contributors to hypoxic vasodilatation in the coronary arteries (Daut et al., 1990; Graser & Rubanyi, 1992; Mellemkjaer & Nielsen-Kudsk, 1994; Liu & Flavahan, 1997; Fukuda et al., 1999). But, neither removal of the endothelium nor preincubation with the KATP channel inhibitor glibenclamide or the cyclooxygenase inhibitor indomethacin changed the concentration–response curves for CGRP under hyperoxia/hypoxia. Neither peptidase inhibitors nor NOS seem to be involved in the mechanism of sensitisation of the vessels in hypoxia.
Using the current classification for CGRP1/CGRP2 receptors (for a review, see Juaneda et al., 2000) the apparent pKB and the pKB values obtained during hyperoxia in this study were in agreement with our previous studies (Hasbak et al., 2001, 2003), indicating that the vasorelaxant effect of αCGRP, AM and AMY can be explained by interaction with the CGRP1 receptor. Interestingly, the αCGRP8–37 antagonist gains affinity against all peptides under hypoxia compared to hyperoxia and normoxia, indicating that this antagonist has variable affinities for the CGRP1 receptor depending on the oxygen level in the experimental setting. Although αCGRP8–37 gains affinity against all peptides, it should be noticed that the apparent pKB for AM/αCGRP8–37 is significantly lower than the pKB values for CGRP/αCGRP8–37 and AMY/αCGRP8–37. Since αCGRP8–37 is a fragment of αCGRP, it is likely that both have the same affinity at the receptor site, and thus that αCGRP has increased affinity at the CGRP1 receptor during hypoxia as well.
When co-expressed with RAMPs, the calcitonin (CT) receptor functions as an AMY receptor (Poyner et al., 2002). But porcine CT receptor mRNA is not present in the LAD coronary arteries (Hasbak et al., 2001). So considering that the AMY vasorelaxant response and the AMY-induced cAMP production are potentiated by hypoxia and blocked by the αCGRP8–37 with identical pKB values as for αCGRP/αCGRP8–37, it is very likely that AMY mediates vasorelaxation via the CGRP1 receptor.
While hypoxia increases both αCGRP and AMY agonist potencies, in producing coronary vasodilation and cAMP generation, the effects produced by AM are not potentiated despite a substantial expression of RAMP2 (Figure 5). Hypoxia also increases the antagonist potency of αCGRP8–37 against all peptide agonists, including AM. The mechanisms underlying these changes remain unclear. In particular: αCGRP and AMY-induced effects are potentiated via increased production of cAMP. Apparently this is not due to increase in the expression of the receptors. On the other hand, the failure of hypoxia to increase AM-mediated effects is probably due to a different signal transduction mechanism activated by AM, as compared to αCGRP or AMY. The mechanisms via which AM can elicit vascular relaxation in the porcine coronary arteries are incomplete understood, but are known to involve both the CGRP and AM receptors as AM has been shown to induce vascular relaxation via either CGRP8–37-sensitive or AM22–52-sensitive mechanisms (Poyner et al., 2002). Furthermore, there is evidence that AM can act via an endothelium-dependent (NO-dependent) mechanism (Yoshimoto et al., 1998), which then relaxes the smooth muscle cells through activation of guanylate cyclase and accumulation of cGMP, which is in contrast to αCGRP and AMY.
This is indirectly supported by our Iso and SNP data. Acting through cAMP Iso shows increased sentisation during hypoxia, confirming the data of Fukuda et al. (1999), whereas SNP (a NO donor acting through cGMP) was unaffected by hypoxia. Perhaps the weak AM response during hypoxia can be explained by interaction with AM receptors activating NO/cGMP-dependent pathways compared to the cAMP-dependent pathway activated by the binding of CGRP/AMY to the CGRP1 receptor. Taken together, these results indicate a complexity of the receptor system(s) for the CGRP superfamily of peptides in contrast to what we have previously suggested (Hasbak et al., 2001).
An explanation for the increase in αCGRP/αCGRP8–37 potency is changes in receptor conformation during hypoxia. It is possible that hypoxia alters the CGRP1 receptor-binding site, thereby increasing the affinity of αCGRP and AMY and maybe also AM for the receptor compared to binding in its normoxic state. A hypoxia-induced modification of the CGRP1 receptor recognition site may be due to change in allosteric structure of the receptor, as suggested by Fritz et al. (1996).
In conclusion, hypoxic incubation potentiates the relaxation effect and cAMP production of CGRP and amylin in rings of porcine coronary arteries in vitro. This is an endothelium-independent effect, thus occurring in the smooth muscle cells. It is neither caused by the release of adenosine nor vasodilator prostanoids, and is not due to KATP channels, NOS, peptidae inhibitors or related to changes in CGRP or AM receptor mRNA. Moreover, αCGRP8–37 showed increased affinity at the CGRP1 receptor during hypoxia possibly due to a conformational change at the CGRP1 receptor site.
Possible physiological/pathophysiological implications
The hypoxia-induced potentiation of the CGRP and AMY vasorelaxant effect in the coronary vascular bed may be a compensatory haemodynamic mechanism to protect the hypoxic myocardium. This phenomenon may be a normal physiological response in the coronary circulation for many vasoactive substances since similar augmentation of the coronary vasorelaxant responses during hypoxia has previously been described, for example, adenosine, noradrenalin (Kwan et al., 1989), neuropeptide Y (Kwan et al., 1990), nitroglycerin (Fukuda et al., 1994) and Iso (Fukuda et al., 1999). Allosteric conformational changes of receptors resulting in increased affinity for signal molecules are possibly a general phenomenon in hypoxic environment.
The increased CGRP-receptor affinity of αCGRP8–37 is not only scientifically interesting but may also have clinical implications as well. Nonpeptide CGRP receptor antagonists are in advanced clinical development for migraine, and it is indeed important to determine if such CGRP receptor antagonists have increased affinity for the CGRP receptor during hypoxia. The implications of hypoxia-induced increased affinity of antagonists must be considered seriously as this may indicate a potential therapeutical problem for patients with ischaemic heart disease.
Acknowledgments
The study was supported by the following foundations: ‘The Danish Heart Foundation', Grant No. 01-2-2-73-22943; ‘The Danish Hospital Foundation for Medical Research', ‘The Danish Medical Association Research Fund', ‘Lundbeck Foundation' and ‘Danish Medical Research Council'. We thank Kirsten Busk, Majken Gudmundsson and Helle Ludvig for excellent technical assistance.
Abbreviations
- AM
adrenomedullin
- AMY
amylin
- cAMP
cyclic adenosine mono-phosphate
- CGRP
calcitonin gene-related peptide
- CL receptor
calcitonin-like receptor
- CT
calcitonin
- DMSO
dimethyl sulphoxide
- Iso
isoproterenol
- KATP channels
ATP-sensitive K-channels
- LAD
left anterior descending
- L-NMMA
NG-monomethyl-L-arginine
- NOS
nitric oxide synthase
- PCR
polymerase chain reaction
- RAMP
receptor-activity-modifying proteins
- RT
reverse transcriptase
- SNP
sodium nitroprusside
References
- BERNE R.M. The role of adenosine in the regulation of coronary blood flow. Circ. Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- BUHLMANN N., LEUTHAUSER K., MUFF R., FISCHER J.A., BORN W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- CASTLE A.L., KUO C.H., IVY J.L. Amylin influences insulin-stimulated glucose metabolism by two independent mechanisms. Am. J. Physiol. 1998;274:E6–E12. doi: 10.1152/ajpendo.1998.274.1.E6. [DOI] [PubMed] [Google Scholar]
- CORNISH-BOWDEN A. Nomenclature for incompletely specified bases in nucleic acid sequences: recommendations 1984. Nucleic Acids Res. 1985;13:3021–3030. doi: 10.1093/nar/13.9.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUT J., MAIER-RUDOLPH W., VON BECKERATH N., MEHRKE G., GUNTHER K., GOEDEL-MEINEN L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- DEEMS R.O., DEACON R.W., YOUNG D.A. Amylin activates glycogen phosphorylase and inactivates glycogen synthase via a cAMP-independent mechanism. Biochem. Biophys. Res. Commun. 1991;174:716–720. doi: 10.1016/0006-291x(91)91476-s. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., HENKE H., LUNDBERG J.M., PETERMANN J.B., HOKFELT T., FISCHER J.A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., KALLNER G., LUNDBERG J.M. Capsazepine-sensitive release of calcitonin gene-related peptide from C-fibre afferents in the guinea-pig heart by low pH and lactic acid. Eur. J. Pharmacol. 1993;238:311–316. doi: 10.1016/0014-2999(93)90862-c. [DOI] [PubMed] [Google Scholar]
- FRITZ K.I., GROENENDAAL F., MCGOWAN J.E., MISHRA O.P., DELIVORIA-PAPADOPOULOS M. Effect of cerebral hypoxia on NMDA receptor binding characteristics after treatment with 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) in newborn piglets. Brain Res. 1996;729:66–74. [PubMed] [Google Scholar]
- FUKUDA S., SAKUMA K., TSUKUI A., FUJIWARA N., TANAKA T., FUJIHARA H., TORIUMI T., SHIMOJI K. Hypoxia modifies the vasodilatory effects of nitroglycerin, prostaglandin E1, and hydralazine on isolated porcine coronary arteries. J. Cardiovasc. Pharmacol. 1994;23:852–858. doi: 10.1097/00005344-199405000-00024. [DOI] [PubMed] [Google Scholar]
- FUKUDA S., TORIUMI T., XU H., KINOSHITA H., NISHIMAKI H., KOKUBUN S., FUJIWARA N., FUJIHARA H., SHIMOJI K. Enhanced beta-receptor-mediated vasorelaxation in hypoxic porcine coronary artery. Am. J. Physiol. 1999;277:H1447–H1452. doi: 10.1152/ajpheart.1999.277.4.H1447. [DOI] [PubMed] [Google Scholar]
- GRASER T., RUBANYI G.M. Different mechanisms of hypoxic relaxation in canine coronary arteries and rat abdominal aortas. J. Cardiovasc. Pharmacol. 1992;20 Suppl 12:S117–S119. doi: 10.1097/00005344-199204002-00033. [DOI] [PubMed] [Google Scholar]
- HASBAK P., OPGAARD O.S., ESKESEN K., SCHIFTER S., ARENDRUP H., LONGMORE J., EDVINSSON L. Investigation of CGRP receptors and peptide pharmacology in human coronary arteries. Characterisation with a non-peptide antagonist. J. Pharmacol. Exp. Ther. 2003;304:326–333. doi: 10.1124/jpet.102.037754. [DOI] [PubMed] [Google Scholar]
- HASBAK P., SAMS A., SCHIFTER S., LONGMORE J., EDVINSSON L. CGRP receptors mediating CGRP-, adrenomedullin- and amylin-induced relaxation in porcine coronary arteries. Characterization with ‘Compound 1' (WO98/11128), a non-peptide antagonist. Br. J. Pharmacol. 2001;133:1405–1413. doi: 10.1038/sj.bjp.0704210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIN T.W., WANG W., ZOGHI B., MUTHUCHAMY M., KUO L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J. Mol. Cell Cardiol. 2001;33:271–282. doi: 10.1006/jmcc.2000.1298. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol. Sci. 1991;12:53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H., BARNARD E.A., HOYER D., HUMPHREY P.P., LEFF P., SHANKLEY N.P. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. IX. Recommendations on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 1995;47:255–266. [PubMed] [Google Scholar]
- JUANEDA C., DUMONT Y., QUIRION R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol. Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M., YANAGISAWA T., TAIRA N. Calcitonin gene-related peptide relaxes porcine coronary arteries via cyclic AMP-dependent mechanisms, but not activation of ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 1993;265:490–497. [PubMed] [Google Scholar]
- KOBAYASHI K., KITAMURA K., HIRAYAMA N., DATE H., KASHIWAGI T., IKUSHIMA I., HANADA Y., NAGATOMO Y., TAKENAGA M., ISHIKAWA T., IMAMURA T., KOIWAYA Y., ETO T. Increased plasma adrenomedullin in acute myocardial infarction. Am. Heart J. 1996;131:676–680. doi: 10.1016/s0002-8703(96)90270-7. [DOI] [PubMed] [Google Scholar]
- KUREISHI Y., KOBAYASHI S., NISHIMURA J., NAKANO T., KANAIDE H. Adrenomedullin decreases both cytosolic Ca2+ concentration and Ca(2+)- sensitivity in pig coronary arterial smooth muscle. Biochem. Biophys. Res. Commun. 1995;212:572–579. doi: 10.1006/bbrc.1995.2008. [DOI] [PubMed] [Google Scholar]
- KWAN Y.W., WADSWORTH R.M., KANE K.A. Responsiveness of sheep isolated coronary artery rings under simulated myocardial ischaemia. Eur. J. Pharmacol. 1989;168:31–38. doi: 10.1016/0014-2999(89)90629-8. [DOI] [PubMed] [Google Scholar]
- KWAN Y.W., WADSWORTH R.M., KANE K.A. Effects of neuropeptide Y and calcitonin gene-related peptide on sheep coronary artery rings under oxygenated, hypoxic and simulated myocardial ischaemic conditions. Br. J. Pharmacol. 1990;99:774–778. doi: 10.1111/j.1476-5381.1990.tb13005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEW M.J., KAO S.W. Examination of adenosine receptor-mediated relaxation of the pig coronary artery. Clin. Exp. Pharmacol. Physiol. 1999;26:438–443. doi: 10.1046/j.1440-1681.1999.03054.x. [DOI] [PubMed] [Google Scholar]
- LIU Q., FLAVAHAN N.A. Hypoxic dilatation of porcine small coronary arteries: role of endothelium and KATP-channels. Br. J. Pharmacol. 1997;120:728–734. doi: 10.1038/sj.bjp.0700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MELLEMKJAER S., NIELSEN-KUDSK J.E. Glibenclamide inhibits hypoxic relaxation of isolated porcine coronary arteries under conditions of impaired glycolysis. Eur. J. Pharmacol. 1994;270:307–312. doi: 10.1016/0926-6917(94)90006-x. [DOI] [PubMed] [Google Scholar]
- MOLLER S., UDDMAN E., WELSH N., EDVINSSON L., ADNER M. Analysis of the time course for organ culture-induced endothelin ET(B) receptor upregulation in rat mesenteric arteries. Eur. J. Pharmacol. 2002;454:209–215. doi: 10.1016/s0014-2999(02)02499-8. [DOI] [PubMed] [Google Scholar]
- MUBAGWA K., MULLANE K., FLAMENG W. Role of adenosine in the heart and circulation. Cardiovasc. Res. 1996;32:797–813. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA M., TAKAHASHI K., MURAKAMI O., SHIRATO K., SHIBAHARA S. Induction of adrenomedullin by hypoxia in cultured human coronary artery endothelial cells. Peptides. 1999;20:769–772. doi: 10.1016/s0196-9781(99)00061-3. [DOI] [PubMed] [Google Scholar]
- PITTNER R.A., ALBRANDT K., BEAUMONT K., GAETA L.S., KODA J.E., MOORE C.X., RITTENHOUSE J., RINK T.J. Molecular physiology of amylin. J. Cell Biochem. 1994;55 Suppl:19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., SEXTON P.M., MARSHALL I., SMITH D.M., QUIRION R., BORN W., MUFF R., FISCHER J.A., FOORD S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- PRIETO D., BENEDITO S., NYBORG N.C. Heterogeneous involvement of endothelium in calcitonin gene-related peptide-induced relaxation in coronary arteries from rat. Br. J. Pharmacol. 1991;103:1764–1768. doi: 10.1111/j.1476-5381.1991.tb09860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGO S., MINAMINO N., KANGAWA K., MIYAMOTO K., KITAMURA K., SAKATA J., ETO T., MATSUO H. Endothelial cells actively synthesize and secrete adrenomedullin [published erratum appears in Biochem Biophys Res Commun 1994;203:1363] Biochem. Biophys. Res. Commun. 1994a;201:1160–1166. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- SUGO S., MINAMINO N., SHOJI H., KANGAWA K., KITAMURA K., ETO T., MATSUO H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem. Biophys. Res. Commun. 1994b;203:719–726. doi: 10.1006/bbrc.1994.2241. [DOI] [PubMed] [Google Scholar]
- UREN N.G., SEYDOUX C., DAVIES G.J. Effect of intravenous calcitonin gene related peptide on ischaemia threshold and coronary stenosis severity in humans. Cardiovasc. Res. 1993;27:1477–1481. doi: 10.1093/cvr/27.8.1477. [DOI] [PubMed] [Google Scholar]
- WINER BJ. McGraw Hill, Inc.: New York; 1971. Statistical principles in experimental design, 2nd edn. [Google Scholar]
- WISSKIRCHEN F.M., GRAY D.W., MARSHALL I. Receptors mediating CGRP-induced relaxation in the rat isolated thoracic aorta and porcine isolated coronary artery differentiated by h(alpha) CGRP(8–37) Br. J. Pharmacol. 1999;128:283–292. doi: 10.1038/sj.bjp.0702764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]